Abstract
Background & Aims:
The vast majority of hepatitis C virus (HCV) infection in Singapore is among those with a history of injecting drug use (IDU), yet harm reduction is not available and what is required to achieve the World Health Organization (WHO) HCV elimination targets (80% incidence reduction, 65% mortality reduction by 2030) is unknown. We model the intervention scale-up required to achieve WHO targets in Singapore.
Methods:
A dynamic model of HCV transmission and progression among those with a history of IDU was calibrated to Singapore, a setting with declining IDU and no harm reduction (~11,000 people with IDU history in 2017, 45% HCV seropositive). We projected HCV treatment scale-up from 2019 required to achieve WHO targets with varying prioritization scenarios, with/without opiate substitution therapy (OST) scale-up (to 40% among people who inject drugs [PWID]).
Results:
We estimated 3855 (95%CI:2635-5446) chronically HCV infected individuals with a history of IDU and 148 (87-284) incident HCV cases in Singapore in 2019. Reaching the HCV incidence target requires 272 (187-384) treatments in 2019, totaling 2444 (1683-3452) across 2019-2030. By prioritizing PWID or PWID and cirrhotics, 60% or 30% fewer treatments are required, respectively, whereas the target cannot be achieved with cirrhosis prioritization. OST scale-up reduces treatments required by 21-24%. Achieving both WHO targets requires treating 631 (359-1047) in 2019, totaling 3816 (2664-5423) across 2019-2030.
Conclusions:
HCV elimination is achievable in Singapore, but even with declining IDU requires immediate treatment scale-up among PWID. Harm reduction provision reduces treatments required and provides additional benefits.
Keywords: people who inject drugs (PWID), hepatitis c virus, treatment, opioid substitution therapy (OST), prevention, elimination, modelling, epidemic, liver disease, public health
INTRODUCTION
Globally, chronic hepatitis C virus (HCV) is a leading cause of liver disease and death.1 The World Health Organization (WHO) has declared an urgent need to eliminate viral hepatitis globally by 2030, with targets to reduce HCV incidence by 80% and HCV-related deaths by 65%.2 Singapore is a country in South East Asia, with a low general population HCV seroprevalence (0.37-0.54%) based on blood donor studies, and where the majority of prevalent and new infections are among those with a history of injecting drug use (IDU)3, 4. In Singapore, drug use is highly criminalized, and roughly 45% of people who inject drugs (PWID) show evidence of current or past HCV infection5. Despite this, harm reduction programs (such as needle and syringe programs and opiate substitution therapy [OST]) which reduce an individual’s risk of HCV6 do not operate in Singapore, and very few PWID have been treated for their HCV. Additionally, there is evidence of a recent shift away from IDU, replaced by non-injecting stimulant use7. Given these complexities, the future HCV epidemic trajectory, and level and targeting of HCV treatment and prevention intervention required to achieve the WHO targets in Singapore is unknown.
We developed a HCV dynamic transmission and disease progression model among PWID and ex-PWID in Singapore, a setting with declining injecting drug use, and use this to determine what level and prioritization of intervention could achieve each of the WHO targets.
METHOD
Mathematical model description
We developed a dynamic, deterministic model of HCV transmission among PWID, and HCV disease progression among PWID and former PWID. The modelled population was stratified by HCV infection state (uninfected, chronic HCV, compensated cirrhosis [CC], decompensated cirrhosis [DC], hepatocellular carcinoma [HCC], PWID status (PWID or ex-PWID), and current OST status for PWID (on/off), see Supplementary Figure S1. PWID enter the population through a time-varying injecting initiation rate, and transition to ex-PWID or permanent cessation from injecting. The model is dynamic, such that an individual’s risk of infection is related to the background prevalence of disease and an individual’s intervention state. Once infected, PWID either develop chronic infection (whereby they proceed to the chronic HCV stage) or spontaneously clear their infection (and remain in the susceptible stage). Unless successfully treated, PWID with chronic HCV transition through the natural history of HCV infection. We neglected HCV-related liver transplant as it is uncommon in Singapore, comprising 7% of the etiologies being wait-listed for liver transplant8, Among those transplanted, all were blood-transfusion related or hemophiliac and none had a history of injecting drug use (personal communication, Tan EK). Background mortality occurs from each stage, with elevated drug related mortality for PWID, and HCV-related mortality from DC/HCC.
A fixed number of individuals can be treated for their HCV, whereupon they either achieve a sustained viral response (SVR) and can be reinfected or fail treatment and remain chronically infected. If the number of infected individuals is less than the number of available treatments, we assume that all infected individuals are treated. Conservatively, we do not assume any behavior change after treatment. We assume PWID who fail treatment or become re-infected can be retreated. Individuals who obtain SVR either cease liver disease progression if in the chronic HCV stage, or progress at a lower rate from compensated cirrhosis to DC or HCC compared to those with cirrhosis who are untreated9, 10. We conservatively do not assume any regression of fibrosis for individuals with SVR.
Model parameterization and calibration.
Calibration procedure:
Key model parameters were estimated based on Singapore specific epidemiological data and global systematic review estimates where Singapore data were lacking (Supplementary Table S1). We incorporated parameter uncertainty (1000 random samples of parameter distributions in Table S1), and calibrated the model to sampled estimates for the ever-PWID (PWID+ex-PWID) population size and HCV chronic prevalence in 2017 using a least-squares global optimization solver in MATLAB. We assume PWID population size and HCV chronic infection prevalence are at steady state prior to 2012. Since 2012, a change in drug use and declines in heroin-related arrests (overall and first-time) have been observed in Singapore7. From 2012-2018, there was a decline in arrests of new heroin users by 33%/year. Coinciding with this decline, there was a shift to non-injecting routes of stimulants (predominantly methamphetamine) since 2012. Consequently, we assumed a time-varying injecting initiation rate which is stable prior to 2012, and reduces by ~30%/year from 2012 to 2018, assuming stable inflow thereafter for our baseline analysis. The model is therefore run until steady state, with a declining PWID injecting initiation rate from 2012-2018, with the model calibrated to ever-PWID population size and HCV chronic prevalence in 2017.
Calibration parameters:
We calibrated to an estimate of 11,000 (6,500-17,000) people with a history of IDU in 2017.11 An estimate amongst individuals from halfway houses (interim accommodation provided to released prisoners with recent incarcerations for substance abuse or voluntary attendees seeking drug rehabilitation) in 2017 found a HCV seroprevalence among ever PWID of 47% (95%CI 40-53%)12, consistent with an older study among PWID at a community addiction program in 2007 (42.5%).5 Therefore, we calibrated to a sampled HCV seroprevalence of 47% (sampled from 40-53%) among ever PWID in 2017, equating to a chronic prevalence among ever PWID of 35% (95%CI 30-40%) based on a spontaneous clearance rate of 26%13.
Intervention parameters:
We assume a 95% SVR rate for those treated with DAAs.14 There is currently no OST provision in Singapore. We simulate scale-up of OST, which we assumed reduces HCV transmission and acquisition by 50% (95%CI 37-60%) based on a recent Cochrane meta-analysis6. We incorporate OST dropout, assuming 7 months average duration on OST, based on other high-income settings15–18.
Model Scenarios:
No national treatment data are available but has been estimated to be very low in the pre-DAA era and likely even lower among people with a history of injecting drug use19. Therefore, we assumed no HCV treatment for PWID at baseline. We projected the required number of treatments required to achieve the WHO targets of reducing HCV incidence by 80% or HCV related death by 65% by 2030 compared to 2019. The model predicts negligible changes in annual HCV-related deaths and HCV incidence rates among all individuals from 2015-2019 (Supplementary Figure S2), so we used 2019 as a baseline given observed initiation of DAA treatment in 2019. Additionally, we projected the impact of the following treatment prioritization scenarios (where treatments are only allocated to the following groups): (1) Scaling-up treatment among all PWID and ex-PWID proportionally, (2) Scaling-up treatment among PWID only, (3) Scaling-up treatment for those with more advanced liver disease (cirrhotic and beyond), or (4) Scaling-up treatment among PWID and among those with more advanced liver disease (cirrhotic and beyond). For each treatment strategy, we also considered the impact on treatments required if OST is scaled-up from 0% to 40% OST coverage among PWID only (based on the WHO recommended coverage). For the OST scale-up scenarios, we implement scale-up through immediate increases in OST recruitment rates to achieve the desired level of coverage by 2021.
Sensitivity analysis.
The baseline analysis incorporates a reduction in injecting initiation from 2012 to 2020 based on observed data, with inflow stabilizing thereafter. In a sensitivity analysis, we also evaluated an alternative scenario to evaluate the impact of a future further decline in PWID prevalence following historical trends (see supplementary information). We additionally performed univariate analyses using lower and upper bounds of our population size estimates, extended duration on OST (18 months) and lower SVR rates (85% and 90%). Finally, because genotype 3 has been associated with increased risk of liver cirrhosis and hepatocellular carcinoma (HCC) compared to other genotypes20–22, and because there is various studies reporting differences in the genotype 3 distribution in Singapore23–25 we also examine lower proportions of genotype 3 (0%, 25%, 50%).
RESULTS
Baseline epidemic projections
In our baseline scenario without treatment, the model estimates a cirrhosis prevalence among HCV infected individuals of 34% in 2019 (95% confidence interval [95%CI] 30-40%), consistent with clinical data indicating 28% (24-32%) cirrhosis among patients with a history of substance use.23 Model projections with no scale up treatment are presented in Supplementary Figure S2.
The baseline model predicts that in 2012 there were an estimated 3432 (1888-5246) PWID, which declined dramatically due to a reduction in new injecting initiations to 2690 (1372-4296) in 2017, and reducing further to a predicted 2285 (1098-3748) PWID in 2019. The model predicts a relatively stable (but uncertain) population of former PWID from 2012-2019, estimated at 8647 (5932-12134) in 2019, with modest future decreases. In total, an estimated 3855 (2635-5446) individuals are chronically infected with HCV in 2019 (among them 1394 [715-2237] PWID and 2461 [1688-3688] ex-PWID), which will decrease to 3330 (2229-4323) in 2030 with no intervention (Supplementary Figures S2 and S13). We estimate 148 (87-284) incident HCV cases in 2019, falling slightly to 113 (64-224) by 2030 with no intervention. Similarly, we estimate 62 (38-92) HCV-related deaths in 2019, which remains relatively flat by 2030, decreasing to 58 (36-85).
Treatment impact with low, moderate, and high treatment rate scenarios
Figure 1 shows the impact of different treatment scenarios on HCV incidence and mortality. If 250 individuals are treated per year (similar to the estimated number of prisoners treated in 201925 the model predicts HCV incidence will reduce by a relative 72% (53-94%) by 2030 to 45 (6-112) new infections relative to 2019. If 500 individuals are treated per year, the model predicts HCV incidence will reduce by a relative 96% (93-97%) by 2030 to 6 (3-14) new infections relative to 2019. Similar impact is achieved if 750 individuals are treated per year starting in 2019.
Fig. 1. Impact with low (250/year), moderate (500/year), and high treatment (750/year) scenarios on the mean annual number of incidence HCV cases (A) and mean number or HCV related deaths (B) in Singapore.
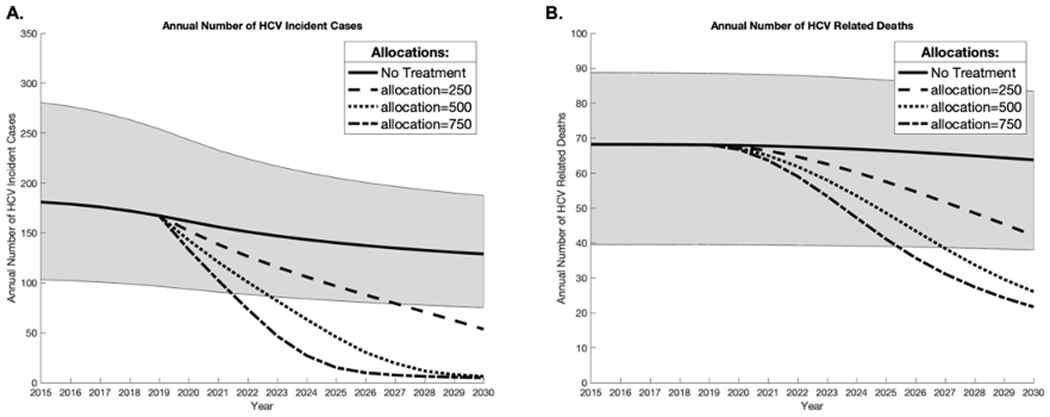
Shaded area represents the 2.5–97.5% interval projections for the ‘no treatment’ baseline scenario
Treatment scale-up needed to achieve WHO targets by 2030
Modeling suggests an 80% decrease in HCV incidence can be achieved by 2030 (Figure 2 and 3). With no scale-up of OST and with all HCV infected individuals eligible for treatment, 272 (187-384) individuals need treatment in 2019 to reach the incidence target (2444 [1683-3452] individuals across 2019-2030). Fewer initial and total treatments are required if treatment is prioritized to PWID: 105 (56-166) treatments required in 2019, 949 (507-1492) PWID over 11 years. Similarly when prioritizing PWID and individuals with cirrhosis or more advanced liver disease, fewer treatments are required, 186 (120-267) in 2019 and 1677 (1080-2399) over 11 years. The WHO incidence reduction target could not be achieved if treatment is prioritized or restricted to individuals with more advanced liver disease (cirrhotic and beyond). Importantly, scale-up of OST could reduce the numbers required to reach the incidence target. If OST is scaled-up to 40% among PWID and treatment allocated to all, then fewer treatments are required (20.7% [15.3-26.0] fewer with random allocation, 23.7% [27.7-30.0] fewer with PWID prioritization, Figure 2 and 3).
Fig. 2. Initial HCV treatment rates required to achieve the WHO targets for incidence (green bars) and mortality (orange bars) with different treatment prioritization scenarios (all, cirrhotic, PWID, PWID and cirrhotic).
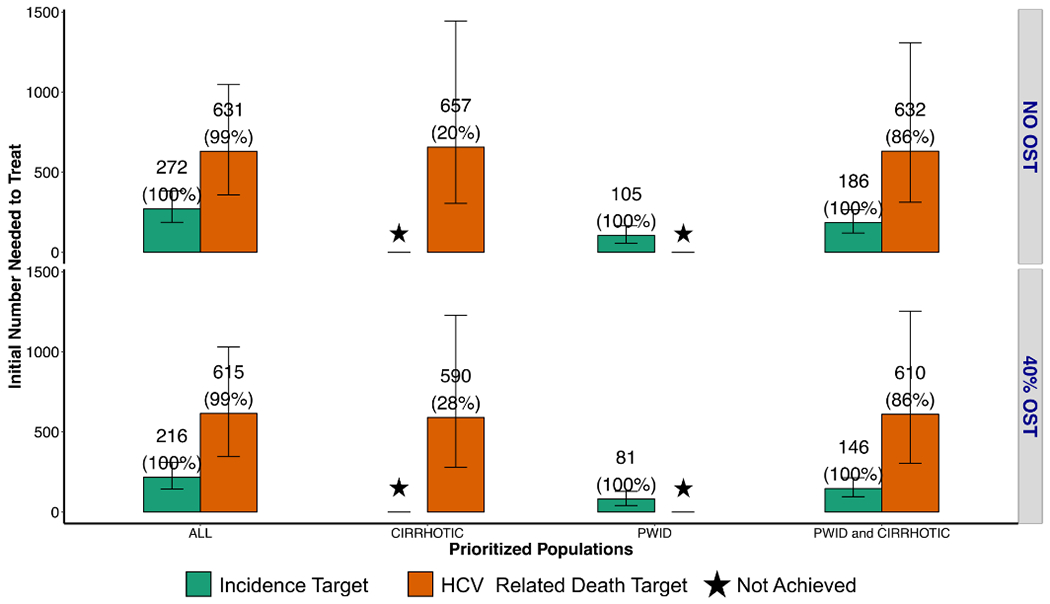
40% OST: scale up of opioid substitution therapy to 40% coverage among PWID. Star: WHO 2030 target not achieved. Mean number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot, with the proportion of runs reaching the target in parentheses
Fig. 3. Total numbers of HCV treatments required over 11 years (2019–2030) to achieve the WHO targets for incidence (green bars) and mortality (orange bars) with different treatment prioritization scenarios (all, cirrhotic, PWID, PWID and cirrhotic).
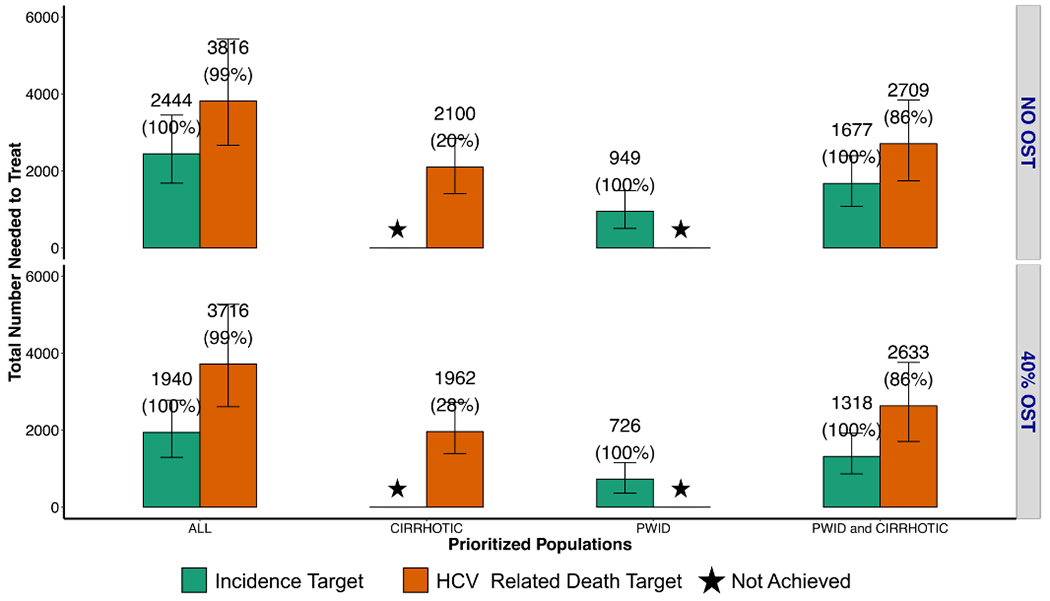
40% OST: scale up of opioid substitution therapy to 40% coverage among PWID. Star: WHO 2030 target not achieved. Mean number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot, with the proportion of runs reaching the target in parentheses
Achieving the 65% reduction in mortality target by 2030 likely requires more treatment than needed to achieve the incidence target. If treatment is randomly allocated, an estimated 631 [359-1047] individuals in 2019, and 3816 [2664-5423] individuals need to be treated over 2019-2030 to achieve the mortality target (which therefore achieves both incidence and mortality targets). A strategy prioritizing PWID and cirrhotics reduced the number of simulations which achieved elimination, and required similar treatment rates in 2019, but in simulations where it was achieved fewer cumulative treatments were required from 2019-2030 (Figure 3).
Sensitivity analysis.
Results were insensitive to assumptions of future IDU initiation patterns, differing by a <10% in an alternative scenario with a continuous decrease in PWID initiation (Supplementary Figures S3 and S4). Other sensitivity analysis results (Supplementary Figures S3–S12) were similarly insensitive to decreasing the SVR rates to 90 and 85% (versus 95% in the baseline scenario), where total treatment numbers increased by 4.7%(to 2,560) and 9.8% (to 2,684), respectively to achieve the incidence target, and increased by <5% to achieve the both targets. With variations in genotype 3 proportion, virtually no difference (<2%) was seen for treatment numbers required to achieve the incidence target. To achieve the mortality target, slightly more treatments were required (13.0%, 7.3% and 3.8%, respectively for 0%, 25%, or 50% genotype 3 prevalences). Results were moderately sensitive to uncertainty in PWID population size. To achieve the incidence target if all are eligible for treatment and no OST, 37.9% fewer and 32.6% more individuals need to be treated in 2019 for PWID population sizes of 6500 and 17000, respectively. Similar relative differences are required to achieve both WHO targets. Finally, when increasing the mean duration on OST from 7 to 18 months with 40% OST, the total number needed to treat decreased by 19% for the incidence target (1,940 vs 1,570) but only decreased by 1% for the mortality target.
DISCUSSION
We find HCV elimination achievable in Singapore, but even in a setting of declining injecting drug use requires an immediate and intensive scale-up of treatment to PWID. Harm reduction implementation, such as OST, could reduce treatments required, and provide additional benefits such prevention of HIV and overdose6. Additionally, prioritization of PWID and those with cirrhosis or more advanced disease can reduce treatments required.
Until recently, DAAs were expensive and inaccessible for the vast majority of Singaporeans due to high cost.19 In 2019, pan-genotypic DAA regimes were introduced into government means-tested subsidy programs. This has paved way for universal access to treatment, however barriers remain with regards to sufficient testing, linkage to care and a lack of a national strategy for HCV elimination. In the absence of a national strategy, treatment remains sporadic and physician directed. Whilst HCV elimination is feasible in Singapore, without a national directive and committed funding to both testing and treatment it is unlikely the scale-up of treatment required would be achievable. Based on a current estimated cost of HCV treatment (including DAAs and delivery) of SGD 9,834 (~$7,000 USD)19 treating 630 patients in 2019 as required by our model to achieve both WHO targets would require SGD 6.2 million (USD $4.47 million), representing 0.05% of the total allocated healthcare expenditure budget.26 This amount would need to be supplemented with funding for diagnostic testing which could be substantial but would be optimized if focused on high risk populations such as those with a history of injecting drug use. It is anticipated that with subsequently years, the numbers needed to treat would reduce as would budgetary demands.
Concomitant OST would reduce the annual cost by reducing the numbers needed to treat to reach HCV elimination, however barriers to scale-up remain in Singapore given problems faced during Buprenorphine availability between 2002-2006 27, which some PWID injected for recreational drug purposes instead, thus eventually resulting in its abandonment. Future schemes should not be ruled out on this basis alone. It is conceivable such schemes could exist if they were centralized and administered by the National Addiction Services under strict protocol. Currently there is a lack of advocacy groups for PWID, which have been instrumental in advocating for harm reduction services in other countries.
Finally, as in countries like Australia, even with broad access to DAAs, HCV case-finding case is a critical issue as the pool of diagnosed individuals is treated28. The prevalence of HCV in the general population in Singapore is low, but high in populations such as PWID who traditionally lack access to HCV-related care. Typically, this population is transitory between the community, halfway houses and correctional facilities within Singapore with 58% of ever PWID with a history of incarceration and overall recidivism rate between 23-30% over 2 years12, 29. This high recidivism rates and their predictable pathway into community halfway houses lends itself to ideal conditions for HCV elimination if universal screening among PWID and appropriate linkage to care measures are instituted. Strategies to improve case finding could include universal screening in foci of high HCV prevalence such as the National Addiction Services, Singapore Prison Services and Halfway Houses across Singapore. Still, many PWID may not be identified through the above existing services; expansion of OST could expand the ability to diagnose PWID. At present, hemodialysis patients are the only group which receive three monthly mandatory universal HCV screening.
Comparisons with literature
Our analysis supports modeling indicating the WHO elimination targets are likely achievable among PWID if treatment is combined with harm reduction scale-up30. Additionally, our analysis is consistent with studies indicating fewer treatments are required if treatment is prioritized to PWID and/or PWID and those with cirrhosis or more advanced disease, as well as if harm reduction is provided in combination.22, 31 Further, our analysis is consistent with findings that achieving the mortality target requires more treatment than achieving the incidence target.22
Strengths and weaknesses
Our analysis is unique in examining what is required for elimination in a setting where HCV is driven by IDU and without harm reduction, while incorporating a recent decline in new PWID. Nevertheless, our analysis has a number of limitations. First, our analysis is restricted to estimating burden among current and former PWID only, and neglects infections among other populations, however these populations are small and unlikely to affect our broader conclusions. For example, routine HCV screening was introduced to all blood products since 1992, and blood donor prevalence estimated at 0.059%24. Among the approximately 6000 patients on hemodialysis Singapore, the seroprevalence of HCV is 2.2% 24, equating to less than 100 patients with chronic HCV, and universal isolation of chronic viral hepatitis patients on hemodialysis has occurred since a small hospital outbreak in 2015.32 Additionally, despite evidence of HCV transmission among HIV-infected men who have sex with men in other major urban centers, this has not been documented in Singapore.
Second, there is uncertainty in our parameters, which we assessed through multivariate uncertainty analysis and one-way sensitivity analyses. As a result, we generate multiple model projections and we note that for some scenarios elimination is not achieved in all of the simulations, which is presented in the results and important to note. As in our previous analysis in other setting, this uncertainty was primary driven by substantial uncertainty in average injecting duration and, relatedly, PWID population size which affected our predictions. Injecting duration until final cessation is difficult to measure as cross-sectional surveys are both right and left censored as they miss those who inject for very short durations, and those surveyed have not yet cessated from injecting. We use the most recent published estimates of ever PWID in Singapore, but note drug use is criminalized and stigmatized in Singapore, so additional estimates would strengthen the evidence base and reduce uncertainty. Further, more comprehensive, routine surveillance among PWID would enable more robust tracking of the epidemiology in this important group.
Third, there is uncertainty in the future trajectory of IDU in Singapore. Although our results were robust to differences in assumptions regarding future trajectories, further work quantifying the dynamics of injecting drug use is warranted. For example, although there is evidence of a recent shift away from injecting drug use to non-injecting stimulant use, it is unclear whether these non-injecting stimulant users will eventually transition to injecting. Indeed, stimulant injection has been associated with elevated HIV and HCV risk among PWID 33, and therefore a transition to stimulant injecting could fuel the HCV epidemic in the future.
CONCLUSION
Urgent scale-up of HCV treatment in combination with harm reduction for PWID is warranted in Singapore, and could achieve the WHO elimination targets. Political will is needed to address the multiple harms associated with injecting drug use in Singapore.
Supplementary Material
SUPPLEMENTARY TABLE S1. Model parameters inputs and sources used in the model.
SUPPLEMENTARY FIGURE S1. Schematic of HCV transmission. PWID who spontaneously clear their infection remain in the susceptible compartment (if never infected) or their current liver disease stage (if cured). (A) Population stratification by PWID and OST status. (B) Population stratification by HCV status.
SUPPLEMENTARY FIGURE S2. Model projections with no scale-up of treatment.
A. Total population of person with HIV (PWID) and ex-PWID
B. Number of Individuals Chronically infected with HCV
C. Annual HCV Related Deaths
D. Annual Number of Incident HCV Cases
E. Incidence Rate among All Individuals
F. Incidence Rate among PWID
SUPPLEMENTARY FIGURE S3. Initial number needed to treat in first year with continued reductions in new PWID from 2018. In this scenario, we assumed that the historical reduction by ~30%/year in the inflow of injectors from 2012 to 2018 continued after 2018 while in our baseline scenario, we assumed that the inflow was stable thereafter.
SUPPLEMENTARY FIGURE S4. Total number needed to treat over 11 years with continued reductions in new PWID from 2018. In this scenario, we assumed that the historical reduction by ~30%/year in the inflow of injectors from 2012 to 2018 continued after 2018 while in our baseline scenario, we assumed that the inflow was stable thereafter.
SUPPLEMENTARY FIGURE S5. INITIAL NUMBER NEEDED TO TREAT IN FIRST YEAR WITH 85% AND 90% SUSTAINED VIROLOGICAL RESPONSE (SVR) RATE
Star: WHO 2030 target not achieved. Mean number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot. In this alternative scenario, we assumed a SVR rate of 85% and 90% respectively (compared to 95% in our baseline model).
SUPPLEMENTARY FIGURE S6. TOTAL NUMBER NEEDED TO TREAT OVER 11 YEARS WITH 85% AND 90% SUSTAINED VIROLOGICAL RESPONSE (SVR) RATE
Star: WHO 2030 target not achieved. Mean total number of HCV PWID and/or ex-PWID needed to treat are indicated above each bar plot. In this alternative scenario, we assumed a SVR rate of 85% and 90% respectively (compared to 95% in our baseline model).
SUPPLEMENTARY FIGURE S7. INITIAL NUMBER NEEDED TO TREAT IN FIRST YEAR FOR A DURATION ON OST OF 18 MONTHS
Star: WHO 2030 target not achieved. Mean number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot. In this alternative scenario, we assumed a mean duration on OST of 18 months (compared to 7 months in our baseline model).
SUPPLEMENTARY FIGURE S8. TOTAL NUMBER NEEDED TO TREAT OVER 11 YEARS FOR A DURATION ON OST OF 18 MONTHS
Star: WHO 2030 target not achieved. Mean total number of HCV PWID and/or ex-PWID needed to treat are indicated above each bar plot. In this alternative scenario, we assumed a mean duration on OST of 18 months (compared to 7 months in our baseline model).
SUPPLEMENTARY FIGURE S9. INITIAL NUMBER NEEDED TO TREAT IN FIRST YEAR FOR VARYING POPULATION SIZE, TREATING ALL.
Mean number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot. In these alternative scenario, we assumed a population of 6,500 and 17,000 individuals.
SUPPLEMENTARY FIGURE S10. TOTAL NUMBER NEEDED TO TREAT IN FIRST YEAR FOR VARYING POPULATION SIZE, TREATING ALL.
Mean total number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot. In these alternative scenario, we assumed a population of 6,500 and 17,000 individuals.
SUPPLEMENTARY FIGURE S11. INITIAL NUMBER NEEDED TO TREAT IN FIRST YEAR FOR VARYING GENOTYPE 3 PREVALENCE, TREATING ALL.
Mean number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot. In these alternative scenario, we assumed a prevalence of genotype 3 of 0%, 25% and 50%.
SUPPLEMENTARY FIGURE S12. TOTAL NUMBER NEEDED TO TREAT IN FIRST YEAR FOR VARYING GENOTYPE 3 PREVALENCE, TREATING ALL.
Mean total number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot. In these alternative scenario, we assumed a prevalence of genotype 3 of 0%, 25% and 50%.
SUPPLEMENTARY FIGURE S13. ANNUAL NUMBER OF TREATED PWID AND EX-PWID.
Funding acknowledgements:
This study was supported by Gilead Sciences. Gilead Sciences had no influence on the design, analysis, and content of the study. NKM acknowledges support from NIAID and NIDA (R01AI147490) and was additionally supported by the University of San Diego Center for AIDS Research (CFAR), a NIH funded program (P30 AI036214). AC acknowledges funding from the National Institute of Health (AI131971-01, AI036214) and from the University of San Diego CFAR.
Footnotes
Compliance and Ethical Requirements: Natasha Martin has received unrestricted research grants and honoraria from Gilead and Merck. Antoine Chaillon, Prem Harichander Thurairajah, and John Hsiang declare have received restricted research grant from Gilead Sciences.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
REFERENCES
- 1.Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–8. Epub 2016/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill AM, Nath S, Simmons B. The road to elimination of hepatitis C: analysis of cures versus new infections in 91 countries. J Virus Erad. 2017;3(3):117–23. Epub 2017/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JE. A study on the epidemiology of hepatitis C infection among blood donors in Singapore. J Public Health Med. 1995;17(4):387–91. Epub 1995/12/01. [PubMed] [Google Scholar]
- 4.Kuperan P, Choon AT, Ding SH, et al. Prevalence of antibodies to hepatitis C virus in relation to surrogate markers in a blood donor population of Singapore. Southeast Asian J Trop Med Public Health. 1993;24 Suppl 1:127–9. Epub 1993/01/01. [PubMed] [Google Scholar]
- 5.Winslow M, Subramaniam M, Ng WL, et al. Seroprevalence of hepatitis C in intravenous opioid users presenting in the early phase of injecting drug use in Singapore. Singapore medical journal. 2007;48(6):504–8. Epub 2007/06/01. [PubMed] [Google Scholar]
- 6.Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database of Systematic Reviews. 2017(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ministry of Home Affairs CNB, Singapore. Demographic Profile of Drug Abusers. 2019. [04/2019]; Available from: https://data.gov.sg/dataset/demographic-profile-of-drug-abusers?resource_id=7bb2d879-c0b7-4887-af83-3486bbcd0b0c. [Google Scholar]
- 8.Tan EK, Goh BKP, Lee SY, et al. Liver Transplant Waitlist Outcomes and the Allocation of Hepatocellular Carcinoma Model for End-Stage Liver Disease Exception Points at a Low-Volume Center. Transplant Proc. 2018;50(10):3564–70. Epub 2018/12/24. [DOI] [PubMed] [Google Scholar]
- 9.Lee YA, Friedman SL. Reversal, maintenance or progression: what happens to the liver after a virologic cure of hepatitis C? Antiviral research. 2014;107:23–30. Epub 2014/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serfaty L Follow-up of patients with chronic hepatitis C and a sustained viral response. Liver international : official journal of the International Association for the Study of the Liver. 2016;36 Suppl 1:67–71. Epub 2016/01/05. [DOI] [PubMed] [Google Scholar]
- 11.Teo AKJ, Prem K, Chen MIC, et al. Estimating the size of key populations for HIV in Singapore using the network scale-up method. Sexually transmitted infections. 2019. Epub 2019/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiang JC, Sinnaswami P, Yok Lee M, et al. A cluster randomized trial of point-of-care hepatitis C screening with direct access referral to improve linkage of care among people with substance misuse. Singapore medical journal. 2019;Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13(1):34–41. Epub 2005/12/21. [DOI] [PubMed] [Google Scholar]
- 14.Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67(10):1477–92. Epub 2018/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–609. Epub 2013/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornish R, Macleod J, Strang J, et al. Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. BMJ. 2010;341:c5475. Epub 2010/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns L, Randall D, Hall WD, et al. Opioid agonist pharmacotherapy in New South Wales from 1985 to 2006: patient characteristics and patterns and predictors of treatment retention. Addiction (Abingdon, England). 2009;104(8):1363–72. Epub 2009/06/25. [DOI] [PubMed] [Google Scholar]
- 18.Nosyk B, Marsh DC, Sun H, et al. Trends in methadone maintenance treatment participation, retention, and compliance to dosing guidelines in British Columbia, Canada: 1996–2006. Journal of substance abuse treatment. 2010;39(1):22–31. Epub 2010/04/27. [DOI] [PubMed] [Google Scholar]
- 19.Wong YJ, Cheen MH, Hsiang JC, et al. Economic evaluation of direct-acting antivirals for the treatment of genotype 3 hepatitis C infection in Singapore. JGH Open. 2019;3(3):210–6. Epub 2019/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanwal F, Kramer JR, Ilyas J, et al. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60(1):98–105. Epub 2014/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Probst A, Dang T, Bochud M, et al. Role of hepatitis C virus genotype 3 in liver fibrosis progression--a systematic review and meta-analysis. J Viral Hepat. 2011;18(11):745–59. Epub 2011/10/14. [DOI] [PubMed] [Google Scholar]
- 22.Lim AG, Qureshi H, Mahmood H, et al. Curbing the hepatitis C virus epidemic in Pakistan: the impact of scaling up treatment and prevention for achieving elimination. Int J Epidemiol. 2018. Epub 2018/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Thurairajah PH, Kumar R, et al. Novel non-invasive score to predict cirrhosis in the era of hepatitis C elimination: A population study of ex-substance users in Singapore. Hepatobiliary & pancreatic diseases international : HBPD INT. 2019;18(2):143–8. Epub 2018/12/19. [DOI] [PubMed] [Google Scholar]
- 24.Soh BYM, Kumar R, Ekstrom VSM, et al. Prevalence of hepatitis C virus infection and the IL28B genotype polymorphism among blood donors and high-risk populations. Singapore medical journal. 2019;60(1):34–9. Epub 2018/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong YJ, Manejero F, Lim K, et al. The impact of universal access to DAA and the real-world treatment outcome amongst genotype 3 hepatitis C virus-infected prisoners. . EASL2020. [Google Scholar]
- 26.Government of Singapore MoF. Singapore Budget 2019. 2019. [Google Scholar]
- 27.Lee CE. Tackling Subutex abuse in Singapore. Singapore medical journal. 2006;47(11):919–21. Epub 2006/11/01. [PubMed] [Google Scholar]
- 28.Hajarizadeh B, Grebely J, McManus H, et al. Chronic hepatitis C burden and care cascade in Australia in the era of interferon-based treatment. J Gastroenterol Hepatol. 2017;32(1):229–36. Epub 2016/05/21. [DOI] [PubMed] [Google Scholar]
- 29.Government of Singapore MoHA. Recidivism Rates Report. 2019. [Google Scholar]
- 30.Trickey A, Fraser H, Lim AG, et al. Modelling the potential prevention benefits of a treat-all hepatitis C treatment strategy at global, regional, and country levels: a modelling study. J Viral Hepat. 2019. Epub 2019/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin NK, Hickman M, Hutchinson SJ, et al. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57 Suppl 2:S39–45. Epub 2013/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singapore Ministry of Health. THE INDEPENDENT REVIEW COMMITTEE REPORT - Executive Summary. 2018. [Google Scholar]
- 33.Farrell M, Martin NK, Stockings E, et al. Responding to global stimulant use: challenges and opportunities. The Lancet. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY TABLE S1. Model parameters inputs and sources used in the model.
SUPPLEMENTARY FIGURE S1. Schematic of HCV transmission. PWID who spontaneously clear their infection remain in the susceptible compartment (if never infected) or their current liver disease stage (if cured). (A) Population stratification by PWID and OST status. (B) Population stratification by HCV status.
SUPPLEMENTARY FIGURE S2. Model projections with no scale-up of treatment.
A. Total population of person with HIV (PWID) and ex-PWID
B. Number of Individuals Chronically infected with HCV
C. Annual HCV Related Deaths
D. Annual Number of Incident HCV Cases
E. Incidence Rate among All Individuals
F. Incidence Rate among PWID
SUPPLEMENTARY FIGURE S3. Initial number needed to treat in first year with continued reductions in new PWID from 2018. In this scenario, we assumed that the historical reduction by ~30%/year in the inflow of injectors from 2012 to 2018 continued after 2018 while in our baseline scenario, we assumed that the inflow was stable thereafter.
SUPPLEMENTARY FIGURE S4. Total number needed to treat over 11 years with continued reductions in new PWID from 2018. In this scenario, we assumed that the historical reduction by ~30%/year in the inflow of injectors from 2012 to 2018 continued after 2018 while in our baseline scenario, we assumed that the inflow was stable thereafter.
SUPPLEMENTARY FIGURE S5. INITIAL NUMBER NEEDED TO TREAT IN FIRST YEAR WITH 85% AND 90% SUSTAINED VIROLOGICAL RESPONSE (SVR) RATE
Star: WHO 2030 target not achieved. Mean number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot. In this alternative scenario, we assumed a SVR rate of 85% and 90% respectively (compared to 95% in our baseline model).
SUPPLEMENTARY FIGURE S6. TOTAL NUMBER NEEDED TO TREAT OVER 11 YEARS WITH 85% AND 90% SUSTAINED VIROLOGICAL RESPONSE (SVR) RATE
Star: WHO 2030 target not achieved. Mean total number of HCV PWID and/or ex-PWID needed to treat are indicated above each bar plot. In this alternative scenario, we assumed a SVR rate of 85% and 90% respectively (compared to 95% in our baseline model).
SUPPLEMENTARY FIGURE S7. INITIAL NUMBER NEEDED TO TREAT IN FIRST YEAR FOR A DURATION ON OST OF 18 MONTHS
Star: WHO 2030 target not achieved. Mean number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot. In this alternative scenario, we assumed a mean duration on OST of 18 months (compared to 7 months in our baseline model).
SUPPLEMENTARY FIGURE S8. TOTAL NUMBER NEEDED TO TREAT OVER 11 YEARS FOR A DURATION ON OST OF 18 MONTHS
Star: WHO 2030 target not achieved. Mean total number of HCV PWID and/or ex-PWID needed to treat are indicated above each bar plot. In this alternative scenario, we assumed a mean duration on OST of 18 months (compared to 7 months in our baseline model).
SUPPLEMENTARY FIGURE S9. INITIAL NUMBER NEEDED TO TREAT IN FIRST YEAR FOR VARYING POPULATION SIZE, TREATING ALL.
Mean number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot. In these alternative scenario, we assumed a population of 6,500 and 17,000 individuals.
SUPPLEMENTARY FIGURE S10. TOTAL NUMBER NEEDED TO TREAT IN FIRST YEAR FOR VARYING POPULATION SIZE, TREATING ALL.
Mean total number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot. In these alternative scenario, we assumed a population of 6,500 and 17,000 individuals.
SUPPLEMENTARY FIGURE S11. INITIAL NUMBER NEEDED TO TREAT IN FIRST YEAR FOR VARYING GENOTYPE 3 PREVALENCE, TREATING ALL.
Mean number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot. In these alternative scenario, we assumed a prevalence of genotype 3 of 0%, 25% and 50%.
SUPPLEMENTARY FIGURE S12. TOTAL NUMBER NEEDED TO TREAT IN FIRST YEAR FOR VARYING GENOTYPE 3 PREVALENCE, TREATING ALL.
Mean total number of HCV PWID and/or ex-PWID needed to treat in the first year are indicated above each bar plot. In these alternative scenario, we assumed a prevalence of genotype 3 of 0%, 25% and 50%.
SUPPLEMENTARY FIGURE S13. ANNUAL NUMBER OF TREATED PWID AND EX-PWID.


