Abstract
Objectives:
The aim of this study was to determine the appropriate propranolol dosing strategy for reducing heart rate in severely burned adults.
Methods:
A total of 26 patients (≥18 years) with burns covering ≥30% of the total body surface area were included in this IRB-approved study. Plasma propranolol concentrations were determined in a placebo group (n=10) or following one of three dosing strategies: Q6 (n=4), Q8 (n=6), and Q24 (n=6). Blood was collected just before dosing and at regular intervals over two dosing periods with corresponding heart rate and blood pressure recordings. Statistical significance was determined by one-way ANOVA followed by the appropriate post-hoc test.
Results:
Heart rate was 86±2 bpm for Q6, 93±3 bpm for Q8, and 90±4 bpm for Q24. The Q8 group had a significantly higher heart rate than the Q6 group (p=0.0001). Plasma propranolol concentrations were significantly higher in the Q6 dosing strategy than in the Q8 dosing strategy (p=0.02).
Conclusions:
Heart rate can be decreased to a similar degree with Q6 and Q24 dosing strategies, with the Q8 dosing strategy being less effective. Q6 dosing is recommended to maintain reduced heart rate throughout dosing periods.
Trial Registration:
NCT01902810 and NCT00675714.
Keywords: burns, β-adrenergic receptor antagonists, adult, heart rate
Introduction
Burn injuries covering greater than 30% of the total body surface area (TBSA) induce a hypermetabolic hypercatabolic state persisting for more than a year after the initial injury [1]. Release of catecholamines such as norepinephrine and epinephrine is significantly increased for a prolonged period [2]. The prolonged increase in circulating catecholamines is purported to be the driving force fueling burn-induced hypermetabolism. The elevations of circulating catecholamine levels and the resultant cardiac effects such as increased heart rate and cardiac work after severe burn injury have been well described [3, 4]. Since the late 1980s, researchers have investigated the use of propranolol, a nonspecific β-adrenergic receptor blocker, to blunt the action of catecholamines post burn [1, 5–7].
The majority of investigations on the use of propranolol to treat hypermetabolism and hypercatabolism after burn injury have been undertaken in pediatric patients [8]. In addition to having the expected effects of reducing heart rate and cardiac work post-burn, propranolol improved burn-induced perturbations in resting energy expenditure, lipolysis, and muscle protein breakdown [5, 7]. Williams et al. determined that a propranolol dose of 4 mg/kg/day was needed to reduce heart rate, cardiac output, and cardiac work without negatively affecting mean arterial pressure in severely burned pediatric patients [9]. In adults with large burns, propranolol administration has been shown to decrease blood loss and improve wound healing [10]. However, a retrospective analysis of propranolol administration in severely burned adults indicated that while relatively low doses of propranolol were required to reduce heart rate by 25%, adults had a greater incidence of hypotensive and bradycardiac episodes than that reported in children [11].
Propranolol is administered as a racemic mixture consisting of two chiral enantiomers: S(−) and R(+). Both enantiomers have beta-blocking abilities, but the S-enantiomer is purported to be 100 times more potent [12]. In healthy volunteers, concentrations of the R-enantiomer are usually lower than that of the S-enantiomer. Some have postulated that the R-enantiomer is metabolized much faster than the S-enantiomer while others have shown that there is no difference. There is also controversy regarding whether the rate of propranolol clearance is inversely proportional to age [13]. Little is known regarding the pharmacokinetics of propranolol in burned adults and the optimal administration frequency to maintain heart rate reduction. This study was undertaken to determine propranolol kinetics in severely burned adults receiving propranolol four times a day (every 6 hours), three times a day (every 8 hours), and once daily (every 24 hours).
Materials and Methods
Patients
This study included 26 adults with burns over at least 30% of TBSA who consented to an IRB-approved experimental protocol between the years of 2012 and 2016 (NCT01902810 and NCT00675714). Patients were at least 18 years old at the time of injury and were randomized to receive either standard of care plus placebo (placebo, n=10) or standard of care plus propranolol (n=16). The University of Texas Medical Branch Institutional Review Board (Galveston, TX) approved this study. Written informed consent was obtained from each patient prior to enrollment.
Plasma propranolol concentrations were determined for patients administered placebo or propranolol (Roxane Laboratories, Columbus, OH) using one of three dosing strategies. In the first dosing strategy, propranolol was given every 6 hours (Q6) as a liquid to decrease heart rate by 15%. In the second strategy, propranolol was given in liquid form every 8 hours (Q8) to reduce heart rate by 20%, with a maximum dose of 1 mg/kg of treatment weight. The final dosing strategy was administration of an extended-release capsule at a dose of 1 mg/kg/day (Q24) to reduce heart rate by 15%. All patients randomized to propranolol received the extended-release capsule when deemed appropriate by the attending physician, generally at a time close to the patient’s discharge from the ICU.
Propranolol Kinetics
After patients had been administered propranolol or placebo for a minimum of 3 days, plasma was collected throughout two dosing periods for determination of drug concentrations. Blood was collected immediately prior to dosing and at varying intervals after dosing (Table 1). Heart rate and blood pressure were recorded at the time of each blood draw. Blood pressure was measured using an arterial line or by blood pressure cuff. Thirteen samples were obtained from patients receiving propranolol Q6 or Q8 while 15 samples were obtained from patients receiving propranolol Q24. Concentrations of both propranolol enantiomers were determined by a high performance liquid chromatography method adapted from Sigma Aldrich [14]. Propranolol was extracted from plasma samples as previously described [9]. Separation and quantification of propranolol enantiomers were performed using a CHIROBIOTIC T column (25 cm × 4.6 mm, 5 μm) with a mobile phase of 15 mM ammonium formate in methanol, a flow rate of 1 ml/min, an injection volume of 5 μl, and a fluorescence detector with an excitation wavelength of 285 nm and emission wavelength of 350 nm. The inter-day coefficient of variation ranged from 0.84% to 6.9% and intra-day coefficient of variation ranged from 0.24% to 0.54%. The limit of detection was 0.02ng/ml and the limit of quantification was 0.05ng/ml.
Table 1.
Propranolol Kinetic Study Sampling Schedule
| Q6 | Q8 | Q24 |
|---|---|---|
| Pre-dose | Pre-dose | Pre-dose |
| 15min | 30min | 2.5hrs |
| 30min | 1hr | 5hrs |
| 1hr | 2hr | 6hrs |
| 2hrs | 4hr | 7hrs |
| 4hrs | 6hr | 12hrs |
| 6hrs (pre-dose 2) | 8hr (pre-dose 2) | 18hrs 24hrs (pre-dose 2) |
Calculations
Mean arterial pressure (MAP) was calculated as follows, and then used to calculate the rate pressure product:
Rate pressure product (RPP) served as an indicator of myocardial oxygen consumption and was calculated as follows:
Statistics
Data are expressed as a percentage or mean ± standard deviation, where appropriate. Statistical analysis was performed by one-way ANOVA followed by Tukey’s post-hoc test or Kruskal-Wallis followed by Dunn’s Comparison. Significance was accepted at p<0.05.
Results
Demographics
Patient characteristics are shown in Table 2. Patient groups were well-matched for age, sex, and burn severity. Due to study design, time between burn and admittance to the burn unit was significantly longer in the group receiving conventional propranolol four times a day as well as that receiving the extended-release tablet once daily (placebo: 1±1 days; Q6: 4±2 days; Q8: 0±0 days; Q24: 3±2 days; p=0.002). The average propranolol dose administered was significantly lower in both the Q8 and Q24 groups than the Q6 group (Q6: 4±1 mg/kg/day; Q8: 2±1 mg/kg/day; Q24: 3±1 mg/kg/day; p=0.02). The time from burn injury to the initial blood collection for pharmacokinetic analysis was significantly shorter in Q8 patients and longer in Q24 patients compared to the placebo group (placebo 11±4 days; Q6: 25±13 days; Q8: 6±1 days; Q24: 27±14 days; p=0.008).
Table 2.
Patient Characteristics
| Characteristic | Placebo (n=10) | Q6 (n=4) | Q8 (n=6) | Q24 (n=6) | P value |
|---|---|---|---|---|---|
| Age | 43±18 | 41±13 | 48±13 | 42±10 | NS |
| Sex, males, n (%) | 8 (88) | 3 (75) | 5 (83) | 6 (86) | NS |
| TBSA burn, % | 51±23 | 48±16 | 41±18 | 47±13 | NS |
| TBSA third, % | 15±17 | 27±23 | 17±15 | 16±22 | NS |
| Inhalation injury, n (%) | 5 (56) | 2 (50) | 1 (16) | 4 (57) | NS |
| Burn to admit, d | 1±1 | 4±2* | 0±0† | 3±2‡ | 0.002 |
| PPL dose, mg/kg/d | – | 4±1 | 2±1† | 3±1† | 0.02 |
| Burn to first PK blood collection, d | 11±4 | 25±13 | 6±1† | 27±14‡ | 0.008 |
Data are presented as mean ± standard deviation. TBSA, total body surface area; PPL, propranolol; PK, pharmacokinetic. Significantly different from
placebo,
Q6, or
Q8.
Drug Kinetics
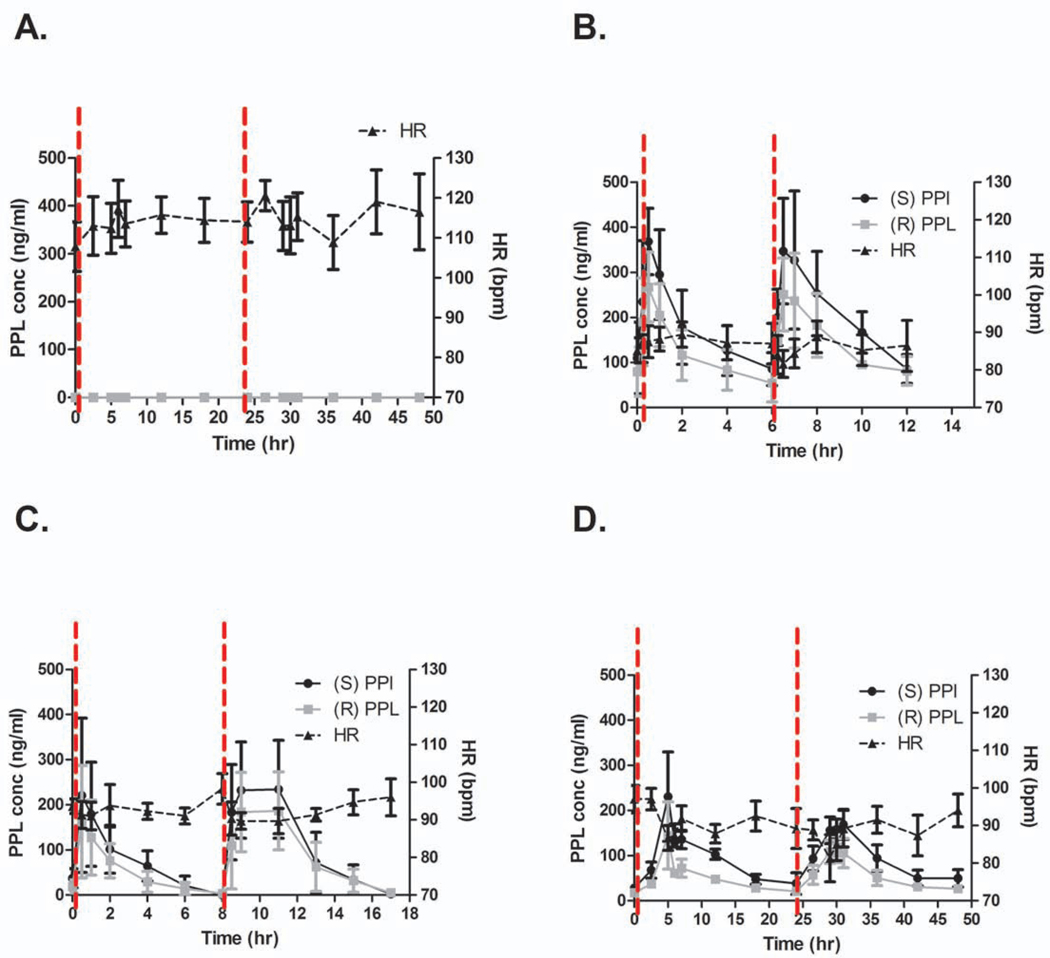
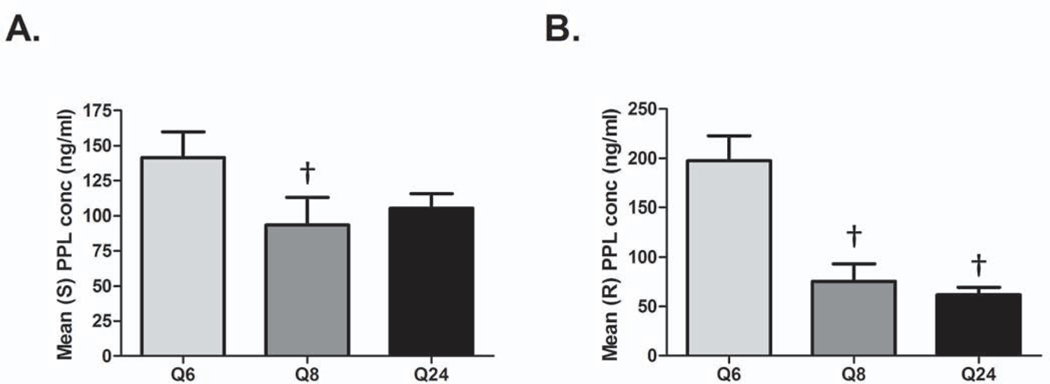
Peak propranolol concentrations were achieved 30 minutes to an hour after propranolol administration in the Q6 and Q8 groups and 4 to 5 hours after dosing in the Q24 group (Fig. 1). The plasma concentrations of both enantiomers were similar throughout the study period. Kinetic profiles for each group are shown in Figure 1. The effective plasma concentration for propranolol is between 30 and 80 ng/ml with maximal beta blockade occurring at 100 ng/ml. Although the peak concentration of propranolol was well above 100 ng/ml in all groups, only the Q6 and Q24 groups remained in the therapeutic window throughout the duration of the dosing period. In the Q8 group, propranolol concentrations fell to sub-therapeutic concentrations approximately 6 hours after dosing with nearly all patients having undetectable concentrations of propranolol prior to the second dose. Additionally, concentrations of both propranolol enantiomers were significantly lower in the Q8 group than the Q6 group (Fig. 2; p=0.02 for S-enantiomer; p<0.0001 for R-enantiomer). The concentration of the S-enantiomer was similar between Q6 and Q24, but the concentration of the R-enantiomer was significantly lower in the Q24 group than the Q6 group (Fig. 2; p=0.02).
Figure 1.
Propranolol decreases heart rate with greatest effect at Q6 and Q24 dosing. Plasma propranolol (PPL) concentrations are shown along with heart rate (HR) for (A) placebo and (B) Q6, (C) Q8, and (D) Q24 dosing strategies. Concentrations of S- and R-enantiomers are included in B-D. Vertical dashed lines indicate administration of the placebo or propranolol. Gray line in (A) shows that propranolol concentrations were undetectable for placebo patients.
Figure 2.
Propranolol enantiomer concentration varies by dosing strategy. Average (S) propranolol (PPL) (A) and (R) propranolol (B) concentration obtained for each dosing strategy is shown. †p<0.05 vs. Q6.
Heart Rate
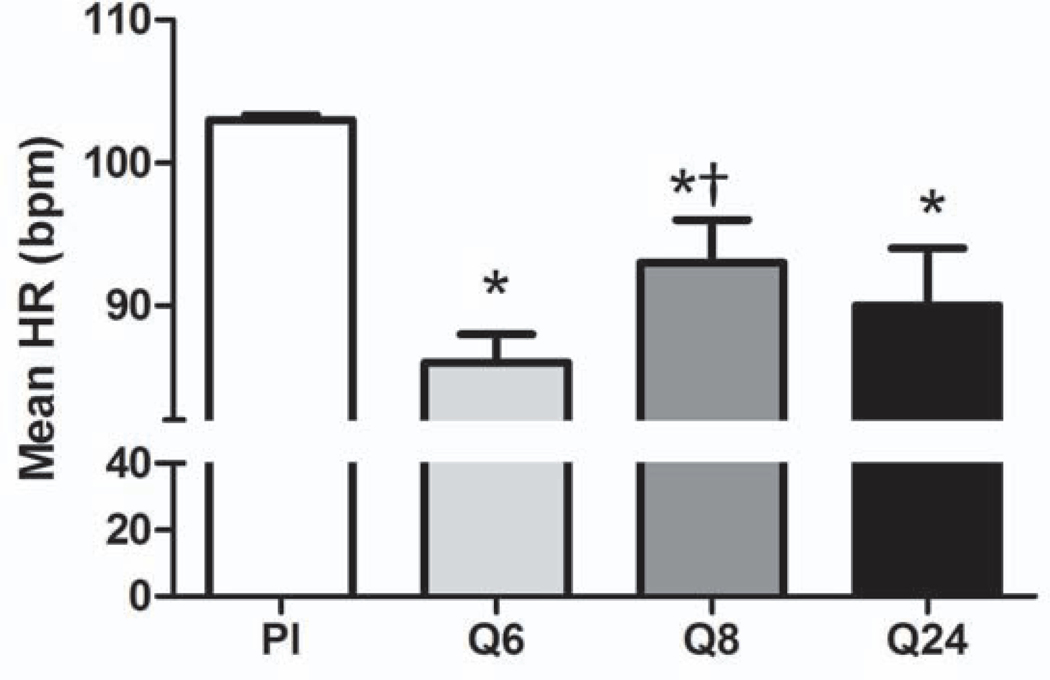
All three dosing strategies significantly reduced heart rate compared to placebo (Fig. 3; p<0.0001). Heart rate was reduced by 16% in the Q6 group, 9% in the Q8 group, and 12% in the Q24 group (placebo: 103±2 bpm; Q6: 86±2 bpm; Q8: 93±3 bpm; Q24: 90±4 bpm). However, heart rate was significantly greater in the Q8 group than in the Q6 group, indicating that this dosing regimen may not be as effective (p=0.0014).
Figure 3.
Heart rate is decreased with all three propranolol dosing strategies. HR, heart rate; BPM, beats per minute; Pl, placebo; *p<0.05 vs. placebo; †p<0.0.5 vs. Q6.
Rate Pressure Product
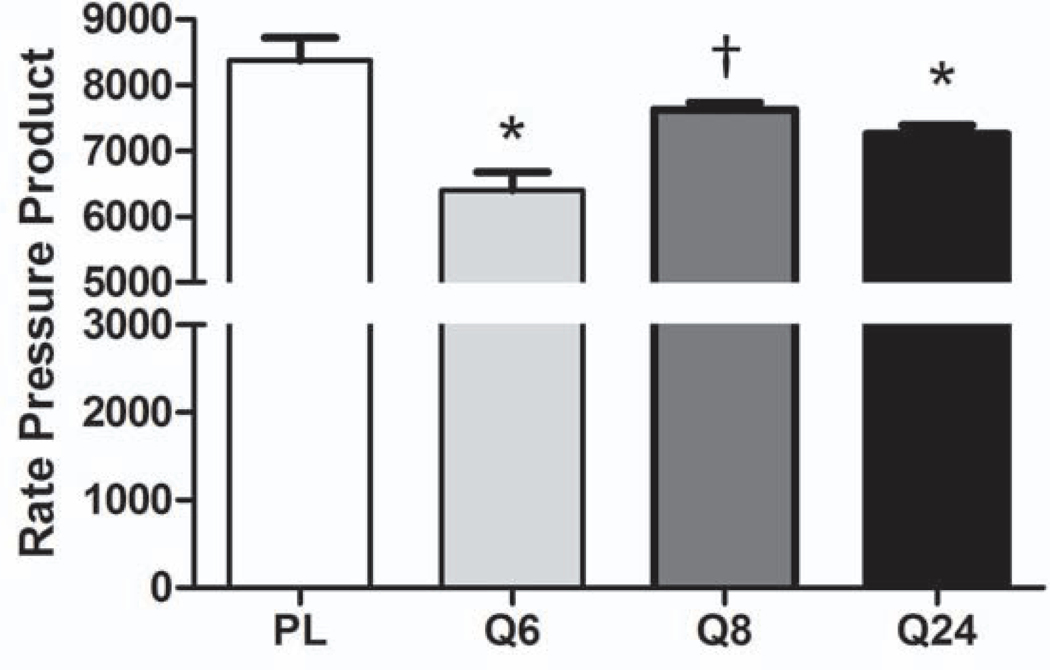
Since propranolol has been shown to reduce cardiac work in pediatric burn patients, we wanted to confirm that the proposed dosing schedules would have similar effects in the severely burned adult population {Williams, 2011 #47}. We used RPP, which is often used as an indicator of myocardial oxygen consumption, as a proxy for cardiac work. RPP was calculated by multiplying the mean arterial pressure, which was calculated from the reported blood pressure values, by the reported heart rate. RPP, was significantly lower in the Q6 and Q24 groups than in the placebo group (Fig. 4). The RPP in Q8 was similar to that in the placebo group and significantly higher than that in Q6 (Fig. 4; placebo: 8377±1350 mmHg*bpm; Q6: 6402±986 mmHg*bpm, p<0.0001 vs placebo; Q8: 7629±374 mmHg*bpm, p=0.005 vs Q6; Q24: 7263±504 mmHg*bpm, p=0.006 vs placebo).
Figure 4.
Rate pressure product is decreased with Q6 and Q24 propranolol dosing strategies. Pl, placebo; BPM, beats per minute; *p<0.05 vs. placebo; †p<0.0.5 vs. Q6.
Discussion
The sequelae that develop after severe burn injury prolong healing and contribute significantly to mortality and morbidity. Post-burn cardiac effects are biphasic. Initially, cardiac function is depressed in response to the shock of the injury. However, 48 to 72 hours post injury, heart rate and cardiac output increase to meet increased oxygen demand of the body [15, 16]. In addition to these changes, severely burned patients have increased resting energy expenditure and muscle wasting [4]. Although most of these metabolic changes have been observed and documented in pediatric patients, many of these sequelae are purported to contribute to morbidity and mortality in adult burn patients as well. It can be postulated that a prolonged increase in heart rate and cardiac work may have severe repercussions in the adult population such as myocardial infarction and heart failure.
We have shown that propranolol administration in pediatric patients mitigates the hypermetabolic and hypercatabolic responses to injury by blocking the over-activation of β-adrenergic receptors by elevated circulating catecholamine levels. Along with reducing heart rate and cardiac work, propranolol administration improves resting energy expenditure, lean body mass, bone mineral content, bone mineral density, lipolysis, and protein synthesis, while reducing inflammatory cytokine levels [7, 17]. These results indicate that propranolol reduces morbidity and may reduce mortality in this patient population. There are limited data on the effects of propranolol in the burned adult population, but propranolol has been shown to improve wound healing and limit blood loss [10].
While there has been a large body of work delineating the early (0 to 5 years post injury) effects of severe burn injury, few have investigated the long-term (>10 years post injury) effects of the perturbation of cardiac function. Duke et al. examined hospital morbidity and death data of adults 45 years and older burned between 1980 and 2012 in Western Australia and determined that mortality, hospital admissions, and length of hospital stay were increased in this population. Of note, these researchers only assessed these outcomes in relation to diseases of the circulatory system [18]. The same group showed that both minor and severe burns are associated with increased musculoskeletal morbidity [19]. These data provide evidence that severe burn injury can influence morbidity and mortality for many years after the injury as healed.
In this study we have shown that propranolol is most effective when administered four times a day (conventional formulation) or once daily (extended-release formulation). When propranolol was administered three times a day, propranolol concentrations were not only lower, but also fell below the therapeutic window prior to the next dosing. Additionally, these patients showed only a 9% reduction in heart rate compared to placebo-treated patients, and their average heart rates were significantly higher than those of patients receiving propranolol four times a day. Q8 administration also failed to significantly reduce RPP. Early studies investigating the pharmacokinetics of propranolol as a hypertensive therapy recommended dosing four times a day to maintain concentrations within the therapeutic window [20]. This dosing regimen should help clinicians avoid having to prescribe high propranolol doses that would result in peak concentrations that may lead to adverse effects.
The kinetic profile of the extended-release capsule was similar to that of the liquid administered four times daily (Q6) despite being given at a significantly lower dose. While the reduction in heart rate was lower in the Q24 group than in the Q6 group, it was not significant, indicating that the extended-release tablet is similarly effective. A similar trend was observed regarding RPP. Our results are in line with those of Serlin et al., who showed that long-acting propranolol was just as effective as conventional propranolol in hypertensive patients despite lower plasma concentrations [21]. Unlike patients receiving the Q8 dosing regimen, the majority of patients receiving propranolol once daily had plasma concentrations within the therapeutic window throughout the dosing period. There is evidence that propranolol’s rate of elimination is reduced when given chronically [20, 22]. Because the extended-release tablet is administered and kinetics are measured after the patient has received multiple doses of propranolol (either Q6 or Q8), it is probable that the decreased rate of elimination contributes to the maintenance plasma concentrations of propranolol despite a lower dose.
There have been numerous studies investigating propranolol pharmacokinetics in healthy patients as well as in patients with various diseases [12, 13, 20–23]. The consensus of the majority of these studies is that it is difficult to correlate the degree of beta blockade with plasma concentrations. Indeed, a given dose can yield dramatically different plasma concentrations across individuals [22, 23]. Thus, plasma propranolol concentrations cannot be used to determine the dose of propranolol required to have the desired effect. Additionally, some have reported that R-enantiomer concentrations can be more than 50% lower than those of the S-enantiomer, but this was not the case in our study. It has also been reported that clearance or elimination of the R-enantiomer is faster than the S-enantiomer, but again this was not observed in this patient population [13].
One limitation of this study is that we did not measure plasma concentrations of 4-hydroxypropranolol. As one of the main metabolites of propranolol, 4-hydroxypropranolol has been shown to have β-adrenergic receptor-blocking capabilities [24]. However, the half-life of this metabolite is much shorter than that of propranolol, and it may not contribute greatly to the reduction of heart rate that we have shown here.
Conclusions
All three dosing propranolol regimens significantly reduce heart rate compared to placebo in this patient population. However, conventional administration of propranolol four times a day more effectively reduces heart rate than administration three times a day. Administration of the extended-release formulation of propranolol is equally effective as conventional administration (4 times daily) in reducing heart rate. Similarly, both Q6 and Q24 regimens, but not Q8, significantly reduce RPP compared to placebo. Thus, to effectively reduce heart rate in severely burned patients, one should administer propranolol either four times a day for the conventional formulation or once daily for the extended-release or long-acting formulation.
Highlights.
Various propranolol dosing strategies and their effect on HR were investigated.
Conventional propranolol dosing Q8 was less effective than Q6 at reducing HR.
The extended release tablet is equally effective as Q6 dosing to reduce HR.
Acknowledgements
The authors thank Chris Nieten, Kaitlin Watson, Liz Montemayor, and Maricela Pantoja for their assistance in sample collection and processing.
Funding: This work was supported by grants from the National Institutes of Health [grant numbers P50GM060338, T32GM008256, R01GM112936, R01GM056687], NIDILRR [grant number 90DP0043-01-00], the Department of Defense [ grant number W81XWH-11-1-0835], and Shriners of North America [ grant numbers 84080, 80100, 84291, 80500, 84202, 71001, 79141, 71008]. CCF is an ITS Career Development Scholar and ANG is a scholar in the Translational Research Scholar Program supported, in part, by NIH KL2RR029875, NIH UL1RR029876, and NIH UL1TR001439.
Abbreviations:
- MAP
mean arterial pressure
- RPP
rate pressure product
- TBSA
total body surface area
Footnotes
Conflicts of interest: none
Reprints will not be ordered.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. The New England journal of medicine. 2001;345:1223–9. [DOI] [PubMed] [Google Scholar]
- [2].Kulp GA, Herndon DN, Lee JO, Suman OE, Jeschke MG. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock. 2010;33:369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Keck M, Herndon DH, Kamolz LP, Frey M, Jeschke MG. Pathophysiology of burns. Wien Med Wochenschr. 2009;159:327–36. [DOI] [PubMed] [Google Scholar]
- [4].Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PloS one. 2011;6:e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Herndon DN, Barrow RE, Rutan TC, Minifee P, Jahoor F, Wolfe RR. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Annals of surgery. 1988;208:484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Minifee PK, Barrow RE, Abston S, Desai M, Herndon DN. Improved myocardial oxygen utilization following propranolol infusion in adolescents with postburn hypermetabolism. Journal of pediatric surgery. 1989;24:806–10; discussion 10–1. [DOI] [PubMed] [Google Scholar]
- [7].Herndon DN, Rodriguez NA, Diaz EC, Hegde S, Jennings K, Mlcak RP, et al. Long-term propranolol use in severely burned pediatric patients: a randomized controlled study. Annals of surgery. 2012;256:402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Finnerty CC, Herndon DN. Is propranolol of benefit in pediatric burn patients? Advances in surgery. 2013;47:177–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Williams FN, Herndon DN, Kulp GA, Jeschke MG. Propranolol decreases cardiac work in a dose-dependent manner in severely burned children. Surgery. 2011;149:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ali A, Herndon DN, Mamachen A, Hasan S, Andersen CR, Grogans RJ, et al. Propranolol attenuates hemorrhage and accelerates wound healing in severely burned adults. Critical care (London, England). 2015;19:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brown DA, Gibbons J, Honari S, Klein MB, Pham TN, Gibran NS. Propranolol Dosing Practices in Adult Burn Patients: Implications for Safety and Efficacy. Journal of burn care & research : official publication of the American Burn Association. 2015. [DOI] [PubMed] [Google Scholar]
- [12].Barrett AM, Cullum VA. The biological properties of the optical isomers of propranolol and their effects on cardiac arrhythmias. British journal of pharmacology. 1968;34:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mehvar R, Brocks DR. Stereospecific pharmacokinetics and pharmacodynamics of beta-adrenergic blockers in humans. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2001;4:185–200. [PubMed] [Google Scholar]
- [14].HPLC Analysis of Propranolol Enantiomers on Astec® CHIROBIOTIC® T application for HPLC | Sigma-Aldrich. 2016. [Google Scholar]
- [15].Papp A, Uusaro A, Parviainen I, Hartikainen J, Ruokonen E. Myocardial function and haemodynamics in extensive burn trauma: evaluation by clinical signs, invasive monitoring, echocardiography and cytokine concentrations. A prospective clinical study. Acta anaesthesiologica Scandinavica. 2003;47:1257–63. [DOI] [PubMed] [Google Scholar]
- [16].Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–902. [DOI] [PubMed] [Google Scholar]
- [17].Baron PW, Barrow RE, Pierre EJ, Herndon DN. Prolonged use of propranolol safely decreases cardiac work in burned children. The Journal of burn care & rehabilitation. 1997;18:223–7. [DOI] [PubMed] [Google Scholar]
- [18].Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM. Understanding the long-term impacts of burn on the cardiovascular system. Burns : journal of the International Society for Burn Injuries. 2016;42:366–74. [DOI] [PubMed] [Google Scholar]
- [19].Randall SM, Fear MW, Wood FM, Rea S, Boyd JH, Duke JM. Long-term musculoskeletal morbidity after adult burn injury: a population-based cohort study. BMJ open. 2015;5:e009395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chidsey CA, Morselli P, Bianchetti G, Morganti A, Leonetti G, Zanchetti A. Studies of the absorption and removal of propranolol in hypertensive patients during therapy. Circulation. 1975;52:313–8. [DOI] [PubMed] [Google Scholar]
- [21].Serlin MJ, Orme ML, MacIver M, Green GJ, Sibeon RG, Breckenridge AM. The pharmacodynamics and pharmacokinetics of conventional and long-acting propranolol in patients with moderate hypertension. British journal of clinical pharmacology. 1983;15:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shand DG. Pharmacokinetics of propranolol: a review. Postgrad Med J. 1976;52 Suppl 4:22–5. [PubMed] [Google Scholar]
- [23].Chidsey C, Pine M, Favrot L, Smith S, Leonetti G, Morselli P, et al. The use of drug concentration measurements in studies of the therapeutic response to propranolol. Postgrad Med J. 1976;52 Suppl 4:26–32. [PubMed] [Google Scholar]
- [24].Von Bahr C, Hermansson J, Tawara K. Plasma levels of (+) and (−)-propranolol and 4-hydroxypropranolol after administration of racemic (+/−)-propranolol in man. British journal of clinical pharmacology. 1982;14:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]