Abstract
Background
The renin-angiotensin-aldosterone system (RAAS) contributes to pulmonary hypertension (PH) pathogenesis. Although animal data suggest that RAAS inhibition attenuates PH, it is unknown if RAAS inhibition is beneficial in PH patients.
Research Question
Is RAAS inhibitor use associated with lower mortality in a large cohort of patients with hemodynamically confirmed PH?
Study Design and Methods
We used the Department of Veterans Affairs Clinical Assessment Reporting and Tracking Database to study retrospectively relationships between RAAS inhibitors (angiotensin converting enzyme inhibitors [ACEIs], angiotensin receptor blockers [ARBs], and aldosterone antagonists [AAs]) and mortality in 24,221 patients with hemodynamically confirmed PH. We evaluated relationships in the full and in propensity-matched cohorts. Analyses were adjusted for demographics, socioeconomic status, comorbidities, disease severity, and comedication use in staged models.
Results
ACEI and ARB use was associated with improved survival in unadjusted Kaplan-Meier survival analyses in the full cohort and the propensity-matched cohort. This relationship was insensitive to adjustment, independent of pulmonary artery wedge pressure, and also was observed in a cohort restricted to individuals with precapillary PH. AA use was associated with worse survival in unadjusted Kaplan-Meier survival analyses in the full cohort; however, AA use was associated less robustly with mortality in the propensity-matched cohort and was not associated with worse survival after adjustment for disease severity, indicating that AAs in real-world practice are used preferentially in sicker patients and that the unadjusted association with increased mortality may be an artifice of confounding by indication of severity.
Interpretation
ACEI and ARB use is associated with lower mortality in veterans with PH. AA use is a marker of disease severity in PH. ACEIs and ARBs may represent a novel treatment strategy for diverse PH phenotypes.
Key Words: aldosterone, angiotensin converting enzyme, epidemiology, pharmacology, pulmonary hypertension
Abbreviations: AA, aldosterone antagonist; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; CART, Clinical Assessment Reporting and Tracking; HR, hazard ratio; PAH, pulmonary arterial hypertension; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RAAS, renin-angiotensin-aldosterone system; RHC, right heart catheterization; RV, right ventricle; VA, Department of Veterans Affairs
FOR EDITORIAL COMMENT, SEE PAGE 1326
Pulmonary hypertension (PH) is a common complication of cardiopulmonary and systemic disorders.1 Regardless of the underlying cause, PH leads to right ventricular failure and predicts or mediates poor outcomes.1, 2, 3, 4 Pulmonary vasodilator therapy is available for the relatively small subgroup of patients with pulmonary arterial hypertension (PAH; group 1 PH), but no specific therapies are available for the large group of patients with PH and increased right ventricle (RV) afterload resulting from chronic heart disease, chronic lung disease, or sleep-disordered breathing (group 2 and 3 PH, respectively).3,4 Unfortunately, even with use of disease-specific therapies in PAH, 3-year survival is only 55%.5 Survival in those with group 2 or 3 PH is similar or even lower.6 An unmet need exists for novel disease-modifying treatments for PAH as well as for those with group 2 or 3 PH.7,8
Renin-angiotensin-aldosterone system (RAAS) inhibition is a widely accepted treatment for heart failure with reduced ejection fraction and systemic hypertension that exerts protective effects on the systemic vasculature and on myocardial remodeling.9,10 Neurohormonal imbalance with increased RAAS activation also is a disease marker and potential treatment target in the RV and pulmonary vasculature in PAH.11, 12, 13, 14 Experimental and clinical studies suggest that RAAS inhibition may prevent PAH development and may attenuate severity.15, 16, 17, 18, 19, 20, 21, 22, 23 Given the benefit of RAAS inhibitors on left ventricular function and remodeling,9 their use also may benefit the RV. This is clinically relevant, because RV function determines survival in PAH and because no RV-specific therapies currently exist.24, 25, 26 Importantly, RV failure is the final common disease pathway not just in PAH, but also in all other forms of PH. Therefore, RV-targeted therapy could be beneficial across the entire spectrum of PH, which is distinct from pulmonary vascular-targeted therapy that is helpful in PAH, but may be harmful in other forms of PH.27, 28, 29, 30, 31, 32 Evidence of RAAS activation also exists in non-PAH PH. For example, increased circulating plasma aldosterone has been reported in patients with group 2 PH33 and in patients with advanced chronic obstructive lung disease and cor pulmonale,34,35 and evidence of increased RAAS signaling exists in patients with pulmonary fibrosis.36
We used the Department of Veterans Affairs (VA) Clinical Assessment Reporting and Tracking (CART) cohort (the single largest database of hemodynamically confirmed PH) to study relationships between RAAS inhibitors (angiotensin converting enzyme inhibitors [ACEIs], angiotensin receptor blockers [ARBs], and aldosterone antagonists [AA]) and mortality in a large cohort of patients with pre-capillary and post-capillary forms of PH. Because most patients in this cohort represent non-PAH PH,37 the CART cohort represents a unique opportunity to study the association of RAAS inhibitors with clinical outcomes in individuals with common and clinically highly relevant types of PH. We hypothesized that ACEI, ARB, and AA use would be associated with improved outcomes in a population with a variety of types of PH.
Methods
See e-Appendix 1 for detailed methods.
Participants
The CART cohort consists of data from a large, national collection of veterans who underwent heart catheterization at a VA medical center since 2005.37,38 For this retrospective analysis, we included all veterans who received a right heart catheterization (RHC) between 2008 and 2016, had a mean pulmonary artery pressure of ≥ 25 mm Hg (which defined PH during the study period), and a recorded value for pulmonary artery wedge pressure (PAWP).
Medications
Medication use was ascertained using the VA medical record. Participants were considered to have used a medication if an outpatient prescription was filled within 90 days of the RHC. Participants were excluded if they died within 90 days of the RHC or underwent a hospital stay lasting > 60 days after that catheterization. The latter provided a minimum of 30 days to detect outpatient medication use. RAAS inhibitor (ACEI/ARB or AA) use was the primary exposure in all analyses. Other baseline medications were considered as potential confounders (e-Table 1).
Covariates
We accounted for possible nonpharmacologic characteristics that may confound the relationship between RAAS inhibitor use and mortality. Specifically, we accounted for (1) demographic characteristics (age [modeled continuously], sex, race or ethnicity, and BMI), (2) markers of socioeconomic status and health behaviors (current or previous history of smoking, current or previous history of alcohol abuse, income, and marital status), (3) comorbid medical conditions, and (4) comedication use (e-Table 1).
Outcomes
The primary outcome was the rate of all-cause mortality. Risk time accrued after the 90-day window that was used to establish exposure status. Separation of exposure ascertainment and outcome assessment was used to avoid immortal time bias and to ensure that all participants had an equal chance for exposure to ACEIs/ARBs or AAs. Mortality was determined using the combined VA vital status file, which has a 97.6% exact agreement with the National Death Index.39
Statistical Analysis
Patient characteristics were compared by RAAS inhibitor use. We used the Kaplan-Meier method to estimate unadjusted associations and Cox proportional hazards regression using a complete case analysis to estimate adjusted associations of RAAS inhibitor use within 90 days of RHC and death. A series of planned a priori adjustments were performed that were consistent with a previous study.40 In the limited model, we adjusted for age, sex, race, and BMI. In the adjusted model, we also accounted for participants’ markers of socioeconomic status and health behaviors.
In separate models, we further adjusted for comorbid medical conditions or comedication use. We did not adjust specifically for systemic hypertension because the adjustment for comedication use addressed confounding by hypertension via inclusion of antihypertensives. Analyses were repeated in a cohort of propensity-matched participants where those included were matched according to their propensity for RAAS inhibitor prescription (e-Appendix 1).
In prespecified exploratory analyses, PAWP of ≤ 15 mm Hg vs > 15 mm Hg was evaluated as an effect modifier in the association between RAAS inhibitor use and mortality. Given the unexpected finding suggesting worse mortality in unadjusted relationships with AA users relative to nonusers and the decreased effect size with inconsistent statistical significances in the propensity-matched cohort, we considered additional exploratory adjustments to understand better the potential role of confounding in this relationship. In particular, we explored whether further adjustment by blood potassium level or markers of disease severity modified the relationships between AA use and mortality as well as between ACEI/ARB use and mortality. B-type natriuretic peptide (BNP) levels and inpatient status at the time of RHC were used as markers of disease severity. Analyses were performed using SAS version 9.4 software (SAS Institute) and R version 3.3.1 software (R Foundation for Statistical Computing). P < .05 was considered statistically significant.
Results
Patient Characteristics
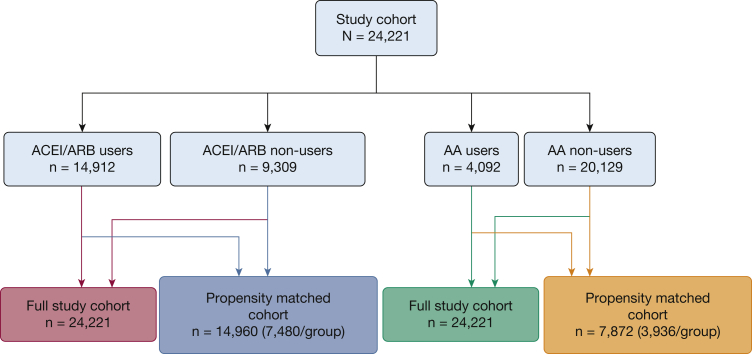
The final cohort included 24,221 patients (Table 1). Mean age was 66.8 years. Mean follow-up was 3.6 ± 2.5 years and total follow-up was 86,632 person-years. Those who used RAAS inhibitors tended to differ qualitatively from nonmedication users and tended to be slightly younger, non-White, heavier, and with a greater burden of comorbidities. In particular, AA users seemed to have a higher prevalence of cirrhosis and alcohol use. AA users also seemed more likely to be inpatients at the time of RHC and had lower cardiac indexes. e-Table 2 details the medication inventory. We identified 14,912 patients with a filled prescription for ACEI/ARB (Fig 1, Table 1). Of these ACEI/ARB users, 7,480 were matched with 7,480 nonusers who were otherwise similar in their propensity to have used these medications (Fig 1, e-Table 3). Four thousand ninety-two patients filled a prescription for AAs (Fig 1, Table 1). Of these, 3,936 AA users were matched to 3,936 nonusers with an otherwise similar propensity to use AAs (Fig 1, e-Table 4).
Table 1.
Characteristics of the Study Cohort
| Variable | All Patients (N = 24,221) | ACEI/ARB |
AA |
||
|---|---|---|---|---|---|
| Users (n = 14,912) | Nonusers (n = 9,309) | Users (n = 4,092) | Nonusers (n = 20,129) | ||
| Age, y | |||||
| <45 | 1.9 (452) | 2.0 (295) | 1.7 (157) | 3.7 (152) | 1.5 (300) |
| 45-54 | 8.1 (1,968) | 8.2 (1,220) | 8.0 (748) | 11.3 (461) | 7.5 (1,507) |
| 55-64 | 34.2 (8,287) | 35.7 (5,322) | 31.9 (2,965) | 41.1 (1,683) | 32.8 (6,604) |
| 65-74 | 35.3 (8,538) | 35.1 (5,239) | 35.4 (3,299) | 31.6 (1,295) | 36.0 (7,243) |
| 75-84 | 17.0 (4,116) | 16.1 (2,406) | 18.4 (1,710) | 10.9 (444) | 18.2 (3,672) |
| ≥85 | 3.6 (860) | 2.9 (430) | 4.6 (430) | 1.4 (57) | 4.0 (803) |
| Male sex | 96.6 (23,395) | 97.2 (14,492) | 95.6 (8,903) | 97.1 (3,972) | 96.5 (19,423) |
| Race | |||||
| White | 76.6 (18,545) | 75.2 (11,218) | 78.7 (7,327) | 70.9 (2,903) | 77.7 (15,642) |
| Black | 21.3 (5,165) | 22.7 (3,379) | 19.2 (1,786) | 27.0 (1,106) | 20.2 (4,059) |
| Other | 2.1 (511) | 2.1 (315) | 2.1 (196) | 2.0 (83) | 2.1 (428) |
| BMI, kg/m2 | |||||
| Underweight (< 18.5) | 0.8 (186) | 0.6 (84) | 1.1 (102) | 0.6 (25) | 0.8 (161) |
| Normal (≥ 18.5-25) | 16.9 (4,090) | 15.8 (2,353) | 18.7 (1,737) | 16.8 (688) | 16.9 (3,402) |
| Overweight (≥ 25-30) | 29.2 (7,068) | 28.5 (4,253) | 30.2 (2,815) | 29.1 (1,189) | 29.2 (5,879) |
| Obese (≥ 30-35) | 25.0 (6,051) | 25.3 (3,766) | 24.5 (2,285) | 24.3 (993) | 25.1 (5,058) |
| Severely obese (≥ 35) | 28.2 (6,826) | 29.9 (4,456) | 25.5 (2,370) | 29.3 (1,197) | 28.0 (5,629) |
| Socioeconomic status | |||||
| Tobacco use | 62.1 (15,048) | 62.3 (9,288) | 61.9 (5,760) | 62.9 (2,572) | 62.0 (12,476) |
| Alcohol use | 11.1 (2,687) | 11.1 (1,657) | 11.1 (1,030) | 14.2 (582) | 10.5 (2,105) |
| Income ($/y) | 49,886 (17,439) | 49,628 (17,215) | 50,299 (17,785) | 49,378 (16,872) | 49,989 (17,551) |
| Marital status | |||||
| Married | 48.7 (11,805) | 48.0 (7,162) | 49.9 (4,643) | 46.4 (1,900) | 49.2 (9,905) |
| Divorced | 29.9 (7,240) | 30.4 (4,529) | 29.1 (2,711) | 31.7 (1,298) | 29.5 (5,942) |
| Single | 13.7 (3,324) | 14.3 (2,132) | 12.8 (1,192) | 15.7 (641) | 13.3 (2,683) |
| Widowed | 7.6 (1,852) | 7.3 (1,089) | 8.2 (763) | 6.2 (253) | 7.9 (1,599) |
| Comorbidities | |||||
| Asthma | 5.4 (1,315) | 5.4 (807) | 5.5 (508) | 5.6 (230) | 5.4 (1,085) |
| Atrial fibrillation/flutter | 34.0 (8,239) | 34.1 (5,082) | 33.9 (3,157) | 38.3 (1,568) | 33.1 (6,671) |
| CHD | 0.6 (146) | 0.5 (81) | 0.7 (65) | 0.4 (17) | 0.6 (129) |
| CHF | 68.7 (16,636) | 73.4 (10,947) | 61.1 (5,689) | 90.5 (3,705) | 64.2 (12,931) |
| Cirrhosis | 7.3 (1,780) | 6.3 (933) | 9.1 (847) | 11.8 (483) | 6.4 (1,297) |
| CKD | 35.0 (8,468) | 32.6 (4,867) | 38.7 (3,601) | 36.3 (1,486) | 34.7 (6,982) |
| COPD | 38.6 (9,358) | 36.8 (5,492) | 41.5 (3,866) | 37.8 (1,546) | 38.8 (7,812) |
| Diabetes | 52.7 (12,771) | 55.8 (8,326) | 47.7 (4,445) | 55.2 (2,257) | 52.2 (10,514) |
| Hypertension | 90.6 (21,944) | 93.3 (13,919) | 86.2 (8,025) | 90.8 (3,716) | 90.6 (18,228) |
| ILD | 0.7 (164) | 0.6 (85) | 0.8 (79) | 0.4 (15) | 0.7 (149) |
| MI, PCI, or CABG | 41.7 (10,103) | 43.3 (6,460) | 39.1 (3,643) | 46.5 (1,901) | 40.7 (8,202) |
| OSA | 15.2 (3,685) | 15.3 (2,280) | 15.1 (1,405) | 17.1 (700) | 14.8 (2,985) |
| Valvular heart disease | 39.4 (9,551) | 37.6 (5,608) | 42.4 (3,943) | 32.9 (1,345) | 40.8 (8,206) |
| Hemodynamics | |||||
| RAP, mm Hg | 12.1 (5.7) | 12.2 (5.6) | 11.9 (5.9) | 13.3 (6.2) | 11.8 (5.5) |
| mPAP, mm Hg | 35.3 (8.5) | 35.3 (8.4) | 35.2 (8.7) | 37.0 (8.7) | 35.0 (8.5) |
| PAWP, mm Hg | 21.5 (7.5) | 22.1 (7.4) | 20.6 (7.6) | 23.6 (7.7) | 21.0 (7.4) |
| Cardiac index, L/min/m2 | 2.4 (0.7) | 2.4 (0.7) | 2.5 (0.7) | 2.2 (0.7) | 2.5 (0.7) |
| PVR, Wood units | 3.0 (2.1) | 2.9 (1.9) | 3.1 (2.3) | 3.1 (2.1) | 2.9 (2.1) |
| MAP, mm Hg | 93.1 (10.2) | 93.6 (10.5) | 92.2 (9.7) | 91.4 (11.3) | 93.4 (10.0) |
| Disease severity | |||||
| Inpatient status | 48.2 (11,685) | 51.5 (7,680) | 43.0 (4,005) | 67.8 (2,036) | 45.5 (9,649) |
| BNP, pg/mL | 1,081 (2,197) | 1,060 (2,111) | 1,119 (2,346) | 1,334 (2,665) | 995 (2,006) |
Values are presented as percent (absolute number), with the exception of income, hemodynamics, and BNP levels, which are presented as mean (SD). AA = aldosterone antagonist; ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BNP = B-type natriuretic peptide; CABG = coronary artery bypass grafting; CHD = congenital heart disease; CHF = congestive heart failure; CKD = chronic kidney disease; ILD = interstitial lung disease; MAP = mean arterial pressure; MI = myocardial infarction; mPAP = mean pulmonary arterial pressure; PAWP = pulmonary artery wedge pressure; PCI = percutaneous coronary intervention; PVR = pulmonary vascular resistance; RAP = right atrial pressure.
Figure 1.
Flow chart showing study sample. Cohorts studied included the full study cohort as well as limited cohorts used for propensity-matched analyses. AA = aldosterone antagonist; ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker.
ACEI/ARB Use Is Associated With Decreased Mortality in Veterans With PH
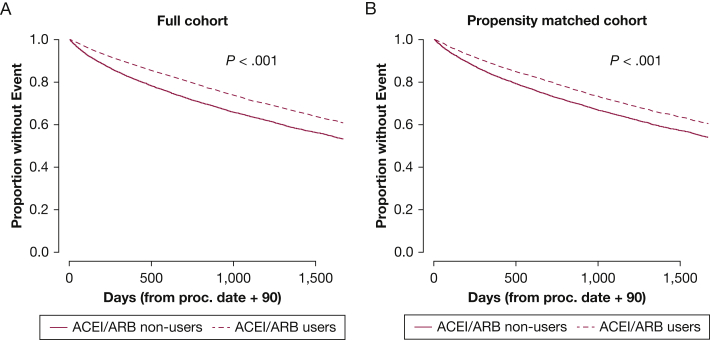
Six thousand one hundred forty-one veterans who used ACEIs/ARBs died over 56,358 person-years (10.9 deaths per 100 person-years), and 4,273 veterans who did not use ACEIs/ARBs died over 30,273 person-years (14.1 deaths per 100 person-years). ACEI/ARB use was associated with improved survival in unadjusted Kaplan-Meier survival analyses in the full and propensity-matched cohorts (Fig 2). ACEI/ARB use was associated with a highly significant 18% to 20% reduction in the hazard of mortality in a series of consistent Cox proportional hazard models that were not dependent on demographics, health behaviors, comorbidities, or comedication use (Table 2). Similar effect sizes and statistical differences were observed in the propensity-matched cohort (Table 2). The ACEI/ARB-mortality relationship was unaltered when accounting for plasma potassium levels or when accounting for BNP and inpatient status at the time of RHC (Table 3), suggesting that potassium levels or disease severity were not confounding the relationship between ACEI/ARB use and decreased mortality. Furthermore, PAWP of more or less than 15 mm Hg did not modify the relationship between ACEI/ARB use and mortality (P > .05, all values for the interaction) (e-Table 5), suggesting that the association with decreased mortality was not limited to individuals with left heart disease.
Figure 2.
A-B, Kaplan-Meier curves showing that ACEI or ARB use is associated with decreased mortality in veterans with pulmonary hypertension. A, Outcome of mortality for ACEI or ARB users and nonusers for the full cohort (14,912 users and 9,309 nonusers). Five-year mortality in ACEI or ARB users was 35.0% (5,212 events) compared with 41.2% in nonusers (3,837 events). χ2 = 154.8. B, Outcome of mortality for the propensity-matched cohort (7,480/group). ACEI or ARB use was defined as use of either medication class within 90 days of right heart catheterization. χ2 = 65.8. Group comparisons were performed by log rank test. ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker.
Table 2.
Cox Proportional Hazard Models for ACEI or ARB Exposure and for AA Exposure
| Variable | Full Cohorta |
Propensity-Matched Cohortb |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Exposure to ACEI or ARB | ||||
| Unadjusted | 0.78 (0.75-0.81) | < .001 | 0.82 (0.78-0.86) | < .001 |
| Limited adjustmentc | 0.81 (0.78-0.84) | < .001 | 0.82 (0.78-0.86) | < .001 |
| Full adjustmentd | 0.81 (0.78-0.84) | < .001 | 0.82 (0.78-0.86) | < .001 |
| Full adjustmentd plus comorbiditye | 0.78 (0.75-0.81) | < .001 | 0.80 (0.76-0.84) | < .001 |
| Full adjustmentd plus comedication usef | 0.80 (0.76-0.83) | < .001 | 0.81 (0.77-0.85) | < .001 |
| Exposure to AA | ||||
| Unadjusted | 1.11 (1.05-1.16) | < .001 | 1.07 (1.01-1.15) | .03 |
| Limited adjustmentc | 1.24 (1.18-1.31) | < .001 | 1.07 (1.00-1.15) | .04 |
| Full adjustmentd | 1.24 (1.18-1.30) | < .001 | 1.07 (1.00-1.14) | .05 |
| Full adjustmentd plus comorbiditye | 1.09 (1.03-1.14) | < .01 | 1.08 (1.01-1.15) | .03 |
| Full adjustmentd plus comedication usef | 1.16 (1.10-1.22) | < .001 | 1.06 (0.99-1.13) | .08 |
Results are presented with and without adjustment in the full cohort and the smaller cohort of participants who used RAAS inhibitors compared with participants with an otherwise similar propensity to use RAAS inhibitors who did not use these medications. Participants in the restricted cohorts were considered in models with full adjustment. AA = aldosterone antagonist; ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; HR = hazard ratio; RAAS = renin-angiotensin-aldosterone system.
n = 24,221.
n = 14,960 for ACEI/ARB analyses and n = 7,872 for AA analyses.
Age, sex, race or ethnicity, and BMI.
Limited model and income, tobacco use, alcohol use, and marital status.
Presence or absence of end-stage renal disease or dialysis; diabetes mellitus; cirrhosis; sleep-disordered breathing; COPD or asthma; interstitial lung disease; prior myocardial infarction; prior percutaneous coronary intervention; prior coronary artery bypass graft; congestive heart failure; valvular heart disease; congenital heart disease; and atrial fibrillation, atrial flutter, or both.
Comedication use included all non-RAAS inhibitor medications included in e-Table 1.
Table 3.
Exploratory Cox Proportional Hazard Models for ACEI/ARB or AA Exposure in Veterans Including Adjustment for Potassium Level or Disease Severity
| Variable | Full Cohort |
Propensity-Matched Cohort |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| ACEI/ARB | ||||
| Full adjustmenta | 0.81 (0.78-0.84) | < .001 | 0.82 (0.78-0.86) | < .001 |
| Full adjustmenta with further adjustment for potassium | 0.80 (0.77-0.83)b | < .001 | 0.81 (0.77-0.85)c | < .001 |
| Full adjustmenta with further adjustment for disease severityd | 0.72 (0.67-0.78)e | < .001 | 0.77 (0.70-0.85)f | < .001 |
| AA | ||||
| Full adjustmenta | 1.24 (1.18-1.30) | < .001 | 1.07 (1.00-1.14) | .05 |
| Full adjustmenta with further adjustment for potassium | 1.23 (1.17-1.30)b | < .001 | 1.08 (1.01-1.15)g | .03 |
| Full adjustmenta with further adjustment for disease severityd | 1.05 (0.96-1.14)e | .28 | 1.04 (0.93-1.16)h | .48 |
Fully adjusted models for entire cohort and propensity-matched cohort (from Table 2) are included for reference. AA = aldosterone antagonist; ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; HR = hazard ratio.
Age, sex, race or ethnicity, BMI, income, tobacco use, alcohol use, and marital status.
n = 21,586.
n = 13,211.
Adjustment for disease severity also accounts for B-type natriuretic peptide level and inpatient admission.
n = 5,823.
n = 3,302.
n = 7,349.
n = 2,676.
ACEI/ARB Use Is Associated With Decreased Mortality in Veterans With Precapillary PH
ACEI/ARB use was also associated with improved mortality in a smaller cohort of participants with precapillary PH (e-Table 6). In 2,875 participants with mean pulmonary artery pressure of ≥ 25 mm Hg, PAWP of ≤ 15 mm Hg, and PVR of >3 Wood units, the hazard of mortality with ACEI/ARB use was 23% to 26% less than in nonusers (HR in the model with limited adjustment, 0.77 [95% CI, 0.69-0.85; P < .001]; HR in the model with full adjustment and accounting for comorbidity, 0.74 [95% CI, 0.66-0.82; P < .001]).
AA Use Is Associated With Worse Survival in Unadjusted Kaplan-Meier Survival Analyses in the Full Cohort, But Only Inconsistently Associated With Mortality in the Propensity-Matched Cohort
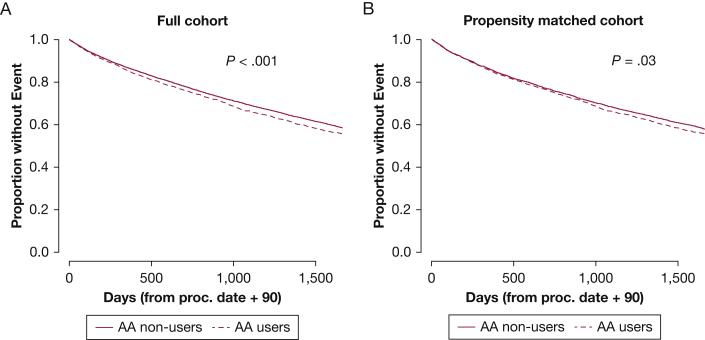
One thousand eight hundred thirty veterans who used AAs died over 13,980 person-years (13.1 deaths per 100 person-years), and 8,584 veterans who did not use AAs died over 72,651 person-years (11.8 deaths per 100 person-years). AA use was associated with worse survival in unadjusted Kaplan-Meier survival analyses (Fig 3). Although statistically significant, the association between AA use and mortality was impacted by adjustment and varied between a 9% and 24% increased risk for mortality depending on the model (Table 2). This relationship was weakest in the model accounting for comorbidities. PAWP did not modify the relationship between AA use and increased mortality (P > .05, all values for the interaction when evaluated in limited and fully adjusted models) (e-Table 5).
Figure 3.
A-B, Kaplan-Meier curves showing that AA use is associated with increased mortality in veterans with pulmonary hypertension, with a less robust association in the propensity-matched cohort. A, Outcome of mortality for AA users and nonusers for the full cohort (4,092 users and 20,129 nonusers). Five-year mortality in AA users was 39.2% (1,606 events) compared with 37.0% in nonusers (7,443 events). χ2 = 14.1. B, Outcome of mortality for the propensity-matched cohort (3,936/group). AA use was defined as use within 90 days of right heart catheterization. χ2 = 4.5. Group comparisons were performed by log rank test. Note the lower χ2 value and less robust statistical significance in the propensity-matched cohort. AA = aldosterone antagonist.
The association further weakened in the propensity-matched cohort. In unadjusted or adjusted analyses of the propensity-matched cohort, only a 6% to 8% increased risk for mortality was found with AA use, and statistical significances were markedly attenuated and not achieved consistently (Table 2). This raises the possibility that the association between AA use and mortality in the full cohort may be confounded by the propensity of use AAs and, as such, may be confounded by indication, disease severity, or both.
AA-Mortality Association Is Insensitive to Adjustment for Potassium Levels, but Is Sensitive to Adjustment for Disease Severity
Because an increase in hyperkalemia-associated morbidity and mortality was noted after publication of the Randomized Aldactone Evaluation Study (RALES),41 we explored preprocedural potassium levels as a potential confounder in a nonplanned analysis after finding increased mortality among AA users. We found that the association between AA use and mortality was independent of potassium levels (Table 3). Finally, given the wide range of effect estimates and sensitivity to adjustment, we remained concerned that differences in disease severity may exist between those who did and did not use AAs. Indeed, accounting for BNP and inpatient status at the time of RHC markedly attenuated the association between AA use and increased mortality in the full cohort (Table 3). This supports the hypothesis that residual confounding related to disease severity was present in the full model and may have accounted for the relationship between AA use and increased mortality.
AA Use Does Not Affect Mortality in Veterans With Precapillary PH
To investigate effects of AA use on mortality in veterans with precapillary PH, we restricted our analyses to participants who used AA and had a mean pulmonary artery pressure of ≥ 25 mm Hg, PAWP of ≤ 15 mm Hg, and PVR of > 3 Wood units. Both BNP level and inpatient status were included as predictors. The low number of deaths as well as exclusion of patients because of missing BNP values precluded confident analysis in this small cohort, and only a model with limited adjustment could be fit. In 529 participants with precapillary PH and a measurement for BNP (e-Table 7), AA use was not associated with an effect on mortality (hazard ratio, 0.98; 95% CI, 0.73-1.31; P = .87).
Discussion
Our data are the first to evaluate the association between RAAS inhibitors and clinical outcomes in a large cohort of PH patients. We demonstrate that use of ACEIs/ARBs is associated with decreased mortality. We observed a relationship between AA use and increased mortality that was no longer present when accounting more robustly for possible differences in disease severity between AA users and nonusers.
Approximately 70 million patients in the United States are estimated to have PH, RV dysfunction, or both.42, 43, 44, 45, 46, 47 PH is a particular problem in veterans, in whom it is common and associated with poor outcomes, yet frequently underrecognized.37,48 In veterans, even mild increases in pulmonary artery pressure are associated with increased morbidity and mortality.37 Furthermore, PH in veterans is not only underrecognized, but frequently also treated inappropriately.32,48 This is important because inappropriate treatment of non-PAH forms of PH with PAH-specific drugs is associated with worse outcomes.27, 28, 29, 30, 31, 32 One factor driving the use of PAH-specific therapy in patients without PAH may be the lack of treatment options for non-PAH forms of PH. Novel therapeutic strategies for these patients are needed.
The large patient volume in the VA, high prevalence of chronic cardiopulmonary diseases, and tracking of hemodynamic data through CART provide a unique opportunity to evaluate the effects of pharmacologic interventions on outcomes in common PH phenotypes in a real-world scenario. For example, we recently showed that use of H2-receptor antagonists in veterans with PH is associated with decreased mortality.40 Survival in this cohort with relatively advanced age and multiple morbidities was relatively well preserved and maybe even better than expected for this population. This may reflect the observation that the VA system performs similarly or better than the non-VA system on most of the nationally recognized measures of inpatient and outpatient care quality.49
We demonstrate several findings that may contribute to understanding novel treatment strategies for PH. First, we show that ACEI/ARB use is associated with decreased risk of mortality. This relationship is highly significant, is relatively insensitive to adjustment, and does not seem to depend on differences in demographics, comorbidity, comedication-use, potassium levels, disease severity, or propensity to use ACEI/ARB. Decreased mortality with ACEI/ARB use also was noted in the cohort with precapillary PH, suggesting that the benefit may not be simply the result of appropriate treatment of left heart disease. Although confounding by indication is always possible in pharmacoepidemiology, insensitivity to a wide range of adjustments and consistency of results in the propensity-matched cohort suggest that the association between ACEI/ARB use and mortality is less likely to be explained by confounding. Although each of these approaches has limitations and opportunities for residual confounding, the strength of the complementary approach is in the mutual reinforcement or disagreement. In the case of ACEI/ARBs, all approaches agreed. Although not totally exculpatory, this is reassuring and adds scientific rigor. This differs from the AA analyses, in which adjustments yielded different effect estimates and suggested that confounding (variably addressed by the method used) strongly influenced the observed relationship. In addition, the high level of statistical significance makes a false-positive result as a consequence of analyzing a large patient population less likely.50
RAAS inhibitors are important treatments for left heart failure and systemic hypertension and have shown promise in experimental PH and in exploratory clinical analyses in PAH patients.9,10,15, 16, 17, 18, 19, 20, 21, 22 Because the RAAS also is activated in group 2 PH, in COPD with RV failure, and other disorders associated with hypoxia, hypercarbia, or both,33, 34, 35, 36 exploring the potential benefit of RAAS inhibitors in a population predominantly characterized by group 2 and 3 PH makes conceptual sense and fills an important clinical knowledge gap. Importantly, ACEIs/ARBs are inexpensive and have a favorable side-effect profile, making them an attractive treatment option.
As hypothesized, we demonstrated benefit of ACEIs/ARBs in postcapillary PH. However, these drugs also were associated with improved survival in participants with precapillary PH. This finding is unusual for PH therapies, because treatment may improve outcomes in selected groups with precapillary PH, but worsen outcomes in those with postcapillary PH.3 Although we could not differentiate between group 1 and 3 PH in our cohort, the burden of comorbidities in the VA suggests that most patients with precapillary disease exhibit a group 3 phenotype (39% of our patients had a diagnosis of COPD; 15% had a diagnosis of OSA). Prior studies suggest that increased RAAS activation may contribute to RV dysfunction in lung disease34,35 and may mediate PAH development.15 As such, the survival benefit observed in our smaller precapillary PH cohort is biologically plausible, especially because RV dysfunction is common in the CART population.51 Future studies will need to corroborate our findings in additional well-phenotyped PH populations and to identify mechanisms of how ACEIs/ARBs exert their potential protective effects. Such studies also should answer the important question of whether protective ACEI/ARB effects target the RV, the pulmonary vasculature, both, or perhaps other organ systems. Such studies will need to address whether benefits of ACEIs/ARBs extend to both group 1 and group 3 PH or are limited to one of these groups. Studies manipulating angiotensin signaling in PAH patients currently are ongoing (ClinicalTrials.gov Identifiers: NCT01181284 and NCT01884051). Our data suggest that additional studies evaluating ACEI/ARB use in group 3 PH may be warranted.
We found an unexpected association between AA use and increased mortality in the full cohort. However, in counterpoint to the findings regarding ACEI/ARB use, this association exhibited a less robust level of statistical significance,50 was variable, and was observed only inconsistently in the propensity-matched cohort. Furthermore, this association was sensitive to adjustment for disease severity. This suggests that residual confounding related to disease severity likely was present in full and unadjusted models using the full cohort. In the CART database, AA use in veterans with PH seems to be a marker of disease severity, rather than a mediator of worse survival. Our results suggest that sicker patients may have received AA, rather than patients with milder disease, which may reflect the typical clinical practice of adding therapies in patients with advanced or refractory symptoms. Two previous studies suggest that AA therapy indeed is prescribed preferentially in patients with greater pulmonary vascular disease burden. First, in the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Study (ARIES) trial of ambrisentan for PAH, enrolled patients were treated with spironolactone based on the discretion of their personal physician.19 Analysis of these patients demonstrated that 61% of patients were prescribed spironolactone to treat “RV failure,” “refractory edema,” or “electrolyte imbalances.” Each of these scenarios suggests spironolactone use to offset or mitigate end-organ damage caused by PAH. Second, in a recent single-center cohort study, patients prescribed AA therapy showed more severe RV contractile dysfunction and a trend toward higher PAWP and more frequent loop diuretic use.52 As a result, care must be taken when interpreting associations between AA use and mortality, and future observational studies should be particularly mindful of the potential for confounding by indication.52
Our study has limitations. Unmeasured or residual confounding can complicate the inference in observational studies. Although a number of the adjustments were intended to isolate RAAS inhibition from a suite of potentially causative health-focused behaviors (eg, a healthy-user paradigm), residual confounding may well persist, and adjustment for health behaviors like exercise and diet was not possible. Confounding by indication also is common in pharmacoepidemiology and seems to have been a particular problem in the association with AA use. This reinforces the need for cautious inference.53 In addition, all-cause mortality is not cause specific.54 Cause-specific mortality was not available for the CART cohort, and noncardiovascular explanations for our findings are possible. As such, we cannot determine if beneficial effects of ACEIs/ARBs were the results of effects on the pulmonary vasculature or the RV or of other mechanisms. Although follow-up RHC data are available for a subgroup of CART patients and could help to answer this question, analysis of these patients outside of a controlled clinical trial would introduce selection bias because repeat RHCs tend to be performed in sicker patients with an unsatisfying treatment response. We used RAAS inhibitor exposure near the time of RHC as a marker of individuals who likely had significant exposure. Documentation of one outpatient prescription fill may not be a robust surrogate marker for consistent RAAS use vs nonuse and represents a limitation of the data source. This misclassification of measured exposure relative to the exposure of interest is likely; however, because this nondifferential misclassification is not conditioned on the outcome, it is likely conservative and may bias observed results toward the null hypothesis. Information on cumulative doses, duration of use, or both would have strengthened the study; however, this was not measured, and relationships using these important biologic gradients are not available to support casual inference on our data. Finally, the strong male predominance in our cohort limits generalizability. Sex differences in the response to spironolactone therapy have been reported,55 and it is possible that specific effects of AAs on subgroups of patients were not captured despite rigorous adjusting.
Nonetheless, to our knowledge, this is the largest single cohort of individuals with invasively confirmed PH, and we believe our data support ongoing study of RAAS signaling in diseases with increased RV afterload. In particular, the association with benefit among those using ACEIs/ARBs was robust in several models and cohorts and, alongside strong preclinical data, may justify randomized controlled study of ACEIs/ARBs in patients with PAH and other forms of PH. ACEIs/ARBs are relatively inexpensive and have a favorable side-effect profile. If our results can be confirmed in additional cohorts and in prospective, randomized studies, ACEIs/ARBs may represent an attractive treatment strategy for the large proportion of patients with non-PAH PH phenotypes.
Conclusions
We demonstrated in a large cohort of veterans with invasively confirmed PH that ACEI/ARB use is associated with a decreased risk of mortality. This relationship was insensitive to adjustment and also was observed in patients with precapillary PH, suggesting that the observed benefit is not explained simply by appropriate treatment of left heart disease. AA use seems to be a marker of disease severity in PH. If these hypothesis-generating results can be confirmed in prospective, randomized clinical studies, ACEIs/ARBs may represent an attractive and novel treatment strategy for diverse PH phenotypes.
Acknowledgments
Author contributions: T. L. had full access to all the data in the study and takes responsibility for its integrity and the data analysis. T. L. contributed to study design, data analysis, and manuscript writing. E. H. contributed to study design, data generation, data analysis, and manuscript writing. A. E. B. contributed to study design, data generation, data analysis, and manuscript editing. T. M. M. contributed to study design, data analysis, and manuscript editing. M. E. P. contributed to study design, data generation, data analysis, and manuscript editing. G. C. contributed to study design, data analysis, and manuscript editing. B. A. M. contributed to study design, data analysis, and manuscript editing. R. T. Z. contributed to study design, data analysis, and manuscript editing. P. J. L. contributed to study design, data analysis, and manuscript writing.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: T. L. has received consultancy fees from Bayer and research reagents from Eli Lilly & Company and is the site primary investigator for a clinical trial funded by Complexa, Inc. T. M. M. has received consultancy fees from Creative Educational Concepts, Inc., and Atheneum Partners; is advising Myia Labs, for which his employer is receiving equity compensation in the company, but is receiving no individual compensation from the company; and is a compensated director for a New Mexico-based foundation, the J. F. Maddox Foundation. G. C. has received an investigator-initiated grant from Novartis. B. A. M. has served as a consultant for Actelion; and is coinventor of patents or patent applications that are related to pulmonary hypertension (U.S. Patent #9,605,047; PCT/US2015/ 029672; Provisional ID: #62475955; Provisional ID: #24624; Provisional ID: #24622). R. T. Z. has received consultancy fees from Morphogen-IX, Vivus, and Actelion; his institution has received research grants from Action, United Therapeutics, and Tenax. P. J. L. has received consultancy fees from Bayer and has been the site principle investigator for trials and registries funded by United Therapeutics, Actelion, and Bayer. None declared (E. H., A. E. B., M. E. P.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: T. L. is supported by the Department of Veterans Affairs [Merit Review Award 1I01BX002042] and the National Institutes of Health [Grants 1R56HL134736-01A1 and 1R01HL144727-01A1]. T. M. M. is supported by the National Institutes of Health National Center for Advancing Translational Sciences [Grant 1U24TR002306-01]. G. C. is supported by the Department of Veterans Affairs [Merit Review Award I01CX001892] and the National Institutes of Health [Grants R01HL128661 and R01HL148727]. B. A. M. is supported by the National Institutes of Health [Grants HL139613-01, HL1535-02, and HL145420], the Cardiovascular Medical Research Education Foundation, the McKenzie Family Charitable Trust, and the Boston Biomedical Innovations Center. P. J. L. is supported by the National Institutes of Health [Grants R33HL142539 and R01HL152724].
Supplementary Data
References
- 1.Simonneau G., Montani D., Celermajer D.S. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vonk Noordegraaf A., Chin K.M., Haddad F. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. 2019;53(1):1801900. doi: 10.1183/13993003.01900-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vachiery J.L., Tedford R.J., Rosenkranz S. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53(1):1801897. doi: 10.1183/13993003.01897-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan S.D., Barbera J.A., Gaine S.P. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53(1):1801914. doi: 10.1183/13993003.01914-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humbert M., Sitbon O., Chaouat A. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122(2):156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 6.Gall H., Felix J.F., Schneck F.K. The Giessen Pulmonary Hypertension Registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant. 2017;36(9):957–967. doi: 10.1016/j.healun.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Newman J.H., Rich S., Abman S.H. Enhancing insights into pulmonary vascular disease through a precision medicine approach. A Joint NHLBI-Cardiovascular Medical Research and Education Fund Workshop report. Am J Resp Crit Care Med. 2017;195(12):1661–1670. doi: 10.1164/rccm.201701-0150WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elinoff J.M., Agarwal R., Barnett C.F. Challenges in pulmonary hypertension: controversies in treating the tip of the iceberg. Am J Resp Crit Care Med. 2018;198(2):166–174. doi: 10.1164/rccm.201710-2093PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16):1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 10.Whelton P.K., Carey R.M., Aronow W.S. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 11.de Man F.S., Handoko M.L., Guignabert C., Bogaard H.J., Vonk-Noordegraaf A. Neurohormonal axis in patients with pulmonary arterial hypertension: friend or foe? Am J Resp Crit Care Med. 2013;187(1):14–19. doi: 10.1164/rccm.201209-1663PP. [DOI] [PubMed] [Google Scholar]
- 12.Maron B.A., Leopold J.A. The role of the renin-angiotensin-aldosterone system in the pathobiology of pulmonary arterial hypertension (2013 Grover Conference series) Pulm Circ. 2014;4(2):200–210. doi: 10.1086/675984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maron B.A., Leopold J.A. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part ii: neurohormonal signaling contributes to the pulmonary vascular and right ventricular pathophenotype of pulmonary arterial hypertension. Circulation. 2015;131(23):2079–2091. doi: 10.1161/CIRCULATIONAHA.114.006980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaillancourt M., Chia P., Sarji S. Autonomic nervous system involvement in pulmonary arterial hypertension. Resp Res. 2017;18(1):201. doi: 10.1186/s12931-017-0679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Man F.S., Tu L., Handoko M.L. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am J Resp Crit Care Med. 2012;186(8):780–789. doi: 10.1164/rccm.201203-0411OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva Goncalves Bos D., Happe C., Schalij I. Renal denervation reduces pulmonary vascular remodeling and right ventricular diastolic stiffness in experimental pulmonary hypertension. JACC Basic Transl Sci. 2017;2(1):22–35. doi: 10.1016/j.jacbts.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maron B.A., Zhang Y.Y., White K. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation. 2012;126(8):963–974. doi: 10.1161/CIRCULATIONAHA.112.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maron B.A., Opotowsky A.R., Landzberg M.J., Loscalzo J., Waxman A.B., Leopold J.A. Plasma aldosterone levels are elevated in patients with pulmonary arterial hypertension in the absence of left ventricular heart failure: a pilot study. Eur J Heart Fail. 2013;15(3):277–283. doi: 10.1093/eurjhf/hfs173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maron B.A., Waxman A.B., Opotowsky A.R. Effectiveness of spironolactone plus ambrisentan for treatment of pulmonary arterial hypertension (from the [ARIES] study 1 and 2 trials) Am J Cardiol. 2013;112(5):720–725. doi: 10.1016/j.amjcard.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maron B.A., Oldham W.M., Chan S.Y. Upregulation of steroidogenic acute regulatory protein by hypoxia stimulates aldosterone synthesis in pulmonary artery endothelial cells to promote pulmonary vascular fibrosis. Circulation. 2014;130(2):168–179. doi: 10.1161/CIRCULATIONAHA.113.007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aghamohammadzadeh R., Zhang Y.Y., Stephens T.E. Up-regulation of the mammalian target of rapamycin complex 1 subunit Raptor by aldosterone induces abnormal pulmonary artery smooth muscle cell survival patterns to promote pulmonary arterial hypertension. FASEB J. 2016;30(7):2511–2527. doi: 10.1096/fj.201500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boehm M., Arnold N., Braithwaite A. Eplerenone attenuates pathological pulmonary vascular rather than right ventricular remodeling in pulmonary arterial hypertension. BMC Pulm Med. 2018;18(1):41. doi: 10.1186/s12890-018-0604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samokhin A.O., Stephens T., Wertheim B.M. NEDD9 targets COL3A1 to promote endothelial fibrosis and pulmonary arterial hypertension. Science Transl Med. 2018;10(445):eaap7294. doi: 10.1126/scitranslmed.aap7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vonk Noordegraaf A., Westerhof B.E., Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69(2):236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 25.Vonk-Noordegraaf A., Haddad F., Chin K.M. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62(25 suppl):D22–D33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 26.van de Veerdonk M.C., Kind T., Marcus J.T. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58(24):2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 27.Vachiery J.L., Delcroix M., Al-Hiti H. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J. 2018;51(2):1701886. doi: 10.1183/13993003.01886-2017. [DOI] [PubMed] [Google Scholar]
- 28.Packer M., McMurray J.J.V., Krum H. Long-Term effect of endothelin receptor antagonism with bosentan on the morbidity and mortality of patients with severe chronic heart failure: primary results of the ENABLE Trials. JACC Heart Fail. 2017;5(5):317–326. doi: 10.1016/j.jchf.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Blanco I, Gimeno E, Munoz PA, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Resp Crit Care Med.181(3):270-278. [DOI] [PubMed]
- 30.Stolz D., Rasch H., Linka A. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J. 2008;32(3):619–628. doi: 10.1183/09031936.00011308. [DOI] [PubMed] [Google Scholar]
- 31.Raghu G., Behr J., Brown K.K. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158(9):641–649. doi: 10.7326/0003-4819-158-9-201305070-00003. [DOI] [PubMed] [Google Scholar]
- 32.Kim D., Lee K.M., Freiman M.R. Phosphodiesterase-5-inhibitor therapy for pulmonary hypertension in the US: actual vs recommended use. Ann Am Thorac Soc. 2018;15(6):693–701. doi: 10.1513/AnnalsATS.201710-762OC. [DOI] [PubMed] [Google Scholar]
- 33.Maron B.A., Stephens T.E., Farrell L.A. Elevated pulmonary arterial and systemic plasma aldosterone levels associate with impaired cardiac reserve capacity during exercise in left ventricular systolic heart failure patients: a pilot study. J Heart Lung Transplant. 2016;35(3):342–351. doi: 10.1016/j.healun.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farber M.O., Weinberger M.H., Robertson G.L., Fineberg N.S. The effects of angiotensin-converting enzyme inhibition on sodium handling in patients with advanced chronic obstructive pulmonary disease. Am Rev Resp Dis. 1987;136(4):862–866. doi: 10.1164/ajrccm/136.4.862. [DOI] [PubMed] [Google Scholar]
- 35.MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part two. Am J Resp Crit Care Med. 1994;150(4):1158–1168. doi: 10.1164/ajrccm.150.4.7921453. [DOI] [PubMed] [Google Scholar]
- 36.Konigshoff M., Wilhelm A., Jahn A. The angiotensin II receptor 2 is expressed and mediates angiotensin II signaling in lung fibrosis. Am J Resp Cell Mol Biol. 2007;37(6):640–650. doi: 10.1165/rcmb.2006-0379TR. [DOI] [PubMed] [Google Scholar]
- 37.Maron B.A., Hess E., Maddox T.M. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking program. Circulation. 2016;133(13):1240–1248. doi: 10.1161/CIRCULATIONAHA.115.020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maddox T.M., Plomondon M.E., Petrich M. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs Clinical Assessment, Reporting, and Tracking program) Am J Cardiol. 2014;114(11):1750–1757. doi: 10.1016/j.amjcard.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 39.Sohn M.W., Arnold N., Maynard C., Hynes D.M. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leary P.J., Hess E., Baron A.E. H2-receptor antagonist use and mortality in pulmonary hypertension: insight from the VA-CART program. Am J Resp Crit Care Med. 2018;197(12):1638–1641. doi: 10.1164/rccm.201801-0048LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juurlink D.N., Mamdani M.M., Lee D.S. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 42.Morrison D.A., Adcock K., Collins C.M., Goldman S., Caldwell J.H., Schwarz M.I. Right ventricular dysfunction and the exercise limitation of chronic obstructive pulmonary disease. J Am Coll Cardiol. 1987;9(6):1219–1229. doi: 10.1016/s0735-1097(87)80459-x. [DOI] [PubMed] [Google Scholar]
- 43.Chaouat A., Weitzenblum E., Krieger J., Oswald M., Kessler R. Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients. Chest. 1996;109(2):380–386. doi: 10.1378/chest.109.2.380. [DOI] [PubMed] [Google Scholar]
- 44.Mohammed S.F., Hussain I., AbouEzzeddine O.F. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130(25):2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redfield M.M., Jacobsen S.J., Burnett J.C., Jr., Mahoney D.W., Bailey K.R., Rodeheffer R.J. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 46.Lloyd-Jones D., Adams R.J., Brown T.M. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 47.Hyduk A., Croft J.B., Ayala C., Zheng K., Zheng Z.J., Mensah G.A. Pulmonary hypertension surveillance—United States, 1980-2002. MMWR Surveill Summ. 2005;54(5):1–28. [PubMed] [Google Scholar]
- 48.Maron B.A., Choudhary G., Khan U.A. Clinical profile and underdiagnosis of pulmonary hypertension in US veteran patients. Circ Heart Fail. 2013;6(5):906–912. doi: 10.1161/CIRCHEARTFAILURE.112.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anhang Price R., Sloss E.M., Cefalu M., Farmer C.M., Hussey P.S. Comparing quality of care in Veterans Affairs and non-Veterans Affairs settings. J Gen Intern Med. 2018;33(10):1631–1638. doi: 10.1007/s11606-018-4433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wayant C., Scott J., Vassar M. Evaluation of lowering the P value threshold for statistical significance from .05 to .005 in previously published randomized clinical trials in major medical journals. JAMA. 2018;320(17):1813–1815. doi: 10.1001/jama.2018.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ventetuolo C.E., Hess E., Austin E.D. Sex-based differences in veterans with pulmonary hypertension: results from the veterans affairs-clinical assessment reporting and tracking database. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corkish ME D.L., Clarke M.M., Murray B.P., Rose-Jones L.J. Rates of hospitalization associated with the use of aldosterone receptor antagonists in patients with pulmonary arterial hypertension. Pulm Circ. 2019;9(3) doi: 10.1177/2045894019868422. 2045894019868422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walker A.M. Confounding by indication. Epidemiology. 1996;7(4):335–336. [PubMed] [Google Scholar]
- 54.Weiss N.S. All-cause mortality as an outcome in epidemiologic studies: proceed with caution. Eur J Epidemiol. 2014;29(3):147–149. doi: 10.1007/s10654-014-9899-y. [DOI] [PubMed] [Google Scholar]
- 55.Merrill M., Sweitzer N.K., Lindenfeld J., Kao D.P. Sex differences in outcomes and responses to spironolactone in heart failure with preserved ejection fraction: a secondary analysis of TOPCAT Trial. JACC Heart Fail. 2019;7(3):228–238. doi: 10.1016/j.jchf.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.