Abstract
Proliferation of pancreatic β-cells has long been known to reach its peak in the neonatal stages and decline during adulthood. However, β-cell proliferation has been studied under the assumption that all β-cells constitute a single, homogenous population. It is unknown whether a subpopulation of β-cells retains the capacity to proliferate at a higher rate and thus contributes disproportionately to the maintenance of mature β-cell mass in adults. We therefore assessed the proliferative capacity and turnover potential of virgin β-cells, a novel population of immature β-cells found at the islet periphery. We demonstrate that virgin β-cells can proliferate but do so at rates similar to those of mature β-cells from the same islet under normal and challenged conditions. Virgin β-cell proliferation rates also conform to the age-dependent decline previously reported for β-cells at large. We further show that virgin β-cells represent a long-lived, stable subpopulation of β-cells with low turnover into mature β-cells under healthy conditions. Our observations indicate that virgin β-cells at the islet periphery can divide but do not contribute disproportionately to the maintenance of adult β-cell mass.
Introduction
More than 30 million Americans currently have diabetes, which results from progressive β-cell loss caused by either autoimmune attack or lifestyle and genetic factors (1). Because β-cells are the only cells in the body capable of secreting insulin, a major therapeutic goal for diabetes has been to replace β-cells lost during disease. Islet or whole-pancreas transplantation has had some success in achievement of insulin independence, but limited donor availability, immunological complications, and transplant survival make this process not a viable option for the majority of patients with diabetes (2). Thus, a great interest continues in strategies that promote the regeneration of β-cells from various cell sources (3,4). Despite these efforts, clinically meaningful restoration of β-cell mass has not yet been achieved, illustrating the ongoing need for strategies to regenerate β-cells.
During late pancreas development, β-cell mass expands rapidly by self-replication of young β-cells. Seminal experiments by Dor et al. (5) established, by pulse-chase labeling of β-cells, that self-replication is the main mechanism to maintain β-cell mass—a conclusion that is noncontroversial (6,7). However, β-cell proliferation declines rapidly with age in mice (8–10), with even lower proliferation rates observed in human islets (11), which complicates the challenge of restoring β-cell mass secondary to diabetes-associated β-cell loss. Against this backdrop, numerous reports of insulin-positive multipotent precursors (12,13), transdifferentiation of α- and δ-cells to β-cells (14–16), transdifferentiation of exocrine cells to β-cells (17,18), and, most recently, the existence of a population of protein C receptor (ProCr)-positive islet-resident stem cells (19) continue to raise the prospect that alternative paths to generation of β-cells exist and could be targeted in diabetes.
Adding to these potential alternative β-cell sources, we recently identified a new population of immature β-cells that we named virgin β-cells, as lineage-tracing studies revealed that these cells express insulin but have never expressed the maturity marker urocortin 3 (Ucn3) (20). Virgin β-cells occur exclusively near the islet periphery, which we therefore referred to as the neogenic niche. They constitute a stable fraction of ∼1.5% of all β-cells and lack key maturity markers in addition to Ucn3, such as G6pc2, MafA, and Ero1lb (20). Virgin β-cells also lack cell-surface expression of Glut2—a feature that they share with β-cells that occur in small clusters outside of conventional islets (21,22). As virgin β-cells share many of the features of immature β-cells during late pancreas development, this raises the possibility that virgin β-cells may possess a relatively high proliferation rate, similar to developing immature β-cells. However, the ability of virgin β-cells to proliferate, the rate at which they do, and their turnover potential all remain unknown.
In this study, we examined the proliferation capacity and the maturation potential of virgin β-cells at the neogenic niche using DNA synthesis labeling and pulse-chase lineage-tracing experiments. We hypothesized virgin β-cells to be more proliferative than mature β-cells based on their resemblance to immature β-cells during pancreas development. However, our data demonstrate that proliferating β-cells do not occur more frequently near the islet periphery but, instead, occur randomly across the cross-sectional surface of the islets. Similarly, when we specifically addressed the proliferation rate of virgin and mature β-cells, we observed that virgin β-cells proliferate but do so at rates similar to those of mature β-cells from the same islets under basal conditions and in response to metabolic challenges such as pregnancy or experimentally induced insulin resistance. Overall, it follows that virgin β-cells in adult islets represent a stable subset of β-cells that demonstrate limited turnover into mature β-cells.
Research Design and Methods
Animals
Mice were maintained in group housing on a 12-h light/12-h dark cycle with free access to water and standard rodent chow. C57BL/6 mice were purchased from Envigo. All mouse procedures were approved by the Institutional Animal Care and Use Committee of University of California, Davis (UC Davis), or the Salk Institute for Biological Studies, and were performed in compliance with the Animal Welfare Act and the Institute for Laboratory Animal Research Guide to the Care and Use of Laboratory Animals.
Administration of 5-Ethynyl-2′-Deoxyuridine and S961
Mice were injected once daily with 5-ethynyl-2′-deoxyuridine (EdU) (Life Technologies) intraperitoneally at 50 mg/kg body wt dissolved at 10 mg/mL in 0.9% saline as indicated. EdU was prepared fresh right before administration. S961 (NNC0069-0961) was generously provided by Novo Nordisk Compound Sharing. Osmotic pumps (Alzet 2001) filled with 10 nmol S961 or PBS only, released at an estimated dose of 0.05 nmol/h, were implanted subcutaneously.
Glucose Tolerance Test
Mice were weighed after an overnight fast and then given a bolus of 2 g/kg dextrose (D9559; Sigma-Aldrich) via intraperitoneal injection. Plasma glucose levels were collected over a 2-h time period with use of tail vein blood with a OneTouch Ultra glucometer.
Islet Isolation
Islets were isolated by injection of 2 mL of 0.8 mg/mL collagenase P (Invitrogen) dissolved in Hanks’ balanced salt solution (HBSS) (Roche Diagnostics) into the pancreas via the common bile duct while the ampulla of Vater was clamped. The injected pancreas was then collected in an additional 2 mL collagenase, incubated at 37°C for 11 min, and dissociated by gentle manual shaking followed by three washes with cold HBSS containing 5% newborn calf serum (NCS). The suspension was passed through a nylon mesh (pore size 425 mm) (Small Parts, Inc.), and islets were isolated by density gradient centrifugation on a Histopaque gradient (1.077 g/mL) (Sigma-Aldrich) for 20 min at 1,400g without brake. Islets were collected from the interface, washed once with cold HBSS containing 5% NCS, and handpicked several times under a dissecting microscope prior to culture in RPMI medium (Roche Diagnostics) containing 5.5 mmol/L glucose, 10% FBS, and penicillin-streptomycin (Gibco).
Flow Cytometry
mIns1-H2b-mCherry × Ucn3-EGFP islets were isolated as described a day prior to dissociation by incubation in 0.25% trypsin-EDTA (Gibco) for 2 min complemented by gentle trituration with a p200 pipette, washed once in HBSS (Roche Diagnostics) containing 5% NCS, and then stained for EdU detection with use of a modified Click-iT Plus EdU Flow Cytometry Assay Kit (C10634; Thermo Fisher Scientific) following the manufacturer’s guidelines and immediately processed on the cytometer. All flow data were collected with the Beckman Coulter CytoFLEX except for the 9-month-old cohort in Fig. 3C due to unexpected technical issues with the CytoFLEX; a Beckman Coulter Astrios EQ equivalent to the CytoFLEX was used for this data collection.
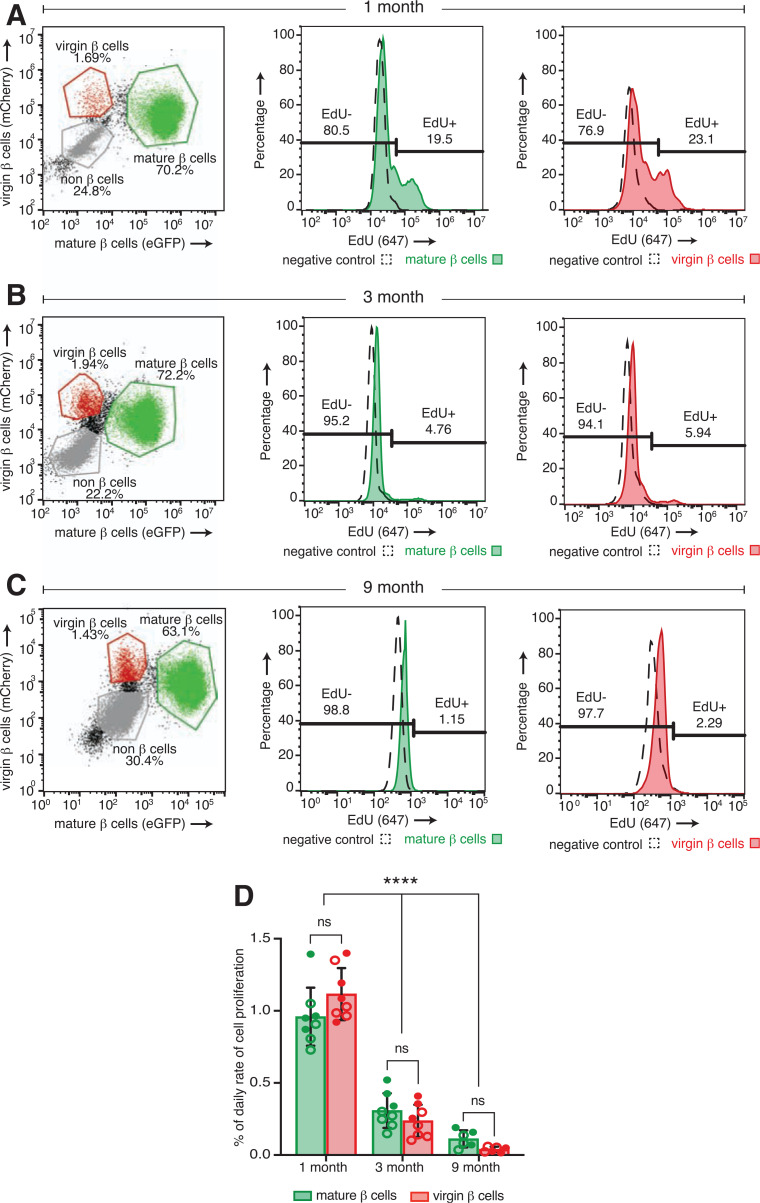
Figure 3.
Virgin β-cell proliferation declines with age at rates similar to those of mature β-cells from the same islet. Flow cytometry strategy to identify and distinguish between mCherry and EGFP copositive mature β-cells, mCherry single-positive virgin β-cells, and all other non-β cells from islets of a representative individual mIns1-H2b-mCherry × Ucn3-EGFP mouse after dissociation into single cell suspensions at 1 month (A), 3 months (B), and 9 months (C) with histogram plots of flow data illustrating the percentage of EdU-positive and EdU-negative virgin and mature β-cells. D: Quantification of the daily proliferation rate of mature (green bars) and virgin (red bars) β-cells across different time points (n ≥ 6 mice per group). Daily proliferation rate was calculated by division of the percentage of EdU-positive mature or virgin β-cells by the labeling period (14 days) and expressed as a percentage total per day (formula adapted from 9). Open circles, females; closed circles, males. Data were analyzed for statistical significance by two-way ANOVA, corrected for multiple comparisons with the Tukey method. Error bars represent ±SD. ns, not significant.
Three-dimensional Imaging of Intact Islets
Islets isolated from mIns1-H2b-mCherry × Ucn3-EGFP mice that received EdU in vivo were cultured on uncoated no. 1.5 glass-bottom 35-mm culture dishes (MatTek Corporation) in RPMI medium (Roche Diagnostics) containing 10% FBS, 5.5 mmol/L glucose, and penicillin-streptomycin. Islets were allowed to attach onto the glass bottom for two nights before fixation with 4% paraformaldehyde (EMD Millipore) for 15 min followed by EdU staining with Click-iT Plus EdU Cell Proliferation Kit (C10640; Thermo Fisher Scientific) according to the manufacturer’s guidelines and then imaged in x, y, and z on a Nikon A1R+ confocal microscope.
Pulse-Chase of mIns-CreER Mice
mIns-CreER × lsl-mT/mG mice were treated by oral gavage with 125 mg/kg body wt tamoxifen (T5648; Sigma-Aldrich) dissolved at 20 mg/mL in sunflower oil. Pancreata were collected at euthanasia, fixed with 4% paraformaldehyde for 5 h on ice, protected in 30% sucrose for 24 h at 4°C, embedded in O.C.T. Compound (Fisher Healthcare), and processed at 14-μm thickness with a Leica CM3050 S Cryostat.
Immunofluorescence
Immunofluorescence was conducted as follows: slides were washed three times for 5 min each in a potassium-based phosphate-buffered saline (KPBS) and then incubated with primary antibodies diluted in donkey block (KPBS supplemented with 2% donkey serum and 0.4% Triton X-100) overnight at 4°C. Slides were then washed three more times in KPBS and incubated with secondary antibodies (Jackson ImmunoResearch) used at a 1:600 dilution in donkey block for 45 min at room temperature. Slides were washed three more times before counterstaining with DAPI where applicable and embedding in ProLong Gold Antifade (Thermo Fisher Scientific). Images were captured with a Nikon A1R+ confocal microscope or an automated Keyence microscope for the data presented in Fig. 1.
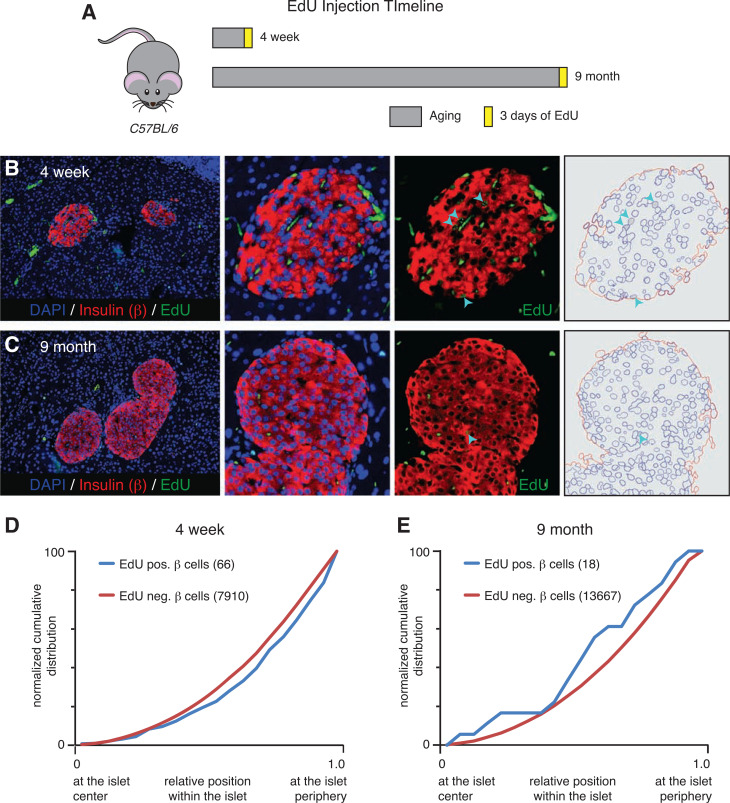
Figure 1.
Proliferating EdU-positive β-cells are randomly distributed throughout the islet. A: Schematic of EdU injection timeline of C57BL/6 mice. Representative immunofluorescence images of pancreatic tissue sections from 4-week-old (B) and 9-month-old (C) mice stained with insulin (red), EdU (green), and DAPI (blue). Arrowheads indicate examples of β-cells that are positive for EdU labeling detection. D: Normalized cumulative distribution of EdU-positive (blue) and EdU-negative (red) β-cells within 4-week-old mouse islets. E: Distribution of EdU-positive (blue) and EdU-negative (red) β-cells within 9-month-old mouse islets. For each distribution graph, n ≥ 3 mice with eight to nine islets counted per animal. neg., negative; pos., positive.
Primary antibodies include guinea pig anti-insulin (cat. no. A0564, 1:500; Dako), rat anti-insulin (MAB1417, 1:500; R&D Systems), guinea pig anti-Ucn3 (044, 1:1,000) and rabbit anti-Ucn3 (7218, 1:1,000) (gifts from Dr. Wylie Vale, Salk Institute for Biological Studies) (23), and rabbit anti-glucagon (2760S, 1:400; Cell Signaling Technology). Click-iT Plus EdU Cell Proliferation Kit (C10637; Thermo Fisher Scientific) was used for EdU detection in pancreatic tissue sections.
Cell Counting and Statistical Analysis
Images were analyzed and manually counted with Fiji/ImageJ. The number of biological replicates (n), measure of central tendency (average, percentage), error bars, and statistical analyses performed with GraphPad Prism, version 8.0, are explained in the figure legends. Differences were considered significant when P < 0.05.
Data and Resource Availability
All data generated or analyzed during this study are included in aggregate in the published article. The underlying data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Results
Proliferating β-Cells Are Randomly Distributed Across the Islet
Because of the preferential location of virgin β-cells at the islet periphery combined with their phenotypic similarities with young β-cells that proliferate relatively fast, we were interested in assessing the spatial distribution of proliferating β-cells within the islet. To do so, we treated eight C57BL/6 mice with the thymidine analog EdU at 4 weeks (n = 3) and 9 months (n = 5) for 3 days before collection (Fig. 1A). The pancreas of each animal was then stained for EdU and insulin to mark all proliferating β-cells (Fig. 1B and C). Next, we quantified the relative position of ∼22,000 individual β-cells from both age-groups and normalized for islet size, a method we previously established using a custom algorithm we developed for this purpose (20). The identity of each β-cell was manually assigned. The normalized cumulative distribution of EdU-positive and EdU-negative β-cells revealed no preferential distribution of dividing β-cells across the islet (Fig. 1D and E), suggesting that proliferating β-cells do not preferentially occur at any particular location within the islet. The spatial location of individual β-cells does not influence their ability to proliferate, which builds on previous observation that all β-cells are homogenous with respect to their proliferative capacity (24).
Virgin β-Cells Proliferate at Rates Similar to Those of Mature β-Cells
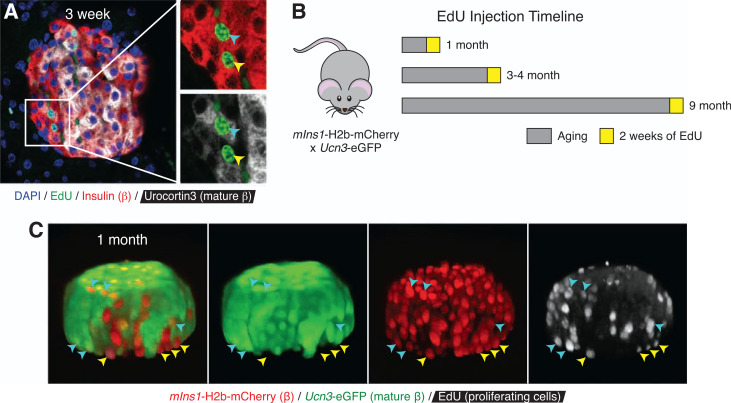
While our initial EdU experiment did not reveal an accumulation of dividing β-cells at the islet periphery where virgin β-cells are found, it also did not directly differentiate between mature and virgin β-cells. We therefore immunostained pancreatic sections of 3-week-old wild-type mice (n = 2) treated with EdU for 3 days before collection. Staining with insulin and Ucn3 allowed us to distinguish between virgin and mature β-cells. EdU-positive virgin β-cells, as shown by their lack of Ucn3 expression and location at the islet edge, were readily detectable in these islets (Fig. 2A). To confirm this independently of immunofluorescence and to overcome the challenges of detecting a relative rare event (β-cell proliferation) in the relatively small virgin β-cell population, we developed a novel approach to quantify β-cell proliferation in a rigorous, quantitative, and high-throughput manner using flow cytometry. Flow cytometry also has the added benefit of avoiding potential biases in detecting proliferating β-cells that come with the detection of β-cell proliferation by immunofluorescence. To achieve this, we crossed mIns1-H2b-mCherry (25) with Ucn3-EGFP reporter mice (20). In bitransgenic offspring of this cross, virgin β-cells are detected as mCherry single-positive β-cells at the islet periphery, while mature β-cells are double positive for EGFP and mCherry (20). This unique mouse model allowed us to quantify whether virgin β-cells proliferate at a rate different from that of mature β-cells from the same islets at a given age. We treated 22 mIns1-H2b-mCherry × Ucn3-EGFP mice at 4 weeks (n = 8), 3–4 months (n = 8), and 9 months (n = 6) with EdU for 2 weeks before collection (Fig. 2B). Visualization of whole islets by confocal microscopy in three dimensions following EdU detection readily revealed multiple mCherry single-positive virgin β-cells and mCherry and EGFP double-positive mature β-cells with nuclear EdU label (Fig. 2C), demonstrating that EdU detection effectively marks proliferating virgin and mature β-cells. To quantify the fraction of EdU-positive and EdU-negative virgin and mature β-cells, we isolated and dissociated the islets at the end of the in vivo EdU labeling, followed by EdU detection and quantification of all β-cells from individual mice by flow cytometry (Fig. 3A–C). Our flow data demonstrated that virgin β-cells proliferate at rates similar to those of mature β-cells from the same islets at 4 weeks, 3 months, and 9 months, and at rates that declined with age (Fig. 3D), in line with previous observations for β-cell proliferation in general (8–10).
Figure 2.
Virgin β-cells can proliferate. A: Two-dimensional representative image of a 3-week-old wild-type mouse islet immunostained with insulin (red), Ucn3 (white), EdU (green), and DAPI (blue). The cyan arrowhead points to a proliferating EdU-positive mature β-cell that expresses Ucn3, while the yellow arrowhead points to a proliferating EdU-positive virgin β-cell that lacks Ucn3 expression. B: Schematic of EdU injection timeline of mIns-H2b-mCherry × Ucn3-EGFP mice. C: Three-dimensional representative image of a 1-month-old mIns-H2b-mCherry × Ucn3-EGFP mouse islet stained for EdU (white) detection. Yellow arrowheads indicate mCherry single-positive dividing virgin β-cells. Cyan arrowheads indicate mCherry and EGFP double-positive dividing mature β-cells.
Virgin β-Cell Proliferation Rates Increase Upon Metabolic Challenge
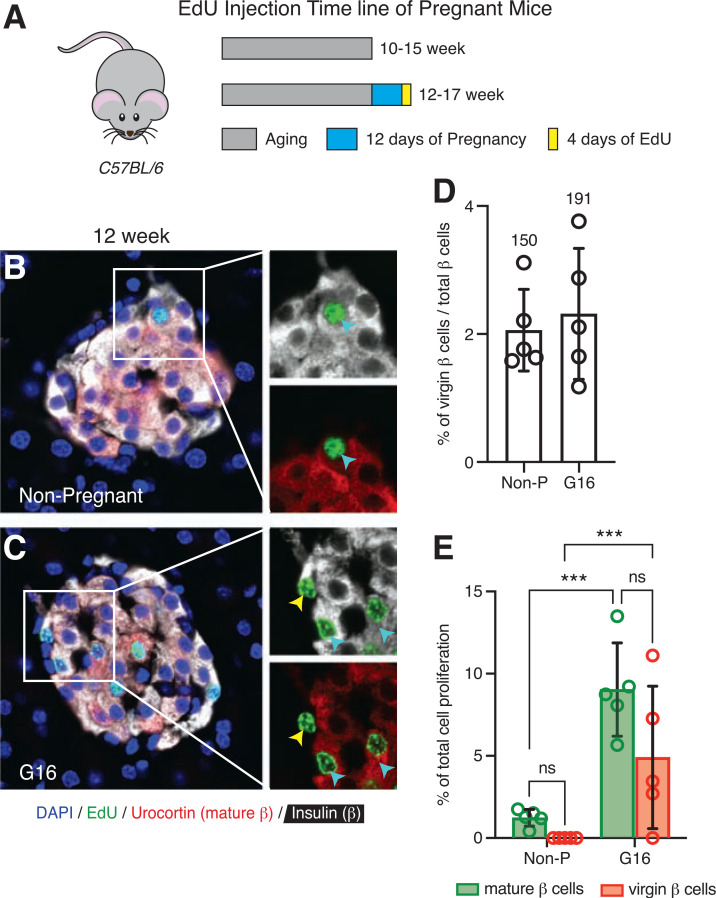
We next investigated the proliferation rates of virgin and mature β-cells under challenged conditions, specifically, during pregnancy and in face of insulin resistance. These are conditions where mouse β-cells are known to expand via self-proliferation (26,27). We wanted to determine whether virgin β-cells would also increase their proliferation and contribute equally as mature β-cells during these challenged circumstances in order to meet the increased demand for insulin. Pregnant C57BL/6 mice (n = 5) were injected with EdU during gestational days 12–16 and then collected and sectioned for histological analyses (Fig. 4A). Proliferating virgin and mature β-cells identified by staining for insulin, Ucn3, and EdU were detected in higher numbers in islets of pregnant compared with nonpregnant mice (Fig. 4B). In addition, the number of EdU-positive virgin and mature β-cells were not different in pregnant islets, which suggests that the proliferation rates of these two β-cell subtypes are similar during pregnancy (Fig. 4C).
Figure 4.
Virgin β-cell proliferation increases similarly to mature β-cell proliferation during pregnancy. A: Schematic of EdU administration timeline of pregnant C57BL/6 female mice. Representative images of islets from a 12-week-old nonpregnant (Non-P) mouse (B) and a gestational-day-16 (G16) pregnant mouse (C) immunostained with insulin (white), Ucn3 (red), EdU (green), and DAPI (blue). The cyan arrowheads point to proliferating EdU-positive mature β-cells, and the yellow arrowhead points to a proliferating EdU-positive virgin β-cell. D: Quantification of the fraction of virgin β-cells between nonpregnant and G16 pregnant mice (n = 5 per group). Values above each bar indicate the total number of virgin β-cells counted per group. E: Quantification of the total percentage of EdU-positive mature (green bars) and virgin (red bars) β-cells between nonpregnant and G16 pregnant mice over 4 days of EdU labeling period (n = 5 per group). Data were analyzed for statistical significance by two-way ANOVA, corrected for multiple comparisons with the Tukey method. Error bars represent ±SD. ***P < 0.001. ns, not significant.
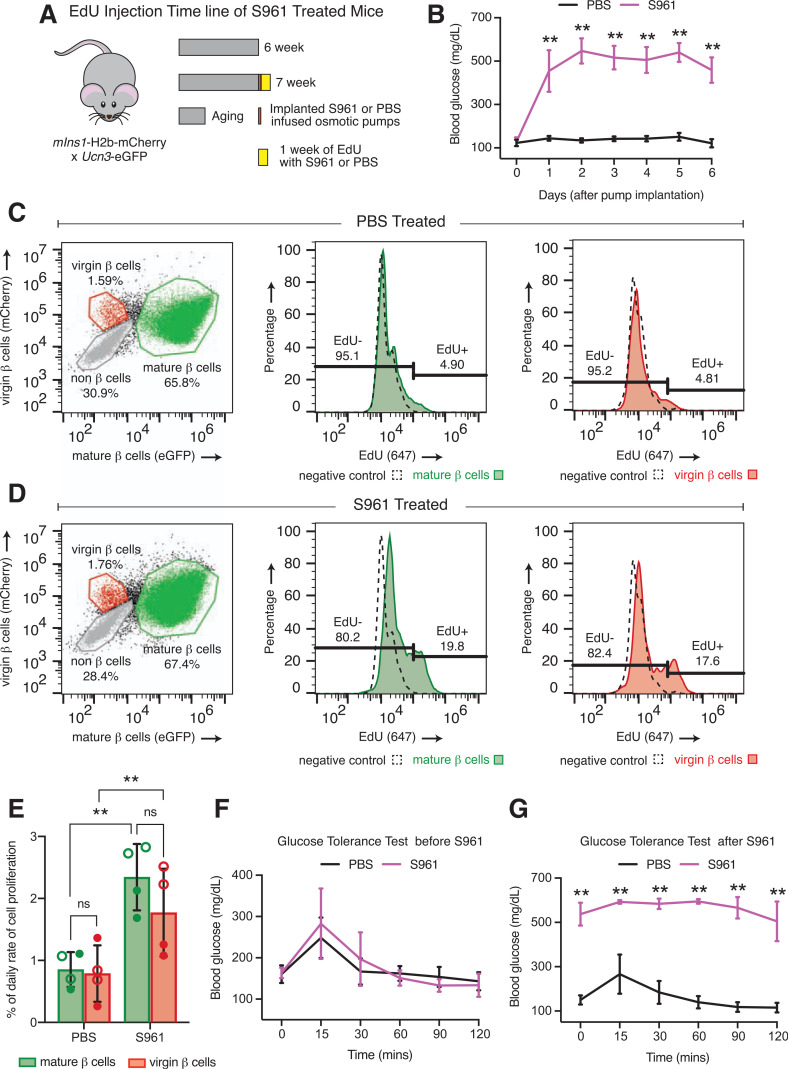
Separately, to determine the proliferation rates of virgin and mature β-cells in the face of acute insulin resistance, we treated eight mIns1-H2b-mCherry × Ucn3-EGFP mice at 6 weeks with either PBS (n = 4) or S961 (n = 4), an insulin receptor antagonist (28), with EdU for 7 days (Fig. 5A). Blood glucose was higher in S961-treated mice than in those treated with PBS after 24 h of S961 infusion as expected (Fig. 5B). Additionally, a glucose tolerance test 2 days prior to S961 treatment showed no difference in blood glucose (Fig. 5F), while a glucose tolerance test 2 days before the end of treatment showed significant difference between PBS- and S961-treated mice (Fig. 5G). Flow analysis of dissociated EdU-positive and EdU-negative virgin and mature β-cells demonstrated that virgin β-cells significantly increased their daily proliferation rate in response to S961 compared with PBS-treated islets—just like mature β-cells. However, the proliferation rates of mature and virgin β-cells did not differ in response to either treatment (Fig. 5C–E). Altogether, our findings indicate that virgin β-cells increase their proliferation rate and, in comparison with mature β-cells, contribute equally to meet the heightened demand for insulin created by pregnancy or insulin resistance in mice.
Figure 5.
Virgin β-cell proliferation increases similarly to mature β-cell proliferation in response to insulin resistance. A: Schematic of EdU administration timeline of PBS- or S961-treated mIns-H2b-mCherry × Ucn3-EGFP mice. B: Daily blood glucose measurement of random-fed PBS- and S961-treated mice. Dot and histogram plots of flow data illustrating the percentage of EdU-positive and EdU-negative virgin and mature β-cells of PBS-treated (C) and S961-treated (D) mice. E: Quantification of the daily proliferation rate of mature (green bars) and virgin (red bars) β-cells between PBS- and S961-treated mice (n = 4 per group). Open circles, females; closed circles, males. Data were analyzed by two-way ANOVA corrected for multiple comparisons with the Tukey method for statistical significance. Glucose tolerance test before S961 treatment (F) and after S961 treatment (G). Data were analyzed by Mann-Whitney test for statistical significance. All error bars represent ± SD. **P < 0.01. mins, minutes; ns, not significant.
Virgin β-Cells Are a Stable, Slowly Maturing Subset of β-Cells
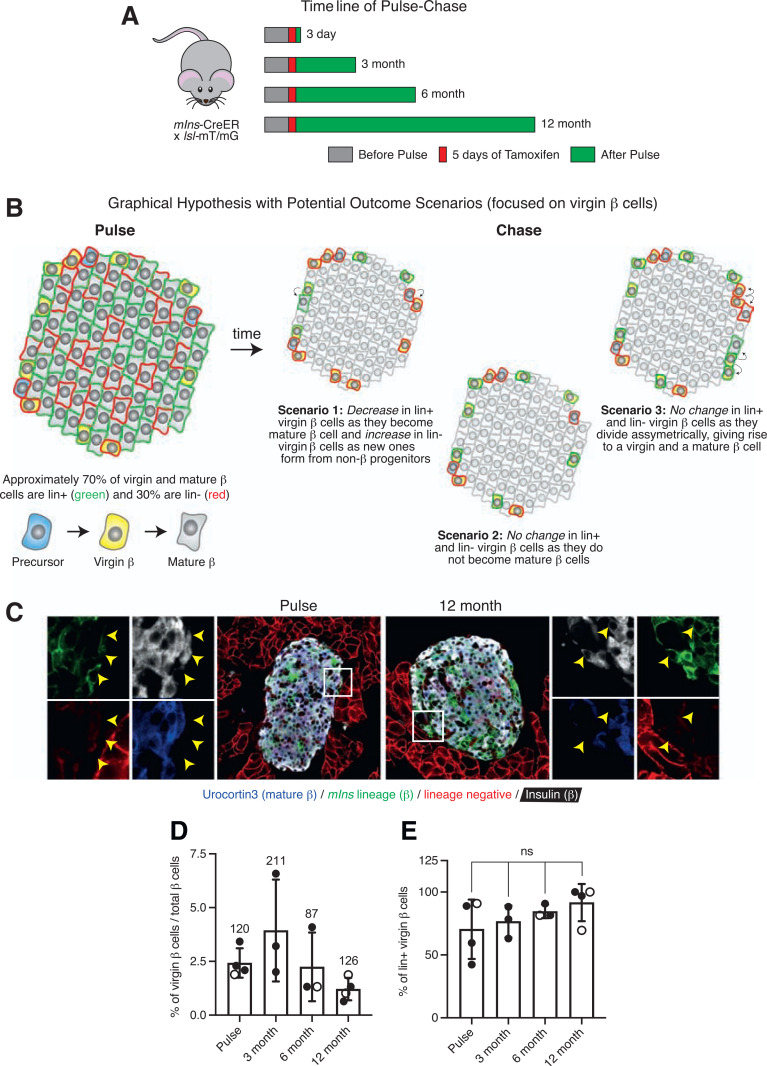
We were next interested in determining the maturation potential of virgin β-cells. To address this question, we performed a pulse-chase experiment where we labeled both mature and virgin β-cells by crossing mIns-CreER (29) to the lsl-mT/mG reporter mouse (30) that switches from membrane-tdTomato (mT) (lineage negative) to membrane-EGFP (mG) (lineage positive) upon inducible Cre expression with tamoxifen. We treated 14 bitransgenic mIns-CreER × lsl-mT/mG mice with tamoxifen once daily for five consecutive days at 4 weeks. We purposefully used oral gavage to administer tamoxifen over intraperitoneal or subcutaneous injections, as these routes of administration can lead to depot formation not conducive to pulse-chase studies (31,32). We collected pancreata for histology 3 days (n = 4) after the last tamoxifen gavage for the pulse cohort or after 3 months (n = 3), 6 months (n = 3), and 12 months (n = 4) for the chase cohorts (Fig. 6A). Lineage-positive virgin and mature β-cells distributed over the islet as previously reported (20) (Fig. 8C). We hypothesized that if virgin β-cells turn into mature β-cells over time, the fraction of lineage-positive virgin β-cells would decrease with time and the fraction of lineage-negative virgin β-cells would increase as new virgin β-cells form from non-β progenitors (Fig. 6B, scenario 1). However, if no maturation occurred within the 12-month chase period, then the fraction of lineage-positive virgin β-cells would remain constant (Fig. 6B, scenario 2). Alternatively, this same outcome could also occur with a scenario where virgin β-cells represent a resident insulin-positive progenitor that self-renews via asymmetric cell division (33) (Fig. 6B, scenario 3). Virgin and mature β-cells were identified based on insulin and Ucn3 expression. Lineage-positive and lineage-negative β-cells were determined by the expression of mG and mT, respectively (Fig. 6C). Virgin β-cells were found at their distinct location near the islet periphery and at the expected average ratio of 1.5–2.0% of all β-cells (Fig. 6D). Quantitative comparison between the fraction of lineage-positive virgin β-cells across the pulse and various chase cohorts showed no differences in the number of lineage-positive virgin β-cells over time (Fig. 6E). This result reflects an outcome consistent with scenarios 2 and 3 (Fig. 6B).
Figure 6.
Virgin β-cells represent a stable, slowly maturing subpopulation of β-cells. A: Schematic of tamoxifen gavage timeline of mIns-CreER × lsl-mT/mG mice for pulse-chase experiment. B: Graphical hypothesis illustrating three possible outcomes from pulse-chase experiment with a focus on the changes in the fraction of lineage-labeled virgin β-cells. In scenario 1, the fraction of lineage-positive virgin β-cells decreases as these cells develop into mature β-cells and the fraction of lineage-negative virgin β-cells increases as new virgin β-cells form from non-β cells (precursors, α-cells, δ-cells, ductal cells, etc.). In scenario 2, virgin β-cells do not mature within the 12-month chase period and thus the fraction of lineage-labeled virgin β-cells remains the same. In scenario 3, the fraction of lineage-labeled virgin β-cells also stays the same, as virgin β-cells act stem cell–like and divide asymmetrically, self-renewing the virgin β-cell pool while yielding daughter cells that mature. C: Representative immunofluorescence images of islets from a mouse in the pulse cohort and a mouse in the 12-month chase cohort stained with insulin (blue) and Ucn3 (white) to discern between virgin and mature β-cells. D: Quantification of the fraction of virgin β-cells across pulse and chase (3, 6, and 12 month) cohorts (n ≥ 3 mice per group). Values above each bar indicate the total number of virgin β-cells counted per cohort. E: Quantification of the average percentage of lineage-labeled virgin β-cells among pulse and chase cohorts (n ≥ 3 mice per group). Open circles, females; closed circles, males. Data were analyzed for statistical significance by Kruskal-Wallis test, corrected for multiple comparisons with the Dunn test. Error bars represent ±SD. ns, not significant.
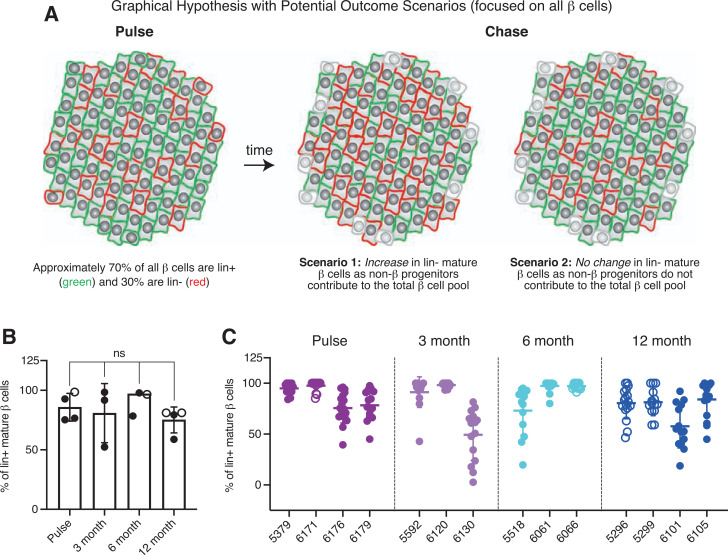
Figure 8.
No detectable contribution of non-β precursors to the maintenance of mature β-cells in adult mice. A: Graphical hypothesis illustrating two possible outcomes for the pulse-chase experiment with a focus on the changes in overall (mature) β-cells. In scenario 1, non-β progenitors contribute to the total pool of β-cells, leading to a gradual increase in the fraction of lineage-negative mature β-cells over time. In scenario 2, non-β progenitors do not contribute to the total pool of β-cells and thus the fraction of lineage-negative mature β-cells remain the same. B: Quantification of the percentage of lineage-positive mature β-cells among pulse and chase cohorts (n ≥ 3 mice per group). C: Distribution of lineage-positive mature β-cells from each individual mouse in the pulse and chase cohorts (n ≥ 3 mice per group, with at least 15 islets and a total of >20 virgin and 800 mature β-cells counted per animal). Open circles, females; closed circles, males. Data were analyzed for statistical significance by Kruskal-Wallis test, corrected for multiple comparisons with the Dunn test. Error bars represent ±SD. ns, not significant.
Virgin β-Cells Persist in Geriatric Mouse Islets
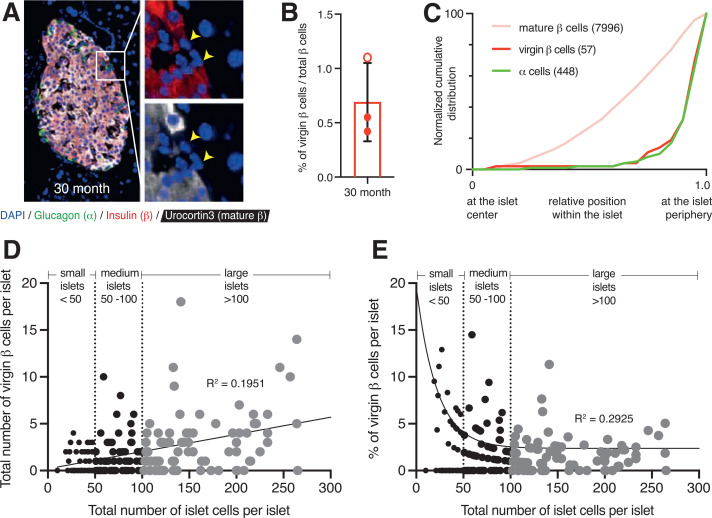
Our pulse-chase experiment result suggests that virgin β-cells constitute a long-lived subpopulation of β-cells. We were therefore interested in determining how long-lived virgin β-cells are. Previously, we reported the presence of virgin β-cells from 3 weeks to 14 months of age in mice (20). To determine whether virgin β-cells persist in islets of geriatric mice, we quantified the fraction of virgin β-cells in wild-type 30-month-old mouse islets (n = 3). Pancreatic sections were stained with insulin and Ucn3 to mark virgin and mature β-cells (Fig. 7A). Ucn3-negative virgin β-cells were readily detected in these geriatric mouse islets at a slightly lower fraction (Fig. 7B) compared with the expected average ratio of 1.5–2.0% of all β-cells in adult mice <1 year old (Fig. 3A–C) but still localized at the islet periphery (Fig. 7C). This supports the notion that virgin β-cells represent a long-lived subset of β-cells that persists in mice up to 30 months of age.
Figure 7.
Virgin β-cells persist in 30-month-old geriatric mouse islets at their distinct location in the islet periphery and correlate positively with islet size. A: Two-dimensional representative image of a 30-month-old wild-type mouse islet immunostained with glucagon (green), insulin (red), Ucn3 (white), and DAPI (blue). The yellow arrowheads point to individual virgin β-cells that lack Ucn3 expression. B: Quantification of the fraction of virgin β-cells from 30-month-old geriatric mice. Open circles, females; closed circles, males. C: Normalized cumulative distribution of α-cells (green), virgin β-cells (red), and mature β-cells (pink) within 30-month-old mouse islets (n = 3 mice with at least 10 islets counted per animal). D: Scatter plot illustrating the distribution of total virgin β-cells in relation to islet size (measured by total β- and α-cells). Each dot represents an islet (n = 193). Relationship analyses performed using Spearman correlation and simple linear regression yielded a positive correlation (r = 0.40, R2 = 0.1951). E: Scatter plot illustrating the distribution of the percentage of virgin β-cells (measured over total β-cells) in relation to islet size. Each dot represents an islet (n = 193). Relationship analyses using Spearman correlation and nonlinear regression yielded an inverse correlation (r = −0.39; R2 = 0.2925). Islets with no virgin β-cells were excluded from this analysis.
Virgin β-Cell Number Correlates with Islet Size
We also wanted to assess whether virgin β-cells occurred equally across all islets or are more prevalent in islets of a certain size, particularly as smaller islets or clusters of β-cells have been suggested to arise by neogenesis and might therefore disproportionally contain younger β-cells (34,35). To address this, we reanalyzed a previously published data set of almost 200 islets from animals of different age-groups. We categorized all of the islets measured by total α- and β-cells per cross-sectional area as small (<50 cells), medium (50–100 cells), and large (>100 cells) and plotted virgin β-cell number against total islet cell number for each islet. Based on our distribution analysis, we observed a positive correlation (r = 0.40; R2 = 0.1951) between the number of virgin β-cells and islet size, which reflects the fact that the chance of observing a virgin β-cell in an islet cross section increases when the islet is larger (Fig. 7D). However, the relative fraction of virgin versus mature β-cells is increased in smaller islets (Fig. 7E). This inverse correlation (r = −0.39; R2 = 0.2925) most likely reflects the fact that smaller islets have a larger surface-to-volume ratio and therefore contain a disproportionate fraction of virgin β-cells because of where these cells are preferentially located across the islet.
Limited Contributions of Non-β Progenitors to the Adult β-Cell Mass
Lastly, we also analyzed our pulse-chase study (Fig. 6A) with a focus on the mature β-cell pool without special regard for virgin β-cells. This enabled us to address the question of whether non-β progenitors contribute to the total β-cell pool over time in healthy adult mice. We reasoned that this was important and valuable, as in essence, the experiment we conducted to determine the turnover of virgin β-cells was also a repeat of the seminal lineage-tracing experiment performed by Dor et al. (5) but with the important distinction that the 70% lineage-labeling efficiency of the mIns-CreER (29) driver that we used is markedly higher than the 30% efficiency of the Rip-CreER (36) driver used in the original experiment. This then would have increased power to detect a minor contribution of non-β progenitors to β-cell mass. We hypothesized that in the event of a contribution from non-β cells to the maintenance of the β-cell pool, the fraction of lineage-positive mature β-cells would diminish over time (Fig. 8A, scenario 1), while in the absence of such contribution, the fraction of lineage-positive β-cells would remain constant (Fig. 8A, scenario 2). Upon quantitative comparison between the pulse and chase cohorts, we observed no dilution of the fraction of lineage-positive mature β-cells over the 12-month duration of our study (Fig. 8B and C). This result is consistent with scenario 2 (Fig. 8A) and with the original observations made by Dor et al. (5) but now repeated with a β-cell–specific CreER driver with a higher lineage-labeling efficiency.
Discussion
Here, we characterized the proliferation capacity and maturation potential of virgin β-cells, a novel population of immature β-cells located at a spatially distinct neogenic niche near the islet periphery (20). First, we showed that proliferating β-cells are located randomly across the islets and do not disproportionately occur at the islet periphery (Fig. 1). Consistent with these data, we then demonstrated that virgin β-cells are able to proliferate (Fig. 2) and do so at rates similar to those of mature β-cells from the same islet at all of the ages we investigated (Fig. 3). The daily proliferation rate of virgin β-cells also followed the age-dependent decline that was previously observed by others in β-cell proliferation rate (Fig. 3). We also demonstrated that the proliferation rate of virgin β-cells can be stimulated during pregnancy (Fig. 4) or upon experimentally induced insulin resistance (Fig. 5) but that their proliferation rates in any of these conditions remain statistically indistinguishable from those of mature β-cells.
Next, we showed that the proportion of lineage-positive virgin β-cells remains constant over a relatively large chase period of a year (Fig. 6), indicating that virgin β-cells constitute a stable subpopulation (Fig. 7) that does not undergo rapid turnover to contribute to the maintenance of adult β-cell mass (Fig. 6B, scenario 2). However, based on our lineage-tracing experiment, we cannot rule out a scenario where virgin β-cells constitute a resident stem-cell like progenitor that contributes and self-renews through asymmetric cell division (Fig. 6B, scenario 3). In addition, because our results focused only on physiological condition with use of healthy mice, we did not account for the possibility that virgin β-cells might be unable to mature due to the lack of important transcription factors such as MafA and the continued presence of other factors like MafB, although we know that the lack of Ucn3 itself does not prevent β-cells from maturing (37). Because of the apparent absence of virgin β-cell maturation over an extensive chase period, the lineage-positive virgin β-cells we observed in our chase cohorts were likely already present during the pulse at 4 weeks and therefore likely represent a long-lived, stable subpopulation of β-cells formed in the perinatal period of life. Isotope-labeling experiments (38) should be able to confirm that virgin β-cells at the islet periphery of older mice represent long-lived β-cells that never fully matured.
Although we initially set out to determine the maturation potential of only virgin β-cells, we lineage-labeled all β-cells using mIns-CreER in our pulse-chase experiment. This thus allowed us to also assess the potential contribution of non-β cells to the β-cell pool over time. In doing so, we did not detect a significant dilution of the pool of lineage-positive mature β-cells (Fig. 8). Our finding is in line with the study conducted by Dor et al. (5), which concluded that self-replication is the major mechanism responsible for the maintenance of β-cell mass, with the important distinction that the β-cell–specific CreER driver that we used here has higher efficiency and is therefore more likely to detect a minor contribution from non-β progenitors to adult β-cell mass (Fig. 8A, scenario 2). This observation contrasts with reports of β-cell transdifferentiation and the presence of potential islet-resident precursors capable of generating new β-cells put forth by some, including ourselves (14,16,19,20). Some studies show lack of β-cell regeneration from any source, including a β or non-β progenitor population after streptozotocin treatment. In contrast, others demonstrate recovery from streptozotocin-mediated diabetes in mice upon engraftment with organoids formed from presumed ProCr-positive islet precursor cells (19). However, our lineage-tracing data did not detect a contribution of such non-β islet-resident progenitors to the mature β-cell pool in healthy mice. These discrepancies in observations highlight the complexity of the cellular cues that may be necessary to elicit a response from facultative stem cells and progenitors under physiological conditions. In conclusion, our observations support the paradigm that β-cell self-replication is the primary source of adult β-cells in mice, with no disproportionate contribution from virgin β-cells. This leaves open the questions of the importance of this distinct and readily identifiable virgin β-cell subpopulation to overall β-cell fate and function and why they locate so distinctly to the islet periphery.
Limitations of Study
Mindful of the potential confounds, such as increased β-cell mass due to the presence of a human growth hormone minigene in the mIns-CreER mouse (39), we only used mice that were all transgenic for mIns-CreER, thus eliminating any confounds of the differential presence of mIns-CreER and its associated transgenic elements. The ideal method to study the turnover potential of virgin β-cells would have been to use a virgin β-cell–specific Cre driver to selectively lineage label only these cells and follow them over time. However, such a tool does not currently exist. Instead, we made use of the best experimental approach available to address this important question. Our data lead us to conclude that there is no detectable contribution from non-β cell progenitors, including virgin β-cells, to the β-cell mass in adult mice under physiological conditions. But, because the labeling of the mIns-CreER driver is 70% efficient, this leaves us underpowered to definitively rule out a minor contribution from potential non-β progenitors to the pool of total β-cells. In addition, our study did not distinguish between virgin and dedifferentiated β-cells, which also lack Ucn3 expression, although the latter are not restricted to the islet periphery in contrast to virgin β-cells. A formal distinction between virgin and dedifferentiated β-cells would have required a lineage trace using the Ucn3-Cre driver, which is not compatible with the use of mIns-CreER in our pulse-chase experiment.
Article Information
Acknowledgments. The authors thank UC Davis Huising Lab members Riya Bansal for help with the manual quantification of cells in geriatric mice and Jessica L. Huang for help with the data presentation in Fig. 1B and C. The authors are also grateful to Bridget McLaughlin and Jonathan van Dyke from the UC Davis Flow Cytometry Shared Resource Laboratory for excellent technical assistance with our flow cytometry experiments and Scott Clarke from Thermo Fisher Scientific for technical assistance with optimizing the use of the Click-iT Plus EdU kits. The geriatric tissues were generously shared by Dr. Celine Rierra (UC Berkeley).
Funding. The research described in this article was supported by grants from the JDRF (CDA-2-2013-54) and American Diabetes Association (1-19-IBS-078). S.L. was supported by the National Science Foundation (NSF) Graduate Research Fellowship Program (1650042), the UC Davis Training Program in Molecular and Cellular Biology (funded in part by National Institutes of Health grant T32-GM007377), and the UC Davis NSF Bridge to Doctorate Program (1612490). This work was also supported by the University of California Davis Flow Cytometry Shared Resource Laboratory with funding from the National Cancer Institute (P30 CA093373) and National Institutes of Health S10 (OD018223) awards. NNC0069-0961 was generously provided by Novo Nordisk Compound Sharing.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.O.H. and S.L. contributed to conceptualization, visualization, and funding acquisition. S.L., J.Z., and T.v.d.M. contributed to research investigation, formal analysis, and data curation. S.S., M.F.F., and D.R.G. contributed to research investigation. J.Z. contributed to novel methodology. S.L. wrote the original draft of the manuscript. M.O.H. contributed to formal analysis, data curation, and manuscript review and editing. M.O.H. was responsible for supervision and project administration. M.O.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 2019 Western Region Islet Study Group (WRISG) Meeting, Victoria, BC, 9–11 October 2019, the 44th Annual West Coast Biological Sciences Undergraduate Research (WCBSUR) Conference, University of San Diego, CA, 6 April 2019, and the 30th Annual Undergraduate Research, Scholarship, and Creative Activities Conference, UC Davis, CA, 26–27 April 2019.
Footnotes
S.L. and J.Z. contributed equally.
References
- 1. Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 2004;53(Suppl. 3):S16–S21 [DOI] [PubMed] [Google Scholar]
- 2. Niclauss N, Meier R, Bédat B, Berishvili E, Berney T. Beta-cell replacement: pancreas and islet cell transplantation. Endocr Dev 2016;31:146–162 [DOI] [PubMed] [Google Scholar]
- 3. Zhong F, Jiang Y. Endogenous pancreatic β cell regeneration: a potential strategy for the recovery of β cell deficiency in diabetes. Front Endocrinol (Lausanne) 2019;10:101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guney MA, Lorberbaum DS, Sussel L. Pancreatic β cell regeneration: to β or not toβ. Curr Opin Physiol 2020;14:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 6. Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007;12:817–826 [DOI] [PubMed] [Google Scholar]
- 7. Meier JJ, Butler AE, Saisho Y, et al. β-Cellreplication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes 2005;54:2557–2567 [DOI] [PubMed] [Google Scholar]
- 9. Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes 2009;58:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tschen S-I, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes 2009;58:1312–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gregg BE, Moore PC, Demozay D, et al. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 2012;97:3197–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smukler SR, Arntfield ME, Razavi R, et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 2011;8:281–293 [DOI] [PubMed] [Google Scholar]
- 13. Razavi R, Najafabadi HS, Abdullah S, Smukler S, Arntfield M, van der Kooy D. Diabetes enhances the proliferation of adult pancreatic multipotent progenitor cells and biases their differentiation to more β-cell production. Diabetes 2015;64:1311–1323 [DOI] [PubMed] [Google Scholar]
- 14. Thorel F, Népote V, Avril I, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010;464:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Courtney M, Gjernes E, Druelle N, et al. The inactivation of Arx in pancreatic α-cells triggers their neogenesis and conversion into functional β-like cells. PLoS Genet 2013;9:e1003934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chera S, Baronnier D, Ghila L, et al. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 2014;514:503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 2008;455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mameishvili E, Serafimidis I, Iwaszkiewicz S, et al. Aldh1b1 expression defines progenitor cells in the adult pancreas and is required for Kras-induced pancreatic cancer. Proc Natl Acad Sci U S A 2019;116:20679–20688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang D, Wang J, Bai L, et al. Long-term expansion of pancreatic islet organoids from resident Procr+ progenitors. Cell 2020;180:1198–1211.e19 [DOI] [PubMed] [Google Scholar]
- 20. van der Meulen T, Mawla AM, DiGruccio MR, et al. Virgin beta cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metab 2017;25:911–926.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beamish CA, Strutt BJ, Arany EJ, Hill DJ. Insulin-positive, Glut2-low cells present within mouse pancreas exhibit lineage plasticity and are enriched within extra-islet endocrine cell clusters. Islets 2016;8:65–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beamish CA, Mehta S, Strutt BJ, Chakrabarti S, Hara M, Hill DJ. Decrease in Ins+Glut2LO β-cells with advancing age in mouse and human pancreas. J Endocrinol 2017;233:229–241 [DOI] [PubMed] [Google Scholar]
- 23. van der Meulen T, Xie R, Kelly OG, Vale WW, Sander M, Huising MO. Urocortin 3 marks mature human primary and embryonic stem cell-derived pancreatic alpha and beta cells. PloS One 2012;7:e52181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brennand K, Huangfu D, Melton D. All β cells contribute equally to islet growth and maintenance. PLoS Biol 2007;5:e163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benner C, van der Meulen T, Cacéres E, Tigyi K, Donaldson CJ, Huising MO. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics 2014;15:620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim H, Toyofuku Y, Lynn FC, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 2010;16:804–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tokumoto S, Yabe D, Tatsuoka H, et al. Generation and characterization of a novel mouse model that allows spatiotemporal quantification of pancreatic β-cell proliferation. Diabetes 2020;69:2340–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schäffer L, Brand CL, Hansen BF, et al. A novel high-affinity peptide antagonist to the insulin receptor. Biochem Biophys Res Commun 2008;376:380–383 [DOI] [PubMed] [Google Scholar]
- 29. Wicksteed B, Brissova M, Yan W, et al. Conditional gene targeting in mouse pancreatic β-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 2010;59:3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis 2007;45:593–605 [DOI] [PubMed] [Google Scholar]
- 31. Reinert RB, Kantz J, Misfeldt AA, et al. Tamoxifen-induced Cre-loxP recombination is prolonged in pancreatic islets of adult mice. PLoS One 2012;7:e33529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ye R, Wang QA, Tao C, et al. Impact of tamoxifen on adipocyte lineage tracing: inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Mol Metab 2015;4:771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Venkei ZG, Yamashita YM. Emerging mechanisms of asymmetric stem cell division. J Cell Biol 2018;217:3785–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bonner-Weir S, Li W-C, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. β-Cellgrowth and regeneration: replication is only part of the story. Diabetes 2010;59:2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic β cell regeneration as a possible therapy for diabetes. Cell Metab 2018;27:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hanahan D. Heritable formation of pancreatic β-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature 1985;315:115–122 [DOI] [PubMed] [Google Scholar]
- 37. Huang JL, Lee S, Hoek P, van der Meulen T, Van R, Huising MO. Genetic deletion of Urocortin 3 does not prevent functional maturation of beta cells. J Endocrinol 2020;246:69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arrojo E Drigo R, Lev-Ram V, Tyagi S, et al. Age mosaicism across multiple scales in adult tissues. Cell Metab 2019;30:343–351.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carboneau BA, Le TDV, Dunn JC, Gannon M. Unexpected effects of the MIP-CreER transgene and tamoxifen on β-cell growth in C57Bl6/J male mice. Physiol Rep 2016;4:e12863 [DOI] [PMC free article] [PubMed] [Google Scholar]