Abstract
Two-thirds of people with type 2 diabetes mellitus (T2DM) have or will develop chronic kidney disease (CKD), which is characterized by rapid renal decline that, together with superimposed T2DM-related metabolic sequelae, synergistically promotes early frailty and mobility deficits that increase the risk of mortality. Distinguishing the mechanisms linking renal decline to mobility deficits in CKD progression and/or increasing severity in T2DM is instrumental both in identifying those at high risk for functional decline and in formulating effective treatment strategies to prevent renal failure. While evidence suggests that skeletal muscle energetics may relate to the development of these comorbidities in advanced CKD, this has never been assessed across the spectrum of CKD progression, especially in T2DM-induced CKD. Here, using next-generation sequencing, we first report significant downregulation in transcriptional networks governing oxidative phosphorylation, coupled electron transport, electron transport chain (ETC) complex assembly, and mitochondrial organization in both middle- and late-stage CKD in T2DM. Furthermore, muscle mitochondrial coupling is impaired as early as stage 3 CKD, with additional deficits in ETC respiration, enzymatic activity, and increased redox leak. Moreover, mitochondrial ETC function and coupling strongly relate to muscle performance and physical function. Our results indicate that T2DM-induced CKD progression impairs physical function, with implications for altered metabolic transcriptional networks and mitochondrial functional deficits as primary mechanistic factors early in CKD progression in T2DM.
Introduction
Type 2 diabetes mellitus (T2DM) is the leading cause of chronic kidney disease (CKD) in the U.S., as nearly two-thirds of people with diabetes have or will develop CKD (1). Moreover, T2DM accounts for >44% of all annual new cases of end-stage renal disease (ESRD), the end point of CKD progression, as diabetes duration, glycemic control, diabetes severity, and proteinuria all significantly exacerbate the rate of renal decline (1–3). This high burden of CKD in T2DM is associated with a litany of comorbidities, reduced quality of life, and significant health care costs (2). More specifically, it is now well established that those with CKD, particularly ESRD, possess lower physical function, impaired physical performance and mobility, and exercise intolerance that contribute to early physical frailty, mobility disability, and increased risk of mortality and poor patient outcomes (4,5). Indeed, patients with an estimated glomerular filtration rate (eGFR) <60 mL/min exhibit deficits of 30% in lower-extremity functional capacity (i.e., gait speed, 6-min walk distances, Timed Up and Go Test), and sixfold higher odds of possessing an exercise capacity <5 METs (moderate-level daily activity). Such deficits increase the risk of mortality and independently and cyclically contribute to renal decline to ESRD (4–6). However, T2DM is independently associated with similar functional and mobility deficits, thus complicating the clinical and mechanistic connections between CKD progression and these comorbidities in this patient population (7). Interestingly, evidence has demonstrated that skeletal muscle strength, as opposed to other physiological systems (i.e., cardiorespiratory), predicts this poor exercise capacity in CKD, implicating intrinsic skeletal muscle factors as a potential area for mechanistic insights (8,9).
The hormonal and systemic milieu in both T2DM and CKD promote a state of protein energy wasting, accelerated contractile protein catabolism, and insulin resistance that contribute to loss of muscle mass (10,11). However, muscle dysfunction and poor strength in ESRD only marginally correlate with muscle atrophy because these patients display poorer muscle function than matched control subjects for a given muscle mass, findings echoed in studies of patients with T2DM alone (12,13). While both the status of muscle function and muscle mass and the underlying mechanisms driving inherent muscle performance impairment in CKD progression in humans remain poorly understood, prior research on the effects of aging has demonstrated that mitochondrial ATP production capacity is significantly and positively associated with muscle strength and contractile power generation per unit muscle volume (14,15). Interestingly, evidence has also demonstrated CKD-associated loss of skeletal muscle mitochondria in both rodent nephrectomy models and renal failure (16,17), while the use of plasma and serum metabolomics has hinted at CKD-associated deficits in whole-body mitochondrial fatty acid oxidation and energetic substrate processing (18). Indeed, most recently, Kestenbaum et al. (19) demonstrated a 25% reduced leg muscle mitochondrial oxidative capacity in participants with CKD compared with age, sex, and BMI-matched control subjects and that leg muscle oxidative capacity is a significant predictor of walking distance. However, a history of diabetes also imparted nearly the same magnitude reduction in mitochondrial function in their population and reduced the magnitude of association between muscle oxidative capacity and walking distance, thus highlighting the challenges in delineating the independent contributions of kidney functional decline and CKD progression upon mitochondrial energetics, muscle performance, and physical function in this population.
Mitochondrial dysfunction, however, may derive from a host of intra- and interorganelle mechanisms, including reduced oxidative capacity secondary to electron transport chain (ETC) deficiencies, altered mitochondrial density, organization and turnover, changes in transcriptional regulation, or uncoupling of respiration from ATP synthesis. This uncoupling can be due to ETC dysfunction but also can stem from excessive H+ proton leak in the inner mitochondrial membrane that promotes redox stress, DNA damage, protein oxidation, and skeletal muscle cell apoptosis (14,20). Interestingly, increased mitochondrial proton leak, or uncoupling, in skeletal muscle has been linked to higher metabolic cost of locomotion, reduced exercise capacity, greater fatigability, and reduced mobility (14). This uncoupling promotes reduced muscle contractile capacity that limits speed and sustainability of functional tasks, yet underlying mitochondrial function and coupling in CKD or its progression in those with T2DM has not been examined (14).
Thus, the purpose of this study is to first determine how CKD progression in T2DM affects the function (coupling efficiency, H+ leak, ETC function), abundance, and transcriptional regulation of skeletal muscle mitochondria and, in turn, subsequently define how this relates to muscle performance and physical function in CKD progression in this patient population. Such insights aim to elucidate, for the first time, the temporal mechanisms associated with skeletal muscle energetics and performance in the course of CKD progression among stages in those most at risk for rapid renal and functional decline: patients with T2DM. In doing so, this work highlights key physiological targets and windows of opportunity for future treatments (e.g., conservative exercise, drug based) to remedy functional deficits and improve activity engagement/tolerance and attenuate CKD progression in patients with T2DM.
Research Design and Methods
Participants
Participants were recruited from the Washington University School of Medicine renal clinic. Measures of glycemic control (HbA1c, oral glucose tolerance test [OGTT]), diabetic medication use, hypertension status, and eGFR (using creatinine) were collected for all participants according to Kidney Disease: Improving Global Outcomes guidelines (see Supplementary Material). Each participant read and signed an institutional review board–approved protocol and informed consent before participation that was approved by the Human Research Protection Office at Washington University in St. Louis.
CKD Staging (eGFR) and Laboratory Measures
CKD staging was performed using serum creatinine concentrations calculated using the Chronic Kidney Disease Epidemiology Collaboration equation and classified into five stages. Participants were collapsed into an early-stage CKD group (eGFR >60 mL/min, stages 1 and 2), middle-stage CKD group (eGFR 30–59 mL/min, stage 3), and late-stage CKD group (eGFR <29 mL/min, stages 4 and 5). Laboratory measures also included blood urea nitrogen (BUN) (mg/dL), HbA1c (%), and urine albumin-to-creatinine ratio (ACR) (mg/g) to quantify degree of proteinuria.
Participant Demographics and Neuropathy Assessment
Participants’ age, duration of T2DM, weight, height, and BMI were collected along with peripheral neuropathy assessment as previously described using a 10-g Semmes-Weinstein monofilament and biothesiometer (7).
Physical Function
Functional physical performance was assessed using the nine-item modified physical performance test (PPT) as previously described (7). Briefly, each item is scored from 0 to 4 on the basis of the time taken to complete the task, with a maximum score of 36 (higher scores indicate better physical function/performance) (see Supplementary Material for task-specific details).
Muscle Performance Assessment
Lower-extremity strength (particularly quadriceps strength deficits) has been demonstrated to more strongly dictate functional mobility capacity and risk of mortality in those with CKD than traditional grip strength assessments (21). Thus, muscle performance of lower-extremity muscle groups (hip and knee extensors) was assessed using a Biodex System 3 Isokinetic Dynamometer as described previously (7). Specifically, isokinetic strength and power (movement speeds of 30°/s and 120°/s), isometric force, and muscle fatigability were assessed as previously described (4,7,22) (see Supplementary Material for additional details).
Fasting/Medication/OGTT
Because renal decline and T2DM severity can contribute to skeletal muscle insulin resistance, an OGTT and fasting glucose and insulin values were obtained to assess for differences in insulin sensitivity as previously described (23,24) (Supplementary Material). Plasma glucose was assayed by an automated glucose analyzer (Yellow Springs Instruments) and plasma insulin by ELISA (23,24).
Body Composition
Each participant underwent a DXA scan (QDR; Hologic, Waltham, MA) to assess composite and regional lean and fat mass and an assessment of muscle strength per unit muscle volume as described previously (13).
Mitochondrial Functional Analysis
Three-milligram subsamples from biopsies of the vastus lateralis muscle were prepared and analyzed for high-resolution respirometry (Oroboros Instruments, Innsbruck, Austria) as described previously (25) (Supplementary Material). O2 flux was calculated as a time derivative of oxygen concentration using DatLab 4.3 analysis software. All O2 flux values are expressed relative to tissue wet weight per second. When possible, all respiration values were further normalized to citrate synthase (CS) activity (measure of mitochondrial number).
mtDNA Copy Number
To determine whether mitochondrial density/abundance contributed to differences found with respiratory function among CKD stage groups, quantitative PCR was used to assess mtDNA copy number as previously described (26) (see Supplementary Material for detailed procedure and primers).
Mitochondrial Enzymatic Testing
Mitochondrial ETC enzyme activity and CS activity were determined spectrophotometrically as described previously (27). Because of the testing requirements of 50 mg of muscle tissue, this and all enzymatic analyses were performed on a subset of 13 participants (n = 5 in CKD stages 1 and 2, n = 5 in stage 3, and n = 3 in stages 4 and 5), with no differences detected between this subset and the remaining study subjects on any outcome measures (Supplementary Material and Supplementary Table 6). CS activity was used as a control factor in respiration measures of a secondary analysis of those 13 participants. Activity was expressed as μmol/min/g of tissue.
Transcriptome Analysis (RNA Sequencing)
One to two micrograms of cDNA were used for Illumina library preparation and sequenced on an Illumina HiSeq 2500 system using single reads that extended 50 bases and targeted 30 million reads per sample as previously described (28) (Supplementary Material). Differentially expressed genes and transcripts were then filtered for false discovery rate (FDR)–adjusted P < 0.05. These results were explored for known Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) biologically relevant pathways, with GO or KEGG terms deemed significant on the basis of changes in observed log2 fold changes for the genes within that term, with an adjusted statistical significance of FDR Q < 0.05. Gene set enrichment analysis (GSEA) methods and subsequent leading edge analysis (LEA) of transcriptomic changes are as described by Mootha et al. (29) and were performed using web-based software (www.broad.mit.edu/gsea) (Supplementary Material).
Statistics
To assess for CKD stage-specific differences in functional, muscle performance, and mitochondrial enzymatic and coupling measures, data were assessed for normality through Shapiro-Wilk test followed by either parametric one-way ANOVA with Tukey multiple comparison performed for associated post hoc analysis or nonparametric Kruskal-Wallis test for measures not normally distributed. For interrelationship measures between continuous variables, Pearson bivariate correlation coefficients were calculated and the associated r value expressed. To assess CKD stage groups for differences in sex composition and medication use, Fisher exact test was used (because of the smaller sample size of the groups). To assess variance of respirometry measures, technical replicates (i.e., measures from each of the two respiration chambers per single muscle sample) were correlated using Pearson bivariate correlation, with the coefficient of variation (CV) calculated using the root mean square method. All statistical analyses were performed using SPSS statistical software (IBM Corporation), with two-tailed testing and α set at 0.05.
Data and Resource Availability
The data supporting these findings are available upon request.
Results
Renal Function and CKD Stage Progression in T2DM Is Associated With Dysregulation of Distinct Metabolic and Mitochondrial Respiratory Transcriptional Pathways in Skeletal Muscle
To probe the unique effects of CKD and its progression/severity on skeletal muscle health and function in T2DM, we enrolled 27 participants with T2DM and clinically diagnosed diabetic nephropathy (diabetes-induced CKD) by a nephrologist. Participants were stratified into early-stage CKD (stages 1 and 2), middle-stage CKD (stage 3), and late-stage CKD (stages 4 and 5) (all n = 9). The groups were matched for all key demographic and potentially confounding metabolic variables (Table 1).
Table 1.
Participant demographics
| Stages 1 and 2 (≥60 mL/min) | Stage 3 (30–59 mL/min) | Stages 4 and 5 (<29 mL/min) | P value | |
|---|---|---|---|---|
| Sex, n | 0.47 | |||
| Male | 7 | 6 | 5 | |
| Female | 2 | 3 | 4 | |
| Age (years) | 55 (9.8) | 61 (6.4) | 62 (7.3) | 0.13 |
| T2DM duration (years) | 14 (6.3) | 13 (7.6) | 17 (7) | 0.10 |
| BMI (kg/m2) | 33.5 (5.6) | 33.7 (5.9) | 38.9 (6.3) | 0.37 |
| HbA1c | 0.88 | |||
| % | 8.01 (2.1) | 7.85 (1.8) | 7.5 (2.1) | |
| mmol/mol | 64.16 (22.8) | 62.5 (19.4) | 58.9 (23.5) | |
| Hemoglobin (g/dL) | 11.84 (1.5) | 11.32 (1.4) | 11.67 (1.1) | 0.72 |
| Hematocrit (%) | 35.13 (4.3) | 34.51 (4.6) | 34.39 (4.1) | 0.92 |
| Neuropathy, n | 0.88 | |||
| Yes | 5 | 5 | 5 | |
| No | 4 | 4 | 4 | |
| Whole-body lean mass (kg) | 59.24 (10.1) | 55.64 (14.7) | 57.08 (9) | 0.80 |
| Whole-body fat (%) | 36.1 (5.4) | 34.1 (7.9) | 40.2 (8.4) | 0.27 |
| Trunk fat (%) | 38.7 (6.3) | 38.3 (7.7) | 43.4 (7) | 0.78 |
| Bone mineral density (SD [z score] from young adult mean) | 1.23 (0.16) | 1.23 (0.16) | 1.20 (0.17) | 0.52 |
| Lipid profile (mg/dL) | ||||
| HDL | 37.9 (14.3) | 36.1 (9.3) | 39.5 (14.6) | 0.86 |
| LDL | 70 (25.7) | 74.9 (16.3) | 80.8 (37.9) | 0.84 |
| Total cholesterol | 152 (44.3) | 146.4 (22.3) | 144.9 (45.3) | 0.92 |
| Triglycerides | 204.6 (150.8) | 175.8 (163.5) | 122.5 (50.2) | 0.39 |
| OGTT AUC (mg/dL/3 h) | 40,180 (10,911) | 47,394 (11,510) | 39,752 (12,010) | 0.37 |
| HOMA (mass units) | 5.9 (3.8) | 3.8 (3.1) | 3.8 (3.6) | 0.5 |
| BUN (mg/dL) | 18 (2.95) | 31.2 (6.55) | 58.2 (21) | <0.001* ^ # |
| ACR (μg/mg) | 279 (530) | 729 (1,100) | 1,086 (790) | 0.16 |
| eGFR (mL/min/1.73 m2) | 87.8 (20.1) | 46.3 (7.4) | 18.9 (7.6) | <0.01* ^ # |
| Diabetes medication, n | ||||
| Biguanides/sulfonylureas | 4 | 5 | 0 | 0.014 |
| Insulin (short and/or long acting) | 3 | 2 | 4 | 0.23 |
| DPP-4/SGLT2 inhibitors | 1 | 0 | 1 | 0.534 |
Data are mean (SD) unless otherwise indicated. Participant demographics were compared using one-way ANOVA, and Fisher exact test was used for sex distribution and peripheral neuropathy status between groups. AUC, area under the curve; DPP-4, dipeptidyl peptidase 4; SGLT2, sodium–glucose cotransporter 2.
P < 0.05, post hoc pairwise comparison between stages 1 and 2 and stage 3.
P < 0.05, post hoc pairwise comparison between stages 1 and 2 and stages 4 and 5.
P < 0.05, post hoc pairwise comparison between stage 3 and stages 4 and 5.
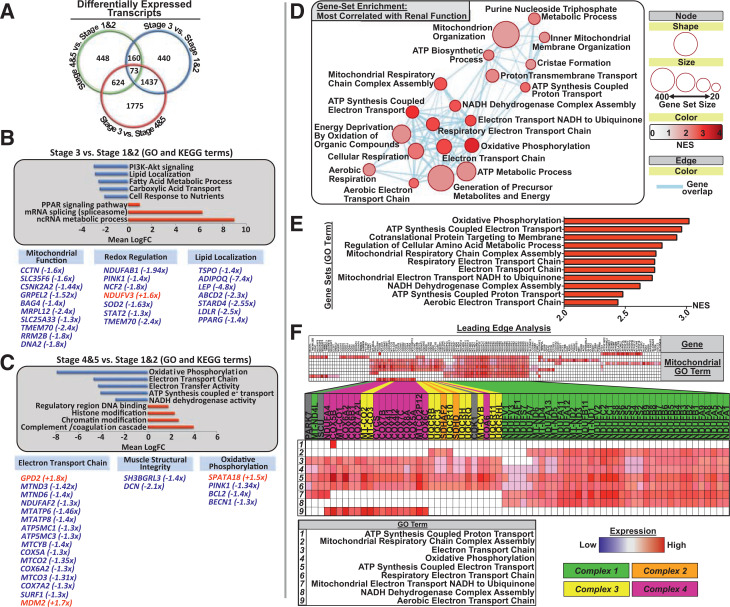
RNA sequencing of skeletal muscle samples from each participant highlighted hundreds of differentially expressed genes among stages of CKD (all P < 0.05) (Fig. 1A). GO and KEGG pathway analyses revealed that upon progression to middle-stage CKD (stage 3), gene networks/pathways governing phosphatidylinositol 3-kinase (PI3K)-Akt signaling, lipid localization, fatty acid metabolism, tricarboxylic acid cycle transport, and cellular nutrient handling became significantly downregulated (Fig. 1B). Indeed, significantly downregulated individual transcripts in stage 3 CKD are involved in a range of mitochondrial regulatory functions from mitochondrial protein import/synthesis and mitochondrial turnover to reactive oxygen species (ROS) buffering and mitochondrial lipid oxidation (Fig. 1B and Supplementary Table 1). In late-stage CKD (stages 4 and 5) compared with early disease, however, the most significantly downregulated gene pathways included general oxidative phosphorylation, ETC-coupled ATP synthesis, and ETC complex 1 activity, with significant downregulation in individual transcripts largely involved in ETC subunit translation and complex assembly, mitochondrial turnover, and ROS handling (all FDR Q < 0.05) (Fig. 1C, Supplementary Table 2, and Supplementary Fig. 1). These findings highlight relatively unique CKD stage-specific alterations to the skeletal muscle transcriptome that may differentially affect mitochondrial function over the course of CKD progression/renal decline in T2DM.
Figure 1.
Skeletal muscle transcriptome in CKD progression in T2DM: RNA sequencing output detailing overall transcript and gene regulatory network differences across CKD stages. A: Venn diagram comparing the number of significantly differentially expressed genes per condition (stages 4 and 5 vs. stage 1: 448 differentially expressed transcripts; stage 3 vs. stages 1 and 2: 440 differentially expressed transcripts; stage 3 vs. stages 4 and 5: 1,775 differentially expressed transcripts). B and C: Selected GO terms and KEGG pathways from a larger list of top 25 down- and upregulated gene networks across stage-wise transcript analysis. Bar charts depict top downregulated (blue bars) or upregulated (red bars) gene networks, with their accompanying network label listed next to its corresponding bar (all GO terms listed have FDR Q < 0.05, and all KEGG terms have P < 0.05). Pathway up- or downregulation is expressed in mean log fold change (LogFC) units and was determined to be significant when compared with background log2 fold change for genes not contained within the network/pathway. Beneath are selected genes from either significant GO or KEGG pathways or with known mitochondrial regulatory functions that are differentially expressed, and the fold change is listed in parentheses next to the gene term. B: Comparison of middle-stage (stage 3) CKD muscle transcriptome with stage 1 and 2 (early CKD is the reference). Metabolic pathways, including PI3K-Akt signaling, lipid handling and localization, and tricarboxylic acid cycle were significantly downregulated in middle-stage CKD compared with early-stage disease. C: Comparison of late-stage CKD with stages 1 and 2 (early CKD is the reference). Oxidative phosphorylation and mitochondrial ATP synthesis components were significantly downregulated in late-stage CKD compared with early, with specific genes listed at the bottom. D: Selected GSEA map depicting biological gene networks most strongly correlated with renal function (eGFR). Circles/nodes indicate biological gene network (node size = number of genes within the network; red scale = NES [0–4, increasing value indicates that the pathway is more strongly enriched/positively related with renal function]; blue lines = individual genes belonging to multiple gene sets [all FDR Q < 0.05]). E: Top biological gene networks most strongly related to renal function (top NES scores, all FDR Q < 0.01). These include networks with considerable overlap in regulating mitochondrial respiration and ETC complex function and assembly. F: LEA of top gene sets (E), highlighting individual genes driving the enrichment of mitochondrial regulatory networks in relationship to renal function. Individual genes driving this relationship are confined to ETC complex subunits and assembly factors, predominately of complex 1 and complex 4.
Upon progression to middle- and late-stage CKD, the most significantly downregulated transcriptional biological pathways shared commonality in their regulation of metabolism and energetics. We thus used further GSEA to elucidate the transcriptional gene sets and individual genes most closely related to renal functional decline. Here, we report that mitochondrial electron transport and ATP proton organization pathway clusters were most strongly related to renal function (average normalized enrichment score [NES] 2.48, FDR Q = 1.0E−4) (Supplementary Fig. 2) and comprised gene sets governing mitochondrial ATP production and ETC complex assembly and function (all FDR Q < 0.01) (Fig. 1D and E and Supplementary Fig. 3). However, because these gene sets overlap, using LEA to identify which specific genes are responsible for this strong association between renal function and mitochondrial pathways, we found that mitochondrial ETC subunit transcripts and assembly factors, most notably of ETC complexes 1 and 4, drive the association between renal function and mitochondrial regulation/function (Fig. 1E and F).
Mitochondrial Respiratory Function and Energetic Coupling Are Incrementally Impaired With Each Stage of CKD Progression in T2DM
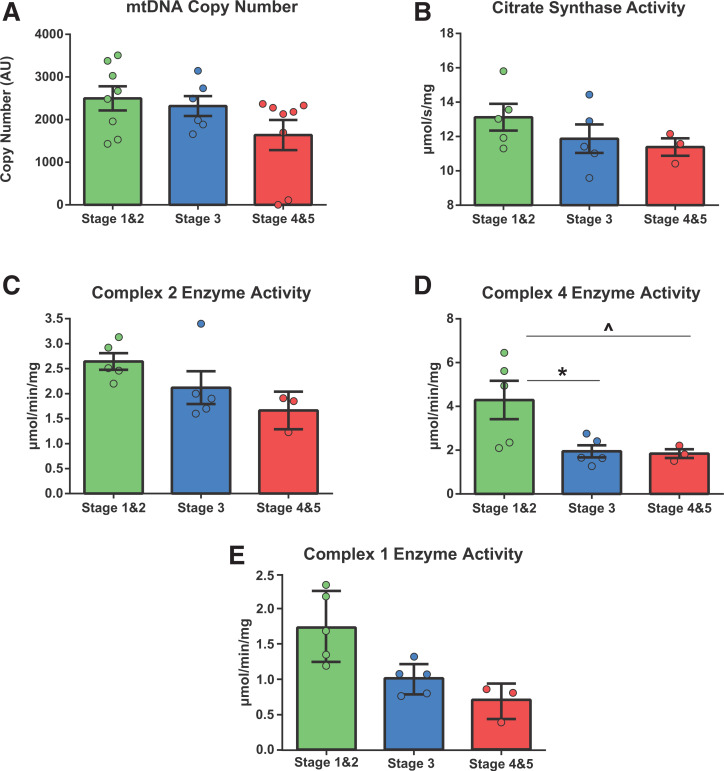
Our transcriptome findings that 1) renal function is most strongly associated with transcription of ETC subunits and assembly factors, notably of complexes 1 and 4, and 2) there were CKD stage-specific differences in the regulation of energetic transcriptional networks spurred us next to determine the relationship between CKD progression and mitochondrial content, ETC coupling, and oxidative function. CKD stage groups did not differ in mitochondrial density as assessed through mtDNA copy number or CS activity (Fig. 2A and B). Interestingly, while ETC complex 2 enzymatic function was not affected by CKD stage progression (Fig. 2C), we found a significant reduction (∼80%) in ETC complex 4 activity with CKD progression to middle and later stages (Fig. 2D), which agrees with our RNA sequencing LEA results.
Figure 2.
Mitochondrial enzymatic activity in CKD progression in T2DM: analysis of mitochondrial density, number, and ETC complex enzyme activity. A: mtDNA copy number (y-axis is unitless [one-way ANOVA F = 3, P = 0.07]) depicting no significant difference between CKD stage groups in mitochondrial abundance. B–D: The remaining analyses were performed on a 13-participant subset of samples (stages 1 and 2, n = 5; stage 3, n = 5; stages 4 and 5, n = 3). B: CS enzyme activity assessment. As in panel A, no significant differences were observed between groups (one-way ANOVA F = 1.2, P = 0.33). C: Mitochondrial ETC complex 2 (succinate dehydrogenase) enzyme activity, an assessment of complex 2–specific function in isolation. Although there appears to be a trend, there is no significant difference between CKD stage groups in complex 2 function (one-way ANOVA F = 3, P = 0.08). D: Mitochondrial ETC complex 4 (cytochrome oxidase) enzyme activity measured as in panel C (one-way ANOVA F = 5.1, P = 0.02). E: Mitochondrial ETC complex 1 (NADH) enzyme activity (one-way ANOVA F = 3.9, P = 0.06). *P < 0.05, post hoc test differences between stages 1 and 2 and stage 3; ^P < 0.05, post hoc test differences between stages 1 and 2 and stages 4 and 5. AU, arbitrary unit.
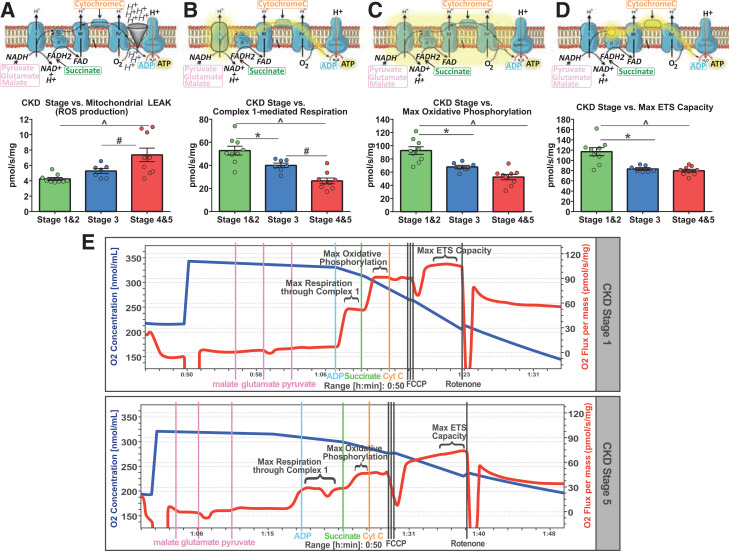
Next, using real-time high-resolution respirometry, we observe progressive increases in mitochondrial H+ LEAK respiration with advancing stage of CKD (state L) (Fig. 3A), and this remained after accounting for mitochondrial number (CS) (Table 2). Interestingly, in agreement with our LEA, we observed significant declines in complex 1–mediated respiration (state 3-C1) with progressive stage of CKD (Fig. 3B). After controlling for CS activity, this relationship remained significant between early- and late-stage CKD (P = 0.018) (Table 2 and Supplementary Results). Similarly, CKD stage progression induced significant declines in maximal oxidative capacity (state 3-C1 + C2, P) (Fig. 3C) and maximal electron transport system (ETS) capacity (state E-ETS) (Fig. 3D and Table 2), with no difference observed between stage 3 and stages 4 and 5 (Fig. 3C and D), and this relationship remained after normalizing to CS (Table 2). An example of respiration traces depicting these differences between early- and late-stage CKD respiration is shown in Fig. 3E. Moreover, H+ LEAK respiration, maximal oxidative phosphorylation capacity, and complex 1–mediated respiration, all normalized to CS, significantly correlated with renal filtration rate (Table 2), while CS-normalized complex 4 enzyme activity significantly positively correlated with renal filtration (r = 0.53, P = 0.02) (Table 2).
Figure 3.
Mitochondrial respiratory functional deficits in CKD progression in T2DM: mitochondrial respiration assessment across CKD stage groups with one-way ANOVAs (Kruskal-Wallis test for complex 1–mediated respiration and maximal ETS capacity). Bar heights indicate group means, with errors bars indicating ± SEM. All respiration measures are depicted in rate of oxygen consumption per second per mg of tissue wet weight (pmol/s/mg) and are not normalized to CS activity. A: LEAK respiration between CKD stages (F = 8, P = 0.002), with stages 1 and 2 (*) and stage 3 (#) exhibiting significantly less LEAK than stages 4 and 5 (P < 0.05). B: Complex 1 respiration (state 3-C1) between CKD stages (F = 21, P < 0.001), with stages 1 and 2 (^) and stage 3 (#) being significantly higher than stages 4 and 5, and stages 1 and 2 (*) being higher than stage 3. C: Maximal oxidative phosphorylation (state 3-C1 + C2) between CKD stages (F = 21, P < 0.001), with stages 1 and 2 (*) being higher than stage 3, and stages 1 and 2 (^) being higher than stages 4 and 5 (P < 0.05). D: Maximal ETS capacity between CKD stages (F = 15.5, P < 0.001), with stages 1 and 2 (*) being higher than stage 3, and stages 1 and 2 (^) being higher than stages 4 and 5 (P < 0.05). E: Representative respiration kinetics depicting mitochondrial respiration (red trace) over the experimental protocol between mitochondria in early (stage 1) CKD and late (stage 5) CKD, with plateaus labeled according to their respective respiration state (complex 1 respiration, maximal oxidative phosphorylation, and maximal ETS capacity). Also depicted are color-coded lines indicating time-specific addition of substrates to the respiration chamber. *P < 0.05, post hoc pairwise comparison between stages 1 and 2 and stage 3; ^P < 0.05, post hoc pairwise comparison between stages 1 and 2 and stages 4 and 5; #P < 0.05, post hoc pairwise comparison between stage 3 and stages 4 and 5. FCCP, carbonyl cyanide-p-trifluoromethoxyphenylhydrazone.
Table 2.
Mitochondrial function and energetic coupling deficits in CKD progression in T2DM
| Stages 1 and 2 (≥60 mL/min) | Stage 3 (30–59 mL/min) | Stages 4 and 5 (<29 mL/min) | P value | |
|---|---|---|---|---|
| Participants, n | 5 | 5 | 3 | |
| LEAK (L) respiration (pmol/s/mg) | 0.325 (0.06) | 0.44 (0.06) | 0.82 (0.12) | <0.05^ # |
| Complex 1 respiration (pmol/s/mg) | 4.3 (0.54) | 3.46 (0.46) | 2.9 (0.6) | 0.018^ |
| Maximal oxidative phosphorylation capacity (P) (pmol/s/mg) | 7.52 (0.87) | 5.7 (0.72) | 5.4 (0.55) | 0.004* ^ |
| Maximal ETS capacity (E) (pmol/s/mg) | 9.3 (1.3) | 6.9 (0.97) | 7.2 (0.31) | 0.001^ # |
| Oxidative phosphorylation control ratio (P/E) | 0.79 (0.07) | 0.81 (0.036) | 0.67 (0.15) | 0.017^ # |
| RCR (P/L) | 22 (4.3) | 13.1 (2.4) | 7.9 (3.2) | <0.001* ^ # |
| eGFR | ||||
| Correlations | r | P value | ||
| LEAK (normalized to CS) | −0.75 | <0.001 | ||
| Complex 4 enzyme activity | 0.53 | <0.001 | ||
| Complex 1 respiration | 0.82 | <0.001 | ||
| Maximal oxidative phosphorylation | 0.81 | <0.001 | ||
Data are mean (SD) unless otherwise indicated. Top: mitochondrial respiration normalized to mitochondrial content (CS activity) performed on a 13-participant subset of the study population was compared across stage groups using one-way ANOVA. Low oxidative phosphorylation control ratio values indicate intrinsic mitochondrial deficits within the phosphorylation system. Bottom: Pearson bivariate correlations were calculated to compare respiration measures/states normalized to mitochondrial content (CS activity) and mitochondrial ETC complex 4 enzyme activity with renal filtration rate (eGFR). RCR is an index in which larger values reflect better coupling between phosphorylation system and ATP production.
P < 0.05, post hoc pairwise comparison between stages 1 and 2 and stage 3.
P < 0.05, post hoc pairwise comparison between stages 1 and 2 and stages 4 and 5.
P < 0.05, post hoc pairwise comparison between stage 3 and stages 4 and 5.
To further probe mitochondrial function and coupling, mitochondrial respiratory flux control ratios were calculated from the aforementioned states and listed in Table 2 and Supplementary Table 3 (see Supplementary Material). There were significant reductions in oxidative phosphorylation control ratio in late-stage CKD (P = 0.017) (Table 2). However, the respiratory control ratio (RCR) was significantly different among stages, with a 39% reduction upon progression to middle-stage CKD (stage 3) and a 63% reduction upon progression to late-stage CKD, indicating that progressive CKD relates to progressively poorer coupling (P < 0.001) (Table 2). This was also true of the ETS coupling efficiency (P < 0.001) (Supplementary Table 3). Conversely, while the phosphorylation control ratio and oxidative phosphorylation coupling efficiency ratios both demonstrated impairment with CKD stage-wise progression (P = 0.001), the phosphorylation control ratio did not differ between middle- and late-stage CKD, while oxidative phosphorylation coupling efficiency did not differ between early- and middle-stage disease (Supplementary Table 3).
Functional Mobility and Skeletal Muscle Performance Are Impaired With Renal Functional Decline and CKD Progression
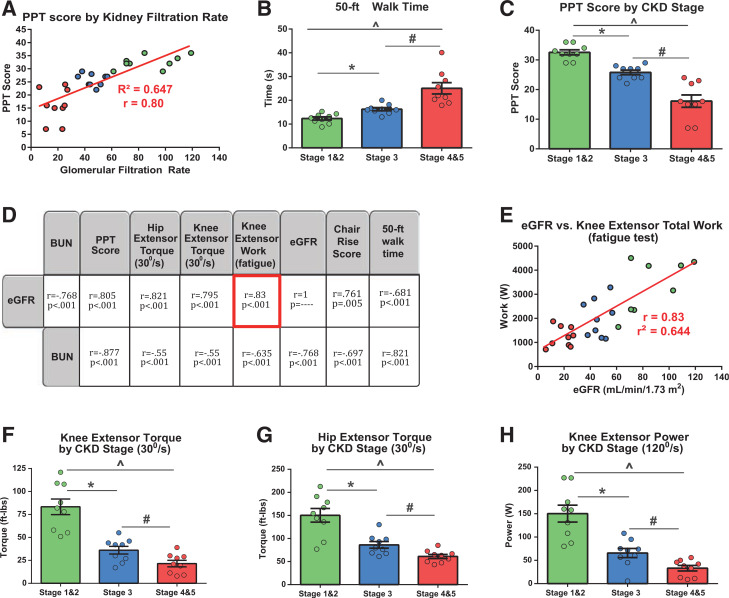
Having established the stage-specific consequences of CKD on skeletal muscle mitochondrial transcriptional regulation and energetic function, we next endeavored to assess the effects of CKD stage progression on physical function and muscle performance. Here, eGFR was significantly positively correlated with physical function (PPT score) (r = 0.8, P < 0.001) (Fig. 4A) and other functional measures, including the ability to repeatedly rise from a chair (P = 0.005) (Fig. 4D). eGFR was also negatively correlated with 50-ft walk time, indicating that worse kidney function was associated with slowed gait (P < 0.001) (Fig. 4B and D). These relationships between renal function and functional mobility were also evident upon correlating BUN (all P < 0.001) (Fig. 4D). Similarly, PPT score significantly declined with progressive stage of CKD (P < 0.001) (Fig. 4C) .
Figure 4.
Impaired physical function and muscle performance in CKD progression in T2DM. A: Pearson bivariate correlation of eGFR and PPT score (0–36) (higher scores indicate better functional performance) (not pictured, P < 0.001). B: Fifty-foot walk time (higher values indicate slower gait speed) (F = 26, P < 0.001). C: PPT score (F = 36, P < 0.001). D: Muscle performance and physical function interrelationships. Pearson bivariate correlation coefficients were calculated among measures of physical function (PPT score, chair rise score, 50-ft walk time), lower-extremity muscle performance (knee and hip extensor torque, knee extensor work over the fatigue task), and renal function–associated serum chemistry values (eGFR, BUN). E: Pearson correlation plotting total knee extensor work over fatigue task (50 repeated contractions; measure of muscle endurance) against renal filtration rate (P < 0.001). F–H: Group differences in knee extensor torque (assessed at isokinetic speed of 30°/s, knee extensor torque measured in ft-lb) (F = 20, P < 0.001), hip extensor torque (assessed at isokinetic speed of 30°/s, hip extensor torque measure in ft-lb) (F = 22, P < 0.001), and knee extensor power (assessed at isokinetic speed of 120°/s, measured in W) (F = 24, P < 0.001). Bar heights represent group mean, and error bars represent ± SEM. Lower-extremity muscle strength (hip extensor torque, knee extensor torque, and knee extensor power and physical function capacity [50-ft walk time, PPT score]) was assessed across CKD stage groups with one-way ANOVAs. E: Data points for each individual participant. *P < 0.05, post hoc pairwise comparison between stages 1 and 2 and stage 3; ^P < 0.05, post hoc pairwise comparison between stages 1 and 2 and stages 4 and 5; #P < 0.05, post hoc pairwise comparison between stage 3 and stages 4 and 5.
With respect to muscle performance, eGFR was significantly positively correlated with knee and hip extensor torque and knee extensor total work during the fatigue task (measure of fatigue resistance) (P < 0.001) (Fig. 4D and E). With progression of CKD stage, there was a significant stepwise decline in hip and knee extensor torque and power production and fatigue resistance, and this remained after controlling for unit muscle mass (P < 0.001 and P < 0.01, respectively) (Fig. 4F–H and Supplementary Fig. 4A). Finally, as a confirmation of the established physiological connection between lower-extremity (quadriceps and hip) muscle performance and functional mobility, we found that knee and hip extensor torque production both significantly positively correlated with PPT scores and functional subtasks (Supplementary Fig. 4).
Mitochondrial Coupling and Respiratory Deficits in CKD Progression Strongly Relate to the Observed Deficits in Muscle Performance and Physical Function
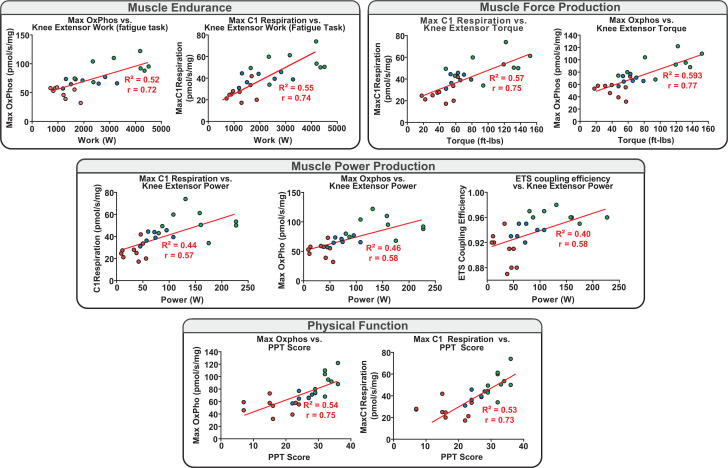
After demonstrating apparent transcriptional and intrinsic mitochondrial coupling and functional deficits with progressive stage of CKD and CKD stage-specific impairments in physical function and muscle contractile performance, we analyzed the relationship between muscle mitochondrial respiration measures and muscle performance and functional mobility. Mitochondrial oxidative capacity, complex-specific function (C1), and electron transport capacity all demonstrated striking relationships with muscle fatigability, muscle force and power production, and physical function measures (all r = 0.5–0.8, P < 0.001) (Fig. 5). Similarly, and in agreement with prior studies, we also found a strong relationship among mitochondrial complex 1 function, oxidative phosphorylation capacity, and muscle force production per unit muscle volume (Supplementary Fig. 5).
Figure 5.
Mitochondrial coupling and oxidative function relationships to muscle performance and physical function. Pearson bivariate correlation plots depicting interrelationships among mitochondrial respiration states (complex 1 respiration [state 3-C1], maximal oxidative phosphorylation [Oxphos] [state 3-C1 +C2], ETS coupling efficiency [ETS capacity/E), knee extensor muscle performance (endurance/work over the fatigue task, force production/torque, power production), and physical function (PPT score). All depicted correlations are significant (P < 0.05). Knee extensor power was assessed as in Fig. 2 (120°/s isokinetic speed), with knee extensor torque measured in ft-lb (30°/s).
Discussion
Respiratory coupling of the mitochondrial ETC to proton motive force and ATP formation directly dictates mitochondrial oxidative capacity and contractile function and muscle force per unit volume, with further evidence demonstrated for its influence over muscle power production and functional mobility (30–32). Respiratory coupling is predictably impaired upon disruption of ETC components. Prior evidence in humans, however, has demonstrated that T2DM impairs ETC complex function (∼40–50%), and this is tightly related to glycemic control, while those with late-stage CKD demonstrate mitochondrial oxidative capacity deficits despite an increase in ETC complex abundance (33,34). Thus, discerning the unique influence of CKD or its progression over mitochondrial coupling and related muscle performance and mobility in T2DM has remained elusive. Here, however, upon matching participants for T2DM severity, metabolic status, and even adiposity (Table 1 and Supplementary Tables 4 and 5) across the spectrum of CKD progression for the first time, we uncovered that genes governing transcription of mitochondrial complex 1 and 4 subunits and assembly factors drive the relationship between renal function and mitochondrial regulatory networks in T2DM (Fig. 1), and CKD stage progression is mirrored by progressive downregulation in oxidative phosphorylation transcriptional networks and genes regulating ETC structure and function (Fig. 1, Supplementary Figs. 1 and 2, and Table 2). These transcriptome findings lend mechanistic support for the observed reduction in complex 4 enzyme activity and coupled respiration in both middle- and late-stage CKD because this may be significantly affected through inadequate complex subunit transcription and/or deficient complex assembly, findings echoed by previous studies in a rodent CKD model (32,35).
Interestingly, animal model studies have demonstrated the consequences of such inadequate complex subunit transcription. Indeed, disruption of just one P- or Q-module ETC complex 1 subunit (MT-ND6 and NDUFS3, respectively) is sufficient to induce excess ROS production and impair skeletal muscle respiratory coupling and state 3-C1 respiration by 36%, consequentially blunting muscle endurance and strength by as much as 80%, all in the absence of muscle atrophy (36,37). These results bear striking similarity to our observed 48% reduction in state 3-C1 respiration in late-stage CKD, with an ∼60% reduction in muscle endurance and reduced expression of these same complex 1 subunits, all in the absence of overt muscle atrophy as well (Figs. 1 and 3, Supplementary Fig. 4, Table 1, and Supplementary Table 1).
While we demonstrate strong relationships between mitochondrial function and muscle performance and physical function deficits in this population (Fig. 5), we also find significant progressive declines in both muscle force per unit volume and mitochondrial coupling with CKD progression (reduced RCR) (Table 2). Notably, our results reflect those reported by Roshanravan et al. (38) of a 34% reduction in mitochondrial coupling ratio of patients without diabetes but with stage 3 CKD. Moreover, our findings remained after normalizing for mitochondrial number (CS activity). Similarly, prior work by Coen et al. (39), using the same muscle mitochondrial respirometry approach and protocol we have used here, found an RCR of ∼11.8 in individuals nearly 80 years of age that was predictive of walking speed and Vo2max. Intriguingly, this respirometry-derived readout of coupling efficiency with old age falls within the RCR range we observe in middle- and late-stage CKD in our study (7.9–13.1). Because our participants in these stages were ∼20 years younger than those in Coen et al., this speaks to the accelerated aging-like phenotype of respiratory coupling in middle- and late-stage CKD. This uncoupling of mitochondrial respiration across stages of CKD in T2DM strongly correlated with, and may partially explain our findings of, reduced muscle force production, power, and endurance and reduced functional mobility (gait speed and increasing difficulty repeatedly rising from a chair) beginning even in early CKD (stage 3) (Fig. 6), as skeletal muscle mitochondrial coupling capacity has been shown to directly affect fatigability, exercise capacity, and muscle force production in other works (14,31,32).
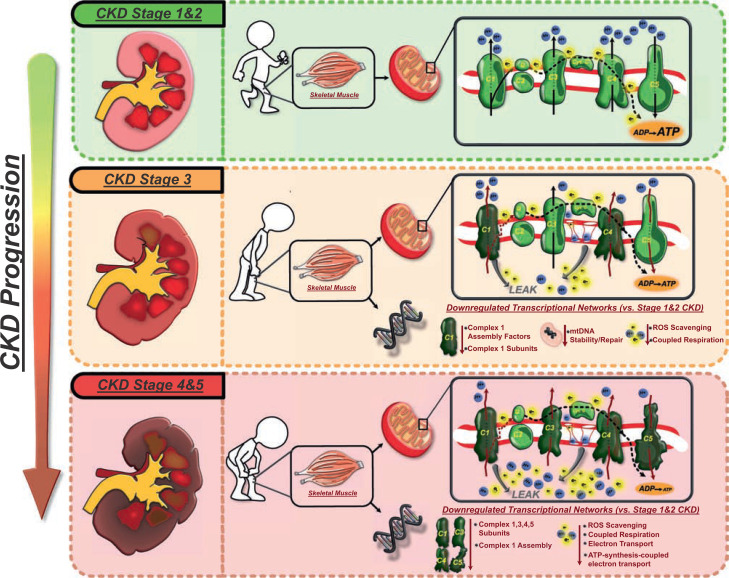
Figure 6.
CKD stage and alterations to mitochondrial coupling and transcriptional regulation: schematic summary and compilation of the findings of this study demonstrating, for the first time, the potential temporal impact of CKD stage transitions (from early to middle and late stages of disease) on skeletal muscle mitochondrial ATP production machinery and transcriptional regulation and its translational impact on functional mobility in those with T2DM. ETC inlay: black arrows indicate proper proton (blue circles) and electron (yellow bursts) pumping and shuttling down the ETC, resulting in ATP production. Kinked red arrows indicate dysfunctional proton and electron pumping by the specific ETC complex. LEAK indicates slippage/backflow of electrons and protons through pore openings or dysfunctional ETC complexes. Early-stage CKD (stages 1 and 2) is characterized by relatively healthy and functional mitochondrial electron transport and coupled ATP production and healthy expression of metabolic transcriptional pathways, which adequately support functional mobility. Stage 3 CKD exhibits the first signs of downregulation in transcriptional networks governing ETC complex 1 assembly and function, ROS scavenging, mtDNA repair, and coupled respiration that combine to impair mitochondrial complex 1–mediated respiration, complex 4 respiration, and ETC-coupled ATP production. These subcellular changes promote early muscle weakness, reduced endurance, and impaired functional mobility. Stage 4 and 5 CKD (predialysis) are marked by more extreme alterations to the mitochondrial system because transcriptional networks governing the production of complex 1, 3, 4, and 5 subunits are all downregulated, while those regulating ROS scavenging and ATP-coupled electron transport are further reduced. This contributes to impairment in mitochondrial ATP production by complexes 1, 3, 4, and 5; increased mitochondrial LEAK/ROS production; and drastically hampered ETC-coupled ATP production. This ultimately induces severe muscle weakness and marked impairment in functional mobility at this stage of disease. Thus, while no causation is definitively proven, this study highlights the stage-specific mechanistic association of CKD on skeletal muscle metabolic transcriptome and accompanying mitochondrial ETC that dictate loss of functional mobility.
However, while the loss of respiratory coupling efficiency may stem from altered expression of ETC subunit–encoding genes, it may also result, in part, from the backward flow of protons toward the matrix through nonspecific leaks, often at ETC complexes or through membrane channels (MPTP, VDAC) (40). LEAK respiration and uncoupling are promoted by inefficient energy transfer and mitochondrial ETC dysfunction, which ultimately culminate in damaging ROS production. Indeed, we report stepwise increases in mitochondrial LEAK respiration with CKD progression that remains after CS normalization. While we acknowledge that LEAK respiration cannot be directly linked to ROS, these findings may parallel evidence in a stage 3 CKD nephrectomy mouse model study that demonstrated that uremic toxin intermediates impair mitochondrial function and exercise performance entirely as a result of ROS stimulation (41). Because mitochondrial LEAK and ROS production are often a function of excess electron accumulation at complex 1 of the ETC, it is plausible that our observed deficiency in complex 1 subunit and assembly factor genes (MTND3, MTND6, NDUFAF2, and NDUFAB1, respectively) and complex 1 respiration with CKD progression may contribute to excessive electron leak and increase ROS production and accumulation implicated in this study. Moreover, the deficiencies in ETS capacity in skeletal muscle directly promote electron leak and superoxide production. This may be further facilitated by the reduced expression of ROS-scavenging SOD2 and ROS-inhibiting TIGAR we observed in middle- and late-stage CKD, respectively (42,43) (Fig. 1 and Supplementary Tables 1 and 2). Additionally, the demonstrated increase in LEAK respiration is potentially detrimental to muscle and mitochondrial function through its promotion of mtDNA and nuclear DNA mutations of genes encoding ETC subunit proteins and through direct alterations of mitochondrial proteins (44,45). While we do not present direct evidence of this process, we observe stepwise increases in LEAK respiration in middle- and late-stage CKD that is accompanied by downregulation in genes governing mtDNA stability, maintenance, and repair of oxidative stress–induced damage (stage 3 CKD: RRM2B, DNA2, and SLC25A33) and an upregulation of SPATA18 indicative of active repair of oxidatively damaged mitochondrial proteins in late-stage CKD (stage 4) (46–48) (Fig. 1 and Supplementary Table 2). Such findings lend potential explanations for the observed negative trend in mtDNA-encoded complex 1 and 2 enzymatic function with CKD progression and in complex 4 activity in middle- and late-stage disease, findings echoed in studies of younger populations with ESRD (49). In this respect, these transcriptional changes are similar to advanced aging and suggest a scenario in which complex 1 respiratory deficiency and overall ETC shuttling impairments in stage 3 CKD may lead to increased LEAK/ROS that is inadequately scavenged, thus exacerbating LEAK/ROS production in later-stage CKD (50). In this respect, complex 1 deficiencies and genomic changes seem to develop early in disease and may contribute to steadily increasing LEAK and associated ROS, which may further exacerbate ETC complex dysfunction and respiratory coupling in CKD stage progression in T2DM, although more direct measures of ROS are required in future studies.
In light of our findings and the principles of mitochondrial dynamics, in the presence of poor coupled respiration in CKD progression shown here (Fig. 3, Table 2, and Supplementary Table 3), mitochondria typically activate signaling pathways to shift the balance between mitochondrial synthesis and degradation (mitophagy) to maintain mitochondrial and muscle health. However, our transcriptome analysis reveals in stage 4 and 5 CKD muscle significant reductions in expression of PINK, BECN1 (Beclin-1), and BCL2, proteins charged with the orchestration of mitophagy of deficient mitochondria (51). This may promote accumulation of defective mitochondria, thus partially explaining the nonsignificant difference in mitochondrial abundance in this stage (Fig. 2) and contributing to poor muscle respiration (51). Similarly, we also observe reduced expression of PINK1 in stage 3 CKD, with additional reduction in BAG4 expression, an antiapoptotic stress protein that negatively regulates PARKIN (52). This downregulation of BAG4 may be a mitophagy signal in response to mitochondrial damage; however, with insufficient PINK transcription, mitophagy may be deficient in stage 3 CKD muscle as well. Further investigation is required to determine the effects of CKD on mitochondrial turnover and intrinsic quality control pathways.
Intervention plans aimed at improving functional mobility often comprise different pharmacological, nutritional, and exercise modalities (e.g., aerobic or anaerobic/resistance exercise) that confer unique adaptive benefits to different physiological systems that affect mobility. These differential effects may necessitate exercise, drug, and nutrition tailoring and prioritization in the setting of chronic disease like T2DM and CKD. Here, we report mitochondrial coupling and complex 1–specific respiratory deficits as being most strongly connected to renal decline in T2DM, with additional evidence for altered mitochondrial turnover pathways with worsening disease. Thus, selection of and emphasis on interventions that address these mechanisms most effectively would theoretically maximize benefits to this patient population.
Indeed, preclinical evidence has suggested that interventions targeted at these identified mechanisms are in development and effective. For instance, systemic delivery of a new class of succinate prodrugs is effective in treating metformin-induced complex 1 deficits because of its ability to bypass skeletal muscle mitochondrial complex 1 to improve respiration (53). Similarly, mitochondrial respiratory improvement has been achieved through the prescription of targeted diet plans, such as a ketogenic diet for people with epilepsy, because this partially bypasses the deficient complex 1 of the respiratory chain characteristics of this disease (54). In the exercise literature, resistance or strength training has been shown to increase skeletal muscle mitochondrial electron flow and the RCR (Table 2) largely through complex 1–mediated adaptations (55). More specifically, others have shown that resistance exercise improves mitochondrial coupling through sensitization of complex 1 to NADH substrate and increasing electron flow from complex 1 to ubiquinone to improve complex 1–coupled respiration and ETS capacity (56). Simultaneously, a large-scale randomized resistance exercise study by Ramel et al. (57), demonstrated the capacity for this exercise modality to improve eGFR by ∼4.5 mL/min in those with stage 3 and 4 CKD. Conversely, aerobic exercise studies have failed to alter skeletal muscle mitochondrial RCR (Table 2) or efficiency of energy transfer (maximal oxidative phosphorylation capacity relative to oxygen consumption) while simultaneously promoting only mild ∼2.39 mL/min improvements in renal filtration (55,58,59). Thus, while our mechanistic findings suggest potential avenues of fruitful targeted interventions for these individuals, future study is warranted to assess the efficacy of coupling- and complex 1-targeted exercise and drug- and nutrition-based treatment approaches in their capacity to improve muscle function and mobility in T2DM and CKD.
This study, however, has some limitations. While our cross-sectional study design was carefully constructed to match participants across CKD stage groups on critical metabolic comorbidities and diabetes status, a longitudinal study design in which participants, serving as their own controls, are followed for multiple years as they progress across CKD stages would allow more direct and definitive conclusions regarding the effects of CKD progression on skeletal muscle health and mitochondrial function. This study is also limited by its sample size, specifically for enzymatic testing analyses. While we were statistically powered to detect CKD stage-specific deficits in mitochondrial respiratory function, this was not the case for the majority of mitochondrial enzymatic analyses because these exhibited moderate to strong effect sizes that appeared to not reach significance as a result of lack of statistical power with 13 total participants. Additionally, recent work by Jacques et al. (60) established that high-resolution respirometry of permeabilized muscle bundles carries strong correlation but moderate variation in respiration measures between technical replicates (CV of ∼15% for maximal oxidative phosphorylation and maximal ETS capacity). However, here we report stronger correlations between technical duplicate samples obtained from the same biopsy specimen (r = 0.77–0.84) (Supplementary Fig. 6A). Subsequently, our data demonstrate a strikingly reduced CV to those obtained by Jacques et al., ranging from as low as 3–7.7% (LEAK respiration and maximal ETS capacity, respectively) (Supplementary Fig. 6B). Moreover, when assessing CS-normalized respiration measures in our 13-participant subset, the CV was still <10% for all respiration measures (Supplementary Fig. 6B). Prior literature using our same respirometry protocol also demonstrated, albeit in a homogeneous healthy young population, that ∼12 muscle samples (i.e., 12 participants) would be required to achieve 80% power and statistical significance (P < 0.05) to detect an ∼20% change in mitochondrial respiration (maximal oxidative phosphorylation and maximal ETS capacity) (60). Similarly, all our 13-particpant, CS-normalized respiration measures (LEAK respiration, complex 1, maximal oxidative phosphorylation, maximal ETS capacity) demonstrated a >20% change between specific CKD stages and achieved statistical significance and at least 80% statistical power (Supplementary Fig. 6C). Thus, despite the smaller sample size of our 13-participant subset, for the listed respiration measures, we demonstrate consistent technical replication, a small CV, and adequate statistical power to detect a significant effect of CKD stage on mitochondrial CS-normalized respiration.
In conclusion, while functional and muscle deficits are most commonly associated with late-stage CKD and ESRD in particular, after controlling for T2DM severity, we demonstrate that CKD-associated decrements in functional mobility and muscle force, power, and endurance strongly relate to intrinsic mitochondrial functional deficits that begin early in stage 3 CKD in T2DM. This may, in turn, derive from altered expression of gene networks involved in mitochondrial respiration, structure, and turnover pathways. These findings highlight the first evidence of potential primary mechanistic factors that characterize CKD progression in a widespread and growing renal disease population (people with T2DM) and inform potential specific interventions geared toward preserving or even improving mobility and muscle health in this population.
Article Information
Funding. This work was supported in part by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant 5T32-HD-007434-26 (Dr. Catherine Lang, principal investigator), National Institute of Diabetes and Digestive and Kidney Diseases grant 1F31-DK-109649-01A1 (to D.C.B.), and Washington University in St. Louis Institute of Clinical and Translational Sciences C (UL1-TR-002345, to D.C.B. and D.R.S.). This work was also supported by the American Physical Therapy Association Foundation for Physical Therapy Research (PODS2 awards 2015–2017, to D.C.B.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.C.B., A.J.B., A.S.V., and T.P. acquired, analyzed, or interpreted data. D.C.B., A.J.B., A.S.V., T.P., and D.R.S. revised/edited the manuscript. D.C.B., A.J.B., and D.R.S. drafted the manuscript. A.S.V. and T.P. provided technical and material support. D.C.B. and D.R.S. conceived and designed the study and obtained funding. D.C.B. and D.R.S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the American Physical Therapy Association Combined Sections Meeting 2018, New Orleans, LA, 21–24 February 2018.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13643477.
References
- 1. Centers for Disease Control and Prevention . National Chronic Kidney Disease Fact Sheet, 2017. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2017. [Google Scholar]
- 2. Bailey RA, Wang Y, Zhu V, Rupnow MF. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) staging. BMC Res Notes 2014;7:415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zoppini G, Targher G, Chonchol M, et al. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol 2012;7:401–408 [DOI] [PubMed] [Google Scholar]
- 4. Roshanravan B, Patel KV, Fried LF, et al.; Health ABC study . Association of muscle endurance, fatigability, and strength with functional limitation and mortality in the Health Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci 2017;72:284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 2013;24:822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robinson-Cohen C, Littman AJ, Duncan GE, et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol 2014;25:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bittel DC, Bittel AJ, Tuttle LJ, et al. Adipose tissue content, muscle performance and physical function in obese adults with type 2 diabetes mellitus and peripheral neuropathy. J Diabetes Complications 2015;29:250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andersen H, Nielsen S, Mogensen CE, Jakobsen J. Muscle strength in type 2 diabetes. Diabetes 2004;53:1543–1548 [DOI] [PubMed] [Google Scholar]
- 9. Diesel W, Noakes TD, Swanepoel C, Lambert M. Isokinetic muscle strength predicts maximum exercise tolerance in renal patients on chronic hemodialysis. Am J Kidney Dis 1990;16:109–114 [DOI] [PubMed] [Google Scholar]
- 10. Roshanravan B, Patel KV, Robinson-Cohen C, et al. Creatinine clearance, walking speed, and muscle atrophy: a cohort study. Am J Kidney Dis 2015;65:737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang XH, Mitch WE. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 2014;10:504–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marcus RL, LaStayo PC, Ikizler TA, et al. Low physical function in maintenance hemodialysis patients is independent of muscle mass and comorbidity. J Ren Nutr 2015;25:371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park SW, Goodpaster BH, Strotmeyer ES, et al.; Health, Aging, and Body Composition Study . Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the Health, Aging, and Body Composition Study. Diabetes Care 2007;30:1507–1512 [DOI] [PubMed] [Google Scholar]
- 14. Conley KE. Mitochondria to motion: optimizing oxidative phosphorylation to improve exercise performance. J Exp Biol 2016;219:243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi S, Reiter DA, Shardell M, et al. 31P Magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 2016;71:1638–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tamaki M, Miyashita K, Wakino S, Mitsuishi M, Hayashi K, Itoh H. Chronic kidney disease reduces muscle mitochondria and exercise endurance and its exacerbation by dietary protein through inactivation of pyruvate dehydrogenase. Kidney Int 2014;85:1330–1339 [DOI] [PubMed] [Google Scholar]
- 17. Conjard A, Ferrier B, Martin M, Caillette A, Carrier H, Baverel G. Effects of chronic renal failure on enzymes of energy metabolism in individual human muscle fibers. J Am Soc Nephrol 1995;6:68–74 [DOI] [PubMed] [Google Scholar]
- 18. Afshinnia F, Rajendiran TM, Soni T, et al.; Michigan Kidney Translational Core CPROBE Investigator Group . Impaired β-oxidation and altered complex lipid fatty acid partitioning with advancing CKD. J Am Soc Nephrol 2018;29:295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kestenbaum B, Gamboa J, Liu S, et al. Impaired skeletal muscle mitochondrial bioenergetics and physical performance in chronic kidney disease. JCI Insight 2020;5:e133289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siegel MP, Kruse SE, Knowels G, et al. Reduced coupling of oxidative phosphorylation in vivo precedes electron transport chain defects due to mild oxidative stress in mice. PLoS One 2011;6:e26963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kirn DR, Reid KF, Hau C, Phillips EM, Fielding RA. What is a clinically meaningful improvement in leg-extensor power for mobility-limited older adults? J Gerontol A Biol Sci Med Sci 2016;71:632–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pincivero DM, Lephart SM, Karunakara RA. Reliability and precision of isokinetic strength and muscular endurance for the quadriceps and hamstrings. Int J Sports Med 1997;18:113–117 [DOI] [PubMed] [Google Scholar]
- 23. Smith GI, Yoshino J, Kelly SC, et al. High-protein intake during weight loss therapy eliminates the weight-loss-induced improvement in insulin action in obese postmenopausal women. Cell Rep 2016;17:849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 25. Greggio C, Jha P, Kulkarni SS, et al. Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle. Cell Metab 2017;25:301–311 [DOI] [PubMed] [Google Scholar]
- 26. Rooney JP, Ryde IT, Sanders LH, et al. PCR based determination of mitochondrial DNA copy number in multiple species. Methods Mol Biol 2015;1241:23–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pestronk A, Keeling R, Choksi R. Sarcopenia, age, atrophy, and myopathy: mitochondrial oxidative enzyme activities. Muscle Nerve 2017;56:122–128 [DOI] [PubMed] [Google Scholar]
- 28. Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014;30:923–930 [DOI] [PubMed] [Google Scholar]
- 29. Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–273 [DOI] [PubMed] [Google Scholar]
- 30. Park SY, Gifford JR, Andtbacka RH, et al. Cardiac, skeletal, and smooth muscle mitochondrial respiration: are all mitochondria created equal? Am J Physiol Heart Circ Physiol 2014;307:H346–H352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rabinovich RA, Vilaró J. Structural and functional changes of peripheral muscles in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med 2010;16:123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vartak R, Deng J, Fang H, Bai Y. Redefining the roles of mitochondrial DNA-encoded subunits in respiratory Complex I assembly. Biochim Biophys Acta 2015;1852:1531–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Antoun G, McMurray F, Thrush AB, et al. Impaired mitochondrial oxidative phosphorylation and supercomplex assembly in rectus abdominis muscle of diabetic obese individuals. Diabetologia 2015;58:2861–2866 [DOI] [PubMed] [Google Scholar]
- 34. Watson EL, Baker LA, Wilkinson TJ, et al. Reductions in skeletal muscle mitochondrial mass are not restored following exercise training in patients with chronic kidney disease. FASEB J 2020;34:1755–1767 [DOI] [PubMed] [Google Scholar]
- 35. Yazdi PG, Moradi H, Yang JY, Wang PH, Vaziri ND. Skeletal muscle mitochondrial depletion and dysfunction in chronic kidney disease. Int J Clin Exp Med 2013;6:532–539 [PMC free article] [PubMed] [Google Scholar]
- 36. Pereira CV, Peralta S, Arguello T, Bacman SR, Diaz F, Moraes CT. Myopathy reversion in mice after restauration of mitochondrial complex I. EMBO Mol Med 2020;12:e10674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arena G, Cissé MY, Pyrdziak S, et al. Mitochondrial MDM2 regulates respiratory complex I activity independently of p53. Mol Cell 2018;69:594–609.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roshanravan B, Kestenbaum B, Gamboa J, et al. CKD and muscle mitochondrial energetics. Am J Kidney Dis 2016;68:658–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci 2013;68:447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal 2010;12:537–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Enoki Y, Watanabe H, Arake R, et al. Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J Cachexia Sarcopenia Muscle 2017;8:735–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci 2000;25:502–508 [DOI] [PubMed] [Google Scholar]
- 43. Geng J, Wei M, Yuan X, et al. TIGAR regulates mitochondrial functions through SIRT1-PGC1α pathway and translocation of TIGAR into mitochondria in skeletal muscle. FASEB J 2019;33:6082–6098 [DOI] [PubMed] [Google Scholar]
- 44. Choksi KB, Nuss JE, Deford JH, Papaconstantinou J. Age-related alterations in oxidatively damaged proteins of mouse skeletal muscle mitochondrial electron transport chain complexes. Free Radic Biol Med 2008;45:826–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fam HK, Choi K, Fougner L, Lim CJ, Boerkoel CF. Reactive oxygen species stress increases accumulation of tyrosyl-DNA phosphodiesterase 1 within mitochondria. Sci Rep 2018;8:4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cho E-C, Kuo M-L, Cheng J-H, et al. RRM2B-mediated regulation of mitochondrial activity and inflammation under oxidative stress. Mediators Inflamm 2015;2015:287345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ronchi D, Di Fonzo A, Lin W, et al. Mutations in DNA2 link progressive myopathy to mitochondrial DNA instability. Am J Hum Genet 2013;92:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Di Noia MA, Todisco S, Cirigliano A, et al. The human SLC25A33 and SLC25A36 genes of solute carrier family 25 encode two mitochondrial pyrimidine nucleotide transporters. J Biol Chem 2014;289:33137–33148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miró O, Marrades RM, Roca J, et al. Skeletal muscle mitochondrial function is preserved in young patients with chronic renal failure. Am J Kidney Dis 2002;39:1025–1031 [DOI] [PubMed] [Google Scholar]
- 50. Mercken EM, Capri M, Carboneau BA, et al. Conserved and species-specific molecular denominators in mammalian skeletal muscle aging. NPJ Aging Mech Dis 2017;3:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tryon LD, Vainshtein A, Memme JM, Crilly MJ, Hood DA. Recent advances in mitochondrial turnover during chronic muscle disuse. Integr Med Res 2014;3:161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hasson SA, Kane LA, Yamano K, et al. High-content genome-wide RNAi screens identify regulators of parkin upstream of mitophagy. Nature 2013;504:291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Protti A. Succinate and the shortcut to the cure of metformin-induced lactic acidosis. Intensive Care Med Exp 2018;6:35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Avula S, Parikh S, Demarest S, Kurz J, Gropman A. Treatment of mitochondrial disorders. Curr Treat Options Neurol 2014;16:292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tonkonogi M, Walsh B, Svensson M, Sahlin K. Mitochondrial function and antioxidative defence in human muscle: effects of endurance training and oxidative stress. J Physiol 2000;528:379–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 2015;47:1922–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ramel A, Arnarson A, Geirsdottir OG, Jonsson PV, Thorsdottir I. Glomerular filtration rate after a 12-wk resistance exercise program with post-exercise protein ingestion in community dwelling elderly. Nutrition 2013;29:719–723 [DOI] [PubMed] [Google Scholar]
- 58. Vanden Wyngaert K, Van Craenenbroeck AH, Van Biesen W, et al. The effects of aerobic exercise on eGFR, blood pressure and VO2peak in patients with chronic kidney disease stages 3-4: a systematic review and meta-analysis. PLoS One 2018;13:e0203662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Young DR, Hivert MF, Alhassan S, et al.; Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; and Stroke Council . Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation 2016;134:e262–e279 [DOI] [PubMed] [Google Scholar]
- 60. Jacques M, Kuang J, Bishop DJ, et al. Mitochondrial respiration variability and simulations in human skeletal muscle: the Gene SMART study. FASEB J 2020;34:2978–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]