Abstract
Background
Patients with COPD who experience pulmonary hypertension (PH) have worse mortality than those with COPD alone. Predictors of poor outcomes in COPD-PH are not well-described. Diffusing capacity of the lung (Dlco) assesses the integrity of the alveolar-capillary interface and thus may be a useful prognostic tool among those with COPD-PH.
Research Question
Using a single center registry, we sought to evaluate Dlco as a predictor of mortality in a cohort of patients with COPD-PH.
Study Design and Methods
This retrospective cohort study analyzed 71 COPD-PH patients from the Johns Hopkins Pulmonary Hypertension Registry with right-sided heart catheterization (RHC)-proven PH and pulmonary function testing data within one year of diagnostic RHC. Transplant-free survival was calculated from index RHC. Adjusted transplant-free survival was modelled using Cox proportional hazard methods; age, pulmonary vascular resistance, FEV1, oxygen use, and N-terminal pro-brain natriuretic peptide were included as covariates.
Results
Overall unadjusted transplant-free 1-, 3-, and 5-year survivals were 87%, 60%, and 51%, respectively. Survival was associated with reduced Dlco across the observed range of pulmonary artery pressures and pulmonary vascular resistance. Severe Dlco impairment was associated with poorer survival (log-rank χ2 13.07) (P < .001); adjusting for covariates, for every percent predicted decrease in Dlco, mortality rates increased by 4% (hazard ratio, 1.04; 95% CI, 1.01-1.07).
Interpretation
Among patients with COPD-PH, severe gas transfer impairment is associated with higher mortality, even with adjustment for airflow obstruction and hemodynamics, which suggests that Dlco may be a useful prognostic marker in this population. Future studies are needed to further investigate the association between Dlco and morbidity and to determine the utility of Dlco as a biomarker for disease risk and severity in COPD-PH.
Key Words: COPD, mortality, pulmonary diffusing capacity, pulmonary gas exchange, pulmonary hypertension
Abbreviations: 6MWD, 6-minute walk distance; Dlco, diffusing capacity of the lung for carbon monoxide; mPAP, mean pulmonary artery pressure; NT-proBNP, N-terminal pro-brain natriuretic peptide; PFT, pulmonary function testing; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RHC, right-sided heart catheterization; RV, residual volume; TLC, total lung capacity
FOR EDITORIAL COMMENT, SEE PAGE 455
COPD is reported to be the fourth leading cause of death in the United States,1 affecting approximately 15.7 million Americans.2 Many patients with COPD experience elevation in pulmonary arterial pressures or pulmonary hypertension (PH).3,4 As compared to those with COPD alone, patients with COPD-PH have increased mortality rates.3,5, 6, 7, 8, 9, 10, 11 Despite this well-documented and profound impact on prognosis, the clinical and physiologic characteristics that predict poor outcomes are poorly understood among patients with COPD-PH.
Historically, COPD-PH was thought to develop as the severity of airflow obstruction, measured by FEV1, and subsequent chronic hypoxemia progressed.3,12,13 However, airflow obstruction increasingly has been noted to be insufficient in the prediction of clinical outcomes in the general population with COPD.14, 15, 16 Studies in COPD-PH have identified hemodynamic measures as better predictors of prognosis,3,6 but these metrics require right-sided heart catheterization (RHC), an invasive procedure that carries its own risks.
An alternative noninvasive measure of interest is diffusing capacity of the lung for carbon monoxide (Dlco). Dlco is a measure of gas exchange reflective of the complex interactions that occur at the alveolar-capillary interface, including morphologic changes in the pulmonary vasculature.17,18 Recent work by our group in a large COPD cohort has demonstrated that Dlco is an indicator of disease morbidity beyond that represented by airflow obstruction or by CT scan evidence of emphysema alone.19 This may be particularly relevant for those with COPD-PH. Among those with World Health Organization groups 1, 2, and undifferentiated 3 PH, Dlco has been associated previously with survival.20, 21, 22, 23 However, to our knowledge, there are no studies that have evaluated Dlco as a predictor of death among patients with well-phenotyped COPD-PH.
This study aimed to examine Dlco as a prognostic marker in a cohort of patients with well-defined COPD-PH with clinical, pulmonary physiologic, and hemodynamics measurements. We hypothesize that lower Dlco will predict increased mortality rates in the COPD-PH population independent of airflow obstruction and pulmonary hemodynamics.
Materials and Methods
Study Population and Design
This retrospective cohort study examined patients with COPD-PH who were enrolled in the Johns Hopkins Pulmonary Hypertension Registry, an Institutional Review Board-approved registry of patients seen at Johns Hopkins University, Baltimore, MD. All patients enrolled in the registry provide written informed consent for data collection.
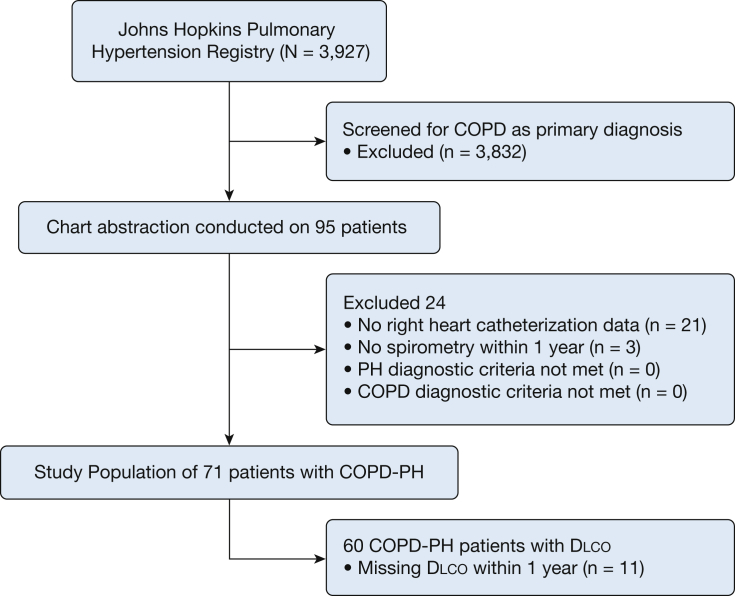
From January 2000 to January 2018, 95 participants were identified on initial screening for patients enrolled with COPD designated as the primary cause for their PH. We excluded patients with incomplete RHC (N = 21) or spirometry (N = 3) data from the final analysis population. PH diagnosis was confirmed by a mean pulmonary artery pressure (mPAP) > 20 mm Hg.24 Importantly, individuals with a pulmonary vascular resistance (PVR) <3 Woods units or a pulmonary capillary wedge pressure (PCWP) > 15 mm Hg were not excluded to better examine the heterogeneity of pulmonary vascular pathophysiology in COPD. COPD diagnosis was confirmed by FEV1/FVC ratio <0.7.25,26 No patients were excluded after verification of both COPD and PH diagnoses, yielding a final analysis population of 71 patients with COPD-PH (Fig 1).
Figure 1.
Patient selection. Dlco = diffusing capacity of the lung for carbon monoxide; PH = pulmonary hypertension.
Baseline characteristics
Demographic data that included age, sex, and ethnicity were abstracted from chart review. Height and weight were obtained from RHC report and used to calculate BMI. Smoking status, pack-years smoked, comorbid medical conditions, and World Health Organization Functional Classification were also collected. Finally, serum lab tests conducted within one month of index RHC were abstracted for creatinine, estimated glomerular filtration rate, and N-terminal pro-Brain Natriuretic Peptide (NT-proBNP).
Medication Use
Prescription of PH-specific medications was treated as a binary variable: ever or never prescribed. Individual classes and formulations that included prostacyclin analogues, prostacyclin receptor agonists, endothelin receptor antagonists, and phosphodiesterase type 5 inhibitors were also abstracted.
Pulmonary Function Testing Data
Absolute and percent predicted FEV1, FVC, total lung capacity (TLC), residual volume (RV), RV/TLC ratio, Dlco, and gas transfer efficiency (Dlco/VA) were abstracted. From 6-minute walk distance (6MWD) testing, the distance walked, Borg dyspnea scores pre- and post-walk, and resting and nadir oxygen saturation were abstracted. Pulmonary function testing (PFT) testing was conducted and interpreted per American Thoracic Society/European Respiratory Society guidelines.27,28 Abstracted data were limited to within one year of index RHC.
Right Heart Catheterization Data
Hemodynamic parameters for RHC included right atrial pressure, mPAP, PCWP, cardiac output, cardiac index, PVR, pulmonary arterial % oxygen saturation (PA Sat%), and arterial oxygen saturation. RHC procedures were performed in the supine position with pressures measured at end expiration.
Survival Data
Transplant-free survival data were obtained from chart review and the Social Security Death Index, with survival time calculated from index RHC. Observations were censored administratively on July 1, 2018. Transplant status was obtained from chart review, and participants were censored by the corresponding date of transplantation.
Statistical Analysis
Participant baseline characteristics were described with means with SDs for continuous variables and percentages for categoric variables. Comparisons in baseline clinical, PFT, and hemodynamic parameters between participants with Dlco above or below 50% predicted were compared with the use of t-tests or Chi-squared tests as appropriate. This Dlco cut-point was based on our prior work demonstrating a strong association between Dlco ≤50% and COPD morbidity.19 Sensitivity analyses that examined the association between Dlco at different cut-points and death were also performed. Baseline PFT and hemodynamic parameters were correlated with the use of Pearson correlation coefficients.
For survival analyses, Dlco percent predicted was treated both as a continuous variable and dichotomized to above or below 50% predicted. Unadjusted transplant-free survival by Dlco group was graphed with the use of the Kaplan-Meier method, and tested with the use of a log rank test. For comparison, unadjusted transplant-free survival by FEV1 percent predicted dichotomized to above or below 50% was similarly tested. Cox proportional hazard methods were used to conduct univariate testing of clinical predictors that included Dlco percent predicted, age at diagnosis, sex, pack years smoked, BMI, 6MWD, FEV1 percent predicted, FVC percent predicted, FEV1/FVC ratio, RV percent predicted, oxygen use, NT-proBNP, creatinine, mPAP, cardiac output, PCWP, PVR, and PH medication use.
Adjusted transplant-free survival was modelled with Cox proportional hazard methods, with Dlco percent predicted, age, and PVR included as prespecified covariates. Additional covariates were selected based on the conceptual framework for the research question and from significant univariate predictors, accounting for collinearity. No more than four covariates were included in any model to avoid over-fitting.29,30 Three models were tested, with the addition of FEV1 percent predicted, oxygen use, or NT-proBNP as covariates to those prespecified. mPAP and cardiac output were not included in multivariable models, given collinearity with PVR; 6MWD was not included because of collinearity with Dlco. Variance inflation factors were used to assess multicollinearity.31,32
To describe the interaction between PFT measures (FEV1 and Dlco) and hemodynamic measures (mPAP and PVR) on mortality rates, each PFT measure was plotted against each hemodynamic measure and stratified by 5-year vital status. Because all death events occurred prior to five years, this was deemed an appropriate time point to use for vital status.
All analyses were performed using STATA version 15.1 (StataCorp). Probability values less than .05 were considered significant.
Results
Characteristics of a Patient With COPD-PH
Baseline characteristics for all 71 patients with COPD-PH are described in Table 1. Patients were on average 65 years old, 66% female, with BMI of 28.3 kg/m2, and 44 pack-years smoked. Approximately three-quarters of them used supplemental oxygen, and nearly 60% were World Health Organization Functional Classification III/IV at index catheterization. PFT demonstrated moderate-severe obstruction (FEV1 52±20%), with severe gas transfer defects (Dlco 43±20%), and severely impaired 6MWD (265±124 m). Among the 48 patients who received PH-specific medications, 34 patients received phosphodiesterase type five inhibitor (PDE-5I) monotherapy; 6 patients received combination PDE-5I and endothelin receptor antagonists therapy, and the remaining 7 patients received combinations of endothelin receptor antagonists and/or PDE-5I and/or prostacyclin analogues therapy.
Table 1.
Clinical Characteristics by Severe Reduction in Diffusing Capacity of the Lung for Carbon Monoxide
| Patient Characteristic | All COPD-PH (N = 71) | Dlco > 50% (n = 20) | Dlco ≤ 50% (n = 40) |
|---|---|---|---|
| Age at diagnosis | 65.1 ± 8.6 | 65.4 ± 8.8 | 64.9 ± 8.3 |
| White, No. (%) | 49 (70) | 16 (80) | 26 (65) |
| Female, No. (%) | 49 (66) | 14 (70) | 27 (68) |
| BMI, kg/m2 | 28.3 ± 6.7 | 30.1 ± 7.9 | 25.9 ± 5.5 |
| Ever smokers, No. (%) | 66 (93) | 17 (85) | 38 (95) |
| Current smoker, No. (%) | 7 (9.8) | 3 (15) | 3 (7.5) |
| Smoking pack years | 44 ± 28 | 36.9 ± 28.7 | 47.1 ± 28 |
| Oxygen use, No. (%) | 53 (73) | 11 (55) | 33 (83) |
| Nadir oxygen saturation % | 84 ± 9 | 89.6 ± 7.5 | 80.5 ± 8.4 |
| NYHA class, No. (%) | |||
| Class I | 2 (2.9) | 1 (5.3) | 1 (2.6) |
| Class II | 27 (39.13) | 11 (57.9) | 12 (30.8) |
| Class III | 31 (44.93) | 6 (31.6) | 21 (53.9) |
| Class IV | 9 (13.04) | 1 (5.3) | 5 (12.8) |
| 6MWD,a m | 265 ± 124 | 314 ± 128 | 251 ± 110 |
| Borg dyspnea | 4.4 ± 2.5 | 2.9 ± 1.4 | 5.2 ± 2.5 |
| Median NT-proBNP,b pg/mL (interquartile range) | 657 (219, 1983) | 229 (176, 311) | 1311 (314, 2176) |
| Creatinine,c mg/dL | 1.1 (0.3) | 1.0 (0.3) | 1.1 (0.4) |
| Median eGFR,d mL/min/1.73 m2 (interquartile range) | 60 (54, 60) | 60 (60, 60) | 60 (46, 60) |
| FEV1% predicted | 52 ± 20 | 56 ± 21 | 51 ± 20 |
| FVC% predicted | 72 ± 21 | 77 ± 25 | 73 ± 20 |
| FEV1/FVC% | 57 ± 14 | 57 ± 17 | 57 ± 12 |
| TLC% predictede | 91 ± 24 | 94 ± 33 | 91 ± 19 |
| RV% predictedf | 119 ± 52 | 119 ± 48 | 121 ± 55 |
| Dlco% predicted | 43 ± 20 | 67 ± 15 | 32 ± 10 |
| Dlco/VA% predicted | 57 ± 28 | 81 ± 27 | 44 ± 18 |
| PH medication use, No. (%) | 48 (68) | 13 (65) | 28 (70) |
| Comorbidities, No. (%) | |||
| Atrial fibrillation/flutter | 10 (14) | 1 (5) | 9 (23) |
| Coronary artery disease | 18 (25) | 4 (20) | 12 (30) |
| Cancer | 13 (18) | 3 (15) | 7 (18) |
| DVT/pulmonary embolism | 8 (11) | 2 (10) | 6 (15) |
| Congestive heart failure | 5 (7) | 1 (5) | 3 (8) |
| Interstitial lung disease | 5 (7) | 2 (10) | 3 (8) |
| OSA | 14 (20) | 5 (25) | 4 (10) |
All values are mean ± SD, unless otherwise specified. 6MWD = 6-minute walk distance; Dlco = diffusing capacity of the lung for carbon monoxide; eGFR = estimated glomerular filtration rate; NT-proBNP = N-terminal pro-brain natriuretic peptide; NYHA = New York Heart Association; PH = pulmonary hypertension; RV = residual volume; TLC = total lung capacity; VA = alveolar volume.
N = 55.
N = 50.
N = 61.
N = 45.
N = 58.
N = 54.
Sixty patients had Dlco measured within one year of diagnostic RHC, 40 of whom had severely impaired gas transfer (Table 1). These patients had lower BMI, higher percentage oxygen use, lower nadir oxygen saturation, increased Borg dyspnea scores, and lower 6MWD than those with Dlco > 50% predicted. The two Dlco groups were similar, however, on degree of obstruction by FEV1 percent predicted, hyperinflation by TLC percent predicted, and air-trapping by RV percent predicted.
Clinical characteristics of those missing Dlco within one year of RHC demonstrated severe limitations by 6MWD and Borg dyspnea score, but crude mortality rate by 5 years was similar to those with a Dlco > 50% (e-Table 1). An additional 6 patients had Dlco measured outside the one-year window and were included in sensitivity analyses (e-Table 2).
A “pulmonary vascular phenotype” has been defined as an individual with severe pre-capillary PH (defined as a PCWP ≤ 15 mm Hg with either mPAP ≥ 35 mm Hg or mPAP ≥ 25 mm Hg in combination with a cardiac index ≤ 2.0 L/min/m2),33,34 mild-moderate airflow obstruction (FEV1 > 60% predicted), and typically a low Dlco.35 Eight patients in this cohort were identified with severe pre-capillary PH and mild-moderate airflow obstruction; five of those eight patients had a Dlco ≤ 50% predicted. Sensitivity analysis that excluded this phenotype is given in e-Table 3.
Dlco Is Associated With PVR
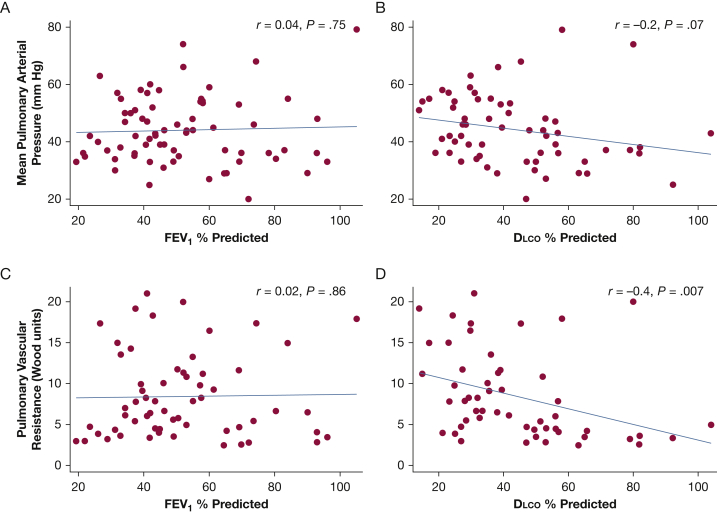
Hemodynamics for the full cohort included an average mPAP of 44 mm Hg, PCWP of 14 mm Hg, and a PVR of 8.4 Woods units, consistent with severe PH (Table 2). Those patients with Dlco ≤ 50% predicted were noted to have significantly lower cardiac index, significantly higher PVR, and tended towards higher mPAP than those with Dlco > 50% predicted. When examined as a continuous variable, percent predicted Dlco was modestly correlated with PVR (r = –0.4; P = .007) and cardiac index (r = 0.6; P < .001) but showed no significant correlation with mPAP (r = –0.2; P = .07). There was no correlation between percent predicted FEV1 and PVR (r = 0.02; P = .86), cardiac index (r = –0.04; P = .76), or mPAP (r = 0.04; P = .75) (Fig 2).
Table 2.
Hemodynamic Characterization by Severe Reduction in Dlco
| Hemodynamic Measure | All COPD-PH (N = 71) | Dlco > 50% (n = 20) | Dlco ≤ 50% (n = 40) | P Value |
|---|---|---|---|---|
| RAP, mm Hg | 9 ± 5 | 8 ± 4 | 9 ± 5 | .38 |
| mPAP, mm Hg | 44 ± 12 | 41 ± 14 | 46 ± 2 | .19 |
| PCWP, mm Hg | 14 ± 7 | 15 ± 7 | 13 ± 7 | .28 |
| Cardiac output, L/min | 4.4 ± 1.5 | 5.0 ± 1.4 | 4.0 ± 1.4 | .02a |
| Cardiac index, L/min/m2 | 2.3 ± 0.6 | 2.7 ± 0.6 | 2.1 ± 0.6 | .002a |
| PVR, Wood units | 8.4 ± 5.2 | 6.2 ± 5.1 | 9.7 ± 5.1 | .03a |
| PA saturation % | 66 ± 8 | 69 ± 9 | 64 ± 8 | .11 |
All values are mean ± SD. mPAP = mean pulmonary arterial pressure; PA saturation % = pulmonary arterial oxygen saturation; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RAP = right atrial pressure.
Probability values correspond to comparison of Dlco > 50% to Dlco ≤ 50%.
Figure 2.
Relationship between pulmonary function and pulmonary hemodynamics in COPD-PH. A-D, Scatterplots show correlation between A, FEV1 and mPAP, B, Dlco and mPAP, C, FEV1 and PVR, and D, Dlco and PVR. Correlation coefficients with corresponding probability values listed in each panel. mPAP = mean pulmonary arterial pressure; PVR = pulmonary vascular resistance. See Figure 1 legend for expansion of the other abbreviation.
Dlco Is a Strong Independent Predictor of Transplant-Free Survival
Overall unadjusted transplant-free 1-, 3-, and 5-year survival was 87%, 60%, and 51%, respectively. During the observed follow-up time, there were 34 deaths (48%) and 3 transplantations (4%); median follow-up time was 3.7 years (interquartile range, 1.7 to 6.8 years). Cause of death was obtained on 23 individuals by primary provider adjudication; 15 of those individuals died of respiratory failure or sudden cardiac death (e-Table 4).
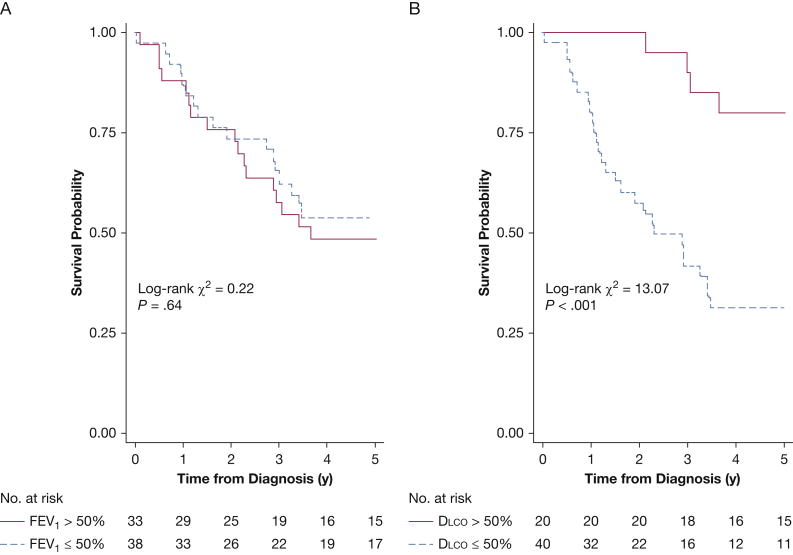
Patients with Dlco ≤50% predicted displayed significantly worse mortality rates than those with Dlco > 50% predicted (log-rank χ2 = 13.07; P = .0003) (Fig 3). In contrast, there was no difference in mortality rates between those patients with an FEV1 above or below 50% predicted (log-rank χ2 = 0.22; P = .64). When categorized with the use of clinically relevant cutoffs of Dlco percent predicted, crude 5-year mortality rates increased with increasing Dlco impairment (e-Fig 1). Given the frequency of events in each category, formal testing of differences in survival across clinical categories was not conducted.
Figure 3.
Severe impairment of Dlco predicts unadjusted tranplant-free survival. A-B, Kaplan-Meier curves of unadjusted transplant free survival by A, FEV1 and B, Dlco dichotomized above and below 50% with associated log-rank testing. See Figure 1 legend for expansion of the abbreviation.
In unadjusted Cox analyses, significant predictors of survival included Dlco percent predicted, 6MWD, oxygen use, NT-proBNP, mPAP, PVR, and cardiac output (Table 3). Notably, aside from Dlco percent predicted and 6MWD, no other PFT measures were significant predictors of death. Additionally, PH-specific medication use was not associated with death in the primary analysis or in sensitivity analyses.
Table 3.
Unadjusted and Adjusted Transplant-Free Survival by Clinical Predictors
| Clinical Predictor | Unadjusted Hazard Ratioa | P Value | Model 1 | P Value | Model 2 | P Value | Model 3 | P Value |
|---|---|---|---|---|---|---|---|---|
| Age at diagnosis | 1.02 (0.98 to 1.06) | .38 | 1.06 (0.99 to 1.13) | .08 | 1.03 (0.98 to 1.09) | .18 | 1.02 (0.96 to 1.11) | .42 |
| Sexb | 0.73 (0.36 to 1.48) | .39 | … | … | … | … | … | … |
| BMI, kg/m2 | 0.95 (0.88 to 1.02) | .16 | … | … | … | … | … | … |
| Smoking pack years | 1.01 (0.99 to 1.02) | .06 | … | … | … | … | … | … |
| 6MWD, m | 0.99 (0.99 to 0.99)c | .048c | … | … | … | … | … | … |
| Borg dyspnea | 1.09 (0.93 to 1.28) | .29 | … | … | … | … | … | … |
| Oxygen use | 2.90 (1.12 to 7.51)c | .028c | … | … | 1.34 (0.45 to 4.02) | .59 | … | … |
| FEV1% predicted | 1.00 (0.98 to 1.02) | .65 | 0.99 (0.98 to 1.01) | .84 | … | … | … | … |
| FVC% predicted | 1.00 (0.99 to 1.02) | .83 | … | … | … | … | … | … |
| FEV1/FVC% | 1.01 (0.99 to 1.04) | .33 | … | … | … | … | … | … |
| RV% predicted | 0.99 (0.99 to 1.00) | .19 | … | … | … | … | … | … |
| Dlco% predicted | 0.96 (0.94 to 0.98)c | < .001c | 0.96 (0.94 to 0.99)c | .003c | 0.96 (0.94 to 0.99)c | .006c | 0.96 (0.94 to 0.99)c | .03a |
| NT-proBNP,d pg/dL | 2.51 (1.32 to 4.79)c | .005c | … | … | … | … | 1.16 (0.51 to 2.63) | .72 |
| Creatinine, mg/dL | 1.37 (0.46 to 4.09) | .58 | … | … | … | … | … | … |
| mPAP, mm Hg | 1.02 (1.00 to 1.06)c | .03c | … | … | … | … | … | … |
| PVR, Wood units | 1.07 (1.01 to 1.14)c | .02c | 1.04 (0.96 to 1.12) | .12 | 1.04 (0.96 to 1.12) | .36 | 1.04 (0.95 to 1.13) | .42 |
| Cardiac output, L/min | 0.76 (0.58 to 0.99)c | .045c | … | … | … | … | … | … |
| Pulmonary capillary wedge pressure, mm Hg | 0.95 (0.89 to 1.02) | .15 | … | … | … | … | … | … |
| PH medication use | 1.85 (0.84 to 4.09) | .13 | … | … | … | … | … | … |
Model 1 (N = 53) included age at diagnosis, FEV1% predicted, Dlco% predicted, and PVR as covariates. Model 2 (N = 53) included age at diagnosis, oxygen use, Dlco% predicted, and PVR. Model 3 (N = 39) included age at diagnosis, Dlco% predicted, NT-proBNP, and PVR. See Table 1 legend for expansion of abbreviations.
Hazard ratios are reported per 1 unit increase in clinical predictor.
Sex hazard ratio is for female vs male.
Denotes statistical significance P < .05.
NT-proBNP was log10-transformed for normality; NT-proBNP is per 10-fold increase in pg/mL.
With adjustment for covariates, Dlco percent predicted remained the only significant predictor of death (Table 3). Three models were analyzed with the addition of FEV1 percent predicted, oxygen use, or NT-proBNP to the base model of prespecified covariates. Additional multivariable models that included 6MWD, mPAP, and cardiac output were not analyzed because of collinearity. In an adjusted model with dichotomous Dlco that included age, FEV1 percent predicted, and PVR as covariates, those patients with severe gas transfer impairment were 5.8 times more likely to die compared with those patients without severe reduction in Dlco (hazard ratio, 5.8; 95% CI, 1.9 to 17.4; P = .002). Similar trends were observed with the use of gas transfer efficiency percent predicted as a predictor in lieu of Dlco percent predicted, although results did not achieve statistical significance (e-Table 5).
Sensitivity analyses that included the data of 6 patients with Dlco that were obtained beyond one year of index RHC (e-Table 2) or excluded the patients with vascular phenotype (e-Table 3) did not alter results; Dlco remained the only significant independent predictor of death. Notably, five of the eight patients (62.5%) who met spirometry and hemodynamic criteria for a pulmonary vascular phenotype had died by five years. Additional sensitivity analysis that excluded individuals with a PCWP of > 15 mm Hg did not alter results (e-Table 6).
Severe Impairment in Dlco Is Associated With Death Across a Wide Range Of Hemodynamics
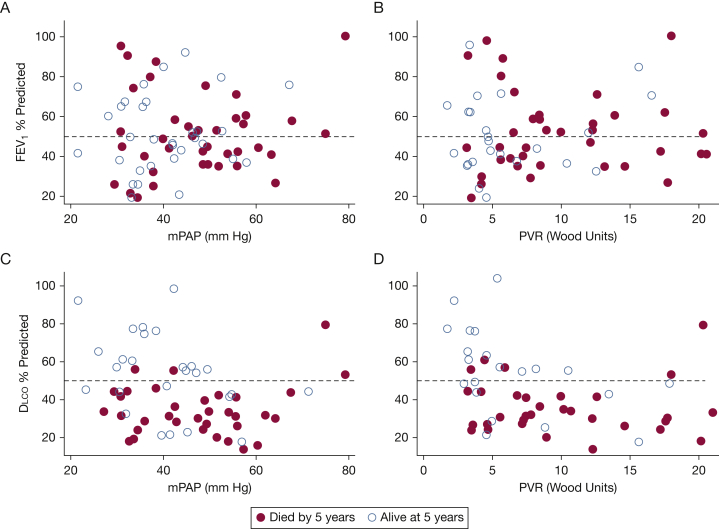
Scatterplots of PFT measures (FEV1 and Dlco) and hemodynamics (mPAP and PVR) were used to explore interactions between these physiologic parameters on survival (Fig 4). FEV1 percent predicted showed no clear pattern with vital status at 5 years; for example, those with an FEV1 > 50% were just as likely to be alive at 5 years as those with an FEV1 ≤50% predicted at any given mPAP (Fig 4A) or PVR (Fig 4B). In contrast, at a fixed value of mPAP or PVR, those patients who had died by 5 years were almost exclusively patients with a Dlco ≤ 50% predicted (Figs 4C, D).
Figure 4.
Dlco% predicted associated with vital status at 5 years across range of mPAP and PVR. A-D, Scatterplots of A, FEV1% predicted vs mPAP, B, FEV1% predicted vs PVR, C, Dlco% predicted vs mPAP, and D, Dlco% predicted vs PVR, categorized by vital status at 5 years. Reference lines for 50% predicted in al four panals. Solid circles represent patients who had died by 5 years. See Figures 1 and 2 legends for expansions of the abbreviations.
Patients with very severe derangements in mPAP and PVR tended to have died by 5 years and tended to have severe reductions in Dlco. However, even among those patients with moderately severe hemodynamic derangements, vital status was associated with Dlco. For example, at mPAP ranging from 40 to 50 mm Hg, those who had died by five years had a Dlco ≤ 50% while those who remained alive predominantly had a Dlco > 50% (Fig 4C).
Discussion
In a cohort of patients with COPD-PH from a PH tertiary referral center, we have demonstrated that (1) overall prognosis is poor among this population, (2) airflow obstruction does not predict death, and (3) gas transfer is a strong independent predictor of death. Gas transfer was observed to be the only significant predictor of death, after accounting for airflow obstruction and hemodynamics, with a 4% increase in mortality rate for every 1% decrease in Dlco. In the context of increasing efforts to better phenotype patients with both COPD and PH, this study highlights gas transfer measurement as a simple, noninvasive tool that may add value to existing prognostic models.
Studies that have examined COPD populations have described worse mortality rates among those patients with COPD-PH as compared with COPD alone.3,5, 6, 7,36 Analogously, PH registry studies have demonstrated increased mortality rates for WHO group 3 PH compared with group 1 PH.37,38 Our results demonstrated an overall 5-year survival of 51%, which is consistent with previously reported survival rates among patients with COPD-PH,6,22,37, 38, 39, 40 and a high degree of functional limitation as evidenced by World Health Organization Functional Classification and 6MWD. Although these findings may reflect selection bias from referral to PH specialists, it also accurately describes poor prognosis at one extreme of the spectrum of pulmonary vascular disease in COPD.
There are few known predictors of survival in COPD-PH; most putative prognostic factors have been extrapolated from the general population with COPD or the population with pulmonary arterial hypertension. In general COPD populations, use of FEV1 and clinical characteristics such as BMI, dyspnea scores, and 6MWD are used in the BODE score to predict death.41 Our study demonstrates that FEV1 is not a strong predictor of death among a population of patients with COPD-PH. Even among patients referred to PH specialists who undergo RHC, we observed a wide range of FEV1 values that do not correlate with hemodynamic disease severity nor with mortality rates. This suggests that, for patients with COPD-PH, airflow obstruction is not the pathophysiologic derangement that drives prognosis. Similar results regarding the prognostic utility of FEV1 have been described previously6,39,42; our study extends these findings by evaluating associations between airflow obstruction, gas transfer, hemodynamics, and outcomes in patients with COPD-PH.
In contrast to airflow obstruction, gas transfer is a strong independent predictor of death. Dlco is a composite measure that reflects the integrity of the alveolar-capillary interface, describing diffusivity of inhaled gas across the alveolar, capillary, and red blood cell membranes, and ultimately reactivity with hemoglobin.18 In patients with COPD, reduced Dlco is often associated with emphysema,43,44 which results in alveolar and capillary destruction. We demonstrate that Dlco is correlated moderately with PVR, which suggests that a component of what Dlco is capturing in this population is related to morphologic changes to the pulmonary vasculature. Prior histologic studies of explanted lungs from patients with end-stage COPD identified increased pathologic lesions in the pulmonary vessels, including decreased pulmonary capillary density, with increasing PH severity.45, 46, 47 Reduction in capillary density represents a plausible pathophysiologic correlate for reduction in Dlco and generates hypotheses that Dlco may be sensitive to early pulmonary vascular disease. Roughly 50 to 70% of the pulmonary vasculature must be obliterated before elevations in pulmonary artery pressures to > 25 mm Hg are evident.48 The independent association between Dlco and death may reflect early reduction in capillary density that is not yet detectable by invasive hemodynamic measures.
Alternatively, there are numerous other comorbidities that occur frequently in individuals with COPD, can alter Dlco measurements, and may be reflected in our findings. Impairment in gas transfer is noted to be frequent among individuals with heart failure, with or without preserved ejection fraction.49, 50, 51, 52 We did not exclude individuals who have left-heart disease, although sensitivity analyses suggest that, even among those without hemodynamic evidence of left-heart disease, Dlco remains a significant predictor of death. We found 11% of the study population (8 participants) had a history of thromboembolic disease, with a greater prevalence among those with lower Dlco; our findings may reflect some degree of thromboembolic burden that is unaccounted for by airflow obstruction or hemodynamics. However, we also obtained ventilation perfusion scan results on these participants, with evidence of very low or low probability scans in six of those eight individuals, which argues against significant contribution of chronic thromboembolic disease to reductions in Dlco. We did not adjust for comorbidities in our models, and our findings note increased prevalence of multiple comorbidities among those with severe reductions in Dlco, so it may be that the association between Dlco and death is reflective of comorbid diseases that can impair gas transfer.
The presented findings confirm and extend prior literature that has assessed Dlco in different populations, including those with group 1 PH,20 group 2 PH,21 and combined group 3 PH.22,40 In idiopathic pulmonary arterial hypertension, studies that have examined gas transfer as a predictor of survival have been discrepant, but a large body of evidence supports Dlco as a predictor of survival.20,53, 54, 55, 56, 57, 58, 59 As a result, Dlco with a cutoff of 40% predicted has been adopted into the REVEAL Risk calculator for pulmonary arterial hypertension.60 The literature surrounding Dlco as a predictor of death in group 3 PH similarly has been conflicted,22,40,42,61,62 but this may be due to the heterogeneity of the populations included in group 3 PH. In the ASPIRE registry, similar to our findings, Dlco was identified as an independent predictor of death when the subgroup with COPD-PH was studied.39 Across these varying disease states, Dlco is likely reflecting vascular alterations.
A subset of patients with COPD-PH deemed the “vascular phenotype,”35 defined as those patients who have minimal airflow obstruction but severe hemodynamic derangement, with either a mPAP of ≥ 35 or mPAP of ≥ 25 with a cardiac index of <2.0, are observed to have a worse prognosis than those who have mild-moderate PH.7,33,39 These patients have also been observed typically to have a low Dlco.7,63 In our cohort, only eight patients (11%) met spirometric and hemodynamic criteria, and five patients (7%) had additionally severe reduction in gas transfer. Sensitivity analysis that excluded these participants, demonstrated no significant difference in results (e-Table 3), confirming that the observed association between Dlco and outcomes was not driven by those patients with the pulmonary vascular phenotype. Gas transfer was found to be a useful predictor both within and outside of the vascular phenotype, strengthening an argument to use Dlco in risk assessment across the spectrum of patients with COPD-PH.
We also explored an altered effect of hemodynamic disease severity on death by Dlco. Most of this study population (78.9%) meet criteria for severe PH by mPAP of ≥ 35 mm Hg criteria.34 Even in such a severe population, gas transfer impairment was closely linked to death across a wide range of mPAP and PVR values. Our analysis suggests that a severe gas transfer deficit confers susceptibility to death at less severe derangement of hemodynamic metrics than would be expected for someone without severe impairment in gas exchange. Future studies of the interaction between hemodynamics and gas exchange on prognosis in a population with milder PH disease severity are especially warranted, given the high prevalence of mild-moderate PH among patients with COPD.4,7,64,65
The findings of this study are limited by the sample size of the population studied. The modest sample size precluded formal testing for interactions between pulmonary physiologic measures, hemodynamic parameters, and vital status. However, a pattern of worse mortality rates in those patients with severe Dlco impairment uncoupled from hemodynamic derangement emerged and warrants further study. The modest sample size further limited the confounders that were included in modeling the outcome; as a result, there is likely unaccounted residual confounding. Additionally, this study population is a select group from a single center who underwent subspecialty referral and invasive testing, thereby limiting generalizability to the larger COPD population. The findings, however, do offer insight into clinical and physiologic characteristics at one extreme of the pulmonary vascular disease spectrum among patients with COPD and generate hypotheses regarding measures that warrant further exploration in the larger COPD population. Smoking status was not assessed at the time of Dlco measurement, which potentially could bias Dlco values. Although this is a limitation, smoking status was assessed at the time of RHC and is not expected to be significantly different for most participants. Additionally, anemia can bias Dlco percent predicted values, but hemoglobin data at the time of Dlco measurement was not obtained. As a result of this limitation, the association identified between Dlco and death could be secondary to underlying anemia, which is also a relevant concern in COPD and warrants further exploration. Cause of death information was not available for most participants, precluding discussion regarding predictors of respiratory causes of death. Concurrent interstitial lung disease was identified by chart diagnosis but without imaging data for verification; the study population may include individuals with both COPD and interstitial lung disease. Finally, because this was a retrospective chart review of clinically obtained data, quantitative imaging that could quantify emphysema in these patients was unavailable. Because Dlco is known to be associated with emphysema in the COPD population, a further evaluation of radiographic degree of emphysema could provide insight into the pathophysiologic condition being captured by Dlco. However, recent work by our group in the general COPD population demonstrated an association between Dlco and increased morbidity independent of CT scan-defined emphysema,19 which suggests that Dlco captures information beyond that obtained from CT emphysema quantification.
This study demonstrates that Dlco, a readily available, inexpensive, noninvasive measurement, is a strong independent predictor of death in patients with COPD with PH. The presented findings suggest that Dlco should be considered for inclusion in prognostic tools for COPD-PH. The underlying pathophysiologic condition being described by Dlco derangements has not been examined directly, but the consistency of these findings across a wide range of spirometric and hemodynamic parameters suggests that Dlco is a robust marker of disease severity. Future studies that will investigate Dlco as a screening tool in the general COPD population for PH and death are warranted. In conclusion, Dlco is useful in predicting death and should be considered as a routine measure in clinical practice for characterization of patients with COPD-PH.
Acknowledgments
Author contributions: S. C. M. contributed to the conception, design of the study, data analysis, interpretation, and preparation of this manuscript and is the guarantor of this paper. A. B. contributed to the conception, design of the study, data analysis, interpretation, and manuscript writing. T. M. K., R. L. D., P. M. H., and M. C. M. contributed to data interpretation and revision of the manuscript. All authors reviewed and approved the manuscript prior to submission for publication.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. C. M. served as a consultant to Glaxo Smith Kline and Celgene and receives royalties from UpToDate for authorship; S. C. M. served as a consultant for Arena, Actelion, Liquidia, and United Therapeutics; served on the Scientific Leadership Council of the Pulmonary Hypertension Association; serves on the Rare Disease Advisory Panel of the Patient Centered Outcomes Research Institute, and has also received research funding from the NIH/NHLBI. None declared (A. B., T. M. K., R. L. D., P. M. H.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Figure and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by the National Heart Lung and Blood Institute [grant T32 HL007534-36].
Supplementary Data
References
- 1.Kochanek K.D., Murphy S., Xu J., Arias E. Mortality in the United States, 2016. NCHS Data Brief. 2017;(293):1–8. [PubMed] [Google Scholar]
- 2.Wheaton A.G., Cunningham T.J., Ford E.S., Croft J.B. Employment and activity limitations among adults with chronic obstructive pulmonary disease: United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(11):289–295. [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler R., Faller M., Weitzenblum E. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(2):219–224. doi: 10.1164/ajrccm.164.2.2006129. [DOI] [PubMed] [Google Scholar]
- 4.Chaouat A., Naeije R., Weitzenblum E. Pulmonary hypertension in COPD. Euro Respir J. 2008;32(5):1371–1385. doi: 10.1183/09031936.00015608. [DOI] [PubMed] [Google Scholar]
- 5.Minai O.A., Chaouat A., Adnot S. Pulmonary hypertension in COPD: epidemiology, significance, and management: pulmonary vascular disease: the global perspective. Chest. 2010;137(6 Suppl):39S–51S. doi: 10.1378/chest.10-0087. [DOI] [PubMed] [Google Scholar]
- 6.Oswald-Mammosser M., Weitzenblum E., Quoix E. Prognostic factors in COPD patients receiving long-term oxygen therapy. Chest. 1995;107(5):1193–1198. doi: 10.1378/chest.107.5.1193. [DOI] [PubMed] [Google Scholar]
- 7.Chaouat A., Bugnet A.-S., Kadaoui N. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189–194. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- 8.Weitzenblum E., Sautegeau A., Ehrhart M., Mammosser M., Hirth C., Roegel E. Long-term course of pulmonary arterial pressure in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1984;130(6):993–998. doi: 10.1164/arrd.1984.130.6.993. [DOI] [PubMed] [Google Scholar]
- 9.Kessler R., Faller M., Fourgaut G., Mennecier B., Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(1):158–164. doi: 10.1164/ajrccm.159.1.9803117. [DOI] [PubMed] [Google Scholar]
- 10.Medrek S.K., Sharafkhaneh A., Spiegelman A.M., Kak A., Pandit L.M. Admission for COPD exacerbation is associated with the clinical diagnosis of pulmonary hypertension: results from a retrospective longitudinal study of a veteran population. COPD. 2017;14(5):484–489. doi: 10.1080/15412555.2017.1336209. [DOI] [PubMed] [Google Scholar]
- 11.Hayes D., Black S.M., Tobias J.D., Mansour H.M., Whitson B.A. Prevalence of pulmonary hypertension and its influence on survival in patients with advanced chronic obstructive pulmonary disease prior to lung transplantation. COPD. 2016;13(1):50–56. doi: 10.3109/15412555.2015.1043425. [DOI] [PubMed] [Google Scholar]
- 12.Minai O.A., Fessler H., Stoller J.K. Clinical characteristics and prediction of pulmonary hypertension in severe emphysema. Respir Med. 2014;108(3):482–490. doi: 10.1016/j.rmed.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Andersen K.H., Iversen M., Kjaergaard J. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant. 2012;31(4):373–380. doi: 10.1016/j.healun.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Han M.K., Muellerova H., Curran-Everett D. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med. 2013;1(1):43–50. doi: 10.1016/S2213-2600(12)70044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agusti A, Calverley PMA, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh D., Agusti A., Anzueto A. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 17.Krogh M. The diffusion of gases through the lungs of man. J Physiol. 1915;49(4):271–300. doi: 10.1113/jphysiol.1915.sp001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roughton F.J., Forster R.E. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11(2):290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- 19.Balasubramanian A., MacIntyre N.R., Henderson R.J. Diffusing capacity of carbon monoxide in assessment of chronic obstructive pulmonary disease. Chest. 2019;156(6):1111–1119. doi: 10.1016/j.chest.2019.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandra S., Shah S.J., Thenappan T., Archer S.L., Rich S., Gomberg-Maitland M. Carbon monoxide diffusing capacity and mortality in pulmonary arterial hypertension. J Heart Lung Transplant. 2010;29(2):181–187. doi: 10.1016/j.healun.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Hoeper M.M., Meyer K., Rademacher J., Fuge J., Welte T., Olsson K.M. Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4(6):441–449. doi: 10.1016/j.jchf.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Rose L., Prins K.W., Archer S.L. Survival in pulmonary hypertension due to chronic lung disease: influence of low diffusion capacity of the lungs for carbon monoxide. J Heart Lung Transplant. 2019;38(2):145–155. doi: 10.1016/j.healun.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamada K., Nagai S., Tanaka S. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest. 2007;131(3):650–656. doi: 10.1378/chest.06-1466. [DOI] [PubMed] [Google Scholar]
- 24.Simonneau G., Montani D., Celermajer D.S. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatt S.P., Balte P.P., Schwartz J.E. Discriminative Accuracy of FEV1:FVC thresholds for COPD-related hospitalization and mortality. JAMA. 2019;321(24):2438–2447. doi: 10.1001/jama.2019.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogelmeier C.F., Criner G.J., Martinez F.J. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 27.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 28.Graham B.L., Brusasco V., Burgos F. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49(1):1600016. doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 29.Harrell F.E., Lee K.L., Mark D.B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Peduzzi P., Concato J., Kemper E., Holford T.R., Feinstein A.R. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 31.Craney T.A., Surles J.G. Model-dependent variance inflation factor cutoff values. Quality Engineering. 2002;14(3):391–403. [Google Scholar]
- 32.O’Brien R.M. A caution regarding rules of thumb for variance inflation factors. Quality Quantity. 2007;41(5):673–690. [Google Scholar]
- 33.Seeger W., Adir Y., Barberà J.A. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25):D109–D116. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 34.Galié N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–975. [DOI] [PubMed]
- 35.Kovacs G., Agusti A., Barberà J.A. Pulmonary vascular involvement in chronic obstructive pulmonary disease. is there a pulmonary vascular phenotype? Am J Respir Crit Care Med. 2018;198(8):1000–1011. doi: 10.1164/rccm.201801-0095PP. [DOI] [PubMed] [Google Scholar]
- 36.Weitzenblum E., Hirth C., Ducolone A., Mirhom R., Rasaholinjanahary J., Ehrhart M. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax. 1981;36(10):752–758. doi: 10.1136/thx.36.10.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gall H., Felix J.F., Schneck F.K. The Giessen Pulmonary Hypertension Registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant. 2017;36(9):957–967. doi: 10.1016/j.healun.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Hurdman J., Condliffe R., Elliot C.A. ASPIRE registry: assessing the spectrum of pulmonary hypertension identified at a referral centre. Eur Respir J. 2012;39(4):945–955. doi: 10.1183/09031936.00078411. [DOI] [PubMed] [Google Scholar]
- 39.Hurdman J., Condliffe R., Elliot C.A. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J. 2013;41(6):1292–1301. doi: 10.1183/09031936.00079512. [DOI] [PubMed] [Google Scholar]
- 40.Brewis M.J., Church A.C., Johnson M.K., Peacock A.J. Severe pulmonary hypertension in lung disease: phenotypes and response to treatment. Eur Respir J. 2015;46(5):1378–1389. doi: 10.1183/13993003.02307-2014. [DOI] [PubMed] [Google Scholar]
- 41.Celli B.R., Cote C.G., Marin J.M. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 42.Dubois P., Machiels J., Smeets F., Delwiche J.P. CO transfer capacity as a determining factor of survival for severe hypoxaemic COPD patients under long-term oxygen therapy. Eur Respir J. 1990;3(9):1042–1047. [PubMed] [Google Scholar]
- 43.Gould G.A., Redpath A., Ryan M. Lung CT density correlates with measurements of airflow limitation and the diffusing capacity. Eur Respir J. 1991;4(2):141–146. [PubMed] [Google Scholar]
- 44.Nambu A., Zach J., Schroeder J. Relationships between diffusing capacity for carbon monoxide (Dlco), and quantitative computed tomography measurements and visual assessment for chronic obstructive pulmonary disease. Eur J Radiol. 2015;84(5):980–985. doi: 10.1016/j.ejrad.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Peinado V.I., Gómez F.P., Barberà J.A. Pulmonary vascular abnormalities in chronic obstructive pulmonary disease undergoing lung transplant. J Heart Lung Transplant. 2013;32(12):1262–1269. doi: 10.1016/j.healun.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Carlsen J., Hasseriis Andersen K., Boesgaard S., Iversen M., Steinbrüchel D., Bøgelund Andersen C. Pulmonary arterial lesions in explanted lungs after transplantation correlate with severity of pulmonary hypertension in chronic obstructive pulmonary disease. J Heart Lung Transplant. 2013;32(3):347–354. doi: 10.1016/j.healun.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Bunel V., Guyard A., Dauriat G. Pulmonary arterial histologic lesions in patients with COPD with severe pulmonary hypertension. Chest. 2019;156(1):33–44. doi: 10.1016/j.chest.2019.02.333. [DOI] [PubMed] [Google Scholar]
- 48.Austin E.D., Kawut S.M., Gladwin M.T., Abman S.H. Pulmonary hypertension: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc. 2014;11(suppl 3):S178–S185. doi: 10.1513/AnnalsATS.201312-443LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andrea R., López-Giraldo A., Falces C. Lung function abnormalities are highly frequent in patients with heart failure and preserved ejection fraction. Heart Lung Circ. 2014;23(3):273–279. doi: 10.1016/j.hlc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Puri S., Baker B.L., Dutka D.P., Oakley C.M., Hughes J.M., Cleland J.G. Reduced alveolar-capillary membrane diffusing capacity in chronic heart failure. Circulation. 1995;91(11):2769–2774. doi: 10.1161/01.cir.91.11.2769. [DOI] [PubMed] [Google Scholar]
- 51.Siegel J.L., Millen A., Brown L.K., DeLuca A., Teirstein A.S. Pulmonary diffusing capacity in left ventricular dysfunction. Chest. 1990;98(3):550–553. doi: 10.1378/chest.98.3.550. [DOI] [PubMed] [Google Scholar]
- 52.Agostoni P., Bussotti M., Cattadori G. Gas diffusion and alveolar-capillary unit in chronic heart failure. Eur Heart J. 2006;27(21):2538–2543. doi: 10.1093/eurheartj/ehl302. [DOI] [PubMed] [Google Scholar]
- 53.Trip P., Nossent E.J., de Man F.S. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J. 2013;42(6):1575–1585. doi: 10.1183/09031936.00184412. [DOI] [PubMed] [Google Scholar]
- 54.Kawut S.M., Horn E.M., Berekashvili K.K. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol. 2005;95(2):199–203. doi: 10.1016/j.amjcard.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Szturmowicz M., Kacprzak A., Franczuk M. Low Dlco in idiopathic pulmonary arterial hypertension: clinical correlates and prognostic significance. Adv Respir Med. 2016;84(2):87–94. doi: 10.5603/PiAP.2016.0006. [DOI] [PubMed] [Google Scholar]
- 56.Jing Z.-C., Xu X.-Q., Badesch D.B. Pulmonary function testing in patients with pulmonary arterial hypertension. Respir Med. 2009;103(8):1136–1142. doi: 10.1016/j.rmed.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Lee I van der, Zanen P., Grutters J.C., Snijder R.J., Bosch JMM van den Diffusing capacity for nitric oxide and carbon monoxide in patients with diffuse parenchymal lung disease and pulmonary arterial hypertension. Chest. 2006;129(2):378–383. doi: 10.1378/chest.129.2.378. [DOI] [PubMed] [Google Scholar]
- 58.D’Alonzo G.E. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med. 1991;115(5):343. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 59.Thenappan T., Shah S.J., Rich S., Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982-2006. Eur Respir J. 2007;30(6):1103–1110. doi: 10.1183/09031936.00042107. [DOI] [PubMed] [Google Scholar]
- 60.Benza R.L., Gomberg-Maitland M., Elliott C.G. Predicting survival in patients with pulmonary arterial hypertension: The REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156(2):323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Chebib N., Mornex J.-F., Traclet J. Pulmonary hypertension in chronic lung diseases: comparison to other pulmonary hypertension groups. Pulm Circ. 2018;8(2) doi: 10.1177/2045894018775056. 2045894018775056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cottin V., Nunes H., Brillet P.-Y. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26(4):586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 63.Thabut G., Dauriat G., Stern J.B. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531–1536. doi: 10.1378/chest.127.5.1531. [DOI] [PubMed] [Google Scholar]
- 64.Weitzenblum E. Chronic cor pulmonale. Heart. 2003;89(2):225–230. doi: 10.1136/heart.89.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barberà J.A., Peinado V.I., Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003;21(5):892–905. doi: 10.1183/09031936.03.00115402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.