Abstract
Background
Phenylalanine hydroxylase (PAH) deficiency is an autosomal recessive disorder that results in elevated concentrations of phenylalanine (Phe) in the blood. If left untreated, the accumulation of Phe can result in profound neurocognitive disability. The objective of this systematic literature review and meta-analysis was to estimate the global birth prevalence of PAH deficiency from newborn screening studies and to estimate regional differences, overall and for various clinically relevant Phe cutoff values used in confirmatory testing.
Methods
The protocol for this literature review was registered with PROSPERO (International prospective register of systematic reviews). Pubmed and Embase database searches were used to identify studies that reported the birth prevalence of PAH deficiency. Only studies including numeric birth prevalence reports of confirmed PAH deficiency were included.
Results
From the 85 publications included in the review, 238 birth prevalence estimates were extracted. After excluding prevalence estimates that did not meet quality assessment criteria or because of temporal and regional overlap, estimates from 45 publications were included in the meta-analysis. The global birth prevalence of PAH deficiency, estimated by weighting regional birth prevalences relative to their share of the population of all regions included in the study, was 0.64 (95% confidence interval [CI] 0.53–0.75) per 10,000 births and ranged from 0.03 (95% CI 0.02–0.05) per 10,000 births in Southeast Asia to 1.18 (95% CI 0.64–1.87) per 10,000 births in the Middle East/North Africa. Regionally weighted global birth prevalences per 10,000 births by confirmatory test Phe cutoff values were 0.96 (95% CI 0.50–1.42) for the Phe cutoff value of 360 ± 100 µmol/L; 0.50 (95% CI 0.37–0.64) for the Phe cutoff value of 600 ± 100 µmol/L; and 0.30 (95% CI 0.20–0.40) for the Phe cutoff value of 1200 ± 200 µmol/L.
Conclusions
Substantial regional variation in the birth prevalence of PAH deficiency was observed in this systematic literature review and meta-analysis of published evidence from newborn screening. The precision of the prevalence estimates is limited by relatively small sample sizes, despite widespread and longstanding newborn screening in much of the world.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13023-021-01874-6.
Keywords: Phenylketonuria, Hyperphenylalaninemia, Prevalence, Newborn screening, Phenylalanine hydroxylase deficiency
Background
Phenylalanine hydroxylase (PAH) deficiency is an autosomal recessive disorder that results in elevated concentrations of the amino acid phenylalanine (Phe) in the blood [1–4]. Over 1000 PAH variants exist [5], and depending on the inherited alleles, affected individuals may have very mild to pronounced elevation of Phe [4]. Phenylalanine hydroxylase catalyzes the conversion of Phe into tyrosine and is key to maintaining a stable concentration of Phe in the blood [7]. When PAH activity is decreased, blood Phe concentration increases from the typical mean of 60 μmol/L [3]. In addition, an estimated 1–2% of cases of hyperphenylalanemia (HPA) are secondary to a deficiency in tetrahydrobiopterin (BH4), a necessary cofactor for PAH and other amino acid-metabolizing enzymes [4, 6]. Cases of mutations in a heat shock co-chaperone family member, DNAJC12 have been also reported to result in HPA [8]. If left untreated, the accumulation of Phe can result in profound neurocognitive disability [2]. Early diagnosis and intervention are essential to preserve cognitive function [1, 3].
Treatment guidelines recommend initiation of treatment as early as possible upon diagnosis of PAH deficiency [3]. Treatment options include dietary and pharmaceutical management. Dietary management involves severely restricted intake of Phe (and protein)-rich foods based on each individual’s maximum Phe tolerance [9, 10] in combination with medical foods to supplement inadequate intake of protein and other essential nutrients due to the Phe-restricted diet. Approved pharmaceutical treatments for PAH deficiency include pegvaliase and sapropterin. While pegvaliase, a Phe-metabolizing enzyme composed of pegylated recombinant phenylalanine ammonia lyase, is approved for use only in adults (United States) and persons aged 16 years and above (Europe) who have uncontrolled Phe in blood (> 600 uM/L) with current treatment [11, 12], sapropterin dihydrochloride, a synthetic form of BH4, is indicated for use in children (> 1 month of age) and adults with BH4-responsive PKU in conjunction with a Phe-restricted diet [2, 13, 14].
Phenylalanine hydroxylase deficiency is classified into mild HPA, mild phenylketonuria (PKU), moderate PKU, and classical PKU based on blood Phe concentration obtained in the neonatal period (Table 1); however, concentrations determined in this period are unlikely to reflect peak untreated levels, as neonates vary in their dietary exposure to Phe before the blood sample is taken, and early treatment often precludes obtaining more definitive Phe concentrations [1].
Table 1.
Current classification and treatment guidelines for PAH deficiency
| Classification | Pretreatment blood phenylalanine concentration | Treatment recommended? | |
|---|---|---|---|
| European guidelinesa | ACMGb | ||
| Classical PKU |
> 1200 µmol/L (> 20 mg/dL) |
Yes | Yes |
| Moderate PKU |
900–1200 µmol/L (15–20 mg/dL) |
Yes | Yes |
| Mild PKU |
600–900 µmol/L (10–15 mg/dL) |
Yes | Yes |
| Mild HPA-gray zone |
360–600 µmol/L (6–10 mg/dL) |
Yes (only if < 12 years or in women before/during pregnancy) | Yesc |
| PAH deficiency not requiring treatment |
120–360 µmol/L (2–6 mg/dL) |
No | No |
Because of the severe consequences of untreated phenylalanine hydroxylase deficiency, many countries currently perform routine newborn screening for elevated blood Phe concentration [15–17]. Methods for measuring Phe have evolved over time, with increasing accuracy, initiating with the bacterial inhibition assay (Guthrie test) in 1963 [18] to the current state-of-the-art tandem mass spectrometry [19]. The Guthrie test has been suggested to miss as many as 1 in 25 affected newborns screened at or before 3 days of age [20].
The accumulation of data from newborn screening programs with varied screening methods employed across the world provides an opportunity to evaluate the birth prevalence of HPA and PKU at the regional and global levels. Here, we systematically review the published literature and analyze regional differences in HPA and PKU birth prevalence, overall and for various clinically relevant blood Phe concentration cutoff values used in confirmatory testing.
Methods
The protocol for this literature review was registered with PROSPERO (International prospective register of systematic reviews: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=156377; ID 156377).
Birth prevalence
For the purpose of this review and to ensure consistent methodology in calculation of birth prevalence estimates across studies, birth prevalence was defined as cases identified during newborn screening divided by the number of newborns screened. This method was most frequently described in studies reporting birth prevalence of PAH deficiency from newborn screening programs.
Literature search
PubMed and Embase were searched using a strategy based on the PICOS (population, intervention, comparison, outcomes, study design) framework (Additional file 1: Table A-1) [21]. The search strategy included terms to identify newborns, prevalence, incidence, newborn screening, Guthrie and other tests, PKU, HPA, and PAH deficiency (Additional file 1: Table A-2 and Table A-3). No language or time limits were implemented. Animal studies, editorials, and commentaries were excluded.
Study selection
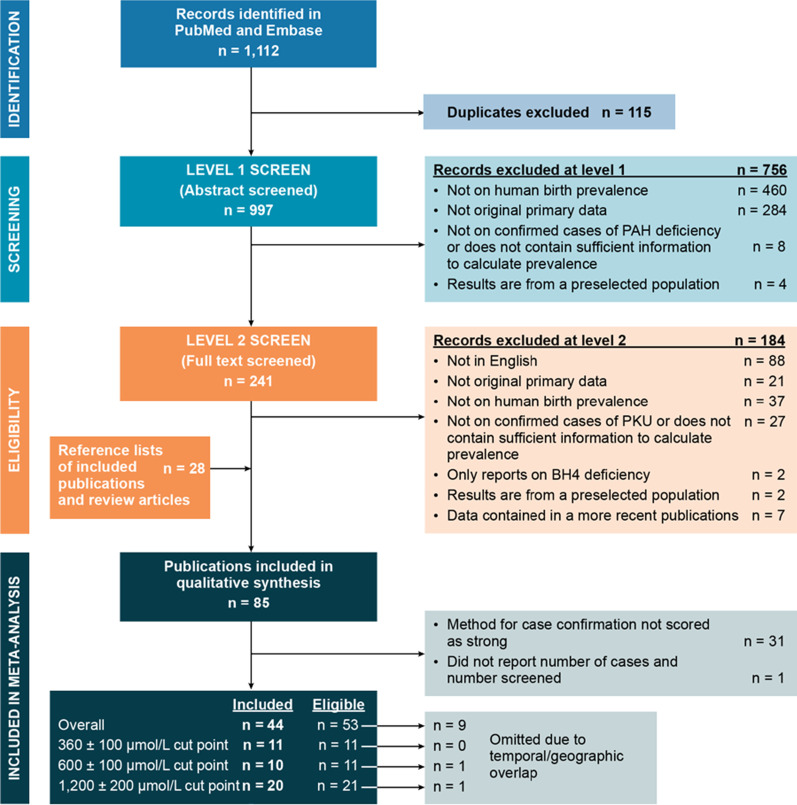
Entries retrieved from PubMed and Embase were screened in two steps (Fig. 1): in level 1 screening, two researchers independently reviewed titles and abstracts; in level 2 screening, two researchers independently reviewed full-text articles. Lack of agreement on inclusion was resolved by discussion and consensus within the research team.
Fig. 1.
Study selection process. PRISMA chart modeled after Moher et al. [21]. BH4 = tetrahydrobiopterin; PAH = phenylalanine hydroxylase deficiency; PKU = phenylketonuria
In level 1 screening (Additional file 1: Table A-4), conference abstracts, studies reporting exclusively on BH4 deficiency but not on PAH deficiency, and studies that focused primarily on assay development and/or validation were excluded. Publications were eligible if the abstract or title indicated that the paper presented original research and contained numeric reports on the birth prevalence of PAH deficiency. Birth prevalence must have been reported on an unselected population (e.g., studies on institutionalized patients were not eligible) and was required to be directly measured (rather than estimated from models). When duplicate records reporting on one study were identified, only one was retained; in this circumstance, records published in English were preferred.
The following additional criteria were applied in level 2 screening (Additional file 1: Table A-5): articles were required to be written in English and birth prevalence was required to be based on confirmed cases. When two or more publications on any given region were identified, both were included if the research had been conducted by different groups, or if both the geography and time frame did not overlap. For reports with geographic and temporal overlap conducted by the same institution, the study covering the largest population was eligible.
Data extraction and quality assessment
Extracted data elements included country and region, dates of data collection, study design, assay method for screening and for case confirmation (when diagnostic methods varied among sites or over time, scoring for the estimate was based on the lowest scoring diagnostic method, per the list in Table 2), diagnosis as reported in the publication (“nominal diagnosis”), Phe concentration used as a positive cutoff value, whether patients with BH4 deficiency were included in the number of cases reported, number of newborns screened, number of cases, and reported birth prevalence. For publications that reported birth prevalence stratified by multiple variables, values for each variable were extracted separately (herein referred to as “estimates”).
Table 2.
Quality assessment tool for birth prevalence estimates
| Scoring domain | Score | ||
|---|---|---|---|
| Strong | Moderate | Weak | |
| Case definitiona | The case definition is complete (including both screening positive and confirmed cases) | The case definition is partially complete (lacks either the definition of screening positive or of confirmed cases) | The case definition is incomplete for both screening positive and confirmed cases |
| Study setting/source population |
Mandatory population-wide newborn screening program General population from a well-defined region and time |
Catchment area of a hospital or other medical facility Hospital or laboratory records or disease registry Surveys (e.g., to health care providers) |
Personal communication Unclear or not reported |
| Statistical methods |
The denominator is the number of newborns screened, and cases in the numerator arise from the population in the denominator If any quantity is estimated rather than directly measured, estimations are in line with the criteria described here |
The denominator is the overall number of births rather than the number screened |
Cases in the numerator do not arise from the population in the denominator Unclear or not reported |
| Precision of prevalence estimateb | Half the width of the 95% confidence interval is less than half of the prevalence | Half the width of the 95% confidence interval is between half of the prevalence and the prevalencec |
Half the width of the 95% confidence interval is greater than the prevalence Confidence interval is not estimable |
| Diagnostic method used for case confirmationd | Tandem mass spectrometry, high-performance liquid chromatography, column chromatography, (rapid) ion exchange chromatography, quantitative amino acid analyzer, positive mutational analysis, or enzymatic assay (including colorimetric, fluorimetric, and ELISA) | Guthrie test, bacterial inhibition assay, thin layer or paper chromatography |
Other methods, or those where urine is used as the assay substrate Unclear or not reported |
aThe case definition was considered complete if the phenylalanine cutoff value was provided
bAdditional file 1 presents the method of calculating the precision of the prevalence estimate
cInclusive of both bounds
dWhen diagnostic methods varied among sites or over time, scoring for the estimate was based on the lowest scoring diagnostic method
Data were extracted by one researcher using a form specifically designed for this study; extracted data were verified by a second researcher. Each estimate was assessed for quality as strong, moderate, or weak in each of five scoring domains (Table 2). The quality assessment tool used in this study was based on existing tools for assessing the quality of studies that report the prevalence of conditions assessed by surveillance [22] or conditions of genetic origin [23].
Meta-analyses
To mitigate errors that may arise from using early, less reliable assays, such as the Guthrie test, only estimates derived from confirmatory diagnostic assays that were assessed as strong in the quality assessment tool (Table 2) were eligible for meta-analysis. Inclusion in the meta-analysis also required that the number of cases and the number of screened newborns were reported. For each region and Phe concentration cutoff value category, at least 2 birth prevalence estimates were required to conduct a meta-analysis. For regions and Phe concentration cutoff value categories with only one published birth prevalence estimate, the single published estimate was used to represent the region (or Phe concentration cutoff value) in the global prevalence estimates. Once the eligible estimates for each planned meta-analysis were identified, estimates with both temporal and geographic overlap were assessed, and the estimate representing the largest geographic coverage or time period was included.
Meta-analyses were performed to determine aggregated regional birth prevalence (Europe, North America, Middle East/North Africa, Latin America, South Pacific, and West Pacific; Additional file 1: Table A-6) and a global birth prevalence. The global birth prevalence was estimated by using two approaches. A “regionally weighted” global prevalence was calculated, in which results from each region were weighted by the region’s relative numerical contribution to the total population of the regions for each analysis. For this determination, country-specific population counts were obtained from 2020 United Nations population estimates [24] and were summed within each region to determine regional totals (weights for analyses incorporating results from six regions: Europe, 0.126; Latin America, 0.097; Middle East/North Africa, 0.125; North America, 0.055; Southeast Asia, 0.303; West Pacific, 0.293). A non-regionally weighted global prevalence was also calculated for comparison to other recently published PKU global birth prevalence estimates that were not regionally-weighted.
For both regional and global birth prevalence determinations, birth prevalence was calculated and stratified by three confirmatory Phe concentration cutoff values (360 ± 100 μmol/L, 600 ± 100 μmol/L, 1200 ± 200 μmol/L). When a publication reported birth prevalence by Phe cutoff interval (e.g., separate birth prevalence values for ≥ 360 ± 100 to 600 μmol/L, ≥ 600 ± 100 μmol/L to 1200 μmol/L and ≥ 1200 ± 200 μmol/L), the sum of all values above the cutoff value was used. Finally, an unstratified meta-analysis was conducted, which additionally included estimates from studies in which Phe cutoff values were not reported, to determine overall (regionally weighted and non-regionally weighted) birth prevalence.
To provide appropriate weights for meta-analysis, birth prevalence estimates were transformed using the double arcsine method [25]; meta-analysis was conducted using a random-effects model with inverse variance weighting. Transformation and calculations were performed using MetaXL (version 5.3, EpiGear International). Heterogeneity was assessed using the I2 statistic [26, 27].
Results
Literature search and review
Searches in PubMed and Embase identified 1112 entries (Fig. 1). Screening of 997 unique PubMed and Embase entries and an additional 28 publications identified from reference lists of screened entries identified 85 publications meeting eligibility criteria, resulting in 238 birth prevalence estimates (Additional file 2).
These 85 publications were published from 1964 [28] to 2019 [29] and reported on data from 1960 [30] to 2018 [29] from 59 countries. Newborn blood or urine samples for screening were taken between the first day of life [31] and age 3–8 weeks [32]; 25 publications (125 birth prevalence estimates) did not report age at screening. Phe concentration used for confirmatory testing ranged from 120 μmol/L [33] to over 2600 μmol/L [34]. Forty-three publications (135 birth prevalence estimates) did not report the cutoff value for confirmatory testing. Nominal diagnoses were inconsistent. For example, classical PKU was defined using confirmatory Phe cutoff values ranging from 726 μmol/L [35] to 1816 μmol/L [36]. Cases with BH4 deficiency were included in 5 publications (6 birth prevalence estimates) and the presence or absence of BH4 deficiency was not reported in 58 publications (186 birth prevalence estimates).
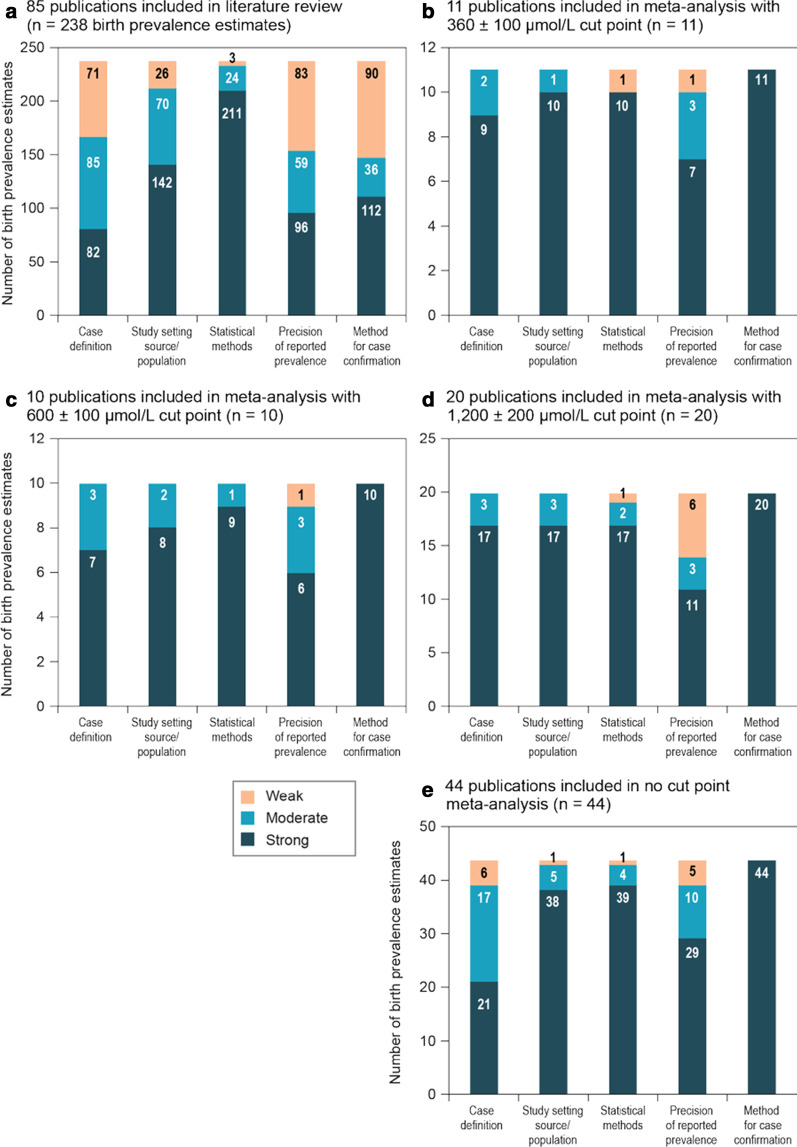
The only domains of the quality assessment tool on which > 50% of the estimates scored strong were statistical methods and study setting/source population. Sixty percent of the estimates scored moderate or weak on precision, and 53% scored moderate or weak on the method for case confirmation (Fig. 2A).
Fig. 2.
a–e Quality of evidence assessments of birth prevalence estimates
Meta-analysis results
A total of 112 birth prevalence estimates (54 publications) scored strong in the quality assessment domain diagnostic method used for case confirmation and were therefore potentially eligible for meta-analysis. One publication (18 estimates) with strong scores in the diagnostic method used for case confirmation reported birth prevalence (in the format 1:8000), but did not provide the number of cases or screened newborns [37] and was not deemed eligible. No birth prevalence estimates from the African region were included in the meta-analysis, and the only estimates eligible for inclusion in Southeast Asia were from Thailand.
Birth prevalence estimates ranged from 0 (Estonia [38], Finland [39], and Thailand [40]) to 2.46 per 10,000 births (Macedonia) [41] (Table 3).
Table 3.
Birth prevalence estimates scoring strong on diagnostic method for case confirmation (n = 54 publications)
| Country | Birth prevalence per 10,000 newborns (95% CI) | Phe cutoff value for confirmatory diagnosis (µmol/L) | Score for additional quality of evidence domainse | References | |||
|---|---|---|---|---|---|---|---|
| Case definition | Study setting source population | Statistical methods | Precision of prevalence estimate | ||||
| Europe | |||||||
| Austria (Eastern, PKUf) | 1.3 (0.92–1.83)d | NR | Weak | Strong | Strong | Strong | Thalhammer [49] |
| Austria (Eastern, HPAf) | 0.49 (0.28–0.85)d | NR | Weak | Strong | Strong | Moderate | Thalhammer [49] |
| Austria (Western, PKUf) | 0.45 (0.23–0.88)d | NR | Weak | Strong | Strong | Moderate | Thalhammer [49] |
| Austria (Western, HPAf) | 0.5 (0.26–0.96)d | NR | Weak | Strong | Strong | Moderate | Thalhammer [49] |
| Estonia | 0 (0–1.02)d | < 600 | Strong | Strong | Strong | Weak | Ounap et al. [38] |
| Estonia | 1.66 (0.76–3.63)c,d | 1000 | Strong | Strong | Strong | Moderate | Ounap et al. [38] |
| Finland | 0 (0–0.52)a,d | 363 | Moderate | Strong | Strong | Weak | Visakorpi et al. [39] |
| Germany | 0.81 (0.66–1)d | NR | Weak | Strong | Strong | Strong | Lindner et al. [50] |
| Germany | 0.78 (0.63–0.97)b,d | 600 | Moderate | Strong | Strong | Strong | Lindner et al. [50] |
| Germany | 0.81 (0.65–1.01)a,d | 363– < 908 | Strong | Strong | Strong | Strong | Mathias and Bickel [51] |
| Germany | 0.99 (0.81–1.21)a,d | 908 | Strong | Strong | Strong | Strong | Mathias and Bickel [51] |
| Germany | 0.96 (0.65–1.43) | 600 | Strong | Strong | Strong | Strong | Schulze et al. [52] |
| Germany | 1.24 (0.87–1.76) | 150–600 | Strong | Strong | Strong | Strong | Schulze et al. [52] |
| Greece | 0.44 (0.05–1.61)d | NR | Moderate | Moderate | Strong | Weak | Loukas et al. [53] |
| Greece | 0.41 (0.31–0.56)c,d | 1211 | Strong | Strong | Strong | Strong | Missiou-Tsagaraki et al. (1988) [54] |
| Hungary | 0.85 (0.39–1.86)d | NR | Weak | Strong | Strong | Moderate | Mehes et al. [55] |
| Italy (PKUf) | 1.38 (0.85–2.23)d | NR | Moderate | Weak | Strong | Moderate | Antonozzi et al. [56] |
| Italy (HPAf) | 0.26 (0.05–0.75)d | NR | Moderate | Weak | Strong | Weak | Antonozzi et al. [56] |
| Italy | 0.22 (0.15–0.32)c,d | 1211 | Strong | Strong | Strong | Strong | Zaffanello et al. [57] |
| Italy | 0.78 (0.63–0.96)d | NR | Moderate | Strong | Strong | Strong | Zaffanello et al. [57] |
| Macedonia | 2.46 (0.06–13.68)d | 151 | Moderate | Strong | Strong | Weak | Kocova and Anastasovska [41] |
| Poland | 0.28 (0.22–0.34)a,d | 363–1211 | Strong | Moderate | Strong | Strong | Cabalska et al. [58] |
| Poland | 1.29 (1.16–1.42)a,c,d | 1211 | Strong | Moderate | Strong | Strong | Cabalska et al. [58] |
| Portugal | 0.82 (0.56–1.2)a,d | 360 | Strong | Strong | Strong | Strong | Vilarinho et al. [59] |
| Portugal | 0.38 (0.22–0.66)d | 150–360 | Strong | Strong | Strong | Moderate | Vilarinho et al. [59] |
| Slovakia | 1.69 (1.45–1.98)d | NR | Weak | Strong | Strong | Strong | Dluholucký et al. [60] |
| Slovenia | 0.98 (0.72–1.35)b,c,d | 1200 | Strong | Strong | Moderate | Strong | Smon et al. [61] |
| Slovenia | 0.39 (0.24–0.64)b,d | 600–900 | Strong | Strong | Moderate | Moderate | Smon et al. [61] |
| Slovenia | 0.1 (0.03–0.27)b,d | 900–1200 | Strong | Strong | Moderate | Weak | Smon et al. [61] |
| Spain | 0.66 (0.22–1.55)d | 240 | Strong | Strong | Strong | Weak | Fernández-Iglesias et al. [62] |
| USSR/Russia | 1.5 (0.98–2.3)b,c,d | 1200 | Moderate | Strong | Strong | Strong | Gerasimova et al. [63] |
| USSR/Russia | 0.36 (0.12–0.84)b,d | 600–1200 | Moderate | Strong | Strong | Weak | Gerasimova et al. [63] |
| United Kingdom | 0.49 (0.36–0.67)c,d | 1200 | Strong | Moderate | Strong | Strong | Walker et al. [64] |
| United Kingdom | 0.19 (0.11–0.31)d | 240 | Strong | Moderate | Strong | Moderate | Walker et al. [64] |
| Yugoslavia | 0.22 (0.1–0.48)b,d | 605–902 | Strong | Moderate | Strong | Moderate | Mardesic et al. [65] |
| Yugoslavia | 0.69 (0.44–1.08)b,d | 908 | Strong | Moderate | Strong | Strong | Mardesic et al. [65] |
| Latin America | |||||||
| Brazil (Laboratory A, 2005) | 0.36 (0.12–0.84)b,d | 605 | Strong | Strong | Strong | Weak | Botler et al. [66] |
| Brazil (Laboratory A, 2006) | 0.59 (0.31–1.12)b,d | 605 | Strong | Strong | Strong | Intermediate | Botler et al. [66] |
| Brazil (Laboratory A, 2007) | 0.35 (0.11–0.82)b,d | 605 | Strong | Strong | Strong | Weak | Botler et al. [66] |
| Brazil (Laboratory B, 2005) | 0.52 (0.06–1.9)b,d | 605 | Strong | Strong | Strong | Weak | Botler et al. [66] |
| Brazil (Laboratory B, 2006) | 0.84 (0.17–2.45)b,d | 605 | Strong | Strong | Strong | Weak | Botler et al. [66] |
| Brazil (Laboratory B, 2007) | 0.91 (0.11–3.28)b,d | 605 | Strong | Strong | Strong | Weak | Botler et al. [66] |
| Brazil | 0.92 (0.25–2.36)a–d | 1211 | Strong | Strong | Strong | Weak | Ramalho et al. [67] |
| Brazil | 0.23 (0.01–1.28)a,d | 302–604 | Strong | Strong | Strong | Weak | Ramalho et al. [67] |
| Brazil | 0.23 (0.01–1.28)a,b,d | 606–1210 | Strong | Strong | Strong | Weak | Ramalho et al. [67] |
| Chile | 0.53 (0.45–0.63)c,d | 1211 | Strong | Strong | Strong | Strong | Cornejo et al. [68] |
| Chile | 0.98 (0.86–1.11)d | NR | Intermediate | Strong | Strong | Strong | Cornejo et al. [68] |
| Middle East/North Africa | |||||||
| Iran | 0.15 (0.07–0.32)b,c,d | 1211 | Strong | Strong | Strong | Intermediate | Abbaskhanian et al. [69] |
| Iran | 0.29 (0.17–0.52)d | 121–605 | Strong | Strong | Strong | Intermediate | Abbaskhanian et al. [69] |
| Iran | 0.22 (0.12–0.42)b,d | 606–1210 | Strong | Strong | Strong | Intermediate | Abbaskhanian et al. [69] |
| Iran | 1.6 (1.11–2.31)a,d | 424 | Strong | Strong | Strong | Strong | Habib et al. [44] |
| Iran | 0.52 (0.14–1.33)d | 121– < 1211 | Strong | Strong | Strong | Weak | Karamifar et al. [70] |
| Iran | 0.39 (0.08–1.14)c,d | 1211 | Strong | Strong | Strong | Weak | Karamifar et al. [70] |
| Iran | 1.92 (1.53–2.41)d | NR | Intermediate | Strong | Intermediate | Strong | Motamedi et al. [71] |
| Saudi Arabia | 0.68 (0.52–0.89)d | 180 | Strong | Strong | Strong | Strong | Alfadhel et al. [72] |
| Turkey (classical PKUf) | 1.35 (1.18–1.54)d | NR | Weak | Strong | Strong | Strong | Ozalp et al. [73] |
| Turkey (mild PKUf) | 0.64 (0.52–0.77)d | NR | Weak | Strong | Strong | Strong | Ozalp et al. [73] |
| Turkey (mild HPAf) | 0.36 (0.28–0.47)d | NR | Weak | Strong | Strong | Strong | Ozalp et al. [73] |
| UAE | 0.76 (0.57–0.99)c,d | 1211 | Strong | Strong | Strong | Strong | Al Hosani et al. [74] |
| North America | |||||||
| Canada (Alberta) | 0.50 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| Canada (Ontario, PKUf) | 0.60 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| Canada (Ontario, HPAf) | 0.29 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| US (NC) | 0.08 (0.01–0.3) | 157 | Intermediate | Strong | Strong | Weak | Frazier et al. [42] |
| US (NC) | 0.46 (0.26–0.82) | 250 | Intermediate | Strong | Strong | Intermediate | Frazier et al. [42] |
| US (NC) | 0.52 (0.39–0.69)a,d | 300 | Intermediate | Strong | Strong | Strong | Frazier et al. [42] |
| US (NY) | 0.1 (0.05–0.2)d | 908– < 1211 | Strong | Strong | Intermediate | Intermediate | Hansen et al. [75] |
| US (NY) | 0.53 (0.39–0.72)c,d | 1211 | Strong | Strong | Intermediate | Strong | Hansen et al. [75] |
| US (NY) | 0.14 (0.07–0.25)d | NR | Intermediate | Strong | Intermediate | Intermediate | Hansen et al. [75] |
| US (NY) | 0.7 (0.52–0.93)d | NR | Intermediate | Strong | Intermediate | Strong | Kelly and Palombi [76] |
| US (MA) | 1.04 (0.62–1.75)d | NR | Intermediate | Strong | Strong | Intermediate | Maccready and Hussey [28] |
| US (CT) | 0.83 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| US (FL) | 1.00 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| US (KS) | 0.80 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| US (KY) | 0.87 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| US (OK) | 0.59 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| US (PA, PKUf) | 0.78 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| US (PA, HPAf) | 0.18 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| US (TX) | 0.38 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| US (VA) | 0.57 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| US (WV) | 0.67 (CI not estimable) | NR | Weak | Intermediate | Strong | Weak | Somers and Favreau [37] |
| US (PA) | 0.43 (0.29–0.64)a,d | 363 | Strong | Strong | Strong | Strong | Wainer and Sideman [43] |
| US (PA) | 0.9 (0.68–1.19)d | NR | Intermediate | Strong | Strong | Strong | Wainer and Sideman [43] |
| US (New England) | 0.27 (0.13–0.56)d | NR | Intermediate | Strong | Strong | Intermediate | Zytkovicz et al. [77] |
| US (New England) | 0.43 (0.24–0.77)d | NR | Intermediate | Strong | Strong | Intermediate | Zytkovicz et al. [77] |
| Southeast Asia | |||||||
| Thailand | 0.04 (0.01–0.08) | NR | Intermediate | Strong | Strong | Weak | Charoensiriwatana et al. [78] |
| Thailand | 0.05 (0.02–0.11) | 1211 | Strong | Strong | Strong | Weak | Pangkanon et al. [79] |
| Thailand | 0.03 (0.02–0.05)c,d | 1200 | Intermediate | Strong | Strong | Intermediate | Pangkanon et al. [80] |
| Thailand | 0 (0–2.12) | NR | Intermediate | Intermediate | Strong | Weak | Ratrisawadi et al. [40] |
| Thailand | 0.04 (0.01–0.1) | NR | Intermediate | Strong | Strong | Weak | Sutivijit et al. [81] |
| West Pacific | |||||||
| Australia | 0.26 (0.09–0.61)d | 200–300 | Intermediate | Intermediate | Strong | Weak | Boneh et al. [34] |
| Australia | 0.37 (0.18–0.76)b,d | 600–1200 | Intermediate | Intermediate | Strong | Intermediate | Boneh et al. [34] |
| Australia | 0.05 (0–0.29)b,d | 2600 | Intermediate | Intermediate | Strong | Weak | Boneh et al. [34] |
| China | 0.17 (0.08–0.36) | 242–1211 | Strong | Strong | Strong | Intermediate | Chen et al. [82] |
| China | 0.59 (0.38–0.89)c | 1211 | Strong | Strong | Strong | Strong | Chen et al. [82] |
| China | 0.38 (0.23–0.64)d | NR | Weak | Strong | Strong | Intermediate | Lin et al. [29] |
| China | 0.1 (0.01–0.36)a,d | 363– < 908 | Strong | Strong | Strong | Weak | Liu and Zuo [83] |
| China | 0.5 (0.27–0.93)a,d | 908 or 1211 | Strong | Strong | Strong | Intermediate | Liu and Zuo [83] |
| China | 0.4 (0.34–0.47) | NR | Intermediate | Strong | Strong | Strong | Maitusong et al. [84] |
| China | 0.91 (0.65–1.28) | NR | Weak | Intermediate | Strong | Strong | Shi et al. [85] |
| China | 0.65 (0.48–0.9)a,c,d | 1200 | Strong | Strong | Weak | Strong | Su et al. [86] |
| China | 0.28 (0.17–0.45)d | 120–360 | Strong | Strong | Weak | Intermediate | Su et al. [86] |
| China | 0.98 (0.76–1.27)a,d | 360–1200 | Strong | Strong | Weak | Strong | Su et al. [86] |
| China | 0.88 (0.46–1.67) | NR | Weak | Strong | Strong | Intermediate | Tu et al. [87] |
| China | 0.07 (0.01–0.21)a–d | 1200 | Strong | Strong | Strong | Weak | Wang et al. [88] |
| China | 0.1 (0.03–0.24)d | 120–360 | Strong | Strong | Strong | Weak | Wang et al. [88] |
| China | 0.05 (0.01–0.17)a,d | 360–600 | Strong | Strong | Strong | Weak | Wang et al. [88] |
| China | 0.14 (0.07–0.31)a,b,d | 600–1200 | Strong | Strong | Strong | Intermediate | Wang et al. [88] |
| China | 0.86 (0.82–0.91)d | NR | Intermediate | Strong | Strong | Strong | Zhan et al. [89] |
| South Korea | 0.51 (0.14–1.29)c,d | 1200 | Strong | Strong | Strong | Weak | Yoon et al. [90] |
| Taiwan | 0.27 (0.2–0.36)d | 120– < 600 | Strong | Strong | Strong | Strong | Niu et al. [91] |
| Taiwan | 0.13 (0.09–0.21)b,d | 600– < 1200 | Strong | Strong | Strong | Strong | Niu et al. [91] |
| Taiwan | 0.03 (0.01–0.08)b–d | 1200 | Strong | Strong | Strong | Weak | Niu et al. [91] |
CI = confidence interval; CT = Connecticut; FL = Florida; HPA = hyperphenylalaninemia; KS = Kansas; KY = Kentucky; MA = Massachusetts; NC = North Carolina; NR = not reported; NY = New York; OK = Oklahoma; PA = Pennsylvania; Phe = phenylalanine; PKU = phenylketonuria; TX = Texas; UAE = United Arab Emirates; US = United States; USSR = Union of Soviet Socialist Republics; VA = Virginia; WV = West Virginia
aEstimate contributes to meta-analysis with diagnostic cutoff value 360 µmol/L
bEstimate contributes to meta-analysis with diagnostic cutoff value 600 µmol/L
cEstimate contributes to meta-analysis with diagnostic cutoff value 1200 µmol/L
dEstimate contributes to overall meta-analysis
eThis table includes only estimates for which the method of diagnosis confirmation was considered “strong” in the quality of evidence scoring tool
fNominal diagnoses as provided in associated reference
Estimates from 45 publications were included in at least one meta-analysis, and the rest were excluded due to temporal and regional overlap. Meta-analysis results are summarized in Table 4 and Additional file 3: Figures A2–A5. The regionally weighted global birth prevalence of PAH deficiency (N = 44 publications, 1 estimate per publication) was 0.64 (95% confidence interval [CI] 0.53–0.75) per 10,000 births (Table 4; quality assessment results shown in Fig. 2E). The lowest regional birth prevalence was observed in Southeast Asia, with 0.03 cases per 10,000 births (95% CI 0.02–0.05); the highest was observed in the Middle East/North Africa, with 1.18 (95% CI 0.64–1.87) cases per 10,000 births.
Table 4.
Meta-analysisa of birth prevalence estimates stratified by region and by phenylalanine diagnostic cutoff value
| Region | Birth prevalence per 10,000 screened (95% CI) | I2 (%) | Number of estimates | Reference(s) | Country |
|---|---|---|---|---|---|
| Confirmatory test phenylalanine cutoff value of 360 ± 100 µmol/L | |||||
| Europe | 0.97 (0.52–1.53) | 93.8 | 4 | Cabalska et al. [58] | Poland |
| Mathias and Bickel [51] | Germany | ||||
| Vilarinho et al. [59] | Portugal | ||||
| Visakorpi et al. [39] | Finland | ||||
| Latin America | 1.38 (0.51–3.01) | NA | 1 | Ramalho et al. [67] | Brazil |
| Middle East/North Africa | 1.60 (1.06–2.31) | NA | 1 | Habib et al. [44] | Iran |
| North America | 0.49 (0.38–0.61) | 0.0 | 2 | Frazier et al. [42] | United States |
| Wainer and Sideman[43] | United States | ||||
| West Pacific | 0.63 (0.03–1.75) | 96.5 | 3 | Liu and Zuo [83] | China |
| Su et al. [86] | China | ||||
| Wang et al. [88] | China | ||||
| Global (non-regionally weighted) | 0.85 (0.51–1.26) | 95.9 | 11 | – | – |
| Global (regionally weighted)b | 0.96 (0.50–1.42) | NA | 11 | – | – |
| Confirmatory test phenylalanine cutoff value of 600 ± 100 µmol/L | |||||
| Europe | 1.18 (0.75–1.69) | 85.8 | 4 | Lindner et al. [50] | Germany |
| Gerasimova et al. [63] | USSR/Russia | ||||
| Mardesic et al. [65] | Yugoslavia | ||||
| Smon et al. [61] | Slovenia | ||||
| Latin America | 0.65 (0.14–1.46) | 64.2 | 2 | Botler et al. [66] | Brazil |
| Ramalho et al. [67] | Brazil | ||||
| Middle East/North Africa | 0.37 (0.21–0.61) | NA | 1 | Abbaskhanian et al. [69] | Iran |
| West Pacific | 0.23 (0.12–0.36) | 55.9 | 3 | Boneh et al. [34] | Australia |
| Niu et al. [91] | Taiwan | ||||
| Wang et al. [88] | China | ||||
| Global (non-regionally weighted) | 0.66 (0.38–1.02) | 94.1 | 10 | – | – |
| Global (regionally weighted)b | 0.50 (0.37–0.64) | NA | 10 | – | – |
| Confirmatory test phenylalanine cutoff value of 1200 ± 200 µmol/L | |||||
| Europe | 0.78 (0.40–1.3) | 96.9 | 7 | Cabalska et al. [58] | Poland |
| Gerasimova et al. [63] | USSR/Russia | ||||
| Missiou-Tsagaraki et al. [54] | Greece | ||||
| Ounap et al. [38] | Estonia | ||||
| Smon et al. [61] | Slovenia | ||||
| Walker et al. [64] | United Kingdom | ||||
| Zaffanello et al. [57] | Italy | ||||
| Latin America | 0.58 (0.30–0.94) | 29.2 | 2 | Cornejo et al. [68] | Chile |
| Ramalho et al. [67] | Brazil | ||||
| Middle East/North Africa | 0.36 (0.04–0.94) | 91.2 | 3 | Abbaskhanian et al. [69] | Iran |
| Karamifar et al. [70] | Iran | ||||
| Al Hosani et al. [74] | United Arab Emirates | ||||
| North America | 0.53 (0.38–0.72) | NA | 1 | Hansen et al. [75] | United States |
| Southeast Asia | 0.03 (0.02–0.05) | NA | 1 | Pangkanon et al. [80] | Thailand |
| West Pacific | 0.22 (0.03–0.56) | 94.6 | 6 | Boneh et al. [34] | Australia |
| Chen et al. [82] | China | ||||
| Niu et al.[91] | Taiwan | ||||
| Su et al. [86] | China | ||||
| Yoon et al. [90] | South Korea | ||||
| Wang (2019) [88] | China | ||||
| Global (non-regionally weighted) | 0.47 (0.26–0.74) | 98.0 | 20 | – | – |
| Global (regionally weighted)b | 0.30 (0.20–0.40) | NA | 20 | – | – |
| Overall analysisc | |||||
| Europe | 1.14 (0.89–1.41) | 92.2 | 19 | Antonozzi et al. [56] | Italy |
| Cabalska et al. [58] | Poland | ||||
| Dluholucký and Knapková [60] | Slovakia | ||||
| Fernández-Iglesias et al. [62] | Spain | ||||
| Gerasimova et al. [63] | USSR/Russia | ||||
| Kocova and Anastasovska [41] | Macedonia | ||||
| Lindner et al. [50] | Germany | ||||
| Loukas et al. [53] | Greece | ||||
| Mardesic et al. [65] | Yugoslavia | ||||
| Mathias and Bickel [51] | Germany | ||||
| Mehes et al. [55] | Hungary | ||||
| Missiou-Tsagaraki et al. [54] | Greece | ||||
| Ounap et al. [38] | Estonia | ||||
| Smon et al. [61] | Slovenia | ||||
| Thalhammer [49] | Austria | ||||
| Vilarinho et al. [59] | Portugal | ||||
| Visakorpi et al. [39] | Finland | ||||
| Walker et al. [64] | United Kingdom | ||||
| Zaffanello et al. [57] | Italy | ||||
| Latin America | 0.98 (0.29–2.03) | 95.8 | 3 | Botler et al. [66] | Brazil |
| Cornejo et al. [68] | Chile | ||||
| Ramalho et al. [67] | Brazil | ||||
| Middle East/North Africa | 1.18 (0.64–1.87) | 96.5 | 7 | Abbaskhanian et al. [69] | Iran |
| Alfadhel et al. [72] | Saudi Arabia | ||||
| Al Hosani et al. [74] | United Arab Emirates | ||||
| Habib et al. [44] | Iran | ||||
| Karamifar et al. [70] | Iran | ||||
| Motamedi et al. [71] | Iran | ||||
| Ozalp et al. [73] | Turkey | ||||
| North America | 0.81 (0.58–1.07) | 82.3 | 6 | Frazier et al. [42] | United States |
| Hansen et al. [75] | United States | ||||
| Kelly and Palombi [76] | United States | ||||
| Maccready and Hussey [28] | United States | ||||
| Wainer and Sideman [43] | United States | ||||
| Zytkovicz et al. [77] | United States | ||||
| Southeast Asia | 0.03 (0.02–0.05) | NA | 1 | Pangkanon et al. [80] | Thailand |
| West Pacific | 0.68 (0.43–0.98) | 94.2 | 8 | Boneh et al. [34] | Australia |
| Lin et al. [29] | China | ||||
| Liu and Zuo [83] | China | ||||
| Niu et al. [91] | Taiwan | ||||
| Su et al. [86] | China | ||||
| Wang et al. [88] | China | ||||
| Yoon et al. [90] | South Korea | ||||
| Zhan et al. [89] | China | ||||
| Global (non-regionally weighted) | 0.96 (0.75–1.19) | 98.0 | 44 | – | – |
| Global (regionally weighted)b | 0.64 (0.53–0.75) | NA | 44 | – | – |
CI = confidence interval; NA = not available
aIncludes only estimates in which the diagnostic method used for case confirmation was considered strong in the quality assessment tool
bGlobal prevalence was calculated by weighting each region by its relative contribution to the total population
cIncludes estimates for which the diagnostic cutoff value was not reported. When a publication reported birth prevalence by Phe cutoff intervals, the value used was for the sum of the intervals
Eleven publications reported birth prevalence estimates (1 estimate per publication) with a confirmatory test Phe concentration cutoff value of 360 ± 100 µmol/L. The regionally weighted global birth prevalence was 0.96 (95% CI 0.50–1.42) per 10,000 births (Table 4 and Fig. 2B). The lowest regional birth prevalence was observed in North America, with 0.49 cases per 10,000 births (95% CI 0.38–0.61), based on two publications that presented very similar results [42, 43], as reflected in the heterogeneity statistic I2 value of 0. The highest birth prevalence was observed in the Middle East/North Africa, 1.60 (95% CI 1.06–2.31) per 10,000 births, based on a single estimate [44].
Ten publications (1 estimate each) reported birth prevalence estimates using a confirmatory test Phe concentration cutoff value of 600 ± 100 µmol/L. The regionally weighted global birth prevalence was 0.50 (95% CI 0.37–0.64) per 10,000 births (Table 4 and Fig. 2C) for this cutoff value.
For the 1200 ± 200 µmol/L cutoff value for a Phe concentration confirmatory test, 20 publications (1 estimate each) were eligible and the regionally weighted global birth prevalence was 0.30 (95% CI 0.20–0.40) per 10,000 births (Table 4 and Fig. 2D).
Discussion
The overall meta-analysis conducted in this systematic review provides a regionally weighted global birth prevalence of PAH deficiency of 0.64 (95% CI 0.53–0.75) per 10,000 births. It is important to weight birth prevalence estimates by region so that the global PAH deficiency birth prevalence reflects both the birth prevalence and population size of each region rather than just the inverse variance (primarily driven by the sample size) of the individual studies (as was done for the calculation of non–regionally weighted birth prevalence). The highest regional birth prevalence in the overall analysis was reported in the Middle East/North Africa, where consanguineous marriages are among the most frequent in the world, with frequencies up to 42% in Saudi Arabia [45].
Among estimates with a confirmatory test Phe concentration cutoff value of 360 ± 100 µmol/L, the regionally weighted global birth prevalence was 0.96 (95% CI 0.50–1.42) per 10,000 births. On the basis of recent European and American College of Medical Genetics and Genomics guidelines (Table 1), this would represent the population for which treatment in children is recommended. Based on the single estimate for Middle East/North Africa, the birth prevalence was again highest in this region [44].
In the meta-analyses based on Phe concentration cutoff values of 600 µmol/L and 1200 µmol/L, the regionally weighted global prevalences were 0.50 (95% CI 0.37–0.64) and 0.30 (95% CI 0.20–0.40), respectively, per 10,000 births. Regional variation in the prevalence of PAH deficiency defined by these cutoff values was observed, with higher prevalences in Europe, Latin America, North America, and the Middle East than was observed globally. In a recent analysis of global variations in PAH genotype [46], genotypes associated with classical PKU (Phe ≥ 1200 µmol/L) tended to be the most common in the Middle East.
As might be expected, in this meta-analysis we observed decreasing pooled birth prevalence as confirmatory test Phe cutoff values increased (Table 4). The decreasing prevalence we observed with increasing Phe cutoff values should be interpreted cautiously. Specifically, this finding does not necessarily reflect differences in the relative frequencies of classical, moderate, mild PKU and HPA, but rather the fact that individuals with higher Phe levels are included in the estimates with lower cutoff values (e.g., the pooled prevalence for the 360 µmol/L cutoff value includes individuals that would be diagnosed as having classical and severe PKU per Table 1). This approach was taken to ascertain the birth prevalence of all individuals whose Phe levels were within the treatable range and the impact different confirmatory Phe cutoff thresholds have on PAH deficiency birth prevalence estimates. The confidence intervals for the various Phe cutoff thresholds had substantial overlap, likely due to heterogeneity of estimates from individual studies.
As evidenced by the high I2 values, heterogeneity of birth prevalence estimates was generally high, even among estimates stratified by region and Phe concentration cutoff values for case confirmation. Heterogeneity may be partly explained by random variation related to sampling, which is supported by the fact that many included studies were small (35% of the 238 estimates scored weak on precision of the prevalence estimate [Fig. 2]). Other reasons for heterogeneity include variations in age at screening and confirmatory testing, and dietary intake prior to sampling.
We found that data elements that are key to understanding the reported birth prevalence estimates were often missing: 30% of the 238 estimates scored weak on case definition (i.e., failed to provide Phe cutoff values for both screening and for case confirmation), and 66% scored moderate on this domain (failed to report on either screening or confirmatory Phe cutoff values); 11% did not report the study setting/source population or derived the information from personal communications. In addition, 126 of 238 reported birth prevalence estimates (53%) scored moderate or weak in diagnostic method used for case confirmation. Thirteen percent of the 238 estimates lacked information on the time period assessed, 3% on the assay used for screening, and 38% on the assay used for case confirmation. Although the frequency of BH4 deficiency is very low (1–2% of HPA cases) [6], it was not reported or not excluded from the reported birth prevalence estimates in 81% of the 238 birth prevalence estimates included in this review.
Substantial inconsistencies were observed in the nominal diagnoses reported, even in recent publications, with poor or inaccurate distinction between PKU, moderate PKU, classical PKU, and HPA (Additional file 2).
We have not found published papers estimating the global prevalence of PAH deficiency. However, two recently published reviews estimated the global prevalence of PKU. Shoraka et al. [47] identified studies reporting the birth prevalence of classical PKU in newborns and meta-analyzed them by region and overall (non-regionally weighted, with no stratification by case confirmation Phe cut off value). Hillert et al. [46] used unpublished information from national screening centers and reports identified through a literature search to estimate a global prevalence of PKU in newborns. Table 5 provides a comparison of the birth prevalence estimates from our analysis with the results from the studies by Shoraka et al. [46] and Hillert et al. [45].
Table 5.
Comparison of birth prevalence estimates among recent literature reviews
| Region | Birth prevalence estimate per 10,000 (95% CI) | ||
|---|---|---|---|
| Hillert et al. [46] | Shoraka et al. [47] | This studyb | |
| Europea | NR | 0.81 (0.65–0.97) | 1.14 (0.89–1.41) |
| Middle East/North Africa | NR | NR | 1.18 (0.64–1.87) |
| Eastern Mediterranean | NR | 0.98 (0.62–1.35) | NR |
| Pan America | NR | 0.53 (0.46–0.61) | NR |
| Latin America | NR | NR | 0.98 (0.29–2.03) |
| North America | NR | NR | 0.81 (0.58–1.07) |
| Southeast Asia | NR | 0.03 (0.02–0.05) | 0.03 (0.02–0.05) |
| West Pacific | NR | 0.29 (0.09–0.50) | 0.68 (0.43–0.98) |
| Global (non-regionally weighted) | NR | 0.60 (0.51–0.69) | 0.96 (0.75–1.19) |
| Global (regionally weighted) | 0.42 (NR) | NR | 0.64 (0.53–0.75)c |
CI = confidence interval; NR = not reported
aShoraka et al. incorrectly classified one included publication as European when it was in fact a North American study
bTable 4 presents the birth prevalence estimates from this analysis in further detail
cGlobal prevalence was calculated by weighting each region by its relative contribution to the total population
The largest differences between the current study and the study by Shoraka et al. were seen in Europe, the Americas, and the West Pacific regions. The similarity between the overall estimate by Shoraka et al. and the currently reported regionally weighted global birth prevalence is likely largely due to chance, as substantially different inclusion criteria and methodologies were employed in the two studies (Additional file 1: Figure A-1). Shoraka et al. excluded publications considered to have a high risk of bias as assessed using an existing 10-point checklist [48], which has some similar elements to the quality of evidence tool used in this publication. There was no requirement that cases be confirmed. The reported prevalence was described as relating to classical PKU, even though the Phe cutoff for confirmatory tests of the included studies ranged from 1.65 mg/dL (equivalent to 100 µmol/L) to 20 mg/dL (1211 µmol/L).
The current study provides a higher estimate of the global birth prevalence of PAH deficiency than Hillert et al. Unfortunately the inclusion and exclusion criteria and the method(s) for combining estimates from individual studies are not fully described in that paper, nor are the sources fully described; the global estimate included data from countries that the study describes as lacking newborn screening programs in parts of Africa, Asia, South America, and the Caribbean [46].
The current findings confirm that regional differences exist in the birth prevalence of PAH deficiency, with higher frequencies of inheritance of this autosomal recessive disease in areas with higher frequencies of consanguineous marriages, as has also been noted by others [46, 47].
Limitations of this study include incomplete reporting of key data elements in many of the included publications. In addition, the precision of the reported prevalence was low for most of the included estimates due to small sample sizes. No articles were identified reporting on the birth prevalence of PAH deficiency in Sub-Saharan Africa and birth prevalence estimates from countries in Southeast Asia were limited, lacking representation of some of the most populous countries in the region such as India. Absence of estimates could be attributed to absence of newborn screening programs for PAH deficiency in specific countries and regions [15], or lack of published estimates from newborn screening programs meeting the inclusion criteria for this review, such as the requirement that the full-text article be written in English. Strengths of this study include the fact that only confirmed cases were included in the qualitative synthesis, and that the meta-analysis only included estimates based on higher quality confirmatory assays. In addition, meta-analyses were undertaken based on clinically relevant diagnostic cutoff values.
Conclusions
In this systematic literature review and meta-analysis, we estimated the regionally weighted global birth prevalence of PAH deficiency to be 0.64 (95% CI 0.53–0.75) per 10,000 births (overall). The estimated regionally weighted global birth prevalence among newborns with Phe level ≥ 360 ± 100 µmol/L at diagnosis was 0.96 (95% CI 0.50–1.42), which is the population for whom treatment is recommended. Substantial regional variation was observed with an elevated birth prevalence of this autosomal recessive disease in regions with higher frequencies of consanguineous births. Despite the fact that newborn screening has been widely implemented in much of the world for decades, the precision of the estimates is limited by the unavailability of publications on large population samples. This observation underscores the need for more comprehensive and systematic data collection as well as improved standards for reporting results. Only with more widespread availability of data from newborn screening programs from large populations will it be possible to obtain robust estimates and truly understand the magnitude of this serious and treatable condition.
Supplementary Information
Additional file 1. Literature search strategy, quality assessment, and country region classification. Description of the literature search strategy, how quality assessments were calculated for precision, and how country regions were classified.
Additional file 2. Data extraction table. 238 birth prevalence estimates from 85 publications.
Additional file 3. Meta-analysis forest plot figures. Figure A-2. Meta-Analysis Results by Region: Confirmatory Test Phenylalanine Cutoff Value of 360 ± 100 μmol/L. Figure A-3. Meta-Analysis Results by Region: Confirmatory Test Phenylalanine Cutoff Value of 600 ± 100 μM/L. Figure A-4. Meta-Analysis Results by Region: Confirmatory Test Phenylalanine Cutoff Value of 1200 ± 200 μM/L. Figure A-5. Meta-Analysis Results by Region: Overall Analysis.
Acknowledgements
Editorial assistance was provided by John Forbes (RTI Health Solutions), medical writing support was provided by Kate Lothman (RTI Health Solutions), and graphical services were provided by Jason Mathes (RTI Health Solutions).
Abbreviations
- ACMG
American College of Medical Genetics and Genomics
- BH4
Tetrahydrobiopterin
- CI
Confidence interval
- HPA
Hyperphenylalaninemia
- NA
Not available
- NR
Not reported
- PAH
Phenylalanine hydroxylase
- Phe
Phenylalanine
- PICOS
Population, intervention, comparison, outcomes, study design
- PKU
Phenylketonuria
- US
United States
Authors' contributions
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: PKF, SL, RS, KA, AVM, BC, MP, AH. Drafting the work or revising it critically for important intellectual content: PKF, SL, RS, KA, AVM, BC, MP, AH. Final approval of the version to be published: PKF, SL, RS, KA, AVM, BC, MP, AH. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: PKF, SL, RS, KA, AVM, BC, MP, AH. All authors read and approved the final manuscript.
Funding
This work was funded by BioMarin Pharmaceutical Inc. RTI’s authors received no compensation other than annual salary from employer.
Availability of data and materials
The dataset supporting the conclusions of this article is within the published manuscript and its appendices.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
RS, KA, and SL are employees of BioMarin Pharmaceutical Inc. PKF was a consultant for BioMarin Pharmaceutical Inc. when this research was conducted. Research team members AH, AVM, BC and MP are full-time employees of RTI Health Solutions. RTI Health Solutions is a unit of RTI International, an independent, nonprofit organization that conducts work for government, public, and private organizations, including pharmaceutical companies. RTI authors participate in this work in the course of employment as work for hire, pursuant to a contract to conduct an independent research study for a client (BioMarin Pharmaceutical Inc.).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van Spronsen FJ, van Wegberg AM, Ahring K, Belanger-Quintana A, Blau N, Bosch AM, et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 2017;5(9):743–756. doi: 10.1016/S2213-8587(16)30320-5. [DOI] [PubMed] [Google Scholar]
- 2.van Wegberg AMJ, MacDonald A, Ahring K, Belanger-Quintana A, Blau N, Bosch AM, et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J Rare Dis. 2017;12(1):162. doi: 10.1186/s13023-017-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vockley J, Andersson HC, Antshel KM, Braverman NE, Burton BK, Frazier DM, et al. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet Med. 2014;16(2):188–200. doi: 10.1038/gim.2013.157. [DOI] [PubMed] [Google Scholar]
- 4.Blau N. Genetics of phenylketonuria: then and now. Hum Mutat. 2016;37(6):508–515. doi: 10.1002/humu.22980. [DOI] [PubMed] [Google Scholar]
- 5.PAHvdb: Phenylalanine Hydroxylase Gene Locus-Specific Database. 2020. http://www.biopku.org/home/pah.asp. Accessed 4 Sept 2020.
- 6.Opladen T, López-Laso E, Cortès-Saladelafont E, Pearson TS, Sivri HS, Yildiz Y, et al. Consensus guideline for the diagnosis and treatment of tetrahydrobiopterin (BH(4)) deficiencies. Orphanet J Rare Dis. 2020;15(1):126. doi: 10.1186/s13023-020-01379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams RA, Mamotte CD, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev. 2008;29(1):31–41. [PMC free article] [PubMed] [Google Scholar]
- 8.Anikster Y, Haack TB, Vilboux T, Pode-Shakked B, Thony B, Shen N, et al. Biallelic mutations in DNAJC12 cause hyperphenylalaninemia, dystonia, and intellectual disability. Am J Hum Genet. 2017;100(2):257–266. doi: 10.1016/j.ajhg.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald A, van Wegberg AMJ, Ahring K, Beblo S, Bélanger-Quintana A, Burlina A, et al. PKU dietary handbook to accompany PKU guidelines. Orphanet J Rare Dis. 2020;15(1):171. doi: 10.1186/s13023-020-01391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDonald A, van Wegberg AMJ, Ahring K, Beblo S, Bélanger-Quintana A, Burlina A, et al. Correction to: PKU dietary handbook to accompany PKU guidelines. Orphanet J Rare Dis. 2020;15(1):230. doi: 10.1186/s13023-020-01486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Medicines Agency. Palynziq—Summary of product characteristics. 2019. https://www.ema.europa.eu/en/documents/product-information/palynziq-epar-product-information_en.pdf. Accessed 4 Sept 2020.
- 12.FDA. Palynziq—US package insert. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761079s000lbl.pdf.
- 13.European Medicines Agency. Kuvan—Summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/kuvan-epar-product-information_en.pdf. Accessed 4 Sept 2020.
- 14.FDA. Kuvan - US package insert. 2007. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/022181lbl.pdf. Accessed 4 Sep 2020.
- 15.Therrell BL, Padilla CD, Loeber JG, Kneisser I, Saadallah A, Borrajo GJ, et al. Current status of newborn screening worldwide: 2015. Semin Perinatol. 2015;39(3):171–187. doi: 10.1053/j.semperi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Zerjav Tansek M, Groselj U, Angelkova N, Anton D, Baric I, Djordjevic M, et al. Phenylketonuria screening and management in southeastern Europe—survey results from 11 countries. Orphanet J Rare Dis. 2015;30(10):68. doi: 10.1186/s13023-015-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrajo GJC. Newborn screening for phenylketonuria: Latin American consensus guidelines. J Inborn Errors Metab Screen. 2016 2016/01/01;4:2326409816682764.
- 18.Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. 1963;32:338–343. [PubMed] [Google Scholar]
- 19.Chace DH, Hannon WH. Technological journey from colorimetric to tandem mass spectrometric measurements in the diagnostic investigation for phenylketonuria. J Inborn Errors Metab Screen. 2016 2016/01/01;4:2326409816671733.
- 20.Blumenfeld CM, Wallace MJ, Anderson R. Phenylketonuria-the guthrie screening test-a method of quantitation, observations on reliability and suggestions for improvement. Calif Med. 1966;105(6):429–434. [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;21(339):b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva LC, Ordunez P, Paz Rodriguez M, Robles S. A tool for assessing the usefulness of prevalence studies done for surveillance purposes: the example of hypertension. Rev Panam Salud Publica. 2001;10(3):152–160. doi: 10.1590/S1020-49892001000900002. [DOI] [PubMed] [Google Scholar]
- 23.Al-Jader LN, Newcombe RG, Hayes S, Murray A, Layzell J, Harper PS. Developing a quality scoring system for epidemiological surveys of genetic disorders. Clin Genet. 2002;62(3):230–234. doi: 10.1034/j.1399-0004.2002.620308.x. [DOI] [PubMed] [Google Scholar]
- 24.United Nations. World population prospects 2019. File POP/1–1: total population (both sexes combined) by region, subregion and country, annually for 1950–2100 (thousands). 2019. https://population.un.org/wpp/. Accessed 28 May 28 2020.
- 25.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Commun Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M. Cochrane handbook for systematic reviews of interventions, version 6.0. 2019. https://training.cochrane.org/handbook. Accessed 5 March 2020. [DOI] [PMC free article] [PubMed]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maccready RA, Hussey MG. Newborn phenylketonuria detection program in Massachusetts. Am J Public Health Nations Health. 1964;54:2075–2081. doi: 10.2105/AJPH.54.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y, Zheng Q, Zheng T, Zheng Z, Lin W, Fu Q. Expanded newborn screening for inherited metabolic disorders and genetic characteristics in a southern Chinese population. Clin Chim Acta. 2019;494:106–111. doi: 10.1016/j.cca.2019.03.1622. [DOI] [PubMed] [Google Scholar]
- 30.Carson NA, Carre IJ, Neill DW. Results of routine screening for phenylketonuria in early infancy, Northern Ireland (1960–1967) Arch Dis Child. 1968;43(228):145–146. doi: 10.1136/adc.43.228.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnopp JJ, Lorey FW, Currier RJ, Eastman JW, Velazquez KB, Morales DR, et al. Results of screening for phenylketonuria using a lower cutoff value in early collected specimens. Screening. 1995;3(4):193–199. doi: 10.1016/0925-6164(94)00022-Z. [DOI] [Google Scholar]
- 32.Martin PH. Six years of newborn PKU screening. J Indiana State Med Assoc. 1968;61(8):1107–1108. [PubMed] [Google Scholar]
- 33.Yang LL, Mao HQ, Zhang WF, Zhao ZY, Yang RL, Zhou XL, et al. Pitfalls in the management of phenylketonuria in China. Hong Kong J Paediatr. 2012;17(3):143–147. [Google Scholar]
- 34.Boneh A, Francis DE, Humphrey M, Upton HJ, Peters HL. Three-year audit of the hyperphenylalaninaemia/phenylketonuria spectrum in Victoria. J Paediatr Child Health. 2006;42(9):496–498. doi: 10.1111/j.1440-1754.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 35.Read KS, Allen RJ, Haddy TB. Phenylketonuria in newborns. Mich Med. 1969;68(13):691–697. [PubMed] [Google Scholar]
- 36.Ervin EN. Hyperphenylalanemia. Incidence in Maine since 1964. J Maine Med Assoc. 1970;61(2):30–32. [PubMed] [Google Scholar]
- 37.Somers DG, Favreau L. Newborn screening for phenylketonuria: incidence and screening procedures in North America. Can J Public Health. 1982;73(3):206–207. [PubMed] [Google Scholar]
- 38.Ounap K, Lilleväli H, Metspalu A, Lipping-Sitska M. Development of the phenylketonuria screening programme in Estonia. J Med Screen. 1998;5(1):22–23. doi: 10.1136/jms.5.1.22. [DOI] [PubMed] [Google Scholar]
- 39.Visakorpi JK, Palo J, Renkonen OV. The incidence of PKU in Finland. Acta Paediatr Scand. 1971;60(6):666–668. doi: 10.1111/j.1651-2227.1971.tb07007.x. [DOI] [PubMed] [Google Scholar]
- 40.Ratrisawadi V, Horpaopan S, Chotigeat U, Sangtawesin V, Kanjanapattanakul W, Ningsanond V, et al. Neonatal screening program in Rajavithi Hospital, Thailand. Southeast Asian J Trop Med Public Health. 1999;30(Suppl 2):28–32. [PubMed] [Google Scholar]
- 41.Kocova M, Anastasovska V. Phenylketonuria screening in the Republic of Macedonia. Orphanet J Rare Dis. 2016;11(1):112. doi: 10.1186/s13023-016-0483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frazier DM, Millington DS, McCandless SE, Koeberl DD, Weavil SD, Chaing SH, et al. The tandem mass spectrometry newborn screening experience in North Carolina: 1997–2005. J Inherit Metab Dis. 2006;29(1):76–85. doi: 10.1007/s10545-006-0228-9. [DOI] [PubMed] [Google Scholar]
- 43.Wainer SC, Sideman L. Nine years of PKU screening in Pennsylvania. Health Lab Sci. 1974;11(4):306–311. [PubMed] [Google Scholar]
- 44.Habib A, Fallahzadeh MH, Kazeroni HR, Ganjkarimi AH. Incidence of phenylketonuria in Southern Iran. Iran J Med Sci. 2010;35(2):137–139. [Google Scholar]
- 45.Hamamy H, Antonarakis SE, Cavalli-Sforza LL, Temtamy S, Romeo G, Kate LP, et al. Consanguineous marriages, pearls and perils: Geneva International Consanguinity Workshop Report. Genet Med. 2011;13(9):841–847. doi: 10.1097/GIM.0b013e318217477f. [DOI] [PubMed] [Google Scholar]
- 46.Hillert A, Anikster Y, Belanger-Quintana A, Burlina A, Burton BK, Carducci C, et al. The genetic landscape and epidemiology of phenylketonuria. Am J Hum Genet. 2020;107(2):234–250. doi: 10.1016/j.ajhg.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoraka HR, Haghdoost AA, Baneshi MR, Bagherinezhad Z, Zolala F. Global prevalence of classic phenylketonuria based on Neonatal Screening Program data: systematic review and meta-analysis. Clin Exp Pediatr. 2020;63(2):34–43. doi: 10.3345/kjp.2019.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Thalhammer O. Distribution and frequency of PKU and hyperphenylalaninemia in eastern and western Austria. Ir Med J. 1976;69(15):396–397. [PubMed] [Google Scholar]
- 50.Lindner M, Gramer G, Haege G, Fang-Hoffmann J, Schwab KO, Tacke U, et al. Efficacy and outcome of expanded newborn screening for metabolic diseases–report of 10 years from South-West Germany. Orphanet J Rare Dis. 2011;6:44. doi: 10.1186/1750-1172-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathias D, Bickel H. Follow-up study of 16 years neonatal screening for inborn errors of metabolism in West Germany. Eur J Pediatr. 1986;145(4):310–312. doi: 10.1007/BF00439408. [DOI] [PubMed] [Google Scholar]
- 52.Schulze A, Lindner M, Kohlmuller D, Olgemoller K, Mayatepek E, Hoffmann GF. Expanded newborn screening for inborn errors of metabolism by electrospray ionization-tandem mass spectrometry: results, outcome, and implications. Pediatrics. 2003;111(6 Pt 1):1399–1406. doi: 10.1542/peds.111.6.1399. [DOI] [PubMed] [Google Scholar]
- 53.Loukas YL, Soumelas GS, Dotsikas Y, Georgiou V, Molou E, Thodi G, et al. Expanded newborn screening in Greece: 30 months of experience. J Inherit Metab Dis. 2010;33(Suppl 3):S341–S348. doi: 10.1007/s10545-010-9181-8. [DOI] [PubMed] [Google Scholar]
- 54.Missiou-Tsagaraki S, Soulpi K, Loumakou M. Phenylketonuria in Greece: 12 years' experience. J Ment Defic Res. 1988;32(Pt 4):271–287. doi: 10.1111/j.1365-2788.1988.tb01416.x. [DOI] [PubMed] [Google Scholar]
- 55.Mehes K, Juhasz E, Ruszinko V. Chromatographic screening of 70,328 neonates for inborn errors of amino acid metabolism. Acta Paediatr Hung. 1985;26(2):147–149. [PubMed] [Google Scholar]
- 56.Antonozzi I, Dominici R, Andreoli M, Monaco F. Neonatal screening in Italy for congenital hypothyroidism and metabolic disorders: hyperphenylalaninemia, maple syrup urine disease and homocystinuria. J Endocrinol Invest. 1980;3(4):357–363. doi: 10.1007/BF03349371. [DOI] [PubMed] [Google Scholar]
- 57.Zaffanello M, Zamboni G, Tatò L. Neonatal screening program for inborn errors of metabolism: a retrospective study from 1978 to 1997 in Northeastern Italy. Ital J Pediatr. 2002;28:479–483. [Google Scholar]
- 58.Cabalska B, Nowaczewska I, Duczynska N, Laskowska-Klita T. Twenty-five years experience with newborn screening for phenylketonuria (PKU) in Poland. Screening. 1993;2(1):29–32. doi: 10.1016/0925-6164(93)90015-B. [DOI] [Google Scholar]
- 59.Vilarinho L, Rocha H, Sousa C, Marcao A, Fonseca H, Bogas M, et al. Four years of expanded newborn screening in Portugal with tandem mass spectrometry. J Inherit Metab Dis. 2010;33(Suppl 3):S133–S138. doi: 10.1007/s10545-010-9048-z. [DOI] [PubMed] [Google Scholar]
- 60.Dluholucký S, Knapková M. Newborn screening in Slovakia—from 1985 till today/Novorodencký skríning na Slovensku—od roku 1985 doposiaľ. Acta Facul Pharm Univ Comen. 2013;60(Suppl VIII):32–6.
- 61.Smon A, Groselj U, Zerjav Tansek M, Bicek A, Oblak A, Zupancic M, et al. Newborn screening in Slovenia. Zdr Varst. 2015;54(2):86–90. doi: 10.1515/sjph-2015-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernández-Iglesias C, Flórez IG, Rodríguez-González MC, Gascón S. Neonatal screening for phenylketonuria and congenital hypothyroidism in Principado de Asturias (Spain) using two types of blood samples. Screening. 1995;4(3):131–138. doi: 10.1016/0925-6164(95)00119-0. [DOI] [Google Scholar]
- 63.Gerasimova NS, Samutin AA, Steklova IV, Tuuminen T. Phenylketonuria screening in Moscow using a microplate fluorometric method. Screening. 1992;1(1):27–35. doi: 10.1016/0925-6164(92)90027-3. [DOI] [PubMed] [Google Scholar]
- 64.Walker V, Clayton BE, Ersser RS, Francis DE, Lilly P, Seakins JW, et al. Hyperphenylalaninaemia of various types among three-quarters of a million neonates tested in a screening programme. Arch Dis Child. 1981;56(10):759–764. doi: 10.1136/adc.56.10.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mardesic D, Gjuric G, Jancikovic J. Screening for phenylketonuria in Yugoslavia (SR Crotia) 1979–1984. J Inherit Metab Dis. 1986;9(Suppl 2):234–236. doi: 10.1007/BF01799715. [DOI] [Google Scholar]
- 66.Botler J, Camacho LA, Cruz MM. Phenylketonuria, congenital hypothyroidism and haemoglobinopathies: public health issues for a Brazilian newborn screening program. Cad Saude Publica. 2012;28(9):1623–1631. doi: 10.1590/S0102-311X2012000900002. [DOI] [PubMed] [Google Scholar]
- 67.Ramalho AR, Ramalho RJ, Oliveira CR, Magalhaes MM, Santos EG, Sarmento PM, et al. Evaluation of effectiveness and outcome of PKU screening and management in the state of Sergipe. Brazil Arq Bras Endocrinol Metabol. 2014;58(1):62–67. doi: 10.1590/0004-2730000002885. [DOI] [PubMed] [Google Scholar]
- 68.Cornejo V, Raimann E, Cabello JF, Valiente A, Becerra C, Opazo M, et al. Past, present and future of newborn screening in Chile. J Inherit Metab Dis. 2010;33(Suppl 3):S301–S306. doi: 10.1007/s10545-010-9165-8. [DOI] [PubMed] [Google Scholar]
- 69.Abbaskhanian A, Zamanfar D, Afshar P, Asadpoor E, Rouhanizadeh H, Jafarnia A, et al. Incidence of neonatal hyperphenylalaninemia based on high-performance liquid chromatography confirmatory technique in Mazandaran province, northern Iran (2007–2015) Int J Prev Med. 2017;8:93. doi: 10.4103/ijpvm.IJPVM_24_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karamifar H, Ordoei M, Karamizadeh Z, Amirhakimi G. Incidence of neonatal hyperphenylalaninemia in Fars province, south Iran. Iran J Pediatr. 2010;20(2):216–220. [PMC free article] [PubMed] [Google Scholar]
- 71.Motamedi N, Godarzi E, Pordanjani SR, Valizadeh R, Moradi Y, Sohrabivafa M, et al. Incidence of phenylketonuria in Lorestan province, west of Iran (2006–2016) Int J Pediatr. 2017;5(4):4713–4721. [Google Scholar]
- 72.Alfadhel M, Al Othaim A, Al Saif S, Al Mutairi F, Alsayed M, Rahbeeni Z, et al. Expanded newborn screening program in Saudi Arabia: incidence of screened disorders. J Paediatr Child Health. 2017;53(6):585–591. doi: 10.1111/jpc.13469. [DOI] [PubMed] [Google Scholar]
- 73.Ozalp I, Coskun T, Tokatli A, Kalkanoglu HS, Dursun A, Tokol S, et al. Newborn PKU screening in Turkey: at present and organization for future. Turk J Pediatr. 2001;43(2):97–101. [PubMed] [Google Scholar]
- 74.Al Hosani H, Salah M, Osman HM, Farag HM, El-Assiouty L, Saade D, et al. Expanding the comprehensive national neonatal screening programme in the United Arab Emirates from 1995 to 2011. East Mediterr Health J. 2014;20(1):17–23. doi: 10.26719/2014.20.1.17. [DOI] [PubMed] [Google Scholar]
- 75.Hansen H, Shahidi A, Stein ZA. Screening for phenylketonuria in New York City Threshold values reconsidered. Public Health Rep. 1978;93(3):246–251. [PMC free article] [PubMed] [Google Scholar]
- 76.Kelly S, Palombi J. Phenylketonuria in New York State. Public Health Rep. 1967;82(10):921–924. doi: 10.2307/4593161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zytkovicz TH, Fitzgerald EF, Marsden D, Larson CA, Shih VE, Johnson DM, et al. Tandem mass spectrometric analysis for amino, organic, and fatty acid disorders in newborn dried blood spots: a two-year summary from the New England Newborn Screening Program. Clin Chem. 2001;47(11):1945–1955. doi: 10.1093/clinchem/47.11.1945. [DOI] [PubMed] [Google Scholar]
- 78.Charoensiriwatana W, Janejai N, Boonwanich W, Krasao P, Chaisomchit S, Waiyasilp S. Neonatal screening program in Thailand. Southeast Asian J Trop Med Public Health. 2003;34(Suppl 3):94–100. [PubMed] [Google Scholar]
- 79.Pangkanon S, Ratrisawadi V, Charoensiriwatana W, Techasena W, Boonpuan K, Srisomsap C, et al. Phenylketonuria detected by the neonatal screening program in Thailand. Southeast Asian J Trop Med Public Health. 2003;34(Suppl 3):179–181. [PubMed] [Google Scholar]
- 80.Pangkanon S, Charoensiriwatana W, Janejai N, Boonwanich W, Chaisomchit S. Detection of phenylketonuria by the newborn screening program in Thailand. Southeast Asian J Trop Med Public Health. 2009;40(3):525–529. [PubMed] [Google Scholar]
- 81.Sutivijit Y, Banpavichit A, Wiwanitkit V. Prevalence of neonatal hypothyroidism and phenylketonuria in Southern Thailand: a 10-year report. Indian J Endocrinol Metab. 2011;15(2):115–117. doi: 10.4103/2230-8210.81941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen RG, Pan XS, Qian DL, Guo H. Twenty-one cases of phenylketonuria out of 358,767 newborns in Shanghai, China. J Inherit Metab Dis. 1989;12(4):485. doi: 10.1007/BF01802047. [DOI] [PubMed] [Google Scholar]
- 83.Liu SR, Zuo QH. Newborn screening for phenylketonuria in eleven districts. Chin Med J (Engl) 1986;99(2):113–118. [PubMed] [Google Scholar]
- 84.Maitusong R, Japaer R, Zhao ZY, Yang RL, Huang XL, Mao HQ. Newborn screening in Zhejiang. China Chin Med J (Engl) 2012;125(4):702–704. [PubMed] [Google Scholar]
- 85.Shi XT, Cai J, Wang YY, Tu WJ, Wang WP, Gong LM, et al. Newborn screening for inborn errors of metabolism in mainland china: 30 years of experience. JIMD Rep. 2012;6:79–83. doi: 10.1007/8904_2011_119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Su Y, Wang H, Rejiafu N, Wu B, Jiang H, Chen H, et al. The molecular epidemiology of hyperphenylalaninemia in Uygur population: incidence from newborn screening and mutational spectra. Ann Transl Med. 2019;7(12):258. doi: 10.21037/atm.2019.05.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tu WJ, Cai J, Shi XD. Newborn screening for inborn errors of metabolism in Beijing, China: 22 years of experience. J Med Screen. 2011;18(4):213–214. doi: 10.1258/jms.2011.011125. [DOI] [PubMed] [Google Scholar]
- 88.Wang X, He Y, Jiang Y, Feng X, Zhang G, Xia Z, et al. Screening and mutation analysis of hyperphenylalaninemia in newborns from Xiamen. China Clin Chim Acta. 2019;498:161–166. doi: 10.1016/j.cca.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 89.Zhan JY, Qin YF, Zhao ZY. Neonatal screening for congenital hypothyroidism and phenylketonuria in China. World J Pediatr. 2009;5(2):136–139. doi: 10.1007/s12519-009-0027-0. [DOI] [PubMed] [Google Scholar]
- 90.Yoon HR, Lee KR, Kang S, Lee DH, Yoo HW, Min WK, et al. Screening of newborns and high-risk group of children for inborn metabolic disorders using tandem mass spectrometry in South Korea: a three-year report. Clin Chim Acta. 2005;354(1–2):167–180. doi: 10.1016/j.cccn.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 91.Niu DM, Chien YH, Chiang CC, Ho HC, Hwu WL, Kao SM, et al. Nationwide survey of extended newborn screening by tandem mass spectrometry in Taiwan. J Inherit Metab Dis. 2010;33(Suppl 2):S295–305. doi: 10.1007/s10545-010-9129-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Literature search strategy, quality assessment, and country region classification. Description of the literature search strategy, how quality assessments were calculated for precision, and how country regions were classified.
Additional file 2. Data extraction table. 238 birth prevalence estimates from 85 publications.
Additional file 3. Meta-analysis forest plot figures. Figure A-2. Meta-Analysis Results by Region: Confirmatory Test Phenylalanine Cutoff Value of 360 ± 100 μmol/L. Figure A-3. Meta-Analysis Results by Region: Confirmatory Test Phenylalanine Cutoff Value of 600 ± 100 μM/L. Figure A-4. Meta-Analysis Results by Region: Confirmatory Test Phenylalanine Cutoff Value of 1200 ± 200 μM/L. Figure A-5. Meta-Analysis Results by Region: Overall Analysis.
Data Availability Statement
The dataset supporting the conclusions of this article is within the published manuscript and its appendices.