Background:
Autoantibodies against glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 (GPIHBP1), a cell-surface protein of capillary endothelial cells, block GPIHBP1-mediated transport of lipoprotein lipase (LPL) to the capillary lumen, resulting in impaired processing of triglyceride-rich lipoproteins (i.e., chylomicrons, VLDL) and severe hypertriglyceridemia (chylomicronemia) (1-4). Chylomicronemia resulting from GPIHBP1 autoantibodies is known as the “GPIHBP1 autoantibody syndrome” (5). Currently, little is known about how to treat this syndrome.
Objective:
To inform internal medicine specialists that some cases of acquired chylomicronemia are caused by GPIHBP1 autoantibodies and that this disorder can be successfully treated with rituximab (resulting in disappearance of GPIHBP1 autoantibodies and normalization of plasma triglyceride levels).
Case Report:
A 27-year-old woman presented to the clinic with chylomicronemia. The serum triglyceride was above 5500 mg/dl, and the cholesterol level was 693 mg/dl. Her general condition was good (BMI: 20.3; 60 kg; 172 cm). She had a history of antiphospholipid syndrome and a cerebral venous sinus thrombosis in 2015. She also had a history of Graves’ disease (treated with a thyroidectomy) and myocarditis. There was no family history of adiposity, diabetes mellitus, autoimmune disease, or hyperlipidemia.
The patient’s serum contained GPIHBP1 autoantibodies; the serum levels of GPIHBP1 were undetectable and the levels of LPL were extremely low (< 10 ng/ml) (Table 1). She had a positive ANA and a positive ENA screen for CENP-B.
TABLE.
Levels of GPIHBP1 antibodies (autoAbs), range of serum triglycerides (TG), and levels of GPIHBP1 and LPL mass in the ~18 months after the patient’s initial presentation with chylomicronemia.
| 6/18 | 8/18 | 11/18 | 12/18 | 1/19 | 2/19 | 2/19 | 5/19 | 7/19 | 8/19 | 8/19 | 9/19 | 10/19 | 12/19 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GPIHBP1 autoAbs (U/ml) | 5414 | 3130 | 3073 | 3011 | 4862 | 787 | 763 | 662 | 474 | 239 | 11 | 11 | 15 | 16 |
| Serum TG range (mg/dl) | 334–472 | 530–935 | 614–5500 | 271–5500 | 180–5500 | 215–1468 | 51–52 | 58–78 | 52 | 55–67 | 42–53 | |||
| GPIHBP1 mass (pg/ml) | 0 | 0 | 0 | 0 | 0 | 5924 | 6660 | 1347 | 914 | 6012 | 2512 | 2583 | 3312 | 3260 |
| LPL mass (ng/ml) | 7 | 7 | 8 | 9 | 7 | 8 | 9 | 11 | 10 | 15 | 64 | 74 | 90 | 122 |
The dates for the first, second, and third (final) rituximab infusions (1/19, 2/19, 8/19) are highlighted in blue, orange, and purple, respectively. Data from 2/19 and 8/19 are split into two columns, before (left) and after (right) the rituximab infusion.
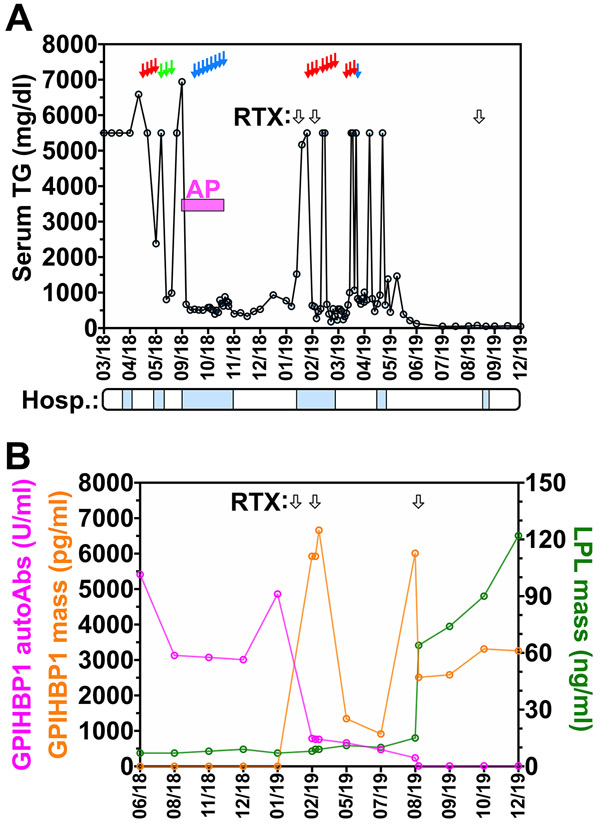
Initially, she was treated with fenofibrate (250 mg/day) and ezetimibe (10 mg/day) and advised to avoid dietary fat. She received a pulse therapy of prednisone 60 mg/day for two weeks, followed by tapering to a maintenance dose of 5 mg per day, but the plasma triglyceride levels remained elevated. She then received four plasma separations for the removal of triglycerides and antibodies and three lipid aphereses for the removal of triglycerides; the triglyceride levels fell but only transiently (Figure, A). Following a bout of acute pancreatitis, she had 10 immunoadsorption treatments for the removal of autoantibodies over a period of two weeks. The serum triglyceride level fell transiently, but serum triglyceride levels remained above 5500 mg/dl. She was then given two 375 mg infusions of rituximab at one-week intervals, followed by a third infusion six months later. Her general condition improved, and the serum triglyceride levels normalized. Normalization of triglyceride levels was accompanied by disappearance of GPIHBP1 autoantibodies and higher serum levels of both GPIHBP1 and LPL (Figure, B; Table).
FIGURE. Clinical and biochemical data on a 27-year-old woman with the GPIHBP1 autoantibody syndrome.
A. Serum triglyceride levels during hospitalizations (“Hosp.”) (blue boxes) following the initial presentation with chylomicronemia, and after plasma separations (red arrows), lipid aphereses (green arrows), immunoadsorptions (blue arrows), and rituximab (RTX, black arrows) treatment. The bout of acute pancreatitis (AP) is indicated in magenta. B. Plasma GPIHBP1 autoantibodies (autoAbs, left y-axis, magenta curve), GPIHBP1 mass (left y-axis, orange curve), and LPL mass (right y-axis, green curve) before and after rituximab (RTX, black arrows) treatments. During calendar year 2020, the plasma triglyceride levels remained normal. We do not understand why the serum GPIHBP1 levels fluctuated following the first two infusions of rituximab.
Discussion:
Our patient with a history of autoimmune diseases (Graves’ disease, antiphospholipid syndrome, myocarditis) presented with chylomicronemia, complicated by a bout of acute pancreatitis. The work-up revealed markedly elevated plasma triglyceride levels, GPIHBP1 autoantibodies, undetectable levels of GPIHBP1 in the serum, and extremely low serum levels of LPL—the hallmark findings of the GPIHBP1 autoantibody syndrome (5). Autoantibodies against GPIHBP1 cause chylomicronemia by blocking the ability of GPIHBP1 to transport LPL to its site of action in the capillary lumen. When LPL is absent from capillaries, the lipolytic processing of chylomicrons and VLDL is markedly impaired, resulting in severe hypertriglyceridemia. The serum levels of GPIHBP1 in patients with GPIHBP1 autoantibodies are generally very low (5, 6), a result of “immunoassay interference—the inability of current immunoassays to detect GPIHBP1 in the presence of GPIHBP1 autoantibodies (7). The low levels of LPL in the plasma reflect the absence of LPL transport to the capillary lumen (3, 5, 8).
The majority of ~10 GPIHBP1 autoantibody syndrome patients described to date (5, 9, 10), like the current case, exhibit clinical or serologic evidence of autoimmune diseases. In several patients, however, GPIHBP1 autoantibodies have been the only manifestation of autoimmune disease (5). For that reason, the GPIHBP1 autoantibody syndrome needs to be considered in any patient with newly acquired and unexplained chylomicronemia. Unfortunately, the GPIHBP1 autoantibody syndrome is sometimes not considered in the differential diagnosis of chylomicronemia, even by authorities with decades of experience in clinical lipidology (11).
In the initial description of the GPIHBP1 autoantibody syndrome (5), there were suggestions that two patients had responded to immunosuppressive drug treatment. However, in those cases, the evidence was inconclusive because the levels of GPIHBP1 autoantibodies were never tested following the initiation of drug therapy. In the current case, lipid-lowering drugs, plasma exchanges, and immunoabsorptions were not helpful, but the plasma triglyceride levels normalized after instituting therapy with rituximab, a CD20-monoclonal antibody that destroys B cells. Rituximab is often used to treat other autoantibody-mediated diseases [e.g., Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis, rheumatoid arthritis, pemphigus vulgaris, thrombotic thrombocytopenic purpura] (12, 13). In our patient, the normalization of plasma triglyceride levels following the rituximab infusions was accompanied by the disappearance of GPIHBP1 autoantibodies, markedly increased serum levels of GPIHBP1 (reflecting the absence of autoantibody-related immunoassay interference), and normalization of LPL levels in the plasma (reflecting restored GPIHBP1-mediated transport of LPL to the capillary lumen). The 2–3-month delay in the disappearance of GPIHBP1 autoantibodies was expected, given that a therapeutic response to rituximab typically occurs after several months (12, 13).
Our hope is that the current case will draw attention to the GPIHBP1 autoantibody syndrome, for two reasons. First, this syndrome is often not considered in the differential diagnosis of chylomicronemia, even by experienced clinical lipidologists (11). Second, the GPIHBP1 autoantibody syndrome carries a high risk of acute pancreatitis and death, yet is eminently treatable, as illustrated by the current case.
Acknowledgments
Financial Support: This work was supported by Grants HL090553, HL087228, and HL125335 from the National Heart, Lung, and Blood Institute; Transatlantic Network Grant 12CVD04 from the Leducq Foundation; Lundbeck Foundation Grant R230-2016-2930, and NOVO Nordisk Foundation Grant NNF17OC0026868.
Footnotes
Disclosures: K.M. is an employee of Immunobiologic Laboratories and holds stock in that company. K.N. holds stock in Immunobiologic Laboratories and serves as a consultant for Skylight and Sysmex.
Contributor Information
Jens Lutz, Medical Clinic, Nephrology-Infectious Diseases, Central Rhine Hospital Group, Koblenz, Germany.
Malgorzata Dunaj-Kazmierowska, Medical Clinic, Nephrology-Infectious Diseases, Central Rhine Hospital Group, Koblenz, Germany.
Sven Arcan, Medical Clinic, Gastroenterology, Central Rhine Hospital Group, Koblenz, Germany.
Ursula Kassner, Medical Clinic, Endocrinology and Metabolism, Charité University Medicine Campus Virchow, Berlin, Germany.
Kazuya Miyashita, Department of Clinical Laboratory Medicine, Gunma University Graduate School of Medicine, Maebashi, Gunma, Japan.
Masami Murakami, Department of Clinical Laboratory Medicine, Gunma University Graduate School of Medicine, Maebashi, Gunma, Japan.
Michael Ploug, Finsen Laboratory, Rigshospitalet, Copenhagen 2220N, Denmark.
Loren G. Fong, Department of Medicine, University of California, Los Angeles, 650 Charles E. Young Dr. South, A2-237 CHS Bldg.; Los Angeles, CA 90095.
Stephen G. Young, Department of Medicine, University of California, Los Angeles, 650 Charles E. Young Dr. South, A2-237 CHS Bldg.; Los Angeles, CA 90095.
Katsuyuki Nakajima, Department of Clinical Laboratory Medicine, Gunma University Graduate School of Medicine, Maebashi, Gunma, Japan.
Anne P. Beigneux, Department of Medicine, University of California, Los Angeles, 650 Charles E. Young Dr. South, A2-237 CHS Bldg.; Los Angeles, CA 90095.
References
- 1.Beigneux AP, Davies B, Gin P, et al. Glycosylphosphatidylinositol-anchored high density lipoprotein–binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5:279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies BSJ, Beigneux AP, Barnes II RH, et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 2010;12:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young SG, Fong LG, Beigneux AP, et al. GPIHBP1 and lipoprotein lipase, partners in plasma triglyceride metabolism. Cell Metab. 2019;30:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birrane G, Beigneux AP, Dwyer B, et al. Structure of the lipoprotein lipase–GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc Natl Acad Sci U S A. 2019;116:1723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigneux AP, Miyashita K, Ploug M, et al. Autoantibodies against GPIHBP1 as a cause of hypertriglyceridemia. N Engl J Med. 2017;376:1647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyashita K, Fukamachi I, Machida T, et al. An ELISA for quantifying GPIHBP1 autoantibodies and making a diagnosis of the GPIHBP1 autoantibody syndrome. Clin Chim Acta. 2018;487:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward G, Simpson A, Boscato L, et al. The investigation of interferences in immunoassay. Clin Biochem. 2017;50:1306–1311. [DOI] [PubMed] [Google Scholar]
- 8.Fong LG, Young SG, Beigneux AP, et al. GPIHBP1 and plasma triglyceride metabolism. Trends Endocrinol Metab. 2016;27:455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eguchi J, Miyashita K, Fukamachi I, et al. GPIHBP1 autoantibody syndrome during interferon beta1a treatment. J Clin Lipidol. 2019;13:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu X, Dallinga-Thie GM, Hovingh GK, et al. GPIHBP1 autoantibodies in a patient with unexplained chylomicronemia. J Clin Lipidol. 2017;11:964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chait A, Eckel RH. The chylomicronemia syndrome is most often multifactorial: A narrative review of causes and treatment. Ann Intern Med. 2019;170:626–634. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet. 2019;394:882–894. [DOI] [PubMed] [Google Scholar]
- 13.Bylsma LC, Fryzek JP, Cetin K, et al. Systematic literature review of treatments used for adult immune thrombocytopenia in the second-line setting. Am J Hematol. 2019;94:118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]