Abstract
Objective:
Angiogenesis plays an important role in the growth and metastasis of non-small cell lung cancer (NSCLC). Bevacizumab is a humanized monoclonal antibody that mainly acts on vascular endothelial growth factor A (VEGFA). Kinase insert domain receptor (KDR) is the most important target of VEGFA. The aim of present study was to investigate the influence of KDR genetic variation on the efficacy and safety of patients with advanced NSCLC receiving first-line bevacizumab plus chemotherapy regimen.
Methods:
A total of 169 patients with advanced NSCLC who received bevacizumab combined with chemotherapy were recruited in this study. Clinical outcome of the regimens was evaluated in the hospital. Peripheral blood and biopsy tissue specimens of patients were collected for the genotyping of KDR genetic variation and KDR mRNA expression, respectively. The association between KDR genotype status and other variables were analyzed. Univariate analysis of genotype status and prognosis was implemented using the Kaplan-Meier survival analysis method. Multivariate Cox regression analysis was performed to adjust the confounding factors.
Results:
Of the polymorphisms analyzed, only V297 L was of clinical significance. The prevalence of V297 L among the study population were as follows: CC genotype 123 cases (72.8%), CT genotype 41 cases (24.3%), TT genotype 5 cases (2.9%). The minimum allele frequency is 0.15. The distribution frequencies of the 3 genotypes corresponded with Hardy-Weinberg equilibrium (P = 0.489). Patients with TT and CT genotypes were merged in the subsequent comparison of clinical outcomes. The analysis of efficacy exhibited that the objective response rates (ORR) of patients with CC genotype and CT/TT genotypes were 52.8% and 47.8% (P = 0.561), respectively. Prognosis indicated that the median progression free survival (PFS) of patients with CC genotype and CT/TT genotype were 8.9 and 5.5 months, respectively (P = 0.006). The median OS of the 2 genotypes were 20.0 and 14.9 months, respectively (P = 0.021). Adjusted in multivariate Cox regression analysis of PFS, CT/TT genotypes were an independent factor for PFS [hazard ratio (HR) = 1.59, P = 0.011). Safety profile according to genotype status of V297 L failed to find significant difference. Interestingly, the expression of KDR mRNA of patients with CT/TT genotype was significantly higher than that of patients with CC genotype in the 58 cancer tissue specimens (P < 0.001).
Conclusion:
The clinical comes of patients with advanced NSCLC receiving first-line bevacizumab plus chemotherapy regimens might be impacted by polymorphism V297 L through mediating the mRNA expression of KDR.
Keywords: non-small cell lung cancer; bevacizumab, kinase insert domain receptor, polymorphism, clinical outcome, safety
Introduction
Lung cancer remains a leading cause of cancer-related death among various malignant tumors in both male and female with the highest morbidity and mortality all over the world. Non-small cell lung cancer (NSCLC) accounts for approximately 85% in lung cancer. Consequently, it is estimated that there are approximately 1.785 million new NSCLC cases and 1.505 million new deaths globally. 1 And it is estimated that there are approximately 0.623 million new NSCLC cases and 0.519 million new deaths in China annually. 2 Recent year have witnessed the dramatic advancement of molecular targeted therapy, which making the advanced NSCLC be the most successful tumor in the trend of precision medicine. 3 Patients with EGFR, ALK, ROS1, BRAF all have effective targeted drugs that bring the patients with survival benefits. 4 Additionally, owning to the development of next-generation sequencing (NGS), some less common or hard-to-target driver oncogenes such as KRAS, MET, RET, HER2 and NTRK were identified to be of clinical significance and the specific targeted drugs were in clinical development and exhibited promising activity for the patients accordingly. 5 Unfortunately, approximately 40% of patients were still lack of clear driver targets and became a research hotspot currently. 6 Amazingly, the anti-angiogenic drug bevacizumab combined with chemotherapy as first-line treatment achieved superior progression-free survival (PFS) and improved the survival of patients in the BEYOND and ECOG 4599 clinical trial. 7,8 Given that the objective response rate (ORR) of first-line platinum-based chemotherapy regimens ranged from 15%-26%, 9 addition of bevacizumab improved the ORR to approximately 50%. 10 Even though, considerable individual differences were observed regarding the efficacy of bevacizumab in clinical application constantly. The predictive biomarkers of bevacizumab were needed urgently. 11
Among all secreted vascular growth factors in tumor cells, vascular endothelial cell growth factor A (VEGFA) was considered as the major activator of angiogenesis, which acted selectively on the vascular endothelial cells, stimulating both normal and abnormal angiogenesis. 12 VEGFA integrated with the receptor VEGFR1 and kinase insert domain receptor (KDR) simultaneously. 13 Interestingly, a previous study investigated the prognostic significance of combining VEGFA, VEGFR1 and KDR mRNA expression in lung cancer and the results suggested that a high level of VEGFA expression and its receptors could be required for cancer progression, which indicated VEGFA/KDR single pathway might serve as one of the most important pathway for angiogenesis. 14 Bevacizumab is a recombinant humanized IgG1 monoclonal antibody that specifically acts on VEGFA to block vascular endothelial growth factor signaling, thereby blocking tumor tissue angiogenesis. 15 Additionally, bevacizumab is also the first antiangiogenic cancer therapy agent that has been used in combination with other anti-cancer agents for patients with metastatic CRC. 16 KDR is the most important receptor for VEGFA, its polymorphisms could be of clinical significance for the efficacy of antiangiogenic targeted drugs. The previous study investigated the influence of KDR gene polymorphism on the efficacy and safety of apatinib in the treatment of patients with advanced NSCLC who progressed after standard therapy and the conclusion exhibited that 4397 T>C polymorphism in KDR gene was associated with the clinical outcomes of the patients. 17 They demonstrated that KDR gene could be of potentially clinical significance. KDR gene is located on chromosome 4q12 and contains 30 exons. Ethnic difference regarding this gene was observed and discrepancies of mRNA expression were noted among different populations. 18 Interestingly, previous study suggested that the 889C>T polymorphism in the coding region of the KDR gene influenced the prognosis of patients with liver cancer treated with sorafenib dramatically. 19 Simultaneously, polymorphism in KDR gene was significantly related to the objective response rate (ORR) of bevacizumab combined with oxaliplatin in the treatment of patients with advanced CRC among European and American population. However, they failed to investigate the potential mechanism underlying. 20
In consequence, the aim of this study was to explore the influence of KDR genetic variation on the efficacy and safety of patients with advanced NSCLC receiving first-line bevacizumab plus chemotherapy regimen.
Materials and Methods
Study Design and Patient Enrollment
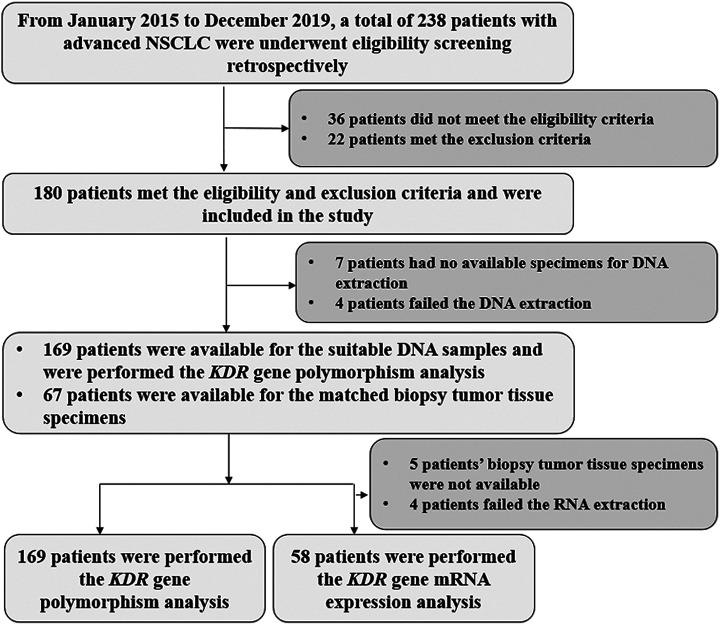
Given that bevacizumab was licensed in China more than 10 years and considerable patients with advanced NSCLC were treated with bevacizumab-based therapy clinically, the present study was designed as a retrospective analysis. Therefore, patients with advanced NSCLC who were treated with the first-line bevacizumab-based regimens from January 2015 to December 2019 in the Department of Respiratory Medicine of The Fourth Hospital of Hebei Medical University were recruited in this study. The detailed inclusion criteria included: (1) a histological or pathological diagnosis of advanced or recurrent NSCLC; (2) age ≥18 years; (3) Eastern Cooperative Oncology Group (ECOG) performance status of 0-2 score; (4) bevacizumab-based therapeutic regimens were administrated as first-line treatment; (5) at least one available measurable target lesion according to response evaluation criteria in solid tumors (RECIST 1.1). 21 The primary exclusion criteria were: (1) lung squamous cell carcinoma; (2) concomitant with another tumor or serious diseases according to the judgment of the investigator; (3) data of efficacy or prognosis assessment of the patients was not available. The flow chart of this retrospective study was illustrated in Figure 1. Eventually, a total of 169 patients with advanced NSCLC was enrolled. The primary endpoint of this study was PFS, the secondary endpoints were ORR, overall survival (OS), safety and the association analysis between KDR genetic variation and prognosis of patients receiving bevacizumab-based therapy. This study was undertaken in accordance with good clinical practice guidelines and approved by the ethics committees of The Fourth Hospital of Hebei Medical University (approval no. 2019 MEC068). All the patients enrolled were provided with the informed consent in accordance with the recommendations of the Declaration of Helsinki.
Figure 1.
The flow chart of the retrospective study for the influence of KDR genetic variation on the efficacy and safety of patients with advanced non-small cell lung cancer receiving first-line bevacizumab plus chemotherapy regimen.
Therapeutic Regimens
Eligible patients were treated with bevacizumab in combination with platinum-based regimens as first-line therapy. The chemotherapy regimens included pemetrexed or paclitaxel or docetaxel combined with platinum drugs (carboplatin or cisplatin). And the dosage of the chemotherapy was the routine 3-week regimens clinically. 22 Pemetrexed 500 mg/m2, paclitaxel 175 mg/m2, docetaxel 75 mg/m2 (the first day). Cisplatin 75 mg/m2 or carboplatin AUC = 5 (the first day), every 21 days was one cycle. The duration of chemotherapy was up to 4-6 cycles or depended on the actual situation when the patients were not tolerable to the chemotherapy. Bevacizumab was administrated with a dosage of 7.5 or 15 mg/kg at first day intravenously, every 21 days was one cycle until disease progression or unacceptable toxicity.
The combined chemotherapy regimens were stopped after 4-6 cycles treatment, and then bevacizumab monotherapy was used for maintenance treatment until progression or intolerable adverse reactions. Therapeutic response was evaluated according to RECIST version 1.1 criteria by investigator assessment. 21 And the change of target lesions was evaluated using CT scans every 2 cycles or depended on the actual situation. Furthermore, the dosage of the chemotherapy was constantly regulated according to the hematological or non-hematological toxicity that occurred during the treatment and the regimens were terminated when potential life-threatening toxic reactions were observed. Adverse reactions during treatment were evaluated using Common Terminology Criteria for Adverse Events (CTCAE) version 4.02 criteria to document toxicity profile that might be drug-related. 23
Collection of Appropriate Specimens and Genotyping of KDR Gene Polymorphism
Approximately 4 ml of peripheral blood specimens were collected from the patients. Genomic DNA was extracted using the phenol-chloroform method and then stored at −20°C for subsequent usage. The single nucleotide polymorphisms of KDR gene were collected from the NCBI database with the minor allele frequency (MAF) >0.1 among Chinese population. Of the polymorphisms analyzed, only rs2305948 (Val297Leu: V297 L) was of clinical significance. Consequently, the subsequent analysis of this study was concentrated on the correlation between V297 L polymorphism and clinical outcomes. The genotypes of V297 L polymorphism in KDR gene was genotyped using restriction fragment length polymorphisms polymerase chain reaction (PCR-RFLP). Genotyping of each sample was operated with the procedures according to the instructions. The PCR products were amplified firstly and then the product was incubated and digested with restriction enzymes. The PCR product was 296 bp. The forward primer was 5′-TTCCAAGACCATAGCTTACCA-3′, the reverse primer was 5′-TGTTTACCAAAGCCCAGATTT-3′. A total of 2 µL PCR products were digested using the restriction enzyme ApaI (Thermo Fisher Scientific, USA). The genotypes were distinguished by the size of the band: CC genotype (one 296 bp band); CT genotype (3 bands: one 296 bp band, one 199 bp band and one 97 bp band); TT genotype (2 bands: one 199 bp band and one 97 bp band). Additionally, a negative reference was set during analysis.
Collection of Biopsy Tissue Specimen and KDR Gene mRNA Expression Analysis
Cancer tissue specimens were relatively difficult to obtain. Therefore, the matched biopsy cancer tissue specimens were only collected from 67 samples among the 169 patients with NSCLC originally. Unfortunately, 5 patients’ biopsy tumor tissue specimens were not available and 4 patients failed the RNA extraction. Finally, a total of 58 specimens were available for KDR mRNA expression analysis and preserved in Liquid nitrogen. Total RNA samples were extracted using Trizol reagents (Takara, Biotechnology, Dalian, China) according to the manufacture’s instruction and stored at −80°C for mRNA expression analysis. A total of 500 ng RNA extracted from the biopsy tissue specimens was used as the templates for reverse-transcription polymerase chain reaction to prepare the first-stand of cDNA using the Prime Script RT reagent Kit. Relative quantitative analysis of KDR gene mRNA expression was carried out using the Light Cycler® 480 (Roche, Shanghai, China) with SYBR Premix EX Taq system. The forward primer of KDR gene was 5′-ATGCAGAGCAAGGTGCTGC-3′, the reverse primer was 5′-TTAAACAGGAGGAGAGCTCAGTG-3′. 17 The real-time quantitative PCR reaction system was as follows: SYBN Premix Ex Tag solution 10μL, KDR F primer (20μM) 0.2μL, KDR R primer (20μM) 0.2μL, ddH2O 7.6µL and cDNA 2μL, the total reaction system is 20μL. GAPDH mRNA expression was used as an internal reference. KDR mRNA was calculated by relative quantification method 2- △△Ct. 24
Statistical Analysis
All variables in this study were analyzed using SPSS software version 25.0 (IBM, Armonk, NY). Whether the genotypes distribution of the polymorphism was in line with Hardy-Weinberg equilibrium was analyzed using chi-square test. Statistical difference of proportion variables and continuous variables according to V297 L genotype status was analyzed using the chi-square test and the Mann-Whitney U nonparametric test, respectively. Kaplan-Meier survival curves were drawn using Stata 14.0 to estimate the differences in PFS and OS of patients with different genotypes and the differences between the curves of different genotypes were calculated using the log-rank test. PFS was calculated from the date of bevacizumab administration to the patients’ tumor progression or death. OS was defined from the date of bevacizumab administration to death of the patients from any cause. For those without survival events by the end of the study, survival end points were censored at the date of data cut-off. Cox regression analysis was introduced for PFS in multivariable analysis. P < 0.05 was considered to be significantly statistical difference.
Results
Baseline Characteristics of the 169 Patients With NSCLC and Genotyping of V297 L
Baseline characteristics of the 169 patients with NSCLC enrolled were exhibited in Table 1. The median age of the patients was 57 years old ranging from 29 to 81 years. 68.0% of the patients were male. 0 score and 1-2 score of ECOG was observed in 63 and 106 patients, respectively. Majority of the patients were noted with disease stage of Ⅳ (89.9%). 72.2% of the patients were nonsmoker/former smoker. A total of 148 patients (87.6%) were observed of adenocarcinoma, 21 patients were of other histology types. Positive driver gene (EGFR/ALK/ROS1) mutation were reported in 24 patients, negative driver gene mutation was noted in 117 patients. In the combined chemotherapy regimen, 78 patients were treated with cisplatin-based regimen and 91 patients were received carboplatin-based chemotherapy. Additionally, 87 patients were treated with pemetrexed regimen, 48 patients were administrated with docetaxel regimen, 34 patients were received paclitaxel regimen. Interestingly, majority of initial dosage of bevacizumab were 7.5 mg/kg (94.1%).
Table 1.
Baseline Characteristics of the 169 Patients With NSCLC According to KDR V297 L Genotype Status.
| Characteristics | Total (n = 169, %) | V297 L genotype status | P | |
|---|---|---|---|---|
| CC (n = 123) | CT/TT (n = 46) | |||
| Age (years) | ||||
| Median (range) | 57 (29-81) | 57 (41-79) | 58 (29-81) | 0.717 |
| Gender | 0.911 | |||
| Male | 115 (68.0) | 84 (68.3) | 31 (67.4) | |
| Female | 54 (32.0) | 39 (31.7) | 15 (32.6) | |
| ECOG score | 0.682 | |||
| 0 | 63 (37.3) | 47 (38.2) | 16 (34.8) | |
| 1-2 | 106 (62.7) | 76 (61.8) | 30 (65.2) | |
| Disease stage | 0.907 | |||
| Ⅲb | 9 (5.3) | 6 (4.9) | 3 (6.5) | |
| Ⅳ | 152 (89.9) | 111 (90.2) | 41 (89.1) | |
| Recurrent | 8 (4.7) | 6 (4.9) | 2 (4.4) | |
| Smoking status | 0.76 | |||
| Nonsmoker/former smoker | 122 (72.2) | 88 (71.5) | 34 (73.9) | |
| Smoker | 47 (27.8) | 35 (28.5) | 12 (26.1) | |
| Histology | ||||
| Adenocarcinoma | 148 (87.6) | 108 (87.8) | 40 (87.0) | 0.882 |
| Other types | 21 (12.4) | 15 (12.2) | 6 (13.0) | |
| Driver gene mutation statusa | 0.943 | |||
| Positive | 24 (14.2) | 17 (13.8) | 7 (15.2) | |
| Negative | 117 (69.2) | 85 (69.1) | 32 (69.6) | |
| NA | 28 (16.6) | 21 (17.1) | 7 (15.2) | |
| Combined chemotherapy regimen | ||||
| Cisplatin based | 78 (46.2) | 59 (48.0) | 19 (41.3) | 0.439 |
| Carboplatin based | 91 (53.8) | 64 (52.0) | 27 (58.7) | |
| Combined chemotherapy regimen | ||||
| Pemetrexed | 87 (51.5) | 64 (52.0) | 23 (50.0) | 0.938 |
| Docetaxel | 48 (28.4) | 34 (27.6) | 14 (30.4) | |
| Paclitaxel | 34 (20.1) | 25 (20.3) | 9 (19.6) | |
| Initial dosage of bevacizumab (mg/kg) | ||||
| 7.5 | 159 (94.1) | 116 (94.3) | 43 (93.5) | 0.839 |
| 15 | 10 (5.9) | 7 (5.7) | 3 (6.5) | |
Abbreviations: NSCLC, non-small cell lung cancer; KDR, kinase insert domain receptor; ECOG, Eastern Cooperative Oncology Group; NA, not available.
a The driver gene were EGFR or ALK or ROS1.
The prevalence of V297 L in the 169 patients with NSCLC were: CC genotype 123 cases (72.8%), CT genotype 41 cases (24.3%), TT genotype 5 cases (2.9%). The minimum allele frequency (MAF) is 0.15. The distribution frequencies of the 3 genotypes corresponded with Hardy-Weinberg equilibrium (P = 0.489). Given that patients with TT genotype were relatively rare, patients with TT and CT genotypes were merged in the subsequent comparison of clinical outcomes. As illustrated in Table 1, the baseline characteristics of patients with CC and CT/TT genotypes were well-balanced and comparable.
Influence of KDR Gene V297 L Polymorphism on the Efficacy and Prognosis
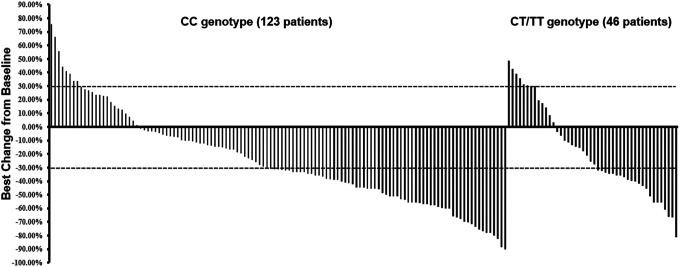
The efficacy of bevacizumab-based first-line regimen was evaluated in this study firstly. The best overall response assessment included complete response (CR) 0 case, confirmed partial response (PR) 87 cases, stable disease (SD) 58 cases and progressive disease (PD) 24 cases. Consequently, ORR and DCR of the 169 patients were 51.5% [95% confidence interval (CI): 43.7%-59.2%) and 85.8% (95%CI: 81.6%-92.1%), respectively. As illustrated in Figure 2, The ORR of patients with CT/TT genotype and CC genotype were 47.8% and 52.8%, respectively (P = 0.561). The DCR of patients with CT/TT genotype and CC genotype were 82.6% and 87.0%, respectively (P = 0.467).
Figure 2.
Waterfall plot for the best percentage change in target lesion size of the 169 patients with advanced non-small cell lung cancer according to KDR V297 L genotype status.
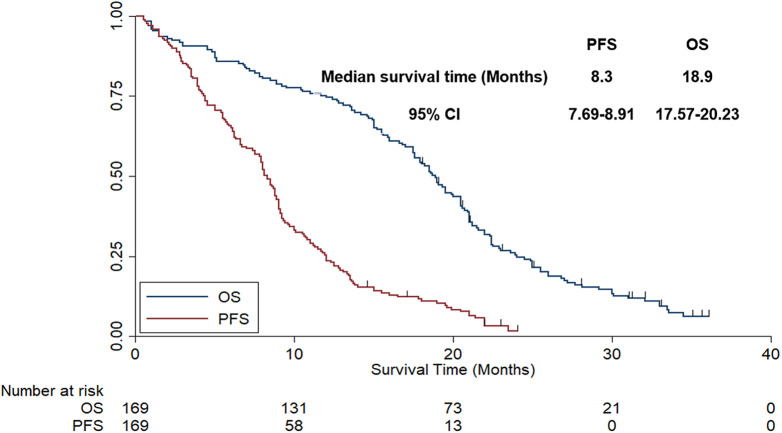
Data cut-off date was October 2020, and the median follow-up time for all patients was 18.5 months (follow-up range: 0.5-36 months). As illustrated in Figure 3, the median PFS of all patients was 8.3 months (95% CI: 7.69-8.91). Given that the follow-up duration of our study was longer enough, OS was evaluated in our study as well. As exhibited in Figure 3, the median OS of the 169 patients with NSCLC was 18.9 months (95%CI: 17.57-20.23).
Figure 3.
The progression-free survival and overall survival of the 169 patients with advanced non-small cell lung cancer.
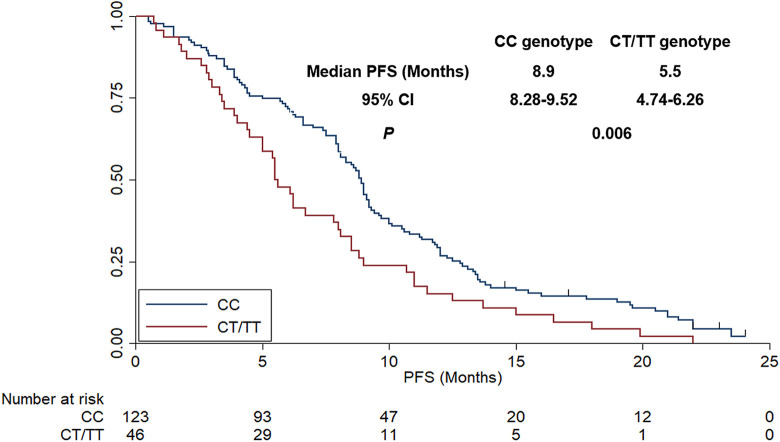
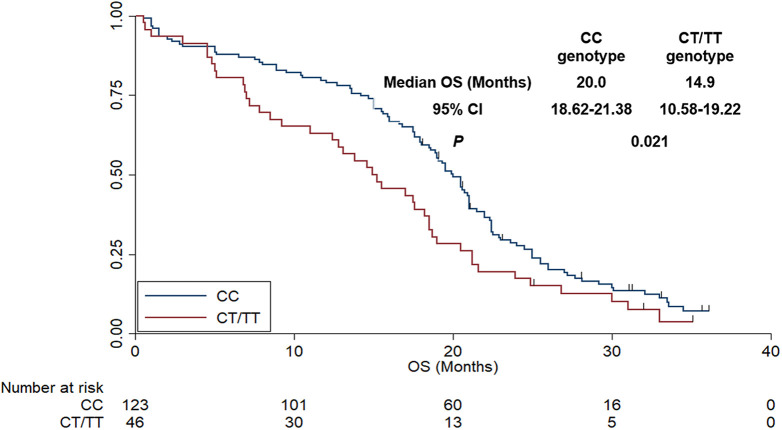
In terms of the association analysis between V297 L polymorphism status and prognosis, as shown in Figure 4, the median PFS of patients with CC and CT/TT was 8.9 months (95%CI: 8.28-9.52) and 5.5 months (95%CI: 4.74-6.26), respectively, which was statistically significant (χ2 = 7.68, P = 0.006). In terms of OS, as exhibited in Figure 5, the median OS of patients with CC and CT/TT genotype were 20.0 months (95%CI: 18.62-21.38) and 14.9 months (95%CI: 10.58-19.22), respectively. The difference was statistically significant (χ2 = 5.31, P = 0.021). Additionally, a multivariate Cox regression model was introduced to adjust the independent significance of V297 L polymorphism for PFS. Therefore, the univariate analysis between baseline characteristics (such as age, gender, ECOG score, smoking status, histology, combined chemotherapy regimen, initial dosage of bevacizumab and V297 L polymorphism) and PFS was carried out firstly. As illustrated in Table 2, age, ECOG score and genotype of V297 L were of significant association with PFS in the univariate analysis. Therefore, there variables were included in the multivariate Cox regression analysis. As exhibited in Table 2, V297 L polymorphism status was an independent factor for PFS [HR = 1.59 (95%CI: 1.22-2.34), P = 0.011] after multivariate adjustment. Interestingly, other statistically significant variables in the multivariate Cox analysis were age (HR = 1.25, P = 0.028) and ECOG score (HR = 1.38, P = 0.021), which indicated that patients of <57 years had longer PFS than that of patients with ≥57 years (median PFS: 9.0 vs 7.2 months) and patients with ECOG 0 score demonstrated superior PFS compared with that of patients with ECOG 1-2 score (median PFS: 9.6 vs 6.9 months).
Figure 4.
The progression-free survival of the 169 patients with advanced non-small cell lung cancer according to KDR V297 L genotype status.
Figure 5.
The overall survival of the 169 patients with advanced non-small cell lung cancer according to KDR V297 L genotype status.
Table 2.
PFS of the 169 Patients With NSCLC According to Baseline Characteristics and Polymorphism in Univariate Analysis and Multivariate Cox Analysis.
| Characteristics | Median PFS (95%CI) | P | Multivariate analysis | |
|---|---|---|---|---|
| (univariate analysis) | HR (95%CI) | P | ||
| Age | 0.017 | 1.25 (1.08-1.89) | 0.028 | |
| <57 | 9.0 (7.55-10.45) | |||
| ≥57 | 7.2 (6.41-7.99) | |||
| Gender | 0.562 | |||
| Male | 8.1 (7.01-9.19) | |||
| Female | 8.6 (7.19-10.01) | |||
| ECOG score | 0.011 | 1.38 (1.11-2.03) | 0.021 | |
| 0 | 9.6 (7.15-12.05) | |||
| 01-Feb | 6.9 (5.91-7.89) | |||
| Smoking status | 0.713 | |||
| Nonsmoker/former smoker | 8.3 (7.27-9.33) | |||
| Smoker | 8.5 (7.11-9.89) | |||
| Histology | 0.434 | |||
| Adenocarcinoma | 8.5 (7.01-9.99) | |||
| Other types | 8.1 (7.12-9.08) | |||
| Combined chemotherapy regimen | 0.718 | |||
| Cisplatin based | 8.3 (7.11-9.49) | |||
| Carboplatin based | 8.3 (7.19-9.41) | |||
| Initial dosage of bevacizumab (mg/kg) | 0.633 | |||
| 7.5 | 8.3 (7.09-9.51) | |||
| 15 | 8.5 (7.12-9.88) | |||
| Genotypes of V297L polymorphism | 0.006 | 1.59 (1.22-2.34) | 0.011 | |
| CC | 8.9 (8.28-9.52) | |||
| CT/TT | 5.5 (4.74-6.26) | |||
Abbreviations: NSCLC, non-small cell lung cancer; OS, overall survival; ECOG, Eastern Cooperative Oncology Group; CI, confidence interval; HR, hazard ratio.
Correlation Analysis Between V297 L Genotype Status and Safety Profile
The regimen of bevacizumab and platinum-based chemotherapy demonstrated the common and specific adverse reactions of chemotherapy drugs and antiangiogenic drugs, respectively. As exhibited in Table 3, the main and common adverse reactions were neutropenia, leukopenia, thrombocytopenia, hypertension, gastrointestinal reaction, proteinuria, hand-foot syndrome and AST/ALT elevation during the treatment of bevacizumab combined with platinum-based chemotherapy. And the incidence was 29.0%, 26.6%, 24.3%, 23.1%, 20.1%, 16.5%, 13.6% and 11.2%, respectively. Correlation analysis between V297 L genotype status and safety profile indicated that no significant correlation was observed between genotype status of V297 L and the common adverse reactions (P > 0.05).
Table 3.
Safety Profile of the 169 Patients With NSCLC Receiving First-Line Bevacizumab Plus Chemotherapy Regimen According to KDR V297 L Genotype Status.
| Safety profile | Total (n = 169, %) | V297 L genotype status | P | |
|---|---|---|---|---|
| CC (n = 123) | CT/TT (n = 46) | |||
| Neutropenia | 49 (29.0) | 37 (30.1) | 12 (26.1) | 0.610 |
| Leukopenia | 45 (26.6) | 34 (27.6) | 11 (23.9) | 0.625 |
| Thrombocytopenia | 41 (24.3) | 30 (24.4) | 11 (23.9) | 0.949 |
| Hypertension | 39 (23.1) | 29 (23.6) | 10 (21.7) | 0.801 |
| Gastrointestinal reaction | 34 (20.1) | 23 (18.7) | 11 (23.9) | 0.452 |
| Proteinuria | 28 (16.5) | 21 (17.1) | 7 (15.2) | 0.773 |
| Hand-foot syndrome | 23 (13.6) | 18 (14.6) | 5 (10.9) | 0.525 |
| AST/ALT elevation | 19 (11.2) | 14 (11.4) | 5 (10.9) | 0.925 |
Abbreviations: NSCLC, non-small cell lung cancer; AST, aspartate amino transferase; ALT, alanine aminotransferase.
Association Analysis Between V297 L Genotype Status and mRNA Expression of KDR
A total of 58 specimens were available for KDR mRNA expression analysis. The expression of KDR mRNA was determined by extracting RNA from 58 biopsy cancer tissue specimens and the correlation between the genotype status of V297 L and mRNA expression was analyzed. Firstly, the prevalence of V297 L polymorphism in the 58 biopsy cancer tissue specimens were: CC genotype 42 cases (72.4%), CT genotype 14 cases (24.1%) and TT genotype 2 cases (3.4%). The MAF was 0.16 and distribution frequency of the 3 genotypes also accorded with the Hardy-Weinberg equilibrium (P = 0.546). Similarly, TT and CT genotypes were merged in the subsequent analysis. As illustrated in Figure 6, compared with patients with CC genotype of V297 L, the relative expression of KDR mRNA in cancer tissues of patients with CT/TT genotype was significantly higher (3.97 ± 0.583 vs 2.80 ± 0.856), and the difference was statistically significant (P < 0.001).
Figure 6.
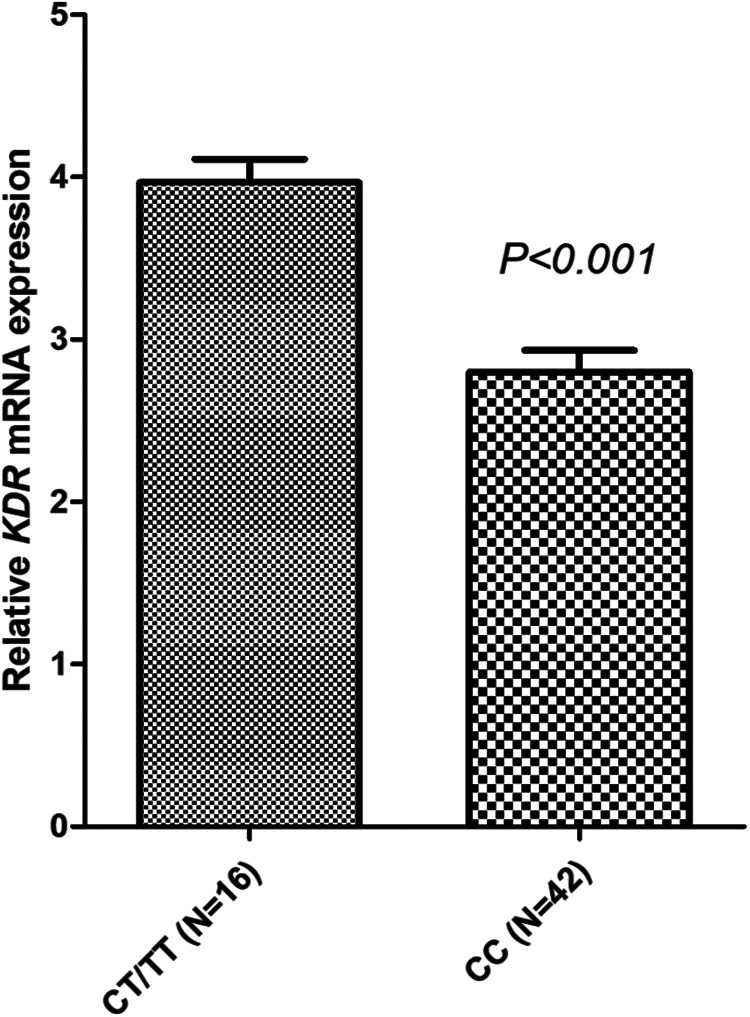
Relative mRNA expression level of KDR gene according to KDR V297 L genotype status.
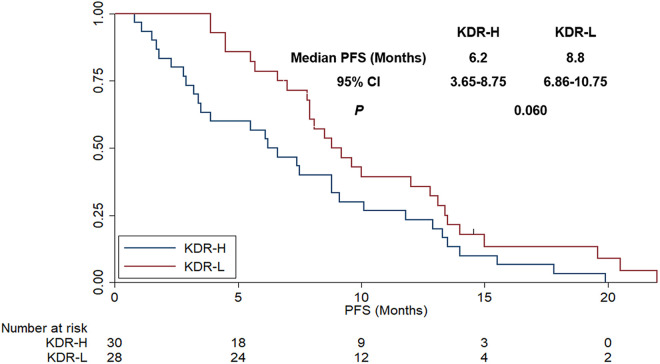
Additionally, KDR mRNA expression status was divided into KDR high expression (KDR-H) and KDR low expression (KDR-L) according to the median KDR mRNA relative expression threshold value to explore the association with PFS. Patients with KDR-H and KDR-L were observed in 30 patients and 28 patients, respectively. As exhibited in Figure 7, patients with KDR-H conferred a trend for worse OS compared with those with KDR-L (median PFS: 6.2 vs 8.8 months), even the difference was not statistically significant (χ2 = 3.543, P = 0.060).
Figure 7.
The progression-free survival of the 58 patients with advanced NSCLC receiving bevacizumab plus chemotherapy regimens according to KDR mRNA expression status.
Discussion
Our retrospective study provided the real-world evidence regarding the efficacy and safety of patients with NSCLC who were treated with first-line bevacizumab plus chemotherapy regimen. Simultaneously, results of correlation analysis between the V297 L genotype status and prognosis indicated that V297 L of KDR could be used as a prognostic biomarker for patients with NSCLC. Additionally, the mRNA expression level of KDR according to different genotypes of V297 L was significantly different. Consequently, clinical comes of patients with advanced NSCLC receiving first-line bevacizumab plus chemotherapy regimens might be influenced by polymorphism V297 L through mediating the mRNA expression of KDR.
NSCLC was a highly heterogeneous and diverse malignant tumor and great individual differences regarding the clinical outcomes and prognosis of NSCLC treatment were observed clinically. 25 On the other hand, to our knowledge, recent years had witnessed that numerous studies regarding the first-line immunotherapy in metastatic non-small cell lung cancer were reported and the results suggested that PD-1/PD-L1 inhibitors-related regimens could cover all the first-line therapy substantially. The Keynote-024 trial exhibited that pembrolizumab monotherapy was better than platinum-based chemotherapy as first-line therapy with superior PFS and OS in NSCLC patients with PD-L1 expression >50% of tumor cells. 26 Additionally, pembrolizumab plus platinum-based chemotherapy could be used as standard first-line setting for NSCLC patients with PD-L1 expression <50% according to the Keynote-189 trial. 27 Furthermore, atezolizumab plus bevacizumab and platinum-based chemotherapy demonstrated significantly improved PFS and OS as first-line therapy among patients with metastatic non-squamous NSCLC according to the Impower 150 trial. 28 The above data suggested that immunotherapy combined with chemotherapy and immunotherapy combined with bevacizumab played a synergetic anti-cancer activity for the patients.
As a humanized monoclonal antibody that specifically targeted on VEGFA, bevacizumab demonstrated superior therapeutic value in different fields such as colorectal cancer and lung cancer. 29 Nevertheless, it should be noted that bevacizumab targeted on VEGF that normally existed in the human body instead of tumor cell directly, 30 which was different from the mechanism of other targeted drugs such as gefitinib. 31 Therefore, polymorphisms of VEGF pathway might be of potential significance on the efficacy of antiangiogenic targeted drugs. 32 Interestingly, we noticed that Takeuchi and colleagues reported in 2020 that polymorphism of SLC22A16 predicted the efficacy of platinum combination chemotherapy and bevacizumab in patients with advanced NSCLC. 33 Crucitta and colleagues investigated in 2019 that Endothelial nitric oxide synthase c.-813C>T predicted the proteinuria in metastatic breast cancer patients who were treated with bevacizumab-based chemotherapy. 34 Berger and colleagues reported in 2018 that rs4588 within the vitamin D transporter gene predicted the OS in patients with metastatic CRC who were treated with FOLFIRI plus bevacizumab regimen. 35 In conclusion, these above data demonstrated the potential value of pharmacogenomics to predict the clinical outcomes or safety of bevacizumab.
The clinical outcomes of the 169 patients with NSCLC who received bevacizumab combined with platinum-based chemotherapy were analyzed and exhibited simultaneously. ORR of the patients was 51.5%, DCR was 85.5%, the median PFS was 8.3 months and the median OS was 18.9 months. It seemed that the clinical outcomes were slightly lower than that of the phase Ⅲ Beyond clinical trial initiated by Zhou and colleagues (ORR = 54%, DCR = 94%, median PFS = 9.2 months and median OS = 24.3 months of bevacizumab arm). 7 We speculated that reason could be attributed to the retrospective design of our study. Management of the patients in retrospective study was not sufficient and normative compared with well-designed phase Ⅲ clinical trial, which was demonstrated in the other retrospective study of bevacizumab in NSCLC. 36 Additionally, it should be noted that patients with ECOG 2 score were also included in our study. However, BEYOND study excluded those with ECOG 2 score. As far as we know, higher ECOG score conferred worse prognosis in patients with NSCLC. 37 And the results of multivariate Cox analysis suggested that patients with ECOG 1∼2 score were associated with worse prognosis compared with those with 0 score. The clinical outcomes of the 169 patients with NSCLC receiving bevacizumab combined with platinum-based chemotherapy in this study provided real-world evidence for bevacizumab administration in patients with NSCLC.
Regarding the clinical significance analysis of KDR gene polymorphism, the present study was the first study focusing on the association between the efficacy of patients with NSCLC receiving bevacizumab treatment and the KDR gene polymorphism analysis in Chinese population. The previous work initiated by Fan and colleagues included a total of 148 patients with advanced NSCLC receiving bevacizumab based first-line regimens and the association analysis between VEGFR2 polymorphism and prognosis indicated that 889C>T polymorphism was of clinical significance to predict the prognosis of patients with NSCLC who were treated with bevacizumab-based regimens. 38 The design and part of results of that study were consistent with our study. However, we included a relatively larger sample size. Interestingly, a recent investigation initiated by Wang and colleagues explored the influence of KDR gene polymorphism on the efficacy and safety of an antiangiogenic tyrosine kinase inhibitor (TKI)-apatinib for patients with advanced NSCLC who were failed after the standard therapy. 17 The conclusion suggested that KDR 4397T>C polymorphism could be used an independent prognostic biomarker for patients with NSCLC when received apatinib treatment. Additionally, another study initiated by Yan and colleagues performed the association analysis between VEGFR2 gene polymorphism and efficacy of apatinib in the treatment of advanced epithelial ovarian carcinoma. 39 Results of their study indicated that the clinical outcomes of apatinib may be influenced by the polymorphism -906T>C of VEGFR2 through mediating the mRNA expression of VEGFR2. In conclusion, these data demonstrated the potential predictive significance of polymorphism in antiangiogenic single pathway for the efficacy and prognosis of patients receiving antiangiogenic targeted drugs therapy. The minor allele frequency (MAF) of V297 L polymorphism in our study was 0.15, which was in line with the genotype frequency of the Chinese population in the NCBI database and the results of previous study initiated by Mario Scartozzi and colleagues. 40 A total of 148 patients with hepatocellular carcinoma who received sorafenib treatment were included and VEGF and VEGFR gene polymorphisms were detected to evaluate the association between genotype status and prognosis of the patients. Conclusion of the study exhibited that patients with CT/TT genotype of rs2305948 were associated with a worse PFS and OS compared with those with CC genotype, which was consistent with the conclusion in our study. Besides, previous study initiated by Babyshkina and colleagues performed a retrospective analysis of 70 women with breast cancer who underwent neoadjuvant chemotherapy and investigated the KDR gene polymorphism. 41 And the results suggested that patients with CT/TT genotype of rs2305948 in KDR gene were associated with worse pathological complete response compared with those with CC genotype, even the difference was not statistically significant. The results were in concert with the conclusion in our study to some extent. Unfortunately, the studies failed to investigate the association between the genotype status of the polymorphism and KDR gene mRNA expression. Notably, a previous study initiated by Matalliotaki and colleagues investigated the implication of KDR in endometriosis through a structural biological and genetic approach. 42 Results suggested that V297 L polymorphism might play an important role in D3-D4 Ig-domain interaction, thus influencing the efficiency of dimerization and contributing to the pathogenesis of endometriosis by impairing VEGF signaling and increasing angiogenesis. It seemed that the structural changes that the SNP might result in the secondary and tertiary structure of the VEGF receptor, thus contributing to the binding kinetics with VEGF molecule blocked of bevacizumab. Therefore, the conclusion in their study could help to explain the clinical significance of V297 L polymorphism in our study to some extent.
Interestingly, mRNA expression of KDR was explored among 58 cancer tissue specimens in our study and the results disclosed that patients with CT/TT genotype of V297 L were associated with higher KDR mRNA expression. KDR is the most important receptor with the strongest binding ability to VEGFA and its expression level plays a vital role in the process of angiogenesis. 43 Besides, it had been demonstrated that higher expression level of KDR in tumor cells made it easier for tumor cells to regenerate blood vessels, thus leading to relapse and metastasize. 44 Consequently, relevant clinical study indicated that higher the expression level of KDR gene was associated with worse PFS and OS of patients with NSCLC. 45 This conclusion was in concert with that the association between KDR mRNA expression and PFS in our study to some extent. In a word, KDR V297 L polymorphism could be used as a potential biomarker for the prediction of patients with advanced NSCLC receiving bevacizumab-based first line regimen.
Additionally, it should be noted that the main and common adverse reactions of the patients with advanced NSCLC receiving the treatment of bevacizumab combined with platinum-based chemotherapy were neutropenia, leukopenia, thrombocytopenia, hypertension, gastrointestinal reaction, proteinuria, hand-foot syndrome and AST/ALT elevation, which are basically consistent with previous study. 46 Interestingly, the correlation analysis between V297 L genotype status and the incidence of adverse reactions demonstrated no statistically significant difference. This could be attributed to the fact that V297 L polymorphism did not contribute to bevacizumab and chemotherapy disposition in vivo.
Limitations were observed in our investigation inevitably. Firstly, the sample size of our study was relatively small and only 169 patients were included. The conclusion could be more objective and representative when larger sample size was adopted. Secondly, it was obvious that some biases could not be avoided in retrospective analysis. For example, we could not design the study with control group randomly, the management and follow-up of the patients were inadequately compared to the clinical trial. Thirdly, another potentially important flaw of our study was that the patients included in our study were all treated with chemotherapy plus bevacizumab first-line regimen in view of the fact that the standard of care for first-line treatment should be PD-1/PD-L1-based regimens currently. Fourthly, our study was limited to Chinese population. It was not representative and suitable for international patients currently. Consequently, the conclusion in our study should be validated large-scale prospective trials further. From the objective view, our study evaluated the prognostic significance of the V297 L polymorphism adequately and revealed part of the reasons for the prognostic difference resulted from this polymorphism from the expression of KDR mRNA appropriately, which could be of clinical guiding significance for the prognostic evaluation of bevacizumab in patients with advanced NSCLC. In the future, polymorphism genotyping could be implemented as routine clinical practice. And when unfavorable genotype such as CT/TT genotype of V297 L was detected, patients should take PD-1/PD-L1-based regimens into consideration instead of bevacizumab-based therapy as the first-line treatment if it was possible.
Conclusion
The clinical comes of patients with advanced NSCLC receiving first-line bevacizumab plus chemotherapy regimens might be impacted by polymorphism V297 L of KDR through mediating the mRNA expression of KDR. Therefore, if we identified the sensitive patients who could really benefit from antiangiogenic targeted drug therapy, then more reasonable medication could be used in the context of precision medicine currently.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Key Program of Medical Science Research of Hebei Province, No. 20160160.
ORCID iD: Wenxia Hu, MD, PhD  https://orcid.org/0000-0002-5825-2695
https://orcid.org/0000-0002-5825-2695
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3. Yang SR, Schultheis AM, Yu H, Mandelker D, Ladanyi M, Büttner R. Precision medicine in non-small cell lung cancer: current applications and future directions. Semin Cancer Biol. 2020;S1044-579X(20):30164–30174. doi:10.1016/j.semcancer.2020.07.009 [DOI] [PubMed] [Google Scholar]
- 4. Janning M, Loges S. Anti-angiogenics: their value in lung cancer therapy. Oncol Res Treat. 2018;41(4):172–180. doi:10.1159/000488119 [DOI] [PubMed] [Google Scholar]
- 5. Lamberti G, Andrini E, Sisi M, et al. Beyond EGFR, ALK and ROS1: current evidence and future perspectives on newly targetable oncogenic drivers in lung adenocarcinoma. Crit Rev Oncol Hematol. 2020;156:103119. doi:10.1016/j.critrevonc.2020.103119 [DOI] [PubMed] [Google Scholar]
- 6. Feng Y, Feng G, Lu X, et al. Exploratory analysis of introducing next-generation sequencing-based method to treatment-naive lung cancer patients. J Thorac Dis. 2018;10(10):5904–5912. doi:10.21037/jtd.2018.09.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou C, Wu YL, Chen G, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33(19):2197–2204. doi:10.1200/jco.2014.59.4424 [DOI] [PubMed] [Google Scholar]
- 8. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi:10.1056/NEJMoa061884 [DOI] [PubMed] [Google Scholar]
- 9. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322(8):764–774. doi:10.1001/jama.2019.11058 [DOI] [PubMed] [Google Scholar]
- 10. Assoun S, Brosseau S, Steinmetz C, Gounant V, Zalcman G. Bevacizumab in advanced lung cancer: state of the art. Future Oncol. 2017;13(28):2515–2535. doi:10.2217/fon-2017-0302 [DOI] [PubMed] [Google Scholar]
- 11. De Mattia E, Bignucolo A, Toffoli G, Cecchin E. Genetic markers of the host to predict the efficacy of colorectal cancer targeted therapy. Curr Med Chem. 2020;27(25):4249–4273. doi:10.2174/0929867326666190712151417 [DOI] [PubMed] [Google Scholar]
- 12. Anderson EM, Mooney DJ. The combination of vascular endothelial growth factor and stromal cell-derived factor induces superior angiogenic sprouting by outgrowth endothelial cells. J Vasc Res. 2015;52(1):62–69. doi:10.1159/000382129 [DOI] [PubMed] [Google Scholar]
- 13. Melincovici CS, Boşca AB, Şuşman S, et al. Vascular endothelial growth factor (VEGF)—key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455–467. [PubMed] [Google Scholar]
- 14. Zhang SD, McCrudden CM, Kwok HF. Prognostic significance of combining VEGFA, FLT1 and KDR mRNA expression in lung cancer. Oncol Lett. 2015;10(3):1893–1901. doi: 10.3892/ol.2015.3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahfouz N, Tahtouh R, Alaaeddine N, et al. Gastrointestinal cancer cells treatment with bevacizumab activates a VEGF autoregulatory mechanism involving telomerase catalytic subunit hTERT via PI3K-AKT, HIF-1α and VEGF receptors. PLoS One. 2017;12(6):e0179202. doi:10.1371/journal.pone.0179202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen CC, Cheng WY, Lee CH, et al. Both p53 codon 72 Arg/Arg and pro/Arg genotypes in glioblastoma multiforme are associated with a better prognosis in bevacizumab treatment. BMC Cancer. 2020;20(1):709. doi:10.1186/s12885-020-07210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song ZZ, Zhao LF, Zuo J, Fan ZS, Wang L, Wang YD. Clinical outcomes and safety of apatinib mesylate in the treatment of advanced non-squamous non-small cell lung cancer in patients who progressed after standard therapy and analysis of the KDR gene polymorphism. Onco Targets Ther. 2020;13:603–613. doi: 10.2147/ott.s222985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lian L, Li XL, Xu MD, et al. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer. 2019;19(1):183. doi:10.1186/s12885-019-5322-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang W, Ma XP, Shi Z, et al. Epidermal growth factor receptor pathway polymorphisms and the prognosis of hepatocellular carcinoma. Am J Cancer Res. 2015;5(1):396–410. [PMC free article] [PubMed] [Google Scholar]
- 20. Gerger A, El-Khoueiry A, Zhang W, et al. Pharmacogenetic angiogenesis profiling for first-line bevacizumab plus oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res. 2011;17(17):5783–5792. doi:10.1158/1078-0432.ccr-11-1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 22. Ettinger DS, Wood DE, Akerley W, et al. Non-small cell lung cancer, version 1.2015. J Natl Compr Canc Netw. 2014;12(12):1738–1761. doi:10.6004/jnccn.2014.0176 [DOI] [PubMed] [Google Scholar]
- 23. Miller TP, Fisher BT, Getz KD, et al. Unintended consequences of evolution of the common terminology criteria for adverse events. Pediatr Blood Cancer. 2019;66(7):e27747. doi:10.1002/pbc.27747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsotetsi TN, Collins NE, Oosthuizen MC, Sibeko-Matjila KP. Selection and evaluation of housekeeping genes as endogenous controls for quantification of mRNA transcripts in Theileria parva using quantitative real-time polymerase chain reaction (qPCR). PLoS One. 2018;13(5):e0196715. doi:10.1371/journal.pone.0196715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–1640. doi:10.1016/j.mayocp.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 26. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 27. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 28. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi:10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 29. Roviello G, Bachelot T, Hudis CA, et al. The role of bevacizumab in solid tumours: a literature based meta-analysis of randomised trials. Eur J Cancer. 2017;75:245–258. doi:10.1016/j.ejca.2017.01.026 [DOI] [PubMed] [Google Scholar]
- 30. Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem. 2006;13(16):1845–1857. doi:10.2174/092986706777585059 [DOI] [PubMed] [Google Scholar]
- 31. Meng S, Wang G, Lu Y, Fan Z. Functional cooperation between HIF-1α and c-Jun in mediating primary and acquired resistance to gefitinib in NSCLC cells with activating mutation of EGFR. Lung Cancer. 2018;121:82–90. doi:10.1016/j.lungcan.2018.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu JY, Zhu BR, Wang YD, Sun X. The efficacy and safety of Apatinib mesylate in the treatment of metastatic osteosarcoma patients who progressed after standard therapy and the VEGFR2 gene polymorphism analysis. Int J Clin Oncol. 2020;25(6):1195–1205. doi:10.1007/s10147-020-01644-7 [DOI] [PubMed] [Google Scholar]
- 33. Takeuchi A, Oguri T, Fukuda S, et al. Variants of SLC22A16 predict the efficacy of platinum combination chemotherapy in advanced non-small-cell lung cancer. Anticancer Res. 2020;40(8):4245–4251. doi:10.21873/anticanres.14426 [DOI] [PubMed] [Google Scholar]
- 34. Crucitta S, Restante G, Del Re M, et al. Endothelial nitric oxide synthase c.-813C>T predicts for proteinuria in metastatic breast cancer patients treated with bevacizumab-based chemotherapy. Cancer Chemother Pharmacol. 2019;84(6):1219–1227. doi:10.1007/s00280-019-03933-z [DOI] [PubMed] [Google Scholar]
- 35. Berger MD, Stintzing S, Heinemann V, et al. A polymorphism within the Vitamin D transporter gene predicts outcome in metastatic colorectal cancer patients treated with FOLFIRI/Bevacizumab or FOLFIRI/Cetuximab. Clin Cancer Res. 2018;24(4):784–793. doi:10.1158/1078-0432.ccr-17-1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duan J, Yang Z, Liu D, Shi Y. Clinical efficacy of bevacizumab combined with gemcitabine and cisplatin combination chemotherapy in the treatment of advanced non-small cell lung cancer. J BUON. 2018;23(5):1402–1406. [PubMed] [Google Scholar]
- 37. Agemi Y, Shimokawa T, Sasaki J, et al. Prospective evaluation of the G8 screening tool for prognostication of survival in elderly patients with lung cancer: a single-institution study. PLoS One. 2019;14(1):e0210499. doi:10.1371/journal.pone.0210499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fan HJ, Yuan J, Wu JJ, Jia YX, Ma YH, Li XY. Influence of polymorphisms of VEGFR2 on clinical outcomes and safety of advanced non-small-cell lung cancer treated by first line bevacizumab plus chemotherapy regimens [in Chinese]. Zhonghua Yi Xue Za Zhi. 2019;99(2):99–104. doi:10.3760/cma.j.issn.0376-2491.2019.02.005 [DOI] [PubMed] [Google Scholar]
- 39. Yan Z, Gu YY, Hu XD, et al. Clinical outcomes and safety of apatinib monotherapy in the treatment of patients with advanced epithelial ovarian carcinoma who progressed after standard regimens and the analysis of the VEGFR2 polymorphism. Oncol Lett. 2020;20(3):3035–3045. doi:10.3892/ol.2020.11857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scartozzi M, Faloppi L, Svegliati Baroni G, et al. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: the ALICE-1 study. Int J Cancer. 2014;135(5):1247–1256. doi:10.1002/ijc.28772 [DOI] [PubMed] [Google Scholar]
- 41. Babyshkina N, Zavyalova M, Tarabanovskaya N, et al. Predictive value of vascular endothelial growth factor receptor type 2 in triple-negative breast cancer patients treated with neoadjuvant chemotherapy. Mol Cell Biochem. 2018;444(1-2):197–206. doi:10.1007/s11010-017-3244-1 [DOI] [PubMed] [Google Scholar]
- 42. Matalliotaki C, Eliopoulos E, Matalliotakis M, et al. Implication of VEGFR2 in endometriosis: a structural biological and genetic approach. World Acad Sci J. 2019;1(6):283–289. doi:10.3892/wasj.2019.29 [Google Scholar]
- 43. Ji L, Wu M, Li Z. Rutacecarpine inhibits angiogenesis by targeting the VEGFR2 and VEGFR2-mediated Akt/mTOR/p70s6 k signaling pathway. Molecules. 2018;23(8):2047. doi:10.3390/molecules23082047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jinesh GG, Manyam GC, Mmeje CO, Baggerly KA, Kamat AM. Surface PD-L1, E-cadherin, CD24, and VEGFR2 as markers of epithelial cancer stem cells associated with rapid tumorigenesis. Sci Rep. 2017;7(1):9602. doi:10.1038/s41598-017-08796-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ding M, Liu L, Hu C, Liu Y, Qiao Y, Jiang X. Expression of VEGFR2 and NRP-1 in non-small cell lung cancer and their clinical significance. Chin J Cancer Res. 2014;26(6):669–677. doi:10.3978/j.issn.1000-9604.2014.12.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsai CM, Au JS, Chang GC, Cheng AC, Zhou C, Wu YL. Safety and efficacy of first-line bevacizumab with chemotherapy in Asian patients with advanced nonsquamous NSCLC: results from the phase IV MO19390 (SAiL) study. J Thorac Oncol. 2011;6(6):1092–1097. doi:10.1097/JTO.0b013e318216687d [DOI] [PubMed] [Google Scholar]