Abstract
Background
Arrhythmogenic cardiomyopathy (AC) is characterized by biventricular dysfunction, exercise intolerance, and high risk of ventricular tachyarrhythmias and sudden death. Predisposing factors for left ventricular (LV) disease manifestation and its prognostic implication in AC are poorly described. We aimed to assess the associations of exercise exposure and genotype with LV dysfunction in AC, and to explore the impact of LV disease progression on adverse arrhythmic outcome.
Methods and Results
We included 168 patients with AC (50% probands, 45% women, 40±16 years old) with 715 echocardiographic exams (4.1±1.7 exams/patient, follow‐up 7.6 [interquartile range (IQR), 5.4–10.9] years) and complete exercise and genetic data in a longitudinal study. LV function by global longitudinal strain was −18.8% [IQR, −19.2% to −18.3%] at presentation and was worse in patients with greater exercise exposure (global longitudinal strain worsening, 0.09% [IQR, 0.01%–0.17%] per 5 MET‐hours/week, P=0.02). LV function by global longitudinal strain worsened, with 0.08% [IQR, 0.05%–0.12%] per year; (P<0.001), and progression was most evident in patients with desmoplakin genotype (P for interaction <0.001). Deterioration of LV function predicted incident ventricular tachyarrhythmia (aborted cardiac arrest, sustained ventricular tachycardia, or implantable cardioverter defibrillator shock) (adjusted odds ratio, 1.1 [IQR, 1.0–1.3] per 1% worsening by global longitudinal strain; P=0.02, adjusted for time and previous arrhythmic events).
Conclusions
Greater exercise exposure was associated with worse LV function at first visit of patients with AC but did not significantly affect the rate of LV progression during follow‐up. Progression of LV dysfunction was most pronounced in patients with desmoplakin genotypes. Deterioration of LV function during follow‐up predicted subsequent ventricular tachyarrhythmia and should be considered in risk stratification.
Keywords: arrhythmogenic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, left ventricular dysfunction, ventricular arrhythmia
Subject Categories: Cardiomyopathy, Sudden Cardiac Death, Arrhythmias, Echocardiography, Genetics
Nonstandard Abbreviations and Acronyms
- AC
arrhythmogenic cardiomyopathy
- GLS
global longitudinal strain
- VTA
ventricular tachyarrhythmia
Clinical Perspective
What Is New?
Greater exercise exposure is associated with left ventricular (LV) dysfunction and is common in patients with arrhythmogenic cardiomyopathy.
Patients with arrhythmogenic cardiomyopathy with desmoplakin mutations have the most pronounced deterioration of LV function during follow‐up.
Deterioration of LV function during follow‐up can predict subsequent ventricular tachyarrhythmias.
What Are the Clinical Implications?
Exercise moderation at the time of diagnosis may not resolve LV dysfunction but may halt the disease progression and should be recommended to all patients with arrhythmogenic cardiomyopathy.
Close attention should be given to emerging signs of LV dysfunction in patients with desmoplakin mutations.
Deterioration of LV function during follow‐up should be recognized as a sign of increased risk of impending ventricular tachyarrhythmias.
Arrhythmogenic cardiomyopathy (AC) is an inheritable heart disease characterized by high risk of ventricular tachyarrhythmias (VTAs) and sudden cardiac death. The disease is primarily acquired by autosomal dominant inheritance of dysfunctional genes encoding cardiac desmosomal proteins, and it exhibits variable penetrance and expressivity. 1
AC has long been referred to as arrhythmogenic right ventricular cardiomyopathy because of the high prevalence of right ventricular (RV) disease manifestation, but left ventricular (LV) disease manifestation is increasingly recognized and has been reported to defer worse clinical prognosis. 2 , 3 Exercise exposure increases disease penetrance and risk of VTA, 4 , 5 , 6 and exercise restriction is recommended to all patients with AC and mutation‐positive family members. 7 However, the association between exercise exposure and LV disease manifestation is not known. Furthermore, it has been suggested that certain genetic mutations, for example, desmoplakin mutations, have more pronounced LV disease manifestation than others, 8 , 9 but no large longitudinal studies have assessed markers of progression of LV disease in patients with AC.
This study aimed to assess the association between recent exercise exposure and LV disease manifestation in patients with AC and to explore whether the disease trajectory is affected by exercise history and different genotypes. We hypothesized that greater exercise exposure would correlate with worse LV function and that progressive LV dysfunction would predict incident VTA.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. Consecutive patients with AC diagnosed at the Unit for Genetic Cardiac Diseases, Department of Cardiology, Oslo University Hospital Rikshospitalet, Oslo, Norway, were included in a longitudinal cohort study. Newly diagnosed patients and further repeated measures were included in the analysis that complemented a previously published database of 598 assessments in 144 patients. 10 The first diagnosed patient in a family was defined as the proband, and all probands fulfilled definite diagnosis according to the Task Force Criteria of 2010. 11 Consenting probands were tested for genetic variants known to be associated with AC, and family members of mutation‐positive probands underwent cascade genetic screening for which mutation‐positive family members were included. Results from clinical follow‐ups were continuously reported back to the genetics department (the Unit for Cardiac and Cardiovascular Genetics, Oslo University Hospital) to improve segregation analysis and continuous reevaluation of mutation pathogenicity. Probands with negative Sanger sequencing were reevaluated using high‐throughput screening when this became available. The pathogenicity of AC‐associated mutations was evaluated according to guidelines from the American College of Medical Genetics and Genomics and the Association for Molecular Pathology with a focus on segregation analysis and, if needed, supplementing functional studies. 12 Class 4 and 5 mutations were considered to be pathogenic. Patient with cardiopulmonary comorbidity were excluded. Patients were also excluded from analysis if their genetic mutation was later reclassified and determined not to be pathogenic.
Exercise exposure during the 3 years before inclusion was assessed by structured interviews, as described previously, 4 and expressed as average metabolic equivalent task hours per week (MET‐ hours/week). In agreement with past and present recommendations, all patients were recommended exercise restriction at the time of enrollment. 13 , 14
All patients provided written informed consent. The study was approved by the Regional Committee for Ethics in Health Research in southeastern Norway and complied with the Declaration of Helsinki.
Cardiac Imaging
Echocardiography is the preferred cardiac imaging modality during follow‐up of patients with AC in our clinic. Comprehensive scans were performed at the time of inclusion in all patients and regularly during follow‐up at our cardiomyopathy clinic (Vivid 7, E9 or E95, subsequently analyzed offline on EchoPac version 202, GE Vingmed, Horten, Norway).
LV ejection fraction (EF) was measured by the biplane Simpson method, and global longitudinal strain (GLS) was assessed by speckle tracking analyses and reported as the average peak negative systolic strain in 16 segments. 15 , 16 , 17 LV dysfunction was defined as abnormal EF, <54% for women and 52% for men, according to the chamber quantification recommendations 18 or abnormal GLS, worse than −18%, according to the expert consensus document for multimodality imaging in patients with AC. 17 Analyses of repeated observations within patients were always performed by a single observer, and all analyses were performed before adjudication of incident VTA.
RV size and function were assessed at baseline by echocardiography in all patients and by cardiac magnetic resonance imaging in a subgroup of patients on clinical indication (1.6‐T unit Magnetom Sonata, Vision Plus or Avanto Siemens, Erlangen, Germany) using a phased‐array body coil. RV dysfunction was defined in concordance with recommendations as fractional area change ≤40% or tricuspid plane annulus systolic excursion <17 mm from an echocardiographic RV‐focused 4‐chamber view, or RV EF<40% by cardiac magnetic resonance. 11 , 17 RV dilation was defined as increased proximal RV outflow tract diameter ≥32 mm or increased RV basal diameter >41 mm by echocardiography 17 or increased indexed RV end‐diastolic volume assessed by cardiac magnetic resonance (>100 mL/m2 for women and >110 mL/m2 for men). 11
Ventricular Tachyarrhythmia
The occurrence of cardiac arrest, documented sustained ventricular tachycardia (>100 beats per minute for >30 seconds 19 ), or appropriate implantable cardioverter defibrillator shock were collectively defined as VTA. These events were adjudicated retrospectively at the time of inclusion and prospectively during follow‐up. The time from baseline to first event during follow‐up was noted, and the time point of the final echocardiographic assessment before an event was defined as the moment of impending VTA.
In a subsequent analysis, we defined a harder arrhythmic end point “life‐threatening VTA” as cardiac arrest, ultra‐fast ventricular tachycardia (>250 beats/min), or ventricular fibrillation terminated by an implantable cardioverter defibrillator shock, or hemodynamically unstable ventricular tachycardia occurring during follow‐up. These events were timed in the same way as VTA. The harder end point is of great interest in the subgroup of patients who present without arrhythmic events.
Statistical Analysis
Values were reported as mean±SD, number with percentages, median with interquartile range (IQR), or regression coefficients with standard error, as appropriate. Data summary measures were compared using generalized estimating equations to accommodate data dependence by patient relatedness. To account for dependence and different number of observations per individual, the association between exercise exposure and LV function at inclusion and the subsequent impact on LV disease trajectory during follow‐up was assessed by linear mixed‐model regression with exchangeable covariance structure and patient relatedness and repeated individual observations as 2 levels of random effects. The impact of desmoplakin mutation and exercise exposure on progression of LV disease manifestation were assessed by interaction analysis in linear mixed‐model regressions. We performed plots of the fitted mean linear response over time of the patient groups of interest to visualize the trajectories of LV function during follow‐up. The impact of LV disease progression on the risk of impending VTA during follow‐up was assessed using generalized estimating equations of the repeated LV functional assessments with binomial family, logit link, and independent correlation structure, adjusting for the effect of patient relatedness, elapsed time, and previous ventricular arrhythmia. The outcome analyses were repeated in the subgroup of patients who presented without previous arrhythmic events together with the harder arrhythmic end point “life‐threatening VTA,” both adjusted for elapsed time. Two‐sided P values <0.05 were considered significant. Statistical analyses were performed using Stata SE 16.0 (StataCorp LLC, College Station, TX).
RESULTS
We included 168 patients (50% probands, 45% women, 40±16 years old, 91 different families) with >1 complete echocardiography exam, in total 715 echocardiographic exams, 4.1±1.7 exams per patient, and with median follow‐up 7.6 (IQR, 5.4–10.9) years. The median time between each exam was 1.6 (IQR, 1.1–2.9) years. The shortest interval was 2 months, and the longest was 17.3 years. Complete exercise data were available in 146 (87%) patients. Median exercise dose at inclusion was 14.5 (IQR, 12–40) MET‐hours/week. Twenty‐five different pathogenic mutations were found in 55 different families (Table S1). Pathogenic mutations in the plakophilin‐2 gene were found in 112 (67%) patients, and 20 (12%) patients had pathogenic mutations in non–plakophilin‐2 genes (10 desmoglein 2, 9 desmoplakin, and 1 cadherin‐2 gene). Among the 84 probands, 34 (38%) had plakophilin‐2 mutations, 5 (6%) had desmoglein‐2 mutations, and 8 (10%) had desmoplakin mutations, and 1 had cadherin‐2 mutation. Thirty‐six (46%) probands with definite AC did not have documented pathogenic variants (22 with negative Sanger sequencing, 12 with negative high‐throughput screening, and 2 who did not consent to genetic testing).
LV Dysfunction
LV dysfunction was evident in 62 (37%) patients at first assessment. Patients presenting with LV dysfunction had greater exercise exposure than those presenting without LV dysfunction (36 [IQR, 12–50] MET‐hours/week versus 14 [IQR, 12–24] MET‐hours/week; P=0.02; Table 1), and LV dysfunction was more prevalent at presentation in male patients than in female patients (60 [57%] versus 16 [26%]; P=0.003; Table 1). RV dysfunction and RV dilation were more prevalent in patients with LV dysfunction than in those without (Table 1). Probands had worse LV function than mutation‐positive family members at first assessment (EF, 55±9 versus 59±4; P<0.001; and GLS, −17.8±3.7 versus −20.1±2.3; P<0.001).
Table 1.
Baseline Characteristics of 168 Patients With Arrhythmogenic Cardiomyopathy Without and With Left Ventricular Dysfunction at Presentation
| All n=168 | No LV Dysfunction n=106 | LV Dysfunction n=62 | P Value | |
|---|---|---|---|---|
| Age, y | 40±16 | 40±16 | 41±16 | 0.87 |
| BSA, m2 | 1.9 (1.8–2.1) | 1.9 (1.8–2.1) | 2.0 (1.9–2.2) | 0.09 |
| Definite diagnosis by TFC, n (%) | 98 (58) | 55 (52) | 43 (69) | <0.001 |
| Female sex, n (%) | 76 (45) | 60 (57) | 16 (26) | 0.003 |
| Exercise dose, MET‐h/wk, n=146 | 15 (12‐40) | 14 (12‐25) | 36 (12‐51) | 0.02 |
| AA medication, n (%) | 23 (14) | 7 (7) | 16 (27) | <0.001 |
|
6 (4) | 2 (2) | 4 (7) | 0.27 |
|
5 (3) | 0 (0) | 5 (8) | 0.002 |
|
12 (7) | 5 (5) | 7 (12) | 0.12 |
| Beta blocker, n (%) | 50 (31) | 30 (29) | 20 (34) | 0.32 |
| Mutation, n (%) | 132 (79) | 85 (80) | 47 (76) | 0.51 |
|
1 (0) | 1 (1) | 0 (‐) | ‐ |
|
10 (6) | 7 (7) | 3 (5) | 0.69 |
|
9 (5) | 4 (4) | 5 (8) | 0.20 |
|
112 (67) | 73 (69) | 39 (63) | 0.46 |
| Probands, n (%) | 84 (50) | 43 (41) | 41 (66) | <0.001 |
| VTA, n (%) | 58 (35) | 30 (28) | 28 (45) | 0.01 |
| Syncope, n (%) | 66 (40) | 36 (34) | 30 (49) | 0.01 |
| Cardiac imaging | ||||
| LV EF, %* | 57±7 | 59±4 | 52±9 | n.a |
| LV GLS, %* | −19.0±3.3 | −20.6±2.0 | −16.3±3.1 | n.a |
| RV FAC, %* | 39±10 | 42±9 | 35±10 | <0.001 |
| TAPSE, mm* | 20±5 | 21±5 | 18±6 | <0.001 |
| RV EF, % † | 47±11 | 49±11 | 42±11 | 0.003 |
| RV dysfunction, n (%) | 94 (56) | 53 (50) | 41 (66) | 0.03 |
| RVOT, mm* | 36±8 | 34±7 | 38±9 | 0.006 |
| RVD, mm* | 42±8 | 40±7 | 45±9 | <0.001 |
| RV EDVi, mL/m † | 107±36 | 103±39 | 114±29 | 0.13 |
| RV dilation, n (%) | 116 (69) | 68 (64) | 48 (77) | 0.008 |
Values are mean±standard deviation, median (interquartile range), or frequency (percentages) compared by generalized estimating equations accounting for dependence by relatedness of patients.
Abbreviations: AA indicates antiarrhythmic; BSA, body surface area; EF, ejection fraction; FAC, fractional area change; GLS, global longitudinal strain; LV, left ventricle; MET‐h/wk, metabolic equivalents task × hours per week; RV, right ventricular; RVD, right ventricular diameter; RVOT, right ventricular outflow tract; TAPSE, tricuspid annular plane systolic excursion; TFC, task force criteria; and VTA, ventricular tachyarrhythmia.
Assessed by echocardiography in all patients.
Assessed by cardiac magnetic resonance imaging in a subgroup of 75 patients.
Among patients with plakophilin‐2 mutations (n=112), LV dysfunction was evident in 39 (35%), and those with LV dysfunction had greater exercise exposure (25 [IQR, 12–56] MET‐hours/week versus 12 [IQR, 10–18] MET‐hours/week; P=0.02). LV dysfunction was evident in 56% (5/9) of patients with desmoplakin mutations at first assessment, versus 43% (48/159) of patients without desmoplakin mutations (P=0.27).
Deterioration of LV Function
GLS was assessed in 648 (91%) exams and demonstrated a subtle but clear decline of LV function (−18.8 [IQR, −19.2 to −18.3]% at presentation, with 0.08% [IQR, 0.05%–0.12%] worsening per year; P<0.001). EF was assessed in 699 (98%) echocardiographic exams. We observed no general decline in EF during follow‐up (57.1 [IQR, 56.0–58.1] at presentation with 0.0% [IQR, −0.1% to 0.1%] worsening per year; P=0.56). There was no difference in the trajectory of LV function between probands and family members (P for interaction 0.85 and 0.83 for deterioration of EF and GLS, respectively).
A total of 628 echocardiographic assessments were analyzed in the 146 patients with complete exercise data. Greater exercise exposure correlated with worse EF and GLS at first echocardiography, but previous exercise habits did not affect the deterioration of LV function during follow‐up, neither by GLS nor by EF (Table 2, Figure 1).
Table 2.
Relationship Between Exercise Exposure and Left Ventricular Function During Long‐Term Follow‐Up With 628 Echocardiographic Exams in 146 Patients With Arrhythmogenic Cardiomyopathy and Known Exercise Habits at Presentation
| Beta | 95% CI | P Value | |
|---|---|---|---|
| GLS (constant), n=584 (93%) | −19.4 | −19.9 to −18.6 | |
| Time, y | 0.07 | 0.02 to 0.12 | 0.008 |
| Exercise dose (5 MET‐h/wk) | 0.09 | 0.01 to 0.17 | 0.02 |
| Interaction: Time×Exercise dose | 0.002 | −0.003 to 0.008 | 0.44 |
| EF (constant), n=616 (98%) | 58 | 57 to 59 | |
| Time, y | 0.02 | −0.10 to 0.14 | 0.72 |
| Exercise dose (5 MET‐h/wk) | −0.18 | −0.36 to −0.003 | <0.05 |
| Interaction: Time×Exercise dose | 0.004 | −0.010 to 0.018 | 0.57 |
Values are regression coefficients with 95% CIs. P values by linear mixed‐model regression with exchangeable covariance structure and random effects by families and individuals.
Abbreviations: EF indicates ejection fraction; GLS, global longitudinal strain; and MET‐h/wk, metabolic equivalents of task×hours per week.
Figure 1. Left ventricular function in 146 patients with arrhythmogenic cardiomyopathy and known exercise habits at presentation.
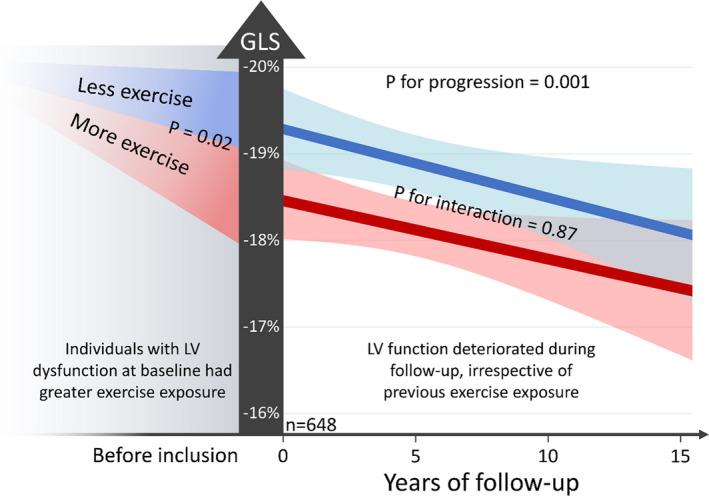
Slopes are fitted mean linear response with 95% CIs for patients with and without desmoplakin mutations. P values for progression and interaction by linear mixed‐model regression with random effects for families and individuals and exchangeable covariance structure. Exercise during 3 immediate years before presentation expressed as average MET‐hours per week, with dichotomization at median exercise exposure. Patients with exercise exposure above median had GLS 1.0% (interquartile range, 0.1%–1.9%; P=0.02) worse than patients with exercise exposure below median. There was no difference in the progression of left ventricular dysfunction in patients with greater or lesser exercise exposure (illustrated by P for interaction=0.87). See Table 2 for coefficients and continuous exercise variable. GLS indicates global longitudinal strain; and LV, left ventricular.
Patients with mutations in the desmoplakin gene had worse progression of LV function during 39 follow‐up echocardiographic exams than patients without desmoplakin mutations (EF, −0.82% [IQR, −1.31% to −0.33%] per year versus 0.05% [−0.04% to 0.14%] per year; P=0.001; and less clear by GLS+0.18% [IQR, −0.01% to 0.38%] per year versus +0.08% [IQR, 0.04%–0.11%] per year; P=0.06; Figure 2). The impact of desmoplakin gene mutations was also independent of exercise exposure, suggesting that EF declined 0.8% more per year in patients with desmoplakin mutations than in other patients independently of exercise exposure (adjusted beta for interaction −0.8 [IQR, −1.3 to −0.3] per year; P=0.003; Table S2).
Figure 2. Left ventricular functional deterioration during follow‐up of 168 patients with arrhythmogenic cardiomyopathy with desmoplakin genotypes (blue curves) and with other genotypes or gene elusive patients (green curves).
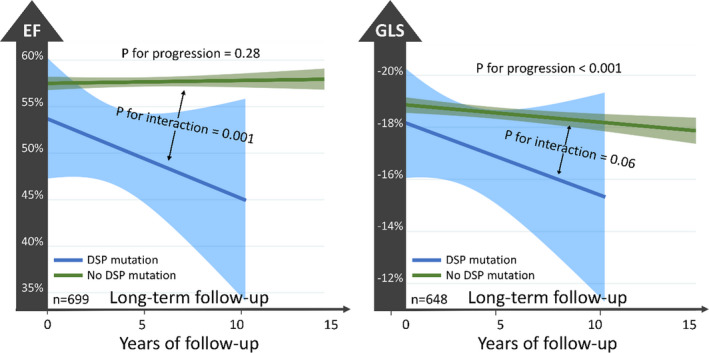
Slopes are fitted mean linear response with 95% CIs for patients with and without desmoplakin mutations. P values for progression and interaction by linear mixed‐model regression with random effects for families and individuals and exchangeable covariance structure. Patients with desmoplakin genotypes had worse progression of left ventricular dysfunction than patients with other genotypes or gene‐elusive patients (illustrated by P for interaction<0.001 for both EF and GLS). See text for regression coefficients. DSP indicates desmoplakin; EF, ejection fraction; and GLS, global longitudinal strain.
Patients with LV dysfunction at first examination did not have more pronounced progression of LV dysfunction during follow‐up than patients presenting with normal LV function. In fact, the analyses demonstrated an opposite trend, with steeper worsening of both EF and GLS in patients presenting with normal LV function (EF, 0.29% [IQR, 0.10%–0.47%] worse per year; P=0.003; and GLS, 0.22% [0.15%–0.30%] worse per year; P<0.001; Figure S1). The progression of LV dysfunction was not affected by the presence of RV dysfunction or RV dilation at baseline (Figure S1).
LV Dysfunction and Prediction of VTAs
VTA occurred in 77 patients, of whom 58 (35% of all patients) had experienced events at or before baseline. Nineteen patients had their first VTA, and 35 (60%) had subsequent incident VTA during follow‐up (in total 54 patients with events after median 1.3 years [IQR, 0.4–3.5). Odds of impending VTA increased by 14% for every 1% absolute deterioration of GLS during follow‐up, adjusted for elapsed time and history of previous VTA (adjusted odds ratio [OR] 1.1 [IQR, 1.0–1.3]; P=0.02; Figure 3).
Figure 3. Left ventricular functional deterioration during follow‐up of 168 patients with arrhythmogenic cardiomyopathy predicted subsequent ventricular tachyarrhythmias.
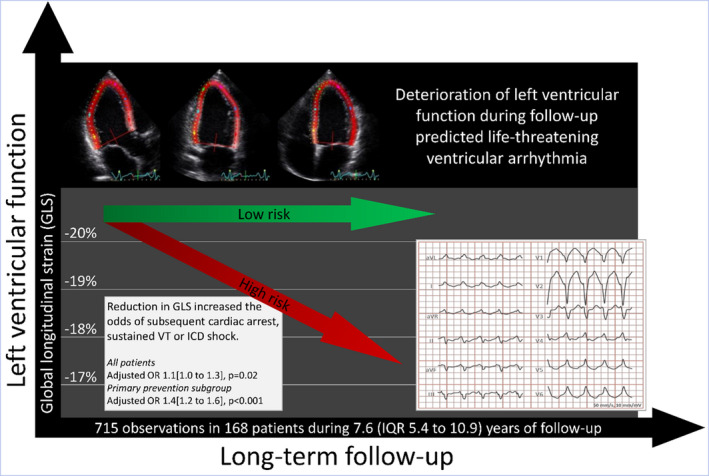
Odds ratios for impending ventricular tachyarrhythmias with 1% decline in GLS presented in the lower left panel were calculated by generalized estimating equations of the repeated left ventricular functional assessments with binomial family, logit link, and independent correlation structure, adjusting for the effect of patient relatedness, elapsed time (primary prevention subgroup) and previous ventricular arrhythmia (all patients). GLS indicates global longitudinal strain; ICD, implantable cardioverter defibrillator; OR, odds ratio; and VT, ventricular tachycardia.
Importantly, this was also evident in the subgroup of patients presenting without previous arrhythmia (n=110; 444 echocardiographic exams), with 35% increased odds of impending VTA for every 1% worsening in GLS (adjusted OR, 1.4 [IQR, 1.2–1.6]; P<0.001, adjusted for time; Figure 3). The effect was less clear for deterioration in EF in the total population (adjusted OR, 1.2 [IQR, 0.9–1.4]; P=0.23, by 5% reduction in EF, adjusted for time and history of previous VTA), but in the subgroup of patients presenting without arrhythmia, 50% increased odds was observed for every 5% fall in EF during follow‐up (adjusted OR, 1.5 [IQR, 1.1–2.2]; P=0.02, adjusted for time). These observations were independent of the underlying pathogenic mutation (Table S3).
Thirteen patients without previous events had life‐threatening VTA during follow‐up. No patients died suddenly during follow‐up. One had resuscitated cardiac arrest. Five had ultra‐fast ventricular tachycardia or ventricular fibrillation that was terminated by an implantable cardioverter defibrillator shock. Seven had hemodynamically unstable ventricular tachycardia. The odds of impending life‐threatening VTA increased by 25% by every 1% worsening in GLS during follow‐up (adjusted OR, 1.3 [1.1–1.5]; P=0.006, adjusted for time) and by 50% by every 5% fall in EF during follow‐up (adjusted OR, 1.5 [IQR, 1.1–2.1]; P=0.02, adjusted for time).
DISCUSSION
This study showed LV dysfunction at the time of presentation in more than one‐third of patients with AC. Greater exercise exposure was associated with LV dysfunction at first visit, highlighting the deleterious effects of exercise on biventricular function in AC. However, we found no effect of previous exercise exposure on the progression of LV dysfunction after established AC diagnosis, indicating no accelerated LV disease progression by previous exercise. LV disease progression was most pronounced in desmoplakin genotype‐positive patients, supporting previous reports of LV vulnerability in this genotype. Deterioration of LV function during follow‐up predicted incident VTA.
Exercise Exposure
Greater exercise exposure was associated with worse LV function at presentation but did not affect the rate of LV deterioration during follow‐up. Interestingly, previous exercise exposure did not seem to accelerate LV disease after exercise was restricted at the time of diagnosis. On the other hand, we found no improvement or normalization of LV function after exercise restriction, suggesting an irreversible exercise‐induced dysfunction.
It is well established that exercise exposure is associated with disease severity in patients with AC, but previous reports have been single‐observation studies focusing on RV disease manifestation. 4 , 5 This study highlights for the first time the association between exercise exposure and LV dysfunction. It is believed that RV disease in exercise‐exposed patients with AC is caused by the mechanical stress imposed on the RV wall by increased loading conditions during exercise. Stretching forces in the context of dysfunctional desmosomes disrupt the cellular signaling and intercellular adhesions, triggering arrhythmias and fibrofatty replacement. 1 While the RV is famously vulnerable to these mechanisms, the LV is also exposed to increased loading conditions during exercise, and therefore exercise‐induced LV alterations must be expected in AC.
Adaptive LV remodeling is frequently encountered in athletes. 20 We do not believe that the differences in LV function at baseline were attributable to physiological exercise adaptations. LV function did not recover in our patients, suggesting irreversible and maladaptive mechanisms in patients with AC, with the result of LV dysfunction probably induced by higher exercise doses.
We demonstrated LV disease progression by repeated assessment of GLS, while EF measurements were relatively stable. GLS provides a more sensitive and accurate estimation of LV function than EF, 21 , 22 and our findings support the use of GLS as the primary index of LV function in patients with AC. 17
Deterioration of LV Function
Previous studies have reported higher prevalence of reduced EF (<55%) in patients with AC with desmoplakin genotypes than in other genotypes. 9 , 23 Our study supports this observation and additionally demonstrates a more pronounced progression of LV dysfunction in patients with desmoplakin mutations. However, and importantly, we observed deterioration of LV function also in patients without desmoplakin highlighting a genotype‐independent biventricular disease.
Our arbitrary definition of LV dysfunction may be subject to debate. Global longitudinal strain is reported to be superior to EF when assessing LV function in several scenarios, but it is not globally implemented in clinical practice. Therefore, we have used a combined definition that may be unusual but aims to detect also early LV dysfunction. Patients with LV dysfunction at the time of diagnosis did progress more rapidly during follow‐up than those presenting with normal LV function. In contrast, we observed a trend toward more pronounced LV progression in patients with normal LV function at first assessment. This observation may have several explanations. First, present data suggested that cessation of high‐intensity exercise may halt progression of LV disease. Second, there may be a component of regression to the sample mean. Most importantly, our data demonstrate that absence of LV dysfunction at first presentation does not exclude subsequent deterioration of LV function.
Presence of RV disease manifestation at the time of diagnosis did not predict LV disease progression. This interesting observation suggests that RV and LV disease manifestation may exist somewhat independent of each other and underscores the importance of careful LV assessment in patients with AC.
Risk of VTA
The odds of impending VTA increased when LV function deteriorated during follow‐up, independently of genotype. The association between LV dysfunction and adverse outcome is both intuitive and previously described, 2 , 3 but this is the first report showing the chronological sequence of LV disease progression leading up to an arrhythmic event. The value of recognizing LV dysfunction was particularly convincing in the subgroup of patients presenting without previous VTA, in whom worsening of both EF and GLS predicted events. Life‐threatening VTA occurred during follow‐up in only 13 patients without previous arrhythmic events. However, this underpowered analysis suggested that deterioration of LV function may also be valuable in predicting harder arrhythmic end points. This must be assessed in future studies, but emerging LV dysfunction should raise awareness for primary preventive implantable cardioverter defibrillator evaluation.
LIMITATIONS
This was a single‐center longitudinal study with uncertain external validity. The observational data do not allow for causal inference. The main limitation is the single‐time‐point ascertainment of exercise habits, and the retrospective assessment of exercise exposure is subject to recall bias and possibly reporting bias. All patients were recommended to abstain from high‐intensity exercise based on the best knowledge at the time, but we do not have data on adherence to exercise restriction recommendations. Future studies should assess exercise exposure as a time‐varying covariate during follow‐up to fully elucidate this interaction. Fulfillment of Task Force Criteria introduced in 2010 was assessed retrospectively in individuals presenting earlier. Our center does not routinely obtain endomyocardial biopsy material from patients with AC, and therefore we cannot firmly exclude differential diagnoses in the mutation‐negative probands. The number of patients with non–plakophilin‐2 mutations was low and was only partially compensated for by the repeated assessments. The pronounced LV functional deterioration in patients with desmoplakin mutations should therefore be interpreted with care. The main arrhythmic outcome was relatively weak, including hemodynamically stable ventricular tachycardia, and hard arrhythmic outcome analysis was underpowered.
CONCLUSIONS
LV dysfunction was detected in one‐third of patients with AC at presentation and was associated with greater exercise exposure. LV function deteriorated during follow‐up and was most pronounced in the desmoplakin genotype positive. LV deterioration was independent of previous exercise exposure, indicating no accelerated effect by greater previous exercise exposure. Exercise cessation did not seem to resolve LV dysfunction but may halt the progression. Deterioration of LV function during follow‐up predicted impending VTAs and should be considered in risk assessments of patients with AC.
Sources of Funding
This work was supported by the South‐Eastern Norway Regional Health Authority (grant #2011094), the Center for Cardiological Innovation supported by the Research Council of Norway (grant #203489) and Precision Health Center for optimized cardiac care (ProCardio) (grant #309762).
Disclosures
None.
Supporting information
Tables–S1–S3
Figure–S1
Acknowledgments
The authors are grateful to all the patients who consented to be included in the present study, and to the staff at the Unit for Genetic Cardiac Disorders and Section of Echocardiography at the Department of Cardiology, Oslo University Hospital, Rikshospitalet.
(J Am Heart Assoc. 2021;10:e018680. DOI: 10.1161/JAHA.120.018680.)
Supplementary material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018680
For Sources of Funding and Disclosures, see page 9.
References
- 1. Corrado D, Basso C, Judge DP. Arrhythmogenic cardiomyopathy. Circ Res. 2017;121:784–802. DOI: 10.1161/CIRCRESAHA.117.309345. [DOI] [PubMed] [Google Scholar]
- 2. Lie ØH, Rootwelt‐Norberg C, Dejgaard LA, Leren IS, Stokke MK, Edvardsen T, Haugaa KH. Prediction of life‐threatening ventricular arrhythmia in patients with arrhythmogenic cardiomyopathy: a primary prevention cohort study. J Am Coll Cardiol Img. 2018;11:1377–1386. DOI: 10.1016/j.jcmg.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 3. Mast TP, Teske AJ, vd Heijden JF, Groeneweg JA, Te Riele ASJM, Velthuis BK, Hauer RNW, Doevendans PA, Cramer MJ. Left ventricular involvement in arrhythmogenic right ventricular dysplasia/cardiomyopathy assessed by echocardiography predicts adverse clinical outcome. J Am Soc Echocardiogr. 2015;28:1103–1113. DOI: 10.1016/j.echo.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 4. Lie ØH, Dejgaard LA, Saberniak J, Rootwelt‐Norberg C, Stokke MK, Edvardsen T, Haugaa KH. Harmful effects of exercise intensity and exercise duration in patients with arrhythmogenic cardiomyopathy. JACC Clin Electrophysiol. 2018;4:744–753. DOI: 10.1016/j.jacep.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 5. Ruwald AC, Marcus F, Estes NA 3rd, Link M, McNitt S, Polonsky B, Calkins H, Towbin JA, Moss AJ, Zareba W. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2015;36:1735–1743. DOI: 10.1093/eurheartj/ehv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H. Exercise increases age‐related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy‐associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62:1290–1297. DOI: 10.1016/j.jacc.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, Daubert JP, de Chillou C, DePasquale EC, Desai MY, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. DOI: 10.1016/j.hrthm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 8. Hoorntje ET, Te Rijdt WP, James CA, Pilichou K, Basso C, Judge DP, Bezzina CR, van Tintelen JP. Arrhythmogenic cardiomyopathy: pathology, genetics, and concepts in pathogenesis. Cardiovasc Res. 2017;113:1521–1531. DOI: 10.1093/cvr/cvx150. [DOI] [PubMed] [Google Scholar]
- 9. Akdis D, Saguner AM, Burri H, Medeiros‐Domingo A, Matter CM, Ruschitzka F, Tanner FC, Brunckhorst C, Duru F. Clinical predictors of left ventricular involvement in arrhythmogenic right ventricular cardiomyopathy. Am Heart J. 2020;223:34–43. DOI: 10.1016/j.ahj.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 10. Chivulescu M, Lie ØH, Popescu BA, Skulstad H, Edvardsen T, Jurcut RO, Haugaa KH. High penetrance and similar disease progression in probands and in family members with arrhythmogenic cardiomyopathy. Eur Heart J. 2019;41:1401–1410. DOI: 10.1093/eurheartj/ehz570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MGPJ, Daubert JP, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. DOI: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier‐Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. DOI: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pelliccia A, Solberg EE, Papadakis M, Adami PE, Biffi A, Caselli S, La Gerche A, Niebauer J, Pressler A, Schmied CM, et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2019;40:19–33. DOI: 10.1093/eurheartj/ehy730. [DOI] [PubMed] [Google Scholar]
- 14. Pelliccia A, Zipes DP, Maron BJ. Bethesda Conference #36 and the European Society of Cardiology consensus recommendations revisited a comparison of U.S. and European criteria for eligibility and disqualification of competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol. 2008;52:1990–1996. DOI: 10.1016/j.jacc.2008.08.055. [DOI] [PubMed] [Google Scholar]
- 15. Voigt J‐U, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1–11. DOI: 10.1093/ehjci/jeu184. [DOI] [PubMed] [Google Scholar]
- 16. Corrado D, van Tintelen PJ, McKenna WJ, Hauer RNW, Anastastakis A, Asimaki A, Basso C, Bauce B, Brunckhorst C, Bucciarelli‐Ducci C, et al. Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J. 2020;41:1414–1429. DOI: 10.1093/eurheartj/ehz669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haugaa KH, Basso C, Badano LP, Bucciarelli‐Ducci C, Cardim N, Gaemperli O, Galderisi M, Habib G, Knuuti J, Lancellotti P, et al. Comprehensive multi‐modality imaging approach in arrhythmogenic cardiomyopathy‐an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017;18:237–253. DOI: 10.1093/ehjci/jew229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. DOI: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 19. Pedersen CT, Kay GN, Kalman J, Borggrefe M, Della‐Bella P, Dickfeld T, Dorian P, Huikuri H, Kim Y‐H, Knight B, et al. EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Europace. 2014;16:1257–1283. DOI: 10.1093/europace/euu194. [DOI] [PubMed] [Google Scholar]
- 20. Galderisi M, Cardim N, D'Andrea A, Bruder O, Cosyns B, Davin L, Donal E, Edvardsen T, Freitas A, Habib G, et al. The multi‐modality cardiac imaging approach to the athlete's heart: an expert consensus of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:353. DOI: 10.1093/ehjci/jeu323. [DOI] [PubMed] [Google Scholar]
- 21. Haugaa KH, Edvardsen T. Global longitudinal strain: the best biomarker for predicting prognosis in heart failure? Eur J Heart Fail. 2016;18:1340–1341. DOI: 10.1002/ejhf.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, Edvardsen T, Remme EW. Geometry as a confounder when assessing ventricular systolic function: comparison between ejection fraction and strain. J Am Coll Cardiol. 2017;70:942–954. DOI: 10.1016/j.jacc.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 23. Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JDH, Murray B, te Riele ASJM, van den Berg MP, Bikker H, et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy‐associated mutation carriers. Eur Heart J. 2015;36:847–855. DOI: 10.1093/eurheartj/ehu509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables–S1–S3
Figure–S1


