Abstract
Background
Randomized clinical trials have failed to show benefit from increasing intensity of renal replacement therapy (RRT) for acute kidney injury, but continue to be frequently used. In addition, intensive RRT is associated with an increase in adverse events potentially secondary to small solute losses, such as phosphate. We hypothesized that, compared with less-intensive RRT, intensive RRT would lead to longer duration of mechanical ventilation.
Research Question
Does more-intensive renal replacement therapy in critically ill patients with acute kidney injury increase time to extubation from mechanical ventilation when compared with less-intensive therapy?
Study Design and Methods
The Acute Renal Failure Trial Network study was a randomized multicenter trial of more-intensive (hemodialysis or sustained low-efficiency dialysis six times per week or continuous venovenous hemodiafiltration at 35 mL/kg per hour) vs less-intensive (hemodialysis or sustained low-efficiency dialysis three times per week or continuous venovenous hemodiafiltration at 20 mL/kg per hour) RRT in critically ill patients with acute kidney injury. Of 1124 patients, 907 who were supported by mechanical ventilation on study initiation were included in this Cox-proportional hazards analysis. The primary outcome was the time to first successful extubation off mechanical ventilation.
Results
Patients who were assigned randomly to more-intensive RRT had a 33.3% lower hazard rate of successful extubation (hazard ratio, 0.67; 95% CI, 0.52-0.88; P < .001) when compared with patients who were assigned to less-intensive RRT. Patients who were assigned to more-intensive RRT had, on average, 2.07 ventilator-free days, compared with 3.08 days in those who were assigned to less-intensive RRT (P < .001) over 14 days from start of the study.
Interpretation
Critically ill mechanically ventilated patients who were assigned randomly to more-intensive RRT had longer duration of mechanical ventilation compared with those who were assigned to less-intensive RRT. The reasons for this, such as excessive phosphate loss from more-intensive RRT, deserve further study to optimize the safety and effectiveness of CRRT delivery.
This was a post hoc analysis of the Acute Renal Failure Trial Network study; clinical trial registration of the original trial is NCT00076219.
Key Words: acute kidney injury, critical care, mechanical ventilation, renal replacement therapy intensity
Abbreviations: AKI, acute kidney injury; ATN, Acute Renal Failure Trial Network; CRRT, continuous renal replacement therapy; CVVHDF, continuous venovenous hemodiafiltration; HR, hazard ratio; IHD, intermittent hemodialysis; RRT, renal replacement therapy; SLED, sustained low-efficiency dialysis
FOR EDITORIAL COMMENT, SEE PAGE 1303
Acute kidney injury (AKI) is a worldwide epidemic that contributes to two million deaths per year and has created a heavy economic burden on society.1 Hospital-acquired AKI is associated independently with increased risks of death and prolonged length of hospital stay.2 Unfortunately, there is no effective treatment beyond supportive care for the majority of cases with established AKI from conditions such as sepsis or hypotension. Severe AKI may require support through intermittent or continuous renal replacement therapy (CRRT) with a goal to temporarily replace some of the life-sustaining functions of the kidneys while the kidneys recover function. In the multinational AKI-Epidemiological Prospective Investigation study of 1802 critically ill patients who were admitted to 97 ICUs from 2009 to 2010, 243 patients (13.5%) experienced AKI-renal replacement therapy (RRT) within one week of ICU admission.3
Both continuous and intermittent therapies have been used for severe AKI. CRRTs are commonly used in the treatment of hemodynamically unstable patients with severe AKI, for whom conventional intermittent dialytic modalities are difficult to administer. Although CRRT was introduced more than three decades ago with anticipated physiologic superiority compared with intermittent therapies, there is a lack of evidence to suggest improvement in clinical outcomes.4, 5, 6, 7, 8 There has also been no demonstrable benefit of increasing RRT dose in randomized controlled trials.9 In fact, increasing intensity of RRT is not innocuous. There are several known adverse consequences that are associated with a greater dose including electrolyte abnormalities such as hypophosphatemia and hypokalemia,10 enhanced elimination of antibiotics that leads to inadequate dosing,11 excessive nutrient losses such as amino acids and proteins,12 and lower urine output.13
We wished to test whether small solute loss through RRT is a potential adverse effect with clinically relevant consequences. We hypothesized that more-intensive RRT would have more adverse effects, such as longer duration of mechanical ventilation, potentially as a result of phosphate or other small solute losses during RRT. Hypophosphatemia is a frequent complication during continuous RRT14, 15, 16 and may contribute to poor patient outcomes due to phosphate's critical role in energy metabolism within every organ system. Hypophosphatemia can impair contractile properties of the diaphragm and may impair tissue oxygen extraction by reducing erythrocyte 2,3 diphosphoglycerate concentrations.17 Through a post hoc analysis of a randomized controlled trial testing intensive vs less-intensive RRT in critically ill patients, we sought to assess the effect of treatment assignment (more-intensive arm vs less-intensive arm) on the duration of mechanical ventilation, as a potential adverse outcome of RRT-induced phosphate depletion.
Methods
Study Population and Design
The Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network (ATN) study was a prospective multicenter randomized clinical trial testing strategies of more-intensive RRT vs less-intensive RRT in critically ill patients with a clinical diagnosis of acute tubular necrosis (N = 1124).10 All patients or their surrogates provided written informed consent, and the study was approved by the Human Rights Committee at the West Haven Veterans Affairs Cooperative Studies Program Coordinating Center and the institutional review boards at each of the participating sites. Eligible patients were ≥18 years old, critically ill with acute renal failure and in need of RRT, and accompanied by sepsis or failure of at least one nonrenal organ system. Patients were assigned randomly to two different strategies of intensity of RRT with the use of a centralized computer-generated adaptive randomization scheme. In the group that received the more-intensive therapy strategy, intermittent hemodialysis (IHD) and sustained low-efficiency dialysis (SLED) were provided six times per week; continuous venovenous hemodiafiltration (CVVHDF) was prescribed to provide a total effluent flow rate of 35 mL/kg of bodyweight per hour. In the less-intensive strategy, IHD and SLED were provided three times per week, and CVVHDF was prescribed to provide a total effluent flow rate of 20 mL/kg per hour. Assigned interventions were delivered for up to 28 days after randomization or until renal recovery, discharge from the hospital, withdrawal of care, or death. Patients were followed for up to 60 days to ascertain the primary endpoint of all-cause death. Patients were excluded if they had received >24 hours of CRRT or more than one session of SLED before randomization, had an expected survival of <28 days secondary to an underlying chronic condition, or had baseline kidney disease (creatinine level >2 mg/dL in male patients and 1.5 mg/dL in female patients).
Exposures and Outcomes
We obtained deidentified data from the National Institute of Diabetes and Digestive and Kidney Diseases ATN study data repository. The study protocol was approved by the Partners Institutional Review Board. We excluded all patients who were not on mechanical ventilation on the first day of study therapy. Demographic data (age, sex, race), comorbid data (high BP, diabetes mellitus, malignancy, Sequential Organ Failure Assessment score, congestive heart failure, previous myocardial infarction, chronic hypoxemia), and weight were recorded at baseline for all study participants. The primary exposure of interest was the randomized treatment assignment of more-intensive vs less-intensive RRT. The primary outcome was the time to first successful extubation off mechanical ventilation in patients from study initiation through study day 14. First extubation was defined as at least 48 consecutive hours independent of ventilator support. Because duration of mechanical ventilation was highly influenced by death and prevented the primary event (extubation) from occurring, we used competing risk regression methods, including estimates of instantaneous risk. This method recognizes that death is related strongly to future prognosis and allows for an association between competing event (death) and outcome of interest (extubation). This is specifically important in our study, given the high rates of mortality in critically ill patients with AKI requiring dialysis.18 Hypophosphatemic event was defined as serum phosphate values <2.5 mg/dL.
Statistical Analyses
We examined categoric variables by frequency distribution, recorded them as proportions, and made comparisons using the chi-square test. We examined continuous variables, which were recorded as the mean (SD) or medians (interquartile range) and compared use of t-tests or Wilcoxon rank sum tests, as appropriate. We used a zero-inflated Poisson regression model with right censoring of data to compare ventilator-free days through day 14 between treatment arms. Terminal extubation was not counted as an event. We calculated ventilator-free days during the first 14 days of study therapy as the days alive minus days receiving mechanical ventilation after study initiation. If a patient died, the number of ventilator-free days were the number of days between extubation and death. We performed all statistical analyses with SAS software (version 9.4; SAS Institute Inc, Cary, NC).
We used the Kaplan-Meier method to estimate survival for both treatment groups and compared time to extubation between groups with the use of two-sided log rank test. We estimated unadjusted hazard ratios (HRs) and corresponding two-sided 95% CIs using the Cox-proportional hazards model (unadjusted model) and fit a multivariable model that adjusted for baseline age, sex, race (black vs non-black), weight, ischemic heart disease, congestive heart failure, chronic hypoxemic condition, hypertension, diabetes mellitus, and fluid balance. Subsequently, the final model was fit with the same covariates as in the multivariable model along with prespecified time-varying covariate of interest (cardiovascular sequential organ failure assessment score (cardiovascular score: 0, 1, 2, 3, 4). We used proportional subdistribution hazards (competing risk) regression as described by Fine and Gray19 to examine the effects of treatment assignment on time to first extubation, treating death as a competing risk. We performed subgroup analyses according to cause of ICU admission and treatment type (CRRT vs IHD) modeled as a time varying covariate. We assessed subgroup effects by testing the interaction of treatment group and subgroup treatment type and used stratified log-rank tests by treatment type to compare the randomized treatment assignment groups. We based model selection on previous clinical knowledge. We assessed the proportional hazards assumptions, verified them using time-varying covariate methods, and found no violations of the proportional hazards assumption used in the model. A probability value of .05 was considered to indicate statistical significance.
Results
A total of 1124 participants were assigned randomly across 27 centers to participate in the ATN study; of these, 907 were on mechanical ventilation on day 1 of the study and constitute the cohort for the current analysis. The cohort had a mean age of 59 ± 15.3 years and a mean weight of 84.5 ± 19 kg. The majority were men (70.4%), and 15.9% were black; 27.3% had diabetes mellitus, and 15.9% reported a known malignancy. Twenty-one percent had known congestive heart failure, and 10% had a history of chronic hypoxemia. There were no significant differences between individuals who were assigned randomly to more-intensive and less-intensive RRT (Table 1).
Table 1.
Baseline Characteristics of Study Patients Who Were Ventilated Mechanically on Day 1 According to Renal Replacement Therapy Intensity Randomized Groups
| Characteristic | Less-Intensive Strategya | More-Intensive Strategyb | P Valuec |
|---|---|---|---|
| Age, mean ± SD, y | 59.3 ± 15.3 | 59.3 ± 15.3 | .95 |
| Male, % | 68.5 | 72.3 | .21 |
| Black, % | 15.5 | 16.4 | .71 |
| Mean weight, mean ± SD, kg | 84.2 ± 19 | 83.6 ± 19.5 | .67 |
| Patients alive at 14 days, n/No. (%) | 288/451 (63.8) | 293/456 (64.2) | .75 |
| Hypertension, % | 2.6 | 2.8 | .20 |
| Diabetes mellitus, % | 24.6 | 30 | .14 |
| Malignancy, % | 13.5 | 18.4 | .11 |
| Ischemic heart disease, % | 21.3 | 21.7 | .73 |
| Congestive heart failure, % | 21 | 20.6 | .80 |
| Primary treating service, n/No. (%) | .65 | ||
| Medical | 198/451 (43.9) | 214/456 (46.9) | |
| Surgical | 201/451 (44.5) | 191/456 (41.8) | |
| Other | 52/451 (11.5) | 51/456 (11.1) | |
| Mode of renal replacement therapy on day 1, n/No. (%) | .14 | ||
| Continuous venovenous hemodiafiltration | 314/388 (80.9) | 313/395 (79.2) | |
| Intermittent hemodialysis | 53/388 (13.6) | 61/395 (15.4) | |
| Sustained low efficiency dialysis | 15/388 (3.9) | 20/395 (5.0) | |
| Isolated ultrafiltration | 6/388 (1.5) | 1/395 (0.2) | |
| Chronic hypoxemia, % | 9 | 10.9 | .39 |
| Cardiovascular Sequential Organ Failure Assessment score (range) | 3 (0 to 4) | 3 (0 to 4) | 1 |
| Apache II score, mean ± SD | 27.5 ± 7.1 | 28.1 ± 6.7 | .2 |
| Serum calcium, mean ± SD, (mg/dL) | 7.5 ± 1.1 | 7.5 ± 1 | .71 |
| Serum creatinine, mean ± SD, mg/dL | 3.6 ± 1.5 | 3.6 ± 1.5 | .72 |
| Serum phosphate, mean ± SD, mg/dL | 5.4 ± 2.2 | 5.4 ± 2.1 | 1 |
| Serum albumin, mean ± SD, g/dL | 2.3 ± 0.3 | 2.3 ± 0.4 | 1 |
No. of participants with events = 154/451.
No. of participants with events = 113/456.
P value for difference; significant testing was done by χ2 for categoric variables and by t- test for continuous variables.
Effect of Treatment Assignment on Mechanical Ventilation
By 14 days, there was no difference in survival between the two treatment assignments (64.3 % of patients were alive in the more-intensive arm compared with 63.9% in the less-intensive arm). Patients who had been assigned randomly to more-intensive RRT had on average 2.07 ventilator-free days, compared with 3.08 days in those assigned to less-intensive RRT (P < .001) over 14 days from start of the study. Results were similar with the use of censor zero-inflated Poisson model (1.51 ± 0.13 fewer ventilator-free days in those assigned to more-intensive vs less-intensive RRT; P < .001). Among the 267 patients who were extubated successfully off ventilation for at least 48 hours in the two groups, reintubation was later performed in 19% of patients in the more-intensive treatment arm compared with 10% in less-intensive arm (P = .03).
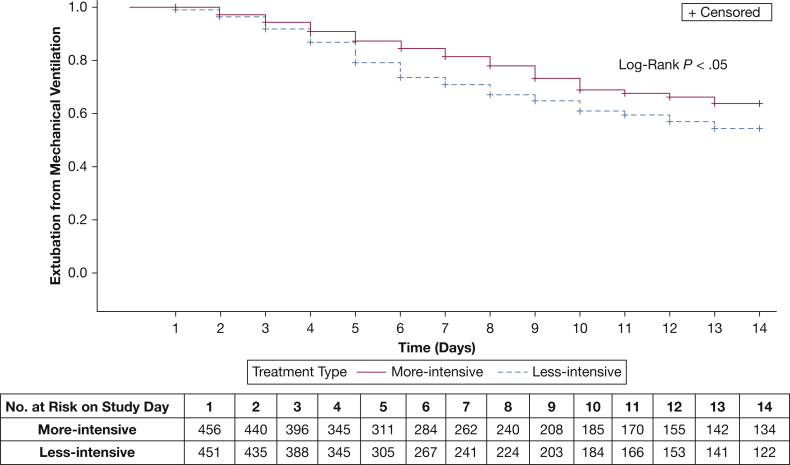
In unadjusted analyses, patients randomly assigned to more-intensive RRT had a 33.3% lower hazard rate of successful extubation (HR, 0.67; 95% CI, 0.53-0.85; P < .001) when compared with patients assigned to the less-intensive RRT (Fig 1). The unadjusted results were not different in the subgroup of patients with sepsis, including pulmonary infections (HR, 0.62; 95% CI, 0.43- 0.85; P < .001). When adjusted for baseline covariates, the effect estimate was unchanged and remained statistically significant (HR, 0.67; 95% CI, 0.52- 0.88; P < .001) (Table 2). Considering death as a competing risk and with the use of proportional sub distribution hazards, the effect estimate was accentuated and remained statistically significant (HR, 0.63; 95% CI, 0.49-0.80; P < .001).
Figure 1.
Kaplan-Meier survival estimates for time to extubation from mechanical ventilation according to treatment intensity. Time to extubation was shorter in the less-intensive arm (blue dashed lines) than the more-intensive arm (red solid lines); the table shows the number of participants who were at-risk.
Table 2.
Cox-Proportional Hazard Modelsa
| Variable | Participants With Events, n/No. | HR (95% CI) |
|
|---|---|---|---|
| Unadjusted Model | Multivariable-Adjusted Modelb | ||
| Overall study sample | 267/907 | 0.67 (0.53-0.85) | 0.67 (0.52-0.88) |
| Subgroup analyses | |||
| Sepsis | 137/495 | 0.62 (0.43-0.86) | 0.60 (0.42-0.85) |
| Baseline serum phosphate | |||
| Tertile 1 (1.2-4.3 mg/dL) | 87/242 | 0.56 (0.36-0.87; P < .05) | 0.57 (0.37-0.88; P < .05) |
| Tertile 2 (4.4-6.3 mg/dL) | 83/257 | 0.76 (0.49-1.1; P = .21) | 0.78 (0.5-1.2; P = .28) |
| Tertile 3 (6.4-15.3 mg/dL) | 56/244 | 0.71 (0.42-1.2; P = .21) | 0.65 (0.37-1.1; P = .13) |
With endpoint of first extubation from mechanical ventilation according to renal replacement therapy intensity randomized groups with adjustment by prespecified covariates along with subgroup analysis; the referent group is the less-intensive arm.
Adjusted for baseline age, sex, race (black vs non-black), weight, fluid balance, ischemic heart disease, congestive heart failure, chronic hypoxemic condition, hypertension, diabetes mellitus, and the cardiovascular component of the sequential organ failure assessment score (0, 1, 2, 3, or 4).
Effect of Treatment Assignment on Phosphate
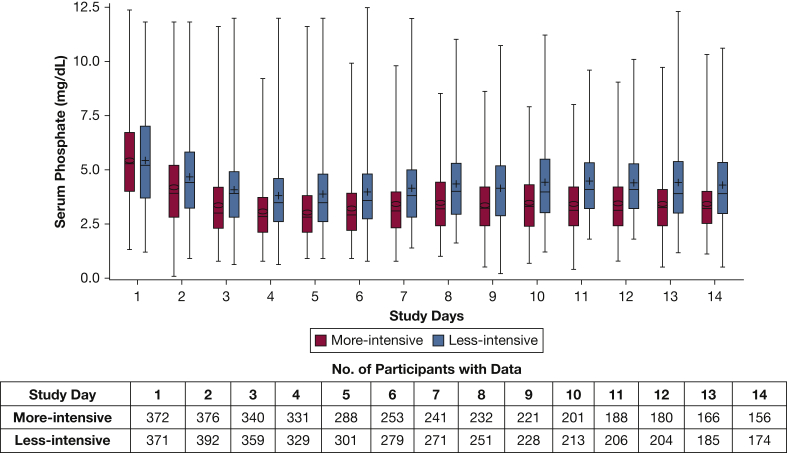
Baseline serum phosphate concentrations were similar in individuals on mechanical ventilation across the two treatment arms (Table 1). Figure 2 shows the daily serum phosphate from days 1 through 14 according to randomized treatment intensity assignment. We used mixed effect models to examine changes in subsequent serum phosphate during the study course between the two treatment arms with considerations of repeated measures. There was a significantly greater reduction in serum phosphate (1.9 ± 0.1 mg/dL) in the more-intensive group as compared with the less-intensive treatment group (1.0 ± 0.1 mg/dL; P < .001). Mean nadir serum phosphate was lower in the more-intensive group compared with the less intensive group (2.6 ± 1.7 mg/dL vs 3.1 ± 1.6 mg/dL; P < .05). Sixty-one percent of the patients who were assigned randomly to more-intensive treatment had at least one hypophosphatemic event (phosphate <2.5 mg/dL) compared with 38% in the less-intensive group over 2 weeks of the study (P < .05).
Figure 2.
Daily phosphate concentrations from day 1 through day 14 according to renal replacement therapy intensity (less intensive is indicated in blue; more intensive is indicated in red); upper and lower lines of the box represent the 75th percentile and 25th percentiles; upper and lower lines represent maximum and minimum values; the Table shows the number of participants with available data on serum phosphate by study day.
Treatment Type and Phosphate-Subgroup Analysis
We conducted subgroup analyses according to baseline serum phosphate concentrations (Table 2). We found evidence of statistical interaction between randomized group and baseline phosphate tertiles (P = .04) for the outcome of time to extubation that showed the presence of effect modification. Within the lowest tertile of baseline phosphate, 78% of patients (189/242) had at least one episode of hypophosphatemia during the study course. Among patients with the lowest concentrations of baseline serum phosphate (1.2 to 4.3 mg/dL), those who had been assigned randomly to more-intensive RRT had a 43% lower hazard rate of successful extubation than patients who had been assigned to less-intensive RRT (HR, 0.56; 95% CI, 0.36-0.87; P < .05). The results in the middle tertile (4.4-6.3 mg/dL) and highest tertile (6.4-15.3 mg/dL) of baseline serum phosphate were in the same direction but did not reach statistical significance, with HR of 0.76 (95% CI, 0.49-1.1; P = .21) and HR 0.71 (95% CI, 0.42-1.2; P = .21), respectively.
We also performed analyses using treatment type (IHD, CVVHDF, and SLED) as a time-dependent variable. Among patients who were on CVVHDF and SLED, there was a 37.1% lower hazard rate of successful extubation (HR, 0.63; 95% CI, 0.42-0.93; P < .05) among patients who were assigned randomly to the more-intensive treatment arm compared with the less-intensive arm. Among patients who were on IHD, there was a statistically nonsignificant 21.4% lower HR of extubation (HR, 0.78; 95% CI, 0.58-1.1; P = .12) in patients assigned randomly to the more-intensive treatment arm compared with the less-intensive arm.
Discussion
In this secondary analysis of a randomized controlled trial that compared strategies of more-intensive and less-intensive RRT in critically ill patients with AKI, we found that mechanically ventilated patients who were assigned randomly to more-intensive RRT had a longer duration of mechanical ventilation compared with those who were assigned to less-intensive RRT. Among patients who were successfully extubated initially, there was a significantly higher probability of reintubation in those who were assigned to more-intensive RRT. Notably, during the study course, we found greater reduction in serum phosphate in the more-intensive treatment arm compared with the less-intensive arm. Furthermore, among those within lowest baseline serum phosphate concentrations, there was less likelihood of being extubated in the more-intensive RRT compared with less-intensive RRT.
There are several potential explanations for our finding that more-intensive RRT may increase the duration of mechanical ventilation. Several iatrogenic complications that are associated with a higher dose of RRT have been cited in RRT literature.20,11 Nutritional status is a key prognostic factor in critically ill patients with AKI. It is plausible that nutritional status is compromised by depletion of vital nutrients with increased dose RRT, particularly in continuous therapies.21 Our finding that mechanical ventilation was more prolonged in those patients on continuous RRT rather than intermittent RRT is consistent with the hypothesis that excessive losses through continuous RRT may be the underlying reason for our observed finding.
Excessive loss of phosphate may be an explanation of our findings. We previously have shown that CRRT leads to net negative phosphate balance (median, 1.2 g/d; range, -0.2 to -6.5 g), despite protocol-driven phosphate repletion strategies if there is a lack of phosphate additives in the dialysate or replacement fluids.22 Unintended loss of phosphate leading to hypophosphatemia has been reported as a complication in more than 10% of patients undergoing CRRT.14,15,20 In the original report of the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network, hypophosphatemia was reported in 17.6% in those patients who were assigned randomly to more-intensive RRT vs 10.9% in those assigned to less-intensive RRT. Our findings, which were restricted to those on mechanical ventilation at the time of study initiation, showed similar results with a clear downward trend of phosphate over time. Although negative phosphate balance may be desirable earlier in the treatment of dialysis-requiring AKI, it may be detrimental to have continuous losses and negative phosphate balance during prolonged CRRT. Our findings were most pronounced in those within the lowest tertile of serum phosphate at baseline, which further highlights the importance of phosphate loss as a potential explanation of our findings.
Phosphate is the most abundant intracellular anion and is essential for multiple biologic functions that include nucleic acid synthesis, energy exchange, membrane transport, and intracellular signal transduction. Phosphate has been shown to have direct effects on the diaphragm and on cellular availability of oxygen. Therefore, obligate phosphate losses may have a direct pathway for impacting pulmonary outcomes. Being primarily intracellular, phosphate orchestrates several vital enzymatic steps that include a key role in RBC function and oxygen transport by influencing the intracellular concentration of 2,3-diphosphoglycerate, a crucial allosteric effector of hemoglobin's affinity for oxygen. We have demonstrated that CRRT-induced phosphate removal is associated with measurable reductions in RBC 2,3-diphosphoglycerate concentration and a corresponding shift in the oxygen hemoglobin affinity curve along with higher risk of in-hospital death.17
Phosphate depletion is a plausible contributor to diaphragmatic and intercostal muscle weakness, which may account for our findings. Aubier et al23 observed significant increase in transdiaphragmatic pressure and response to phrenic nerve stimulation after correction of hypophosphatemia in eight patients who were ventilated mechanically ventilated. In a prospective study of 23 patients with hypophosphatemia, Gravelyn et al24 found respiratory muscle weakness (defined as reduction in maximal inspiratory pressure or maximal expiratory pressure) in 16 patients. In addition, the mean initial respiratory pressures were significantly lower in the hypophosphatemic group compared to the control group with normal phosphate concentrations.
Other studies have also found that hypophosphatemia may be associated with worse pulmonary outcomes. In a single-center observational study of 321 patients with AKI on continuous dialysis, Demirjian et al25 found hypophosphatemia to be associated with higher incidence of prolonged respiratory failure that required tracheostomy (OR, 1.81; 95% CI, 1.07-3.08). In a single center prospective cohort study of 96 patients in intensive care with AKI that required RRT, Lim et al26 found that hypophosphatemia during RRT was associated independently with prolonged mechanical ventilation (≥7 days; adjusted OR, 14.0; 95% CI, 1.37-143.90; P = .03). The finding that hypophosphatemia was associated with poor outcomes in patients on mechanical ventilation in observational studies may be confounded by other factors such as malnutrition. A key strength of our analysis is that we used randomized treatment assignment as a proxy for unregulated solute loss.
Several studies have examined outcomes associated with hypophosphatemia outside the context of AKI-RRT. In a retrospective analysis of 118 pediatric patients who were admitted to the ICU, Kilic et al27 found a significant difference in the duration of mechanical ventilation: 7.7 days in the hypophosphatemia group compared with 4.1 days in the normophosphatemia group. In addition, length of ICU stay was 9.7 days in the hypophosphatemia group compared with 5 days in the normophosphatemia group. Alsumrain et al28 conducted a prospective analyses on 66 patients in ICU and found hypophosphatemia to be associated with a 18% greater risk of failure to extubate from mechanical ventilation.
The implications of our study deserve to be highlighted, particularly given that more- intensive RRT continues to be prescribed frequently, which is evidenced by surveys from critical care physicians worldwide29,30 More-intensive RRT has not been shown to improve outcomes and may, as we have shown, have deleterious effects. If our findings are from excessive losses of small solutes such as phosphate, careful monitoring and repletion strategies should be implemented.
Our study had several strengths. We assessed the outcome of time on mechanical ventilation in critically ill patients who were enrolled in a randomized controlled trial with detailed data collection on demographics, comorbid conditions, laboratory data, and clinical outcomes. The ATN study allowed us to adjust our analyses to account for the number of covariates, which were evenly balanced in our post hoc analyses, minimizing residual confounding.
Our study has several important limitations. Although the data were generated from 27 centers, recruitment was predominantly in tertiary-care academic medical centers and Veterans Administration hospitals, which limited generalizability. Even though this is a secondary analysis of a well-designed clinical trial, it was not designed primarily to assess the effect of different levels of serum phosphorus during the ICU stay on the outcomes. Serum phosphorus was not measured uniformly or consistently for all participants, so we were not able to adjust for daily phosphate or test for mediation. In addition, we also lacked data on phosphorus supplementation.
In conclusion, more-intensive RRT may lead to a greater risk of failure to extubate from mechanical ventilation, potentially from a loss of small solutes such as phosphate. More aggressive repletion strategies of phosphate or other small solutes, potentially as additions to replacement or dialysate solutions,31, 32, 33 should be tested to optimize the safety and effectiveness of CRRT delivery.
Acknowledgments
Author contributions: S. S. designed the study, performed the statistical analyses, interpreted the data, and wrote the manuscript. Y. P. K. assisted with data interpretation. P. M. P. was responsible for overseeing patient enrollment, sample collection, and assisted with data interpretation. S. S. W. participated in study design, statistical analysis, and data interpretation. All authors provided assistance in critically revising the manuscript, approving the final version to be published, and agreeing to be accountable for all aspects of the work.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. S. W. reports personal fees from Public Health Advocacy Institute, CVS, Roth Capital Partners, Kantum Pharma, Mallinckrodt, Wolters Kluewer, GE Health Care, GSK, Mass Medical International, Barron and Budd (vs. Fresenius), JNJ, Venbio, Strataca, Takeda, Cerus, Pfizer, Bunch and James, and grants and personal fees from Allena Pharmaceuticals. P. M. P. reports grant support from National Institutes of Diabetes and Digestive and Kidney Diseases [grants: UH3 DK114861 and R01 DK106256], VA Office of Research & Development Cooperative Studies Program, Dascena and BioPorto and personal fees from Baxter, outside the submitted work. None declared (S. S., Y. P. K.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank Drs Earl Francis Cook, ScD and Finnian McCausland, MBBCh, MMSc, for important discussions and comments. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
Footnotes
FUNDING/SUPPORT: This work was conducted with support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic healthcare centers.
References
- 1.Ali T., Khan I., Simpson W. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18(4):1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 2.Zeng X., McMahon G.M., Brunelli S.M., Bates D.W., Waikar S.S. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12–20. doi: 10.2215/CJN.02730313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoste E.A., Bagshaw S.M., Bellomo R. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 4.Rabindranath K., Adams J., Macleod A.M., Muirhead N. Intermittent versus continuous renal replacement therapy for acute renal failure in adults. Cochrane Database Syst Rev. 2007;(3):CD003773. doi: 10.1002/14651858.CD003773.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Fieghen H.E., Friedrich J.O., Burns K.E. The hemodynamic tolerability and feasibility of sustained low efficiency dialysis in the management of critically ill patients with acute kidney injury. BMC Nephrol. 2010;11:32. doi: 10.1186/1471-2369-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta R.L., McDonald B., Gabbai F.B. A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int. 2001;60(3):1154–1163. doi: 10.1046/j.1523-1755.2001.0600031154.x. [DOI] [PubMed] [Google Scholar]
- 7.Schefold J.C., von Haehling S., Pschowski R. The effect of continuous versus intermittent renal replacement therapy on the outcome of critically ill patients with acute renal failure (CONVINT): a prospective randomized controlled trial. Crit Care. 2014;18(1):R11. doi: 10.1186/cc13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lins R.L., Elseviers M.M., Van der Niepen P. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant. 2009;24(2):512–518. doi: 10.1093/ndt/gfn560. [DOI] [PubMed] [Google Scholar]
- 9.Jun M., Heerspink H.J., Ninomiya T. Intensities of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Clin j Am Soc Nephrol. 2010;5(6):956–963. doi: 10.2215/CJN.09111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Network VNARFT. Palevsky P.M., Zhang J.H. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller B.A., Pasko D.A., Sowinski K.M. Higher renal replacement therapy dose delivery influences on drug therapy. Artif Organs. 2003;27(9):808–814. doi: 10.1046/j.1525-1594.2003.07283.x. [DOI] [PubMed] [Google Scholar]
- 12.Btaiche I.F., Mohammad R.A., Alaniz C., Mueller B.A. Amino Acid requirements in critically ill patients with acute kidney injury treated with continuous renal replacement therapy. Pharmacotherapy. 2008;28(5):600–613. doi: 10.1592/phco.28.5.600. [DOI] [PubMed] [Google Scholar]
- 13.McCausland F.R., Asafu-Adjei J., Betensky R.A., Palevsky P.M., Waikar S.S. Comparison of urine output among patients treated with more intensive versus less intensive RRT: results from the Acute Renal Failure Trial Network Study. Clin j Am Soc Nephrol. 2016;11(8):1335–1342. doi: 10.2215/CJN.10991015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santiago M.J., Lopez-Herce J., Urbano J., Bellon J.M., del Castillo J., Carrillo A. Hypophosphatemia and phosphate supplementation during continuous renal replacement therapy in children. Kidney Int. 2009;75(3):312–316. doi: 10.1038/ki.2008.570. [DOI] [PubMed] [Google Scholar]
- 15.Ratanarat R., Brendolan A., Volker G. Phosphate kinetics during different dialysis modalities. Blood Purif. 2005;23(1):83–90. doi: 10.1159/000082016. [DOI] [PubMed] [Google Scholar]
- 16.Schiffl H., Lang S.M. Severe acute hypophosphatemia during renal replacement therapy adversely affects outcome of critically ill patients with acute kidney injury. Int Urol Nephrol. 2013;45(1):191–197. doi: 10.1007/s11255-011-0112-x. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S., Brugnara C., Betensky R.A., Waikar S.S. Reductions in red blood cell 2,3-diphosphoglycerate concentration during continuous renal replacment therapy. Clin j Am Soc Nephrol. 2015;10(1):74–79. doi: 10.2215/CJN.02160214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uchino S., Kellum J.A., Bellomo R. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 19.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 20.RENAL Replacement Therapy Study Investigators. Bellomo R., Cass A. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 21.Finkel K.W., Podoll A.S. Complications of continuous renal replacement therapy. Semin Dial. 2009;22(2):155–159. doi: 10.1111/j.1525-139X.2008.00550.x. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S., Waikar S.S. Phosphate balance in continuous venovenous hemofiltration. Am J Kidney Dis. 2013;61(6):1043–1045. doi: 10.1053/j.ajkd.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aubier M., Murciano D., Lecocguic Y. Effect of hypophosphatemia on diaphragmatic contractility in patients with acute respiratory failure. N Engl J Med. 1985;313(7):420–424. doi: 10.1056/NEJM198508153130705. [DOI] [PubMed] [Google Scholar]
- 24.Gravelyn T.R., Brophy N., Siegert C., Peters-Golden M. Hypophosphatemia-associated respiratory muscle weakness in a general inpatient population. Am J Med. 1988;84(5):870–876. doi: 10.1016/0002-9343(88)90065-4. [DOI] [PubMed] [Google Scholar]
- 25.Demirjian S., Teo B.W., Guzman J.A. Hypophosphatemia during continuous hemodialysis is associated with prolonged respiratory failure in patients with acute kidney injury. Nephrol dial transplant. 2011;26(11):3508–3514. doi: 10.1093/ndt/gfr075. [DOI] [PubMed] [Google Scholar]
- 26.Lim C., Tan H., Kaushik M. Hypophosphatemia in critically ill patients with acute kidney injury treated with hemodialysis is associated with adverse events. Clin Kidney J. 2017;10(3):341–347. doi: 10.1093/ckj/sfw120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilic O., Demirkol D., Ucsel R., Citak A., Karabocuoglu M. Hypophosphatemia and its clinical implications in critically ill children: a retrospective study. J Crit Care. 2012;27(5):474–479. doi: 10.1016/j.jcrc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Alsumrain M.H., Jawad S.A., Imran N.B., Riar S., DeBari V.A., Adelman M. Association of hypophosphatemia with failure-to-wean from mechanical ventilation. Ann Clin Lab Sci. 2010;40(2):144–148. [PubMed] [Google Scholar]
- 29.Clark W.R., Ding X., Qiu H. Renal replacement therapy practices for patients with acute kidney injury in China. PloS One. 2017;12(7) doi: 10.1371/journal.pone.0178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Legrand M., Darmon M., Joannidis M., Payen D. Management of renal replacement therapy in ICU patients: an international survey. Intensive Care Med. 2013;39(1):101–108. doi: 10.1007/s00134-012-2706-x. [DOI] [PubMed] [Google Scholar]
- 31.Troyanov S., Geadah D., Ghannoum M., Cardinal J., Leblanc M. Phosphate addition to hemodiafiltration solutions during continuous renal replacement therapy. Intensive Care Med. 2004;30(8):1662–1665. doi: 10.1007/s00134-004-2333-2. [DOI] [PubMed] [Google Scholar]
- 32.Broman M., Carlsson O., Friberg H., Wieslander A., Godaly G. Phosphate-containing dialysis solution prevents hypophosphatemia during continuous renal replacement therapy. Acta Anaesthesiol Scand. 2011;55(1):39–45. doi: 10.1111/j.1399-6576.2010.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morabito S., Pistolesi V., Tritapepe L. Continuous venovenous hemodiafiltration with a low citrate dose regional anticoagulation protocol and a phosphate-containing solution: effects on acid-base status and phosphate supplementation needs. BMC Nephrol. 2013;14:232. doi: 10.1186/1471-2369-14-232. [DOI] [PMC free article] [PubMed] [Google Scholar]