Abstract
Rationale: Single-center studies demonstrated that methamphetamine use is associated with pulmonary arterial hypertension (Meth-APAH). We used the Pulmonary Hypertension Association Registry to evaluate the national distribution of Meth-APAH and to compare its impact on patient-reported and clinical outcomes relative to idiopathic PAH.
Objectives: To determine if patients with Meth-APAH differ from those with idiopathic PAH in demographics, regional distribution in the United States, hemodynamics, health-related quality of life, PAH-specific treatment, and health care use.
Methods: The Pulmonary Hypertension Association Registry is a U.S.-based prospective cohort of patients new to care at a Pulmonary Hypertension Care Center. The registry collects baseline demographics, clinical parameters, and repeated measures of health-related quality of life, World Health Organization functional class, 6-minute walk distance, therapy, and health care use. Repeated measures of functional class, health-related quality of life, type of therapy, emergency department visits, and hospitalizations were compared using generalized estimating equations.
Results: Of 541 participants included, 118 had Meth-APAH; 83% of Meth-APAH arose in the western United States. The Meth-APAH group was younger and had a poorer socioeconomic status and lower cardiac index than the idiopathic PAH group, despite no difference in mean pulmonary artery pressure or pulmonary vascular resistance. The Meth-APAH group had a more advanced functional class in longitudinal models (0.22 points greater; 95% confidence interval [CI], 0.07 to 0.37) and worse PAH-specific (emPHasis-10) health-related quality of life (−5.4; 95% CI, −8.1 to −2.8). There was no difference in dual combination therapy; however, participants with Meth-APAH were less likely to be initiated on triple therapy (odds ratio [OR], 0.43; 95% CI, 0.24 to 0.77) or parenteral therapy (OR, 0.10; 95% CI, 0.04 to 0.24). Participants with Meth-APAH were more likely to seek care in the emergency department (incidence rate ratio, 2.30; 95% CI, 1.71 to 3.11) and more likely to be hospitalized (incidence rate ratio, 1.42; 95% CI, 1.10 to 1.83).
Conclusions: Meth-APAH represents a unique clinical phenotype of PAH, most common in the western United States. It accounts for a notable proportion of PAH in expert centers. Assessment for methamphetamine use is necessary in patients with PAH.
Keywords: Pulmonary Hypertension Association Registry, methamphetamine-associated pulmonary arterial hypertension, idiopathic pulmonary arterial hypertension, health-related quality of life, drug- and toxin-induced pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is characterized by an elevated pulmonary vascular resistance, leading to right ventricular failure, poor health-related quality of life (HRQL), and death (1–3). Drug- and toxin-induced PAH occurs with various drugs including aminorex, fenfluramine, dexfenfluramine, benfluorex, and dasatinib (4, 5). Single-center studies and anecdotal evidence also implicate methamphetamine, a prescription stimulant and an illicit drug of abuse, in the development of PAH (6–9). Indeed, the proceedings from the 2018 World Symposium on Pulmonary Hypertension now lists methamphetamine use as a definite cause of PAH (methamphetamine-associated pulmonary arterial hypertension [Meth-APAH]) (5, 10, 11). The popularity of methamphetamine is now increasing, with a lifetime U.S. prevalence of 5.4% (12–15). The epidemic also extends beyond the United States; the United Nations Office on Drugs and Crime estimates the annual global prevalence of methamphetamine use at 0.7%, with the highest non-U.S. prevalence occurring in Poland, the Netherlands, Australia, Thailand, and Nigeria (16).
The first descriptions of an association between methamphetamine use and PAH came from a retrospective study that demonstrated that patients with idiopathic PAH were more likely to use methamphetamine, amphetamine, or cocaine than patients with other forms of PAH (8). In the largest study to date, a single-center study from Stanford University, patients with Meth-APAH had more advanced symptoms of heart failure, higher right atrial pressure, and lower stroke volume index (9). Patients with Meth-APAH also had a lower event-free survival, even after adjusting for confounders. Furthermore, patients with Meth-APAH were less likely to be initiated on parenteral PAH-specific therapy or prostacyclin derivatives. Although this study paved the way in characterizing the association between methamphetamine use and PAH, a knowledge gap remains regarding the impact of Meth-APAH on HRQL. Additionally, it is not known if there are differences in patient-level socioeconomic demographics and the geographic distribution of Meth-APAH, and if the findings can be replicated in a multicenter cohort.
We sought to compare Meth-APAH with idiopathic PAH using data from the Pulmonary Hypertension Association Registry (PHAR), a prospective multicenter U.S.-based registry of patients new to care at a Pulmonary Hypertension Care Center. We hypothesized that we would find differences in World Health Organization (WHO) functional class, demographics, regional distribution throughout the United States, hemodynamics, HRQL, treatment, and health care use.
Methods
Study Design, Participants, and Setting
We performed a prospective cohort study in the PHAR, which included 47 Pulmonary Hypertension Care Centers at the time of this analysis (Table E1 and Figure E1 in the online supplement). The PHAR began enrollment in 2015, and the inclusion criteria have previously been reported (17, 18). Of note, although Stanford University is one of the enrolling centers, this study has no overlap with their previously reported cohort (9).
At the enrollment visit after informed consent, each participant completed a tablet-based survey that included the collection of demographics, socioeconomic status, anthropomorphic data, pulmonary hypertension diagnostic history, social history, symptoms, current PAH-specific therapy, HRQL, and clinical outcomes (17). This included the question “have you ever used methamphetamines in your life, even once”? Baseline right heart catheterization hemodynamics were included in the database at the time of enrollment. Participants typically underwent repeat study assessments every 6 months, with some variability in the interval based on the center and the patient. At each repeat clinical visit, repeated measures of symptoms, WHO functional class, 6-minute walk distance, current PAH-specific therapy, HRQL, and clinical outcomes were collected. The current data set from PHAR was locked on March 23, 2020. All study participants provided informed consent at enrollment. The University of Pennsylvania serves as the single institutional review board for the PHAR (Protocol Number: 822830).
The diagnosis and the etiology of pulmonary hypertension was determined by the enrolling PHAR center, as part of standard clinical practice. Diagnostic groupings available in the registry include idiopathic PAH, heritable PAH, drug- and toxin-induced PAH, and connective tissue disease–associated PAH, among others. Within the drug- and toxin-induced PAH classification, further subgroups include if the inciting drug is fenfluramine, methamphetamine, amphetamine, or dasatinib. No central guidelines were provided to PHAR investigators quantifying sufficient exposure to methamphetamine necessary to make a diagnosis of Meth-APAH, and the enrolling clinician, who determined the diagnostic category, did not have access to the results of the survey question about prior methamphetamine use. Given the similarities between amphetamine and methamphetamine, we combined the six participants listed as amphetamine-associated PAH with the group containing participants listed as Meth-APAH.
Given the goal of this study, we restricted our analysis to participants with PAH from methamphetamine or amphetamine use, and, as a comparator group, participants with idiopathic PAH. We also restricted our analysis to participants older than 18 years at enrollment.
Analytic Approach
We compared baseline demographic, hemodynamic, clinical variables, and HRQL between groups using Student’s t test or Wilcoxon rank-sum test for continuous variables and the chi-squared test for categorical variables.
We used generalized estimating equations to determine the association between Meth-APAH and WHO functional class, 6-minute walk distance, HRQL, choice of therapy, referral for transplantation, emergency department visits, and hospitalizations (19). We chose to use generalized estimating equations, clustered by participant, for our modeling of repeated measures over time because our primary predictor variable is immutable within each participant. For each model, we ran an unadjusted analysis and a multivariate adjusted analysis. To determine the impact of Meth-APAH on survival, we used multivariate adjusted Cox proportional hazards models. More details on the justification behind which covariates were chosen are included in the online supplement.
Sensitivity Analyses and Missing Data
To determine if hemodynamics in the database represented baseline hemodynamics or not, we ran a hemodynamic sensitivity analysis using incident cases only. We were also concerned that our lack of centralized definition of what constituted a sufficient exposure to methamphetamine in order for a participant to be diagnosed with Meth-APAH may bias our results. As a result, we performed a series of five sensitivity analyses on our functional status, HRQL, treatment, and healthcare use models. These sensitivity analyses included 1) assessing ever-users of methamphetamine versus never-users, 2) including participants with a registry diagnosis of idiopathic PAH who ever used methamphetamine in the Meth-APAH group, 3) removal of participants with idiopathic PAH who ever used methamphetamine, 4) removal of participants with a diagnosis of amphetamine-associated PAH, and 5) removing all participants with a registry diagnosis of Meth-APAH who did not admit to using methamphetamine on the PHAR survey. Additionally, we imputed missing data using 10-fold multiple imputation with chained equations and reran our primary analyses after imputation (20, 21). Finally, to ensure that our results were not impacted by unmeasured confounders, we calculated an e-value for the main finding in each of our primary models.
For all analyses, we used a P value of 0.05 as the cutoff for determining statistical significance.
Analyses were conducted using Stata 15.1 (Stata Corp).
A more detailed description of the study population, included variables, justification for adjustment, and imputation methods are included in the online supplement.
Results
Study Population
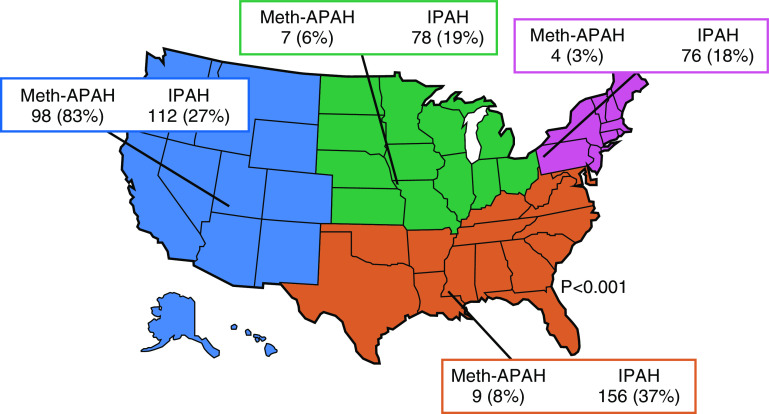
Of the 541 participants included in the analysis, 118 (22%) had Meth-APAH (Figure E2) (Table 1), which represents 9% of the entire PHAR cohort. Participants with Meth-APAH were younger than those with idiopathic PAH and were less likely to be insured, college graduates, married, or employed (all P < 0.03). The vast majority of participants with a diagnosis of Meth-APAH (91%) reported prior use of methamphetamines, whereas only a small minority of participants with idiopathic PAH (6%) reported having ever used methamphetamines. Participants with Meth-APAH reported a lower taxable income per year than participants with idiopathic PAH (P < 0.001). In both the Meth-APAH and the idiopathic PAH participants, the predominant race/ethnicity was white, and the predominant sex was female. Notably, there was a higher percentage of men with Meth-APAH than idiopathic PAH. Participants with Meth-APAH had a greater 6-minute walk distance at enrollment; however, there was no difference in WHO functional class or REVEAL Lite 1 Risk Score between groups (22). There were notable differences in diagnostic etiology by U.S. Census Region, with the overwhelming majority of Meth-APAH occurring in one census region (P < 0.001). Fully 83% of Meth-APAH participants received care at PHAR centers in the Western U.S. Census Region, whereas the distribution of idiopathic PAH was more even throughout the U.S. Census Regions (Figure 1).
Table 1.
Baseline demographics of the Pulmonary Hypertension Association Registry
| Meth-APAH (n = 118) | IPAH (n = 423) | |
|---|---|---|
| Age, yr (n = 541) | 47.5 ± 9.2 | 55.5 ± 17.2 |
| Body mass index, kg/m2 (n = 522) | 30.4 ± 6.5 | 31.1 ± 7.8 |
| Sex, M (n = 541) | 44 (37) | 100 (24) |
| Uninsured (n = 541) | 6 (5) | 7 (2) |
| Never married (n = 531) | 35 (30) | 72 (17) |
| College graduate (n = 533) | 20 (17) | 141 (34) |
| Unemployed (n = 541) | 52 (44) | 88 (21) |
| Ever-smoker (n = 535) | 84 (72) | 200 (48) |
| Current alcohol use (n = 532) | 38 (32) | 152 (37) |
| Ever use of methamphetamines (n = 539) | ||
| Yes | 106 (91) | 27 (6) |
| No | 8 (7) | 389 (92) |
| Declined to answer | 3 (3) | 6 (1) |
| Mean follow up time, d (n = 437) | 390.9 ± 352.7 | 414.3 ± 358.3 |
| Incident diagnosis of PAH (n = 540) | 54 (46) | 212 (51) |
| Race/ethnicity (n = 509) | ||
| Non-Hispanic white | 79 (73) | 300 (75) |
| Hispanic | 18 (17) | 43 (11) |
| Black | 6 (6) | 51 (13) |
| Asian | 5 (5) | 7 (2) |
| Taxable income per year (n = 442) | ||
| Less than $50,000 | 84 (84) | 172 (50) |
| $50,000–$100,000 | 12 (12) | 90 (26) |
| $100,000–$150,000 | 2 (2) | 47 (14) |
| Greater than $150,000 | 2 (2) | 33 (10) |
| U.S. census region (n = 541) | ||
| Northeast | 4 (3) | 76 (18) |
| Midwest | 7 (6) | 79 (19) |
| South | 9 (8) | 156 (37) |
| West | 98 (83) | 112 (27) |
| WHO functional class (n = 506) | ||
| Class 1 | 10 (9) | 41 (10) |
| Class 2 | 32 (28) | 134 (34) |
| Class 3 | 62 (54) | 193 (49) |
| Class 4 | 11 (10) | 23 (6) |
| REVEAL lite 1 risk category (n = 231) | ||
| Low risk | 27 (59) | 94 (51) |
| Intermediate risk | 12 (26) | 45 (24) |
| High risk | 7 (15) | 46 (25) |
| Six-minute walk distance, m (n = 451) | 375.8 ± 118.0 | 343.3 ± 139.3 |
| B-type natriuretic peptide, pg/ml (n = 304) | 104 [32.5–370] | 114 [41–332] |
| Creatinine, mg/dl (n = 523) | 0.9 [0.8–1.1] | 0.9 [0.8–1.1] |
| Therapy at enrollment (n = 538) | ||
| Digoxin | 13 (11) | 33 (8) |
| On PAH-specific therapy | 100 (86) | 370 (88) |
| Dual combination therapy | 60 (52) | 216 (52) |
| Triple therapy | 13 (11) | 86 (20) |
| Parenteral prostacyclin | 7 (6) | 117 (28) |
| Oxygen supplementation | 30 (26) | 203 (48) |
Definition of abbreviations: IPAH = idiopathic pulmonary arterial hypertension; Meth-APAH = methamphetamine-associated pulmonary arterial hypertension; PAH = pulmonary arterial hypertension; WHO = World Health Organization.
Baseline demographic and clinical parameters of Meth-APAH versus IPAH in the Pulmonary Hypertension Association Registry at the time of enrollment in the registry. Data are presented as mean ± standard deviation for normally distributed continuous variables, as median [interquartile range] for nonnormally distributed continuous variables, and n (%) for categorical variables. REVEAL Lite 1 risk score calculated from the REVEAL risk calculator (22).
Figure 1.
Distribution of Meth-APAH and IPAH in the Pulmonary Hypertension Association Registry. IPAH = idiopathic pulmonary arterial hypertension; Meth-APAH = methamphetamine-associated pulmonary arterial hypertension.
Hemodynamics
We found that participants with Meth-APAH had less favorable hemodynamics than participants with idiopathic PAH. At the baseline clinical visit, participants with Meth-APAH had a higher heart rate, a lower systolic blood pressure, and a higher diastolic blood pressure. On diagnostic right heart catheterization done before enrollment, participants with Meth-APAH had a higher right atrial pressure, a lower cardiac output, a lower cardiac index, and a lower right ventricular stroke volume index than participants with idiopathic PAH (all P < 0.04) (Table 2). We found no differences in mean pulmonary artery pressure, pulmonary vascular resistance, pulmonary capillary wedge pressure, or transpulmonary gradient between participants with Meth-APAH and participants with idiopathic PAH. Our hemodynamic sensitivity analysis using only incident cases was not substantially different from the complete cohort (Table E2).
Table 2.
Baseline hemodynamics of the Pulmonary Hypertension Association Registry
| Meth-APAH (n = 118) | IPAH (n = 432) | |
|---|---|---|
| Heart rate, beats/min | 85.2 ± 17.4 | 79.0 ± 15.6 |
| Systolic blood pressure, mm Hg | 122.4 ± 15.8 | 129.8 ± 26.1 |
| Diastolic blood pressure, mm Hg | 81.1 ± 13.0 | 76.5 ± 13.1 |
| Right atrial pressure, mm Hg | 11.9 ± 7.4 | 10.2 ± 6.1 |
| Mean pulmonary artery pressure, mm Hg | 51.8 ± 12.7 | 50.9 ± 14.2 |
| Pulmonary vascular resistance, Wood Units | 11.1 ± 5.0 | 10.4 ± 5.7 |
| Pulmonary capillary wedge pressure, mm Hg | 11.5 ± 7.6 | 11.4 ± 5.7 |
| Transpulmonary gradient, mm Hg | 41.0 ± 12.5 | 39.2 ± 13.8 |
| Cardiac output, L/min | 3.99 ± 1.45 | 4.24 ± 1.39 |
| Cardiac index, L/min/m2 | 2.06 ± 0.66 | 2.22 ± 0.70 |
| Stroke volume index, ml/beat/m2 | 25.3 ± 10.4 | 29.1 ± 10.6 |
Definition of abbreviations: IPAH = idiopathic pulmonary arterial hypertension; Meth-APAH = methamphetamine-associated pulmonary arterial hypertension.
Hemodynamics at baseline for participants in the Pulmonary Hypertension Association Registry stratified by Meth-APAH and IPAH. Data are presented as mean ± standard deviation.
Functional Status
In longitudinal assessments of functional status, there were differences in functional class, but not 6-minute walk distance, between groups. In unadjusted and adjusted longitudinal models, participants with Meth-APAH had more advanced WHO functional class (Table 3). Six-minute walk distance was greater in participants with Meth-APAH using unadjusted longitudinal models; however, the difference did not remain in models adjusted only for age or in fully adjusted models.
Table 3.
Effect estimate for impact of methamphetamine-associated PAH on functional capacity
| Outcome | Mean Difference (95% CI) | P Value |
|---|---|---|
| Unadjusted | ||
| World Health Organization functional class | 0.15 (0.01 to 0.29) | 0.032 |
| Six minute walk distance, m | 34.31 (8.96 to 59.66) | 0.008 |
| | ||
| Adjusted for age alone | ||
| World Health Organization functional class | 0.22 (0.08 to 0.36) | 0.002 |
| Six minute walk distance, m | 6.6 (−18.8 to 32.1) | 0.610 |
| | ||
| Adjusted for age, sex, race/ethnicity, education, and the time-dependent covariates of body mass index and whether the participant was on PAH-specific therapy or not | ||
| World Health Organization functional class | 0.22 (0.07 to 0.37) | 0.004 |
| Six-minute walk distance, m | 9.5 (−16.5 to 35.5) | 0.474 |
Definition of abbreviations: CI = confidence interval; PAH = pulmonary arterial hypertension.
Data are presented as mean difference with 95% CIs between participants with methamphetamine-associated PAH as compared with participants with idiopathic PAH, as quantified by generalized estimating equations using a Gaussian distribution. This represents the adjusted mean increase in outcome over the entire study that is seen in participants with methamphetamine-associated PAH as compared with those with idiopathic PAH. Models adjusted for age, sex, race/ethnicity, education, and the time-dependent covariates of body mass index and whether the participant was on PAH-specific therapy or not.
Health-Related Quality of Life
We found differences in HRQL that varied by instrument. At baseline, participants with Meth-APAH had poorer generic-mental HRQL (44.8 ± 9.1 vs. 48.9 ± 8.4, P < 0.001) and PAH-specific HRQL (reverse coded) (20.8 ± 12.7 vs. 25.7 ± 12.4, P < 0.001) compared with participants with idiopathic PAH. In contrast, participants with Meth-APAH reported better generic-physical HRQL when compared with participants with idiopathic PAH (35.7 ± 6.2 vs. 33.5 ± 6.7, P = 0.003) (Figure 2). This pattern held true over serial visits (Table 3). In adjusted models, participants with Meth-APAH still reported worse generic-mental HRQL and worse PAH-specific HRQL. The association between Meth-APAH and better generic-physical HRQL did not remain after adjustment. The association of Meth-APAH with differences in HRQL approached the minimally clinically important difference for the PAH-specific instrument but not the generic-mental or the generic-physical instruments.
Figure 2.
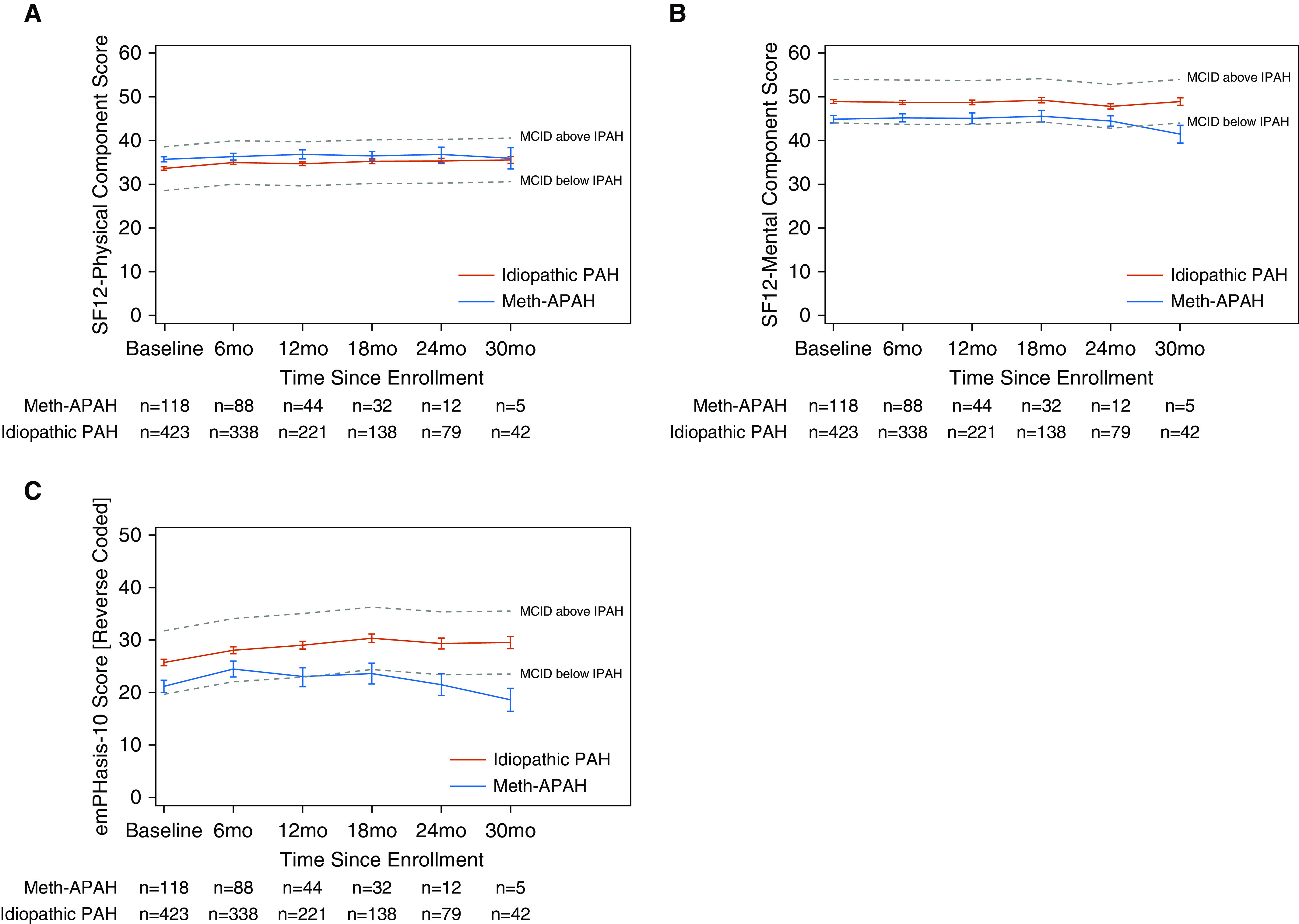
Unadjusted plots of health-related quality of life (HRQL) from time of enrollment in registry separated by diagnostic grouping of methamphetamine-associated pulmonary arterial hypertension (Meth-APAH) versus idiopathic pulmonary arterial hypertension (PAH). (A) Short Form 12 (SF12) Physical Component Score (generic-physical HRQL). (B) SF12 Mental Component Score (generic-mental HRQL). (C) emPHasis-10 Score reverse coded so that higher scores denote better HRQL (PAH-specific HRQL). Blue lines represent participants with Meth-APAH. Red lines represent participants with idiopathic PAH. Dashed gray lines represent the MCID above or below the HRQL in idiopathic PAH. Whiskers represent standard error. MCID = minimally clinically important difference.
Treatment
Participants with Meth-APAH were prescribed PAH-specific therapy but were less likely to receive triple therapy or parenteral therapy. In adjusted models, there was no difference in the odds of being started on digoxin, on any PAH-specific therapy, or on dual combination oral therapy with a phosphodiesterase 5 inhibitor or riociguat, combined with an endothelin receptor antagonist, between participants with Meth-APAH and participants with idiopathic PAH (Table 4). Participants with Meth-APAH were less likely to be started on triple combination therapy, on parenteral prostacyclin therapy, or on supplemental oxygen.
Table 4.
Association between methamphetamine-associated PAH and HRQL
| Instrument | Instrument Type | MCID | Mean Difference (95% CI) | P Value |
|---|---|---|---|---|
| Unadjusted | ||||
| SF12-PCS | Generic-physical | 5 | 1.7 (0.7 to 2.8) | 0.001 |
| SF12-MCS | Generic-mental | 5 | −3.8 (−5.3 to −2.4) | <0.001 |
| emPHasis-10 (reverse coded) | PAH specific | 6 | −5.0 (−7.4 to −2.6) | <0.001 |
| | ||||
| Adjusted for age, sex, race/ethnicity, education, body mass index, and the time-dependent covariate of whether the participant was on PAH-specific therapy or not | ||||
| SF12-PCS | Generic-physical | 5 | 0.8 (−0.4 to 2.0) | 0.175 |
| SF12-MCS | Generic-mental | 5 | −2.7 (−4.4 to −1.1) | 0.001 |
| emPHasis-10 (reverse coded) | PAH specific | 6 | −5.4 (−8.1 to −2.8) | <0.001 |
Definition of abbreviations: CI = confidence interval; HRQL = health-related quality of life; MCID = minimally clinically important difference; PAH = pulmonary arterial hypertension; SF12-MCS = Short Form 12-Mental Component Summary; SF12-PCS = Short Form 12-Physical Component Summary.
Data are presented as mean difference with 95% CIs between participants with methamphetamine-associated PAH as compared with participants with idiopathic PAH, as quantified by generalized estimating equations using a Gaussian distribution. Models were adjusted for age, sex, race/ethnicity, education, body mass index, and the time-dependent covariate of whether the participant was on PAH-specific therapy or not. HRQL instruments used include the SF12-PCS, the SF12-MCS, and the PAH-specific emPHasis-10. The emPHasis-10 instrument was reverse coded so that higher scores denote better HRQL in all HRQL instruments. Each instrument has its own MCID.
Healthcare Use, Referral for Transplantation, and Survival
We found differences in healthcare use between groups. Participants with Meth-APAH were more likely to be seen in the emergency department and more likely to be hospitalized than participants with idiopathic PAH (Table 5). We found no difference in the rate of referral for lung transplantation between participants with Meth-APAH and participants with idiopathic PAH (odds ratio, 0.54 [0.09–3.42]; P = 0.517). No patients in either group underwent transplantation during the study period. In adjusted Cox proportional hazards models, survival was similar between groups (hazard ratio for death, 1.07 [0.33–3.44]; P = 0.912).
Table 5.
Therapeutic decision making in participants with methamphetamine-associated PAH
| Treatment | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Unadjusted | ||
| Digoxin | 1.90 (1.04–3.48) | 0.038 |
| On PAH-specific treatment | 0.77 (0.41–1.43) | 0.403 |
| Dual combination | 1.36 (0.95–1.95) | 0.094 |
| Triple therapy | 0.59 (0.36–0.95) | 0.031 |
| Parenteral prostacyclin | 0.20 (0.10–0.39) | <0.001 |
| Supplemental oxygen | 0.47 (0.32–0.70) | <0.001 |
| | ||
| Adjusted for age, sex, race/ethnicity, education, body mass index, and the time-dependent covariates of 6-minute walk distance and World Health Organization functional class | ||
| Digoxin | 1.83 (0.91–3.70) | 0.091 |
| On PAH-specific treatment | 0.73 (0.31–1.71) | 0.465 |
| Dual combination | 1.23 (0.79–1.92) | 0.361 |
| Triple therapy | 0.43 (0.24–0.77) | 0.005 |
| Parenteral prostacyclin | 0.10 (0.04–0.24) | <0.001 |
| Supplemental oxygen | 0.49 (0.30–0.81) | 0.005 |
Definition of abbreviations: CI = confidence interval; PAH = pulmonary arterial hypertension.
Data are presented as odds ratio of being on specific therapy with 95% CIs in participants with methamphetamine-associated PAH as compared with participants with idiopathic PAH, as quantified by generalized estimating equations using a Binomial distribution. Models were adjusted for age, sex, race/ethnicity, education, body mass index, and the time-dependent covariates of World Health Organization functional class and 6-minute walk distance. Dual combination therapy represents combination therapy with a phosphodiesterase 5 inhibitor or riociguat, and an endothelin receptor antagonist. Triple therapy represents combination therapy with a phosphodiesterase 5 inhibitor or riociguat, an endothelin receptor antagonist, and any prostacyclin pathway agent.
Table 6.
Healthcare use in participants with methamphetamine-associated PAH
| Outcome | Incidence Rate Ratio (95% CI) | P Value |
|---|---|---|
| Unadjusted | ||
| Emergency department visit | 2.30 (1.71–3.10) | <0.001 |
| Hospitalization | 1.39 (1.10–1.76) | 0.005 |
| | ||
| Adjusted for age, sex, race/ethnicity, education, body mass index, and the time-dependent covariate of whether the participant was on PAH-specific therapy or not | ||
| Emergency department visit | 2.30 (1.71–3.11) | <0.001 |
| Hospitalization | 1.42 (1.10–1.83) | 0.007 |
Definition of abbreviations: CI = confidence interval; PAH = pulmonary arterial hypertension.
Data are presented as incidence rate ratio of emergency department visits/hospitalizations in the preceding 6 months with 95% CI between participants with methamphetamine-associated PAH as compared with participants with idiopathic PAH, as quantified by generalized estimating equations using a Poisson distribution. Models adjusted for age, sex, race/ethnicity, education, body mass index, and the time-dependent covariate of whether the participant was on PAH-specific therapy or not.
Our results did not substantially change in any of the five sensitivity analyses on functional status, HRQL, treatment, or healthcare use (Tables E3–E6). In addition, imputation of missing data did not substantially change our results (Table E7). The point estimates for the e-value of our primary findings of greater WHO functional class, impaired PAH-specific and generic-mental HRQL, differences in treatment, and higher incidence of healthcare use were all greater than 1.93 (Table E8), indicating that our results are unlikely to have resulted from an unmeasured confounder.
Discussion
In a prospective multicenter U.S.-based registry of patients with PAH, we found that Meth-APAH represents a distinct clinical phenotype when compared with idiopathic PAH. Notably, the diagnosis of pulmonary vascular disease due to methamphetamine use appears to be a regional phenomenon, with the vast majority of diagnoses arising from centers located in the western United States. Participants in the registry with Meth-APAH are younger and have a lower socioeconomic status, with a lower level of education achieved, a lower income, and a lower rate of employment. Participants with Meth-APAH suffer from a more advanced functional class in longitudinal analyses, and clinically significant impairments in PAH-specific HRQL. Upon presentation to expert centers, Meth-APAH reflects a phenotype with less favorable hemodynamics. These participants have increased healthcare use, with a higher rate of emergency department visits and a higher rate of hospitalizations. Providers in the PHAR are less likely to prescribe triple combination or parenteral therapy to participants with Meth-APAH.
This is the first time that regional variation of Meth-APAH has been shown. This finding parallels the U.S. patterns of drug use. The primary source of methamphetamine in the U.S. market comes from Mexico, which is in closest proximity to the Western U.S. Census Region (23). According to local law enforcement agencies, methamphetamine is the greatest drug threat in the Pacific, West Central United States, and Southwest (24). Although seizures of methamphetamine exceed cocaine nationally, and are second only to marijuana in frequency, there are regional differences. Roughly, 44% of all methamphetamine seizures occur in the Western U.S. Census Region (25). Furthermore, the epidemic is spreading throughout the United States as clandestine laboratories are most common in the Midwest (24). As such, we expect the regionalization of Meth-APAH to change as the epidemic spreads throughout the United States and the world.
Understanding the hemodynamic differences between Meth-APAH and idiopathic PAH informs us about the potential cardiopulmonary toxicities caused by methamphetamine use. We found notable differences in the right atrial pressure, right ventricular stroke volume index, and cardiac index without any differences in mean pulmonary artery pressure or pulmonary vascular resistance. These findings suggest that the direct myocardial toxicity from methamphetamine use may play an important role in the functional limitations of this population. Indeed, in animal models of methamphetamine use, there is evidence of myocardial damage and cellular apoptosis (26, 27). Similar results are seen in postmortem human studies, where cardiovascular pathology is present in the majority of cases in which methamphetamine is listed as the cause of death (28). Furthermore, the presence of left and biventricular failure in methamphetamine users is well characterized (29–32). The concomitant cardiac toxicity may also explain why participants with Meth-APAH suffer from limitations in functional class and PAH-specific HRQL, despite no extra limitations in 6-minute walk distance, a younger age, and similar generic-physical HRQL. These results also confirm the findings of the Stanford cohort, in which patients with Meth-APAH had a lower stroke volume index than patients with idiopathic PAH, despite similar pulmonary artery pressures (9). Conceptually, this may be because the presence of myocardial toxicity may precipitate uncoupling of the right ventricle and the pulmonary artery (33).
It is not entirely surprising that there were differences in HRQL, even after adjusting for confounders. We believe that the lower generic-mental HRQL in Meth-APAH reflects the concomitant psychosocial issues of drug abuse, rather than the mechanisms driving the PAH. Furthermore, the poorer PAH-specific HRQL likely reflects the less favorable hemodynamics, causing more respiratory limitations. It is unclear why Meth-APAH is associated with better generic-physical HRQL in unadjusted models; however, the difference failed to meet the minimally clinically important difference, and did not remain after multivariate adjustment, so it is likely not clinically meaningful.
There are several possible explanations for the different use of PAH-specific therapy in participants with Meth-APAH. Psychosocial issues that many drug users face, including lack of insurance, drug relapse, social isolation, and decreased medical literacy, could lead to less adherence with complicated medical treatment (34, 35). Although some may argue that PAH-specific therapy should not be offered to this population owing to high costs (36), the PHAR centers that deal with Meth-APAH offer dual combination therapy at a similar rate to what is seen in idiopathic PAH. Although the patient with Meth-APAH who seeks medical care and is willing to enroll in a clinical registry may not be truly representative of the entire population of methamphetamine abusers, our findings of decreased generic-mental HRQL, lower rate of insurance, decreased education, decreased employment, lower income, and higher healthcare use argue that the registry captures some of these high-risk patients. That we found a hesitancy in prescribing triple and parenteral therapy, similar to the Stanford cohort, is not entirely surprising. Triple therapy involves oral medications with an increased side-effect profile that requires more frequent follow-up, and parenteral therapy is often administered via an indwelling catheter, which is of concern when caring for a patient with a drug-induced disease (37, 38). Of note, aggressive therapy was not entirely avoided, as 11 of 118 participants with Meth-APAH were started on parenteral prostacyclin therapy, 6 of whom were ultimately transitioned to intravenous formulations.
The increased number of emergency department visits and hospitalizations seen in participants with Meth-APAH is concerning. Obvious reasons for this increased healthcare use may include noncompliance, inappropriate use of emergency services, and relapse on illicit drugs. Although some of the hesitancy to prescribe parenteral therapy may be from attempts to limit costs in this high-risk population, the increased healthcare use may also be from undertreatment of PAH, possibly contradicting the argument of cost reduction when making treatment decisions. Although the Stanford cohort demonstrated a lower event-free survival for patients with Meth-APAH, we did not find the same results. This may be explained because our registry is quite young with very few deaths, and a difference in survival may emerge as the registry ages.
Our study should inform future research objectives. The prevalence of Meth-APAH in our cohort is higher than the prevalence of anorexigen-associated PAH found in other registries (39, 40). Although some of this difference may be due to increased recognition of Meth-APAH as a disease entity, much of the increase also likely reflects the increasing popularity of methamphetamine as an illicit drug. Despite better understanding of the association between methamphetamine use and the development of PAH, the pathogenesis remains unclear. Although there was a higher percentage of males with Meth-APAH, we still found a female predominance, similar to idiopathic PAH, which argues that methamphetamine use might be unmasking PAH in higher-risk individuals. Future studies defining how methamphetamine use causes PAH, and the epigenetic mechanisms driving the disease, are of crucial importance moving forward. One potential area of exploration to help understand the mechanism behind the development of Meth-APAH could be to determine if there is interaction between methamphetamine use and mutations in the bone morphogenetic protein receptor type 2, as a high prevalence of mutations in patients with PAH from fenfluramine, but not dasatinib, has previously been described (41, 42). Furthermore, the concomitant myocardial dysfunction these patients experience highlights the potential for different treatment avenues in this population. It is possible that adjunctive therapies for right ventricular remodeling, such as spironolactone, may be more beneficial in Meth-APAH than other etiologies (43). Finally, describing the association is merely the first step and does not imply causation. A knowledge gap remains in how to define the diagnosis of Meth-APAH. It remains unclear if methamphetamine use causes PAH via intravenous administration or via all types of administration. Definitions of sufficient cause are also unclear; that is, how much methamphetamine abuse is enough to lead to development of PAH? Future studies should focus on methamphetamine abusers without PAH to help identify risk factors for the development of Meth-APAH (44). Additionally, future studies should focus on prescription methamphetamine and amphetamine, given to patients with attention-deficit/hyperactivity disorder, to determine if they also can lead to the development of PAH (11). Finally, a better understanding of the impact of screening and drug rehabilitation in patients with Meth-APAH is necessary to determine if the hemodynamic changes can improve with cessation of methamphetamine use.
Strengths and Limitations
Our study is not without limitations. The most notable limitation is that participants were not asked about methamphetamine use during each follow-up clinical visit, and that we did not perform routine drug screens on all participants in the study. As such, we do not know if participants with Meth-APAH were still using methamphetamine during the study period, or if participants with idiopathic PAH started using methamphetamine during the study period. Potentially confounding this problem is that the diagnosis of Meth-APAH was made by each individual center, without any central definition of what is a sufficient exposure necessary for the diagnosis. Notably, the lack of standardized definition of sufficient exposure means that some participants with minimal or distant methamphetamine use might be included in the Meth-APAH group. Although this does introduce bias into our sample, we believe that this would bias the results to the null. Furthermore, it is our hope that our sensitivity analyses mitigate some of the limitations of not having a centralized definition of Meth-APAH; however, it is important to recognize as a limitation of this registry. Another important limitation is that we do not have any information on time from symptom onset. As a result, we do not know if the hemodynamic differences represent a later presentation to care rather than a more aggressive phenotype. The PHAR also suffers from a potential for a selection bias because participants must opt in to the registry. This potential bias may overestimate the use of PAH-specific therapy in Meth-APAH because patients who are deemed unreliable may not be approached for the registry or may not consent to participate. This may also explain why we found no difference in survival, contrary to the Stanford cohort. Additionally, as the PHAR enrolls from highly specialized centers, it is also possible that our results do not reflect the practice patterns in nonspecialized centers and the general population. Furthermore, our study is limited by the extent of the data collected in the registry; although the PHAR includes comprehensive measurements of clinical outcomes and baseline demographics, it did not include pulmonary function data and echocardiographic parameters. Future studies should assess left ventricular function and right ventricular function on echocardiogram to better understand the contribution of methamphetamine on the development of myocardial toxicity. Finally, our recordings of deaths were not verified with external sources, such as the Social Security Death Master File. Therefore, it is possible that some of the deaths may not have been captured.
Despite our limitations, our study has notable strengths. It is the largest, and the only multicenter registry, to report on the prevalence of Meth-APAH. We confirmed the findings of the Stanford cohort in a multicenter manner, showing that participants in the registry with Meth-APAH have a lower stroke volume index and the same mean pulmonary artery pressure as participants with idiopathic PAH (9). We also report novel findings related to cardiac index, HRQL, patient-level demographics, the regional distribution of the care of these patients, and the therapeutic approaches taken by PHAR investigators who practice in a variety of different patterns/locations.
Conclusions
Methamphetamine is an addictive substance with high abuse potential. Given the increasing use of methamphetamine across the United States and the world, we expect Meth-APAH to become an important clinical entity in PAH. Providers should be aware of this high-risk patient population given the less favorable hemodynamics and the increased healthcare use.
Acknowledgments
Acknowledgment
The authors thank the other investigators, the staff, and particularly participants of the PHAR for their valuable contributions. A full list of participating PHAR sites and institutions can be found at www.PHAssociation.org/PHAR.
Footnotes
The Pulmonary Hypertension Association Registry is supported by Pulmonary Hypertension Care Centers, Inc., a supporting organization of the Pulmonary Hypertension Association.
Author Contributions: N.A.K. is the guarantor. He is responsible for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. N.A.K., S.M.K., D.V.G., J.P.S., and T.D.M. made substantial contributions to the conception and design of the work. N.A.K., R.T.Z., V.A.d.J.P., D.B.B., R.L.B., C.D.B., M.M.C., J.M.E., J.F., M.R.L., S.C.M., J.W.M., K.W.P., J.C.R., J.S., O.A.S., M.A.S., S.M.K., D.V.G., J.P.S., and T.D.M. made substantial contributions to the acquisition, analysis, or interpretation of data for the work. N.A.K. wrote the first draft of the manuscript. R.T.Z., V.A.d.J.P., D.B.B., R.L.B., C.D.B., M.M.C., J.M.E., J.F., M.R.L., S.C.M., J.W.M., K.W.P., J.C.R., J.S., O.A.S., M.A.S., S.M.K., D.V.G., J.P.S., and T.D.M. revised the manuscript for important intellectual content.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the Pulmonary Hypertension Association Registry Investigators
References
- 1.McGoon MD, Benza RL, Escribano-Subias P, Jiang X, Miller DP, Peacock AJ, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62(25) Suppl:D51–D59. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol. 2015;65:1976–1997. doi: 10.1016/j.jacc.2015.03.540. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, De Marco T, Kobashigawa EA, Katz PP, Chang VW, Blanc PD. Comparison of cardiac and pulmonary-specific quality-of-life measures in pulmonary arterial hypertension. Eur Respir J. 2011;38:608–616. doi: 10.1183/09031936.00161410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montani D, Seferian A, Savale L, Simonneau G, Humbert M. Drug-induced pulmonary arterial hypertension: a recent outbreak. Eur Respir Rev. 2013;22:244–250. doi: 10.1183/09059180.00003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaiberger PH, Kennedy TC, Miller FC, Gal J, Petty TL. Pulmonary hypertension associated with long-term inhalation of “crank” methamphetamine. Chest. 1993;104:614–616. doi: 10.1378/chest.104.2.614. [DOI] [PubMed] [Google Scholar]
- 7.van Wolferen SA, Vonk Noordegraaf A, Boonstra A, Postmus PE. [Pulmonary arterial hypertension due to the use of amphetamines as drugs or doping] [in Dutch] Ned Tijdschr Geneeskd. 2005;149:1283–1288. [PubMed] [Google Scholar]
- 8.Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest. 2006;130:1657–1663. doi: 10.1378/chest.130.6.1657. [DOI] [PubMed] [Google Scholar]
- 9.Zamanian RT, Hedlin H, Greuenwald P, Wilson DM, Segal JI, Jorden M, et al. Features and outcomes of methamphetamine-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;197:788–800. doi: 10.1164/rccm.201705-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galiè N, McLaughlin VV, Rubin LJ, Simonneau G. An overview of the 6th World Symposium on pulmonary hypertension. Eur Respir J. 2019;53:1802148. doi: 10.1183/13993003.02148-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez RL, III, Perez VJ, Zamanian RT. Methamphetamine and the risk of pulmonary arterial hypertension. Curr Opin Pulm Med. 2018;24:416–424. doi: 10.1097/MCP.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothman RB, Baumann MH. Methamphetamine and idiopathic pulmonary arterial hypertension: role of the serotonin transporter. Chest. 2007;132:1412–1413. doi: 10.1378/chest.07-0235. [DOI] [PubMed] [Google Scholar]
- 13.Mattson ME. The CBHSQ Report. Rockville, MD: Substance Abuse and Mental Health Services Administration;; 2013. Emergency department visits involving methamphetamine: 2007 to 2011. pp. 1-2. [PubMed] [Google Scholar]
- 14.Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, et al. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129:167–179. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Center for Behavioral Health Statistics and Quality. 2017 National survey on drug use and health: detailed tables. Rockville, MD: Substance Abuse and Mental Health Services Administration, editor.
- 16.United Nations Office on Drugs and Crime World Drug Report 2016 (United Nations publication, Sales No. E.16.XI.7). 2016. [Google Scholar]
- 17.Gray MPKSM. The pulmonary hypertension association registry: rationale, design, and role in quality improvement. Adv Pulm Hypertens. 2018;16:185–188. [Google Scholar]
- 18.DesJardin JT, Kolaitis NA, Kime N, Kronmal RA, Benza RL, Elwing JM, et al. PHAR Investigators. Age-related differences in hemodynamics and functional status in pulmonary arterial hypertension: baseline results from the Pulmonary Hypertension Association Registry. J Heart Lung Transplant. 2020;39:945–953. doi: 10.1016/j.healun.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Colditz GA. Optimal design of longitudinal data analysis using generalized estimating equation models. Biom J. 2017;59:315–330. doi: 10.1002/bimj.201600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 22.Benza RL, Kanwar M, Raina A, Lohmueller LC, Pasta DJ, La R, et al. Comparison of risk discrimination between the REVEAL 2.0 calculators, the French Pulmonary Registry Algorithm and the Bologna method in patients with Pulmonary Arterial Hypertension (PAH) [abstract] Am J Respir Crit Care Med. 2019;199:A2512. [Google Scholar]
- 23.Artigiani EE, Hsu MH, McCandlish D, Wish ED. Methamphetamine: a regional drug crisis. NDEWS Sentinel Community Epidemiologists, editor. College Park, MD: University of Maryland; 2018. [Google Scholar]
- 24.DEA. 2017 National Drug Threat Assessment. U.S. Department of Justice, National Drug Intelligence Center; 2017 [accessed 2021 Feb 22]. Available from: https://www.dea.gov/documents/2017/10/01/2017-national-drug-threat-assessment.
- 25.Substance Abuse and Mental Health Services Administration. Treatment episode data set: national admissions to substance abuse treatment services. Substance Abuse and Mental Health Services Administration, editor; Rockville, MD: Substance Abuse and Mental Health Services Administration; 2017. [Google Scholar]
- 26.Sun X, Wang Y, Xia B, Li Z, Dai J, Qiu P, et al. Methamphetamine produces cardiac damage and apoptosis by decreasing melusin. Toxicol Appl Pharmacol. 2019;378:114543. doi: 10.1016/j.taap.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Tomita M, Katsuyama H, Watanabe Y, Hidaka K, Yoshitome K, Miyaishi S, et al. Water-restraint stress enhances methamphetamine-induced cardiotoxicity. Chem Biol Interact. 2011;190:54–61. doi: 10.1016/j.cbi.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 28.Akhgari M, Mobaraki H, Etemadi-Aleagha A. Histopathological study of cardiac lesions in methamphetamine poisoning-related deaths. Daru. 2017;25:5. doi: 10.1186/s40199-017-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schürer S, Klingel K, Sandri M, Majunke N, Besler C, Kandolf R, et al. Clinical characteristics, histopathological features, and clinical outcome of methamphetamine-associated cardiomyopathy. JACC Heart Fail. 2017;5:435–445. doi: 10.1016/j.jchf.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Kaye S, Darke S, Duflou J, McKetin R. Methamphetamine-related fatalities in Australia: demographics, circumstances, toxicology and major organ pathology. Addiction. 2008;103:1353–1360. doi: 10.1111/j.1360-0443.2008.02231.x. [DOI] [PubMed] [Google Scholar]
- 31.Darke S, Duflou J, Kaye S. Prevalence and nature of cardiovascular disease in methamphetamine-related death: a national study. Drug Alcohol Depend. 2017;179:174–179. doi: 10.1016/j.drugalcdep.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Zhao SX, Kwong C, Swaminathan A, Gohil A, Crawford MH. Clinical characteristics and outcome of methamphetamine-associated pulmonary arterial hypertension and dilated cardiomyopathy. JACC Heart Fail. 2018;6:209–218. doi: 10.1016/j.jchf.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in Pulmonary hypertension. J Am Coll Cardiol. 2017;69:236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 34.Rommel N, Rohleder NH, Wagenpfeil S, Haertel-Petri R, Kesting MR. Evaluation of methamphetamine-associated socioeconomic status and addictive behaviors, and their impact on oral health. Addict Behav. 2015;50:182–187. doi: 10.1016/j.addbeh.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 35.Cumming C, Troeung L, Young JT, Kelty E, Preen DB. Barriers to accessing methamphetamine treatment: a systematic review and meta-analysis. Drug Alcohol Depend. 2016;168:263–273. doi: 10.1016/j.drugalcdep.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Dranitsaris G, Mehta S. Oral therapies for the treatment of pulmonary arterial hypertension: a population-based cost-minimization analysis. Appl Health Econ Health Policy. 2009;7:43–59. doi: 10.1007/BF03256141. [DOI] [PubMed] [Google Scholar]
- 37.Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, et al. GRIPHON Investigators. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522–2533. doi: 10.1056/NEJMoa1503184. [DOI] [PubMed] [Google Scholar]
- 38.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 39.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 40.Farber HW, Miller DP, Poms AD, Badesch DB, Frost AE, Muros-Le Rouzic E, et al. Five-year outcomes of patients enrolled in the REVEAL registry. Chest. 2015;148:1043–1054. doi: 10.1378/chest.15-0300. [DOI] [PubMed] [Google Scholar]
- 41.Humbert M, Deng Z, Simonneau G, Barst RJ, Sitbon O, Wolf M, et al. BMPR2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Respir J. 2002;20:518–523. doi: 10.1183/09031936.02.01762002. [DOI] [PubMed] [Google Scholar]
- 42.Weatherald J, Chaumais MC, Savale L, Jaïs X, Seferian A, Canuet M, et al. Long-term outcomes of dasatinib-induced pulmonary arterial hypertension: a population-based study. Eur Respir J. 2017;50:1700217. doi: 10.1183/13993003.00217-2017. [DOI] [PubMed] [Google Scholar]
- 43.Maron BA, Waxman AB, Opotowsky AR, Gillies H, Blair C, Aghamohammadzadeh R, et al. Effectiveness of spironolactone plus ambrisentan for treatment of pulmonary arterial hypertension (from the [ARIES] study 1 and 2 trials) Am J Cardiol. 2013;112:720–725. doi: 10.1016/j.amjcard.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng Y, Tung CK, Chung AKK, Liu WW, Huang D, Chan PH, et al. Screening of Pulmonary Hypertension in Methamphetamine Abusers (SOPHMA): rationale and design of a multicentre, cross-sectional study. BMJ Open. 2019;9:e027193. doi: 10.1136/bmjopen-2018-027193. [DOI] [PMC free article] [PubMed] [Google Scholar]