Abstract
Background
Lung cancer screening, despite its proven mortality benefit, remains vastly underutilized. Previous studies examined knowledge, attitudes, and beliefs to better understand the reasons underlying the low screening rates. These investigations may have limited generalizability because of traditional participant recruitment strategies and examining only subpopulations eligible for screening. The current study used crowdsourcing to recruit a broader population to assess these factors in a potentially more general population.
Methods
A 31-item survey was developed to assess knowledge, attitudes, and beliefs regarding screening among individuals considered high risk for lung cancer by the United States Preventive Services Task Force. Amazon’s crowdsourcing platform (Mechanical Turk) was used to recruit subjects.
Results
Among the 240 respondents who qualified for the study, 106 (44%) reported knowledge of a screening test for lung cancer. However, only 36 (35%) correctly identified low-dose CT scanning as the appropriate test. A total of 222 respondents (93%) reported believing that early detection of lung cancer has the potential to save lives, and 165 (69%) were willing to undergo lung cancer screening if it was recommended by their physician. Multivariable regression analysis found that knowledge of lung cancer screening, smoking status, chronic pulmonary disease, and belief in the efficacy of early detection of lung cancer were associated with willingness to screen.
Conclusions
Although a minority of individuals at high risk for lung cancer are aware of screening, the majority believe that early detection saves lives and would pursue screening if recommended by their primary care physician. Health systems may increase screening rates by improving patient and physician awareness of lung cancer screening.
Key Words: early-detection cancer, lung cancer, smoking
Abbreviations: CFIR, Consolidated Framework for Implementation Research; CMS, Centers for Medicare & Medicaid Services; LDCT, low-dose CT; MTurk, Mechanical Turk; NCCN, National Comprehensive Cancer Network; USPSTF, United States Preventive Services Task Force
Lung cancer is the leading cause of cancer-related death in the United States.1 Screening with low-dose CT (LDCT) imaging, despite its proven mortality benefit,2 remains vastly underutilized.3,4 Knowledge, attitudes, and beliefs of individuals at high risk for lung cancer may contribute to the low screening rates.5 A better understanding of these factors will inform the design of interventions that aim to increase lung cancer screening rates.
Several areas of uncertainty remain. Lack of knowledge of screening may be an important barrier to screening,6, 7, 8, 9, 10, 11, 12 but awareness of screening among individuals at high risk of lung cancer is not well characterized. Another area of uncertainty is whether there is a relationship between willingness to screen and one’s self-perceived risk of lung cancer or belief in the efficacy of lung cancer screening. Previous studies have reported a relationship between these factors13, 14, 15, 16, 17; however, the findings may not be generalizable because the studies surveyed population subgroups (eg, veterans, socioeconomically disadvantaged populations) and in some cases screen-ineligible individuals (eg, nonsmokers). Measuring knowledge, attitudes, and beliefs in a broad population of high-risk individuals is challenging. Crowdsourcing platforms may be one way to obtain this information.18, 19, 20 Although previous studies have used crowdsourcing to study knowledge, attitudes, and beliefs in various contexts,20, 21, 22, 23 this method has not been used for lung cancer screening.
Using Amazon’s Mechanical Turk (MTurk) crowdsourcing platform, we characterized knowledge, attitudes, and beliefs regarding lung cancer screening among individuals at high risk for lung cancer based on United States Preventive Services Task Force (USPSTF)24 criteria. We hypothesized that a minority of high-risk individuals know about lung cancer screening and more specifically about LDCT imaging. We also hypothesized that a self-perceived high risk of lung cancer and a belief in the efficacy of screening are both associated with a greater willingness to be screened. Because little is known about how individuals prefer to learn about screening, an exploratory aspect of the current study was to generate preliminary data on the learning preferences of this population.
Materials and Methods
Survey Development
We developed survey questions informed by the Consolidated Framework for Implementation Research (CFIR)25 and the Lung Cancer Screening Extended Health Belief Model,5 which is based on the psychometric study that developed the Lung Cancer Screening Health Belief scales.6 CFIR is a meta-theoretical framework (based on published theories) that provides a pragmatic structure for identifying barriers to or facilitators of implementation at the system-level using five domains. Using the “Characteristics of Individuals” domain, we focused on the constructs of the intervention’s “relative advantage” and the influence of “patient needs and resources” in this survey.26 In addition, the Lung Cancer Screening Extended Health Belief Model identified cognitive variables (eg, knowledge of a screening test) and health beliefs (eg, perceived benefits of screening) to be factors affecting lung cancer screening.5 An exploratory component of our survey examined respondents’ preferences among different modalities of receiving information on lung cancer screening; this was done with the goal of potentially generating ideas for future research on screening communication. Our survey was developed with input from all co-authors over 18 iterations. Early iterations focused on aligning with existing lung cancer screening-related surveys and the CFIR. Later iterations were reviewed for conciseness, clarity, and timing. Our survey was tested among research staff, although none of the staff was eligible for screening. The final survey consisted of 31 items (e-Appendix 1).
Study Population
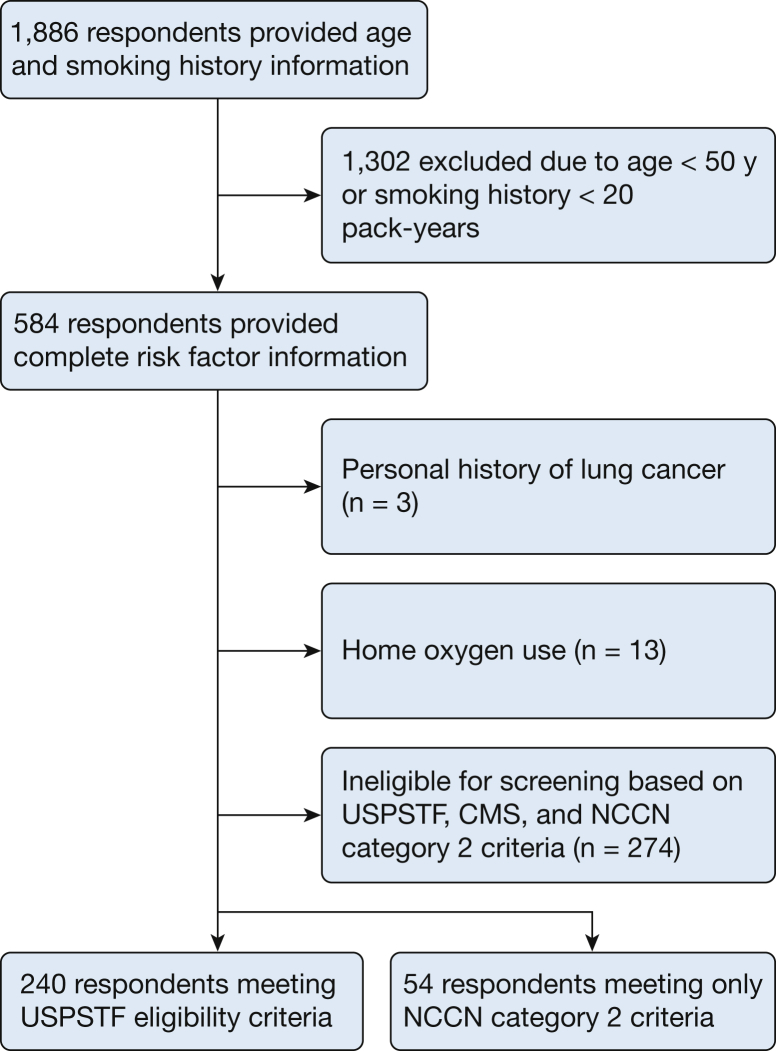
The study population represents a convenience sample of registered participants in the Amazon MTurk crowdsourcing marketplace between January and February 2018 who lived in the United States. We first asked five questions to determine participant age and smoking history (e-Appendix 2) and provided $0.25 as compensation. We then offered respondents aged ≥ 50 years with a ≥ 20 pack-year smoking history $2.00 to complete the full survey. We primarily analyzed respondents at high risk for lung cancer who met the USPSTF eligibility criteria for screening (age 55-80 years, former or current 30 pack-year smokers, former smokers who quit within the last 15 years).24 The reason we offered the survey to a broader and younger group was to conduct a planned sensitivity analysis to determine if responses varied according to different eligibility criteria. Respondents were excluded who reported requiring home oxygen or a history of lung cancer (Fig 1). This study was exempted from human subjects review by the University of Washington Human Subjects Division Institutional Review Board (STUDY00003234).
Figure 1.
Flowchart for inclusion and analysis of Amazon Mechanical Turk respondents. CMS = Centers for Medicare & Medicaid Services; NCCN = National Comprehensive Cancer Network; USPSTF = United States Preventive Services Task Force.
Survey Deployment
Participants completed the survey using Qualtrics. We prevented respondents from taking the survey multiple times by using Internet browser-specific “cookies” that blocked multiple attempts from the same Internet device or account. To avoid automated survey responses, we also used a Completely Automated Public Turing Test to Tell Computers and Humans Apart verification test at the beginning and end of the survey.27 The survey included forced responses; however, respondents had the option of selecting “prefer not to answer.”
Statistical Analysis
Respondent characteristics were summarized by using means or medians for normally distributed and nonnormally distributed continuous variables, respectively. Frequencies were calculated for categorical variables. We also conducted a sensitivity analysis comparing responses across varying eligibility criteria, including the Center for Medicare & Medicaid Services (CMS) and the National Comprehensive Cancer Network (NCCN) category 2 criteria (ie, individuals ≥ 50 years of age, with a ≥ 20 pack-year smoking history, and additional risk factors that increase the estimated 6-year lung cancer risk to > 1.3%).28 We used χ2 and Student t tests to compare demographic characteristics and knowledge, attitude, and belief variables. Respondent rankings of preferred methods of learning about screening were reported as mean rank with SD. A post hoc multivariable logistic regression analysis was conducted to evaluate factors associated with willingness to screen. All statistical analysis was conducted by using R version 3.4.4. (R Foundation for Statistical Computing).
Results
A total of 1,886 individuals accepted our initial invitation. We invited 584 current or former smokers aged ≥ 50 years with at least a 20 pack-year smoking history to take the full survey. The final cohort included 240 USPSTF-defined high-risk individuals (Fig 1).
Table 1 summarizes the study population. The median age was 60 years; the majority of respondents were white and current smokers, and had health insurance, at least a high school education, and income greater than $25,000 per year. Thirty-three (14%) respondents reported that their primary care provider had discussed lung cancer screening, and 24 (10%) reported having been screened. Of these 24 subjects, one-half reported undergoing CT scanning and one-half had a chest radiograph.
Table 1.
Amazon MTurk Respondent Characteristics
| Characteristic | MTurk Respondents Meeting USPSTF Criteria for High Risk (N = 240) |
|---|---|
| Age, y | 60 (7) |
| Female sex | 173 (72%) |
| Race | |
| White | 219 (91%) |
| American Indian/Alaska Native | 4 (1.7%) |
| Asian | 2 (0.4%) |
| Native Hawaiian/Pacific Islander | 0 |
| Black | 9 (3.7%) |
| Other | 6 (2.5%) |
| Hispanic | 4 (1.7%) |
| Highest education | |
| Less than high school | 5 (2.1%) |
| High school graduate/GED | 37 (15%) |
| Post-high school training, excluding college | 57 (24%) |
| Associate’s degree or some college | 80 (33%) |
| Bachelor's degree | 44 (18%) |
| Graduate school | 17 (7.0%) |
| BMI, kg/m2 | 26 (7) |
| Current smoker | 148 (62%) |
| Pack-years | 42 (12) |
| Years quita | 5 (8) |
| Previous malignancy (excluding lung cancer, BCC, or SCC) | 7 (2.9%) |
| Chronic pulmonary disease | |
| Chronic bronchitis/emphysema | 41 (17%) |
| Pulmonary fibrosis | 1 (0.4%) |
| Exposures | |
| Silica | 7 (2.9%) |
| Asbestos | 41 (17%) |
| Family history of lung cancer | 60 (25%) |
| Insurance | |
| Employer-based commercial | 83 (35%) |
| Non-employer-based commercial | 29 (12%) |
| Medicare | 55 (2%) |
| Medicaid/other state program | 26 (11%) |
| TRICARE/VA/military | 13 (6.3%) |
| Alaska Native/Indian/Tribal Health Services | 0 |
| Other | 2 (0.8%) |
| None | 28 (12%) |
| Income | |
| < $25,000 | 67 (28%) |
| $25,000-$49,999 | 75 (3%) |
| $50,000-$74,999 | 52 (22%) |
| $75,000-$99,999 | 26 (11%) |
| $100,000-$149,999 | 13 (5.4%) |
| $150,000-$199,999 | 2 (0.8%) |
| ≥ $200,000 | 0 |
| Declined to answer | 5 (2.1%) |
Data are presented as median (interquartile range) or No. (%). BCC = basal cell carcinoma; GED = General Education Development; MTurk = Mechanical Turk; SCC = squamous cell carcinoma; USPSTF = United States Preventive Services Task Force; VA = Veterans Affairs.
Calculated among former smokers (n = 92).
A total of 106 respondents (44%) reported knowledge of a screening test. Among those claiming such knowledge, 36 (35%) correctly identified LDCT imaging as the appropriate test (Table 2), whereas 28 (27%) and 16 (15%) identified chest radiograph and MRI as the correct test, respectively. Twenty-two respondents (9.2%) did not think a screening test exists. Most respondents (n = 114 [48%]) reported being unsure about the existence of a test. In a post hoc analysis excluding the 24 individuals who reported prior screening, 85 (39%) reported knowledge of a screening test. Of those claiming knowledge of screening, 28 (33%) correctly identified LDCT scanning (e-Table 1).
Table 2.
Knowledge, Attitudes, and Beliefs
| Variable | MTurk Respondents Meeting USPSTF Criteria for High Risk (N = 240) |
|---|---|
| Knowledge | |
| Responded “there is a test to screen for lung cancer” | 44 [38-51] |
| Correctly identified LDCT scan as the lung cancer screening testa | 35 [26-45] |
| Attitudes | |
| Willing to undergo lung cancer screening | 69 [63-75] |
| Beliefs | |
| Believe that early detection of cancer has the potential to save lives | 89 [85-93] |
| Believe that early detection of lung cancer has the potential to save lives | 93 [89-96] |
| Believe they are at high risk for lung cancer | 69 [62-74] |
Data are presented as % [95% CI]. LDCT = low-dose CT. See Table 1 legend for expansion of other abbreviations.
Proportion calculated among individuals who responded that there is a test to screen for lung cancer.
Regarding attitudes, 165 respondents (69%) reported being willing to undergo lung cancer screening if recommended by their physician (Table 2). Of the 75 (31%) unwilling to participate in screening, 69 (92%) reported wanting more information prior to deciding.
In terms of beliefs, 222 respondents (93%) reported believing that early detection of lung cancer has the potential to save lives. The proportion who believed that early detection of any cancer is life-saving was similarly high (89%). The majority of respondents believed they were high risk for lung cancer (69%) (Table 2). Respondents who reported a willingness to be screened were more likely to believe they were at high risk for lung cancer (P = .01) and believe in the efficacy of lung cancer screening (P < .001) compared with those who were undecided or unwilling to be screened (Table 3).
Table 3.
Relation Between Willingness to Screen and Perceived Risk of Lung Cancer and Efficacy of Screening Among Respondents Meeting USPSTF Criteria for High Risk
| Variable | Unwilling to Be Screened (n = 6) | Want More Information Prior to Deciding on Screening (n = 69) | Willing to Be Screened (n = 165) | P Valuea |
|---|---|---|---|---|
| Knowledge of lung cancer screening | 1 (17) | 18 (26) | 85 (52) | < .01 |
| Belief that one is at high risk of lung cancer | 2 (33) | 41 (59) | 121 (73) | .01 |
| Belief in the efficacy of lung cancer screening | 1 (17) | 61 (88) | 160 (97) | < .01 |
Data are presented as No. (%). See Table 1 legend for expansion of abbreviation.
P values refer to comparison of the “willing” group vs the combination of the “unwilling” and “want more information” groups.
A post hoc multivariable logistic regression analysis found that former smoker status, chronic pulmonary disease, reported knowledge of lung cancer screening, and belief in the efficacy of early detection of lung cancer were significantly associated with the odds of willingness to screen; the respective estimated ORs (95% CIs) were 2.47 (1.20-5.08), 19.06 (2.45-148.54), 3.40 (1.70-6.79), and 5.05 (1.54-16.62) (e-Table 2).
Fifty-four individuals met NCCN category 2 criteria for being high risk but did not meet USPSTF criteria (e-Table 3). Our sensitivity analysis of these individuals revealed similar results (e-Table 4). All 240 respondents who met USPSTF criteria also met CMS criteria. A total of 274 individuals did not meet CMS, USPSTF, or NCCN category 2 criteria. A sensitivity analysis revealed that these individuals were less likely to believe they were at high risk for lung cancer (e-Table 5).
An exploratory analysis examining some of the learning preferences of USPSTF-eligible respondents found that primary care physicians and electronic health record messages were more preferred than other forms of communication such as social media (e-Table 6).
Discussion
In this study of high-risk individuals on a crowdsourcing platform, we found that a minority of individuals are aware of lung cancer screening. However, knowledge of screening, former smoker status, chronic pulmonary disease, and belief in the efficacy of early detection are associated with willingness to screen.
Qualitative studies of high-risk individuals identify lack of knowledge as a barrier to lung cancer screening.7, 8, 9, 10, 11, 12 Rates of awareness of lung cancer screening vary across studies, ranging from 10% to 41%.9,12,29,30 These studies were conducted in mixed populations of screen-eligible and ineligible subjects,9,29 or in segments of screen-eligible individuals (eg, black subjects, white subjects, those who have undergone lung cancer screening).12,30 Although important, these studies limit our ability to apply their findings broadly. They may also be limited by traditional recruitment strategies. Regardless, the rate of awareness in our investigation is consistent with these studies and is generally low. Because there is little messaging of lung cancer screening overall, subgroups of high-risk individuals and screen-ineligible individuals are similarly likely to have knowledge of screening compared with broader populations. One unique contribution of the current study is that we disentangle individuals who claim knowledge of a screening test from those who know the actual test used to screen. Knowledge of LDCT scanning as the actual screening test is substantially lower than knowledge of a screening test in general. Unfortunately, we do not know if individuals who think they know the “right” test will be responsive to information about the correct test. Nonetheless, the significance of our findings is that there is little ability for individuals to self-advocate without knowledge of screening. One way to overcome this barrier is to develop patient-facing education interventions that increase knowledge.
The Lung Cancer Screening Extended Health Belief Model identified belief in the efficacy of early detection as an important facilitator of screening.5 Because only one other study has reported a relation between this factor and willingness to screen,31 the current study provides additional empirical support for the model. Other studies report on the prevalence of a belief in the efficacy of early detection without linking this factor to willingness to screen. Other studies have found that most individuals believe that early detection of lung cancer saves lives, but belief ranges from 66% to 94%.9,15,31, 32, 33, 34 Reasons for this variability may include the sampling of different populations or ambiguity over the definition of “life saved” (eg, a reduction in all-cause and lung cancer mortality). Our post hoc logistic regression found that belief in the efficacy of lung cancer screening was associated with willingness to screen. In addition, the current regression identified smoking status as associated with willingness to screen. One explanation for this finding is that former smokers have been shown to be more engaged in risk-reducing behavior. This hypothesis is further supported by studies showing that smokers use motivated reasoning to reduce their perceived risk. These findings suggest patient-facing interventions highlighting the efficacy of early detection and providing smoking cessation counseling may increase willingness to screen.
This study has important limitations. The Amazon MTurk population may not be representative of high-risk individuals. For instance, the current cohort differed from the National Lung Screening Trial population in a few ways: MTurk had a lower proportion of men, former smokers, individuals whose highest education level was high school, some college, or a graduate degree, and a higher proportion of American Indian/Alaska Native individuals and asbestos exposure. Others note differences in the demographic characteristics of MTurk and warn of underestimating/overestimating prevalence rates.35 However, our prevalence rates were similar to those of other studies.
Another limitation of MTurk is the inability to determine how many individuals received our survey invitation. This precludes a response rate calculation and/or evaluation for nonresponse bias. From a study cost perspective, MTurk does not provide a means of offering surveys to individuals in a prespecified age range, such as those outlined by screening guidelines. To overcome this limitation, we used our initial survey to “screen” for respondents meeting age criteria, which, however, led to increased study costs. We were also limited by the accuracy of respondent-provided information; we relied on self-reported sociodemographic and clinical risk factors that we cannot confirm. Online compensation mechanisms may incentivize creation of computer programs or “bots” that rapidly complete surveys. We took measures to prevent this by using Completely Automated Public Turing Test to Tell Computers and Humans Apart tests to ensure surveys were completed by a human. We found no evidence of automated activity in the free-text survey responses. An important limitation of our study is that we did not ask individuals about how they would want to receive information on screening from their physician and whether a discussion aid should be included. Finally, respondents’ stated preference to participate in screening may differ from their revealed preference in actual clinical settings.
A strength and novel aspect of the current study is the use of a crowdsourcing platform to conduct survey research in individuals at high risk for lung cancer. This approach allows researchers to potentially reach a broad range of individuals, and it may lower the threshold for survey participation. In addition, it is relatively inexpensive and allows for efficient and timely collection of data (ie, we completed our survey data collection within 2 months at a cost of $3,000). Crowdsourcing platforms may be an important adjunct to conventional methods of surveying individuals at high risk for lung cancer.
Conclusions
Our findings support patient and physician-facing interventions to increase knowledge pertaining to lung cancer screening. Specifically, among individuals meeting USPSTF eligibility criteria, efforts to increase knowledge of the availability of LDCT screening and the efficacy of screening, as well as providing smoking cessation information, may increase willingness to screen.
Acknowledgments
Author contributions: F. F. takes responsibility for (is the guarantor of) the content of the manuscript, including data and analysis. All authors made substantial contributions to the conception and design and interpretation of data; revised it critically and substantially for important intellectual content; provided final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. J. M. and F. F. also made substantial contributions to acquisition of data and analysis.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. M. was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health [Award Number T32DK070555]. D. E. W. was a consultant for Olympus Respiratory America and GRAIL; the vice-chair of the American Cancer Society National Lung Cancer Roundtable; an advisory board member for the GO2 Lung Cancer Foundation; and expert advisor for the BMS Foundation. None declared (M. T., E. M. W., D. C. L., D. R. F., F. F.).
Other contributions: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information: The e-Appendixes and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Supplementary Data
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.The National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A., Fedewa S.A. Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncology. 2017;3(9):1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pham D., Bhandari S., Oechsli M., Pinkston C., Kloecker G. Lung cancer screening rates: data from the Lung Cancer Screening Registry [abstract] J Clin Oncol. 2018;36(suppl):6504. [Google Scholar]
- 5.Carter-Harris L., Davis L.L., Rawl S.M. Lung cancer screening participation: developing a conceptual model to guide research. Res Theory Nurs Pract. 2016;30(4):333–352. doi: 10.1891/1541-6577.30.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter-Harris L., Slaven J.E., II, Monohan P., Rawl S.M. Development and psychometric evaluation of the Lung Cancer Screening Health Belief Scales. Cancer Nurs. 2017;40(3):237–244. doi: 10.1097/NCC.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra S.I., Sussman A.L., Murrietta A.M. Patient perspectives on low-dose computed tomography for lung cancer screening, New Mexico, 2014. Prev Chronic Dis. 2014;13(E108):1–10. doi: 10.5888/pcd13.160093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter-Harris L., Ceppa D.P., Hanna N., Rawl S.M. Lung cancer screening: what do long-term smokers know and believe? Health Expect. 2015;20(1):59–68. doi: 10.1111/hex.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crothers K., Kross E.K., Reisch L.M. Patients' attitudes regarding lung cancer screening and decision aids. A survey and focus group study. Ann Am Thorac Soc. 2016;13(11):1992–2001. doi: 10.1513/AnnalsATS.201604-289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardarelli R., Roper K.L., Cardarelli K. Identifying community perspectives for a lung cancer screening awareness campaign in Appalachia Kentucky: the Terminate Lung Cancer (TLC) Study. J Cancer Educ. 2017;32(1):125–134. doi: 10.1007/s13187-015-0914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simmons V.N., Gray J.E., Schabath M.B., Wilson L.E., Quinn G.P. High-risk community and primary care providers knowledge about and barriers to low-dose computed topography lung cancer screening. Lung Cancer. 2017;106:42–49. doi: 10.1016/j.lungcan.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Roth J.A., Carter-Harris L., Brandzel S., Buist D.S.M., Wernli K.J. A qualitative study exploring patient motivations for screening for lung cancer. PLoS ONE. 2018;13(7) doi: 10.1371/journal.pone.0196758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanner N.T., Egede L.E., Shamblin C. Attitudes and beliefs toward lung cancer screening among US veterans. Chest. 2013;144(6):1783–1787. doi: 10.1378/chest.13-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delmerico J., Hyland A., Celestino P. Patient willingness and barriers to receiving a CT scan for lung cancer screening. Lung Cancer. 2014;84(3):307–309. doi: 10.1016/j.lungcan.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonnalagadda S., Bergamo C., Lin J.J. Beliefs and attitudes about lung cancer screening among smokers. Lung Cancer. 2012;77(3):526–531. doi: 10.1016/j.lungcan.2012.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lillie S.E., Fu S.S., Fabbrini A.E. What factors do patients consider most important in making lung cancer screening decisions? Findings from a demonstration project conducted in the Veterans Health Administration. Lung Cancer. 2017;104:38–44. doi: 10.1016/j.lungcan.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Silvestri G.A., Nietert P.J., Zoller J., Carter C., Bradford D. Attitudes towards screening for lung cancer among smokers and their non-smoking counterparts. Thorax. 2007;62(2):126–130. doi: 10.1136/thx.2005.056036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buhrmester M., Kwang T., Gosling S. Amazon's Mechanical Turk: a new source of inexpensive, yet high-quality, data? Perspect Psychol Sci. 2011;6(1):3–5. doi: 10.1177/1745691610393980. [DOI] [PubMed] [Google Scholar]
- 19.Simons D.J., Chabris C.F. Common (mis)beliefs about memory: a replication and comparison of telephone and mechanical turk survey methods. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0051876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartek M.A., Truitt A.R., Widmer-Rodriguez S. The promise and pitfalls of using crowdsourcing in research prioritization for back pain: cross-sectional surveys. J Med Internet Res. 2017;19(10):e341. doi: 10.2196/jmir.8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrell L., Brennan L., Lanham M. Knowledge, attitudes, and perceptions of infertility: a national survey. Fertility Sterility. 2018;110(4):e6. [Google Scholar]
- 22.Naylor W., Parsons E.C.M. An online survey of public knowledge, attitudes, and perceptions toward whales and dolphins, and their conservation. Frontiers Marine Sci. 2018;5:153. [Google Scholar]
- 23.Taylor L.E., Antshel K.M. Factors associated with parental treatment attitudes and information-seeking behaviors for childhood ADHD. J Atten Disord. 2019 doi: 10.1177/1087054718821734. 108705471882173. [DOI] [PubMed] [Google Scholar]
- 24.US Preventive Services Task Force Lung cancer: screening. http://www.uspreventiveservicestaskforce.org/uspstf/uspslung.htm
- 25.Damschroder L., Aron D. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CFIR The Consolidated Framework for Implementation Research. https://cfirguide.org
- 27.Ahn V.L., Blum M., Langford J. Telling humans and computers apart automatically. Communications ACM. 2004;47(2):56–60. [Google Scholar]
- 28.Wood D.E., Kazerooni E.A., Baum S.L. Lung cancer screening, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(4):412–441. doi: 10.6004/jnccn.2018.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Percac-Lima S., Ashburner J.M., Atlas S.J. Beliefs about lung cancer, knowledge, and interest in lung screening among community health center patients. Park J Clin Oncol. 2016;34(15) [Google Scholar]
- 30.Carter-Harris L., Slaven J.E., Jr., Monahan P.O., Shedd-Steele R., Hanna N., Rawl S.M. Understanding lung cancer screening behavior: racial, gender, and geographic differences among Indiana long-term smokers. Prev Med Rep. 2018;10:49–54. doi: 10.1016/j.pmedr.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cataldo J. High-risk older smokers' perceptions, attitudes, and beliefs about lung cancer screening. Cancer Med. 2016;5(4):753–759. doi: 10.1002/cam4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flynn A.E., Peters M.J., Morgan L.C. Attitudes towards lung cancer screening in an Australian high-risk population. Lung Cancer Int. 2013;2013 doi: 10.1155/2013/789057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bui N.C., Lee Y.Y., Suh M. Beliefs and intentions to undergo lung cancer screening among Korean males. Cancer Res Treat. 2017;50(4):1096–1105. doi: 10.4143/crt.2017.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smits S.E., McCutchan G.M., Hanson J.A., Brain K.E. Attitudes towards lung cancer screening in a population sample. Health Expect. 2018;21(6):1150–1158. doi: 10.1111/hex.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong M., Zhang D., Morgan J.C. Similarities and differences in tobacco control research findings from convenience and probability samples. Ann Behav Med. 2019;53(5):476–485. doi: 10.1093/abm/kay059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.