Abstract
Background
Traditional methods for the quantification of OSA severity may not encapsulate potential relationships between hypoxemia in OSA and cardiovascular risk.
Research Question
Do novel nocturnal oxygen saturation (Spo2) metrics have prognostic value in patients with OSA and high cardiovascular event risk?
Study Design and Methods
We conducted post hoc analyses of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial. In 2687 individuals, Cox proportional hazards models that were stratified for treatment allocation were used to determine the associations between clinical characteristics, pulse oximetry-derived metrics that were designed to quantify sustained and episodic features of hypoxemia, and cardiovascular outcomes. Metrics included oxygen desaturation index, time <90% Spo2, average Spo2 for the entire recording (mean Spo2), average Spo2 during desaturation events (desaturation Spo2), average baseline Spo2 interpolated across episodic desaturation events (baseline Spo2), episodic desaturation event duration and desaturation/resaturation-time ratio, and mean and SD of pulse rate.
Results
Neither apnea-hypopnea index, oxygen desaturation index, nor any of the novel Spo2 metrics were associated with the primary SAVE composite cardiovascular outcome. Mean and baseline Spo2 were associated with heart failure (hazard ratio [HR], 0.81; 95% CI, 0.69-0.95; P = .009; and HR, 0.78; 95% CI, 0.67-0.90; P = .001, respectively) and myocardial infarction (HR, 0.86; 95% CI, 0.77-0.95; P = .003; and HR, 0.81; 95% CI, 0.73-0.90; P < .001, respectively). Desaturation duration and desaturation/resaturation time ratio, with established risk factors, predicted future heart failure (area under the curve, 0.86; 95% CI, 0.79-0.93).
Interpretation
Apnea-hypopnea index and oxygen desaturation index were not associated with cardiovascular outcomes. In contrast, the pattern of oxygen desaturation was associated with heart failure and myocardial infarction. However, concomitant risk factors remained the predominant determinants for secondary cardiovascular events and thus deserve the most intensive management.
Key Words: cardiovascular risk, heart failure, hypoxemia, SAVE, sleep apnea
Abbreviations: AHI, apnea-hypopnea index; ODI, oxygen desaturation index; SAVE, Sleep Apnea Cardiovascular Endpoints; Spo2, oxygen saturation
Sleep-disordered breathing, particularly OSA, is common in patients with cardiovascular disease, and is associated with significant morbidity and death.1,2 CPAP treatment is the main therapy for OSA, effectively reducing hypopneas and apneas when used appropriately. Despite a clear association between CPAP treatment and reduced cardiovascular event rates and mortality rates in nonrandomized studies,1 CPAP therapy did not prevent secondary cardiovascular events in the randomized Sleep Apnea Cardiovascular Endpoints (SAVE) study3 or a subsequent meta-analysis.4
Various theories have been advanced to explain discrepancies between epidemiologic and randomized controlled study findings, which included the theories that residual confounding factors caused observational studies to overestimate OSA-associated cardiovascular risk and that low CPAP adherence contributed to neutral findings in randomized studies.5 Alternatively, current sleep study metrics, namely frequency of respiratory or desaturation episodes, may be less informative for cardiovascular disease risk than other pathophysiologic features of OSA.6 Recent retrospective analyses of data from cohort studies have identified new candidate markers of OSA-associated cardiovascular risk, which include degree of daytime sleepiness,7 length of obstructive events,8 and cumulative extent of oxygen desaturation.9, 10, 11, 12, 13, 14, 15, 16, 17
In practice, a disturbance in oxygen saturation (Spo2) generally is quantified as the oxygen desaturation index (ODI) (ie, the number of desaturation episodes ≥3% to 4% per hour of sleep. The apnea-hypopnea index (AHI) (ie, number of hypopneas and apneas per hour of sleep) provides similar information because it requires that hypopnea events are accompanied by ≥3% to 4% Spo2 or arousal.18 Neither of these event-based metrics capture features of episodic or sustained changes in Spo2, such as severity of episodic desaturations and the baseline Spo2 level from which they occur, for which cumulative effects may be clinically important.6,19 The clinical utility of other measures of nocturnal hypoxemia compared with conventional indexes of sleep apnea severity is unclear. Thus, to help clarify potential impacts of different patterns of hypoxemia on cardiovascular outcomes, this study aimed to examine overnight pulse oximetry data from participants in the SAVE study to compare the predictive value of conventional and novel metrics of nocturnal Spo2 and patterns of episodic and sustained episodic desaturations and pulse rate data to predict longer term cardiovascular outcomes that include stroke, myocardial infarction, and heart failure. We hypothesized that oximetry-derived metrics of nocturnal hypoxemic burden have predictive utility for future cardiovascular events.
Methods
Study Design
This is an ancillary study of the SAVE trial. This study used existing data from the SAVE clinical trial (NCT00738179/ACTRN 126080000409370) to assess new methods, but it is not itself a clinical trial. SAVE was an international, multicenter, prospective, randomized, open-label, blinded outcome event assessed (PROBE) trial of CPAP treatment for secondary prevention of cardiovascular events in patients with co-occurring moderate or severe OSA and cardiovascular disease. For this analysis, all individuals, irrespective of CPAP treatment allocation, were included to examine the association of novel oximetry metrics at baseline with longitudinal outcomes. Details are outlined elsewhere.3,20, 21, 22 SAVE was conducted in accordance with the amended Declaration of Helsinki. The protocol of the SAVE clinical trial was approved by regulatory authorities and ethics committees at participating centers; participants provided written informed consent (e-Table 1).
Data Collection
Screening for participants involved overnight home use of an ApneaLink device (ResMed) to record nasal pressure (a surrogate of airflow) and fingertip oximetry (Nonin 8000AA-Adult; Nonin Medical Inc). Eligibility for SAVE required an ODI of ≥12 events/h, defined by ≥4% Spo2 drops. Participants who showed a predominantly Cheyne-Stokes respiration pattern of central sleep apnea were excluded, as were those who had very severe oxygen desaturation (resting awake Spo2 of ≤90% or >10% of recording time with Spo2 of ≤80%). Clinically significant heart failure (New York Heart Association categories III-IV) was also excluded. Eligible participants were assigned randomly to receive either CPAP treatment plus usual cardiovascular care (CPAP group) or usual cardiovascular care alone (usual care group). Medical history, demographic, and anthropometric information was collected at baseline and follow-up appointments (1, 3, 6, and 12 months, and annually thereafter). Clinical outcomes and serious adverse events were recorded according to Medical Dictionary for Regulatory Activities classifications (MedDRA version 14). The primary end point for the SAVE study was a composite of cardiovascular death, myocardial infarction, stroke, or hospitalization for heart failure, acute ischemic cardiac event (unstable angina), or cerebral event (transient ischemic event).23 For nonfatal events, participants could continue in the trial, and any subsequent events of the same or other types could be recorded.
Oximetry Analyses
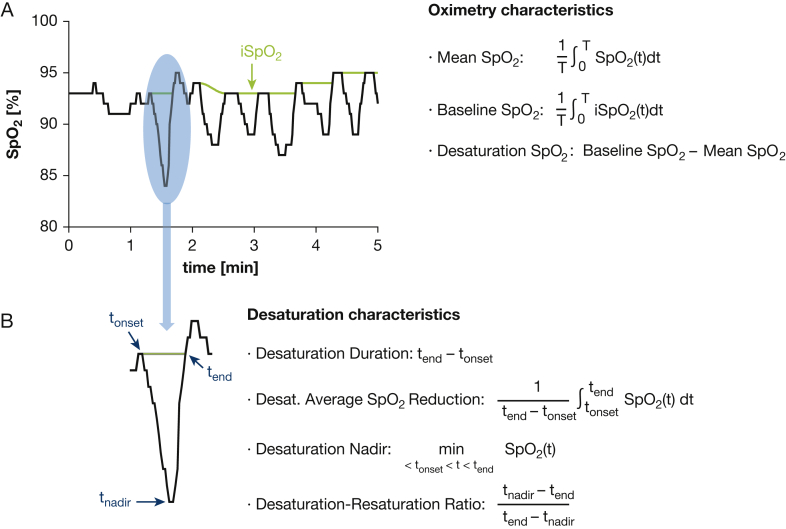
Novel metrics calculated to characterize oximetry features included (1) average Spo2 across the recording (mean Spo2), (2) average baseline Spo2 interpolated across acute desaturation events (baseline Spo2), and (3) average Spo2 desaturation below the interpolated baseline Spo2 during acute events (desaturation Spo2) (Fig 1). Acute desaturations were defined as episodic, monotonic drops in Spo2 that reach a peak amplitude of ≥4%, followed by resaturation of at least two-thirds of the preceding drop, within 150 seconds from desaturation onset.10,23 ODI was calculated as the number of such events per hour. Total accumulated time spent at <90% Spo2 (T90) was also calculated.
Figure 1.
A-B, Descriptive diagram and definition of oximetry measures (A) and desaturation characteristics (B) obtained from raw oximetry data (recorded by ApneaLink [ResMed, San Diego, CA]) with the use of a custom MATLAB software algorithm (MathWorks). dt = integrated with respect to time; iSpo2 = Spo2 signal with desaturation periods interpolated; Spo2 = oxygen saturation; T = total recording duration.
To further characterize acute episodic desaturations, we determined (1) median event duration (from onset of deoxygenation to completion of resaturation), (2) median of the average reduction in Spo2 over the duration of identified events, (3) median nadir of all desaturation events, and (4) median desaturation/resaturation time ratio.
Using pulse rate traces from ApneaLink software output, we calculated the average pulse rate and its SD as surrogate measures of mean heart rate and heart rate variability. Methods information and artefact detection have been published10,23 (e-Fig 1).
Statistical Analyses
Statistical analyses used SPSS software (version 23; IBM). Given the number of comparisons and the exploratory nature of the analyses, a probability value of <.01 was considered statistically significant.
Relationships between standard and novel metrics and outcome events were investigated with the use of Cox proportional hazards regression. Outcomes that were assessed were the primary composite as described in the main SAVE trial3 and each component event type. Each variable was first assessed individually with only a stratification for treatment allocation (CPAP vs usual care), which permitted different baseline hazard according to treatment arm but a single estimate of the regression coefficient for the variable of interest. For multivariable Cox regression analyses, two stages (blocks) were used to generate predictive equations. Block 1 included common exposures that are also associated with OSA-related outcomes recorded at baseline as predictor variables: diabetes mellitus and hypertension diagnoses, age, waist:hip ratio, smoking status (never/past/current), ODI, ethnicity category (Caucasian/European, Asian, other), treatment allocation (CPAP/usual care), cardiovascular disease history (coronary/cerebrovascular/both), and total number of prescribed cardiovascular and diabetes mellitus medications. Block 2 included oximetry and pulse metrics:pulse mean and SD, desaturation/resaturation time ratio, desaturation average Spo2 reduction and duration, and baseline Spo2. For the composite outcome and separately for each component event type, block 1 was assessed first, and variables were retained after stepwise assessment proceeded to the second stage, in which block 2 variables were added and a second round of stepwise assessment was carried out to generate a final model. Regression coefficients that were generated from block 1 in the first phase alone, and from both blocks 1 and 2 in the second phase, were applied as predictive equations to assess the relative contribution of known and novel variables and presented as receiver-operating characteristic curves.
Results
General Characteristics
Of 2687 participants who were included in the SAVE follow up,3 38 participants could not be included due to technical problems with their recording files, which left 2649 participants with complete overnight oximetry raw data for analysis. Table 1 and e-Table 2 outline their general characteristics. Most were middle-aged, overweight men, with median follow up of 3.54 years (range, 1 day to 6.89 years). There were no significant differences between treatment allocation groups in pulse or oximetry metrics (e-Table 3).
Table 1.
Baseline Demographic and Anthropometric Features of Participants Included in the Study
| Patient Characteristics | CPAP (n = 1,329) | Usual Care (n = 1,320) | Total (N=2,649) |
|---|---|---|---|
| Sex: female, No. (%) | 250 (18.8) | 254 (19.2) | 504 (19.0) |
| Ethnicity, No. (%) | |||
| Causcasian/European | 326 (24.5) | 332 (25.2) | 658 (24.8) |
| Asian | 851 (64.0) | 831 (63.0) | 1,682 (63.5) |
| Other | 152 (11.4) | 157 (11.9) | 309 (11.7) |
| Country, No. (%) | |||
| Australia | 104 (7.8) | 117 (8.9) | 221 (8.3) |
| Brazil | 139 (10.5) | 140 (10.6) | 279 (10.5) |
| China | 845 (63.6) | 823 (62.3) | 1,668 (63.0) |
| India | 59 (4.4) | 58 (4.4) | 117 (4.4) |
| New Zealand | 53 (4.0) | 45 (3.4) | 98 (3.7) |
| Spain | 128 (9.6) | 134 (10.2) | 262 (9.9) |
| United States | 1 (0.1) | 3 (0.2) | 4 (0.2) |
| Smoking status,a No. (%) | |||
| Never | 580 (43.7) | 595 (45.2) | 1175 (44.5) |
| Past | 536 (40.4) | 528 (40.1) | 1,064 (40.3) |
| Current | 210 (15.8) | 194 (14.7) | 404 (15.3) |
| Age at randomization, median (IQR), y | 61.4 (55.6-67.2) | 61.6 (55.3-67.6) | 61.4 (55.4-67.4) |
| Height, median (IQR), cm | 168 (163-173) | 168 (162-173) | 168 (162-173) |
| Weight, median (IQR), kg | 80 (70-89) | 79 (70-88) | 80 (70-89) |
| BMI, median (IQR), kg/m2 | 28.2 (25.8-31.0) | 28.0 (25.7-30.9) | 28.1 (25.7-30.9) |
| Waist, median (IQR), cm | 100 (93-108) | 100 (93-107) | 100 (93-108) |
| Hip, median (IQR), cm | 105 (100-110) | 105 (100-111) | 105 (100-110) |
| Waist:hip ratio, median (IQR) | 0.96 (0.91-1.00) | 0.95 (0.91-1.00) | 0.95 (0.91-1.00) |
| Neck circumference, median (IQR), cm | 41 (38-43) | 41 (38-43) | 41 (38-43) |
| Systolic BP, median (IQR), mm Hg | 130 (120.5-140) | 130 (120-140) | 130 (120-140) |
| Diastolic BP, median (IQR), mm Hg | 80 (72.5-87) | 80 (71.5-86) | 80 (72-86.5) |
| Heart rate, median (IQR), beats/min | 70 (63-78) | 71 (64-79) | 71 (64-78) |
Information missing for six participants (three in each treatment group). IQR = interquartile range.
Predictive Value of Measures for the Composite Endpoint and Its Component Cardiovascular Outcomes
Unadjusted Cox proportional hazard models stratified by treatment allocation (CPAP vs usual care) showed no significant predictive value of AHI, ODI, or T90 for the primary composite outcome (Table 2) or any component cardiovascular outcomes (Table 3).
Table 2.
Summary of Unadjusted Univariate Cox Regression Analyses for the Primary Composite End Point of the Sleep Apnea Cardioascular Endpoints (SAVE) Study
| Variable | Hazard Ratioa | 95% CIa | P Value |
|---|---|---|---|
| Apnea-hypopnea index, events/h | 0.99 | 0.98-1.00 | .066 |
| Patterns of nocturnal hypoxemia (custom MATLAB [MathWorks] algorithm) | |||
| Oxygen desaturation index, events/h | 0.99 | 0.98-1.00 | .072 |
| T<90%, min (log transformed) | 1.00 | 0.94-1.07 | .988 |
| Mean Spo2, % | 0.99 | 0.94-1.04 | .652 |
| Baseline Spo2, % | 0.96 | 0.91-1.01 | .141 |
| Desaturation Spo2, % (log transformed) | 0.93 | 0.81-1.06 | .262 |
| Desaturation duration, s | 1.01 | 1.00-1.02 | .039 |
| Desaturation/resaturation time ratio | 0.93 | 0.80-1.08 | .329 |
| Desaturation average Spo2 reduction, % | 0.94 | 0.86-1.03 | .166 |
| Desaturation nadir, % | 1.00 | 0.97-1.04 | .886 |
| Pulse mean, beats/min | 0.99 | 0.99-1.01 | .774 |
| Pulse SD, beats/min | 1.00 | 0.95-1.05 | .987 |
From crude (univariate) Cox regressions for the primary composite end point of SAVE, with the use of individual measures as univariate continuous covariates. Regression analyses were stratified according to CPAP treatment allocation, with no other adjustments. Spo2 = oxygen saturation.
Table 3.
Summary of Unadjusted Univariate Cox Regression Analyses for Component Outcomes of the Sleep Apnea Cardioascular Endpoints (SAVE) Study
| Variable | Hazard Ratio (95% CI)a; P Value |
Myocardial Infarction (n = 81) | Stroke (n = 132) | Heart Failure (n = 32) | Angina (n = 187) | Transient Ischemic Event (n = 25) |
|---|---|---|---|---|---|---|
| Cardiovascular Death (n = 44) | ||||||
| Apnea-hypopnea index, events/h | 0.98 (0.96-1.00); .085 | 0.99 (0.97-1.00); .057 | 1.00 (0.99-1.01); .970 | 0.99 (0.97-1.01); .476 | 0.99 (0.98-1.00); .108 | 0.99 (0.97-1.02); .632 |
| Oxygen desaturation index, events/h | 0.99 (0.97-1.01); .293 | 0.99 (0.97-1.00); .137 | 0.99 (0.99-1.01); .840 | 1.00 (0.98-1.03); .729 | 0.99 (0.98-1.00); .131 | 0.98 (0.96-1.01); .286 |
| T<90%, min (log transformed) | 0.81 (0.67-0.99); .035 | 1.17 (0.99-1.37); .066 | 0.98 (0.89-1.10); .717 | 1.27 (0.97-1.66); .081 | 0.98 (0.89-1.09); .722 | 0.89 (0.68-1.16); .374 |
| Mean Spo2, % | 1.15 (0.97-1.36); .119 | 0.86 (0.77-0.95); .003 | 1.09 (1.00-1.20); .065 | 0.81 (0.69-0.95); .009 | 0.97 (0.90-1.05); .456 | 1.13 (0.90-1.41); .295 |
| Baseline Spo2, % | 1.05 (0.88-1.26); .563 | 0.81 (0.73-0.90); .001 | 1.13 (1.02-1.26); .020 | 0.78 (0.67-0.90); .001 | 0.94 (0.87-1.01); .092 | 1.07 (0.85-1.34); .583 |
| Desaturation Spo2 (log transformed), % | 0.73 (0.48-1.12); .145 | 0.81 (0.59-1.10); .178 | 1.08 (0.86-1.37); .509 | 0.89 (0.55-1.45); .657 | 0.87 (0.71-1.07); .191 | 0.86 (0.49-1.49); .581 |
| Desaturation duration, s | 1.02 (0.99-1.06); .124 | 1.01 (0.98-1.03); .595 | 1.01 (0.99-1.02); .565 | 1.03 (1.00-1.07); .059 | 1.00 (0.99-1.02); .931 | 1.05 (1.01-1.08); .011 |
| Desaturation/resaturation time ratio | 0.81 (0.50-1.01); .397 | 0.65 (0.44-0.96); .032 | 1.13 (0.88-1.45); .352 | 0.35 (0.17-0.74); .005 | 0.94 (0.75-1.18); .609 | 0.87 (0.46-1.64); .658 |
| Desaturation average Spo2 Reduction, % |
0.71 (0.50-1.01); .055 | 0.82 (0.65-1.03); .094 | 1.06 (0.92-1.23); .410 | 0.78 (0.53-1.14); .202 | 0.94 (0.82-1.08); .390 | 0.77 (0.50-1.19); .243 |
| Desaturation nadir, % | 1.16 (1.01-1.34); .033 | 0.97 (0.89-1.05); .430 | 1.02 (0.95-1.09); .622 | 0.93 (0.82-1.06); .279 | 0.99 (0.93-1.04); .631 | 1.16 (0.97-1.40); .104 |
| Pulse mean, beats/min | 1.02 (0.99-1.05); .249 | 1.00 (0.98-1.03); .890 | 1.02 (1.00-1.04); .035 | 1.01 (0.98-1.05); .472 | 0.99 (0.97-1.00); .087 | 0.98 (0.94-1.03); .375 |
| Pulse SD, beats/min | 1.08 (1.04-1.12); .001 | 0.90 (0.79-1.04); .161 | 1.06 (1.02-1.09); .001 | 0.95 (0.76-1.18); .625 | 0.85 (0.78-0.94); .001 | 0.98 (0.78-1.23); .847 |
P values < .01 indicating comparisons considered significant are in bold.
From crude (univariate) Cox regressions for component outcomes of SAVE, with the use of individual measures as univariate continuous covariates. Regression analyses were stratified according to CPAP treatment allocation, with no other adjustments. Spo2 = oxygen saturation.
With the use of the same approach to assess novel Spo2 metrics and acute episodic desaturation patterns, none were strongly predictive of the SAVE composite outcome (Table 2). However, lower mean Spo2 and baseline Spo2, but not desaturation Spo2, were associated with increased risk of heart failure and myocardial infarction (Table 3). The hazard ratio for desaturation/resaturation time ratio indicated an inverse relationship with heart failure risk (0.35 [0.17-0.74]; P = .005), which indicates a higher risk with lower desaturation/resaturation ratios, as would be expected in central rather than OSA (Table 3). Pulse rate SD, which is indicative of high heart rate variability, was associated with a higher risk of cardiovascular death and stroke but showed a protective relationship with angina (Table 3).
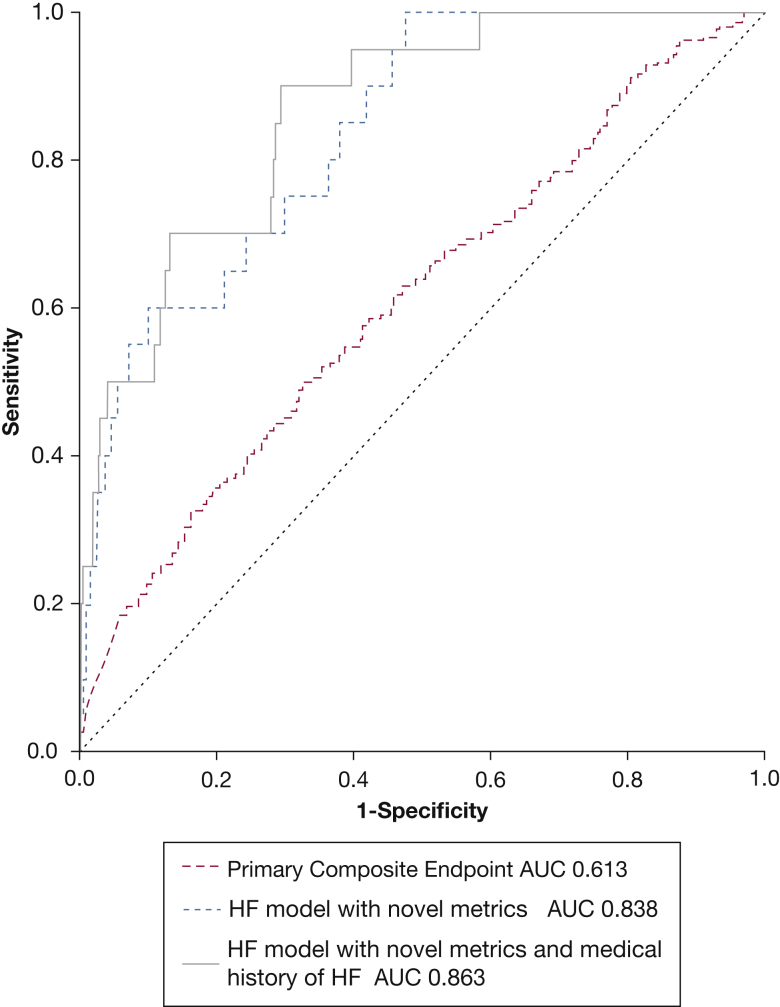
Multivariable Cox regression analysis adjusted for demographic, clinical, and anthropometric risk variables was used to generate a predictive equation for the composite outcome (Table 4). The resultant receiver-operating characteristic curve showed statistically significant (area under the curve, 0.61; 95% CI, 0.58-0.65; P < .001) but poor predictive utility for the primary composite outcome at 2 years (sensitivity, 70%; specificity, 41% at threshold value of 2.95) (Fig 2).
Table 4.
Final Model From Two Block Backwards Stepwise (Conditional) Multivariable Cox Regression Analysis for Predictors of the Primary Composite End Point of the Sleep Apnea Cardioascular Endpoints (SAVE) Study Outcome at 2 Years
| Variable | Hazard Ratio (95% CI) | Hazard Ratio, Z-Normalized Value for Scale Variables (95% CI) | P Value |
|---|---|---|---|
| Diabetes mellitus | 1.50 (1.23-1.83) | … | < .001 |
| Ethnicity (simplified) | |||
| White/European | Reference | … | .092 |
| Asian | 1.23 (0.95-1.59) | … | .111 |
| Other | 0.85 (0.56-1.28) | … | .436 |
| Hypertension | 1.43 (1.10-1.86) | … | .008 |
| Cardiovascular disease history | |||
| Cardiac only | Reference | … | < .001 |
| Cerebrovascular only | 0.78 (0.63-0.97) | … | .028 |
| Both | 2.74 (1.98-3.79) | … | < .001 |
| Age at baseline | 1.01 (1.00-1.02) | 1.07 (0.97-1.18) | .167 |
| Waist:hip ratio | 5.73 (1.69-19.45) | 1.15 (1.04-1.26) | .005 |
| Oxygen desaturation index | 1.00 (0.99-1.00) | 0.92 (0.82-1.02) | .125 |
| Median desaturation duration, s | 1.01 (1.00-1.02) | 1.09 (0.99-1.21) | .073 |
Regression formula for the primary composite outcome: 0.003 + 0.405∗1(if diabetic, 0 if not) + 0.209∗1(if Asian, 0 if not) + 0.355∗1(if hypertension, 0 if no) + 1.746∗waist:hip ratio + -0.005∗ODI + 1.008∗1(if both cardiac and cerebrovascular disease history, 0 if not) + 0.009∗median desaturation duration.
Figure 2.
Receiver-operator characteristic curves that use regression formulae from stepwise multivariable Cox regression analyses applied to 2-year outcomes for the primary composite end point (Table 4) and the heart failure component (for the regression formulae see e-Tables 5, 6).
Stepwise backwards conditional multivariable Cox regression analyses indicated distinctive patterns that were associated with each component outcome type (e-Table 4). Several novel oximetry metrics were predictive of cardiovascular death. None of the novel oximetry metrics retained independent significance as predictors of stroke or myocardial infarction. Receiver-operating characteristic curves that were derived from these multivariable regression equations for component cardiovascular outcomes are shown in e-Figure 2; except for heart failure, these were poorly predictive.
Desaturation/resaturation time ratio, desaturation duration, and mean pulse rate were retained as predictors of heart failure risk in multivariable analyses. The regression formula that was produced by stepwise conditional multivariable Cox regression analysis for heart failure (e-Table 5) was significant and strongly predictive of outcomes at 2 years (area under the curve, 0.84; 95% CI, 0.76-0.91; P < .001; sensitivity, 75%; specificity, 70% at threshold value of 11.18) (Fig 2).
The 2-year heart failure outcome was reanalyzed in a second backwards stepwise conditional analysis, in which the general category of cardiovascular disease history in block 1 was replaced with a variable specific for history of heart failure (e-Table 5). Although a history of heart failure was important, desaturation duration and desaturation/resaturation time ratio retained predictive value in this second model; the resultant equation was similarly strongly predictive of heart failure outcomes at 2 years (area under the curve, 0.86; 95% CI, 0.79-0.93; P < .001; sensitivity 90%, specificity 70% at threshold value of 7.39) (Fig 2).
Discussion
In this ancillary study of the SAVE trial, conventional metrics such as AHI and ODI predicted neither the primary composite cardiovascular outcome nor its components in individuals with moderate-to-severe OSA and high cardiovascular risk. Novel oximetry-derived Spo2 metrics had some prognostic value, but concomitant risk factors, which are highly prevalent in patients with OSA, remain the predominant determinants for secondary cardiovascular events.
Other features of nocturnal hypoxemic burden, such as the degree of sustained hypoxemia and/or the severity of intermittent desaturation events, may provide additional prognostic information. Punjabi et al24 first showed that prevalent cardiovascular disease was related to the extent of hypopnea-associated oxygen desaturation during sleep. More recently, T90 was shown to be an independent predictor of all-cause death in patients with stable chronic heart failure9 and was associated with increased incidence of cardiovascular death11 and fatal stroke in older men.10 Another recent study showed that hypoxemic burden due to sleep apnea is strongly predictive of cardiovascular death.12 In contrast, in this SAVE study cohort, neither T90 nor the component of hypoxemic burden due to acute desaturation events (desaturation Spo2) were associated with the primary end point and were associated only weakly with risk of myocardial infarction and heart failure risk. There are several potential reasons for these disparate findings. First, follow up in SAVE was shorter, with relatively few deaths. Second, because SAVE included only participants with moderate-to-severe OSA, the mean T90 in SAVE was 3- to 4-times higher than other cohort studies that investigated the association between T90 and mortality rates in individuals who were not preselected for OSA status.10, 11, 12 Although nocturnal hypoxemia may be a key variable that affects cardiovascular death and morbidity,9, 10, 11, 12, 13, 14, 15, 16, 17 it may be less evident in the SAVE population that is preselected by OSA diagnosis because of the narrower range of T90 values. Instead, other cardiovascular risk factors, which were prevalent in the population recruited to SAVE, appear to predominate.
The contribution of acute desaturation events (desaturation Spo2) to overall hypoxemia, with the incorporation of cumulative severity of desaturation events, did not determine cardiovascular risk in SAVE participants. Mean Spo2, and baseline Spo2 comprising sustained changes in Spo2 independent of episodic desaturation events failed to predict the primary composite outcome in SAVE but were associated with myocardial infarction and heart failure in univariate analyses; hazard ratios of <1 indicated worse prognosis with lower mean Spo2 and baseline Spo2. Baseline Spo2 was retained in predictive models for some outcome components. Although patients with clinical lung disease, awake Spo2 of ≤90% or very severe nocturnal hypoxemia were excluded from the SAVE study, alterations in mean and baseline Spo2 may reflect mild subclinical lung disease, pulmonary congestion, or V̇/Q̇ mismatches. Additionally, some individuals with severe sleep apnea may be at risk of “micro” lung injury due to OSA-related mechanical or oxidative stressors.25
We cannot exclude a potential bidirectional cause-effect relationship for which not only hypoxemic burden may contribute to component cardiovascular outcomes, but also additional mechanisms that involve pulmonary congestion, prolonged circulation time, and subclinical lung disease in patients with cardiovascular disease may also increase hypoxemic burden. Pooling pathophysiologically distinct end points such as strokes and myocardial infarction with different cause-effect relationships into one composite outcome may partially mask the effects of a specific exposure such as hypoxemia on individual outcomes. This has important implications for interventions that target or are being guided by a specific variable that may be associated heterogeneously with a component of the composite outcome. It also affects the selection of components in composite outcomes and sample size requirements in future clinical studies.
The duration of respiratory events and cycle length of hypopneas have been studied previously in central sleep apnea and Cheyne-Stokes respiration.26, 27, 28 Our study examined additional features of episodic desaturation events to determine amplitudes and durations of desaturation and reoxygenation phases. The desaturation/reoxygenation relationship may provide important information about hemodynamics and cardiac performance. Increased sensitivity of peripheral and central chemoreceptors and prolonged circulation time may contribute to dysregulation of respiratory control and prolong respiratory events.29, 30, 31 Obstructive respiratory events typically show a slower desaturation phase followed by steeper resaturation, although central apneas show a more symmetric pattern.6 In this study, a lower desaturation/resaturation time ratio, which is suggestive of a more central pattern of breathing instability, potentially indicates preexisting subclinical or emerging cardiac dysfunction despite exclusion of participants with frank heart failure and predicted high risk of heart failure during follow up. Subclinical heart failure in some study participants may have escaped the SAVE exclusion criteria and have contributed to a central sleep apnea component in some of the participants with predominant OSA in SAVE. Theoretically, the desaturation/resaturation time ratio may be helpful to separate the cause of desaturations by detecting subtle “central-like” events in overnight oximetry recordings.
Heart (or pulse) rate provided little additional predictive information about future cardiovascular risk. However, heart (pulse) rate variability, which is an indicator of autonomic regulation,32 did provide predictive value in multivariable prediction of some of the component outcomes of SAVE.
Although broad inclusion criteria were used, SAVE was a clinical trial that purposefully recruited patients in stable condition with comorbid cardiovascular disease and untreated OSA with a relatively short follow-up time and excluded those with very severe hypoxemia (ie, >10% overnight recording with Spo2 of <80%). As such, we were unable to assess outcomes in a broader range of individuals with OSA or to undertake comparisons with individuals without OSA. Moreover, we do not have detailed information about participants’ lung function or lung volume. Because the regression models often involved small numbers of events, our data are mainly hypothesis-generating and need cautious interpretation. Given the large number of comparisons made in our analyses, some statistically significant findings may reflect chance. However, particularly strong predictive utility in patients with heart failure clearly warrants validation in an independent sample and with larger numbers of participants.
Interpretation
Conventional metrics such as AHI and ODI had limited value for the prediction of future cardiovascular events in our population with moderate-to-severe OSA and preexisting cardiovascular disease. Novel oximetry-derived Spo2 metrics and shapes of episodic desaturation events had some prognostic value, but concomitant risk factors, which are highly prevalent in patients with OSA, remain the predominant determinants for secondary cardiovascular events and thus deserve intensive management. Future studies that will measure and target alternative measures of OSA severity should clarify whether nocturnal hypoxemic burden metrics represent meaningful risk markers or modifiable cardiovascular risk factors.
Acknowledgments
Author contributions: C. S. A. and R. D. M. were the primary investigators. D. L., K. A. L., P. C., R. D. M., and M. Baumert planned the analyses. K. A. L. and M. Baumert had access to raw data. M. Baumert completed all custom quantification of the oximetry data; K. A. L. carried out statistical analyses. D. Z. and M. Barnes provided advice on statistical methods. D. L., P. C., P. S., C. S. A., W. Q., S. R., and R. D. M. provided insight into the interpretation of the results. D. L., K. A. L., R. D. M., and M. Baumert drafted the manuscript. All of the authors read and approved the manuscript for submission.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: D. L. reports having served on the advisory board of LivaNova and Medtronic, having received lecture and/or consulting fees from Bayer, LivaNova, Medtronic, and ResMed, and having received research funding from Sanofi, ResMed and Medtronic. P. S. reports having served on the advisory board of Biosense-Webster, Medtronic, Abbott, Boston Scientific, and CathRx; he also reports that the University of Adelaide has received, on his behalf, lecture and/or consulting fees from Biosense-Webster, Medtronic, Abbott, and Boston Scientific and that the University of Adelaide has received, on his behalf, research funding from Medtronic, St Jude Medical, Boston Scientific, Biotronik, and LivaNova. R. D. M. reports receiving research funding from Philips Respironics, ResMed, and Fisher & Paykel. None declared (C. S. A., S. R., P. C., K. A. L., D. Z., W. Q., M. Barnes, and M. Baumert).
∗SAVE (Sleep Apnea Cardiovascular Endpoints) Investigators: For list of investigators, see e-Appendix 1.
Other Contributions: The authors thank David Tickner for exporting ApneaLink data and all SAVE investigators, collaborators, and participants.
Additional Information: The e-Appendix, e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
D. Z. is currently at Centre for Big Data Research in Health, University of New South Wales (Sydney, NSW, Australia).
Drs Linz and Loffler share first authorship.
FUNDING/SUPPORT: The SAVE (Sleep Apnea Cardiovascular Endpoints) trial was funded by project grants 1006501 and 1060078 from the National Health and Medical Research Council (NHMRC) of Australia and by the Respironics Sleep and Respiratory Research Foundation and Philips Respironics. Supplementary trial funding was provided by Fisher & Paykel Healthcare and the Australasian Sleep Trials Network (enabling grant 343020 from the NHMRC). In-kind donations were provided by Philips Respironics for CPAP equipment and by ResMed for sleep apnea diagnostic devices. S. R. was supported in part by NIH R35HL135818. P. S. and R. D. M. were supported by Practitioner Fellowships from the National Health and Medical Research Council of Australia.
Supplementary Data
References
- 1.Linz D., Woehrle H., Bitter T. The importance of sleep-disordered breathing in cardiovascular disease. Clin Res Cardiol. 2015;104(9):705–718. doi: 10.1007/s00392-015-0859-7. [DOI] [PubMed] [Google Scholar]
- 2.Marin J.M., Carrizo S.J., Vicente E., Agusti A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 3.McEvoy R.D., Antic N.A., Heeley E. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 4.Yu J., Zhou Z., McEvoy R.D. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156–166. doi: 10.1001/jama.2017.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drager L.F., McEvoy R.D., Barbe F., Lorenzi-Filho G., Redline S., INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists) Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840–1850. doi: 10.1161/CIRCULATIONAHA.117.029400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linz D., Baumert M., Catcheside P. Assessment and interpretation of sleep disordered breathing severity in cardiology: clinical implications and perspectives. Int J Cardiol. 2018;271:281–288. doi: 10.1016/j.ijcard.2018.04.076. [DOI] [PubMed] [Google Scholar]
- 7.Mazzotti DR, Keenan BT, Lim DC, Gottlieb DJ, Kim J, Pack AI. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493-506. [DOI] [PMC free article] [PubMed]
- 8.Butler M.P., Emch J.T., Rueschman M. Apnea-hypopnea event duration predicts mortality in men and women in the Sleep Heart Health study. Am J Respir Crit Care Med. 2019;199(7):903–912. doi: 10.1164/rccm.201804-0758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oldenburg O., Wellmann B., Buchholz A. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J. 2016;37(21):1695–1703. doi: 10.1093/eurheartj/ehv624. [DOI] [PubMed] [Google Scholar]
- 10.Baumert M., Immanuel S.A., Stone K.L. Composition of nocturnal hypoxemic burden and its prognostic value for cardiovascular mortality in older community-dwelling men. Eur Heart J. 2020;41(4):533–541. doi: 10.1093/eurheartj/ehy838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone K.L., Blackwell T.L., Ancoli-Israel S. sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep. 2016;39(3):531–540. doi: 10.5665/sleep.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azarbarzin A., Sands S.A., Stone K.L. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health study. Eur Heart J. 2019;40(14):1149–1157. doi: 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gellen B., Canouï-Poitrine F., Boyer L. Apnea-hypopnea and desaturations in heart failure with reduced ejection fraction: are we aiming at the right target? Int J Cardiol. 2016;203:1022–1028. doi: 10.1016/j.ijcard.2015.11.108. [DOI] [PubMed] [Google Scholar]
- 14.Asano K., Takata Y., Usui Y. New index for analysis of polysomnography, ‘integrated area of desaturation’, is associated with high cardiovascular risk in patients with mild to moderate obstructive sleep apnea. Respiration. 2009;78(3):278–284. doi: 10.1159/000202980. [DOI] [PubMed] [Google Scholar]
- 15.Xie J., Sert Kuniyoshi F.H., Covassin N. Nocturnal hypoxemia due to obstructive sleep apnea is an independent predictor of poor prognosis after myocardial infarction. J Am Heart Assoc. 2016;5(8):e003162. doi: 10.1161/JAHA.115.003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linz D., Colling S., Nußstein W. Nocturnal hypoxemic burden is associated with epicardial fat volume in patients with acute myocardial infarction. Sleep Breath. 2018;22(3):703–711. doi: 10.1007/s11325-017-1616-0. [DOI] [PubMed] [Google Scholar]
- 17.Khoshkish S., Hohl M., Linz B. The association between different features of sleep-disordered breathing and blood pressure: a cross-sectional study. J Clin Hypertens (Greenwich) 2018;20(3):575–581. doi: 10.1111/jch.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry R.B., Brooks R., Gamaldo C. AASM Scoring Manual updates for 2017 (version 2.4) J Clin Sleep Med. 2017;13(5):665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowie M.R. Sleep-disordered breathing: how should we judge its severity? Eur Heart J. 2016;37(21):1704–1706. doi: 10.1093/eurheartj/ehv691. [DOI] [PubMed] [Google Scholar]
- 20.Antic N.A., Heeley E., Anderson C.S. The Sleep Apnea cardioVascular Endpoints (SAVE) trial: rationale, ethics, design, and progress. Sleep. 2015;38(8):1247–1257. doi: 10.5665/sleep.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEvoy R.D., Anderson C.S., Antic N.A. The Sleep Apnea Cardiovascular Endpoints (SAVE) trial: rationale and start-up phase. J Thorac Dis. 2010;2(3):138–143. doi: 10.3978/j.issn.2072-1439.2010.02.03.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heeley E., Billot L., Anderson C.S. Statistical analysis plan for the Sleep Apnea cardioVascular Endpoints study: an international randomised controlled trial to determine whether continuous positive airways pressure treatment for obstructive sleep apnea in patients with CV disease prevents secondary cardiovascular events. Int J Stroke. 2016;11(1):148–150. doi: 10.1177/1747493015607504. [DOI] [PubMed] [Google Scholar]
- 23.Linz D., Kadhim K., Brooks A.G. Diagnostic accuracy of overnight oximetry for the diagnosis of sleep-disordered breathing in atrial fibrillation patients. Int J Cardiol. 2018;272:155–161. doi: 10.1016/j.ijcard.2018.07.124. [DOI] [PubMed] [Google Scholar]
- 24.Punjabi N.M., Newman A.B., Young T.B., Resnick H.E., Sanders M.H. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177(10):1150–1155. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J.S., Podolanczuk A.J., Borker P. Obstructive sleep apnea and subclinical interstitial lung disease in the Multi-Ethnic Study of Atherosclerosis (MESA) Ann Am Thorac Soc. 2017;14(12):1786–1795. doi: 10.1513/AnnalsATS.201701-091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efken C., Bitter T., Prib N., Horstkotte D., Oldenburg O. Obstructive sleep apnoea: longer respiratory event lengths in patients with heart failure. Eur Respir J. 2013;41(6):1340–1346. doi: 10.1183/09031936.00082212. [DOI] [PubMed] [Google Scholar]
- 27.Hall M.J., Xie A., Rutherford R., Ando S., Floras J.S., Bradley T.D. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med. 1996;154(2 Pt 1):376–381. doi: 10.1164/ajrccm.154.2.8756809. [DOI] [PubMed] [Google Scholar]
- 28.Wedewardt J., Bitter T., Prinz C., Faber L., Horstkotte D., Oldenburg O. Cheyne-Stokes respiration in heart failure: cycle length is dependent on left ventricular ejection fraction. Sleep Med. 2010;11(2):137–142. doi: 10.1016/j.sleep.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Floras J.S. Sleep apnea and cardiovascular disease: an enigmatic risk factor. Circ Res. 2018;122(12):1741–1764. doi: 10.1161/CIRCRESAHA.118.310783. [DOI] [PubMed] [Google Scholar]
- 30.Javaheri S., Barbe F., Campos-Rodriguez F. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linz D., McEvoy R.D., Cowie M.R. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3(6):532–540. doi: 10.1001/jamacardio.2018.0095. [DOI] [PubMed] [Google Scholar]
- 32.Nikolovska Vukadinović A., Vukadinović D., Borer J. Heart rate and its reduction in chronic heart failure and beyond. Eur J Heart Fail. 2017;19(10):1230–1241. doi: 10.1002/ejhf.902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.