GPR84 is an understudied Gi-coupled G-protein-coupled receptor (GPCR) that is expressed on the surface of immune cells. Recently, the synthesis and discovery of chemical agonists and antagonists have begun to reveal the role of GPR84 in modulating the innate immune response in conditions such as fibrotic disorders and highlighted its potential as a drug target.
Biological functions
GPR84 has been associated with inflammation as well as the regulation of metabolism and energy sensing for around 15 years. GPR84 has also has recently been shown to be highly expressed in skeletal muscle, and its absence leads to detrimental effects in mitochondrial function1.
Although medium-chain fatty acids (9–14 carbons) can activate GPR84, their low concentrations in vivo have prompted questions regarding the true endogenous ligands and the downstream effects of GPR84. Various chemical tools have recently been developed in order to study the activation of GPR84 and illuminate its downstream cell signalling effects.
Chemical tools
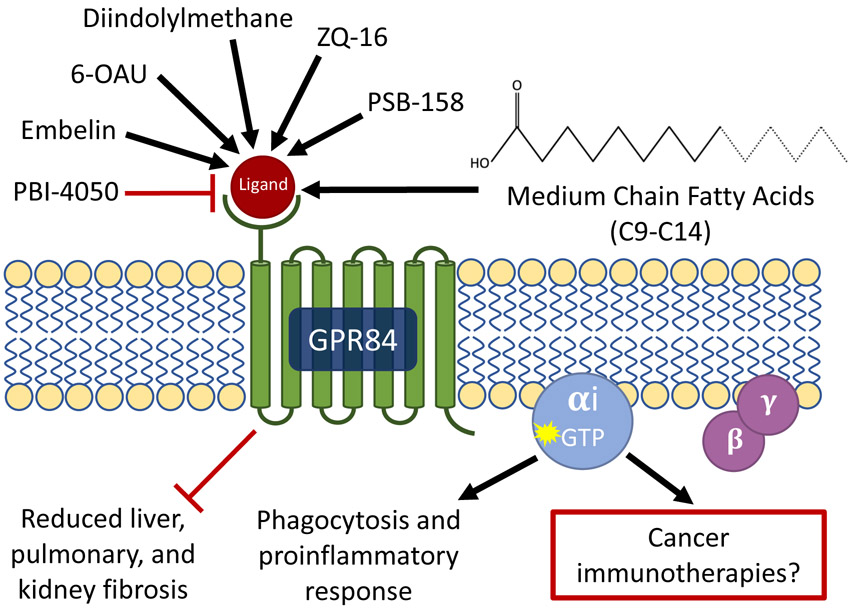
In 2013, Suzuki and colleagues provided strong evidence that agonists targeting GPR84 induce a pro-inflammatory response, and identified 6-n-octylaminouracil (6-OAU) as a surrogate GPR84 agonist2. This prompted the development of other small-molecule modulators for GPR84 (Fig. 1). 6-hexylamino-2,4(1H,3H)-pyrimidinedione (PSB-1584) is another ligand for GPR84, and by adding a radiolabel to it, it was possible to visualize GPR84 localization in live cells and tissues, study competition between different ligands, identify allosteric ligands and develop a molecular model of GPR843. Non-lipid agonists such as diindolylmethane and its derivatives, as well as embelin and 2-(hexylthio)pyrimidine-4,6-diol (ZQ-16) were suggested as GPR84 agonists4.
Fig. 1 ∣. Chemical tools to target GPR84 and their downstream effects.
Examples of agonists that activate the GPR84 receptor include embelin, 6-n-octylaminouracil (6-OAU), diindolylmethane, 2-(hexylthio)pyrimidine-4,6-diol (ZQ-16), 6-hexylamino-2,4(1H,3H)-pyrimidinedione (PSB-1584), and medium-chain fatty acids (9–14 carbons). GPR84 activation using the selective biased agonist DL-175 has been shown to lead to phagocytosis and a pro-inflammatory response4. PBI-4050, an antagonist of GPR84 as well as an agonist of GPR40, reduced liver, pulmonary and kidney fibrosis in mouse and rat models5.
Different GPR84 agonists have been demonstrated to induce different downstream pathways. For example, the selective biased agonist DL-175 resulted in less arrestin signalling when compared with 6-OAU in GPR84-CHO cells4. Additionally, DL-175 resulted in similar phagocytotic activity but less chemotaxis when compared with 6-OAU in macrophages.
The most advanced and promising drug candidates that target GPR84 are the antagonists PBI-4050 and GLPG1250. PBI-4050 is a dual-action modified fatty-acid derivative that is also an agonist of GPR405. Both GPR40 and GPR84 have been associated with induction of fibrosis in mice. Knockout mice models show that GPR40 has a protective effect against fibrosis while GPR84 enhances fibrosis5. GLPG1250 is a potent and specific GPR84 antagonist6. Both PBI-4050 and GLPG1250 are currently in phase II trials for pulmonary fibrosis after beneficial effects were observed in animal models. PBI-4050 is also tested in phase II trials for Alström syndrome, a rare autosomal recessive genetic disorder that results in multiple organ dysfunction and obesity. We speculate that other applications for GPR84 modulators may emerge in the future, such as promoting immune responses in order to boost cancer immunotherapies.
Acknowledgements
This article is part of a series from the NIH Common Fund Illuminating the Druggable Genome (IDG) programme. The goal of IDG is to catalyse research on understudied proteins from druggable gene families by providing reagents, phenotypes and a mineable database; focusing on GPCRs, kinases and ion channels. For more information, see https://druggablegenome.net/
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Montgomery MK et al. Regulation of mitochondrial metabolism in murine skeletal muscle by the medium-chain fatty acid receptor Gpr84. The FASEB Journal 33, 12264–12276, doi: 10.1096/fj.201900234R (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki M et al. Medium-chain Fatty Acid-sensing Receptor, GPR84, Is a Proinflammatory Receptor. Journal of Biological Chemistry 288, 10684–10691, doi: 10.1074/jbc.M112.420042 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Köse M et al. An Agonist Radioligand for the Proinflammatory Lipid-Activated G Protein-Coupled Receptor GPR84 Providing Structural Insights. Journal of Medicinal Chemistry 63, 2391–2410, doi: 10.1021/acs.jmedchem.9b01339 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Lucy D et al. A Biased Agonist at Immunometabolic Receptor GPR84 Causes Distinct Functional Effects in Macrophages. ACS Chemical Biology 14, 2055–2064, doi: 10.1021/acschembio.9b00533 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Gagnon L et al. A Newly Discovered Antifibrotic Pathway Regulated by Two Fatty Acid Receptors: GPR40 and GPR84. The American Journal of Pathology 188, 1132–1148, doi: 10.1016/j.ajpath.2018.01.009 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Saniere L et al. in A19. LESS IDIOPATHIC: STRUCTURAL AND FUNCTIONAL ABNORMALITIES IN IPF American Thoracic Society International Conference Abstracts A1046–A1046 (American Thoracic Society, 2019). [Google Scholar]