Purpose
This prospective study was aimed at assessing the ability of 99mTc-PSMA scan to detect bone metastases in prostate cancer (PCa) against 99mTc-MDP scan as a standard and assess the correlation of these modalities in PCa staging of bone involvement.
Patients and Methods
Forty-one patients (41) with histologically confirmed PCa were scanned using both methods. Planar imaging was performed with additional regional SPECT/CT 3 to 4 hours posttracer injection. Scans were reported as positive, negative, or equivocal. In the case of positive scans, lesions were quantified by each of the 3 reporters separately. Planar and SPECT/CT images were reported together to obtain the final report on each scan.
Results
Our preliminary results showed no significant difference in the detection of bone metastases between the 2 scans. 99mTc-PSMA detected 52 of the 55 bone lesions detected on 99mTc-MDP. However, 99mTc-PSMA provided extra information by reporting lymph nodal metastases in 7 patients and residual disease in the prostate in 2 patients with biochemical progression after radical therapy. In 1 patient, the PSMA scan resulted in change in management with patient now on 177Lu-PSMA radioligand therapy. Equivocal findings were reported in 4 patients on 99mTc-MDP and none on 99mTc-PSMA.
Conclusions
99mTc-PSMA was comparable to 99mTc-MDP in detection of bone metastases and demonstrated an additional benefit of providing information on visceral disease. 99mTc-PSMA may be a better alternative to 99mTc-MDP in staging, restaging, and assessment of patients with biochemical progression after radical therapy of PCa in a resource-limited setup like ours while also assisting to detect patients eligible for PSMA-labeled radioligand therapy.
Key Words: PSMA scan, bone scan, SPECT/CT, urology, radioligand therapy
Prostate cancer (PCa) is the most common noncutaneous cancer in men with a lifetime risk of 12.5%.1 Although only 6% of men with PCa have metastatic disease at diagnosis, 90% of men who die of PCa have metastatic disease to bone.2 The high rate of bone metastases has led to the incorporation of bone imaging in most published national and international treatment guidelines.3–6 Imaging of bone metastases in PCa has traditionally involved the use of the bone-seeking 99mTc-MDP, which is highly sensitive for detection of bone lesions.7 99mTc-labeled prostate-specific membrane antigen (PSMA) is a newer agent used for imaging in PCa. It has the advantage of being able to demonstrate both visceral and bone lesions.8,9 To our knowledge, there is currently only 1 study comparing the sensitivity of 99mTc-MDP bone scan with that of 99mTc-PSMA in detecting bone metastases in PCa.8
99mTc-MDP bone scintigraphy is the cornerstone of skeletal nuclear medicine imaging and has been regarded as the standard of reference in detection of bone metastases in PCa patients.10 99mTc-MDP is a bisphosphonate derivative, which localizes within the hydroxyapatite portion of the bony matrix by chemical adsorption (chemisorption).11 It is highly sensitive, readily available, and cost-effective, and it has been the standard method for nuclear imaging of the skeletal system for decades.12 There is a need to affordably improve imaging of metastases in PCa as scintigraphy with 99mTc-MDP is associated with limited sensitivity in patients with low prostate-specific antigen (PSA),13,14 long PSA doubling time,15 lytic bone lesions,16 and in assessing biochemical progression after radical prostatectomy.17
Modern clinical management of PCa increasingly relies on exploiting the PSMA as a molecular target both for imaging and for treatment of PCa.6,18–21 PSMA is a type II integral membrane glycoprotein with an intracellular component, a transmembrane component, and a large extracellular domain.22 99mTc-PSMA is able to detect both soft tissue and skeletal metastases, and it has been reported in a study by Rathke et al8 that 99mTc-PSMA scintigraphy demonstrates a reduction of the number of equivocal findings in comparison to 99mTc-MDP bone scan. The limitation to the available literature is that, in many instances, a comparison is made between PET/CT and SPECT/CT.23–26 The higher spatial resolution on PET27,28 is a confounding factor, which this study eliminated by comparing the 2 tracers using SPECT/CT imaging. The SPECT/CT-to-SPECT/CT comparison in this study is also important because many centers in our setting can only afford SPECT/CT scanners due to the high cost of PET/CT scanners and tracers.
The aim of this prospective study was to compare the detection rate of bone metastases of 99mTc-PSMA to that of 99mTc-MDP in PCa and correlate the findings with patient factors such as age, disease stage, Gleason score, and PSA.
To achieve this, male patients with PCa in KwaZulu-Natal were recruited to do both scans within a period of 28 days. The scans were scrutinized for the comparative detection rate of positive, equivocal, and negative findings by experienced certified nuclear medicine physicians overall having more than 7 years’ experience in the field.
PATIENTS AND METHODS
Forty-one male patients referred for staging/restaging of PCa with either 99mTc-MDP or 99mTc-PSMA were recruited for the second scan to be performed within 28 days of the first. Participants underwent both scans with whole-body and regional SPECT/CT scans. The scans were performed within a mean time interval of 21 days of each other. The study was undertaken in the Nuclear Medicine Department of Inkosi Albert Luthuli Central Hospital in KwaZulu-Natal. Patients with histologically confirmed PCa, regardless of disease stage and prior interventions, were included. All patients signed a written informed consent form. The BREC (Biomedical Research Ethics Committee) approved this evaluation (BREC reference number BE381/19).
Radiopharmaceuticals
The labelling of 99mTc was done using the supplier-provided HYNIC-IPSMA ligand kit according to the provided protocol. Quality control (QC) for 99mTc-labeled PSMA was performed by thin-layer chromatography, and the only radiopharmaceutical with radiochemical purity of greater than 95% was used. For 99mTc-MDP, the QC is performed by the suppliers, and only kits that have passed QC are supplied to the departments.
Imaging Protocol
For the 99mTc-MDP scan, each candidate was injected with a standard dose of 740 MBq (20 mCi) of 99mTc-MDP followed by 3 to 4 hours postinjection delayed whole-body imaging. The patients were imaged in the supine position on the camera bed with both arms along the sides and feet slightly internally rotated. Camera matrix size was set at 256 × 1024 for both detectors, and the zoom was set 1.00 and image processing was done with planar enhancement processor at 30% enhancement. SPECT/CT imaging was done in selected regions of interest determined by the tracer uptake on the planar/whole-body images. Regional SPECT/CTs were matched in both scans, and the region of interest was guided by the first scan in sequence. For CT, 3.0-mm slices with an extended field of view (650) were used (iterative reconstruction was used for both SPECT and CT).
For 99mTc-PSMA scan, a standard dose of 555 MBq (15 mCi) of 99mTc-PSMA was injected intravenously followed by delayed 3 to 4 hours imaging. Whole-body planar imaging and regional SPECT/CT scans were obtained as above. The patients positioning and camera settings were the same as that of 99mTc-MDP scan. In addition to the above regional SPECT/CT, 99mTc-PSMA scans had a mandatory pelvic SPECT/CT scans for assessment of the prostatic bed and lymph nodes.
Reporting of Findings
The studies were evaluated by 3 experienced certified nuclear medicine physicians to evaluate the number, location, and characteristics of the skeletal metastases. All the scans were anonymized and reviewed by each physician independently to avoid subjective bias. Concordance in discrepant results was achieved by consensus. The findings were reported as positive, negative, or equivocal. Uptake was considered to be positive for metastasis if it was seen in an area less likely to be due to trauma, contamination, or degenerative, widespread pattern and/or having typical sclerotic/lytic changes on CT. Uptake was considered to be negative for metastases if it localized to areas of benign change or contamination. Uptake was considered to be equivocal for metastasis if it localized to areas that did not display typical benign or metastatic changes. In the case of positive findings, the total number of identified lesions was recorded. The CT was used (fused with a SPECT) for further characterization of equivocal findings to determine the likelihood of metastasis. In addition, results were correlated with age, disease stage, serum PSA, and Gleason score.
Best Valuable Comparator
To assess the performance of the 2 scans independently, a criterion standard would be required. Owing to ethical and practical reasons, bone histology was not performed as the criterion standard, and a best valuable comparator (BVC) was used as a standard of comparison. The BVC was defined as in previous investigations,8,23,25 using a combination of all available information including 99mTc-PSMA and 99mTc-MDP bone scans (initial and interval scans), SPECT/CT, PET/CTs, CT scans, and clinical data.
Statistical Analysis
Patient demographic characteristics were summarized using descriptive statistics. Furthermore, testing the differences in mean (±SD) number of lesions observed on 99mTc-MDP to that of 99mTc-PSMA was performed using exact Wilcoxon matched pairs signed rank test. To accommodate the high frequency of zero lesions among several patients in the study, a zero-inflated negative binomial regression was used to model the association between the number of soft tissue metastases on 99mTc-PSMA and several patient-related factors. Furthermore, a logistic model was used to determine factors associated with 99mTc-PSMA uptake in the prostate gland. Sensitivity, specificity, and area under the curve (AUC) were determined by receiver operating characteristics for both 99mTc-MDP and 99mTc-PSMA, and their 95% confidence interval (CI) were calculated binomial exact. Comparisons of AUC were performed by using the DeLong method.29 Data analysis was performed using Stata IC 15 (Stata Statistical Software Release 15; StataCorp LLC, College Station, TX).
RESULTS
A total of 41 patients were included in the study. Three patients were excluded from analysis due to having unquantifiable diffuse metastases (albeit matching). Table 1 shows a summary of selected patient-related characteristics of the 38 patients. The median age of the participants was 68.5 years (interquartile range [IQR], 11), and the median age at diagnosis was 67 years (IQR, 11). The stage of cancer diagnosis for most patients (n = 13, 36.11%) was 3A, and the median PSA was 28.95 ng/mL (IQR, 72) at the time of the study. On biopsy, 55.26% (n = 21) of the patients had a Gleason score of 7 (intermediate risk), whereas the 26.32% (n = 10) had a Gleason score of 8 to 10 (high risk). In addition, the mean time interval between the taking of PSA level and the first scan was 5.5 month (±5.41). The median time was 4 months (IQR, 5 months) with the maximum interval time being 20 months and the minimum being 1 month. On the other hand, the mean time between 99mTc-MDP and 99mTc-PSMA scans was 22 days (±20.8). The median time was 18 days (IQR, 10 days) with the maximum interval time being 96 days and the minimum being 4 days.
TABLE 1.
Selected Patient Characteristics
| Patient Characteristics | Frequency (n) | % |
|---|---|---|
| Age category | ||
| 46–56 y | 4 | 10.53 |
| 57–67 y | 13 | 34.21 |
| 68–78 y | 19 | 50 |
| 79–89 y | 2 | 5.26 |
| Age at diagnosis | ||
| 45–55 y | 3 | 9.68 |
| 56–66 y | 12 | 38.71 |
| 67–77 y | 16 | 51.61 |
| Stage of cancer at diagnosis | ||
| 1 (unspecified) | 1 | 2.78 |
| 2 (unspecified) | 1 | 2.78 |
| 2A | 5 | 13.89 |
| 2B | 5 | 13.89 |
| 2C | 1 | 2.78 |
| 3 (unspecified) | 2 | 5.56 |
| 3A | 13 | 36.11 |
| 3B | 2 | 5.56 |
| 4 (unspecified) | 3 | 8.33 |
| 4A | 1 | 2.78 |
| 4B | 2 | 5.56 |
| PSA level | ||
| 1 (0–9.9 ng/mL) | 6 | 15.79 |
| 2 (10–20 ng/mL) | 10 | 26.32 |
| 3 (>20 ng/mL) | 22 | 57.89 |
| Gleason score | ||
| ≤6 (low risk) | 7 | 18.42 |
| 7 (intermediate risk | 21 | 55.26 |
| 8–10 (high risk) | 10 | 26.32 |
There were 38 patients assessed for bone metastases by 99mTc-MDP and 99mTc-PSMA. Of these, 13/38 (34.2%) patients were referred for primary staging, 16/38 (42.1%) for restaging, and 9/38 (23.6%) for biochemical progression after definitive therapy. Of the 9 patients referred for biochemical progression after definitive therapy, 8/25 had received a combination of pharmacotherapy, surgery, and radiotherapy, and only 1/25 reported having had a prostatectomy only. All the patients referred for restaging were on pharmacotherapy, and none of the patients received further therapy between the 2 scans. A cumulative total of 56 lesions were reported on BVC with 99mTc-MDP detecting 55/56 (98%) and 99mTc-PSMA detecting 52/56 (92%). On 99mTc-MDP, 4 lesions were classified as equivocal, and all these were reported as negative based on BVC. None of the equivocal MDP lesions was seen on 99mTc-PSMA, and there were no equivocal findings on 99mTc-PSMA at all. Based on the BVC, 11 patients (28.9%) were classified as having bone metastases, whereas 27 patients (71.1%) were classified as having no bone metastases. The mean number of lesions reported under BVC was 1.5 (±3.5). The maximum number of lesions was 16, whereas the minimum was zero. On 99mTc-MDP, 26.3% (n = 10) of the patients had bone metastases. The mean number of lesions observed on 99mTc-MDP was 1.45 (±3.4) (Table 2); the maximum was 15, whereas the minimum was zero. On the other hand, 23.68% (n = 9) of the patients had bone metastases under 99mTc-PSMA. The mean number of lesions observed under 99mTc-PSMA was 1.36 (±3.4).
TABLE 2.
Number of Lesions and Number of Patients With or Without Lesions Observed Under 99mTc-MDP and 99mTc-MDP
| No. Lesions | No. Patients | ||||
|---|---|---|---|---|---|
| Mean | SD | With Lesions | No Lesions | Equivocal | |
| BVC | 1.5 | 3.5 | 11 (28.95%) | 27 (71.05%) | 0 |
| 99mTc-PSMA | 1.36 | 3.4 | 9 (76.32%) | 29 (76.32%) | 0 |
| 99mTc-MDP | 1.45 | 3.4 | 10 (26.32%) | 28 (73.68) | 4 |
In a univariate logistical analysis, the study showed a relationship (P < 0.05) among the presence of bone metastases on MDP with cancer stage, PSA level, and no relationship (P > 0.05) with age and Gleason score. There was a relationship among bone metastases seen on 99mTc-PSMA with cancer stage, PSA level, and Gleason score. In a bivariate analysis, the study showed that there was no relationship (P > 0.05) among Gleason score, PSA level, age category, and age category at diagnosis with the number of soft tissue metastases on 99mTc-PSMA. However, further analysis in a zero-inflated negative binomial regression showed that Gleason score (coefficient, 1.81; 95% CI, 0.48–3.15), age at diagnosis (coefficient, 0.84; 95% CI, 0.17–1.52), and stage of the cancer (coefficient, 0.48; 95% CI, 0.20–0.76) had a positive influence on the number of soft tissue metastases on 99mTc-PSMA. Furthermore, the study showed that 99mTc-PSMA uptake in the prostate gland was influenced by the form of interventions used (OR, 0.28; 95% CI, 0.12–0.63). The study showed that the odds of 99mTc-PSMA uptake in the prostate gland among those who had mixed interventions (OR, 0.027; 95% CI, 0.002–0.371) were lower than those who had no intervention. On the other hand, there were no statistical differences in the 99mTc-PSMA uptake between those who had no intervention and those who had medical intervention (OR, 0.361; 95% CI, 0.032–3.962). The Hosmer-Lemeshow test used to assess the goodness-of-fit showed that the model was sufficiently specified (P = 0.831). There was no statistical difference in the rate of detection of bone lesions between 99mTc-MDP and 99mTc-PSMA based the different stages (P = 0.23) and risk as defined by Gleason score (P = 0.27) and PSA value (P = 0.42), with both scans detecting the most lesions in stage 4 disease, intermediate- and high-risk Gleason score group, and high-risk PSA group (Table 3). The rate of detection of bone lesions between the 2 methods was comparable regardless of the indication, that is, staging/restaging and biochemical progression after radical therapy (P = 0.66) (Table 3).
TABLE 3.
Number of Bone Lesions According to Patient Characteristics and Indications
| Patient Characteristics and Indications | No. Bone Lesions Seen on 99mTc-MDP Scan | No. Bone Lesions Seen 99mTc-PSMA Scan | Paired t test P |
|---|---|---|---|
| Initial stage | |||
| Stage 1–2 | 3 | 3 | |
| Stage 3 | 9 | 7 | |
| Stage 4 | 43 | 42 | |
| 0.225 | |||
| Gleason score | |||
| ≤6 (low risk) | 1 | 0 | |
| 7 (intermediate risk | 44 | 41 | |
| 8–10 (high risk) | 11 | 11 | |
| 0.27 | |||
| PSA | |||
| 1 (0–9.9 ng/mL) | 0 | 0 | |
| 2 (10–20 ng/mL) | 1 | 1 | |
| 3 (>20 ng/mL) | 54 | 51 | |
| 0.42 | |||
| Indication | |||
| Primary staging/restaging | 32 | 28 | |
| Biochemical progression after radical therapy | 23 | 24 | |
| 0.66 |
Correlation Between 99mTc-MDP and 99mTc-PSMA
A Wilcoxon matched pairs signed rank test between mean number of lesions observed under 99mTc-MDP and 99mTc-PSMA showed no statistical difference (z = 1.63, P = 0.103). Based on the BVC as a standard of reference, there was no significant difference (P = 0.317) in sensitivity between 99mTc-MDP (90.91%, SE = 4.5%) and 99mTc-PSMA (81.82%, SE = 6.1%) in the detection of bone lesions.
Sensitivity and Specificity Analysis
In 38 patients involved in the overall bone lesion assessment by BVC, 27 patients (64%) were correctly classified as having no lesions, whereas 10 patients (24%) were also classified as having lesions under 99mTc-MDP. On the other hand, 1 patient classified as having bone lesions on BVC was not classified as such under 99mTc-MDP resulting in a detection of 10/11. Furthermore, analysis showed that, of the 27 patients classified as having no lesions under BVC, 4 patients were classified as equivocal under 99mTc-MDP. The sensitivity of 99mTc-MDP SPECT/CT was 90.91%, and the specificity was 100%. Receiver operating characteristics analysis revealed an accuracy measured as AUC of 0.95% (95% CI, 0.86%–1%) for 99mTc-MDP (Table 3). Of the 11 patients classified as having bone metastases under BVC, 9 (23.6%) were correctly assigned as having bone metastases under 99mTc-PSMA scan. There were no equivocal findings under 99mTc-PSMA. The sensitivity of 99mTc-PSMA SPECT/CT was 81.82%, and the specificity was 100%. Receiver operating characteristics analysis revealed an accuracy measured as AUC of 0.90.0% (95% CI, 0.79%–1%) (Table 4). The time interval among the scans that were negative on 99mTc-PSMA but positive on 99mTc-MDP was 9 days and 11 days, respectively.
TABLE 4.
Patient-Based Analysis of Lesions on 99mTc-MDP SPECT/CT and 99mTc-PSMA SPECT/CT
| 99mTc-MDP | 99mTc-PSMA | |
|---|---|---|
| Sensitivity | 90.91% | 81.82% |
| Specificity | 100% | 100% |
| AUC | 0.955 | 0.909 |
| SE | 0.045 | 0.061 |
| 95% CI | 0.865–1 | 0.789–1 |
DISCUSSION
When correlating the mean number of bone lesions detected by 99mTc-MDP bone scan with that of 99mTc-PSMA scan in detection of bone metastases in PCa patients, it was determined that there was no statistically significant difference between the 2 tracers (z = 1.63, P = 0.103). 99mTc-PSMA detected 52/55 (94.5%) of the lesions seen on 99mTc-MDP bone scan. The discordance seen in the 3/55 lesions not picked up on 99mTc-PSMA but reported on Tc-MDP bone scan is unusual. Possible reasons considered for this discordance include false-positive report on 99mTc-MDP considering that it is noted to have low specificity with uptake seen in benign pathology,30–32 the possibility that these lesions were in the healing phase as 99mTc-MDP is known to remain positive for a as long as 6 months after resolution of skeletal metastases,33,34 or else a PSMA-negative tumor phenotype could be an alternative explanation.35,36 Based on the BVC as a standard of reference, the study still demonstrated no significant difference (P = 0.317) in sensitivity between 99mTc-MDP and 99mTc-PSMA in the detection of bone lesions, with 99mTc-PSMA demonstrated a sensitivity of 81.82% and specificity of 100% compared with 99mTc-MDP bone scan with a sensitivity of 90.9% and specificity of 100%. The time interval among the scans, which were negative on 99mTc-PSMA but positive on 99mTc-MDP, was 9 days and 11 days, respectively. A study done by Rathke et al8 reported superior detection of bone metastases on 99mTc-PSMA with a sensitivity and specificity of 92% and 90% compared with 99mTc-MDP at 76% and 86%, respectively. The difference between this and our study could be due to the smaller sample size in our study and the nonstandardization of the BVC. The specificity of bone scan reported in this study is unusually high, and this was thought due to the small sample size with only 11 patients having metastases that were analyzed; then, the final interpretation of the scan involved the use of SPECT/CT in any foci that did not demonstrate typical findings resulting in the elimination of potential false-positives (Fig. 1).37 As observed in other studies,8 some 99mTc-MDP scans demonstrated equivocal findings, especially in areas more commonly associated with degenerative change while none were observed on the 99mTc-PSMA scan (Fig. 2).
FIGURE 1.
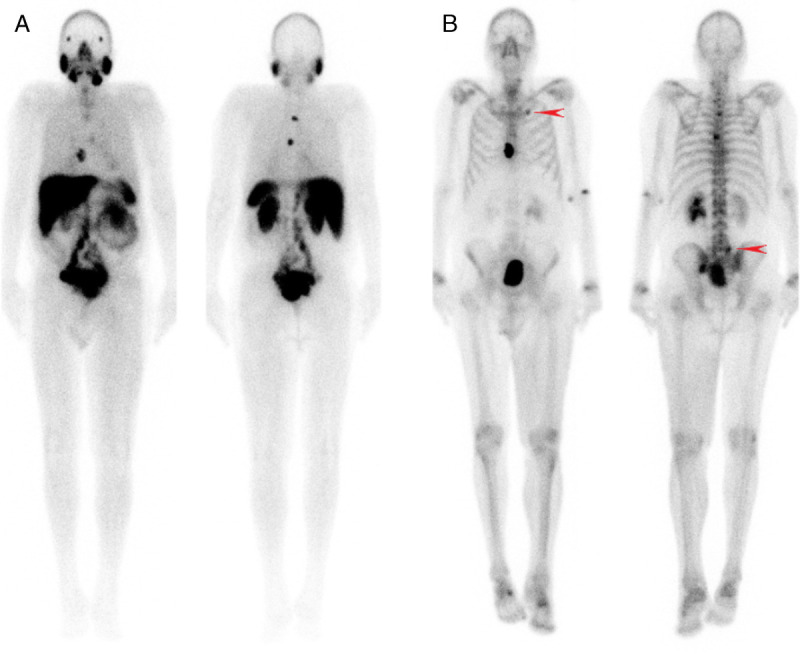
Planar 99mTc-PSMA (A) and 99mTc-MDP (B) demonstrating 2 matching typical bone lesions in the thoracic spine and sternum. However, 99mTc-MDP (B) also demonstrates uptake in areas of degenerative changes (arrowhead), requiring SPECT/CT to increase specificity.
FIGURE 2.
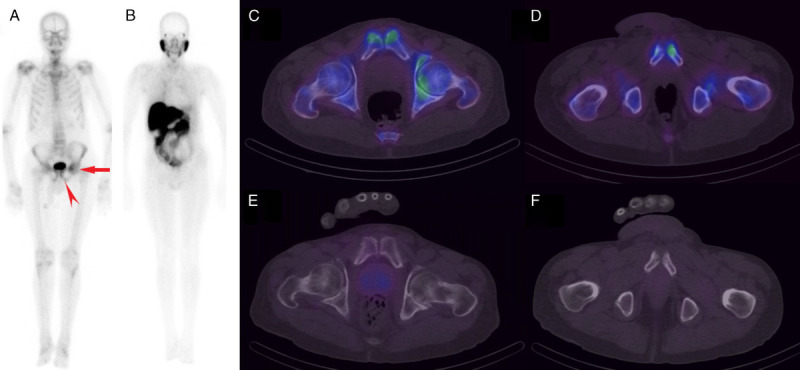
Whole-body 99mTc-MDP scan (A) showing asymmetrically increased uptake in the left pubis (arrowhead) and iliac region (arrow), whereas no obvious increased uptake is seen in the corresponding areas on 99mTc-PSMA scan (B). SPECT/CT through 2 different slices highlighting the above changes localizes the iliac findings to the left femoroacetabular joint region (C) in keeping with degenerative change. The focal uptake seen in the left pubis (D) localizes to an area of sclerosis adjacent to the pubic symphysis. This was of concern for metastasis; however, degenerative change was also a consideration due to the proximity to the joint (rendering the finding equivocal). On 99mTc-PSMA SPECT/CT scans (E and F), there was no pathological uptake in the corresponding areas to suggest osseous metastases.
Many studies comparing PSMA to 99mTc-MDP have used PMSA-labeled PET tracers such as 18F-DCFPyL PET/CT38 and 68Ga.25,39 These studies also reported a higher sensitivity in PSMA-labeled PET tracers compared with 99mTc-MDP, with sensitivity ranging from 96% to 100% and that of MDP being as low as 73%. One of the limitations of comparing our study to the above is the use of different imaging modalities with different resolutions (superior resolution on PET/CT27,28). However, the good detection rate of bone metastases by 99mTc-PSMA reported by this study and Rathke et al8 makes a good argument for the use of 99mTc-PSMA in areas that have no access to PET scan. Further to this, studies comparing 99mTc-PSMA SPECT/CT to 68Ga-PSMA PET/CT have demonstrated how 99mTc-PSMA SPECT/CT could be a potential substitute for 68Ga-PSMA PET/CT.9,40
A similar study comparing the 2 tracers focused on patients with known bone metastases,8 whereas our study included both new patients and patients being followed up. The risk profile in our study included low-, intermediate-, and high-risk patients. Although the use of 99mTc-PSMA is mostly reserved for detection of occult disease in patients with low PSA41,42 and primary staging of high-risk patients43,44 and bone scan to intermediate- to high-risk patients,12 we were also interested in seeing how 99mTc-PSMA performs in patients across the risk spectrum, including patients typically reserved for staging bone scan.6 Thomsen et al45 reported a correlation between advanced disease stage and high PSA with bone metastases, and we report similar findings with patients having advanced disease stage and a high PSA more likely to have metastases on both 99mTc-PSMA and 99mTc-MDP scans and with the 2 methods having no statistically significant difference in detection rate (Table 3). In a study involving 106 PCa patients, Al-Ghazo et al46 reported that PSA level >20 ng/mL and Gleason score >7 were independently predictive of positive bone scan. In our study, 98% of the bone lesions were observed in patients with PSA level >20 ng/mL and Gleason score >7. Of the patients referred for staging or restaging, only those with intermediate to high PSA levels demonstrated bone lesions. Interestingly, patients referred for biochemical progression after radical therapy had intermediate to high PSA and demonstrated comparable number of lesions on both scans (Table 3).
According to a report by Bechis et al,47 with increasing age, men were significantly more likely to have high-risk PCa, this study demonstrated that age at diagnosis had a positive influence on the presence of soft tissue metastases on 99mTc-PSMA with a mean age at diagnosis of 69.2.
99mTc-PSMA scan detected soft tissue metastases to the lymph nodes in 7 patients; of these, 3 patients also had bone metastases and the 99mTc-PSMA scan detected the same number of bone lesions as 99mTc-MDP bone scan. This is an important finding as it demonstrates the ability of 99mTc-PSMA to give extra information on soft tissue disease without compromising on the bone findings. Uptake of 99mTc-PSMA in the prostate was seen in 28 patients. This was an important finding in 2 of these patients referred for biochemical progression after radical therapy as these findings represent either residual disease or recurrence and thus explained the nonresolving PSA (Fig. 3). In 26/28 patients, the uptake in the prostate was nonspecific because they did not have a history of prostatectomy. The lack of specificity is due to the normal uptake of 99mTc-PSMA expected even in nonmalignant prostate glands. 99mTc-PSMA also demonstrated superiority in determining the significance of equivocal findings seen on bone scan.
FIGURE 3.
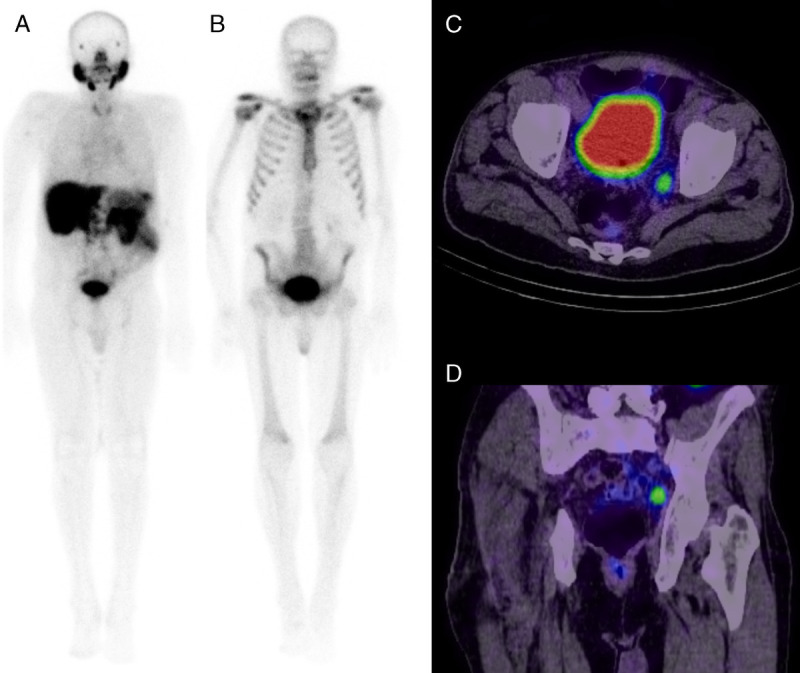
Whole-body planar 99mTc-PSMA (A) and 99mTc-MDP (B) images negative for bone metastases in a patient with a raised PSA. 99mTc-PSMA pelvic axial (C) and coronal (D) SPECT/CT demonstrate increased uptake in a left internal iliac lymph node in keeping with metastasis.
Some studies reported a mean interval between the 2 scans from as few as 10 days8 and others as many as 80 days.48 Our target interval was a maximum of 28 days, and we achieved a mean of 21 days. Our decision for this method was due to the impracticality of shorter intervals and the concern for disease change in longer intervals. It is interesting to note that, despite the differences in the mean interval between this study and that reported by investigators with fewer days,8 the findings are comparable.
The findings in our study make a good argument for the use of 99mTc-PMSA as an alternative to 99mTc-MDP for both staging and follow-up in patients who are not for palliative radioligand bone therapy. Therefore, in resource-limited situations where a patient can only get a single scan (such as our setup where patients who live far from the center may not afford to travel for multiple scans), it would be more beneficial if that scan was a 99mTc-PSMA regardless of the stage.
Among the limitations of this study was a lack of bone histology as the criterion standard. This has the potential of having some false-positive results on both scans. The smaller sample size has the potential to shift results away from what is expected in the represented population. The 3 patients who demonstrated diffuse disease were analyzed separately because their lesions were not quantifiable due to their diffuse extent. The findings in diffuse bone disease were comparable between the 2 tracers. The detection of 99mTc-PSMA–avid disease resulted in recommending 177Lu-PSMA radioligand therapy in a case of failed chemotherapy.
When taken beyond the context of staging and assessment of the therapy response, the uptake seen on both scans does not always represent the same thing, especially when it comes to radioligand therapy where both still have a role in planning. Patients demonstrating intense 99mTc-PSMA uptake are good candidates for PSMA radioligand therapy such as 177Lu-PSMA,3,49 whereas those demonstrating reduced 99mTc-PSMA uptake, but increased MDP uptake, may benefit from radioligand palliative bone therapy.50 In these cases, the 2 scans cannot replace each other.
CONCLUSIONS
Our preliminary results show that the ability of 99mTc-PSMA to detect bone metastases in PCa is comparable to that of 99mTc-MDP but with the additional benefit of providing information on soft tissue disease in both early and advanced disease. Therefore, in patients who can only afford a single scan, 99mTc-PSMA scan would be a better choice. With regards to therapy, 99mTc-PMSA scan might have utility to select candidates for PSMA radioligand therapy such as 177Lu-PSMA, whereas 99mTc-bone scan may have similar utility in palliative radioligand bone therapy with bisphosphonates.
Footnotes
Conflicts of interest and sources of funding: none declared.
Contributor Information
Lerato Gabela, Email: leratogabela@icloud.com.
Chester Kalinda, Email: ckalinda@gmail.com.
Colleen Aldous, Email: aldousc@ukzn.ac.za.
Venesen Pillay, Email: venesenpillay@gmail.com.
Nozipho Nyakale, Email: n.nyakale@yahoo.com.
REFERENCES
- 1.American Cancer Society . Key Statistics for Prostate Cancer. Available at: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html#:~:text=Risk%20of%20prostate%20cancer,rare%20in%20men%20under%2040. Published 2021. Updated January 12, 2021. Accessed March 16, 2021.
- 2.National Cancer Institute . Cancer Stat Facts: Prostate Cancer. Available at: https://seer.cancer.gov/statfacts/html/prost.html. Published 2020. Accessed March 16, 2021.
- 3.Kratochwil C Fendler WP Eiber M, et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. 2019;46:2536–2544. [DOI] [PubMed] [Google Scholar]
- 4.Römer W. Prostate cancer imaging and therapy, a technologist’s guide. Soc Nucl Med Imaging. 2018;27. Available at: https://www.eanm.org/content-eanm/uploads/2018/10/EANM_2018_TechGuide_ONLINE.pdf. Accesed May 30, 2021. [Google Scholar]
- 5.National Comprehensive Cancer Network (NCCN) . NCCN Guidelines for Prostate Cancer Version 2, February 2021. Plymouth Meeting, PA: nccn.org; 2020. [Google Scholar]
- 6.Mottet N Bellmunt J Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–629. [DOI] [PubMed] [Google Scholar]
- 7.Van den Wyngaert T Strobel K Kampen WU, et al. , EANM Bone & Joint Committee and the Oncology Committee. The EANM practice guidelines for bone scintigraphy. Eur J Nucl Med Mol Imaging. 2016;43:1723–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rathke H Afshar-Oromieh A Giesel FL, et al. Intraindividual comparison of 99m Tc-methylene diphosphonate and prostate-specific membrane antigen ligand 99m Tc-MIP-1427 in patients with osseous metastasized prostate cancer. J Nucl Med. 2018;59:1373–1379. [DOI] [PubMed] [Google Scholar]
- 9.Albalooshi B Al Sharhan M Bagheri F, et al. Direct comparison of 99mTc-PSMA SPECT/CT and 68Ga-PSMA PET/CT in patients with prostate cancer. Asia Ocean J Nucl Med Biol. 2020;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mettler FA, Guiberteau MJ. Essentials of Nuclear Medicine Imaging. 5th ed. Philadelphia, PA: Saunders Elsevier; 2006. [Google Scholar]
- 11.Ziessman HA, O’Malley JP, Thrall JH. Nuclear Medicine: The Requisites. 4th ed. Philadelphia, PA: Saunders Elsevier; 2014. [Google Scholar]
- 12.Chong A Hwang I Ha JM, et al. Application of bone scans for prostate cancer staging: which guideline shows better result? Can Urol Assoc J. 2014;8:E515–E519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Özgür BC Gültekin S Ekici M, et al. A narrowing range of bone scan in newly diagnosed prostate cancer patients: a retrospective comparative study. Urol Ann. 2015;7:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka N Fujimoto K Shinkai T, et al. Bone scan can be spared in asymptomatic prostate cancer patients with PSA of <=20 ng/ml and Gleason score of <=6 at the initial stage of diagnosis. Jpn J Clin Oncol. 2011;41:1209–1213. [DOI] [PubMed] [Google Scholar]
- 15.Okotie OT Aronson WJ Wieder JA, et al. Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. J Urol. 2004;171:2260–2264. [DOI] [PubMed] [Google Scholar]
- 16.Ozcan Kara P Kara T Kara Gedik G, et al. Comparison of bone scintigraphy and 18F-FDG PET-CT in a prostate cancer patient with osteolytic bone metastases. Rev Esp Med Nucl. 2011;30:94–96. [DOI] [PubMed] [Google Scholar]
- 17.Kane CJ Amling CL Johnstone PA, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61:607–611. [DOI] [PubMed] [Google Scholar]
- 18.Kiess AP Banerjee SR Mease RC, et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q J Nucl Med Mol Imaging. 2015;59:241–268. [PMC free article] [PubMed] [Google Scholar]
- 19.Lowrance WT Breau RH Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline PART II. J Urol. 2021;205:22–29. [DOI] [PubMed] [Google Scholar]
- 20.Schmidkonz C Cordes M Beck M, et al. SPECT/CT with the PSMA ligand 99mTc-MIP-1404 for whole-body primary staging of patients with prostate cancer. Clin Nucl Med. 2018;43:225–231. [DOI] [PubMed] [Google Scholar]
- 21.Reinfelder J Kuwert T Beck M, et al. First experience with SPECT/CT using a 99mTc-labeled inhibitor for prostate-specific membrane antigen in patients with biochemical recurrence of prostate cancer. Clin Nucl Med. 2017;42:26–33. [DOI] [PubMed] [Google Scholar]
- 22.Bouchelouche K, Choyke PL. PSMA PET in prostate cancer—a step towards personalized medicine. Curr Opin Oncol. 2016;28:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sathekge M Lengana T Maes A, et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate carcinoma: preliminary results on differences between Black and White South-Africans. Eur J Nucl Med Mol Imaging 2018;45:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuminaga Y Rothe C Kam J, et al. 68Ga-PSMA PET/CT versus CT and bone scan for investigation of PSA failure post radical prostatectomy. Asian J Urol. 2020. Available at: https://www.sciencedirect.com/science/article/pii/S2214388220300047. Accessed May 30, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyka T Okamoto S Dahlbender M, et al. Comparison of bone scintigraphy and (68)Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2114–2121. [DOI] [PubMed] [Google Scholar]
- 26.Simsek DH Sanli Y Civan C, et al. Does bone scintigraphy still have a role in the era of 68 Ga-PSMA PET/CT in prostate cancer? Ann Nucl Med. 2020;34:476–485. [DOI] [PubMed] [Google Scholar]
- 27.Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun. 2008;29:193–207. [DOI] [PubMed] [Google Scholar]
- 28.Spanoudaki VC, Ziegler SI. PET & SPECT instrumentation. Handb Exp Pharmacol. 2008;(185 Pt 1):53–74. [DOI] [PubMed] [Google Scholar]
- 29.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 30.Turpin A Girard E Baillet C, et al. Imaging for metastasis in prostate cancer: a review of the literature. Front Oncol. 2020;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen G Deng H Hu S, et al. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol. 2014;43:1503–1513. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J Gou Z Wu R, et al. Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a systematic review and meta-analysis. Skeletal Radiol. 2019;48:1915–1924. [DOI] [PubMed] [Google Scholar]
- 33.O’Sullivan GJ, Carty FL, Cronin CG. Imaging of bone metastasis: an update. World J Radiol. 2015;7:202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fogelman I. The flare phenomenon: still learning after 35 years. Eur J Nucl Med Mol Imaging. 2011;38:5–6. [DOI] [PubMed] [Google Scholar]
- 35.Noto B Auf der Springe K Huss S, et al. Prostate-specific membrane antigen-negative metastases—a potential pitfall in prostate-specific membrane antigen PET. Clin Nucl Med. 2018;43:e186–e188. [DOI] [PubMed] [Google Scholar]
- 36.Keidar Z Gill R Goshen E, et al. Positive and negative Ga68-PSMA PET/CT studies in prostate cancer patients with biochemical failure - comparison of patients’ characteristics. J Nucl Med. 2018;59(supplement 1):1448. Available at: https://jnm.snmjournals.org/content/59/supplement_1/1448.short. Accessed May 30, 2021. [Google Scholar]
- 37.Ahmadzadehfar H Marx C Ebert A, et al. Diagnostic accuracy of SPECT-CT in comparison to whole body scintigraphy and SPECT in patients with suspected osseous metastases: results of a two center study. J Nucl Med. 2012;53(supplement 1):336. Available at: https://jnm.snmjournals.org/content/53/supplement_1/336.short. Accessed May 30, 2021.22178626 [Google Scholar]
- 38.Rowe SP Mana-ay M Javadi MS, et al. PSMA-based detection of prostate cancer bone lesions with 18F-DCFPyL PET/CT: a sensitive alternative to (99m)Tc-MDP bone scan and Na18F PET/CT? Clin Genitourin Cancer. 2016;14:e115–e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zacho HD Ravn S Afshar-Oromieh A, et al. Added value of 68Ga-PSMA PET/CT for the detection of bone metastases in patients with newly diagnosed prostate cancer and a previous 99mTc bone scintigraphy. EJNMMI Res. 2020;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallahi B Khademi N Karamzade-Ziarati N, et al. 99mTc-PSMA SPECT/CT versus 68Ga-PSMA PET/CT in the evaluation of metastatic prostate cancer. Clin Nucl Med 2021;46:e68–e74. [DOI] [PubMed] [Google Scholar]
- 41.Eiber M Maurer T Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. [DOI] [PubMed] [Google Scholar]
- 42.Afshar-Oromieh A Zechmann CM Malcher A, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maurer T Gschwend JE Rauscher I, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–1443. [DOI] [PubMed] [Google Scholar]
- 44.Herlemann A Wenter V Kretschmer A, et al. 68Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. 2016;70:553–557. [DOI] [PubMed] [Google Scholar]
- 45.Thomsen FB Westerberg M Garmo H, et al. Prediction of metastatic prostate cancer by prostate-specific antigen in combination with T stage and Gleason grade: nationwide, population-based register study. PLoS One. 2020;15:e0228447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Ghazo MA Ghalayini IF Al-Azab RS, et al. Do all patients with newly diagnosed prostate cancer need staging radionuclide bone scan? A retrospective study. Int Braz J Urol. 2010;36:685–691; discussion 691–692. [DOI] [PubMed] [Google Scholar]
- 47.Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janssen J-C Meißner S Woythal N, et al. Comparison of hybrid 68Ga-PSMA-PET/CT and 99mTc-DPD-SPECT/CT for the detection of bone metastases in prostate cancer patients: additional value of morphologic information from low dose CT. Eur Radiol. 2018;28:610–619. [DOI] [PubMed] [Google Scholar]
- 49.Fendler WP Rahbar K Herrmann K, et al. (177)Lu-PSMA radioligand therapy for prostate cancer. J Nucl Med. 2017;58:1196–1200. [DOI] [PubMed] [Google Scholar]
- 50.Handkiewicz-Junak D Poeppel TD Bodei L, et al. EANM guidelines for radionuclide therapy of bone metastases with beta-emitting radionuclides. Eur J Nucl Med Mol Imaging. 2018;45:846–859. [DOI] [PMC free article] [PubMed] [Google Scholar]


