Abstract
We describe the case of a baby-girl affected by the Koolen-de Vries syndrome (KdVS), with epilepsy. Our patient has microdeletions in the 17q21.31 region (array CGH). Therapy with Levetiracetam showed a relatively lack of efficacy, while adding a low dose of Topiramate in the therapy allowed the complete seizures control. KdVS is a rare syndrome and its epilepsy features and seizures treatment are not well known by pediatric neurologists. However, with broader use of array CGH, an increasing number of cases are likely to be identified and their description, including response to specific medications, can facilitate recognition and treatment. The complex treatment of epilepsy should be part of the global management and counseling in these composite patients.
Keywords: epilepsy, antiepileptic drugs, quality of life, genetics, electroencephalography
Introduction
Koolen-de Vries syndrome (KdVS) should be suspected in individuals presenting with mild or moderate intellectual disability affecting particularly speech and language development, in combination with other clinical findings, such as epileptic seizures, muscle hypotonia, joint hypermobility or joint dysplasia. 1,2 Other findings may include: behavioral disorders (attention-deficit/hyperactivity disorder or autistic behavior), structural brain abnormalities, dysmorphic facial features, heart defects and renal or urologic anomalies (25%-50%). 2 The epilepsy phenotype in KdVS is broad, but most children have focal to bilateral tonic-clonic seizures that can be prolonged and have prominent autonomic features. 2 Approximately half of patients with KdVS have seizures in their lifetime, which can be refractory to initial drug treatment. 1 Use of rescue benzodiazepines in the home setting should be strongly considered because of the high risk of status epilepticus. Despite its distinctive clinical features, KdVS is not widely recognized by pediatric neurologists, and epilepsy-related aspects of the syndrome, including its responsiveness to specific antiseizure medications (ASMs), remain poorly understood.
Case Report
We report the case of a three-year old girl with KdVS presenting with epilepsy and a MRI phenotype very similar to that described by Myers et al. 1
Genetic Diagnosis
The girl shows a “de novo” microdeletion in the 17q21.31 region (array CGH) including the KANSL1 gene. Array CGH revealed a CNV that corresponds to a deletion of the long arm of chromosome 17, at 17q21.31, 465 kb in size. The CNV was defined as: arr [GRCh37] 1721.31 (43706895_44171916) x1. The microdeletion contains 3 OMIM genes: KANSL1 (OMIM 612452), MAPT (OMIM 157140), CRHR1(OMIM 122561). The identified CNV is absent in the DNA of the parents who are clinically normal.
Development
The patient presented with muscle hypotonia and developmental impairment. She ambulated independently at 16 months; in the first year she required a brace for hypotonia and congenital hip dysplasia. She had mild dysmorphic facial features and a severe deficit in expressive language: at 2 years and 6 months she could not say referential words. Her behavioral manifestations were consistent with mild autism spectrum disorder, but it was not possible to perform an exhaustive evaluation. She also showed attention-deficit/hyperactivity disorder and a severe intellectual disability with an IQ <50 (Griffiths III Scale, evaluated in a psychomotor setting).
Neuroimaging
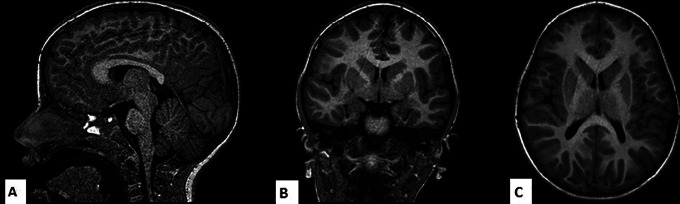
Her MRI showed abnormality in ventricles, in the posterior body of corpus callosum and a dysplastic hippocampus (Figure 1).
Figure 1.
A, T1 volumetric sagittal image showing a corpus callosum with ribbon-like appearance, mild posterior body/splenial hypogenesis and mild structural abnormalities of the brainstem. B, T1 coronal image showing dysplastic hippocampi with malrotated-inverted morphology. C, T1 axial image showing dysplasia of lateral ventricle with squared occipital horns and thinned white matter in the parietal subcortical region.
Seizures Types and EEG
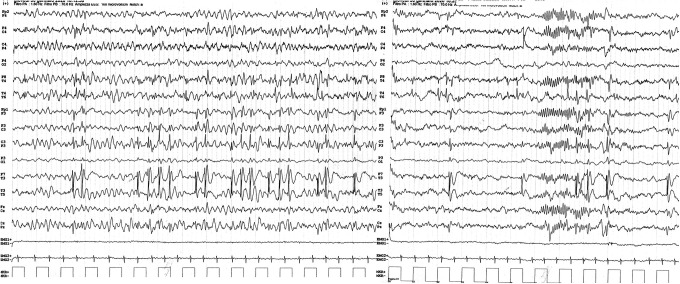
The onset of the girl’s epileptic manifestations was at age 2 years and one month, when she had 2 seizures characterized by diffuse hypotonia, confusion, drooling, vomiting and pallor. Each seizure occurred after waking up, lasted for up to 20 minutes and stopped spontaneously. Subsequently, she presented with weekly focal impaired awareness seizures evolving to bilateral tonic-clonic seizures, with starring, drooling, impaired awareness for 15-20 minutes and vomiting. The interictal EEG showed multifocal epileptiform discharges activated by drowsiness and sleep (Figure 2). The patient based on the clinical findings and EEG findings was diagnosed with focal epilepsy 3 and there was no electrical status epilepticus during slow-wave sleep (ESES) to account for the developmental impairment.
Figure 2.
Interictal EEG with multifocal epileptiform discharges activated by drowsiness and sleep with prevailing centrotemporal spikes.
Response to ASMs and Follow Up
The girl was started initially on Levetiracetam (titrated over 8 months up to 700 mg/day, corresponding to 46,63 mg/kg/day on a b.i.d. schedule) but her epilepsy remained uncontrolled, with monthly seizures lasting approximately 15 min. Addition of Topiramate (50 mg/day, corresponding to 3.33 mg/kg/day in 2 divided daily doses) resulted in rapid disappearance of all seizures. The Topiramate dose was increased gradually to 150 mg/day over 9 months for the weight growth and, mostly, to prevent new prolonged seizures. Later the Levetiracetam dose was reduced to 500 mg/day. After 14 months from starting Topiramate she had recurrence of one brief seizure and the dose of Topiramate was increased to 162.5 mg/day (9.4 mg/kg/day). Since then, she has remained seizure-free over a follow-up period of another 4 months. Our patient underwent regular periodic clinical and EEG follow up. While remaining seizure free, her last EEG follow up was unchanged, with persistence of epileptiform discharges.
During follow-up, the mother reported that seizure control was associated with improved language and behavior. However further follow-up is required to determine long-term outcome, including the possibility of persisting seizure-freedom after discontinuation of drug therapy, as noted by other authors 1 .
Discussion
The phenotype of KdVS is broad. 2 Our patient had a microdeletion in the 17q21.31 region (array CGH) including the KANSL1 gene. She presented mild dysmorphic facial features, muscle hypotonia and joint dysplasia and behavioral disorders, as described by others, 1,2 but she had no congenital anomalies affecting the heart, kidney and urinary tract, reported by Koolen only in 25%-50% of patients. 2 On the other hand, her epileptic manifestations were very similar to those described by Myers et al. 1 Therefore, our case is similar to those described by Koolen 2 and Myers et al. 1 The pathophysiological mechanisms of epilepsy in KdVS are probably complex because of the broad role of KANSL1 in regulating multiple genes. In KdVS MRI shows structural brain abnormality reported in approximately half patients: corpus callosum dysgenesis, abnormal hippocampal shape, cortical dysplasia, subependymal heterotopia. 1 Pathological neuroblast migration and abnormal axonal guidance may be potentially the cause of multiple epileptogenic foci caused by cortical nodular heterotopia. 1 Epilepsy seizures are usually prolonged, with multifocal onset and prominent autonomic features. As for response to specific ASM: benzodiazepines, valproic acid, and phenobarbital were previously reported as possibly effective 1 , possibly due their GABAergic actions. 1 In our patient, Levetiracetam was ineffective in controlling the seizures, as documented by others. 1 On the contrary, addition of Topiramate (50 mg/day) resulted in rapid disappearance of seizures. Topiramate has multiple mechanisms of action, which include: enhancement of GABA-mediated transmission, blockade of voltage-gated sodium channels, AMPA receptor antagonism, and inhibition of carbonic anhydrase. 4 Which specific actions contributed to its effectiveness in this patient remains unclear. In our study we present preliminary findings. A critical evaluation of the reported case suggests that there are some limitations in our study, and it is necessary caution in the judgement, indeed: 1) child had an isolated brief seizure recurrence one year after starting Topiramate at low dose and after a small reduction of Levetiracetam; 2) the child is on dual therapy and so it is hard to know if and how much the 2 medications are working together; 3) this is the first report that identifies the potential role of low dose Topiramate in controlling prolonged seizures, refractory to initial drug treatment, in KdVS; 4) it is difficult to generalize findings to other patients with KdVS given that we described only one child who responded to Topiramate.
Conclusion
KdVS is a rare syndrome, and its epileptic phenotype is not well known by child neurologists. 1 However, with broader use of array CGH, an increasing number of cases are likely to be identified and their description, including response to specific medications, can facilitate recognition and treatment. In our case, Topiramate was effective in controlling seizures, although there was a single brief seizure breakthrough after one year. No side effects were reported from the patient and her family. Even with these limitations, our observations provide support to the concept that Topiramate may be an antiepileptic drug with good efficacy in focal epilepsy in KdVS syndrome. The first step to better study whether Topiramate is helpful in KdVS patients may be a prolonged follow up with gradually tapering Levetiracetam aiming for Topiramate monotherapy. The second step may be to replicate the study in a larger population of KdVS children in a multicenter study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Neither of the authors has any conflict of interest to disclose. Parent consent was obtained for description and publication of clinical history and EEG or RM images in anonymous form.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Myers KA, Mandelstam SA, Ramantani G, et al. The epileptology of Koolen-de Vries syndrome: electro-clinico-radiologic findings in 31 patients. Epilepsia. 2017;58(6):1085–1094. doi:10.1111/epi.13746. [DOI] [PubMed] [Google Scholar]
- 2. Koolen DA, Morgan A, de Vries BBA. Koolen-de Vries syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al. eds. GeneReviews®. University of Washington; 2010. [Google Scholar]
- 3. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):512–521. doi:10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shank RP, Gardocki JF, Streeter AJ, Maryanoff BE. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41(S1):3–9. [PubMed] [Google Scholar]