To the Editor: In a recent study, Greinacher et al.1 reported thrombotic complications, mostly cerebral vein thrombosis, associated with thrombocytopenia in 11 patients after they had been vaccinated with ChAdOx1 nCoV-19 (AstraZeneca). Although none of these patients had received heparin, the authors detected high titers of anti–platelet factor 4 (PF4)–heparin antibodies that strongly activated platelets in vitro without heparin and in the presence of PF4. This syndrome, which resembles autoimmune heparin-induced thrombocytopenia, was called vaccine-induced immune thrombotic thrombocytopenia (VITT), and an algorithm for the management of this syndrome was proposed on the basis of immunoassays detecting anti–PF4–heparin antibodies.
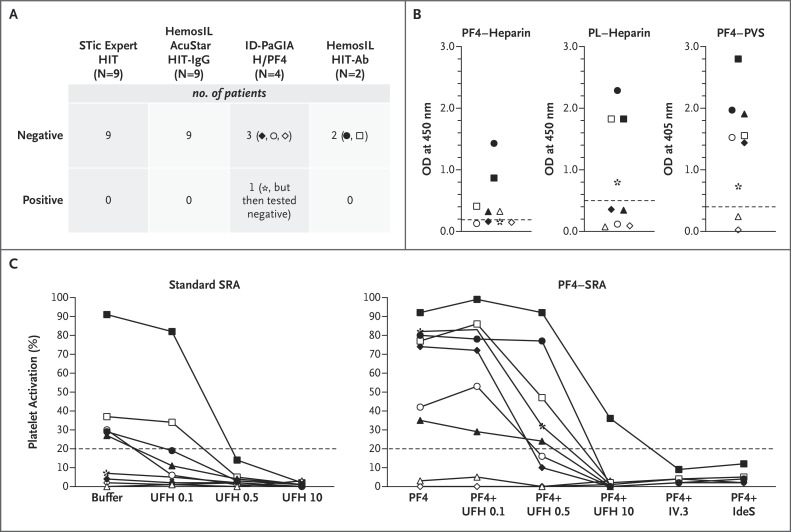
Between March 19 and April 1, 2021, plasma samples from nine patients (median age, 44 years) with suspected VITT after vaccination with ChAdOx1 nCoV-19 were analyzed in our laboratory (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). Cerebral vein thrombosis (in six patients) and splanchnic vein thrombosis (in five patients) were the most common events. All the patients had severe thrombocytopenia (median platelet count nadir, 29,000 per cubic millimeter; range, 9 to 61,000) except for one woman with both cerebral vein thrombosis and splanchnic vein thrombosis. Two rapid immunoassays widely used for the diagnosis of heparin-induced thrombocytopenia (STic Expert HIT and HemosIL AcuStar HIT-IgG) were performed on plasma samples to detect PF4-specific antibodies, and the results were negative in all the patients. Two other rapid tests had been performed in some patients by the referring laboratories and were negative, except in one patient, who had an equivocal result (Figure 1A).
Figure 1. PF4-Specific Immunoassays and SRA in Patients with Suspected VITT.
Panel A shows that rapid immunoassays do not detect antibodies to platelet factor 4 (PF4) associated with vaccine-induced immune thrombotic thrombocytopenia (VITT). All the plasma samples from the patients with findings suggestive of VITT were tested with two rapid immunoassays (STic Expert HIT, Stago, and HemosIL AcuStar HIT-IgG, Werfen), and negative results were obtained in every case. Two other rapid assays had also been performed in some patients (each represented by a specific symbol) in the referring centers (ID-PaGIA H/PF4, DiaMed, and HemosIL HIT-Ab, Werfen), and they also showed negative or doubtful (in patient *, who had an initial positive result but then tested negative) results.
Panel B shows that the sensitivity of enzyme-linked immunosorbent assays (ELISAs) to detect PF4-specific IgG antibodies depends on the antigen target. Levels of PF4-specific IgG antibodies were evaluated in plasma samples from the nine patients with suspected VITT (each represented by a specific symbol) with the use of three different ELISAs with varying antigen targets (PF4–heparin complexes [Asserachrom HPIA, Stago], PF4 released from a platelet lysate [PL] and complexed with heparin [Zymutest HIA IgG, Hyphen], and PF4–poly[vinyl sulfonate] [PVS] complexes [Lifecodes PF4 IgG, Immucor]). OD denotes optical density.
Panel C shows that a serotonin release assay (SRA) should be performed with the use of PF4 to detect platelet-activating antibodies to VITT. SRA was performed by incubating 75 μl of washed platelets obtained from healthy persons with 20 μl of plasma obtained from each patient, either in the absence (standard SRA) or the presence (PF4–SRA) of 10 μg per milliliter of PF4. All tests were performed with or without unfractionated heparin at 0.1 IU per milliliter (UFH 0.1), 0.5 IU per milliliter (UFH 0.5), and 10 IU per milliliter (UFH 10). Clinically significant and strong platelet activation, with maximum release ranging from 36 to 99%, was measured in seven of the nine patients only when PF4 was present in the reaction mixture. Moreover, platelet activation was not inhibited by therapeutic concentrations of UFH 0.1 or UFH 0.5. In contrast, platelet activation was completely abolished by 10 μg per milliliter of IV.3, a monoclonal antibody specific for FcγRIIA, or 6 U of IdeS, an IgG-degrading enzyme of Streptococcus pyogenes, preincubated for 15 minutes at 37°C in the plasma sample of each patient tested (five patients) before SRA.
We also tested all plasma samples with three different PF4-specific enzyme-linked immunosorbent assays and obtained variable results (Figure 1B). Significant levels of IgG antibodies to PF4 were detected in seven patients only by the assay that used PF4–poly(vinyl sulfonate) (PVS) complex as the antigenic target. In addition, optical density values were variable and lower than those previously reported with a similar test.2 The diagnosis of VITT was confirmed by PF4–serotonin release assay3 in all seven patients with IgG antibodies to PF4–PVS (Figure 1C), whereas a standard serotonin release assay was negative in two patients. Platelet activation was suppressed by IV.3, a monoclonal antibody that binds FcγRIIA receptors, but also by IdeS (IgG-degrading enzyme derived from Streptococcus pyogenes) (Figure 1C), a protease that also inactivates heparin-induced thrombocytopenia IgG antibodies.4 Intravenous immune globulins may be inappropriate for severe cerebral vein thrombosis with intracranial hypertension. IdeS (imlifidase) may be an effective treatment and needs to be evaluated.
Our results provide further support to show that rapid immunoassays should be avoided in the detection of PF4-specific antibodies in patients with suspected VITT. Therefore, the use of a sensitive, quantitative, immunologic test is strongly recommended, because according to the recently proposed algorithm,1,5 nonheparin anticoagulants should be preferred when clinically significant levels of anti-PF4 antibodies are detected.
Supplementary Appendix
Disclosure Forms
This letter was published on May 19, 2021, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. DOI: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. DOI: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vayne C, Guery EA, Kizlik-Masson C, et al. Beneficial effect of exogenous platelet factor 4 for detecting pathogenic heparin-induced thrombocytopenia antibodies. Br J Haematol 2017;179:811-819. [DOI] [PubMed] [Google Scholar]
- 4.Kizlik-Masson C, Deveuve Q, Zhou Y, et al. Cleavage of anti-PF4/heparin IgG by a bacterial protease and potential benefit in heparin-induced thrombocytopenia. Blood 2019;133:2427-2435. [DOI] [PubMed] [Google Scholar]
- 5.Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie 2021. April 01 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.