Abstract
Background
Pulmonary arterial hypertension (PAH) is a progressive disease associated with significant morbidity and mortality. Despite the negative impact of PAH on quality of life and survival, data on use of specialty palliative care services (PCS) is scarce.
Research Question
We sought to evaluate the inpatient use of PCS in patients with PAH.
Study Design and Methods
Using the National (Nationwide) Inpatient Sample, 30,495 admissions with a primary diagnosis of PAH were identified from 2001 through 2017. The primary outcome of interest was temporal trends and predictors of inpatient PCS use in patients with PAH.
Results
The inpatient use of PCS was low (2.2%), but increased during the study period from 0.5% in 2001 to 7.6% in 2017, with a significant increase starting in 2009. White race, private insurance, higher socioeconomic status, hospital-specific factors, higher comorbidity burden (Charlson Comorbidity Index), cardiac and noncardiac organ failure, and use of extracorporeal membrane oxygenation and noninvasive mechanical ventilation were independent predictors of increased PCS use. PCS use was associated with a higher prevalence of do-not-resuscitate status, a longer length of stay, higher hospitalization costs, and increased in-hospital mortality with less frequent discharges to home, likely because these patients were also sicker (higher comorbidity index and illness acuity).
Interpretation
The inpatient use of PCS in patients with PAH is low, but has been increasing over recent years. Despite increased PCS use over time, patient- and hospital-specific disparities in PCS use continue. Further studies evaluating these disparities and the role of PCS in the comprehensive care of PAH patients are warranted.
Key Words: critical care cardiology, end-of-life, National (Nationwide) Inpatient Sample, outcomes research, palliative care, pulmonary arterial hypertension, pulmonary vascular disease
Abbreviations: DNR, do-not-resuscitate; HCUP, Healthcare Quality and Utilization Project; ICD, International Classification of Diseases; NIS, National (Nationwide) Inpatient Sample; PAH, pulmonary arterial hypertension; PCS, palliative care services; QOL, quality of life; SES, socioeconomic status
FOR EDITORIAL COMMENT, SEE PAGE 2260
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by elevated pulmonary artery pressure and pulmonary vascular resistance with normal left-sided filling pressures.1 PAH has an estimated prevalence of 10 to 50 per 1 million and can be idiopathic, familial, or associated with conditions such as congenital heart disease, connective tissue disease, drugs or toxin use, and portal hypertension.2, 3, 4, 5 Symptoms of PAH include dyspnea, fatigue, chest pain, and dizziness. After right heart failure develops, symptoms also can include edema, weight gain, and abdominal bloating. PAH negatively impacts physical, social, and emotional health-related quality of life (QOL) and survival.6, 7, 8 PAH-specific medications improve symptoms, slow the progression of disease, and reduce hospitalizations, but are not curative and commonly are associated with significant side effects.3,4
Despite improved survival over the last few decades related to advances in pharmacologic treatment options, PAH remains a progressive and fatal disease with 1-year mortality rates as high as 10% to 20%.9, 10, 11, 12 Hospitalization is associated with a particularly poor prognosis; patients with PAH and right heart failure have in-hospital mortality rates from 14% to as high as 30% to 48% if they are admitted to an ICU.13 Mortality also remains high after hospitalization. In patients who survive hospitalization, 35% die within 1 year of hospital discharge.14
Palliative care is a medical specialty of interdisciplinary clinicians aimed at improving QOL for patients with a serious or life-limiting illness, or both, as well as support for patients and families. Specialty palliative care services (PCS) are present in the vast majority of hospitals with more than 300 beds, and PCS use for patients who are facing serious illness with potentially life-limiting prognoses increasingly is becoming the standard of care.15,16 Experts have advocated for earlier integration of PCS in the management of PAH, but small survey-based studies have found that PCS are underrecognized and underused.17, 18, 19
A paucity of studies have evaluated the role of palliative care in patients with PAH, particularly those who are admitted to the hospital.17,19,20 Therefore, we sought to investigate the national use of PCS by hospital admissions with PAH in the United States. We evaluated the temporal trends of PCS use, predictors of PCS use, and outcomes associated with the use of PCS in hospital admissions for PAH.
Methods
Study Population, Variables, and Outcomes
The National (Nationwide) Inpatient Sample (NIS) is the largest all-payer database of hospital inpatient stays in the United States. The NIS contains discharge data from a 20% stratified sample of hospitals and is a part of the Healthcare Quality and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality.21 Information regarding each discharge includes patient demographics, primary payer, hospital characteristics (region; rural, urban nonteaching, or urban teaching; and small, medium, or large), principal diagnosis, up to 24 secondary diagnoses, and procedural diagnoses. The HCUP and NIS do not evaluate individual patients, but rather evaluate a single inpatient admission. Because the NIS is publicly available deidentified database, institutional review board approval was waived.
Using the NIS data from 2001 through 2017, a retrospective cohort of hospital admissions (≥ 18 years of age) with a primary discharge diagnosis of PAH (International Classification of Diseases [ICD], 9th Edition, Clinical Modification, code 416.0; ICD, 10th Edition, Clinical Modification, code I27.0) were identified.22 PCS use was identified using ICD, 9th Edition, Clinical Modification, code V66.7 or ICD, 10th Edition, Clinical Modification, code Z51.5, consistent with prior literature.23, 24, 25 The ICD, 9th Edition, Clinical Modification, code for palliative care encounter (V66.7) showed moderate sensitivity and high specificity (> 90%) in prior validation studies.26,27 Prior validation studies demonstrated that when ICD, 9th Edition, Clinical Modification, code V66.7 is documented, > 90% patients receive specialist PCS consultation during an inpatient hospitalization.26,27 This methodology was used previously in studies from the NIS database. Demographic characteristics, hospital characteristics, primary payer, acute organ failure, and cardiac and noncardiac procedures associated with each discharge were identified using previously validated methodology by our group (e-Table 1).25,28, 29, 30, 31, 32, 33, 34 The Deyo’s modification of the Charlson Comorbidity Index was used to identify the burden of comorbid diseases.35 In accordance with HCUP definitions, we used median household income of the patient’s zip code of residence as a proxy measure of a patient’s socioeconomic status (SES).36
The primary outcome was the use of PCS in admissions with PAH during this 17-year period. Secondary outcomes included temporal trends of PCS use; predictors of PCS use; and association of PCS use with do-not-resuscitate (DNR) status, length of stay, costs, in-hospital mortality, and discharge disposition. Costs were adjusted for inflation.
Statistical Analysis
As recommended by the HCUP and NIS, survey procedures using discharge weights provided with the NIS database were used to generate national estimates. Using the trend weights provided by the HCUP and NIS, samples from 2001 through 2011 were reweighted to adjust for the 2012 HCUP and NIS redesign.37 To compare categorical and continuous variables, χ 2 and t tests, respectively, were used. Logistic regression was used to analyze trends in PCS use over time (referent year, 2001). Univariate analysis for trends and outcomes was performed and outcomes were represented as OR with 95% CI. To account for differences in available PAH therapies over time, we also analyzed outcomes stratified by decade (2001-2010 and 2010-2017) (e-Tables 1-3). Multivariate logistic regression analysis incorporating age, sex, race, primary payer status, SES, hospital characteristics, comorbidities, acuity of illness, cardiogenic shock, acute organ failure, and cardiac and noncardiac procedures was performed for predictors of PCS use. For the multivariate modeling, regression analysis with purposeful selection of statistically (liberal threshold, P < .20 in univariate analysis) and clinically relevant variables was conducted.
The inherent restrictions of the NIS database related to research design, data interpretation, and data analysis were reviewed and addressed.37 Pertinent considerations include not assessing individual hospital-level volumes (because of the changes to sampling design detailed previously), treating each entry as an admission as opposed to individual patients, restricting the study details to inpatient factors because the NIS database does not include outpatient data, and limiting administrative codes to those previously validated and used for similar studies. Two-tailed P values of < .05 were considered statistically significant. All statistical analyses were performed using SPSS version 25.0 software (IBM Corp.).
Results
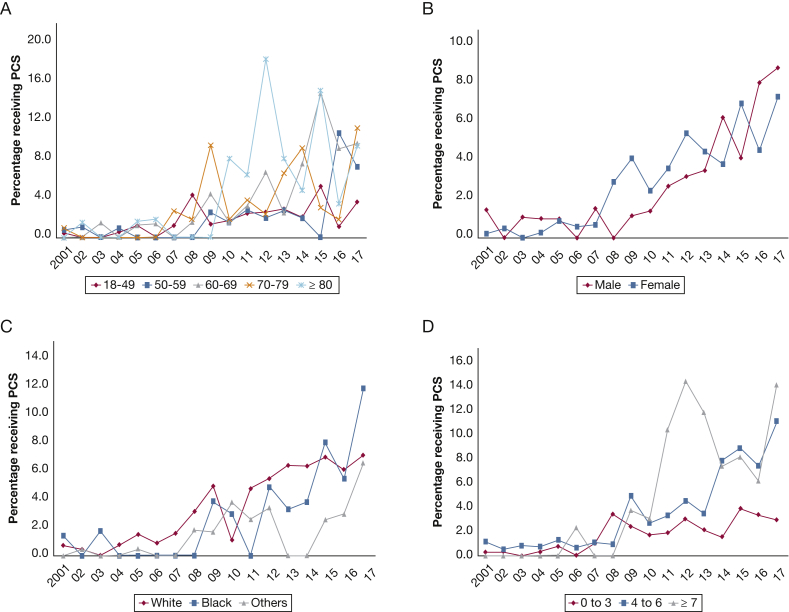
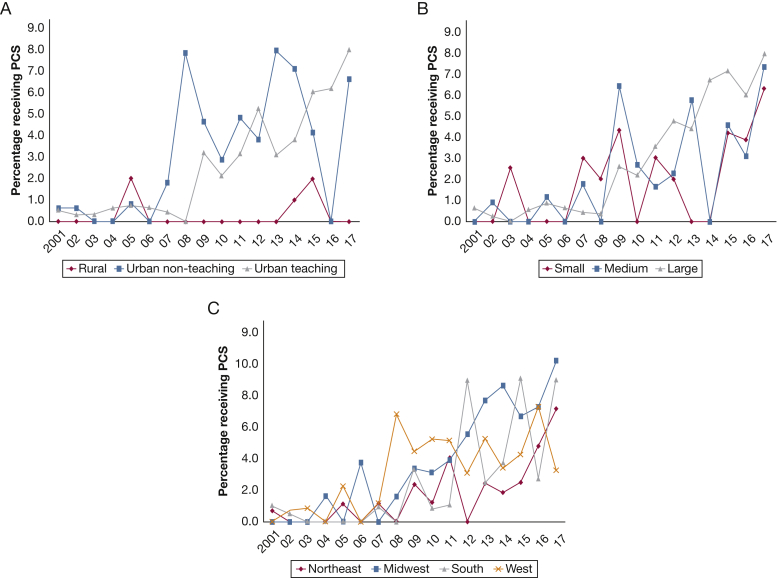
In the period between January 1, 2001, and December 31, 2017, an estimated 30,495 hospital admissions with a primary diagnosis of PAH. PCS use was noted in 682 (2.2%) of all admissions. In the period from 2001 through 2017, a steady increase in PCS use occurred from 0.5% in 2001 to 7.6% in 2017 (Fig 1A). In a multivariate logistic regression analysis incorporating age, sex, race, primary payer status, SES, hospital characteristics, comorbidities, acuity of illness, cardiogenic shock, acute organ failure, and cardiac and noncardiac procedures, a nearly five-fold increase in PCS use occurred in 2017 (OR, 5.27; 95% CI, 2.94-9.44; P < .001) compared with 2001 (Fig 1B). The use of PCS stratified by patient and hospital characteristics is presented in Figures 2 and 3. A steady increase in PCS referrals was found, with a notable inflection point around 2009 across most patient and hospital characteristics.
Figure 1.
Graphs showing temporal trends in PCS referrals in pulmonary arterial hypertension (PAH) hospital admissions. A, Unadjusted temporal trends of PCS use in PAH hospital admissions between 2001 and 2017 (P < .001 for trend over time). B, Adjusted ORs (with 95% CIs) for PCS use in all PAH hospital admissions between 2001 and 2017 (with 2001 as the referent). Adjustment for age, sex, race, primary payer status, socioeconomic status, hospital characteristics, comorbidities, acuity of illness, cardiogenic shock, acute organ failure, and cardiac and noncardiac procedures (P < .001 for trend over time). PCS = palliative care services.
Figure 2.
A-D, Graphs showing temporal trends in PCS referrals in hospital admissions with pulmonary arterial hypertension (PAH) stratified by patient characteristics. Unadjusted temporal trends of PCS use in hospital admissions with PAH between 2001 and 2017 stratified by age (A), sex (B), race (C), and Charlson Comorbidity Index (D) (P < .001 for trend over time). See Figure 1 legend for expansion of abbreviation.
Figure 3.
A-C, Graphs showing temporal trends in PCS referrals hospital admissions with pulmonary arterial hypertension (PAH) stratified by hospital characteristics. Unadjusted temporal trends of PCS use in PAH admissions between 2001 and 2017 stratified by hospital location and teaching status (A), bed size (B), and region (C) (P < .001 for trend over time). See Figure 1 legend for expansion of abbreviation.
Compared with those not receiving PCS, the cohort receiving PCS was older and more likely to be White, and significant differences were found in primary payer and SES. Patients receiving PCS demonstrated a higher comorbidity burden and were more likely to be admitted during the weekend to medium and large urban teaching hospitals with geographic variation (all P < .001) (Table 1).
Table 1.
Baseline and In-Hospital Characteristics of PAH Admissions Stratified by PCS Use
| Characteristic | PCS Use (n = 682) | No PCS Use (n = 29,813) | P Value |
|---|---|---|---|
| Age, y | 62.2 ± 16.2 | 55.9 ± 17.4 | < .001 |
| Female sex | 77.1 | 76.6 | .78 |
| Race | < .001 | ||
| White | 74.1 | 65.1 | |
| Black | 17.9 | 17.8 | |
| Othersa | 7.9 | 17.1 | |
| Primary payer | < .001 | ||
| Medicare | 55.5 | 45.9 | |
| Medicaid | 10.7 | 16.5 | |
| Private | 31.6 | 31.3 | |
| Othersb | 2.2 | 6.3 | |
| Weekend admission | 21.6 | 14.3 | < .001 |
| Quartile of median household income for zip code | < .001 | ||
| 0-25th | 16.5 | 23.7 | |
| 26th-50th | 30.3 | 24.8 | |
| 51st-75th | 28.9 | 24.7 | |
| 75th-100th | 24.3 | 26.8 | |
| Charlson Comorbidity Index | < .001 | ||
| 0-3 | 33.1 | 58.3 | |
| 4-6 | 42.8 | 31.9 | |
| ≥ 7 | 24.0 | 9.8 | |
| Hospital teaching status and location | < .001 | ||
| Rural | 2.1 | 7.0 | |
| Urban nonteaching | 23.5 | 22.4 | |
| Urban teaching | 74.4 | 70.6 | |
| Hospital size by no. of beds | < .001 | ||
| Small | 7.9 | 12.5 | |
| Medium | 18.6 | 14.4 | |
| Large | 73.4 | 73.1 | |
| Hospital region | < .001 | ||
| Northeast | 15.1 | 23.3 | |
| Midwest | 27.5 | 19.5 | |
| South | 29.1 | 32.7 | |
| West | 28.3 | 24.5 | |
| Heart failure | 9.5 | 3.7 | < .001 |
| Cardiogenic shock | 10.1 | 1.4 | < .001 |
| Acute pulmonary embolism | 2.2 | 1.8 | .38 |
| Acute noncardiac organ failure | |||
| Respiratory | 39.3 | 11.0 | < .001 |
| Renal | 40.1 | 13.1 | < .001 |
| Hepatic | 1.5 | 1.1 | .36 |
| Hematologic | 21.6 | 9.1 | < .001 |
| Neurologic | 12.5 | 1.1 | < .001 |
| Coronary angiography | 28.9 | 20.8 | < .001 |
| Percutaneous coronary intervention | 0.0 | 1.2 | < .001 |
| Right heart catheterization | 24.5 | 29.1 | .01 |
| Pulmonary artery catheterization (continuous monitoring) | 9.9 | 10.9 | .40 |
| Extracorporeal membrane oxygenation | 1.5 | 0.3 | < .001 |
| Fiber-optic bronchoscopy | 2.9 | 2.4 | .38 |
| Invasive mechanical ventilation | 9.5 | 4.0 | < .001 |
| Noninvasive mechanical ventilation | 16.7 | 3.7 | < .001 |
| Acute hemodialysis | 2.3 | 0.8 | < .001 |
Data are presented as percentage or mean ± SD unless otherwise indicated. PAH = pulmonary arterial hypertension; PCS = palliative care services.
Hispanic, Asian, Native American, others.
Uninsured, no charge, others.
The PCS cohort demonstrated higher acuity of illness as noted by higher rates of heart failure; cardiogenic shock; acute noncardiac organ failure; and use of cardiac, respiratory, and renal support systems (Table 1). Multivariate predictors of PCS use are detailed in Table 2. In a multivariate logistic regression analysis, White race, private insurance, higher SES, admission to an urban hospital, small hospital by number of beds, hospital region (Midwest, South, and West), higher comorbidity burden (Charlson Comorbidity Index), heart failure and cardiogenic shock, acute noncardiac organ failure, and use of extracorporeal membrane oxygenation and noninvasive mechanical ventilation independently were predictive of increased PCS use, whereas right heart catheterization, invasive mechanical ventilation, and bronchoscopy were associated with decreased PCS use (Table 2).
Table 2.
Multivariate Predictors of PCS Use in Admissions With PAH
| Predictor (Total N = 30,495) | OR | 95% CI |
P Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Age group, y | ||||
| 18-49 | Reference category | |||
| 50-59 | 0.68 | 0.51 | 0.90 | .01 |
| 60-69 | 1.23 | 0.92 | 1.64 | .16 |
| 70-79 | 0.86 | 0.61 | 1.22 | .41 |
| ≥80 | 0.91 | 0.62 | 1.34 | .65 |
| Female sex | 1.20 | 0.99 | 1.46 | .06 |
| Race | ||||
| White | Reference category | |||
| Black | 1.09 | 0.87 | 1.38 | .45 |
| Othersa | 0.40 | 0.32 | 0.50 | < .001 |
| Primary payer | ||||
| Medicare | Reference category | |||
| Medicaid | 1.16 | 0.85 | 1.58 | .36 |
| Private | 1.43 | 1.15 | 1.79 | .002 |
| Othersb | 0.54 | 0.31 | 0.95 | .03 |
| Quartile of median household income for zip code | ||||
| 0-25th | Reference category | |||
| 26th-50th | 1.92 | 1.49 | 2.48 | < .001 |
| 51st-75th | 1.74 | 1.35 | 2.26 | < .001 |
| 75th-100th | 1.21 | 0.92 | 1.59 | .17 |
| Hospital teaching status and location | ||||
| Rural | Reference category | |||
| Urban nonteaching | 6.33 | 3.14 | 12.77 | < .001 |
| Urban teaching | 5.74 | 2.87 | 11.46 | < .001 |
| Hospital size by no. of beds | ||||
| Small | Reference category | |||
| Medium | 0.50 | 0.37 | 0.68 | < .001 |
| Large | 0.65 | 0.51 | 0.84 | .001 |
| Hospital region | ||||
| Northeast | Reference category | |||
| Midwest | 2.55 | 1.95 | 3.33 | < .001 |
| South | 1.58 | 1.21 | 2.05 | .001 |
| West | 2.35 | 1.80 | 3.07 | < .001 |
| Charlson Comorbidity Index | ||||
| 0-3 | Reference category | |||
| 4-6 | 2.08 | 1.63 | 2.66 | < .001 |
| ≥ 7 | 3.91 | 2.90 | 5.27 | < .001 |
| Heart failure | 1.84 | 1.36 | 2.49 | < .001 |
| Cardiogenic shock | 2.06 | 1.46 | 2.91 | < .001 |
| Acute organ failure | ||||
| Respiratory | 2.98 | 2.47 | 3.58 | < .001 |
| Renal | 2.07 | 1.71 | 2.49 | < .001 |
| Hepatic | 0.47 | 0.24 | 0.95 | .04 |
| Hematologic | 2.27 | 1.83 | 2.80 | < .001 |
| Neurologic | 5.56 | 4.04 | 7.65 | < .001 |
| Right heart catheterization | 0.78 | 0.64 | 0.95 | .01 |
| Pulmonary artery catheterization (continuous monitoring) | 0.86 | 0.65 | 1.14 | .29 |
| Extracorporeal membrane oxygenation | 3.61 | 1.51 | 8.64 | .004 |
| Invasive mechanical ventilation | 0.66 | 0.46 | 0.93 | .02 |
| Noninvasive mechanical ventilation | 2.78 | 2.17 | 3.56 | < .001 |
| Fiber-optic bronchoscopy | 0.46 | 0.25 | 0.85 | .01 |
| Acute hemodialysis | 0.73 | 0.41 | 1.30 | .29 |
See Table 1 legend for expansion of abbreviations.
Hispanic, Asian, Native American, others.
Uninsured, no charge, others.
The cohort with PCS consultation showed greater use of DNR status (46.2% vs 1.8%), longer length of stay (12.9 ± 20.4 days vs 7.2 ± 9.8 days), higher hospitalization costs ($130,434 ± $172,417 vs $56,499 ± $144,316), and higher in-hospital mortality (52.8% vs 6.4%; all P < .001). PCS consultation remained an independent predictor of mortality; other significant predictors were age, race, payer, hospital characteristics (size and region), patient comorbidities, and use of mechanical circulatory and noncardiac organ support (e-Table 2). The PCS cohort had fewer discharges to home (25% vs 68.9%) and more frequent discharges to skilled nursing facilities (27.9% vs 7.7%; all P < .001). PCS use was associated independently with greater use of DNR status and fewer discharges to home even after adjusting for age, race, primary payer status, SES, hospital characteristics, comorbidities, acuity of illness, cardiogenic shock, out-of-hospital cardiac arrest, acute organ failure, and cardiac and noncardiac procedures (adjusted OR, 25.49 [95% CI, 20.63-31.48; P < .001] and 0.12 [95% CI, 0.09-0.15; P < .001], respectively). When stratified by decades (2001-2010 and 2010-2017), results overall were similar. The cohorts with PCS consultation had higher prevalence of DNR status, longer length of stay, increased hospital costs, and increased in-hospital mortality (e-Table 3). Notably, a higher prevalence of DNR status, decreased length of stay, higher costs, and increased discharges to nursing homes was found in more recent years (e-Table 3).
Discussion
In this nationally representative descriptive study evaluating PCS use in PAH, we noted very limited use of PCS in only 2% of hospital admissions. A serial increase in PCS use was found during the study period across all patient and hospital categories, but the overall rate remained low over time. White race, higher comorbidity burden, higher SES, private insurance, admission to an urban hospital, small hospital by number of beds, geographic region (Midwest, South, and West), heart failure and cardiogenic shock, acute noncardiac organ failure, and use of extracorporeal membrane oxygenation and noncardiac organ support were independent predictors of PCS use. PCS use was associated with a higher prevalence of DNR status and identified a higher risk cohort with greater in-hospital resource use, higher in-hospital mortality, and less frequent discharges to home, suggesting delayed PCS consultation and higher rates of therapeutic failure in this population.
Despite the poor prognosis and significant symptom burden associated with PAH, our study found a low rate of PCS use in PAH hospital admissions. PAH is a progressive disease associated with impaired QOL and survival.6,7,38,39 Experts have advocated for early implementation of PCS to improve QOL in PAH, but data on use of palliative care in PAH are very scarce.17,19,20 Prior survey-based studies have provided some insight into PCS use in PAH, but to our knowledge, this is the first study to characterize use of PCS in PAH during hospital admissions. A survey of PAH specialists showed low PCS referral (40%) for impaired QOL and symptoms, whereas another survey of PAH patients described a high prevalence of impaired QOL, but low patient awareness and use of PCS.17,19 PAH hospitalizations are increasing, and patients admitted have a higher comorbidity burden with more advanced right heart failure and often die in the hospital setting.20,22,40 Although the ideal timing of PCS consultation is not known, PCS use during hospitalizations can be helpful to address issues related to symptoms, QOL, and advanced care planning, particularly if these issues have not been addressed in the outpatient setting. Given the high morbidity and mortality associated with PAH even after hospitalization, hospital admissions without PCS use represent a missed opportunity.14
The low inpatient use of PCS of < 10% of PAH admissions to hospital is similar to that found in studies of patients with other cardiopulmonary conditions, such as acute myocardial infarction (4.5%), heart failure (1.2%), and COPD (1.7%).25,41,42 Concordant with prior studies, an increase in PCS use occurred with time, even after adjusting for baseline demographics and comorbidities.25,42 This may be related to an increased awareness of the benefit of PCS in other diseases such as heart failure and cancer or increased availability of PCS.43,44 Interestingly, a notable inflection point occurred in 2009. Although the exact reason for this observation is not known, it may be related to recognition of palliative care and hospice as a medical subspecialty with board certification in 2008 or identification of palliative care by the National Priorities Partnership as one of six priority areas in 2008.45,46
Discrepancies in PCS use were present and varied according to type of hospital practice, geographic region, insurance status, comorbidity burden, SES, and race. Similar disparities have been observed in prior studies of heart failure and cardiogenic shock resulting from myocardial infarction.25,42,47 We identified specific groups with lower rates of PCS use. Patients with worse SES and “other” race, for example, showed lower rates of PCS use. These groups may benefit from advocacy efforts to increase access to PCS. In contrast, patients with private insurance showed higher rates of PCS use. Potential reasons for these disparities cannot be elucidated from this study, but may include differential access to PCS, unconscious biases related to usefulness of PCS, and misconception of PCS as end-of-life care.25,42 Further studies to explore reasons for these disparities in PCS use are needed to minimize inequalities and to ensure uniform access to PCS.
We suspect that some differences in PCS use, such as the lower use in rural vs urban hospitals, is related to availability of PCS services. The Pulmonary Hypertension Association recently encouraged accredited Pulmonary Hypertension Care Centers to incorporate PCS into clinical practice, emphasizing its importance in PAH management. Access to expert quality care and specialty services such as PCS are an additional reason for patients with PAH to be managed at an accredited Pulmonary Hypertension Care Center.
Patients who received PCS had a higher acuity of illness; they were more likely to have heart failure and cardiogenic shock and showed a higher use of extracorporeal membrane oxygenation. These findings highlight selective and late use of PCS, which also has been described in other conditions.25,42,47 Despite multiple studies highlighting the critical role of palliative care in patients with chronic medical conditions and higher benefit with early involvement,44,48 palliative care consultation often is considered synonymous with end-of-life cares and is often underused.49 In contrast, some invasive procedures associated with more aggressive care, such as right heart catheterization and invasive ventilation, were associated with lower PCS use, whereas less aggressive measures, such as noninvasive ventilation, were associated with higher PCS use. We suspect that these differences may be reflective of differences in goals of care and potentially may be related to differences in preferences associated with intubation.
PCS use was associated with a higher prevalence of DNR status, a longer length of stay, higher hospitalization costs, and increased likelihood of being discharged to a skilled nursing facility. Studies of heart failure have described a similar association between PCS use and DNR status.25,47 Palliative care specialists help patients establish goals of care aligned with their wishes through advanced communication skills, and this may account for differences in DNR status.50 Similar to some studies in heart failure and cardiogenic shock using the same database, but contrary to others, we noted a longer length of stay and higher hospitalization costs in patients with PCS use.25,42,47,51 This is likely because the patients who received PCS were sicker (higher comorbidity index and increased need for advanced organ support) and older than those who did not.
Patients who received PCS showed higher mortality, which is most likely multifactorial and related to higher illness acuity and comorbidity burden, higher prevalence of DNR status, and other unmeasured confounding factors. In multivariate analysis, the association between PCS use and mortality persisted after adjusting for comorbidity burden, organ failure, and need for advanced organ support, which suggests that unmeasured factors such as frailty and treatment failure confound the relationship between PCS and mortality. Because of limitations of the database, it is not possible to assess the timing of PCS use, so it is not known whether PCS was used late by patients with prolonged hospitalizations. Given the high mortality rates associated with PCS use, it is clear that PCS was used more at the end of life and less in patients who survived hospitalization. This is consistent with a prior survey of PAH specialists in which providers stated that they used PCS primarily for patients who were actively dying at the end of life.17 Although it is possible that patients would benefit from early PCS in the outpatient setting or during a hospitalization to improve symptoms and QOL and to decrease overall health-care costs, these outcomes cannot be assessed within this database.
In summary, to our knowledge, our study is the first to evaluate the use of PCS in patients admitted with PAH and reports a low overall rate of use. A significant increase in PCS use occurred from 2001 through 2017, which is encouraging. Although the appropriate timing for PCS consultation in PAH is unclear because of a paucity of data, experts recommend early PCS use to improve symptoms and QOL and to address unmet needs of PAH patients.52,53 To implement these recommendations, it is clear that improved and systematic PCS use in patients with PAH is needed.
Study Limitations
This study has several limitations, some of which are inherent to the analysis of a large administrative database. Because the NIS relies on discharge diagnosis for identifying patients, errors in coding could have led to some hospitalizations to be missed and possibly other causes of pulmonary hypertension to be included, particularly in early years of the study because of a lack of PAH awareness. Data on medication and PAH cause are not available in this database, so the effect of these on PCS use and patient outcomes could not be evaluated. The HCUP attempts to mitigate potential errors in the NIS by using internal and external quality control measures. Important factors such as terminal extubation, QOL, and mortality after hospitalization could not be identified reliably in this hospital admissions database. Also, the reasons for obtaining a PCS referral, the role of PCS on symptom mitigation, and improvement in QOL and relationships could not be assessed in this administrative database. The timing of PCS consultation and whether patients already had undergone a consultation with palliative care before admission is unclear and also cannot be ascertained through this database. It is possible that these patients could have undergone PCS previously in the outpatient setting or during a different inpatient encounter that was not captured on the NIS admission. Finally, ICD codes, which were used for inclusion, may not be specific for the diagnosis of PAH. Despite these limitations, this study addressed an important knowledge gap highlighting the national use of PCS in PAH.
Conclusions
In this large, nationally representative contemporary cohort study, use of PCS was documented in only 2% of all admissions for PAH despite the high morbidity and mortality associated with this condition. Despite increasing trends in the adoption of PCS, significant patient- and hospital-specific disparities in implementation remain. Further dedicated studies evaluating these disparities and the integration of PCS into the comprehensive care of PAH patients are warranted.
Acknowledgments
Author contributions: S. V., V. A., and H. M. D. designed the study, reviewed the literature, analyzed the data, and performed the statistical analysis. S. V. managed the data and data analysis and had access to the data. S. V., V. A., and H. M. D. drafted the manuscript. S. V., V. A., H. M. D., W. C., R. P. F., H. R. C., and J. J. S. revised the manuscript, made intellectual revisions, and provided mentorship. S. V., V. A., H. M. D., W. C., R. P. F., H. R. C., and J. J. S. gave final approval.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Anand and Vallabhajosyula contributed equally to this manuscript.
FUNDING/SUPPORT: Dr Vallabhajosyula is supported by the National Institutes of Health [the National Center for Advancing Translational Sciences Clinical and Translational Science Award UL1 TR000135]. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of National Institutes of Health.
Supplementary Data
References
- 1.Simonneau G., Montani D., Celermajer D.S. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1–13. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoeper M.M., Simon R.G.J. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev. 2014;23(134):450–457. doi: 10.1183/09059180.00007814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galie N., Humbert M., Vachiery J.L. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 4.Maron B.A., Galie N. Diagnosis, treatment, and clinical management of pulmonary arterial hypertension in the contemporary era: a review. JAMA Cardiol. 2016;1(9):1056–1065. doi: 10.1001/jamacardio.2016.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijeratne D.T., Lajkosz K., Brogly S.B. Increasing incidence and prevalence of World Health Organization groups 1 to 4 pulmonary hypertension: a population-based cohort study in Ontario, Canada. Circ Cardiovasc Qual Outcomes. 2018;11(2) doi: 10.1161/CIRCOUTCOMES.117.003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delcroix M., Howard L. Pulmonary arterial hypertension: the burden of disease and impact on quality of life. Eur Respir Rev. 2015;24(138):621–629. doi: 10.1183/16000617.0063-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taichman D.B., Shin J., Hud L. Health-related quality of life in patients with pulmonary arterial hypertension. Respir Res. 2005;6:92. doi: 10.1186/1465-9921-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubenfire M., Lippo G., Bodini B.D., Blasi F., Allegra L., Bossone E. Evaluating health-related quality of life, work ability, and disability in pulmonary arterial hypertension: an unmet need. Chest. 2009;136(2):597–603. doi: 10.1378/chest.08-1260. [DOI] [PubMed] [Google Scholar]
- 9.Benza R.L., Miller D.P., Gomberg-Maitland M. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 10.Hoeper M.M., Kramer T., Pan Z. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2):1–10. doi: 10.1183/13993003.00740-2017. [DOI] [PubMed] [Google Scholar]
- 11.Tonelli A.R., Arelli V., Minai O.A. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188(3):365–369. doi: 10.1164/rccm.201209-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benza R.L., Gomberg-Maitland, Ellitt C.G. Comparison of three risk assessment strategies as predictors of one-year survival in US pulmonary arterial hypertension (PAH) patients. Am J Respir Crit Care Med. 2018;197:A7649. [Google Scholar]
- 13.Huynh T.N., Weigt S.S., Sugar C.A., Shapiro S., Kleerup E.C. Prognostic factors and outcomes of patients with pulmonary hypertension admitted to the intensive care unit. J Crit Care. 2012;27(6):739 e737–e713. doi: 10.1016/j.jcrc.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Campo A., Mathai S.C., Le Pavec J. Outcomes of hospitalisation for right heart failure in pulmonary arterial hypertension. Eur Respir J. 2011;38(2):359–367. doi: 10.1183/09031936.00148310. [DOI] [PubMed] [Google Scholar]
- 15.Kavalieratos D., Corbelli J., Zhang D. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA. 2016;316(20):2104–2114. doi: 10.1001/jama.2016.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley A.S., Morrison R.S. Palliative care for the seriously ill. N Engl J Med. 2015;373(8):747–755. doi: 10.1056/NEJMra1404684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenstad E.R., Shanafelt T.D., Sloan J.A. Physician attitudes toward palliative care for patients with pulmonary arterial hypertension: results of a cross-sectional survey. Pulm Circ. 2014;4(3):504–510. doi: 10.1086/677365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khirfan G., Tonelli A.R., Ramsey J., Sahay S. Palliative care in pulmonary arterial hypertension: an underutilised treatment. Eur Respir Rev. 2018;27(150):1–9. doi: 10.1183/16000617.0069-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swetz K.M., Shanafelt T.D., Drozdowicz L.B. Symptom burden, quality of life, and attitudes toward palliative care in patients with pulmonary arterial hypertension: results from a cross-sectional patient survey. J Heart Lung Transplant. 2012;31(10):1102–1108. doi: 10.1016/j.healun.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Grinnan D.C., Swetz K.M., Pinson J., Fairman P., Lyckholm L.J., Smith T. The end-of-life experience for a cohort of patients with pulmonary arterial hypertension. J Palliat Med. 2012;15(10):1065–1070. doi: 10.1089/jpm.2012.0085. [DOI] [PubMed] [Google Scholar]
- 21.Introduction to the HCUP Nationwide Inpatient Sample 2009. http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_2009_INTRODUCTION.pdf Healthcare Quality and Utilization Project website.
- 22.Anand V., Roy S.S., Archer S.L. Trends and outcomes of pulmonary arterial hypertension-related hospitalizations in the United States: analysis of the Nationwide Inpatient Sample database from 2001 through 2012. JAMA Cardiol. 2016;1(9):1021–1029. doi: 10.1001/jamacardio.2016.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murthy S.B., Moradiya Y., Hanley D.F., Ziai W.C. Palliative care utilization in nontraumatic intracerebral hemorrhage in the United States. Crit Care Med. 2016;44(3):575–582. doi: 10.1097/CCM.0000000000001391. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee K., Goyal A., Kakkera K., Harrington S., Corwin H.L. National trends (2009-2013) for palliative care utilization for patients receiving prolonged mechanical ventilation. Crit Care Med. 2018;46(8):1230–1237. doi: 10.1097/CCM.0000000000003182. [DOI] [PubMed] [Google Scholar]
- 25.Vallabhajosyula S., Prasad A., Dunlay S.M. Utilization of palliative care for cardiogenic shock complicating acute myocardial infarction: a 15-year national perspective on trends, disparities, predictors, and outcomes. J Am Heart Assoc. 2019;8(15) doi: 10.1161/JAHA.119.011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feder S.L., Redeker N.S., Jeon S. Validation of the ICD-9 diagnostic code for palliative care in patients hospitalized with heart failure within the Veterans Health Administration. Am J Hospice Palliat Care. 2018;35(7):959–965. doi: 10.1177/1049909117747519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hua M., Li G., Clancy C., Morrison R.S., Wunsch H. Validation of the V66.7 code for palliative care consultation in a single academic medical center. J Palliat Med. 2017;20(4):372–377. doi: 10.1089/jpm.2016.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallabhajosyula S., Prasad A., Bell M.R. Extracorporeal membrane oxygenation use in acute myocardial infarction in the United States, 2000 to 2014. Circ Heart Fail. 2019;12(12) doi: 10.1161/CIRCHEARTFAILURE.119.005929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallabhajosyula S., Vallabhajosyula S., Bell M.R. Early vs. delayed in-hospital cardiac arrest complicating ST-elevation myocardial infarction receiving primary percutaneous coronary intervention. Resuscitation. 2019;148:242–250. doi: 10.1016/j.resuscitation.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Vallabhajosyula S, Prasad A, Sandhu GS, et al. Mechanical circulatory support-assisted early percutaneous coronary intervention in acute myocardial infarction with cardiogenic shock: 10-year national temporal trends, predictors and outcomes [published online ahead of print November 19, 2019]. EuroIntervention. 10.4244/EIJ-D-19-00226. [DOI] [PMC free article] [PubMed]
- 31.Vallabhajosyula S., Dunlay S.M., Barsness G.W., Rihal C.S., Holmes D.R., Jr., Prasad A. Hospital-level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol. 2019;124(4):491–498. doi: 10.1016/j.amjcard.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 32.Vallabhajosyula S., Dunlay S.M., Prasad A. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2019;73(14):1781–1791. doi: 10.1016/j.jacc.2019.01.053. [DOI] [PubMed] [Google Scholar]
- 33.Vallabhajosyula S., Kumar V., Vallabhajosyula S. Acute myocardial infarction-cardiogenic shock in patients with prior coronary artery bypass grafting: a 16-year national cohort analysis of temporal trends, management and outcomes. Int J Cardiol. 2020;310:9–15. doi: 10.1016/j.ijcard.2020.02.033. [DOI] [PubMed] [Google Scholar]
- 34.Vallabhajosyula S., Patlolla S.H., Dunlay S.M. Regional variation in the management and outcomes of acute myocardial infarction with cardiogenic shock in the United States. Circ Heart Fail. 2020;13(2) doi: 10.1161/CIRCHEARTFAILURE.119.006661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 36.HCUP facts and figures 2008. https://www.hcup-us.ahrq.gov/reports/factsandfigures/2008/definitions.jsp Healthcare Quality and Utilization Project website. Accessed September 4, 2020.
- 37.Khera R., Krumholz H.M. With great power comes great responsibility: big data research from the National Inpatient Sample. Circ Cardiovasc Qual Outcomes. 2017;10(7):e003846. doi: 10.1161/CIRCOUTCOMES.117.003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathai S.C., Suber T., Khair R.M., Kolb T.M., Damico R.L., Hassoun P.M. Health-related quality of life and survival in pulmonary arterial hypertension. Ann Am Thorac Soc. 2016;13(1):31–39. doi: 10.1513/AnnalsATS.201412-572OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cenedese E., Speich R., Dorschner L. Measurement of quality of life in pulmonary hypertension and its significance. Eur Respir J. 2006;28(4):808–815. doi: 10.1183/09031936.06.00130405. [DOI] [PubMed] [Google Scholar]
- 40.Gin-Sing W. Palliative care in pulmonary arterial hypertension. Curr Opin Support Palliat Care. 2017;11(1):7–11. doi: 10.1097/SPC.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 41.Rush B., Hertz P., Bond A., McDermid R.C., Celi L.A. Use of palliative care in patients with end-stage COPD and receiving home oxygen: national trends and barriers to care in the United States. Chest. 2017;151(1):41–46. doi: 10.1016/j.chest.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alqahtani F., Balla S., Almustafa A., Sokos G., Alkhouli M. Utilization of palliative care in patients hospitalized with heart failure: a contemporary national perspective. Clin Cardiol. 2019;42(1):136–142. doi: 10.1002/clc.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward C. The need for palliative care in the management of heart failure. Heart. 2002;87(3):294–298. doi: 10.1136/heart.87.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parikh R.B., Kirch R.A., Smith T.J., Temel J.S. Early specialty palliative care—translating data in oncology into practice. N Engl J Med. 2013;369(24):2347–2351. doi: 10.1056/NEJMsb1305469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lutz S., Lupu D., Johnstone P., Howell D.D., Janjan N. The influence of the newly formed hospice and palliative medicine subspecialty on radiation oncology and end-of-life care. J Am Coll Radiol. 2008;5(11):1102–1105. doi: 10.1016/j.jacr.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 46.National Quality Forum, NQF; National Priorities Partnership. National priorities and goals: aligning our efforts to transform America’s health care. December 3, 2008. Agency for Healthcare Research and Quality website. Accessed September 4, 2020.
- 47.Patel B., Secheresiu P., Shah M. Trends and predictors of palliative care referrals in patients with acute heart failure. Am J Hosp Palliat Care. 2019;36(2):147–153. doi: 10.1177/1049909118796195. [DOI] [PubMed] [Google Scholar]
- 48.Qureshi D., Tanuseputro P., Perez R., Pond G.R., Seow H.Y. Early initiation of palliative care is associated with reduced late-life acute-hospital use: a population-based retrospective cohort study. Palliat Med. 2019;33(2):150–159. doi: 10.1177/0269216318815794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McIlfatrick S., Noble H., McCorry N.K. Exploring public awareness and perceptions of palliative care: a qualitative study. Palliat Med. 2014;28(3):273–280. doi: 10.1177/0269216313502372. [DOI] [PubMed] [Google Scholar]
- 50.Kelley A.S., Meier D.E. Palliative care—a shifting paradigm. N Engl J Med. 2010;363(8):781–782. doi: 10.1056/NEJMe1004139. [DOI] [PubMed] [Google Scholar]
- 51.Isenberg S.R., Lu C., McQuade J. Impact of a new palliative care program on health system finances: an analysis of the palliative care program inpatient unit and consultations at Johns Hopkins medical institutions. J Oncol Pract. 2017;13(5):e421–e430. doi: 10.1200/JOP.2016.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klinger J.R., Elliott C.G., Levine D.J. Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report. Chest. 2019;155(3):565–586. doi: 10.1016/j.chest.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 53.McGoon M.D., Ferrari P., Armstrong I. The importance of patient perspectives in pulmonary hypertension. Eur Respir J. 2019;53(1):1–15. doi: 10.1183/13993003.01919-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.