Abstract
Currently there is no established guidance on how to process and evaluate resected lung cancer specimens following neoadjuvant therapy in the setting of clinical trials and clinical practice. There is also a lack of precise definitions on the degree of pathologic response, including major pathologic response (MPR) or complete pathologic response (CPR). In other cancers such as osteosarcoma, colorectal, breast and esophageal carcinomas, there have been multiple studies investigating pathologic assessment of the effects of neoadjuvant therapy including some detailed recommendations on how to handle these specimens. A comprehensive mapping approach to gross and histologic processing of osteosarcomas following induction therapy has been used for over 40 years.
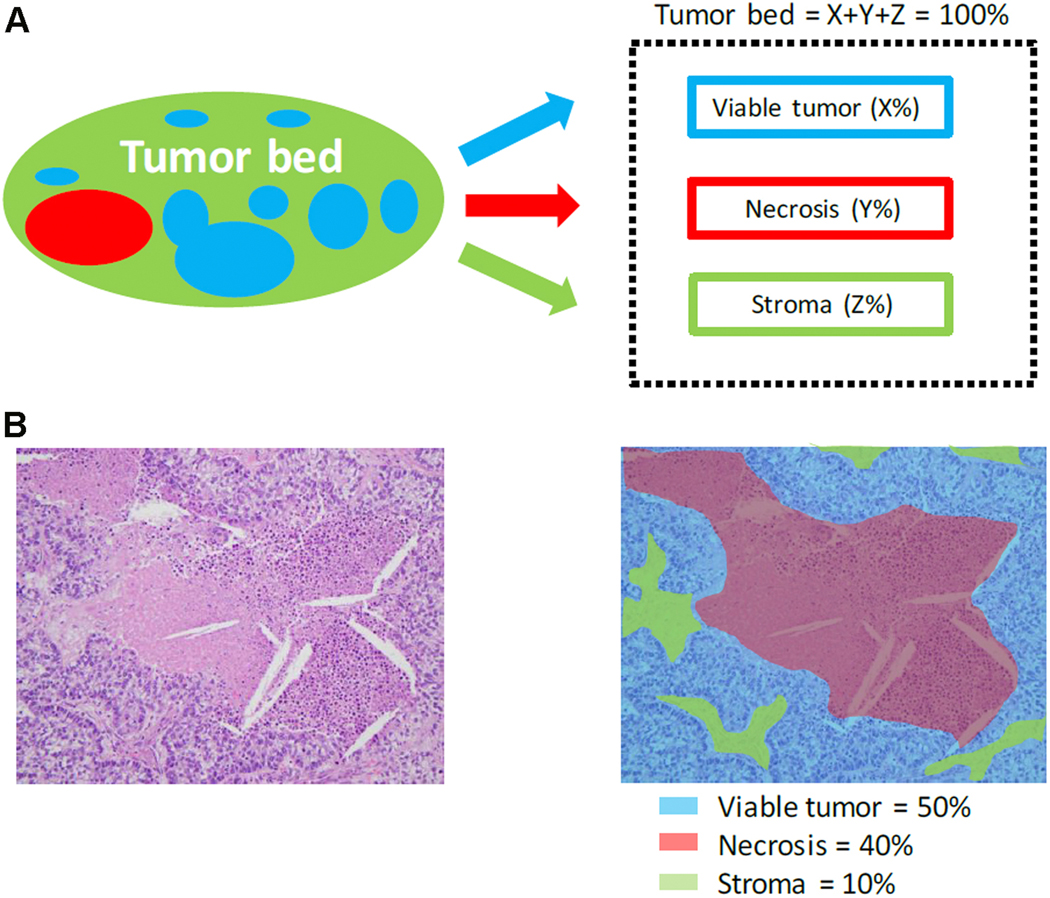
The purpose of this article is to outline detailed recommendations on how to process lung cancer resection specimens and to define pathologic response including MPR and CPR following neoadjuvant therapy. A standardized approach is recommended to assess the percentages of: 1) viable tumor, 2) necrosis and 3) stroma (including inflammation and fibrosis) with a total adding up to 100%. This is recommended for all systemic therapies including chemotherapy, chemoradiation, molecular targeted therapy, immunotherapy or any future novel therapies yet to be discovered whether administered alone or in combination. Specific issues may differ for certain therapies such as immunotherapy, but the grossing process should be similar and the histologic evaluation should contain these basic elements. Standard pathologic response assessment should allow for comparisons between different therapies and correlations with disease free survival and overall survival in ongoing and future trials. The International Association for the Study of Lung Cancer (IASLC) has an effort to collect such data from existing and future clinical trials. These recommendations are intended as guidance for clinical trials, although it is hoped they can be viewed as suggestion for good clinical practice outside of clinical trials, to improve consistency of pathologic assessment of treatment response.
INTRODUCTION
Currently there is no established guidance on how to process and evaluate resected lung cancer specimens following neoadjuvant therapy in the setting of clinical trials and clinical practice. There is also a lack of precise definitions on the degree of pathologic response, including major pathologic response (MPR) or complete pathologic response (CPR). In other cancers such as osteosarcoma,1–3 breast,4–8 colorectal9–11 and esophageal carcinomas,12, 13 there have been multiple studies investigating pathologic assessment of the effects of neoadjuvant therapy including some detailed recommendations on how to handle these specimens.14–17 A comprehensive mapping approach to gross and histologic processing of osteosarcomas following induction therapy has been used for over 40 years.1, 2 Additionally, new treatment modalities including immunotherapy and targeted molecular therapies may change the tumor microenvironment and the manner in which specimens are scored pathologically.
The Food and Drug Administration (FDA) made recommendations for pathology specimen processing for neoadjuvant trials in high risk early stage breast cancer, which provides some perspective on what might be needed for lung cancer.18 The purpose of this article is to outline detailed recommendations on how to process lung cancer resection specimens and to define pathologic response including MPR and CPR following neoadjuvant therapy (Table 1). The protocol can then be applied to all systemic therapies including chemotherapy, chemoradiation, molecular targeted therapy, immunotherapy or any future novel therapies yet to be discovered whether administered alone or in combination. Standard pathologic response assessment should allow for correlations with disease free survival and overall survival in ongoing and future trials. The International Association for the Study of Lung Cancer (IASLC) has a goal to collect such data from existing and future clinical trials. These recommendations are intended as guidance for clinical trials, although it is hoped they can be viewed as suggestion for good clinical practice outside of clinical trials, to improve the consistency of pathologic assessment of treatment response.19
TABLE 1:
PRINCIPLES OF PATHOLOGIC ASSESSMENT OF PRIMARY TUMORS†
| LUNG TUMOR BED | |
| How to Recognize the Tumor bed, which is the area where the original pre-treatment tumor was considered to be located. | |
| 1. | To identify the tumor bed, look for the presence of pleural retraction and palpate the intact specimen. |
| 2. | In cases where identification and/or orientation of the tumor are difficult, review of the pretherapy and preoperative CT can be helpful. |
| 3. | Look for any identifying marks/stiches placed by the surgeon. |
| 4. | After the tumor bed has been identified, lung specimens should be sectioned in the plane that demonstrates the maximum dimension and best reveals the tumor bed and its relationship to the surrounding structures relevant for staging and the surgical resection margin(s). |
| 5. | Photograph the cut surface demonstrating the tumor bed and the adjacent structures. Save the images in the pathology electronic records. |
| 6. | The gross size of the tumor bed should be assessed using a ruler to measure three-dimensional size. |
| 7. | Document the distance between tumor bed and surgical resection margins in the gross description. |
| 8. | Estimate percentage of gross necrosis that will be correlated with the estimated necrosis on the microscopic slides. |
| SAMPLING | |
| How to sample the suitable area for assessment of response to neoadjuvant therapy | |
| 1. | Following the tumor bed measurement, the surgical specimens may be processed fresh or with routine fixation in 10% neutral buffered formalin least six hours and no longer than 48 hours. |
| 2. | Cases with marked necrosis and cavitation are difficult to cut fresh. Overnight fixation may be helpful in such cases |
| 3. | If the tumor is small (<3 cm) it should be entirely sampled. |
| 4. | If the tumor is larger than 3 cm, an approximately 0.5 cm thick cross section of tumor in its maximum dimeson should be made and photographed. |
| 5. | On this gross photograph, a map should be superimposed of the complete histologic sectioning corresponding to the submitted blocks (See Figure 5. These photographs should be saved with the pathology report, electronically if possible. Additional histologic sections can be submitted if desired. |
| 6. | Histologic sections at the periphery of the tumor should include the border of the tumor with at least 1 centimeter of the surrounding nonneoplastic lung parenchyma in order to define the edge of the tumor. |
| HISTOLOGIC ASSESSMENT OF PRIMARY TUMORS | |
| How to define the Border of the Tumor Bed from Surrounding Non-neoplastic Lung | |
| 1. | Identify reactive changes in surrounding non-neoplastic lung (for example: organizing pneumonia, interstitial fibrosis, hemorrhage, marked type II pneumocyte hyperplasia /reactive atypia and inflammatory infiltrates). |
| 2. | Inflammatory cells that are part of the reactive changes surrounding the tumor bed must be distinguished from tumor stromal inflammation where the inflammatory cells should be confined to the tumor bed. |
| 3. | The true tumor bed should consist only of viable tumor along with concurrent necrosis and stroma which includes both fibrosis and inflammation. The size of tumor bed should be adjusted for histologic changes related to neoadjuvant treatment in the surrounding lung. |
| 4. | Correlate with the gross photograph with mapping of histologic sections in order to determine whether the gross measurement of the tumor bed size is an accurate assessment or if it includes non-neoplastic reactive changes. |
| How to Record the Histologic Features in Tumor Bed | |
| 1. | The percentages of viable tumor, stromal tissue, and necrosis should be estimated on the basis of the review of the microscopic sections on each slide and then the total percentage of viable tumor is estimated. The percentage should total 100% of the tumor bed. |
| 2. | Each component should be assessed in 10% increments unless the amount is below 5% when an estimate of single percentages should be recorded. |
| 3. | There are two stromal tissue components: fibrosis and inflammation. Although more detailed assessment of stroma can be made (Fibrosis: dense hyalinized connective tissue, fibroelastotic scarring and loose or myxoid connective tissue. Inflammation: chronic inflammation, acute inflammation, histiocytes, xanthogranulomatous, cholesterol clefts and granulomatous reaction), until there is sufficient validation that any of these are clinically relevant, recording of these features is not needed for routine clinical purposes. |
| DETERMINATION OF THE PATHOLOGIC RESPONSE TO NEOADJUVANT THERAPY | |
| 1) | The final pathological response should be determined on the basis of the histological features correlated with the gross findings. Particularly the mapped gross photograph and corresponding histological sections can be helpful, especially in markedly necrotic and cavitated tumors. |
| 2) | Until digital and/or computational approaches are routinely available, a semiquantitative approach can be done. |
For processing of lymph nodes, see recommendation 9.
This document and the recommendations are based upon expert opinion from an international panel of members of the IASLC, primarily the Pathology Committee but also from an international group of expert thoracic medical oncologists, surgeons and radiologists. In addition, pertinent literature was reviewed on the topic of neoadjuvant therapy as it relates to pathologic assessment. Face to face meetings of the IASLC Pathology Committee were held in National Harbor, Maryland in March 2019 and in Barcelona, Spain in September 2019. During these meetings this project was planned (National Harbor). Following this meeting writing assignments were made and after the draft document was assembled it was sent out for review to the co-authors, the entire IASLC Pathology Committee and to the membership of the Pulmonary Pathology Society (PPS). After comments from the co-authors, IASLC Pathology Committee and PPS membership were incorporated, another revised draft was prepared and distributed back to the IASLC Pathology Committee. This revised document was circulated again to all coauthors and the proposed recommendations were discussed by the Pathology Committee in Barcelona. After revisions were made following the Barcelona meeting, the document was distributed electronically by the IASLC office for public comment. Throughout this process multiple conference calls and email communications occurred with the project leaders (IW, SD, LS and WT). This document was also reviewed by the Food and Drug Administration, Maryland, USA (G. Blumenthal), European Medicines Agency, The Netherlands (R. Herold), Office of New Drug, Pharmaceuticals and Medical Devices Agency, Japan (K. Kiyohara) and Office of Clinical Evaluation, Center for Drug Evaluation, National Medical Products Administration, P.R. China (Z Yang).
CLINICAL RELEVANCE OF PATHOLOGIC RESPONSE
Platinum-doublet adjuvant chemotherapy became the standard treatment option for patients with resectable lung cancer two decades ago.20 Preoperative chemotherapy became a standard option when a meta-analysis of neoadjuvant chemotherapy trials demonstrated that preoperative platinum-doublet chemotherapy improved survival over surgery alone in resectable early stage non-small cell lung cancer (NSCLC).21 The magnitude of improvement of survival outcome is thought to be almost equal to postoperative adjuvant platinum-doublet chemotherapy with hazard ratios of 0.8721 for neoadjuvant therapy and 0.89 for adjuvant therapy.20
While large and lengthy adjuvant studies assessing new strategies beyond chemotherapy yielded negative results,22 systemic therapy for advanced lung cancer has evolved such that platinum-doublet chemotherapy alone has become less common, due to the use of tyrosine kinase inhibitors in patients with molecular drivers, immunotherapy alone in patients with high PD-L1 tumor proportion scores (TPS) and chemotherapy plus checkpoint inhibitors in the remaining NSCLC patients. Conversely, there have been no changes to the standard of care in resectable disease.23, 24 Studies are needed to move these advances into the curative setting. In adjuvant trials, the efficacy of the therapy cannot be determined until years later when disease free survival (DFS) and overall survival (OS) are available whereas neoadjuvant trials allow efficacy endpoints such as clinical and pathologic response to be determined in several months. Neo-adjuvant treatments offer potential advantages over postoperative treatments, including the ability to treat micrometastatic disease and analyze the treatment-related effect on the primary tumor. Neoadjuvant treatment with surrogate measures of efficacy such as treatment response have the potential to accelerate curative therapies for the general lung cancer patient population.19
After neoadjuvant treatment with chemotherapy, multiple studies demonstrated that patients with lung cancers that show a major pathologic response (MPR) defined as 10% or less viable tumor have a significantly improved survival.25–32 These previous studies have lumped histologic types together, particularly adenocarcinoma and squamous cell carcinoma. The 2017 College of American Pathologists (CAP) Synoptic Template for assessment of resected lung cancers following neoadjuvant therapy recommended - in line with the work of Junker et al,25, 30 recording the presence of greater than, or less than or equal to 10% residual viable tumor.33 A similar threshold is recommended by the Royal College of Pathologists.34 This recommendation has led to design of lung cancer neoadjuvant therapy clinical trials where MPR is a primary endpoint.35
RADIOLOGIC ASSESSMENT OF PATHOLOGIC RESPONSE
Use of CT and PET to Predict Pathologic Response and Prognosis
Computer tomography (CT) is typically used to assess the response to neoadjuvant therapy in patients with advanced NSCLC. William et al., reported that CT response by Response Evaluation Criteria in Solid Tumors (RECIST) was a significant predictor of OS in patients with NSCLC after neoadjuvant chemotherapy.29 However, the discordant rate between histopathologic response and CT RECIST response (histopathologic response with stable disease or progressive disease by CT criteria, no histopathologic response with complete response or partial response by CT criteria) was 41% to 45%.29, 36 This discrepancy in assessing histopathologic response after neoadjuvant chemotherapy has also been observed in other malignancies such as breast cancer and sarcomas.37–39 Alterations in the inflammatory, stromal or fibrotic components of the tumor rather than cancer cell death may confound the radiographic interpretation of tumor size, contributing to the inability of CT to accurately predict histopathologic response after neoadjuvant therapy.29, 40 In this regard, while CT can identify gross necrosis, features such as stromal, inflammatory or fibrotic changes are similar in appearance to viable cancer cells. Several studies have suggested that there may be more accurate response criteria than RECIST such as volumetric response measurements with automatic deformable image registration.41, 42 In a fluorodeoxyglucose (FDG) positon emission tomography (PET) study, metabolic activity predicted pathological response.43 Other authors have suggested that monitoring response with apoptosis molecular imaging or contrast-enhanced MRI may be more accurate than conventional CT assessment.40, 44–46 Recently the phenomenon of nodal immune flare has been described with the clinical impression of nodal progression by CT and PET, but only noncaseating granulomas found on pathologic examination.47, 48
The assessment of therapeutic response has also been complicated by newer treatment options such as molecular-targeted therapy and immunotherapy where the anti-tumor effect may not cause reduction of the tumor size and where inflammatory effects may influence tumor size on imaging.46 Because of the inherent limitation of assessing response using changes in serial measurements of tumor size in these patients, determination of response may require functional and molecular imaging. [18F] FDG-PET can be used to assess the effectiveness of neoadjuvant therapy as FDG uptake by the tumor is related to proliferative activity as well as the number of viable cancer cells remaining. Although FDG-PET can be useful in identifying viable tumor, confounding factors include tumor cell differentiation and competitive uptake of FDG by macrophage/monocyte infiltration. In fact, therapy-induced inflammatory response after neoadjuvant therapy can lead to a considerable number of false-positive findings, especially when residual tumor volumes are large (> 10 cm3).49 The prediction of histopathologic response in patients with NSCLC after neoadjuvant chemotherapy may be more accurate when defined by using both CT and FDG-PET together (73% to 82%) rather than separately. 40, 49
To address the limitations of CT and FDG-PET in the determination of histopathologic response to therapy, there has been an interest in applying computational approaches such as machine learning in patients with lung cancer. A large set of advanced quantitative imaging features can be extracted mathematically from conventional imaging and used to create a unique phenotypic map of the tumor. These morphologic characteristics, commonly referred to as radiomic features, have the potential to predict histopathologic response to neoadjuvant therapy. Coroller et al., report that radiomic analysis of the CT prior to neoadjuvant therapy in patients with advanced NSCLC was a significant predictor of pathologic gross residual disease and CPR.50 In fact, radiomic features available from conventional CT, performed better than CT evaluation of response. However, while radiomics has the potential to improve patient stratification prior to the initiation of neoadjuvant therapy and to assess histopathologic response following neoadjuvant therapy, there are potential limitations to clinical applicability including lack of image acquisition standardization, variations in analysis and reproducibility.
PATHOLOGIC ASSESSMENT OF RESPONSE TO THERAPY
Even though MPR in lung cancer patients treated with neoadjuvant therapy has been recognized as a predictor of survival and potential surrogate endpoint in clinical trials, few studies have described approaches for gross and microscopic assessment of the lung resection specimens. Junker et al. grossly evaluated formalin fixed lung resection specimens and sampled areas of viable tumor or regressed tumor tissue. Depending on the size of the tumor, up to 58 paraffin blocks have been prepared in one study.30 Blaauwgeers et al. evaluated on average 7 blocks per case (range 3–15).51 Other studies mostly focused on the histologic features and little or no detail was provided on the methods of gross processing of tumors.26–28, 52 Pataer et al. identified histologic heterogeneity among submitted sections and suggested to submit at least one section per cm of resected tumor to adequately assess MPR.26 Numerous histologic criteria were reviewed, and the major three features include necrosis, stromal fibrosis and viable tumor. The percent of viable tumor has consistently been shown to be the only prognostically significant histologic indicator. It was also noticed that the same histologic changes attributed to treatment effect can be seen in resection specimens without history of neoadjuvant treatment, and therefore it is essential for the pathologists to be aware of the treatment history. Historic definition of MPR as ≤ 10% of residual viable tumor in NSCLC regardless of histologic subtype has been recently challenged. Qu et al. suggested based on resected lung cancers following platinum based neoadjuvant chemotherapy that the optimal cutoff for predicting survival may be different according to histologic type with 10% and 65% cut-offs for squamous cell carcinoma and adenocarcinoma, respectively.53 However, this observation needs to be validated and investigated in samples treated with other types of neoadjuvant therapies.
The impact of evaluating lymph nodes,54 diagnostic reproducibility of the response criteria among pathologists,51, 53 and the potential role of immunohistochemistry,55 digital imaging55 and molecular studies following neoadjuvant treatment are largely unknown.
Similar Pathologic Changes Also Occur without Preoperative Therapy
Two of the treatment related findings commonly recognized as part of the response to therapy are necrosis and fibrosis. Paradoxically, these have the opposite prognostic implications in patients who have not undergone neoadjuvant therapy. In the neoadjuvant setting, data suggests that they are markers of favorable prognosis when these two features in combination exceed 90% with resulting 10% or less viable tumor.26–28, 30, 32 However, in treatment naïve lung adenocarcinomas, poor prognosis has been associated with the size of the fibrous scar and the presence of tumor necrosis.56–59 In contrast necrosis is not clearly associated with poor prognosis in squamous cell carcinoma, but the presence of stromal fibrosis is sometimes associated with worse outcome.60, 61 Cavitary necrosis may be seen in pathology specimens following neoadjuvant therapy but it is not been shown to be a poor prognostic factor in this setting. However, in patients without preoperative therapy, it has been shown to be an independent predictor of poor prognosis in patients with resected p-stage I-IIA primary lung cancer.62
After neoadjuvant chemotherapy in lung cancer, resected tumors show variable amounts of necrosis, fibrosis and inflammation with cholesterol cleft formation. However, in chemotherapy-naïve lung cancer resections, such changes are not uncommon (Figure 1A-D). So, a small proportion of resected lung cancers show features reminiscent of those seen after neoadjuvant therapy, but these patients received no such treatment. These changes have been likened to those described in so called spontaneous partial regression of cancers such as renal cell carcinoma and cutaneous malignant melanoma, and have been rarely described in lung cancer.26, 30, 63, 64 The mechanism is presumed to be immunological, the same outcome intended by the use of immune checkpoint blockade.
Figure 1:
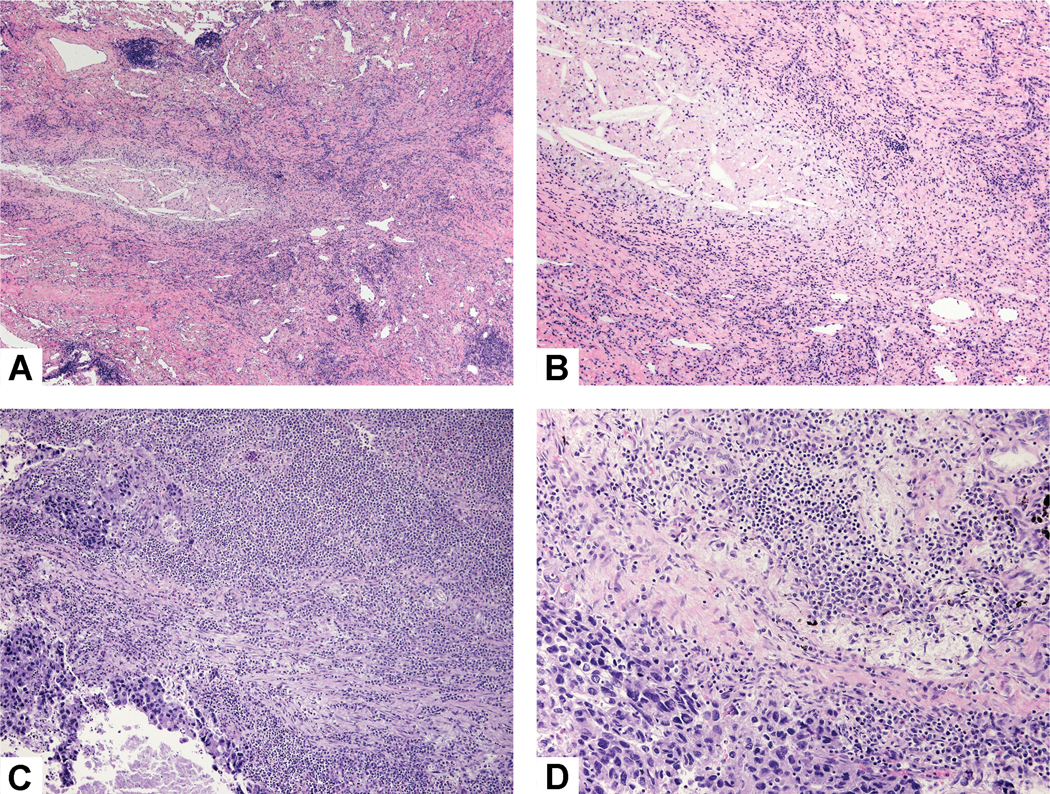
Histologic changes without neoadjuvant therapy: A) Only 10% of this tumor was viable with 90% showing fibrosis, chronic inflammation and focal necrosis with cholesterol clefts. B) Higher power shows dense fibrosis with a mild chronic inflammatory infiltrate and an area of necrosis with cholesterol clefts. C) This lung cancer was associated with a large chronic inflammatory infiltrate that overshadowed a small focus of adenocarcinoma. D) This area of solid nests of tumor cells (bottom left) are surrounded by an extensive mixed inflammatory infiltrate of lymphocytes, plasma cells and histiocytes.
Lung cancers showing this phenomenon represented about 3% of cases in the largest described series.63 Regressing tumors were more likely to be undifferentiated and conferred better post-operative survival compared to cases without this phenomenon. Tumors were characterized by marked lymphoid infiltrates (Figure 1A and B) and variable fibrosis, often obliterating large segments of the tumor area, sometimes with residual tumor cell islands. Macrophages were numerous, often aggregates and frequently seen surrounding and ‘eroding’ tumor cell nests. Granulomas were sometimes present and cholesterol clefts could be found in areas of scar tissue (Figure 1C and D). Tumors usually had a fibro-cellular pseudo-capsule at the interface with surrounding lung. This phenomenon is qualitatively different from regular stromal inflammation in lung cancer, giving the impression of replacement of part or all the tumor mass by this mixed fibro-inflammatory reaction. The distribution of these changes may be patchy. By immunohistochemistry, these tumors had a greater density of CD3, CD68 and CD57 expressing cells and S100 positive dendritic cells, when compared to lung cancers showing either high or low levels of ‘regular’ chronic inflammation. In some cases, it was possible to document, from serial imaging prior to surgery, growth and then shrinkage or retarded expansion of the tumor.63
Junker et al. proposed that the histologic changes associated with neoadjuvant therapy typically occur in the tumor periphery while in tumors undergoing spontaneous regression, the histologic inflammatory and/or fibrotic changes are seen more in the center of the tumor.30 However, this finding was contradicted in one study where the viable tumor cells in neoadjuvant cases were found at the periphery.51 Pataer et al. also suggest that the histologic findings such as coagulation necrosis, foam cell infiltration and inflammatory infiltrates that they observed in the resected tumors of patients who underwent neoadjuvant chemotherapy are nonspecific as they were also observed in the tumors of patients who underwent surgical resection alone.26
The significance of these findings is that tumor regression may rarely occur in the absence of therapy and thus for a small percentage of patients could potentially confound the assessment of resected tumors from patients receiving neoadjuvant therapy. The term regression bed has been introduced in the neoadjuvant immunotherapy setting suggesting this can be distinguished from tumor bed stroma.65 However, we prefer to use the term tumor bed and to avoid the term regression bed. One reason is because we favor to only have one rather than two categories of tumor bed stroma. Another reason is that it can be extremely difficult to distinguish histologic changes related to therapy from those that may have been present without therapy.
PATHOLOGIC ASSESSMENT
Communication from thoracic surgeon and operative issues that impact pathologic assessment
To facilitate accurate and complete pathologic reporting of lung cancer resection specimens from patients who received neoadjuvant therapy, thoracic surgeons should inform pathologists on whether the patient received neoadjuvant therapy when the specimen is delivered to the pathology laboratory (Table 2). In countries where infections such as tuberculosis are endemic, the impact of these on specimen assessment, particularly possible tumor bed overestimation should be considered.66–68 Current treatments should reflect the type of neoadjuvant therapy (chemo-, immuno-, radiation, targeted therapy, or combinations), date of last treatment, and any pneumonitis associated with therapy. The site of the specimen, type of surgery, adherent tissue included (in case of extended resection), and N1 and N2 lymph nodes (separately indicated by station) should be noted on the paperwork submitted to pathology with the specimen. In addition to placing appropriate markers (i.e. sutures) to orient the specimen for the pathologist, surgeons should report pertinent intraoperative findings such as difficulty in hilar dissection due to fibrosis69 or adherent peribronchial lymph nodes and mark the area of suspicion, such as an area of adhesion to the parietal pleura. Cutting into the specimen in the operating room should be avoided, and researchers should be encouraged to obtain tissue from the pathology department. In complicated cases, it can be helpful for the surgeon to come to the pathology frozen section and/or gross room to orient the specimen. If the surgical resection is incomplete it may not be possible to fully assess the tumor bed for extent of pathologic response unless correlation between the operative and pathologic findings indicates there is only a microscopically positive margin. However, the definition of a positive margin in the context of a MPR is an unexplored issue. More data is needed to address this.
TABLE 2:
RECOMMENDED SYNOPTIC TEMPLATE FOR RECORDING LUNG CANCERS FOLLOWING NEOADJUVANT THERAPY
| PRIMARY TUMOR |
| Type of Neoadjuvant Therapy |
| a. No known presurgical therapy |
| b. Type of neoadjuvant therapy: |
| a. Chemotherapy _____ |
| b. Radiotherapy _____ |
| c. Immunotherapy (Please specify) _____ |
| d. TKI (please specify) _____ |
| e. Other (please specify) _____ |
| Treatment Effect in Primary Tumor |
| a. Percentage of viable tumor: % (record in 10% increments except below 10%, then record single digits between 1–5%) † |
| b. No residual viable tumor identified |
| c. Percentage of necrosis: % |
| d. Percentage of stroma (includes fibrosis and inflammation): % |
| Grade of Inflammation (choose the appropriate grade) |
| ______ Mild |
| ______ Moderate |
| ______ Marked |
| Method (Choose all what was used for evaluation) |
| ______ Correlation was made with a gross photograph of tumor cut surface: Yes ____ No ____ |
| ______ Evaluation was aided by use of tumor mapping to match a gross photograph to histologic sections: Yes ____ No ____ |
| ______ Evaluation was aided by radiologic pathologic correlation: Yes ____ No ____ |
| TREATMENT EFFFECT IN LYMPH NODE METASTASES |
| a. Total number of lymph node stations examined: ____ |
| b. Total number of lymph nodes examined: ____ |
| c. No carcinoma present: ____ |
| d. Total number of lymph nodes with metastatic carcinoma: ____ |
| e. Lymph node stations involved by tumor with treatment related changes: ____ |
| f. Lymph node stations with treatment related changes without viable tumor: ____ |
| g. Largest tumor focus: mm at station number: ____ |
| h. Extracapsular extension present: ____ |
| i. No extracapsular extension: Comment: ____ |
| Comment: _______________________________ |
The three components: % viable tumor, % necrosis and % stroma should add up to 100%.
Gross Assessment and Processing
Lung Tumor Bed
A thorough and consistent approach to grossing lung cancer resection specimens is critical in the assessment of pathologic response. The goal is to provide a comprehensive histologic assessment of the tumor treatment response.
The first step in the gross assessment of the lung specimen is to identify the tumor bed, which is the area where the original pre-treatment tumor was located. This can often be achieved by identifying pleural retraction and palpation of the specimen. The surgeon can aid in the gross evaluation by marking the tumor location with a suture on the gross specimen and notifying the pathologist on the specimen requisition form the meaning of this designation. In general, it is a good practice to review the most recent CT scans of the chest to localize the tumor in the gross resection specimens (Figure 4A). This is particularly helpful in the uncommon cases with a complete response, where the tumor bed cannot be visualized or palpated.
Figure 4:
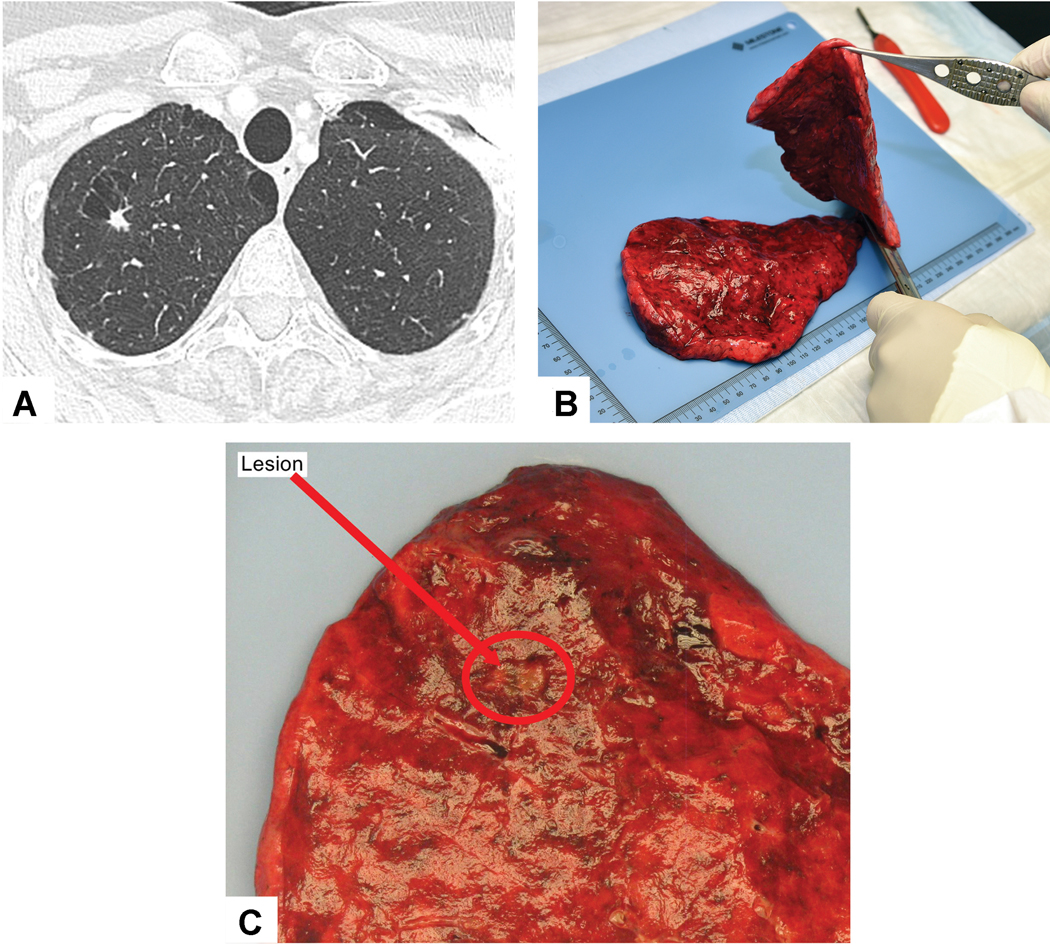
Gross Assessment to identify the tumor bed: A) Review of the CT scan shows a small 0.8 cm right upper lobe mass. B) After the tumor was identified on palpation, the specimen is cut with a knife through the tumor bed along its maximal dimension. C) This tumor bed measured 0.8 cm and corresponded well to the nodule seen on CT (Red circle highlights the tumor bed).
The gross size of the tumor bed is best assessed on the fresh, unfixed lung resection specimens. However, formalin fixed specimens can also be utilized. Although formalin fixation can result in some tumor shrinkage after fixation, this would not be expected to alter the percentage of viable tumor.70 The tumor should be palpated prior to sectioning to try to cut across the middle of the tumor bed along the maximum dimension (Figure 4B). When possible it is helpful to also demonstrate the tumor bed’s relationship to the surrounding structures, particularly those relevant for staging such as the visceral pleura, chest wall, interlobar fissures, the bronchus, and the surgical resection margin(s) (Figure 5A). After each cut across the tumor bed the knife should be wiped clean. After making a cross section of the tumor, a ruler should be used to measure three-dimensional sizes.71 Photographic images should be taken of the cut surface demonstrating the maximum dimension of the tumor bed (Figure 4C) and if possible including adjacent structures and these should be saved in the pathology electronic records. The distance between tumor bed and surgical resection margins should be documented in the gross description. The pathology gross descriptions should also contain an estimate of the percentage of any necrosis present. The initial gross and microscopic estimates may be inaccurate, requiring some adjustments, particularly in large necrotic tumors where histologic sections cannot be obtained from cavitary areas. So the final assessment of the amount of necrosis then can be determined by correlating the gross and microscopic findings at the time of final evaluation of the case incorporating any available gross photographs.
Figure 5:
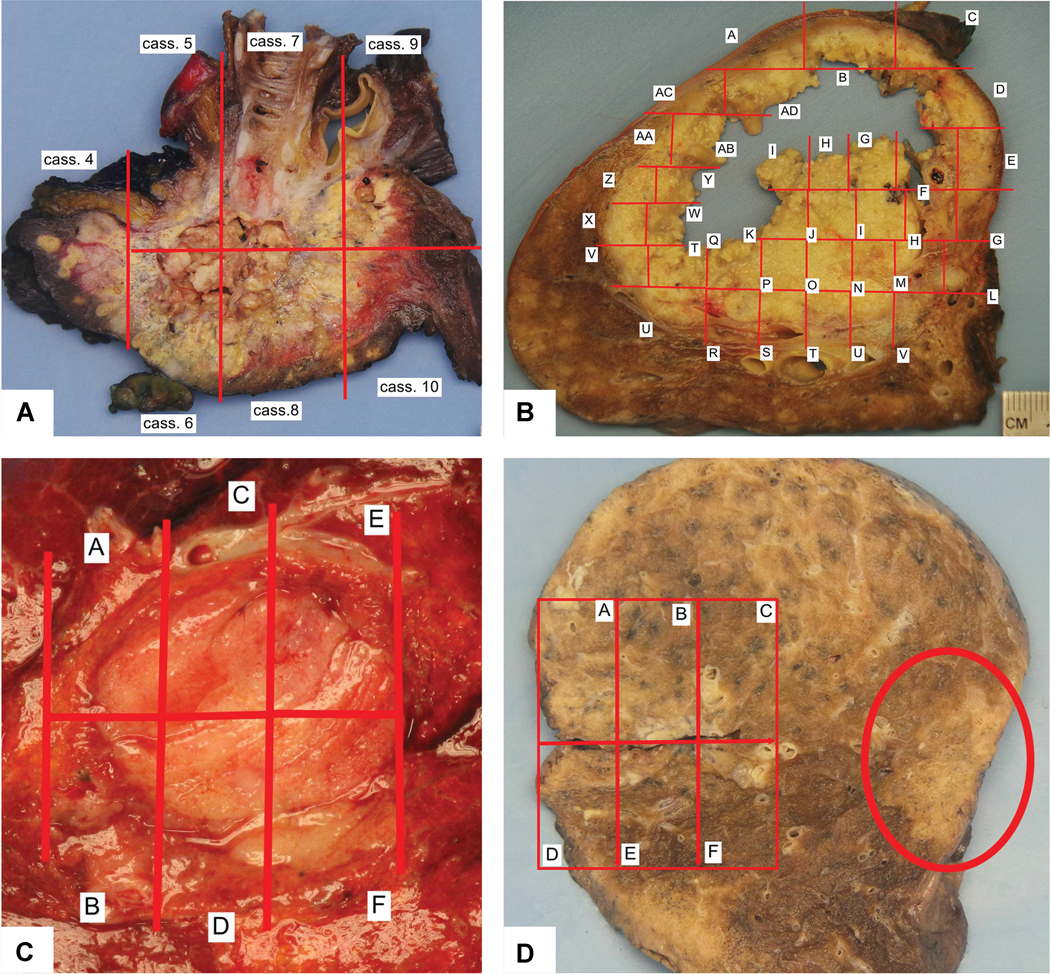
Gross Appearance and Mapping. A) This adenocarcinoma shows extensive necrosis with both yellow areas and some chalky white tissue within areas of cavitation. In addition, this photograph demonstrates the anatomic relationship of the tumor to the overlying pleura and the proximal bronchus. B) This large necrotic tumor shows a large central area of cavitation. In the cavitated area it is impossible to submit blocks of tissue sections, so this area is not mapped. C) This small tumor is mapped but due to its small size the entire tumor was sampled histologically. D) In this case the mapped area turned out to be granulomatous inflammation, and the tumor was on the opposite side of the specimen (red oval shaped circle). So additional sections from the non-mapped area of tumor needed to be submitted to evaluate for treatment effect.
Gross findings in neoadjuvant treated lung cancers can vary widely with the entire spectrum of gross manifestations of resected lung cancers. In tumors where there was little response to the neoadjuvant therapy the gross appearance may show little difference from untreated tumors. However, when there is a response, the gross appearance of the tumor may be altered due to fibrosis, inflammation and necrosis. Large tumors are easily identified (Figure 5A), but small tumors, particularly those that achieve MPR, can be difficult to visualize or palpate on gross exam as they can be small (Figure 4C) or soft when the reaction is mostly due to inflammation and/or necrosis rather than a firm fibrous scar. Necrosis often consists of yellow to brown soft granular material or it can appear white and pasty. Necrosis can result in cavitation which can be extensive (Figure 5B).
If the tumor bed is small (≤ 3 cm) a cross section documenting the maximum dimension should be made and the tumor should be entirely sampled (Figure 5C). If the tumor bed is larger than 3 cm, an approximately 0.5 cm thick cross section of tumor should be taken that can be mapped for complete histologic sectioning (Figure 5A and B). Blocks should be submitted from the entire cross section of the tumor bed.
A gross picture should be made of the cross section whether the tumor is small or large and a map of the complete histologic sectioning matching areas on the specimen corresponding to the submitted blocks should be superimposed upon the gross photograph (Figure 5A-C). If the technology is not available to insert the map electronically onto a digital picture, the picture can be printed, the block designation hand written on the picture and this can then be photographed and stored with the pathology report.
In cases where the tumor bed is larger than 3.0 cm a minimum of one section per diameter of the tumor bed should be submitted. More histologic sections of the tumor bed can be submitted beyond this minimum recommendation, depending on the level of interest and individual institutional resources, While all histologic sections of the tumor bed should be assessed for percentage of viable tumor, necrosis and stroma usually the final percentages reported are obtained from the mapped sections. If there is a discrepancy between the percentage of viable tumor in the mapped tissue sections and the additional tissue sections, adjustments can be made to reflect what appears to be the overall percentages of viable tumor, necrosis and stroma. If no tumor is seen in the initial histologic sampling, additional sections should be submitted. There is no clear guidance for further sampling. This could include sampling the rest of the tumor if this does not result in what is regarded as an unreasonable number of sections. However, if the tumor bed is very large, only representative sampling is acceptable. Histologic sections at the periphery of the tumor bed should include the border of the tumor with at least 1 centimeter of the surrounding nonneoplastic lung parenchyma to define the edge of the tumor.
In some cases, the mapped area originally thought to be tumor turns out to be a nonneoplastic lesion such as granulomatous inflammation and the specimen needs further evaluation to search for sample the area of the tumor bed (Figure 5D). In addition, sometimes the tumor bed shows multiple discontinuous areas of viable tumor alternating with areas of inflammation and fibrosis (Figures 6A-C). In such cases the question may be raised whether there were multiple synchronous primary tumors or intrapulmonary metastases. In addition, in this setting it is impossible to measure tumor size with a ruler (See Staging Issues below). Review of the pretherapy CT scan can help to sort out whether there was a single tumor or whether the multiple separate areas of viable tumor represent a heterogenous response to therapy (Figure 6D). The presence of a single mass on the pre-therapy CT along with similar histology of the multiple discrete areas of viable tumor within the tumor bed favors a single lung carcinoma with a heterogeneous response to therapy.
Figure 6:
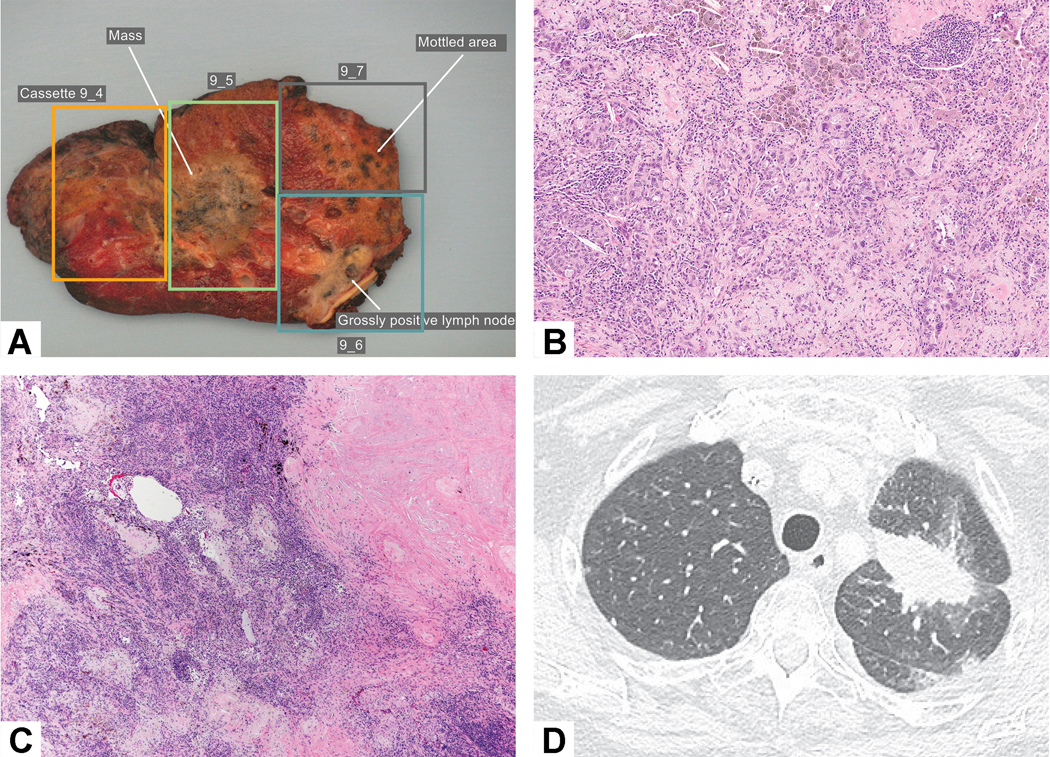
Tumor bed with multiple areas of viable tumor. The viable tumor alternated with stromal inflammation and fibrosis that led the person grossing the specimen to describe three different areas of tumor (in blocks 9–4, 9–5 and 9–7) raising the question whether there were intrapulmonary metastases. An area in block 9–6 shows grossly positive lymph nodes reflecting metastatic carcinoma. B) Residual viable tumor consists of acinar glands and the adjacent stroma shows prominent chronic inflammation and loose myxoid connective tissue. C) The nodules of residual viable tumor alternated with intervening fibroinflammatory stroma in the tumor bed. D) Prechemotherapy CT scan shows a solitary mass confirming that there is a single tumor with a heterogeneous response to chemotherapy.
A small sample of tumor can be procured for various research purposes such as banking of frozen tissue, genetic studies or flow cytometry. However, this should be done in the pathology laboratory coordinated with the pathology processing of the gross specimen in a way that does not compromise assessment of the specimen according to the protocol described herein. If no viable tumor is identified in the permanent sections, the tissue sample procured for research should be examined histologically to see if any viable tumor is present. In order to know whether the procured research tissue represents tumor, stroma, necrosis or peritumoral reaction, it would be ideal to perform a frozen section at the time of usage for research studies or make a corresponding adjacent paraffin block to confirm whether the tissue being studied for research is actually tumor, reactive changes in the tumor bed, or other non-neoplastic lung tissue from the tumor border, or some other lesion such as granulomas (Figure 5D).
Following the tumor bed measurement, the surgical specimens may be processed fresh in laboratories with experience in processing of unfixed large resection specimens. Otherwise, a routine fixation in 10% neutral buffered formalin after inflation of the lung via the bronchi should be performed for at least six hours and no longer than 48 hours.72 Cases with marked necrosis and cavitation are difficult to cut fresh. In such cases overnight fixation may be helpful.
Use of CT by Pathologists to Assess Gross Specimens
Pathologic assessment of lung specimens resected in the neoadjuvant setting can benefit in several ways by radiologic pathologic correlation. First, in some cases the tumor can be hard to identify on gross examination. In such cases, review of CT scans can help identify where in the resected lung specimen the tumor bed should be located. Second, determination of the tumor bed size can be helped in some cases by review of the after neoadjuvant therapy some tumors show a heterogeneous response and on gross and/or histologic examination of the resection specimen it may appear there are multiple tumors. In this setting, review of the CT prior to therapy can help to determine if the original tumor was solitary or if there were multiple tumors.
When reviewing post-neoadjuvant therapy imaging studies, the tumor size may not reflect the tumor bed but also reactive changes surrounding the tumor. In addition, the tumor size seen on CT is not a reliable way to know the amount of viable tumor versus stromal inflammation and fibrosis and necrosis.19, 73 Detailed CT pathologic correlation to maintain three dimensional orientation can be helpful in certain cases.74
Histologic Assessment of Primary Tumors
Defining the Border of the Tumor Bed from Surrounding Non-neoplastic Lung
The distinction of the border of the tumor bed from the surrounding non-neoplastic lung is important to establish the exact size of the tumor bed. Inflammation and fibrosis that are part of the reactive changes surrounding the tumor bed must be distinguished from tumor stromal inflammation where the inflammatory cells should be confined to the tumor bed. However, that distinction can be challenging as there are frequently extensive reactive changes in the adjacent non-neoplastic lung parenchyma beyond the edge of the tumor bed (Figure 6A-D). Dense fibrosis or organizing pneumonia can appear white or tan on gross exam and difficult to distinguish from viable tumor. In those cases, the presence of adjacent organizing pneumonia and/or interstitial fibrosis and inflammation may preclude a reliable assessment of the tumor bed size on gross exam alone. Therefore, correlation of the gross photograph with mapping of histologic sections is important in order to determine whether the gross measurement of the tumor bed size is an accurate assessment or if it includes non-neoplastic reactive changes. The true tumor bed should consist only of viable tumor along with concurrent necrosis and stroma which includes both fibrosis and inflammation.26, 53 This can also result in discrepancies between size measurements in pathology and radiology. The initial gross measurements of the tumor bed should be re-evaluated at the time of microscopic examination. At that time the size of tumor bed should be adjusted, taking into account non-tumor related histologic changes in the surrounding lung parenchyma (see below), if these were included in the initial gross measurement.
Histologically at low power, one can usually see the border of the tumor surrounded by a rim of abnormal lung parenchyma that extends with an ill-defined edge that interfaces with the normal lung parenchyma (Figure 7A). In the reactive non-neoplastic parenchyma the architecture of the lung is generally preserved with interstitial thickening by fibrosis and inflammation (Figure 7B, C and D). The changes in the tumor border can also include organizing pneumonia (Figure 7E), marked type II pneumocyte hyperplasia/reactive atypia (Figure 7C) and various types of inflammatory infiltrates including chronic (Figure 7D) or acute inflammation, histiocytes, giant cell reaction and granulomas. In addition to organizing pneumonia, post-obstructive pneumonia can be characterized by extensive lymphoid aggregates within the interstitium of alveolar walls. The main way to distinguish the tumor bed from the reactive changes in the surrounding lung parenchyma is to identify preserved underlying alveolar architecture while in the tumor bed the lung architecture is destroyed (Figure 7A-F). The presence of entrapped and hyperplastic epithelium such as pneumocytes or bronchiolar epithelium in an evenly spaced distribution is helpful to confirming the reactive changes are in the nonneoplastic lung parenchyma (Figure 7A-F).
Figure 7:
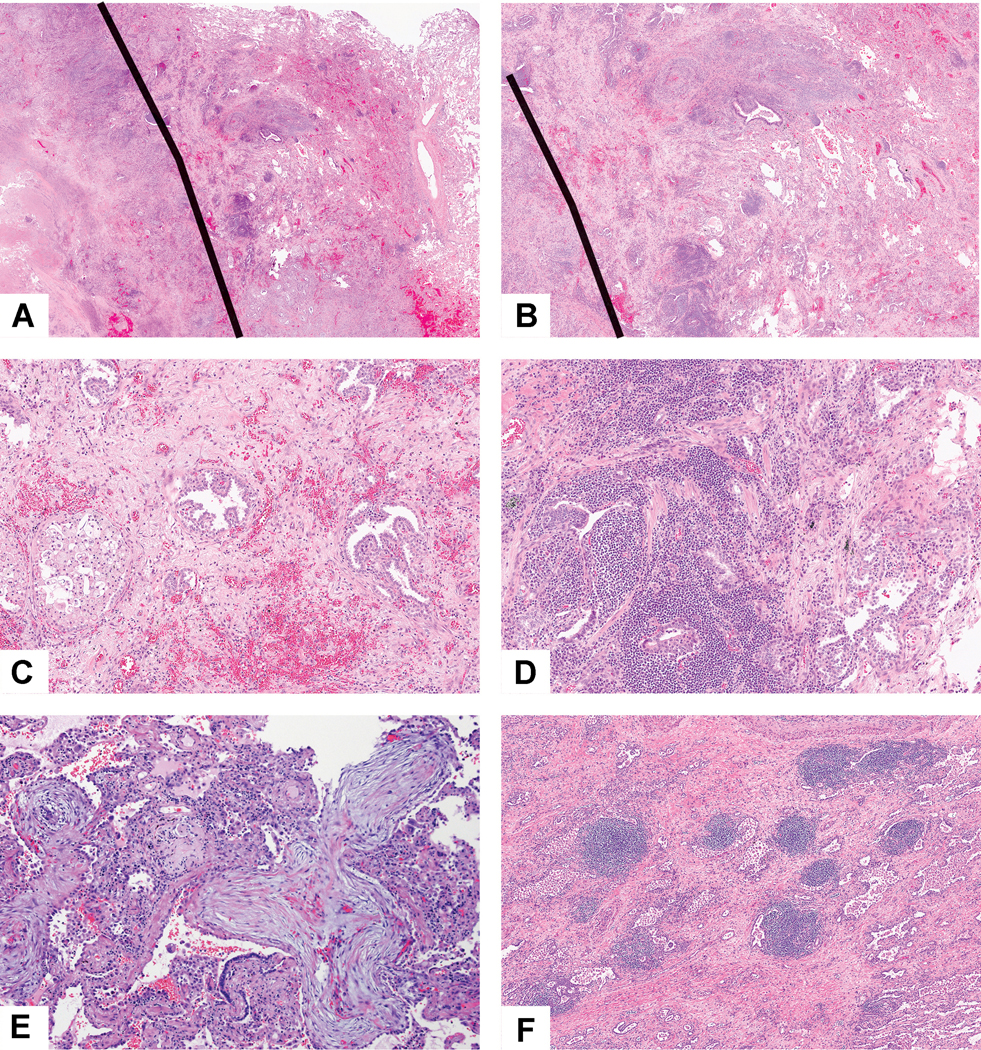
Border of tumor with adjacent non-neoplastic lung: A) This tumor shows a thick rim of reactive change beyond the edge of the tumor bed. The black line demarcates the edge of the tumor bed to the left and the nonneoplastic lung to the right. B) In the rim of reactive lung parenchyma between the tumor border and the surrounding normal lung tissue, there is marked interstitial inflammation, fibrosis and prominent reactive pneumocyte proliferation, however, the overall alveolar architecture is preserved. C) This area shows foamy macrophages within an air space consistent with post obstructive endogenous lipoid pneumonia and prominent hyperplastic pneumocytes. The nodular areas of hyperplastic pneumocytes are situated in alveolar spaces altered by the intervening interstitial fibrosis indicating these are reactive changes rather than direct tumor involvement. D) Focally the nonneoplastic lung shows a marked interstitial lymphoid infiltrate surrounding reactive and hyperplastic epithelial cells. E) Organizing pneumonia at the margin of the tumor bed and in the adjacent lung consists of polypoid plugs of loose connective tissue within distal air spaces. F) In this post-obstructive pneumonia setting there are prominent lymphoid aggregates within the nonneoplastic alveolar parenchyma adjacent to the tumor.
Recording Histologic Features in Tumor Bed
Numerous histologic features have been reported in lung resection specimens from patients treated with neoadjuvant therapy. However, the histologic changes can be divided into: 1) necrosis, 2) stromal tissue, and 3) viable tumor (Figure 8).53,25–28, 30 As recommended by Pataer et al., these three features should be estimated based on review of the microscopic sections and should total 100% of the tumor bed.26 Stromal tissue includes the following components: fibrosis and inflammation (Figure 8). These two components are morphologically heterogenous and frequently intimately admixed together and most previous studies reported them together as a percentage of stromal tissue.25–28, 30 The inflammation can be graded as mild, moderate or marked, however, this is included as part of the stroma, as it is very difficult to separate and quantify inflammation versus stromal fibrosis. In the study by Qu et al., pathologic assessment of these three components were made in 5% increments as continuous variables, 53 however it seems practical to use 10% increments unless the amount is 5% or less where estimates in single digits can be recorded. There is no established grading system for the extent of inflammation in the neoadjuvant setting based on light microscopic review of hematoxylin and eosin stained slides, although several approaches have been proposed in studies without neoadjuvant therapy.75–77 Junker described marked swelling of tumor cells after neoadjuvant therapy more often in adenocarcinomas than in squamous cell carcinomas.30 In rare cases it may be difficult to differentiate single tumor cells or small clusters of tumor cells after neoadjuvant therapy from cells of a histiocytic reaction based on H&E alone. In such cases immunohistochemistry with broad spectrum cytokeratins and macrophage markers may be helpful, but immunohistochemistry is not recommended for routine cases. In addition, it can be difficult to separate viable tumor cells from necrotic tumor cells where there are ghosts of tumor cells with shrunken cytoplasm, fragmentation and apoptotic bodies (Figure 9A and B). However, only definitely well-preserved tumor cells should be regarded as positive for viable tumor (Figure 9C and D).
Figure 8:
Histologic components of the tumor bed. A) Schematic image showing how percentage compositions are assigned. The tumor bed is divided into viable tumor area, necrosis and stroma. Stroma includes inflammation and fibrosis. B) A representative hematoxylin-and-eosin stained slide image (left) and a corresponding color illustration of the distribution of the components (right). The blue, red and black areas represent viable tumor, necrosis and stroma, respectively. Figure 1 from Qu et al. with permission.53
Figure 9:
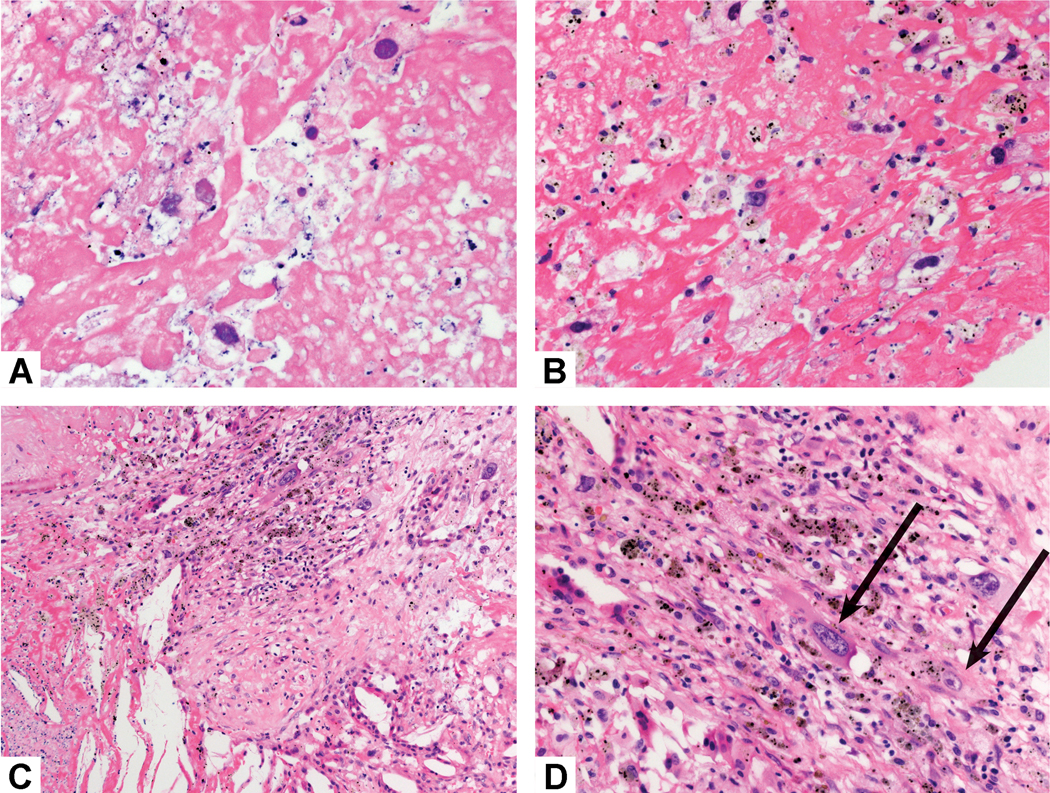
Necrotic versus Viable Tumor Cells. A and B) These necrotic tumor cells are not viable. Although there are nuclear ghosts, the tumor cell cytoplasm is not visible. C) Adjacent to this area of necrosis (bottom) there are rare viable tumor cells (top), highlighted in part D wher the tumor cell cytoplasm is more clearly evident.
The fibrosis can consist of dense hyalinized connective tissue (Figure 10A), fibroelastotic scarring (Figure 10B) and loose or myxoid connective tissue (Figure 10C). In addition, there can be prominent capillary vascularity in the stroma (Figure 10D). The inflammation can consist of chronic inflammation with lymphocytes, plasma cells, or lymphoid aggregates, (Figure 11A and 11B), neutrophils (Figure 11C) histiocytes (Figure 11D), or xanthogranulomatous reaction (Figure 11E and 11F). The latter consists of an exuberant accumulation of foamy macrophages and multinucleated giant cells of the foreign body type often associated with cholesterol clefts. Necrosis can consist of completely necrotic tissue (Figure 2B and 2C, 3B) or it can be filled with neutrophils (Figure 11C), other inflammatory cells or it can be full of cholesterol clefts (Figure 11E and 11F).
Figure 10:
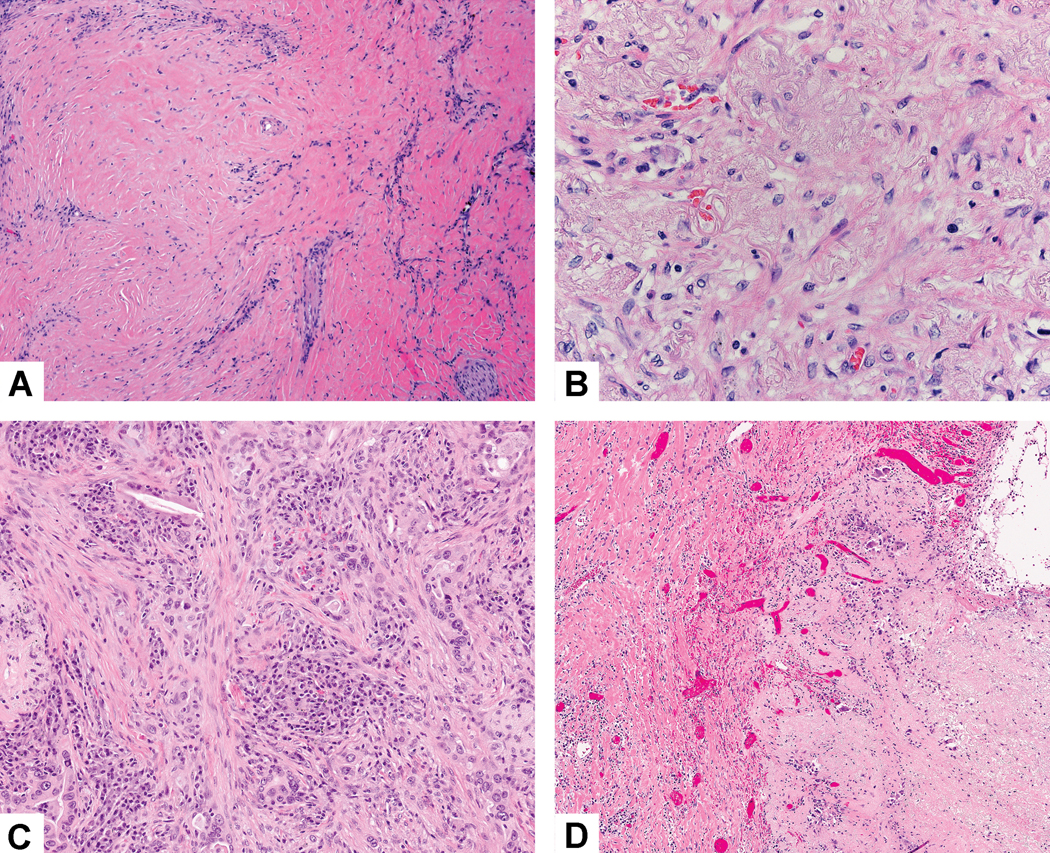
Stromal fibrosis. A) This tumor stroma consists of dense hyalinized fibrosis. B) The fibrosis in this area has prominent elastic fibers forming a fibroelastotic scar. C) The fibrosis in this area consists of loose spindle shaped myofibroblastic cells with little collagen. D) In this area of fibrotic scarring there are numerous small capillary sized blood vessels.
Figure 11:
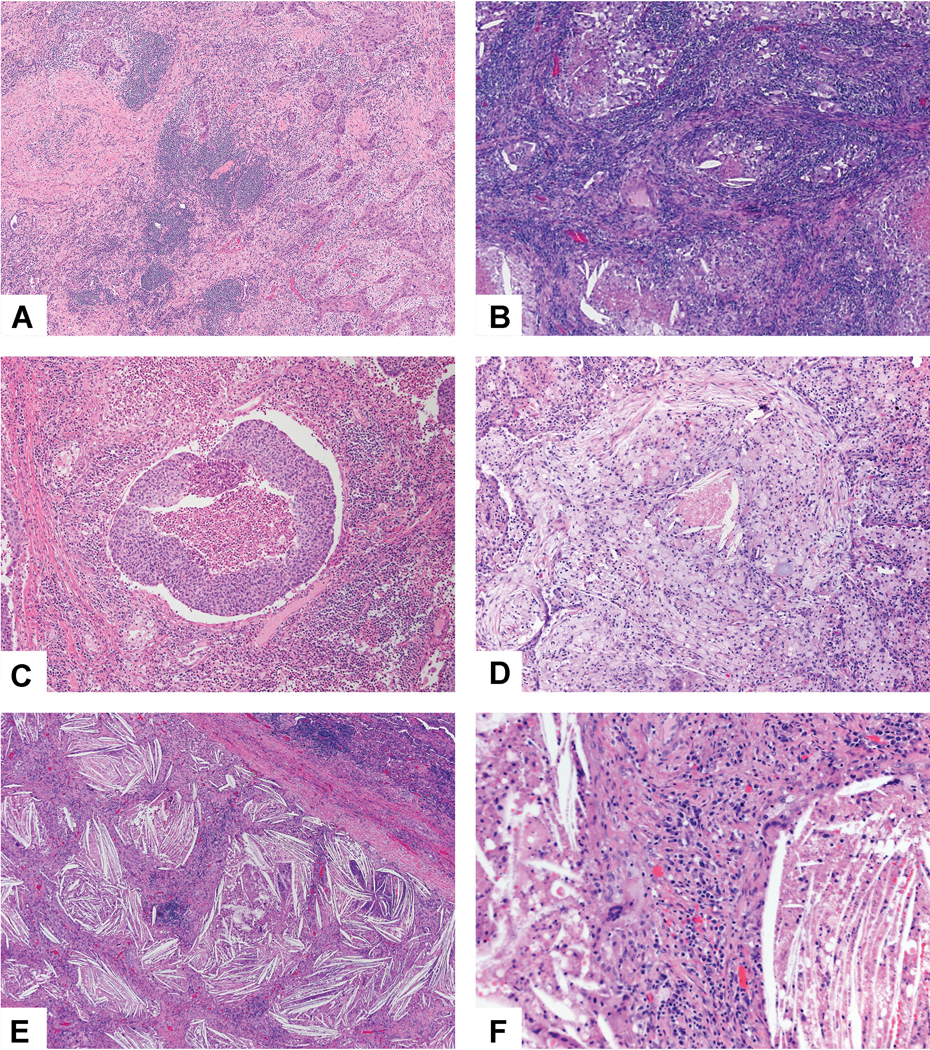
Stromal inflammation and necrosis. A) This squamous cell carcinoma has numerous lymphoid aggregates in the stroma. B) This adenocarcinoma shows a dense lymphoplasmacytic stromal infiltrate. C) Numerous neutrophils are seen not only within the focus of tumor necrosis in the center of the image, but also within the surrounding stroma. D) The tumor stroma is infiltrated by numerous histocytes which show a small area of necrosis in the center. E) Prominent cholesterol clefts are seen in this area of the tumor bed. F) The cholesterol clefts are surrounded by bands of stroma with prominent chronic inflammation and giant cells, some of which are associated with the cholesterol clefts.
Figure 2:
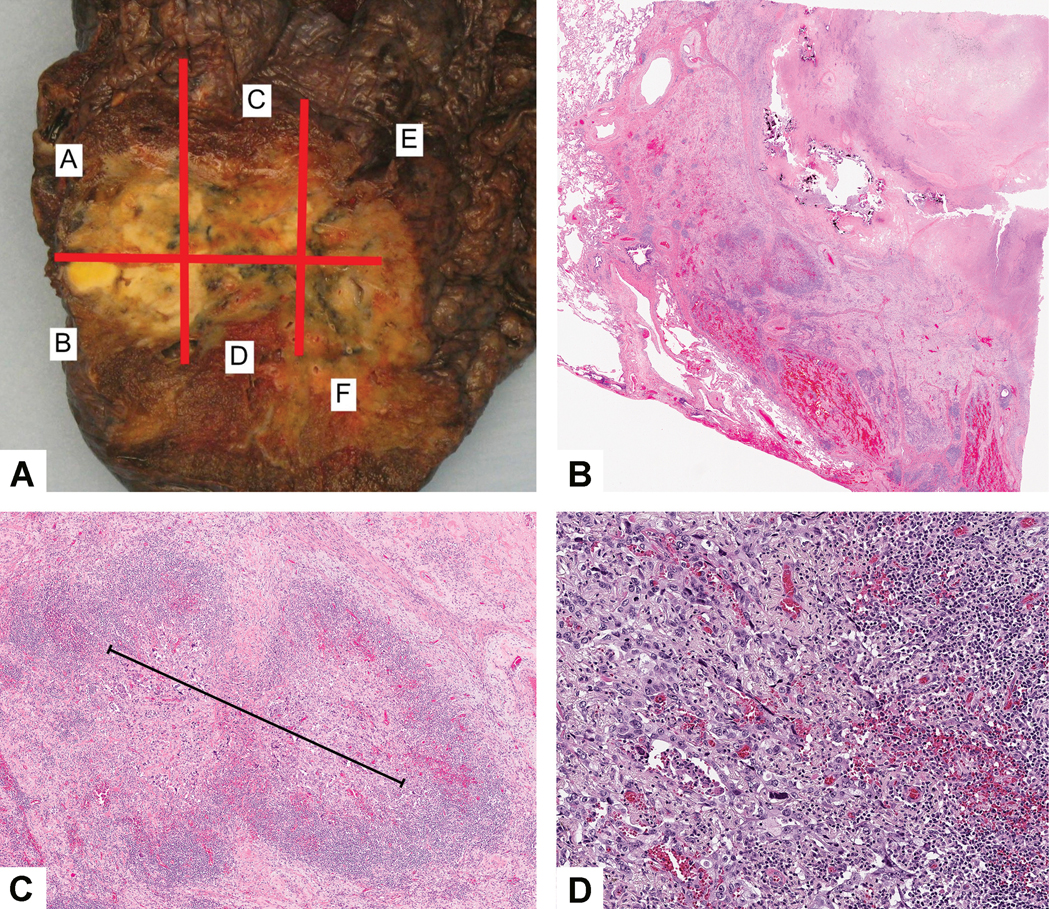
Major pathologic response: A) This tumor shows a variegated cut surface with yellow and white necrotic areas. B) Low power shows a large area of necrosis surrounded by dense fibrosis and chronic inflammation. C) Only a single 2 mm focus of viable adenocarcinoma was seen. Black line indicates the tumor size measurement. D) This focus of viable tumor cells surrounded by stroma with marked chronic inflammation consists of solid adenocarcinoma that was TTF-1 positive.
Figure 3:
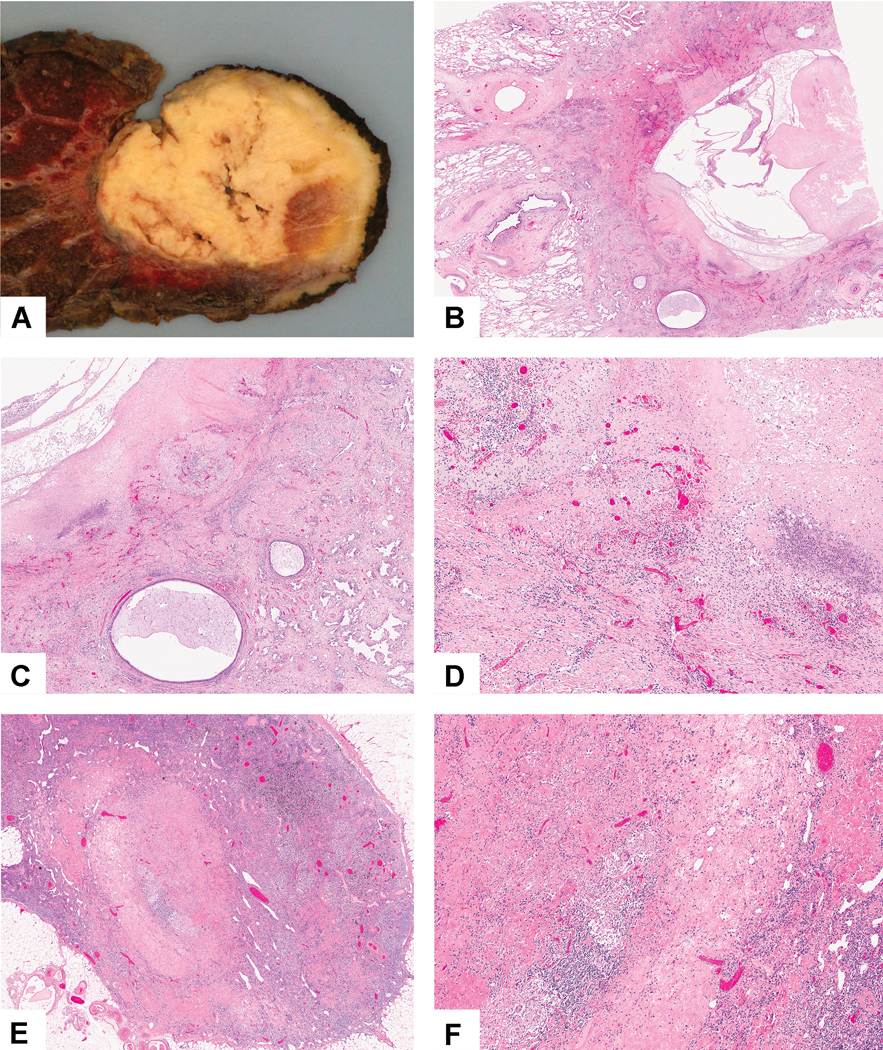
Complete pathologic response: A) This tumor is almost entirely necrotic with yellow soft areas and small firm tan areas. B) Low power shows a large necrotic cavitated area surrounded by dense fibrosis. C) The stroma shows marked chronic inflammation, foamy histiocytes and fibrosis with some adjacent alveolar parenchyma showing reactive pneumocytes (bottom) D) High power shows numerous chronic inflammatory cells, foamy histiocytes and dense fibrosis. No viable tumor was seen. E) In addition, no tumor was seen in two lymph nodes with changes consistent with treatment effect. This lymph node shows nodular scar and granulomatous inflammation. F) The tumor bed shows dense fibrosis with focal chronic inflammation and giant cells.
For colloid adenocarcinomas, where tumor cells are only focal, the mucin pools should be included in the percentage of viable tumor. However, if there are only areas of extracellular mucin without any apparent viable tumor cells within the mucin, we suggest regarding this as stroma. Further study is needed to address this point, however this approach has been used in esophageal adenocarcinoma with mucin pools following neoadjuvant therapy. 78
Literature is rather scant on what methodology should be used in estimating the percentages of each histologic component in tumor bed. For example, it has been proposed to estimate the percentage of viable tumor on each slide and then to determine a total percentage of viable tumor by averaging the results for all slides.26, 28 The problem with this approach is that it does not adjust for the fact that each slide typically has a different amount of tumor on it (Figure 12A and 12B), so this method is not completely accurate. So, some adjustment for amount of tumor on the various slides is needed. Although not expressly stated, in most studies the estimation of treatment effect has been done with an informal semiquantitative or “eyeball” approach.25–27, 29, 53 As pointed out by Raymond et al., the semiquantitative or eyeball approach to assessment of pathologic response is crude and subjective.2 However, this approach has been used in all prior studies that have shown the clinical relevance of major pathologic response in lung cancer.25–27, 29 Opportunities for more sophisticated approaches using digital imaging or artificial intelligence are addressed below. However, for pathology specimens to be signed out in an expeditious fashion, until digital and/or computational approaches are routinely available, this approach is practical and can be done by pathologists in most if not all laboratories.
Figure 12:
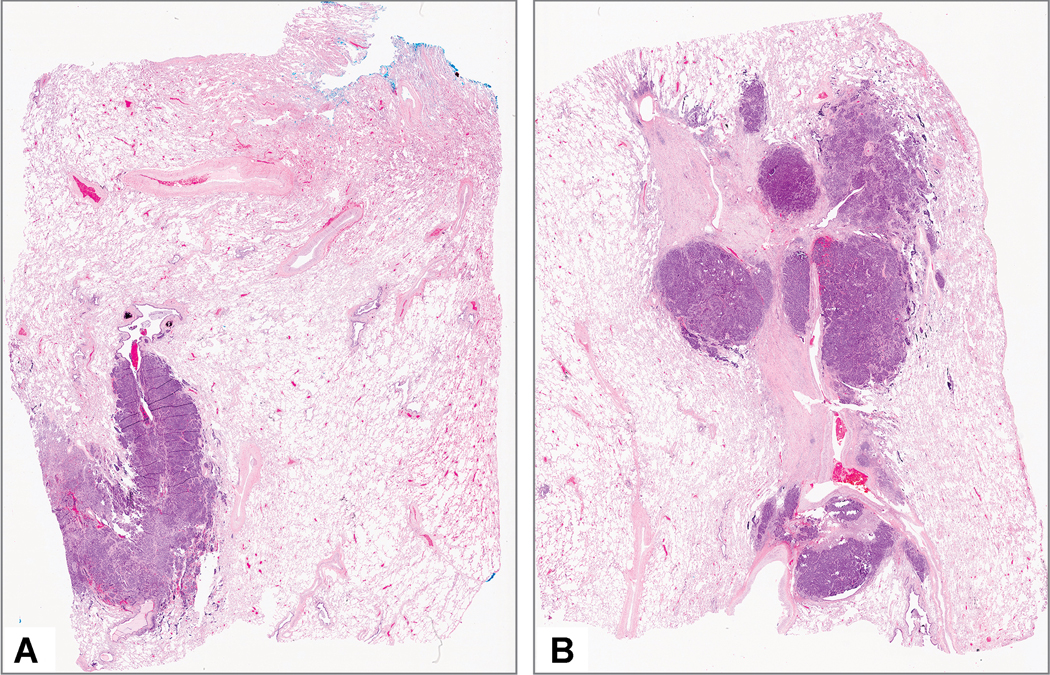
Different amount of tumor on separate slides. These two slides A and B show different amounts of tumor with at least twice as much tumor on the slide in part B compared to part A.
Although historically MPR in NSCLC has been analyzed lumping adenocarcinoma and squamous cell carcinoma together, recent data suggests that the optimal cutoff for predicting survival may be different according to histologic type with squamous cell carcinoma showing a 10% threshold, but adenocarcinoma a much higher optimal cutoff of 65%.53 This needs further validation. If research studies going forward only use 10% increments, it will be difficult to validate the 65% cutoff.
While there is limited data on reproducibility of assessment of MPR, it is remarkable that all previous studies lumping all NSCLC histologic types have consistently demonstrated that ≤ 10% viable tumor is the optimal prognostically significant cut off for MPR. Two studies suggest there is excellent reproducibility in assessment of pathologic response. In the study by Qu et al. using intraclass coefficients (ICC), there was a high degree of interobserver reproducibility between two pathologist’s assessments for both adenocarcinoma (ICC 0.97; 95% CI: 0.93–0.99) and squamous cell carcinoma estimating percent viable tumor in 5% increments (ICC=0.99; 95% CI: 0.96–1.00).53 Also, although no statistical methods were used, in the Blaauwgeers study where a 10% cut-off for viable tumor was used, it was stated that there were no discrepancies between the scores of two observers.51
The following recommendations provide definitions of major pathologic response and complete pathologic response.
Special Features according to specific types of therapy
It is expected that there may be differences in morphologic features and clinical relevance of histologic patterns of response according to the various specific types of neoadjuvant therapy including chemotherapy, radiotherapy, molecular targeted therapy, immunotherapy and various combinations of these approaches. Many detailed histologic features have been examined in various research studies,25, 26, 51, 53, 65 but at this time we have kept our recommendations as simple as possible to be useful in routine clinical practice as well as clinical trials. It is intended that a uniform approach will facilitate comparison of the impact of MPR and CPR between different types of neoadjuvant therapies. Recommendations requiring more detailed histologic analysis await further validation in independent studies. However, future research examining the clinical significance of various histologic details and proposed scoring systems is encouraged.
Chemotherapy and chemoradiotherapy
Until recently all previous pathologic studies only showed prognostic significance according to the percent viable tumor, mostly using either CPR or MPR with a cut-off of 10% or less viable tumor.25, 26, 30, 51 In none of these studies were prognostically significant associations identified with individual histologic parameters in any of these studies. However, in the study by Qu, et al., prognostic associations were identified for some of the individual histologic features and they differed according to histologic type.53
Qu et al. demonstrated a variety of the histologic factors beyond percentage of viable tumor were prognostically significant in univariate analysis and that they differed in adenocarcinoma compared to squamous cell carcinoma.53 However, for both squamous cell carcinoma and adenocarcinoma only percent viable tumor was significant in univariate analysis with different cut-offs according to histologic type as mentioned above.53
Fibroelastotic scars are frequent findings associated with lung cancers, particularly adenocarcinomas in the absence of neoadjuvant therapy. 79–81 They are also found in benign conditions such as subpleural fibroelastotic scars of apical caps and associated with infarcts.82, 83 However, in the setting of neoadjuvant therapy the presence of prominent elastic fibers has been noted consistently (Figure 9B).25, 26, 30, 51, 53, 84 This creates a dilemma in determining whether the fibroelastotic changes are native underlying fibrosis or therapy induced. Prominent elastic fiber rich fibrosis is the signature histologic finding of a rare form of interstitial lung disease called pleuroparenchymal fibroelastosis (PPFE) and it is reported following chemotherapy and transplantation in the setting of bone marrow, stem cell or lung transplantation.85–88 This raises the possibility that some of the fibroelastotic changes found in the neoadjuvant setting is actually therapy induced.
Vascular changes including inflammation of blood vessel walls or vasculitis, medial fibrotic thickening sometimes obliterating vascular lumens and recanalization can be seen. However, these may not be specific to neoadjuvant therapy.
Cytologic atypia of the tumor cells can occur with bizarre nuclei and in one study this finding was higher in the neoadjuvant therapy group compared to the surgery alone group.84 It is difficult to know if tumors undergoing neoadjuvant therapy might inherently have more pleomorphic cells than those only requiring surgery.
Data from studies addressing the pathologic changes associated with neoadjuvant chemotherapy alone26, 53 versus combination chemotherapy and radiation therapy25, 30, 51, 54 suggest the histologic changes are similar, although there are no detailed histologic comparisons with statistical analyses.
Immunotherapy
Several dozen clinical trials are currently examining the effects of immunotherapy in the neoadjuvant setting for patients with NSCLC (clinicaltrials.gov). Despite this proliferation of studies, the published literature on the pathologic features of surgically-resected, immunotherapy-treated tumors remains relatively sparse. Once ongoing trials reach maturity, we will likely have a substantial body of data to draw upon.
Some pathologic response patterns have been described based on early data from a trial of anti-PD-1 monotherapy in the neoadjuvant setting. Twenty-two surgically-resectable NSCLC patients received two doses of nivolumab followed by surgical resection approximately 4 weeks after the first dose. Following pathologic review of the resected tumor bed, the authors described a 45% rate of MPR (10% or less residual viable tumor) including three patients with CPR.36 Remarkably, chest CT performed within the week prior to surgery showed partial response in only 2 (10%) of patients, a substantial discrepancy with the pathologic findings. Cottrell and colleagues systematically evaluated the pathologic findings of the resected tumors and identified co-localization of the following features adjacent to tumor in responders: proliferative fibrosis, neovascularization, cholesterol clefts, high numbers of tumor infiltrating lymphocytes, and tertiary lymphoid structures.65 While some of these individual features are not specific to immunotherapy, the authors suggest that the overall pathologic immunoarchitecture of responsive tumors appears to reflect a state of immune activation. This observation may explain the discrepancy between radiologic and pathologic response: namely, a unique feature of responders following anti-PD-1 was the combination of these features to form a “regression bed” replacing the tumor without necessarily leading to a decrease in the volume of the tumor bed. Based on these findings, the authors propose immune-related pathologic response criteria (irPRC), where percent response is calculated as residual volume of tumor (RVT) / tumor bed where the tumor bed includes RVT+regression bed+necrosis.65 These criteria have also been used in a proposed Pan-Tumor pathologic scoring system.89 However, these need validation in other patient cohorts.
According to our proposal, we include in stroma what has been called the regression bed in the immunotherapy setting,65 but we also include stroma that does not meet the proposed criteria for regression bed. At present, there is limited data to suggest that pathologists can consistently distinguish regression versus native tumor stroma, although we acknowledge there are suggestive histologic features. Furthermore, we do not think this distinction can be made in every case and may be confounded by other factors, such as combination therapy with cytotoxic agents.
Additionally, the suggestion as defined in the Cottrell paper (Table S1), to include intratumoral stroma as residual viable tumor in cases without features of “regression”,65 differs from what is proposed in the current paper. According to our proposal we only count viable tumor cells as residual viable tumor and intratumoral stroma is counted as stroma rather than viable tumor. While early data indicates that this approach is associated with good inter-pathologist agreement around percent response,65, 89 we feel there is too little published data to support the use of this approach in routine practice and encourage studies examining reproducibility and predictive strength of these and other response scoring systems.
Other studies are currently examining the role of neoadjuvant PD-1/PD-L1 inhibitors either as single agents48, 90 or in combination with CTLA-4 inhibition48 or platinum-based chemotherapy.91 In the Lung Cancer Mutation Consortium 3 (LCMC3) study, two doses of neoadjuvant atezolizumab produced a 19% rate of MPR in the resected population, including four CPRs.90 In the NEOSTAR trial, Cascone et al. observed a 17% rate of MPR following three doses of nivolumab and a 33% MPR rate following the combination nivolumab plus ipilimumab in the intention-to treat population of patients (including both resected and not resected patients).48 Six patients in the combination arm had CPR, while only two patients had no residual viable tumor following nivolumab monotherapy.48 Both the LCMC3 and NEOSTAR studies reported a positive association between tumor shrinkage at imaging and MPR at the time of surgery.48, 90 These observations suggest that several factors, including the type of neoadjuvant immunotherapies, the number of doses administered and the time from last systemic treatment to surgery, may influence the degree of pathologic tumor regression at surgery and its relationship with radiographic change in tumor size following neoadjuvant immunotherapy. Preliminary reports suggest that neoadjuvant chemotherapy and immunotherapy may produce histopathological changes consistent with those of upfront surgically resected tumors, but with lower amounts of viable tumor and higher fibrosis.92 These observations require validation in larger cohorts.
Molecular targeted therapy
Few Phase 2 neoadjuvant targeted therapy trials in NSCLC patients have been conducted, mainly using EGFR (6 studies) and ALK (1 study) tyrosine kinase inhibitors (TKIs).52, 93–100 Four studies were conducted mainly in clinical early stage patients, while four others were in stage III (N2) patients. Since several studies were performed prior to EGFR mutation testing became a routine clinical test, they included patients who were treated with targeted therapy but did not harbor the corresponding driver oncogene. In addition, few case reports have also been reported.101–109 Among the cohort studies, only one study included detailed assessment of the histopathological parameters and molecular markers, and their correlation with radiological responses. Several studies have noted in the tumors of patients who demonstrated clinical response, the presence of extensive areas of fibrosis or necrosis with only focal residual tumor cells.52, 94, 97, 103 Fibrotic areas are characterized by their low cellularity and low tumor proliferative (Ki-67) index. Interestingly, surviving tumor areas often demonstrate prominent chronic inflammatory cell infiltrates,52, 94 however, this needs further validation. While currently there are no histopathological features that have been definitively associated with therapeutic response and survival outcome, future neoadjuvant trials of targeted therapies should include careful documentation of tumor histological features, including adenocarcinoma pattern, percent areas of fibrosis, necrosis, tumor cellularity and grade, and degree of inflammatory cell infiltrate.
ASSESSMENT OF METASTASES TO LYMPH NODES OR OTHER SITES
In patients who demonstrate significant response to neoadjuvant therapy, metastatic tumor cells in the lymph nodes may be mostly eliminated leading to tumor down-staging. Therefore, complete pathological examination of the lymph nodes in post-neoadjuvant lobectomy or pneumonectomy specimens is critical. Junker et al. proposed that lymph nodes from lung cancer patients be assessed in the same way as primary tumors.54 However, there has been little further attention to the clinical significance of pathologic assessment of treatment effect in lymph node metastases in the neoadjuvant setting in the lung cancer literature since this was recommended. For this reason, we have no data to indicate whether this is clinically important.
To investigate this possibility, we suggest that a systematic approach to evaluation of metastatic sites be utilized in clinical trials. In most cases, the lymph nodes are small enough to completely sample, but if there is a very large metastasis or tumor bed (>2cm) the lymph node can be bisected and the central slice through the tumor can be submitted in designated cassettes. This should also be done during grossing of intraoperative frozen sections of lymph nodes. Depending on individual laboratory resources, more extensive or even complete sampling can also be done. Then the same approach can be used for histologic evaluation that is used for the resected lung cancer reporting percent viable tumor, necrosis and stroma. A synoptic template for recording lymph node assessment is proposed in Table 2. In some cases, either the entire or the majority of viable tumor may be seen in the lymph nodes and the primary lung tumor may show minimal or no viable tumor. Complete pathologic response in a lymph node can be recognized if there is a well-defined scar and/or area of tumor necrosis in the absence of identifiable viable tumor cells. In lymph node metastases it can be difficult to assess tumor stromal inflammation due to the background lymphocytes (Figure 13A). In addition, when there is metastatic mucinous adenocarcinoma with only mucin but no viable tumor cells, the lymph node can be regarded to have no metastatic tumor or ypN0 (Figure 13B). 78 It is important to distinguish burnt out granulomas and silicoanthracotic changes from a histiocytic reaction to lymph node metastases (Figure 13C and 13D). Identification of prominent carbon pigment and polarizable silica-like particles can help to make this distinction. A recent report of clinically suspected nodal immune flare in patients receiving nivolumab described only noncaseating granulomas rather than metastatic tumor on pathologic exam.47
Figure 13:
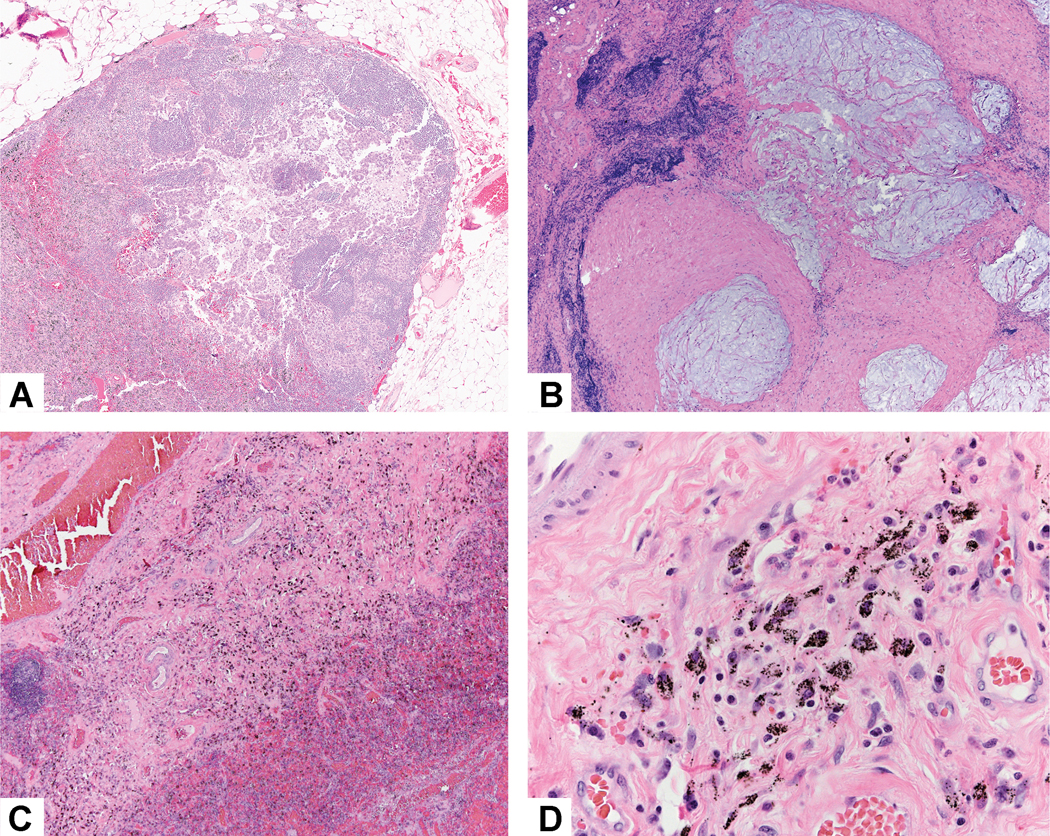
Lymph node assessment. A) This lymph node contains a focus of metastatic adenocarcinoma. It is difficult to be certain what is inflammation in the stroma because of the background lymphocytes in the lymph node. However there appears to be approximately 40–50% tumor necrosis and no stromal fibrosis is seen. B) In this lymph node metastatic mucinous adenocarcinoma had only pools of mucin but no viable tumor cells. Therefore, this was regarded as no viable tumor and the lymph nodes for this case were classified as ypN0. C) Changes related to metastatic tumor related treatment effect need to be distinguished from silicoanthracotic changes as seen in this lymph node where there are numerous histiocytes. D) On close inspection the histiocytes are filled with anthracotic pigment and on polarization microscopy silica-like particles are frequently seen.
In assessing lymph node metastases, the same approach can be used that is recommended in the primary lung tumor by estimating the percent 1) viable tumor, 2) necrosis, and 3) stroma so these add up to 100%. For simplicity of reporting pathologic response in the setting of multiple metastases in multiple lymph node stations, pathologists may report this information for each separately submitted specimen at a minimum for the size of the largest lymph node metastasis. In research settings, attempts to quantitate the extent of therapeutic changes in all lymph nodes can be investigated. Although clinical nodal responses have been documented previously,110 the clinical significance of detailed histologic features associated with response to neoadjuvant therapy in metastatic lung cancer involving lymph nodes has not been determined and needs further study. The total number of positive lymph nodes would be reported in the same way as in the non-neoadjuvant setting but staged with the ypN designation.
The clinical significance of assessment of pathologic response in resected metastases from other sites such as the brain, liver or adrenal gland is not known. However, it is suggested that a comprehensive approach similar to that recommended for the primary tumors may be useful.
Clinical over staging may occur when enlarged lymph nodes are detected by CT and may be PET positive while pathologic assessment can have features of treatment response but no viable tumor. In benign lymph nodes, it can be difficult to be certain whether the presence of fibrosis and necrosis actually represent treatment response by a previous metastasis or unrelated benign changes caused by necrotizing granulomas or silicoanthracotic changes. In necrotic tumor there are usually discrete irregular foci of necrosis, fibrosis and/or inflammation, sometimes with chronic hemorrhage and foamy macrophages.
The response to neoadjuvant treatment may vary between the primary tumor and metastases to lymph nodes or other sites. It is not known how to define MPR for the primary tumor when there is no or little viable tumor in the lung primary but substantial viable metastatic tumor in lymph nodes. Similarly, it is not clear how to regard cases where the primary tumor did not respond well to induction therapy, but mediastinal lymph nodes showed complete pathologic response. More investigation is needed on such cases.
STAGING ISSUES
Tumor size measurement can be challenging in resected lung cancers in the neoadjuvant setting. If there is a discrete measurable mass it can be measured with a ruler. In some cases, the tumor is not a discrete measurable mass on gross or microscopic exam as the treatment response is heterogeneous leaving multiple separate islands of viable tumor surrounded by necrosis and stroma with inflammation and fibrosis. In such cases one can estimate the tumor size by multiplying the percentage of viable tumor times the maximum dimension of tumor bed as proposed previously.111 Although this provides a practical approach to tumor size estimation this has not been validated in other studies. Moreover T-factor size has not been shown to be an independent prognostic factor in the neoadjuvant setting as it is in patients undergoing surgical resection without neoadjuvant therapy.
Even though histologic changes indicate that the tumor bed with fibrosis or necrosis extends into adjacent structures such as the chest wall suggesting there was previous PL3 or T3 preoperative stage, if there is no viable tumor in the chest wall the ypT factor should be determined only by the extent of viable tumor spread documented in the resected specimen.
If there is more than one tumor within a lobe, the pathological response should be reported for each tumor unless the quantity is too numerous to count. There is no data to suggest how to estimate overall pathologic response combining the results of multiple tumors in such cases.
In tumors that have a component of lepidic growth, tumor size estimation should use the principles introduced in the 8th Edition TNM classification that record both total size and invasive size, but only use invasive size for T-factor determination. 111 Thus in the neoadjuvant setting, viable tumor size estimation for such cases may need two adjustments: one for invasive size excluding the lepidic component and a second for the percent viable tumor as outlined above.
In the clinical setting where there is no residual viable tumor in the lung primary, but lymph nodes are positive for tumor, the staging should be ypT0 ypN1–3, depending on which lymph nodes are involved. Such tumors can qualify for MPR, although there are no good data to address this issue. Staging would be classified as Stage 0 in the absence of viable tumor in the lung primary, if the lymph node is ypN0, Stage IIB if the lymph nodes are ypN1 and Stage IIIA if the lymph nodes are ypN2.
Lesions of AIS or squamous cell carcinoma in situ that are separate from the main tumor for which neoadjuvant therapy was administered should be staged separately.
CLINICAL TRIALS
The development of innovative neoadjuvant strategies for resectable NSCLC has been hampered by a lack of surrogate endpoints. Surrogate endpoints can be measured much faster than the hard endpoints they predict (e.g., DFS and OS)112 and thus have the potential to spare clinical and financial resources associated with drug development.7, 8, 28
MPR has been proposed as a surrogate endpoint in neoadjuvant trials for resectable NSCLC.28 In a retrospective study of 192 patients treated with neoadjuvant chemotherapy and 166 patients treated with surgery upfront, the authors demonstrated a 19% MPR rate to neoadjuvant chemotherapy and improved survival in patients who achieved MPR at surgery compared to those who did not.26 These findings have since been reproduced in other retrospective29, 113 and prospective studies of neoadjuvant chemotherapy32 and chemotherapy plus antiangiogenic agents.27
Several studies have reported initial MPR rates to neoadjuvant PD-1/PD-L1 inhibitors either as monotherapy or in combination with CTLA-4 blockade or platinum-based chemotherapy. Two doses of neoadjuvant nivolumab induced a 45 % MPR rate in 20 resected NSCLC patients with no major delays in surgery.36 In the LCMC3 study two cycles of neoadjuvant atezolizumab induced a 19% MPR rate in patients with resected NSCLC and 4% of evaluable patients had a CPR.90
Results of the first phase 2 randomized study testing neoadjuvant nivolumab and nivolumab plus ipilimumab (NEOSTAR) in 44 resectable NSCLC patients were recently reported.48 In the intention to treat population nivolumab monotherapy produced a 17% MPR rate, including two patients with CPR, and the combination a 33% rate of MPR in the combination group, including six patients with CPR. Neoadjuvant chemotherapy has been shown to enhance PD-L1 expression and promotes immune infiltration of tumors 55 generating enthusiasm for testing neoadjuvant PD-1/PD-L1 inhibitors in combination with platinum-based chemotherapy in early trials. Atezolizumab combined with chemotherapy has induced MPR rates of 50% including three patients with CPR (21%)114 and in the NADIM study the combination of nivolumab plus chemotherapy resulted in a MPR rate of 83% and a CPR rate of 59% in the resected population of patients.91
The initial studies of neoadjuvant immunotherapy and immunotherapy plus chemotherapy are promising but indicate that some intertrial variability in MPR is present. Whether this variability is due to differing sample sizes, tumor burden, tumor histologies, timing and types of neoadjuvant therapies remains unknown.53, 113 Intertrial variability may also be affected by reporting rates of MPR and CPR in all resected patients as compared to all treated patients across different trials. It also remains unclear whether adopting a standardized approach for MPR evaluation would alleviate some of the intertrial variability. It has been suggested that using histopathologic features of immune-mediated regression may be beneficial for evaluating characteristics of pathologic tumor regression following neoadjuvant immune checkpoint inhibitors.65 Other preliminary reports indicate that neoadjuvant chemotherapy and immunotherapy may produce similar histopathological changes compared to untreated and resected tumors albeit with lower proportions of viable tumor and higher fibrosis,92 illustrating the importance of standardizing the methods of MPR assessment in larger cohorts.
Experience with targeted therapies for resectable NSCLC patients is limited, but initial testing suggests that the approach is safe and capable of producing MPRs.115 The LCMC has proposed an umbrella trial for resectable NSCLC that will identify oncogenic drivers at diagnosis and then target them with the corresponding therapy prior to tumor resection.19 Analysis of tumor specimens before and after surgical resection may identify molecular, genetic and protein pathway changes that may lead to rational combination therapy in the future.
Adopting a rigorous protocol for tissue sample harvesting and processing will be critical in that resected specimens are usually parceled out for additional analyses. The unique requirements and scope of the neoadjuvant trials necessitate dedicated personnel and services in the medical oncology, surgical, and pathology teams. Therefore, it is critical to provide funding support to pathology and surgical departments in institutions participating in neoadjuvant clinical trials to coordinate appropriate tissue sampling and processing. Also, clinical trials need to be performed in such a way so that MPR is assessed in a uniform manner, realizing that it may differ according to tumor heterogeneity, tumor histology and type and duration of neoadjuvant therapy administered.7, 8, 28, 53
FUTURE DIRECTIONS
Much future work is needed involving the pathology of lung cancers resected in a neoadjuvant setting. These recommendations are intended to be the basis for future work so they can be used for clinical care and clinical trials to pursue research in order to validate and/or modify these proposals.
There is a need for further pathology reproducibility studies in assessing major pathologic response in the neoadjuvant setting. These studies are currently being planned by the IASLC and we encourage other groups to perform these as well.
Despite standardization efforts, estimating the percentage of necrosis and stromal reaction after neoadjuvant therapy remains a time-consuming process prone to observer bias and inaccuracy. In osteosarcoma, there are promising data showing that computer aided approaches using image segmentation and self-learning algorithms by neural networks might be efficient and accurate for differentiating necrotic tissue from viable tumor on scanned whole slide images.116, 117. Similar studies on lung cancer have not yet been done. However, as digital pathology is slowly entering diagnostic practice, computational pathology might become a useful tool to measure pathological response across different tumor types in the future.
Further work is needed to evaluate the clinical significance of this IASLC proposal in comparison with any other proposed scoring systems.
Studies to explore the role of immunohistochemistry and molecular studies on resected tumors in the neoadjuvant setting are encouraged.
Optimized banking protocols and/or incorporation of special techniques such as flow cytometry, single cell sequencing and transcriptional profiling will be essential to fully understand the features of response and non-response. Careful specimen handling, adhering to the guidelines set forth for molecular testing, should be employed as part of routine clinical processing.118
As the multiple ongoing and planned clinical studies of neoadjuvant therapies in lung cancer enroll, it is imperative that the processing, analysis, and data collections are done systemically across trials and with commitment to centralization of data for large scale review. These data are essential to compare to clinical outcomes for future assessment of surrogacy. The recommendations made in this paper need to be tested in these clinical trials.
Inclusion of pathologists in grant review and as co-investigators on clinical trials is necessary for accurate evaluation, similar to central review of CT images in some clinical trials.
Evaluate opportunities for radiologic/pathologic correlation and scoring for tumor response.
Develop an IASLC international database of trials collecting pathologic response, DFS, and OS to prove whether or not pathologic response can be used as a surrogate endpoint in the future with involvement and concurrence of regulatory agencies.
RECOMMENDATION 1.
The term “tumor bed” is the area where the original pretreatment tumor was considered to be located. It can be challenging to determine whether necrosis and stromal inflammation and/or fibrosis are due to regression secondary to neoadjuvant therapy, native tumor characteristics or a combination. For this reason we favor the term tumor bed. It is suggested to simply describe the major components of the tumor bed as 1) viable tumor, 2) necrosis or 3) stroma (which can include inflammation or fibrosis).
RECOMMENDATION 2:
It is essential that information be provided from the surgical team to the pathology laboratory on whether the patient received neoadjuvant therapy in order for this specimen processing protocol to be followed. If there is more than one tumor in the specimen, it is of critical importance to also provide this information. It is good clinical practice to correctly label the specimen with the lobe(s) resected and to clarify any issues that may be needed for pathologic staging such as the pericardium, diaphragm or chest wall.
RECOMMENDATION 3:
Lung cancer resection specimens following neoadjuvant therapy should be sampled to optimize comprehensive gross and histologic assessment of the lung tumor bed for pathologic response. The tumor should be cut in its greatest dimension to maximize the tumor bed cross section. In cases where identification and/or orientation of the tumor are difficult, review of the preoperative CT scan can be helpful. Tumors 3 cm or less in size should be completely sampled. For larger tumors greater than 3 cm the tumor should be cut across in serial sections 0.5 cm thick and after gross inspection the most representative cross section showing viable tumor should be sampled. At least one cross section of the entire tumor (0.5 cm thick) with a gross photograph and histologic mapping should be made. Histologic sections at the tumor periphery should include 1 cm of adjacent lung parenchyma. Pathologic response cannot be assessed in small biopsies; a resection specimen is required.
RECOMMENDATION 4:
To determine the border of the tumor bed the edge of the tumor needs to be distinguished from the surrounding non-neoplastic lung parenchyma. This can be facilitated by review of the gross specimen and the histologic slides from the periphery of the tumor bed.
RECOMMENDATION 5:
Determination of the pathologic response to therapy should be made after review of all H&E slides of tumor by estimating the percentages of 1) viable tumor, 2) necrosis and 3) stroma which includes both fibrosis and inflammation so each of these three components add up to 100%. Each component should be assessed in 10% increments unless the amount is below 5% when an estimate of single percentages should be recorded. While this is primarily done by review of histologic sections of the tumor bed, correlation with the gross findings, in some cases facilitated by a gross photograph, may be important in markedly necrotic and/or cavitated tumors where it is not possible to reflect this change in histologic sections.
Note: Although it may be useful to record the amount of each of these components on each individual histologic slide, it needs to be kept in mind that the amount of tumor bed varies on each slide so these percentages cannot be summed and averaged as if they were in equal amounts. This is a semiquantitative process. There is no validated quantitative method that is available that can be implemented in a timely fashion for clinical decisions.
A proposed synoptic template for reporting pathologic findings for resected lung cancers following neoadjuvant therapy is summarized in Table 2.
RECOMMENDATION 6.
Definition of Major Pathologic Response
Major Pathologic Response (MPR) is defined as the reduction of viable tumor to the amount beneath an established clinically significant cutoff based on prior evidence according to the individual histologic type of lung cancer and a specific therapy.
The historical definition of MPR for all histologic types of lung cancer is ≤ 10% of viable tumor with no viable tumor required for complete pathologic response (CPR). MPR is calculated as the estimated size of viable tumor divided by the size of the tumor bed. For the moment, this is the cutoff being used in multiple active clinical trials. However, recent data suggests the MPR in the conventional chemotherapy setting may differ according to histologic type: i.e. adenocarcinoma versus squamous cell carcinoma.
If after review of histologic sections, the percentage of viable tumor is near the cutoff for major pathologic response, additional histologic sections should be submitted. The pathology report should record the total number of blocks of tumor bed that were examined even if the blocks did not consist entirely of tumor bed but also included some uninvolved lung.
For colloid adenocarcinomas, where tumor cells are only focal, the mucin pools should be included in the percentage of viable tumor. However, if there are only areas of extracellular mucin without any apparent viable tumor cells within the mucin, we suggest regarding this as stroma. Further study is needed to address this point.
Major pathologic response can also be classified for the lung primary in the setting where the lung primary shows little or no viable tumor, but lymph nodes show viable metastatic carcinoma (ypT0, N1,2 or 3). However, the prognostic and therapeutic implications of this clinical setting are not known.
RECOMMENDATION 7:
Definition of Complete Pathologic Response
Complete pathologic response (CPR) is defined as lack of any viable tumor cells on review of H&E slides after complete evaluation of a resected lung cancer specimen including all sampled regional lymph nodes. Such tumors would be staged as ypT0N0 according to the 8th edition AJCC and UICC staging systems.
Note:
If no tumor is seen in the initial sections and tissue from the tumor bed remains, additional histologic sections should be made. The number of additional sections should be whatever seems reasonable in the individual setting depending on the size of the tumor bed and the capacity of the individual pathology laboratory. If the histologic changes in the initial sections obtained do not show findings that fit for the effects of therapy, the possibility that the wrong area was sampled should be considered. In such cases the gross specimen may need to be re-evaluated using radiologic pathologic correlation and if additional lesions are identified, these should be sampled. The pathology report should record the total number of blocks of tumor bed that were examined even if the entire block did not consist of tumor bed.
The identification of incidental lesions of squamous cell carcinoma in situ, atypical adenomatous hyperplasia, adenocarcinoma in situ or minimally invasive adenocarcinoma in the surrounding lung parenchyma that are clearly separate from the main tumor for which neoadjuvant therapy was administered does not disqualify a case for classification as MPR or CPR. This proposal is based on clinical judgement as currently no clinical data exists to make a specific recommendation.
In the setting of multiple tumors where a second invasive predominant lung carcinoma, is present that was regarded preoperatively to be an intrapulmonary metastasis however, it is determined to be a second synchronous primary after clinical, radiologic, pathologic and/or molecular assessment, it is questionable whether the terms MPR or CPR should be used if the main tumor otherwise meets the above criteria. No data exists currently to address this question.
RECOMMENDATION 8:
In the absence of more systemic data regarding evaluation of tumors following immunotherapy and molecular targeted therapy the same approach to pathologic assessment of resected lung cancers in the neoadjuvant setting in evaluating the percentage of viable tumor, necrosis and stroma should be used regardless of the type of neoadjuvant therapy administered whether it was radiation, chemotherapy, targeted therapy, immunotherapy, chemoradiation, chemoimmunotherapy, or chemotherapy-targeted therapy. There also may be different features that can be addressed depending on the type of therapy such as immune cell infiltrates in patients who received immunotherapy.
RECOMMENDATION 9:
In most cases, the lymph nodes are small enough to completely sample, but if there is a very large metastasis or tumor bed (>2cm) the lymph node can be bisected and the central slice through the tumor can be submitted in designated cassettes. This should also be done during grossing of intraoperative frozen sections of lymph nodes. Depending on individual laboratory resources, more extensive or even complete sampling can also be done. Then the same approach can be used for histologic evaluation that is used for the resected lung cancer reporting percent viable tumor, necrosis and stroma. Complete pathologic response in a lymph node can be recognized if there is a well-defined scar and/or area of tumor necrosis in the absence of identifiable viable tumor cells.
RECOMMENDATION #10:
The following recommendations are made for T factor staging of neoadjuvant lung cancer resection specimens.
Tumor size:
If the viable tumor forms a discrete mass where the size can be measured with a ruler either grossly or microscopically (where it can be measured on a single H&E slide) this is the preferred approach (Figure 2A-D). However, if the viable tumor cannot be measured with a ruler either due to grossly indistinct borders, multiple foci interspersed among necrosis and/or stroma or if it is present on multiple slides, the viable invasive tumor size should be estimated using the following formula.
Viable invasive tumor size (cm) = tumor bed size X percentage viable invasive tumor Estimating invasive size by adjusting for lepidic component
In tumors that have a component of lepidic growth, tumor size estimation should use the principles introduced in the 8th Edition TNM classification that record both total size and invasive size, but only use invasive size for T-factor determination. Thus, in the neoadjuvant setting, viable tumor size estimation for such cases may need two adjustments: one for invasive size excluding the lepidic component and a second for the percent viable tumor as outlined above. However, the clinical implications of this adjustment for the lepidic component are not known in the neoadjuvant setting.
T3 – multiple tumors considered to represent intrapulmonary metastases
If there is more than one tumor within a lobe, the pathological response or percent viable tumor should be reported for each tumor unless the number of intrapulmonary metastases are too numerous to count.
RECOMMENDATION 11:
Ongoing neoadjuvant studies with targeted therapies and immunotherapies in resectable NSCLC represent a unique source of information and the International Association for the Study of Lung Cancer strongly recommends and will promote the design and implementation of an international database to collect uniformly clinical and pathologic information with the ultimate goal of fostering collaboration and to facilitate the identification of surrogate end points of long-term survival.
Acknowledgments
DISCLOSURES:
Dr. Dacic reports personal fees from Bayer HealthCare Pharmaceuticals, Inc., personal fees from Takeda Pharmaceutical Company, outside the submitted work.
Dr. Wistuba reports grants and personal fees from Genentech/Roche, during the conduct of the study; grants and personal fees from Genentech/Roche, grants and personal fees from Bayer, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Astra Zeneca/Medimmune, grants and personal fees from Pfizer, grants and personal fees from HTG Molecular, grants and personal fees from Asuragen, grants and personal fees from Merck, personal fees from GlaxoSmithKline, grants and personal fees from Guardant Health, personal fees from MSD, personal fees from Oncoplex, personal fees from DepArray, personal fees from Adaptive, personal fees from Adaptimmune, personal fees from EMD Serono, personal fees from Takeda,, personal fees from Amgen, personal fees from Johnson & Johnson, personal fees from Iovance, personal fees from 4D, personal fees from Novartis, personal fees from Akoya, outside the submitted work.
Dr. Sholl reports grants from Genentech, personal fees from LOXO Oncology, personal fees from AstraZeneca, personal fees from Foghorn Therapeutics, outside the submitted work.
Dr. Bubendorf reports grants and personal fees from Roche, personal fees from Boehringer Ingelheim, personal fees from BMS, grants from AbbVie, from Astra Zeneca, grants and personal fees from MSD, personal fees from Bayer, outside the submitted work.
Dr. Bunn reports personal fees from AstraZeneca, personal fees from Merck, personal fees from Genentech, personal fees from Lilly, personal fees from BMS, personal fees from Takeda, outside the submitted work.
Dr. Cascone has received speaker’s fees from the Society for Immunotherapy of Cancer and Bristol-Myers Squibb, receives consultant fees from MedImmune and Bristol-Myers Squibb, and research funding to MD Anderson Cancer Center from Boehringer Ingelheim, MedImmune and Bristol-Myers Squibb
Dr. Chaft reports personal fees and other from AstraZeneca, personal fees and other from BMS, personal fees and other from Merck, personal fees and other from Genentech, outside the submitted work.
Dr. Cooper reports non-financial support from Roche, non-financial support from Pfizer, non-financial support from AstraZeneca, outside the submitted work.
Dr. Goo reports grants from Lunit Inc., grants from Infinitt Healthcare, outside the submitted work.
Dr. Heymach reports grants and other from Bristol Myers Squibb, grants and other from AstraZeneca, other from Merck, other from EMD Serono, other from Genentech, other from Takeda, grants and other from Spectrum, other from Sanofi US Services, outside the submitted work.
Dr. Horinouchi reports grants and personal fees from BMS, grants and personal fees from MSD, grants and personal fees from Chugai, grants and personal fees from Taiho, grants and personal fees from Astra Zeneca, grants from Astellas, grants from Merck Serono, grants from Genomic Health, grants and personal fees from Lilly, grants and personal fees from Ono, outside the submitted work.
Dr. Kris reports grants from Free to Breathe, during the conduct of the study; personal fees from AstraZeneca, personal fees from Pfizer, personal fees from Regeneron, outside the submitted work; and Mark G. Kris has received honoraria for participation in educational programs from WebMD, Physicians Education Resources, Prime Oncology, Intellisphere, Creative Educational Concepts,Health
Research Incorporated, Vindico, Peerview, i3 Health, Paradigm Medical Communications, AXIS, Carvive Systems, AstraZeneca, and Research to Practice. Funds for travel and lodging, and food and beverage have been provided by AstraZeneca, Pfizer, Regeneron, and Genentech.
Dr. Kris is an employee of Memorial Sloan Kettering. Memorial Sloan Kettering has received research funding from The National Cancer Institute (USA), The Lung Cancer Research Foundation, Genentech Roche, and PUMA Biotechnology for research conducted by Dr. Kris. Memorial Sloan Kettering has an institutional agreement with IBM for Watson For Oncology and receives royalties from IBM.
Dr. Lopez-Rios reports grants from Roche, grants from Thermo Fisher, during the conduct of the study; grants and personal fees from Abbvie, personal fees from Bayer, grants and personal fees from Roche, personal fees from Astra Zeneca, grants and personal fees from BMS, personal fees from MSD, grants and personal fees from Pfizer, grants and personal fees from Thermo Fisher, outside the submitted work.
Dr. Mitsudomi reports personal fees from AstraZeneca, grants and personal fees from MSD, grants and personal fees from Chugai, grants and personal fees from Bristol Myers Squibb, grants and personal fees from Ono pharmaceutical, during the conduct of the study; grants and personal fees from Boehringer Ingelheim, grants and personal fees from pfizer, grants and personal fees from Eli-Lilly, grants and personal fees from Taiho, grants and personal fees from Ethicon, grants and personal fees from Medtronic, outside the submitted work.
Dr. Motoi reports personal fees from Bristol-Myers Squibb, personal fees from MSD, grants and personal fees from Ono, personal fees from Chugai, personal fees from Astra Zeneca, personal fees and non-financial support from Novartis, personal fees from Agilent, personal fees from Cook Japan, personal fees from Miraca Life Sciences, personal fees from Roche Diagnostics, personal fees from Taiho, outside the submitted work.
Dr. NICHOLSON reports personal fees from MERCK, personal fees from BOEHRINGER INGELHEIM, grants and personal fees from PFIZER, personal fees from NOVARTIS, personal fees from ASTRA ZENECA, personal fees from BRISTOL MYER SQUIB, personal fees from ROCHE, personal fees from ASTRA ZENECA, personal fees from ABBVIE, personal fees from ONCOLOGICA, outside the submitted work.
Dr. Oliveira reports personal fees from AstraZeneca, outside the submitted work.
Dr. PAPOTTI reports personal fees from AstraZeneca, personal fees from Roche, personal fees from Pfizer, personal fees from MSD, personal fees from AbbVie, outside the submitted work.
Dr. Paz-Ares reports personal fees from Roche, personal fees from Lilly, personal fees from Novartis, personal fees from BMS, personal fees from MSD, personal fees from Amgen, personal fees from Boehringuer Ing., personal fees from AstraZeneca, personal fees from Sanofi, personal fees from Merck, personal fees from Pharmamar, outside the submitted work.
Dr. Provencio reports grants from BMS, grants from Roche, grants from MSD, grants from Takeda, grants from Astra Zeneca, outside the submitted work
Dr. Scagliotti reports personal fees from Eli Lilly, personal fees from Astrazeneca, personal fees from Roche, personal fees from Takeda, personal fees from MSD, other from Bayer, outside the submitted work.
Dr. Tsao reports grants and personal fees from Merck, grants and personal fees from AstraZeneca, personal fees from BMS, grants and personal fees from Pfizer, grants and personal fees from Bayer, personal fees from Hoffmann La Roche, personal fees from Takeda, outside the submitted work.
Dr. YATABE reports personal fees from Chugai-pharma, personal fees from MSD, personal fees from Novartis, personal fees from AstraZeneca, personal fees from Pfizer, personal fees from Roche Diagnostics, personal fees from Agilent Dako, personal fees from ThermoFisher, outside the submitted work.
Appendix of Reviewers
Frederick Askin, Chapel Hill, NC, USA
Sabina Berezowska, Bern, Switzerland (IASLC Pathology Panel member)
Mary Beth Beasley, New York, NY, USA (IASLC Pathology Panel member)
Elisabeth Brambilla
Kelly Butnor, Portland, ME, USA
Allen Burke, Baltimore, MD, USA
Lina Carvalho, Coimbra, Portugal
Nicolas Girard, Paris, France
Meera Hameed, New York, USA
Philippe Joubert, Quebec, Canada (IASLC Pathology Panel member)
Klaus Junker, Bremen, Germany
Apar Pataer, Houston, TX, USA
Anjali Saqi, New York, NY, USA
Frank Schneider, Atlanta, GA, USA
Janis Taube, Baltimore, MD, USA
Paul VanderLaan, Boston, MA USA
Annikka Weissferdt, Houston, TX, USA
Regulatory Agencies
Koshin Kiyohara: Director Office of New Drug, Pharmaceuticals and Medical Devices Agency, Japan
Gideon Blumenthal: Division of Oncology Products, Food and Drug Administration, Silver Spring Maryland
Ralf Herold MD PhD: European Medicines Agency, Amsterdam, The Netherlands.
Zhiming Yang: Office of Clinical Evaluation 1, Center for Drug Evaluation, National Medical Products Administration, Beijing; P.R. China
Footnotes
NOTHING TO DISCLOSE:
William D. Travis, MD
Prasad Adusumilli, MD
Carlos Gil Ferreira, MD
Ugo Pastorino, MD
Giuseppe Pelosi, MD
Erik Thunnissen, MD
Walter Weder, M.D
Gang Chen, MD
Teh-Ying Chou, MD
Jeremy J. Erasmus, MD Fred R. Hirsch M.D.
Deepali Jain, MD
Keith Kerr, MD
Young Tae Kim, MD Shun Lu, MD
Andre Moreira, MD
Claudia Poleri, MD
Anja C. Roden, MD
Stephen G. Swisher, MD
Johan Vansteenkiste, MD
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med 1977;101:14–18. [PubMed] [Google Scholar]
- 2.Raymond AK, Chawla SP, Carrasco CH, et al. Osteosarcoma chemotherapy effect: a prognostic factor. Seminars in diagnostic pathology 1987;4:212–236. [PubMed] [Google Scholar]
- 3.Chui MH, Kandel RA, Wong M, et al. Histopathologic Features of Prognostic Significance in High-Grade Osteosarcoma. Arch Pathol Lab Med 2016;140:1231–1242. [DOI] [PubMed] [Google Scholar]
- 4.Ali HR, Dariush A, Provenzano E, et al. Computational pathology of pre-treatment biopsies identifies lymphocyte density as a predictor of response to neoadjuvant chemotherapy in breast cancer. Breast cancer research : BCR 2016;18:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peintinger F, Sinn B, Hatzis C, et al. Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2015;28:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas JSJ, Provenzano E, Hiller L, et al. Central pathology review with two-stage quality assurance for pathological response after neoadjuvant chemotherapy in the ARTemis Trial. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2017;30:1069–1077. [DOI] [PubMed] [Google Scholar]
- 7.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25–32. [DOI] [PubMed] [Google Scholar]
- 8.Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016;17:791–800. [DOI] [PubMed] [Google Scholar]
- 9.Shia J, Guillem JG, Moore HG, et al. Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. The American journal of surgical pathology 2004;28:215–223. [DOI] [PubMed] [Google Scholar]
- 10.Gomez Dorronsoro ML, Vera R, Ortega L, et al. Recommendations of a group of experts for the pathological assessment of tumour regression of liver metastases of colorectal cancer and damage of non-tumour liver tissue after neoadjuvant therapy. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico 2014;16:234–242. [DOI] [PubMed] [Google Scholar]
- 11.Reggiani Bonetti L, Lionti S, Domati F, et al. Histological grading based on poorly differentiated clusters is predictive of tumour response and clinical outcome in rectal carcinoma treated with neoadjuvant chemoradiotherapy. Histopathology 2017;71:393–405. [DOI] [PubMed] [Google Scholar]
- 12.Ott K, Blank S, Becker K, et al. Factors predicting prognosis and recurrence in patients with esophago-gastric adenocarcinoma and histopathological response with less than 10 % residual tumor. Langenbeck’s archives of surgery 2013;398:239–249. [DOI] [PubMed] [Google Scholar]
- 13.Karamitopoulou E, Thies S, Zlobec I, et al. Assessment of tumor regression of esophageal adenocarcinomas after neoadjuvant chemotherapy: comparison of 2 commonly used scoring approaches. The American journal of surgical pathology 2014;38:1551–1556. [DOI] [PubMed] [Google Scholar]
- 14.Bossuyt V, Provenzano E, Symmans WF, et al. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann Oncol 2015;26:1280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchio C, Maletta F, Annaratone L, et al. The Perfect Pathology Report After Neoadjuvant Therapy. Journal of the National Cancer Institute Monographs 2015;2015:47–50. [DOI] [PubMed] [Google Scholar]
- 16.Bossuyt V, Symmans WF. Standardizing of Pathology in Patients Receiving Neoadjuvant Chemotherapy. Ann Surg Oncol 2016;23:3153–3161. [DOI] [PubMed] [Google Scholar]
- 17.Bossuyt V. Processing and Reporting of Breast Specimens in the Neoadjuvant Setting. Surgical pathology clinics 2018;11:213–230. [DOI] [PubMed] [Google Scholar]
- 18.Administration FaD. Guidance for Industry. Pathologic Complete Response in Neoadjuvant Treatment of High-Risk Early Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. In: Services USDoHaH, ed.Silver Spring, MD: FDA, Center for Drug Evaluation and Research; 2014;https://www.fda.gov/downloads/drugs/guidances/ucm305501.pdf:1-21. [Google Scholar]
- 19.Blumenthal GM, Bunn PA Jr., Chaft JE, et al. Current Status and Future Perspectives on Neoadjuvant Therapy in Lung Cancer. J Thorac Oncol 2018;13:1818–1831. [DOI] [PubMed] [Google Scholar]
- 20.Pignon J-P, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 2008;26:3552–3559. [DOI] [PubMed] [Google Scholar]
- 21.Group NM-aC. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakelee HA, Dahlberg SE, Keller SM, et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2017;18:1610–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines: Non-small cell lung cancer. National Comprehensive Cancer Network; [serial online].Version 4.2019:1–225. Available from National Comprehensive Cancer Network, Plymouth Meeting, PS. Accessed 02/04/2019. [Google Scholar]
- 24.Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [DOI] [PubMed] [Google Scholar]
- 25.Junker K, Langner K, Klinke F, et al. Grading of tumor regression in non-small cell lung cancer : morphology and prognosis. Chest 2001;120:1584–1591. [DOI] [PubMed] [Google Scholar]
- 26.Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaft JE, Rusch V, Ginsberg MS, et al. Phase II trial of neoadjuvant bevacizumab plus chemotherapy and adjuvant bevacizumab in patients with resectable nonsquamous non-small-cell lung cancers. J Thorac Oncol 2013;8:1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hellmann MD, Chaft JE, William WN, Jr., et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.William WN, Jr., Pataer A, Kalhor N, et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2013;8:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Junker K, Thomas M, Schulmann K, et al. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy. Histological assessment. J Cancer Res Clin Oncol 1997;123:469–477. [DOI] [PubMed] [Google Scholar]
- 31.Dooms C, Verbeken E, Stroobants S, et al. Prognostic stratification of stage IIIA-N2 non-small-cell lung cancer after induction chemotherapy: a model based on the combination of morphometric-pathologic response in mediastinal nodes and primary tumor response on serial 18-fluoro-2-deoxyglucose positron emission tomography. JClinOncol 2008;26:1128–1134. [DOI] [PubMed] [Google Scholar]
- 32.Cascone T, Gold KA, Swisher SG, et al. Induction Cisplatin Docetaxel Followed by Surgery and Erlotinib in Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105:418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butnor K, Beasley MB, Dacic S, et al. Protocol for the Examination of Specimens From Patients With Primary Non-Small Cell Carcinoma, Small Cell Carcinoma, or Carcinoid Tumor of the Lung. Accessed March 3, 2018, www.cap.org/cancerprotools) [DOI] [PubMed]
- 34.Nicholson AG, Kerr K, Gosney J. Dataset for histopathological reporting of lung cancer. In: Pathologists Royal College of Pathologists, ed.United Kingdom: 2018. [Google Scholar]
- 35.Owen D, Chaft JE. Immunotherapy in surgically resectable non-small cell lung cancer. Journal of thoracic disease 2018;10:S404–s411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. The New England Journal of Medicine 2018;378:1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canter RJ, Martinez SR, Tamurian RM, et al. Radiographic and histologic response to neoadjuvant radiotherapy in patients with soft tissue sarcoma. Ann Surg Oncol 2010;17:2578–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Namura M, Tsunoda H, Yagata H, et al. Discrepancies Between Pathological Tumor Responses and Estimations of Complete Response by Magnetic Resonance Imaging After Neoadjuvant Chemotherapy Differ by Breast Cancer Subtype. Clin Breast Cancer 2018;18:128–134. [DOI] [PubMed] [Google Scholar]
- 39.Kuerer HM, Vrancken Peeters M, Rea DW, et al. Nonoperative Management for Invasive Breast Cancer After Neoadjuvant Systemic Therapy: Conceptual Basis and Fundamental International Feasibility Clinical Trials. Ann Surg Oncol 2017;24:2855–2862. [DOI] [PubMed] [Google Scholar]
- 40.Lee HY, Lee HJ, Kim YT, et al. Value of combined interpretation of computed tomography response and positron emission tomography response for prediction of prognosis after neoadjuvant chemotherapy in non-small cell lung cancer. J Thorac Oncol 2010;5:497–503. [DOI] [PubMed] [Google Scholar]
- 41.Kozak MM, Murphy JD, Schipper ML, et al. Tumor volume as a potential imaging-based risks-tratification factor in trimodality therapy for locally advanced non-small cell lung cancer. J ThoracOncol 2011;6:920–926. [DOI] [PubMed] [Google Scholar]
- 42.Nishino M, Jagannathan JP, Krajewski KM, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol 2012;198:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bahce I, Vos CG, Dickhoff C, et al. Metabolic activity measured by FDG PET predicts pathological response in locally advanced superior sulcus NSCLC. Lung Cancer 2014;85:205–212. [DOI] [PubMed] [Google Scholar]
- 44.de Langen AJ, van den Boogaart V, Lubberink M, et al. Monitoring response to antiangiogenic therapy in non-small cell lung cancer using imaging markers derived from PET and dynamic contrast-enhanced MRI. J Nucl Med 2011;52:48–55. [DOI] [PubMed] [Google Scholar]
- 45.Graves EE, Maity A, Le QT. The tumor microenvironment in non-small-cell lung cancer. Seminars in radiation oncology 2010;20:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishino M, Hatabu H, Hodi FS . Imaging of Cancer Immunotherapy: Current Approaches and Future Directions. Radiology 2019;290:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sepesi B, Godoy MC, William WN, et al. Nodal Immune Flare (NIF) Following Neoadjuvant Anti-PD-1 and Anti-CTLA-4 Therapy in Non-Small Cell Lung Cancer. J Thoracic Oncol 2019;14:S745. [Google Scholar]
- 48.Cascone T, Nassib W, Weissferdt A, et al. Neoadjuvant nivolumab (N) or nivolumab plus ipilumab (NI) for resectable non-small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. J Clin Oncol 2019;37:Abstract 8504. [Google Scholar]
- 49.Poettgen C, Theegarten D, Eberhardt W, et al. Correlation of PET/CT findings and histopathology after neoadjuvant therapy in non-small cell lung cancer. Oncology 2007;73:316–323. [DOI] [PubMed] [Google Scholar]
- 50.Coroller TP, Agrawal V, Huynh E, et al. Radiomic-Based Pathological Response Prediction from Primary Tumors and Lymph Nodes in NSCLC. J Thorac Oncol 2017;12:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blaauwgeers JL, Kappers I, Klomp HM, et al. Complete pathological response is predictive for clinical outcome after tri-modality therapy for carcinomas of the superior pulmonary sulcus. Virchows Arch 2013;462:547–556. [DOI] [PubMed] [Google Scholar]
- 52.Lara-Guerra H, Chung CT, Schwock J, et al. Histopathological and immunohistochemical features associated with clinical response to neoadjuvant gefitinib therapy in early stage non-small cell lung cancer. Lung Cancer 2012;76:235–241. [DOI] [PubMed] [Google Scholar]
- 53.Qu Y, Emoto K, Eguchi T, et al. Pathologic Assessment After Neoadjuvant Chemotherapy for NSCLC: Importance and Implications of Distinguishing Adenocarcinoma From Squamous Cell Carcinoma. J Thorac Oncol 2019;14:482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Junker K. Histopathologic evaluation of mediastinal lymph nodes in lung cancer. Lung Cancer 2004;45 Suppl 2:S79–83.:S79-S83. [DOI] [PubMed] [Google Scholar]
- 55.Parra ER, Villalobos P, Behrens C, et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. Journal for immunotherapy of cancer 2018;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki K, Yokose T, Yoshida J, et al. Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. AnnThoracSurg 2000;69:893–897. [DOI] [PubMed] [Google Scholar]
- 57.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2012;25:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Makinen JM, Laitakari K, Johnson S, et al. Histological features of malignancy correlate with growth patterns and patient outcome in lung adenocarcinoma. Histopathology 2017;71:425–436. [DOI] [PubMed] [Google Scholar]
- 59.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2011;24:653–664. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi Y, Ishii G, Taira T, et al. Fibrous stroma is associated with poorer prognosis in lung squamous cell carcinoma patients. J ThoracOncol 2011;6:1460–1467. [DOI] [PubMed] [Google Scholar]
- 61.Weichert W, Kossakowski C, Harms A, et al. Proposal of a prognostically relevant grading scheme for pulmonary squamous cell carcinoma. Eur Respir J 2015;47:938–946. [DOI] [PubMed] [Google Scholar]
- 62.Tomizawa K, Shimizu S, Ohara S, et al. Clinical significance of tumor cavitation in surgically resected early-stage primary lung cancer. Lung Cancer 2017;112:57–61. [DOI] [PubMed] [Google Scholar]
- 63.Kerr KM, Johnson SK, King G, et al. Partial regression in primary carcinoma of the lung: does it occur? Histopathology 1998;33:55–63. [PubMed] [Google Scholar]
- 64.Leo F, Nicholson AG, Hansell DM, et al. Spontaneous regression of large-cell carcinoma of the lung--a rare observation in clinical practice. Thorac Cardiovasc Surg 1999;47:53–55. [DOI] [PubMed] [Google Scholar]
- 65.Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 2018;29:1853–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bae JM, Li ZM, Shin MH, et al. Pulmonary tuberculosis and lung cancer risk in current smokers: the Seoul Male Cancer Cohort Study. Journal of Korean medical science 2013;28:896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health O. Global Tuberculosis Report. Geneva: World Health Organization; 2018. [Google Scholar]
- 68.Werutsky G, Hochhegger B, Lopes de Figueiredo Pinto JA, et al. PET-CT has low specificity for mediastinal staging of non-small-cell lung cancer in an endemic area for tuberculosis: a diagnostic test study (LACOG 0114). BMC Cancer 2019;19:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chaft JE, Hellmann MD, Velez MJ, et al. Initial Experience With Lung Cancer Resection After Treatment With T-Cell Checkpoint Inhibitors. Ann Thorac Surg 2017;104:e217–e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsu PK, Huang HC, Hsieh CC, et al. Effect of formalin fixation on tumor size determination in stage I non-small cell lung cancer. AnnThoracSurg 2007;84:1825–1829. [DOI] [PubMed] [Google Scholar]
- 71.Isaka T, Yokose T, Ito H, et al. Comparison between CT tumor size and pathological tumor size in frozen section examinations of lung adenocarcinoma. Lung Cancer 2014;85:40–46. [DOI] [PubMed] [Google Scholar]
- 72.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J ThoracOncol 2013;8:823–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ichikawa T, Hattori A, Suzuki K, et al. Clinicopathological characteristics of lung cancer mimicking organizing pneumonia on computed tomography-a novel radiological entity of pulmonary malignancy. Jpn J Clin Oncol 2016;46:681–686. [DOI] [PubMed] [Google Scholar]
- 74.Radonic T, Dickhoff C, Mino-Kenudson M, et al. Gross handling of pulmonary resection specimen: maintaining the 3-dimensional orientation. Journal of thoracic disease 2019;11:S37–s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brambilla E, Le Teuff G, Marguet S, et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hendry S, Salgado R, Gevaert T, et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv Anat Pathol 2017;24:311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia D, Casanova R, Machiraju D, et al. Computationally-Guided Development of a Stromal Inflammation Histologic Biomarker in Lung Squamous Cell Carcinoma. Scientific reports 2018;8:3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hornick JL, Farraye FA, Odze RD. Prevalence and significance of prominent mucin pools in the esophagus post neoadjuvant chemoradiotherapy for Barrett’s-associated adenocarcinoma. The American journal of surgical pathology 2006;30:28–35. [DOI] [PubMed] [Google Scholar]
- 79.Shimosato Y, Suzuki A, Hashimoto T, et al. Prognostic implications of fibrotic focus (scar) in small peripheral lung cancers. AmJ SurgPathol 1980;4:365–373. [DOI] [PubMed] [Google Scholar]
- 80.Kung IT, Lui IO, Loke SL, et al. Pulmonary scar cancer. A pathologic reappraisal. AmJ SurgPathol 1985;9:391–400. [DOI] [PubMed] [Google Scholar]
- 81.Edwards C, Carlile A. Scar adenocarcinoma of the lung: a light and electron microscopic study. J ClinPathol 1986;39:423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yousem SA. Pulmonary apical cap: a distinctive but poorly recognized lesion in pulmonary surgical pathology. AmJSurgPathol 2001;25:679–683. [DOI] [PubMed] [Google Scholar]
- 83.Lagstein A. Pulmonary Apical Cap-What’s Old Is New Again. Arch Pathol Lab Med 2015;139:1258–1262. [DOI] [PubMed] [Google Scholar]
- 84.Yamane Y, Ishii G, Goto K, et al. A novel histopathological evaluation method predicting the outcome of non-small cell lung cancer treated by neoadjuvant therapy: the prognostic importance of the area of residual tumor. JThoracOncol 2010;5:49–55. [DOI] [PubMed] [Google Scholar]
- 85.Mariani F, Gatti B, Rocca A, et al. Pleuroparenchymal fibroelastosis: the prevalence of secondary forms in hematopoietic stem cell and lung transplantation recipients. Diagnostic and interventional radiology (Ankara, Turkey) 2016;22:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matsui T, Maeda T, Kida T, et al. Pleuroparenchymal fibroelastosis after allogenic hematopoietic stem cell transplantation: important histological component of late-onset noninfectious pulmonary complication accompanied with recurrent pneumothorax. Int J Hematol 2016;104:525–530. [DOI] [PubMed] [Google Scholar]
- 87.Namkoong H, Ishii M, Mori T, et al. Clinical and radiological characteristics of patients with late-onset severe restrictive lung defect after hematopoietic stem cell transplantation. BMC pulmonary medicine 2017;17:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Higo H, Miyahara N, Taniguchi A, et al. Cause of pleuroparenchymal fibroelastosis following allogeneic hematopoietic stem cell transplantation. Respiratory investigation 2019;57:321–324. [DOI] [PubMed] [Google Scholar]
- 89.Stein JE, Lipson EJ, Cottrell TR, et al. Pan-Tumor Pathologic Scoring of Response to PD-(L)1 Blockade. Clin Cancer Res 2019;epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kwiatkowski D, Rusch VW, Chaft JE, et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): Interim analysis and biomarker data from a multicenter study (LCMC3). J Clin Oncol 2019;37:Abstract 8503. [Google Scholar]
- 91.Provencio M, Nadal E, Insa A, et al. NADIM Study: Updated Clinical Research and Outcomes. J Thoracic Oncol 2019;14:S241. [Google Scholar]
- 92.Weissferdt A, Sepesi B, Pataer A, et al. 1928P Pathologic assessment following neoadjuvant immunotherapy or chemotherapy demonstrates similar patterns in non-small cell lung cancer (NSCLC). Annals of Oncology 2018;29:viii 680. [Google Scholar]
- 93.Lara-Guerra H, Waddell TK, Salvarrey MA, et al. Phase II study of preoperative gefitinib in clinical stage I non-small-cell lung cancer. J Clin Oncol 2009;27:6229–6236. [DOI] [PubMed] [Google Scholar]
- 94.Ning Y, Bao M, Yan X, et al. Surgery for advanced non-small cell lung cancer patient after epidermal growth factor receptor tyrosine kinase inhibitor neoadjuvant therapy. Annals of translational medicine 2018;6:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rizvi NA, Rusch V, Pao W, et al. Molecular Characteristics Predict Clinical Outcomes: Prospective Trial Correlating Response to the EGFR Tyrosine Kinase Inhibitor Gefitinib with the Presence of Sensitizing Mutations in the Tyrosine Binding Domain of the EGFR Gene. ClinCancer Res 2011;17:3500–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sacher AG, Le LW, Lara-Guerra H, et al. A window of opportunity study of potential tumor and soluble biomarkers of response to preoperative erlotinib in early stage non-small cell lung cancer. Oncotarget 2016;7:25632–25639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schaake EE, Kappers I, Codrington HE, et al. Tumor response and toxicity of neoadjuvant erlotinib in patients with early-stage non-small-cell lung cancer. J Clin Oncol 2012;30:2731–2738. [DOI] [PubMed] [Google Scholar]
- 98.Xiong L, Li R, Sun J, et al. Erlotinib as Neoadjuvant Therapy in Stage IIIA (N2) EGFR Mutation-Positive Non-Small Cell Lung Cancer: A Prospective, Single-Arm, Phase II Study. Oncologist 2019;24:157–e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang C, Li SL, Nie Q, et al. Neoadjuvant Crizotinib in Resectable Locally Advanced Non-Small Cell Lung Cancer with ALK Rearrangement. J Thorac Oncol 2019;14:726–731. [DOI] [PubMed] [Google Scholar]
- 100.Zhong WZ, Chen KN, Chen C, et al. Erlotinib Versus Gemcitabine Plus Cisplatin as Neoadjuvant Treatment of Stage IIIA-N2 EGFR-Mutant Non-Small-Cell Lung Cancer (EMERGING-CTONG 1103): A Randomized Phase II Study. J Clin Oncol 2019:Jco1900075. [DOI] [PubMed] [Google Scholar]
- 101.Dumont D, Do P, Lerouge D, et al. Off-Label Use of Crizotinib as a Neoadjuvant Treatment for a Young Patient When Conventional Chemotherapy Gave No Benefits in Stage IIIA Non-Small Cell Lung Cancer. The American journal of case reports 2017;18:890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu M, Jiang G, He W, et al. Surgical resection of locally advanced pulmonary adenocarcinoma after gefitinib therapy. AnnThoracSurg 2011;92:e11–e12. [DOI] [PubMed] [Google Scholar]
- 103.Lopez-Gonzalez A, Almagro E, Salas C, et al. Use of a tyrosine kinase inhibitor as neoadjuvant therapy for non-small cell lung cancer: A case report. Respiratory medicine case reports 2013;9:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ong M, Kwan K, Kamel-Reid S, et al. Neoadjuvant erlotinib and surgical resection of a stage iiia papillary adenocarcinoma of the lung with an L861Q activating EGFR mutation. Current oncology (Toronto, Ont) 2012;19:e222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rotow JK, Woodard GA, Urisman A, et al. Pathologic Complete Response to Neoadjuvant Crizotinib in a Lung Adenocarcinoma Patient With a MET Exon 14 Skipping Mutation. Clin Lung Cancer 2019;20:e137–e141. [DOI] [PubMed] [Google Scholar]
- 106.Imanishi N, Yoneda K, Taira A, et al. Major pathologic response to alectinib in ALK-rearranged adenocarcinoma of the lung. Surgical case reports 2018;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kilickap S, Onder S, Dizdar O, et al. Short-time use of crizotinib as neoadjuvant in ALK-positive non-small cell lung carcinoma can be a chance for resectability. Cancer Chemother Pharmacol 2019;83:1195–1196. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Z, Lin J, Peng S, et al. Radical surgical resection after neoadjuvant targeted therapy in non-small cell lung cancer: a single-center retrospective study of 6 cases. Journal of thoracic disease 2019;11:248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parikh AB, Hammons L, Gomez JE. Neoadjuvant Tyrosine Kinase Inhibition in Locally-advanced Non-small Cell Lung Cancer: Two Cases and a Brief Literature Review. Anticancer Res 2019;39:897–902. [DOI] [PubMed] [Google Scholar]
- 110.Betticher DC, Hsu Schmitz SF, Totsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol 2003;21:1752–1759. [DOI] [PubMed] [Google Scholar]
- 111.Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204–1223. [DOI] [PubMed] [Google Scholar]
- 112.Mauguen A, Pignon JP, Burdett S, et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: a re-analysis of meta-analyses of individual patients’ data. Lancet Oncol 2013;14:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Masip JR, Martinez-Marti A, Costa EC, et al. Major pathological response after preoperative chemotherapy as a surrogate marker of survival in early-stage non-small cell lung cancer. Ann Oncol 2017;28:v453–456. [Google Scholar]
- 114.Shu CA, Grigg C, Chiuzan C, et al. Neoadjuvant atezolizumab + chemotherapy in resectable non-small cell lung cancer (NSCLC). J Clin Oncol 2018;36:8352. [Google Scholar]
- 115.Zhong W-Z, Wu Y, Chen K, et al. CTONG 1103: Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment for stage IIIA-N2 EGFR-mutation Non-small cell lung cancer (EMERGING). Ann Oncol 2018;29:LBA48-PR. [DOI] [PubMed] [Google Scholar]
- 116.Arunachalam HB, Mishra R, Armaselu B, et al. COMPUTER AIDED IMAGE SEGMENTATION AND CLASSIFICATION FOR VIABLE AND NON-VIABLE TUMOR IDENTIFICATION IN OSTEOSARCOMA. Pacific Symposium on Biocomputing Pacific Symposium on Biocomputing 2017;22:195–206. [DOI] [PubMed] [Google Scholar]
- 117.Mishra R, Daescu O, Leavey P, et al. Convolutional Neural Network for Histopathological Analysis of Osteosarcoma. J Comput Biol 2018;25:313–325. [DOI] [PubMed] [Google Scholar]
- 118.Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol 2018;13:323–358. [DOI] [PubMed] [Google Scholar]