Abstract
Background
NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) is widely used to diagnose and manage patients with heart failure. We aimed to investigate associations between NT‐proBNP levels and development of global and regional myocardial impairment, dyssynchrony, and risk of developing myocardial scar over time.
Methods and Results
We included 2416 adults (45–84 years) without baseline clinical cardiovascular disease from MESA (Multi‐Ethnic Study of Atherosclerosis). NT‐proBNP was assessed at baseline (2000–2002). Cardiac magnetic resonance–measured left ventricular parameters were assessed at baseline and year 10 (2010–2012). Tagged cardiac magnetic resonance and myocardial dyssynchrony were assessed. We used linear and logistic regression models to study the relationships between quartiles of NT‐proBNP levels and outcome variables. Left ventricular parameters decreased over time. After 10‐year follow‐up and adjusting for cardiovascular disease risk factors, people in the highest quartile had significantly greater decline in left ventricular ejection fraction (−1.60%; 95% CI, −2.26 to −0.94; P<0.01) and smaller decline in left ventricular end systolic volume index (−0.47 mL/m2; 95% CI, −1.18 to 0.23; P<0.01) compared with those in the lowest quartile. Individuals in the highest quartile had more severe risk factor adjusted global, mid, and apical regional dyssynchrony compared with those in the lowest, second, and third quartiles (all P‐trend<0.05). Compared with the lowest‐quartile group, the adjusted odds ratios for having myocardial scar was 1.3 (95% CI, 0.7–2.2) for quartile 2; 1.2 (95% CI, 0.6–2.3) for quartile 3; and 2.7 (95% CI, 1.4–5.5) for quartile 4 (P‐trend=0.012) for the total sample.
Conclusions
Among participants without baseline clinical cardiovascular disease, higher baseline NT‐proBNP concentration was significantly associated with subclinical changes in developing myocardial dysfunction, more severe cardiac dyssynchrony, and higher odds of having myocardial scar over a 10‐year period independent of traditional cardiovascular disease risk factors.
Keywords: cardiac magnetic resonance imaging, longitudinal study, NT‐proBNP, scar
Subject Categories: Imaging, Magnetic Resonance Imaging (MRI)
Nonstandard Abbreviations and Acronyms
- ECC
circumferential strain
- MESA
Multi‐Ethnic Study of Atherosclerosis
- TPS
time to peak systole
Clinical Perspective
What Is New?
NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) is a marker of cardiac remodeling and is widely used to diagnose and manage patients with heart failure.
We investigated the association of NT‐proBNP levels; markers of cardiac remodeling and heart failure; and development of global and regional myocardial impairment, dyssynchrony, and risk of developing myocardial scar over 10 years.
After 10‐year follow‐up and adjusting for cardiovascular disease risk factors, higher levels of NT‐proBNP were significantly associated with reduced left ventricular ejection fraction; more severe global, mid, and apical regional dyssynchrony; and higher risk of having myocardial scar.
What Are the Clinical Implications?
Our findings lend support to consider earlier screening using biomarkers such as NT‐proBNP in identifying persons with subclinical cardiovascular disease who may benefit most from early therapeutic intervention and provide measures for cardiovascular disease prevention.
Elevated NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels are associated with many cardiovascular pathologies, including heart failure, atrial fibrillation, and stroke, and they have also been linked to preclinical states of vascular damage and reduced myocardial perfusion. 1 , 2 Moreover, it was previously reported in the MESA (Multi‐Ethnic Study of Atherosclerosis) participants that elevated NT‐proBNP is associated with increment in extracellular volume fraction and native T1 value, imaging markers of microscopic fibrosis. 3
Cardiac magnetic resonance (CMR) imaging is the current reference standard for the assessment of myocardial structure and function. 4 CMR with myocardial tissue tagging provides quantitative and highly reproducible myocardial data that are largely operator and patient independent, as well as characterization of myocardial scar and scar distribution. 5 , 6 In addition to myocardial strain, myocardial dyssynchrony is another index of regional myocardial contractility dysfunction. CMR is also able to accurately evaluate myocardial tissue for presence of scar. In the MESA study, prevalence of myocardial scar was reported to be 7.9% at year 10 among participants who were free of clinical CVD at the time of enrollment, while 78% of these participants were unrecognized by ECG or clinical adjudication. 7
To date, few studies have evaluated the direct relationship of NT‐proBNP levels at baseline to changes in CMR‐measured myocardial structure/function, dyssynchrony, and myocardial scarring over time, and data are even more scarce for large, ethnically diverse prospective cohorts. Therefore, the purpose of this study is to investigate the potential mechanisms and implications behind the associations between NT‐proBNP levels at baseline and changes in global and regional myocardial function, dyssynchrony, development of myocardial scar tissue, and extent of scar burden during 10‐year follow‐up using data collected from the MESA study.
Methods
Data Availability Statement
The MESA cohort participates in the National Heart, Lung, and Blood Institute's Biologic Specimen and Data Repository. The MESA data are available upon request, including data from examinations 1 to 5 used in this analysis. Requests for data can be made through the following Web site: https://biolincc.nhlbi.nih.gov/studies/mesa/.
Study Sample
The design of MESA has been described in detail previously. 8 Briefly, MESA is a multicenter, population‐based cohort study of 6 communities in the United States (Illinois, North Carolina, Maryland, California, New York, and Minnesota). Participants were recruited between July 2000 and August 2002. Participants defined themselves as White (38%), African‐American (28%), Hispanic (22%), or Chinese American (12%). All MESA participants were free of clinical cardiovascular disease (CVD) at baseline. The MESA study was approved by the institutional review boards of all participating centers, and all participants gave written, informed consent. For the present study, we included 2416 MESA participants who had plasma NT‐proBNP measured at baseline, underwent CMR imaging, had CMR measures of left ventricular (LV) parameters at baseline and year 10 (2010–2012, follow‐up), and had no missing data on covariates of interest. Sample sizes varied for each outcome studied and are as follows: baseline LV parameters, n=2416; change in LV parameters over time, n=2416; LV dyssynchrony/strain parameters at follow‐up, n=2211; presence of late gadolinium enhancement imaging during follow‐up, n=1498 (Figure).
Figure 1. Flow diagram characteristics of MESA participants who contributed to each of the analysis cohorts evaluating the relationships between NT‐proBNP and baseline LV parameters, change in LV parameters over time, LV dyssynchrony/strain parameters, and having myocardial scar over time.
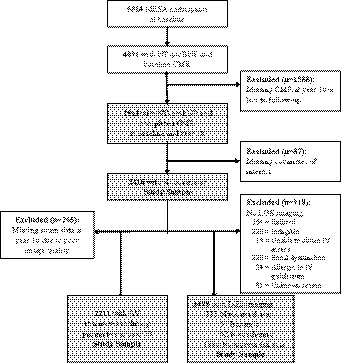
Renal dysfunction=estimated glomerular filtration rate of less than 45 mL/min per 1.73 m2. CMR indicates cardiac magnetic resonance imaging; LGE, late gadolinium enhancement; LV, left ventricular; MESA, Multi‐Ethnic Study of Atherosclerosis; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Assessment of NT‐proBNP
Serum NT‐proBNP levels were measured at the core lab (University of California, San Diego) using a highly sensitive and specific Elecsys electrochemiluminescence immunoassay based on the double‐antibody sandwich method (Roche Diagnostics Corporation, Indianapolis, IN), as previously described. 9 A previously unthawed or only thawed once 250‐μL serum sample was used for analysis. The intra‐ and interassay coefficients of variation were as follows: at 175 pg/mL, 2.7% and 3.2%; at 355 pg/mL, 2.4% and 2.9%; at 1068 pg/mL, 1.9% and 2.6%; and at 4962 pg/mL, 1.8% and 2.3%, respectively. 10
Assessment of Cardiovascular Risk Factors
All participants underwent comprehensive baseline testing including documentation of comorbidities; measurement of heart rate and blood pressure; laboratory testing (including fasting glucose and fasting lipid profile); and computed tomography of the chest for the determination of coronary artery calcium (CAC) score. Body mass index was calculated as weight divided by the square of height (kg/m2). Blood pressure at rest was measured 3 times in the seated position, and the last 2 measurements were averaged and used for these analyses. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or current use of antihypertensive medications. Diabetes mellitus was defined as fasting glucose ≥126 mg/dL or the use of diabetic medications. Physical activity was defined as the number of metabolic equivalent minutes per week spent doing intentional leisure‐time exercise (eg, moderate walking exercise, dance, and vigorous sports). Computed tomography scanning (for CAC) in MESA have been described in detail previously. 11 For the present study, we included data on CAC score as a marker of subclinical atherosclerosis at baseline.
Assessment of LV Structure and Function
The MESA CMR protocol has been described in detail previously. 12 Briefly, CMR examinations were performed at 6 MESA field centers using 1.5‐Tesla scanners. All images were read at the central MESA CMR review center at Johns Hopkins University (Baltimore, MD). LV mass, LV end‐diastolic and LV end‐systolic volumes, and LV ejection fraction (LVEF) were calculated by drawing endocardial and epicardial contours at end systole and end diastole on the short‐axis stack of slices in each subject. The papillary muscle mass was excluded from LV mass. LV mass and volume index were defined as LV mass and volume divided by body surface area. A detailed explanation of the development and application of calibration curves between the baseline and follow‐up exams are presented in Data S1. 13
Assessment of Myocardial Strain, Dyssynchrony, and Scar
Strain analysis has been described in detail previously. 14 Peak systolic circumferential strain (ECC) was measured using the HARP method embedded in MATLAB software (HARP 1.15, Diagnosoft, Palo Alto, CA). 15 ECC regional strain was determined at 3 levels (basal, mid, and apical) for 4 LV regions (septum, anterior, posterior, and lateral), expressed as a percentage, with negative values representing circumferential shortening. Peak “global” systolic strain was calculated as the averaged peak ECC across all 12 segments. Indexes of cardiac dyssynchrony were derived from CMR strains. Time to peak systole (TPS) was defined as time intervals from end diastole to peak systolic circumferential strain. SD of time to peak systolic strain across all 12 segments was used as the index of global myocardial dyssynchrony, as previously described by Rosen et al. 6 Higher SD of TPS means greater myocardial dyssynchrony.
Late gadolinium enhancement–CMR imaging was performed 15 minutes after intravenous contrast‐agent administration (Magnevist; Bayer Healthcare Pharmaceuticals, Wayne, NJ) at a dose of 0.15‐mmol/kg using inversion‐recovery fast gradient‐echo pulse sequences. Scar measurements were performed using Qmass (version 7.2; Medis, Leiden University Medical Center, Leiden, Netherlands) using a semi‐automated technique, as previously described. 16 Myocardial scars were classified as either ischemic (involving the subendocardium in a coronary artery distribution) or nonischemic (predominantly midwall or subepicardium location without subendocardial involvement in a noncoronary distribution). 7 The extent of scarring was quantified as a percentage of LV mass.
Statistical Analysis
Baseline and year 10 data for the CMR‐derived LV parameters and major cardiovascular risk factors were computed for the study sample, with results expressed as the mean (SD) for normally distributed continuous variables, the median (interquartile range) for nonnormally distributed continuous variables, and the counts and percentages for categorical variables. Differences in mean values between baseline and year 10 data were compared using paired t‐tests. When departures from normality were detected in the continuous variables, differences in distributions were compared with the Wilcoxon rank‐sum test. Differences in proportions for the categorical variables were compared using the chi‐square or McNemar test.
Participants were classified into 4 NT‐proBNP groups according to quartiles of NT‐proBNP distribution within the entire cohort (Q1 [low], 5 to <24 pg/mL; Q2, 24 to <55 pg/mL; Q3, 55 to <112 pg/mL; Q4 [high], ≥112 pg/mL). NT‐proBNP values were log‐transformed using the base‐10 log (log) before modeling as a continuous variable (because of highly skewed distribution). General linear models were used to compute NT‐proBNP group mean values and 95% CIs for each LV structure/function measure at baseline and changes in these parameters over 10 years, LV global and regional myocardial function at year 10, and extent of dyssynchrony at year 10. Multivariable logistic regression analyses were used to model the odds for developing scar at 10‐year follow‐up using NT‐proBNP categorically (included in the models as 3 dummy variables, with the lowest quartile as the omitted reference group). Standard techniques were used to select models and to test for model validity (eg, goodness of fit, interactions, and collinearity). 17 There was no evidence of nonlinearity in the relationship between log (NT‐proBNP) and the log odds of myocardial scar. The Hosmer–Lemeshow statistical test for goodness of fit was also considered and found to be nonsignificant. Test for linear trend was performed with the continuous log (NT‐proBNP) in appropriate models (ie, general linear models for continuous outcomes and logistic regression for binary outcomes).
Covariates identified a priori on the basis of previously published NT‐proBNP and subclinical CVD studies were screened for inclusion into the multivariable models. The final model included race/ethnicity (included as 4 indicator variables with White people as the omitted reference group), sex, and baseline variables: age (per year), education more than high school, physical activity (sex‐specific tertiles: low, middle, high), current cigarette smoking, diabetes mellitus, body mass index, systolic blood pressure, antihypertensive medication use, high‐density lipoprotein cholesterol, total cholesterol, statin medication use, prevalence of CAC score >10, LV parameter outcome of interest (in models of LV change only), and LV mass (in models for global and regional LV strain and dyssynchrony only). In addition, effect modification by sex was examined by including interaction term between log (NT‐proBNP) with sex, adjusted for age. For models with significant interaction terms, the regression analysis was repeated separately by stratifying sex. The potential for effect modification by race/ethnicity with NT‐proBNP was not investigated because of the small number of Chinese people in the NT‐proBNP highest quartile.
The Shapiro–Wilk test or Q‐Q plot was used to check for normality of distribution for the CMR outcomes in relation to log (NT‐proBNP), and no departures from normality were found. Variance inflation factors were also calculated for the independent variable (log [NT‐proBNP]), and all aforementioned covariates and found to be all <1.7, indicating no excessive multicollinearity in our data that warranted any corrective measures. All analyses were conducted using SAS statistical software version 9.4 (SAS Institute Inc, Cary, NC). The statistical testing was 2‐sided. P<0.05 was considered statistically significant.
Results
Study Population Characteristics
Among 6814 MESA participants at baseline, 4091 had both NT‐proBNP and CMR measures of LV parameters, 1675 were excluded because of missing data or were lost to follow‐up. According to baseline data, the cohort of individuals (n=2416) included in this analysis was more likely to be younger and healthier, with lower NT‐proBNP levels, lower rates of CAC score >10, lower values for systolic blood pressure, less diabetes mellitus, and lower rates of antihypertensive medication use; a higher proportion of White participants and a lower proportion of Hispanic participants; and also more commonly nonsmokers, more educated, and participated in more physical activity than individuals who were excluded in the study (Table S1).
Our study sample included 2416 MESA participants (51.1% women; 43% White, 22% African‐American, 21% Hispanic, and 14% Chinese) with a mean age of 59.6 years, and the median (interquartile range) NT‐proBNP was 46.9 (20.5–91.9) pg/mL at baseline. The characteristics of the participants at baseline and year 10 are displayed in Table 1. Participants at year 10 were more likely to be taking antihypertensive or lipid‐lowering therapies, increased heart rate, and higher prevalence of diabetes mellitus. However, body mass index was similar between baseline and year 10. Increase in LV mass index and decrease in LV volume indices were observed at year 10.
Table 1.
Participant Characteristics at Baseline and Year 10, the MESA Study (2001–2011)
| N=2416 | Baseline | Year 10 | P Value* |
|---|---|---|---|
| Age, mean (SD), y | 59.6 (9.3) | ||
| Women, n (%) | 2470 (51.1) | ||
| Race/ethnicity, n (%) | |||
| White | 1043 (43.2) | ||
| African‐American | 532 (22.0) | ||
| Hispanic | 510 (21.1) | ||
| Chinese | 331 (13.7) | ||
| NT‐proBNP, pg/mL, median (IQR) | 46.9 (20.5–91.9) | ||
| Education > HS graduate, n (%) | 1723 (71.3) | ||
| BMI, kg/m2, mean (SD) | 27.6 (4.9) | 27.8 (5.1) | 0.119 |
| Smoking status, n (%) | |||
| Never | 1283 (53.1) | 1135 (47.0) | <0.001 |
| Former | 867 (35.9) | 1108 (45.8) | |
| Current | 226 (11.0) | 173 (7.2) | |
| Physical activity, † MET‐min/wk, median (IQR) | 945.0 (210.0–2100.0) | 1710.0 (720.0–3540.0) | <0.001 |
| Systolic BP, mm Hg, mean (SD) | 122.9 (20.0) | 122.3 (20.0) | 0.305 |
| Diastolic BP, mm Hg, mean (SD) | 71.7 (10.2) | 68.0 (9.9) | <0.001 |
| Heart rate, beats/min, mean (SD) | 62.3 (9.1) | 66.6 (21.3) | <0.001 |
| Antihypertensive medication, n (%) | 739 (30.6) | 1267 (52.4) | <0.001 |
| Diabetes mellitus, n (%) | 201 (8.3) | 390 (16.1) | <0.001 |
| HDL‐C cholesterol, mg/dL, mean (SD) | 51.3 (14.8) | 55.9 (16.7) | <0.001 |
| Total cholesterol, mg/dL, mean (SD) | 193.6 (33.9) | 183.6 (36.8) | <0.001 |
| Statin medication, n (%) | 326 (13.5) | 858 (35.5) | <0.001 |
| CAC score >10, Agatston units, n (%) | 863 (36) | ||
| Left ventricle, mean (SD) | |||
| End‐diastolic mass index, g/m2 | 64.2 (11.2) | 66.3 (13.7) | <0.001 |
| End‐diastolic volume index, mL/m2 | 69.9 (12.1) | 64.9 (14.1) | <0.001 |
| End‐systolic volume index, mL/m2 | 26.1 (6.4) | 24.9 (8.7) | <0.001 |
| Stroke volume index, mL/m2 | 43.8 (8.4) | 40.0 (8.6) | <0.001 |
| Ejection fraction, n % | 62.7 (5.8) | 62.0 (7.3) | <0.001 |
| Cardiac output, L/min, mean (SD) | 5.3 (1.4) | 4.8 (1.7) | <0.001 |
Results are expressed as mean (SD), number (%), or median (IQR). All LV volume and mass measurements were adjusted for body surface area. BMI indicates body mass index; BP, blood pressure; CAC, coronary artery calcium; HDL, high‐density lipoprotein; HS, high school; IQR, interquartile range (Q1–Q3); MESA, Multi‐Ethnic Study of Atherosclerosis; MET, metabolic equivalent of task; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
P values for test of difference between baseline and year 10; paired t‐test for continuous variables; chi‐square or McNemar for categorical variables; or Wilcoxon rank‐sum test for nonparametric variables.
Includes moderate walking exercise, dance, and vigorous sports.
The log (NT‐proBNP) level had significant positive correlations with baseline risk factors: age, high physical activity, systolic blood pressure, antihypertensive medication use, high‐density lipoprotein cholesterol, and CAC score >10. Significant negative correlations of the log (NT‐proBNP) level were observed for baseline risk factors: sex, race/ethnicity, education more than high school, body mass index, current smoking, and diabetes mellitus (Table S2).
Baseline NT‐proBNP and LV Structure and Function
Higher levels of NT‐proBNP were positively and significantly associated with higher multivariable‐adjusted mean values for the baseline LV mass index, end‐diastolic volume index, end‐systolic volume index, and stroke volume index (all P‐trend<0.001), but not with ejection fraction or cardiac output (Table 2, model 2).
Table 2.
Baseline Left Ventricular Structure/Function in Relation to NT‐proBNP Quartiles (N=2416)
| Models* | NT‐proBNP Quartiles (pg/mL) | P Trend † | |||
|---|---|---|---|---|---|
|
Q1 (Low, 5 to <24) N=704 |
Q2 (24 to <55) N=648 |
Q3 (55 to <112) N=608 |
Q4 (High, ≥112) N=456 |
||
| Mean (95% CI) | |||||
| LV end‐diastolic mass index, g/m2 | |||||
| Unadjusted | 65.5 (64.7–66.4) | 64.2 (63.4–65.1) | 62.8 (61.9–63.7) | 64.0 (63.0–65.0) ‡ | 0.001 |
| Model 1 | 62.3 (61.5–63.1) | 64.0 (63.3–64.8) | 64.3 (63.5–65.0) | 67.4 (66.4–68.3)‖ | <0.001 |
| Model 2 | 63.1 (62.3–63.8) | 64.1 (63.4–64.8) | 64.1 (63.4–64.9) | 66.2 (65.3–67.2)‖ | <0.001 |
| LV end‐diastolic volume index, mL/m2 | |||||
| Unadjusted | 68.9 (68.0–69.8) | 69.5 (68.5–70.4) | 69.7 (68.7–70.6) | 72.2 (71.1–73.3)‖ | <0.001 |
| Model 1 | 67.3 (66.4–68.2) | 69.3 (68.5–70.2) | 70.3 (69.4–71.2) | 74.0 (72.8–75.1)‖ | <0.001 |
| Model 2 | 67.9 (67.0–68.8) | 69.4 (68.6–70.3) | 70.2 (69.3–71.1) | 73.1 (72.0–74.2)‖ | <0.001 |
| LV end‐systolic volume index, mL/m2 | |||||
| Unadjusted | 26.8 (26.4–27.3) | 26.0 (25.5–26.5) | 25.5 (25.0–26.0) | 25.8 (25.3–26.4)§ | 0.010 |
| Model 1 | 25.3 (24.8–25.7) | 25.8 (25.4–26.3) | 26.2 (25.7–26.6) | 27.7 (27.1–28.3)‖ | <0.001 |
| Model 2 | 25.5 (25.0–26.0) | 25.9 (25.4–26.3) | 26.1 (25.6–26.6) | 27.4 (26.8–28.0)‖ | <0.001 |
| LV stroke volume index, mL/m2 | |||||
| Unadjusted | 42.6 (42.0–43.3) | 43.5 (42.9–44.2) | 44.0 (43.3–44.6) | 45.6 (44.8–46.4)‖ | <0.001 |
| Model 1 | 42.0 (41.4–42.7) | 43.5 (42.9–44.1) | 44.2 (43.5–44.8) | 46.3 (45.5–47.1)‖ | <0.001 |
| Model 2 | 42.4 (41.8–43.1) | 43.6 (42.9–44.2) | 44.1 (43.4–44.7) | 45.7 (44.9–46.5)‖ | <0.001 |
| LV ejection fraction, % | |||||
| Unadjusted | 61.8 (61.4–62.2) | 62.6 (62.2–63.1) | 63.1 (62.6–63.5) | 63.4 (62.9–63.9)‖ | <0.001 |
| Model 1 | 62.3 (61.9–62.8) | 62.7 (62.3–63.1) | 62.8 (62.4–63.3) | 62.9 (62.3–63.4) | 0.525 |
| Model 2 | 62.4 (61.9–62.8) | 62.7 (62.3–63.1) | 62.8 (62.3–63.2) | 62.8 (62.3–63.4) | 0.783 |
| Cardiac output, L/min | |||||
| Unadjusted | 5.5 (5.4–5.6) | 5.4 (5.3–5.5) | 5.1 (5.0–5.2) | 5.1 (5.0–5.2)‖ | <0.001 |
| Model 1 | 5.3 (5.2–5.4) | 5.4 (5.3–5.5) | 5.2 (5.1–5.3) | 5.4 (5.3–5.5) | 0.911 |
| Model 2 | 5.3 (5.2–5.4) | 5.4 (5.3–5.5) | 5.2 (5.1–5.3) | 5.4 (5.2–5.5) | 0.825 |
All LV volume and mass measurements were adjusted for body surface area. CAC indicates coronary artery calcium; LV, left ventricular; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Model 1: adjusted for age, sex, and race (White [reference], African‐American, Chinese, Hispanic); Model 2: adjusted for all variables in model 1 plus education more than high school, physical activity (sex‐specific tertiles: low, middle, high), current cigarette smoking, diabetes mellitus, body mass index, systolic blood pressure, antihypertensive medication use, high‐density lipoprotein cholesterol, total cholesterol, statin medication use, and prevalence of CAC score >10.
P for linear trend tested by entering log (NT‐proBNP) as a continuous variable in the same model.
P<0.05, § P<0.01, ‖ P<0.001 for comparisons between the highest quartile relative to the lowest quartile (reference category).
Among participants who completed both CMRs at baseline and year 10, LV end‐diastolic volume index decreased, end‐systolic volume index decreased, stroke volume index decreased, ejection fraction decreased, and mass index increased over time (Table 3). Compared with the lowest quartile group, individuals in the highest NT‐proBNP quartile had a smaller risk factor–adjusted decline in LV end‐systolic volume index (−0.47 mL/m2; 95% CI, −1.18 to 0.23; P<0.01), and a greater decline in LV ejection fraction (−1.60%; 95% CI, −2.26 to −0.94; P<0.01) over time. No significant associations were noted between baseline NT‐proBNP levels and changes in LV mass, end‐diastolic volume, stroke volume, and cardiac output over time (model 2). In addition, there was no significant interaction between baseline NT‐proBNP levels and sex in relation to the association of any of the baseline LV parameters and 10‐year change in each LV parameter, adjusted for age (all P for interaction >0.05).
Table 3.
10‐Year Changes in LV Structure/Function in Relation to Baseline NT‐proBNP Quartiles (N=2416)
| Models* | NT‐proBNP Quartiles (pg/mL) | P Trend † | |||
|---|---|---|---|---|---|
|
Q1 (Low, 5 to <24) N=704 |
Q2 (24 to <55) N=648 |
Q3 (55 to <112) N=608 |
Q4 (High, ≥112) N=456 |
||
| Mean (95% CI) | |||||
| Δ LV end‐diastolic mass index, g/m2 | |||||
| Unadjusted | 3.27 (2.55 to 3.98) | 2.49 (1.75 to 3.24) | 1.74 (0.97 to 2.50) | 0.23 (−0.65 to 1.12)‖ | <0.001 |
| Model 1 | 2.52 (1.77 to 3.28) | 2.45 (1.73 to 3.18) | 2.13 (1.37 to 2.88) | 0.91 (−0.02 to 1.84)§ | 0.001 |
| Model 2 | 1.92 (1.19 to 2.65) | 2.38 (1.69 to 3.06) | 2.16 (1.45 to 2.88) | 1.90 (1.00 to 2.81) | 0.399 |
| Δ LV end‐diastolic volume index, mL/m2 | |||||
| Unadjusted | −4.50 (−5.39 to −3.61) | −4.55 (−5.47 to −3.62) | −4.69 (−5.64 to −3.73) | −6.60 (−7.71 to −5.49)§ | 0.001 |
| Model 1 | −4.81 (−5.77 to −3.85) | −4.59 (−5.50 to −3.67) | −4.48 (−5.44 to −3.52) | −6.34 (−7.52 to −5.16) ‡ | 0.036 |
| Model 2 | −5.56 (−6.47 to −4.64) | −4.78 (−5.63 to −3.92) | −4.40 (−5.29 to −3.50) | −5.02 (−6.15 to −3.89) | 0.532 |
| Δ LV end‐systolic volume index, mL/m2 | |||||
| Unadjusted | −1.61 (−2.16 to −1.06) | −1.11 (−1.67 to −0.55) | −1.01 (−1.59 to −0.43) | −0.76 (−1.44 to −0.08) ‡ | 0.046 |
| Model 1 | −1.45 (−2.03 to −0.87) | −1.14 (−1.69 to −0.58) | −1.00 (−1.58 to −0.42) | −0.98 (−1.70 to −0.27) | 0.071 |
| Model 2 | −1.69 (−2.26 to −1.13) | −1.23 (−1.77 to −0.70) | −0.99 (−1.56 to −0.43) | −0.47 (−1.18 to 0.23)§ | 0.030 |
| Δ LV stroke volume index, mL/m2 | |||||
| Unadjusted | −3.40 (−4.06 to −2.74) | −3.47 (−4.16 to −2.78) | −3.47 (−4.18 to −2.76) | −5.29 (−6.11 to −4.47)‖ | 0.001 |
| Model 1 | −3.36 (−4.08 to −2.64) | −3.45 (−4.14 to −2.76) | −3.49 (−4.20 to −2.77) | −5.35 (−6.24 to −4.47)‖ | 0.002 |
| Model 2 | −4.12 (−4.74 to −3.50) | −3.58 (−4.16 to −3.00) | −3.34 (−3.95 to −2.74) | −4.18 (−4.95 to −3.42) | 0.890 |
| Δ LV ejection fraction, % | |||||
| Unadjusted | −0.24 (−0.81 to 0.33) | −0.63 (−1.20 to −0.05) | −0.44 (−1.04 to 0.15) | −1.69 (−2.39 to −0.99)§ | 0.009 |
| Model 1 | −0.29 (−0.89 to 0.30) | −0.59 (−1.16 to −0.01) | −0.50 (−1.09 to 0.10) | −1.60 (−2.34 to −0.87)§ | 0.031 |
| Model 2 | −0.40 (−0.94 to 0.13) | −0.53 (−1.03 to −0.03) | −0.44 (−0.96 to 0.09) | −1.60 (−2.26 to −0.94)§ | 0.010 |
| Δ Cardiac output, L/min | |||||
| Unadjusted | −0.43 (−0.57 to −0.29) | −0.53 (−0.67 to −0.38) | −0.39 (−0.54 to −0.24) | −0.71 (−0.88 to −0.53)§ | 0.047 |
| Model 1 | −0.45 (−0.60 to −0.30) | −0.53 (−0.68 to −0.39) | −0.38 (−0.53 to −0.23) | −0.68 (−0.87 to −0.49) | 0.181 |
| Model 2 | −0.45 (−0.58 to −0.32) | −0.49 (−0.62 to −0.36) | −0.45 (−0.58 to −0.32) | −0.65 (−0.82 to −0.48) | 0.068 |
All LV volume and mass measurements were adjusted for body surface area. Change (Δ) in LV structure/function was characterized by changes over 10 years from baseline to follow‐up (year 10−baseline). CAC indicates coronary artery calcium; LV, left ventricular; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Model 1: adjusted for age, sex, and race (White [reference], African‐American, Chinese, Hispanic); Model 2: adjusted for all variables in model 1 plus education more than high school, physical activity (sex‐specific tertiles: low, middle, high), current cigarette smoking, diabetes mellitus, body mass index, systolic blood pressure, antihypertensive medication use, high‐density lipoprotein cholesterol, total cholesterol, statin medication use, prevalence of CAC score >10, and baseline LV structure/function of outcome of interest.
P for linear trend tested by entering log (NT‐proBNP) as a continuous variable in the same model.
P<0.05, § P<0.01, ‖ P<0.001 for comparisons between the highest quartile relative to the lowest quartile (reference category).
Baseline NT‐proBNP and Subsequent LV Strain, Dyssynchrony, Myocardial Scar and Scar Extent
Table 4 shows the adjusted mean values of year 10 LV strain and dyssynchrony parameters at the global and regional (basal, mid, and apical) levels in relation to NT‐proBNP quartiles. Higher levels of NT‐proBNP were independently associated with higher subsequent risk factor–adjusted mean SD of TPS at the global (3.9 ms; 95% CI, 3.8–4.0; P‐trend=0.016), mid and apical regions (P‐trend=0.043 and 0.041, respectively), as well as higher ECC at the basal region (P‐trend=0.014). The associations between NT‐proBNP levels and the other ECC regions were largely nonsignificant.
Table 4.
Global and Regional LV Strain and Dyssynchrony at Year 10 in Relation to NT‐proBNP Quartiles (N=2211)
| Models* | NT‐proBNP Quartiles (pg/mL) | P Trend † | |||
|---|---|---|---|---|---|
|
Q1 (Low, 5 to <24) N=641 |
Q2 (24 to <55) N=582 |
Q3 (55 to <112) N=564 |
Q4 (High, ≥112) N=424 |
||
| Adjusted Mean (95% CI) | |||||
| Peak systolic circumferential strain (ECC), % | |||||
| Global | |||||
| Model 1 | −18.1 (−18.3 to −17.9) | −18.1 (−18.3 to −17.9) | −18.1 (−18.3 to −17.9) | −18.0 (−18.2 to −17.8) | 0.637 |
| Model 2 | −18.2 (−18.4 to −18.0) | −18.1 (−18.3 to −17.9) | −18.0 (−18.2 to −17.8) | −17.9 (−18.2 to −17.7) | 0.132 |
| Basal | |||||
| Model 1 | −17.0 (−17.2 to −16.7) | −16.9 (−17.2 to −16.7) | −16.8 (−17.1 to −16.6) | −16.4 (−16.8 to −16.3) ‡ | 0.106 |
| Model 2 | −17.0 (−17.2 to −16.8) | −17.0 (−17.2 to −16.7) | −16.7 (−17.0 to −16.5) | −16.4 (−16.7 to −16.2)§ | 0.014 |
| Mid | |||||
| Model 1 | −18.1 (−18.3 to −17.9) | −18.1 (−18.3 to −17.9) | −18.2 (−18.4 to −17.9) | −18.1 (−18.4 to −17.8) | 0.746 |
| Model 2 | −18.1 (−18.4 to −17.9) | −18.1 (−18.3 to −17.9) | −18.1 (−18.3 to −17.9) | −18.0 (−18.3 to −17.8) | 0.716 |
| Apical | |||||
| Model 1 | −19.3 (−19.5 to −19.0) | −19.2 (−19.4 to −19.0) | −19.2 (−19.4 to −19.0) | −19.2 (−19.4 to −19.1) | 0.965 |
| Model 2 | −19.3 (−19.5 to −19.1) | −19.2 (−19.5 to −19.0) | −19.2 (−19.5 to −19.0) | −19.1 (−19.4 to −18.9) | 0.046 |
| Standard deviation of time to peak systolic circumferential strain, ms | |||||
| Global | |||||
| Model 1 | 3.7 (3.6 to 3.9) | 3.8 (3.7 to 3.8) | 3.8 (3.7 to 3.9) | 3.9 (3.8 to 4.0) ‡ | 0.014 |
| Model 2 | 3.7 (3.6 to 3.8) | 3.8 (3.7 to 3.8) | 3.8 (3.7 to 3.9) | 3.9 (3.8 to 4.0) ‡ | 0.016 |
| Basal | |||||
| Model 1 | 3.6 (3.5 to 3.8) | 3.6 (3.5 to 3.7) | 3.6 (3.5 to 3.7) | 3.7 (3.6 to 3.9) | 0.447 |
| Model 2 | 3.7 (3.5 to 3.8) | 3.6 (3.5 to 3.7) | 3.6 (3.5 to 3.7) | 3.7 (3.5 to 3.9) | 0.758 |
| Mid | |||||
| Model 1 | 3.6 (3.5 to 3.7) | 3.6 (3.4 to 3.7) | 3.6 (3.5 to 3.7) | 3.7 (3.5 to 3.9) | 0.080 |
| Model 2 | 3.6 (3.5 to 3.7) | 3.6 (3.4 to 3.7) | 3.6 (3.5 to 3.7) | 3.7 (3.5 to 3.8) | 0.043 |
| Apical | |||||
| Model 1 | 3.3 (3.2 to 3.4) | 3.4 (3.3 to 3.5) | 3.4 (3.3 to 3.6) | 3.4 (3.2 to 3.5) | 0.073 |
| Model 2 | 3.3 (3.2 to 3.4) | 3.4 (3.3 to 3.5) | 3.4 (3.3 to 3.6) | 3.5 (3.3 to 3.6) ‡ | 0.041 |
ECC denotes circumferential shortening and is normally negative; less negative values of ECC reflect decreased regional function. CAC, coronary artery calcium; LV, left ventricular; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Model 1: adjusted for age, sex, and race (White [reference], African‐American, Chinese, Hispanic); Model 2: adjusted for all variables in model 1 plus education more than high school, physical activity (sex‐specific tertiles: low, middle, high), current cigarette smoking, diabetes mellitus, body mass index, systolic blood pressure, antihypertensive medication use, high‐density lipoprotein cholesterol, total cholesterol, statin medication use, prevalence of CAC score >10, and LV mass.
P for linear trend tested by entering log (NT‐proBNP) as a continuous variable in the same model.
P<0.05, § P<0.01.
In this sample, the overall prevalence of CMR‐defined myocardial scar at year 10 was 7.5% (113/1498; Table 5). Table 5 presents the odds ratios and 95% CIs of developing myocardial scar for the total group and by women and men separately over 10‐year follow‐up periods, adjusted for baseline cardiovascular risk factors. In age‐, race‐, and sex‐adjusted analyses for the total sample, the odds ratio of developing scar at year 10 for individuals in the highest quartile was 2.9 (95% CI, 1.5–5.6) versus the lowest quartile (Table 5, model 1). Adjustment for baseline CVD risk factors only slightly attenuated the results (model 2). For the total sample, the association of NT‐proBNP levels with risk of myocardial scar was positive, significant, and graded (P‐trend=0.012; Table 5, model 2).
Table 5.
Risk of Myocardial Scar at Year 10 in Relation to Baseline NT‐proBNP Quartiles, Total and by Sex
| Models* | NT‐proBNP Quartiles (pg/mL) | P Trend † | |||
|---|---|---|---|---|---|
|
Q1 (5 to <24) Reference |
Q2 (24 to <55) | Q3 (55 to <112) | Q4 (≥112) | ||
| Odds Ratio (95% CI) ‡ | |||||
| Total myocardial scar | |||||
| No. of cases/total (113/1498) | 30/492 | 30/406 | 24/358 | 29/242 | |
| Unadjusted | 1.0 | 1.2 (0.7–2.1) | 1.1 (0.6–1.9) | 2.1 (1.2–3.6) ‖ | 0.018 |
| Model 1 | 1.0 | 1.3 (0.8–2.3) | 1.3 (0.7–2.5) | 2.9 (1.5–5.6)¶ | 0.003 |
| Model 2 | 1.0 | 1.3 (0.7–2.2) | 1.2 (0.6–2.3) | 2.7 (1.4–5.5) ‖ | 0.012 |
|
Lower 3 quartiles combined (5 to <112) # Reference |
Q4 (≥112) | P Trend † | |
|---|---|---|---|
| Women | |||
| No. of cases /total (16/708) | 5/535 | 11/173 | |
| Unadjusted | 1.0 | 7.2 (2.4–21.0)¶ | <0.001 |
| Model 1 | 1.0 | 6.9 (2.2–21.0)¶ | <0.001 |
| Model 2 | 1.0 | 7.2 (2.1–24.4)¶ | 0.001 |
| Men | |||
| No. of cases/total (97/790) | 79/721 | 18/69 | |
| Unadjusted | 1.0 | 2.9 (1.6–5.1) | <0.001 |
| Model 1 | 1.0 | 1.7 (0.9–3.1) | 0.109 |
| Model 2 | 1.0 | 1.8 (0.9–3.5) | 0.101 |
Association of NT‐proBNP with risk of scar differed by sex (P for interaction=0.018; adjusted for age). NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide.
Model 1: adjusted for age, sex (total only), and race (White [reference], African‐American, Chinese, Hispanic); Model 2: adjusted for all variables in model 1 plus education more than high school, physical activity (sex‐specific tertiles: low, middle, high), current cigarette smoking, diabetes mellitus, body mass index, systolic blood pressure, antihypertensive medication use, high‐density lipoprotein cholesterol, total cholesterol, statin medication use, and prevalence of coronary artery calcium score >10.
P for linear trend tested by entering log (NT‐proBNP) as a continuous variable in the same model.
Odds ratio represents the odds of having scar at year 10 in the NT‐proBNP quartile relative to the reference category, adjusting for all other covariates in the model.
P<0.01, ¶ P<0.001 for comparisons between the highest quartile relative to the reference category.
Because the rate of myocardial scar was low in the lower 3 NT‐proBNP categories in women, the lower 3 NT‐proBNP categories were combined and served as the reference group in these analyses.
Significant interaction was observed between baseline NT‐proBNP levels and sex for risk of myocardial scar during the 10‐year follow‐up period (P for interaction<0.018; adjusted for age). Because the rate of myocardial scar was low in the lower 3 NT‐proBNP categories for women, the lower 3 NT‐proBNP categories were combined and served as the reference group in these analyses for women and men. Women in the highest quartile of NT‐proBNP also had higher odds of having scar (odds ratio, 7.2; 95% CI, 2.1–24.4; P<0.001) compared with those in the reference quartile after accounting for CVD risk factors (Table 5, model 2), but no significant association was observed among men (Table 5, model 2). Of note, estimates for women were not as stable as those for other groups, as indicated by the wide CIs, potentially attributable to small numbers of cases of myocardial scar in this group.
In stratified analyses, we found that the association between baseline NT‐proBNP and risk of developing scar varied by myocardial scar groups (Table 6). This positive and significant association was also found for ischemic scar group (P‐trend=0.04; Table 6, model 2). No significant association was observed between baseline NT‐proBNP levels and nonischemic scar.
Table 6.
Risk of Myocardial Scar at Year 10 in Relation to Baseline NT‐proBNP Quartiles, by Myocardial Scar Groups
| Models* | NT‐proBNP Quartiles (pg/mL) | P Trend † | |||
|---|---|---|---|---|---|
|
Q1 (5 to <24) Reference |
Q2 (24 to <55) | Q3 (55 to <112) | Q4 (≥112) | ||
| Odds Ratio (95% CI) ‡ | |||||
| Ischemic scar | |||||
| No. of cases/total (51/1498) | 13/492 | 8/406 | 14/358 | 16/242 | |
| Unadjusted | 1.0 | 0.7 (0.3–1.8) | 1.5 (0.7–3.2) | 2.6 (1.2–5.5)‖ | 0.006 |
| Model 1 | 1.0 | 0.7 (0.3–1.8) | 1.6 (0.7–3.6) | 2.8 (1.2–6.9) § | 0.013 |
| Model 2 | 1.0 | 0.7 (0.3–1.7) | 1.3 (0.6–3.2) | 2.5 (1.1–6.8) § | 0.040 |
| Nonischemic scar | |||||
| No. of cases/total (62/1498) | 17/492 | 22/406 | 10/358 | 13/242 | |
| Unadjusted | 1.0 | 1.6 (0.8–3.1) | 0.8 (0.4–1.8) | 1.6 (0.8–3.3) | 0.494 |
| Model 1 | 1.0 | 1.8 (0.9–3.6) | 1.1 (0.5–2.5) | 2.3 (1.0–5.5) § | 0.103 |
| Model 2 | 1.0 | 1.8 (0.9–3.7) | 1.0 (0.4–2.4) | 2.3 (0.9–5.9) | 0.148 |
NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide.
Model 1: adjusted for age, sex, and race (White [reference], African‐American, Chinese, Hispanic); Model 2: adjusted for all variables in model 1 plus education more than high school, physical activity (sex‐specific tertiles: low, middle, high), current cigarette smoking, diabetes mellitus, body mass index, systolic blood pressure, antihypertensive medication use, high‐density lipopeotein cholesterol, total cholesterol, statin medication use, and prevalence of coronary artery calcium score >10.
P for linear trend tested by entering log (NT‐proBNP) as a continuous variable in the same model.
Odds ratio represents the odds of having scar at year 10 in the NT‐proBNP quartile relative to the reference category, adjusting for all other covariates in the model.
P<0.05, ‖ P<0.01.
Among the 113 participants with myocardial scar at year 10, the median total extent of scarring was 3.3% (interquartile range, 1.4–7.2%). The average extent of myocardial scarring of ischemic scar was 9.0% (95% CI, 6.7–11.3), whereas that of nonischemic scar was 3.2% (95% CI, 1.9–4.5; P<0.001). Continuous percentage of LV scars were not investigated because of the low prevalence of scar in this relatively healthy adult cohort without concurrent overt diseases and the skewness of the data.
Additional Analyses
To assess the potential role of cardiovascular risk factor progression over time, adjustment for 10‐year change in baseline cardiovascular risk factors were examined in additional analyses, and similar results were observed (data not shown). We also repeated the fully adjusted model for each outcome after excluding individuals with clinical CVD (myocardial infarction, resuscitated cardiac arrest, angina, congestive heart failure, and stroke) between baseline and follow‐up (N=126; 5.2% of the study population) and did not yield materially different results (data not shown).
Discussion
In this longitudinal study of a cohort free of clinical CVD at baseline, we observed that elevated baseline NT‐proBNP concentration was associated with significant longitudinal decrease in LVEF, more severe cardiac dyssynchrony, and higher odds of developing myocardial scar.
Our findings that higher baseline NT‐proBNP levels are significantly associated with lower LVEF, and more residual blood at end systole over 10‐year follow‐up reflect a possible increase in LV filling pressure, a marker of diastolic dysfunction. A positive association between high BNP (B‐type natriuretic peptide) levels and diastolic LV filling pressure has been reported previously in patients with recent myocardial infarction. 18 In a community‐based cohort of individuals free of heart failure, diastolic dysfunction was more common among those with greater BNP levels. 19 Furthermore, increased BNP levels were previously suggested to serve as the hallmark of diastolic heart failure independent of LV hypertrophy. 20
Association of NT‐proBNP with diastolic dysfunction is also reported in a cross‐sectional study of the MESA cohort 3 that showed elevated log–NT‐proBNP is related to increment of native T1 and extracellular volume fraction, imaging markers of microscopic myocardial fibrosis that play a crucial role in the development of diastolic dysfunction. These results suggest that increased fibrosis leads to decreased LV relaxation, increased stiffness, and elevated LV filling pressures, with a subsequent increase in NT‐proBNP levels.
Previously, Wang et al 2 reported in participants without heart failure from the Framingham study a significant increase in the risk of death, first cardiovascular event, heart failure, atrial fibrillation, and stroke with increase in log‐BNP level during a mean follow‐up of 5.2 years. Moreover, it was previously shown that a single measure of NT‐proBNP predicts incidence of heart failure in asymptomatic individuals beyond traditional cardiovascular risk factors and LV hypertrophy in a multiethnic population. 21
Our study was conducted among a relatively healthier cohort of adults without prevalent CVD at baseline. Although the overall prevalence of myocardial scar at year 10 was low in our study (7.5%) compared with other higher‐risk cohorts, the association of NT‐proBNP levels with risk of myocardial scar was positive, significant, and graded. In a previous MESA study, the prevalence of myocardial scar was reported 7.9% at year 10, while 78% of these participants were unrecognized by ECG or clinical assessment. 7 In the ICELAND study, 22 in elderly participants, the prevalence of recognized and unrecognized myocardial scar detected by CMR were 9.7% and 17%, respectively. After adjustment for risk factors, unrecognized myocardial scar remained associated with mortality, 22 emphasizing the importance of early identification of patients with myocardial scar. We found that the higher chance of developing myocardial scar is sex specific, and in women, higher NT‐proBNP level was associated with higher odds of myocardial scar at year 10. This is in keeping with prior findings from a MESA study, which reported that a higher extent of dyssynchrony in women but not in men predicted major adverse cardiovascular events, including myocardial infarction, heart failure, stroke, and death. 23
Our results further support the concept that how NT‐proBNP is related to cardiovascular events or death reported in prior community‐based studies. 2 , 24 Elevated NT‐proBNP levels are related to a higher chance of developing macroscopic scar over time as seen in our study, as well as having microscopic scar (indicated by high native T1 and extracellular volume fraction values) as reported in another cross‐sectional MESA study. 3 The mechanism of involvement of NT‐proBNP in CVD was not well understood. Replacement of viable myocardial tissue with fibrotic noncontractile tissue can subsequently result in decreased LVEF and ECC, less synchronized myocardial contraction, or more severe dyssynchrony and a higher chance of arrythmia, which all predisposes the patients to a higher risk of major cardiovascular events or death. These results further reiterate the importance of prior suggestions that measurement of NT‐proBNP can be part of the patient's evaluation and help to establish the diagnosis and prognosis of heart failure in outpatient settings. 25
NT‐proBNP is the metabolically inactive cleavage product of the prohormone proBNP. The active hormone BNP was not measured in MESA, but previous studies have shown that inactive NT‐proBNP is believed to be secondary to increased myocardial stretch. 26 The significant association between higher baseline NT‐proBNP levels and smaller decline in LV end‐systolic volume over 10‐year follow‐up seen here means more residual blood at end systole, which can cause increased myocardial stretch and subsequently triggers synthesis of proBNP. Elevated BNP levels were related to cardiac geometric remodeling and increasing sphericity indices, and a lower LVEF in patients with stable chronic ischemic cardiomyopathy. 27 Higher NT‐proBNP concentrations were also significantly associated with larger infarct size and lower LVEF in patients with ST elevation myocardial infarction. 28 Reduced myocardial perfusion was also associated with increased NT‐proBNP in a cross‐sectional MESA study, which probably reflects microvascular dysfunction, 1 and presence of fibrotic tissue needless of blood flow. Elevated baseline NT‐proBNP and its significant association with more severe dyssynchrony seen in our 10‐year follow‐up may indicate changes in both regional and global cardiac contractility, which are likely secondary to underlying fibrosis. Moreover, NT‐proBNP is reported to be strongly associated with the incidence of atrial fibrillation in a population‐based cohort. 9
The association of NT‐proBNP with impaired ECC and more severe dyssynchrony is important. Development of dyssynchrony can be used as the surrogate of asymptomatic LV function impairment, since alterations in the synchronization of LV myocardial contraction precede impairment in global LV function. 16 Inverse association of myocardial perfusion at rest with greater extent of myocardial dyssynchrony and increased time to peak systolic deformation has been reported previously in a MESA study. 6 Moreover, in asymptomatic individuals, lower myocardial flow reserve was correlated with reduced regional function, expressed as lower ECC. 29 Even in patients with prior myocardial infarction, although LV scar burden was associated with the occurrence of ventricular tachycardia during follow‐up, but LV dyssynchrony was shown to be independently correlated with occurrence of ventricular tachycardia. 30
We found that individuals with elevated NT‐proBNP levels had higher odds of having myocardial scar and systolic and possibly diastolic function impairment independent of traditional CVD risk factors. Our results further support the hypothesis that both systolic and diastolic dysfunction represent development of myocardial mechanical impairment, and both are likely present in varying degrees in patients with heart failure. 6
Our longitudinal analysis may have been subject to selection bias by limiting analyses to individuals who were able to complete both the baseline and follow‐up CMR. The baseline characteristics of MESA participants included in this analysis demonstrated a healthier cohort compared with those excluded. However, we do not believe selection bias would alter the longitudinal results qualitatively. If selection bias was absent and if the study cohort was less healthy, we would expect to observe directionally similar NT‐proBNP–related longitudinal changes in LV structure/function, dyssynchrony, and development of myocardial scar to an even greater degree than what we observed.
Strengths and Limitations
The strengths of our study include the ability to conduct longitudinal analyses relating baseline NT‐proBNP levels to change in CMR‐assessed LV structure/function, dyssynchrony, and developing of myocardial scar in a well‐characterized, multiethnic cohort, after adjustment for baseline traditional CVD risk factors and CVD risk factor progression over time.
Our study was subject to some limitations. Although we were able to adjust for several covariates given our large sample size, residual confounding is an issue that cannot be ruled out in observational study. Furthermore, the use of correction equations to account for differences in CMR pulse sequence technology and software that occurred between baseline and year 10 to make the data comparable may have also contributed some measurement errors. Although not a limitation per se, the MESA cohort was free of clinical CVD at baseline, our findings cannot be extrapolated to individuals with symptoms or known coronary artery disease.
Conclusions
Among people without prevalent CVD, a higher level of NT‐proBNP was prospectively associated with development of subclinical changes in developing myocardial dysfunction, more severe myocardial dyssynchrony, and higher odds of developing myocardial scar independent of established cardiovascular risk factors. More importantly, our findings lend support to consider earlier screening using biomarkers such as NT‐proBNP in identifying people with subclinical CVD who may benefit most from early therapeutic intervention and provide measures for CVD prevention.
Sources of Funding
This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01‐HC‐95159, 75N92020D00005, N01‐HC‐95160, 75N92020D00002, N01‐HC‐95161, 75N92020D00003, N01‐HC‐95162, 75N92020D00006, N01‐HC‐95163, 75N92020D00004, N01‐HC‐95164, 75N92020D00007, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168 and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute; and by grants UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420 from the National Center for Advancing Translational Sciences.
Disclosures
None.
Supporting information
Acknowledgments
The authors sincerely thank Cheeling Chan, MS, former biostatistician at Northwestern University, for statistical analysis and support. The authors also thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa‐nhlbi.org.
(J Am Heart Assoc. 2021;10:e019243. DOI: 10.1161/JAHA.120.019243.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019243
For Sources of Funding and Disclosures, see page 12.
References
- 1. Mitchell A, Misialek JR, Folsom AR, Duprez D, Alonso A, Jerosch‐Herold M, Sanchez OA, Watson KE, Sallam T, Konety SH. Usefulness of N‐terminal pro‐brain natriuretic peptide and myocardial perfusion in asymptomatic adults (from the Multi‐Ethnic Study of Atherosclerosis). Am J Cardiol. 2015;115:1341–1345. DOI: 10.1016/j.amjcard.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. DOI: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 3. Liu CY, Heckbert SR, Lai S, Ambale‐Venkatesh B, Ostovaneh MR, McClelland RL, Lima JAC, Bluemke DA. Association of elevated NT‐proBNP with myocardial fibrosis in the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2017;70:3102–3109. DOI: 10.1016/j.jacc.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two‐dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. DOI: 10.1016/S0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 5. Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. DOI: 10.1161/01.CIR.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 6. Rosen BD, Fernandes VR, Nasir K, Helle‐Valle T, Jerosch‐Herold M, Bluemke DA, Lima JA. Age, increased left ventricular mass, and lower regional myocardial perfusion are related to greater extent of myocardial dyssynchrony in asymptomatic individuals: the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2009;120:859–866. DOI: 10.1161/CIRCULATIONAHA.108.787408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turkbey EB, Nacif MS, Guo M, McClelland RL, Teixeira PBRP, Bild DE, Barr RG, Shea S, Post W, Burke G, et al. Prevalence and correlates of myocardial scar in a US cohort. JAMA. 2015;314:1945–1954. DOI: 10.1001/jama.2015.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, et al. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. DOI: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 9. Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G, Kronmal RA. N‐terminal pro‐b‐type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi‐Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;99:1832–1836. DOI: 10.1136/heartjnl-2013-304724. [DOI] [PubMed] [Google Scholar]
- 10. Daniels LB, Laughlin GA, Clopton P, Maisel AS, Barrett‐Connor E. Minimally elevated cardiac troponin t and elevated N‐terminal pro‐B‐type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52:450–459. DOI: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi‐Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113:30–37. DOI: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 12. Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch‐Herold M, Pearson G, Sinha S, Arai A, Lima JAC, et al. Cardiovascular function in Multi‐Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–365. DOI: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 13. Ambale Venkatesh B, Volpe GJ, Donekal S, Mewton N, Liu C‐Y, Shea S, Liu K, Burke G, Wu C, Bluemke DA, et al. Association of longitudinal changes in left ventricular structure and function with myocardial fibrosis: the Multi‐Ethnic Study of Atherosclerosis study. Hypertension. 2014;64:508–515. DOI: 10.1161/HYPERTENSIONAHA.114.03697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fernandes VR, Cheng S, Cheng YJ, Rosen B, Agarwal S, McClelland RL, Bluemke DA, Lima JA. Racial and ethnic differences in subclinical myocardial function: the Multi‐Ethnic Study of Atherosclerosis. Heart. 2011;97:405–410. DOI: 10.1136/hrt.2010.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castillo E, Osman NF, Rosen BD, El‐Shehaby I, Pan L, Jerosch‐Herold M, Lai S, Bluemke DA, Lima JA. Quantitative assessment of regional myocardial function with MR‐tagging in a multi‐center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7:783–791. DOI: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 16. Sharma RK, Donekal S, Rosen BD, Tattersall MC, Volpe GJ, Ambale‐Venkatesh B, Nasir K, Wu CO, Polak JF, Korcarz CE, et al. Association of subclinical atherosclerosis using carotid intima‐media thickness, carotid plaque, and coronary calcium score with left ventricular dyssynchrony: the Multi‐Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;239:412–418. DOI: 10.1016/j.atherosclerosis.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. London: John Wiley&Sons; 2000. [Google Scholar]
- 18. Paelinck BP, Vrints CJ, Bax JJ, Bosmans JM, De Hert SG, de Roos A, Lamb HJ. Relation of B‐type natriuretic peptide early after acute myocardial infarction to left ventricular diastolic function and extent of myocardial damage determined by magnetic resonance imaging. Am J Cardiol. 2006;97:1146–1150. DOI: 10.1016/j.amjcard.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 19. McKie PM, Rodeheffer RJ, Cataliotti A, Martin FL, Urban LH, Mahoney DW, Jacobsen SJ, Redfield MM, Burnett JC Jr. Amino‐terminal pro‐B‐type natriuretic peptide and B‐type natriuretic peptide: biomarkers for mortality in a large community‐based cohort free of heart failure. Hypertension. 2006;47:874–880. DOI: 10.1161/01.HYP.0000216794.24161.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamaguchi H, Yoshida J, Yamamoto K, Sakata Y, Mano T, Akehi N, Hori M, Lim YJ, Mishima M, Masuyama T. Elevation of plasma brain natriuretic peptide is a hallmark of diastolic heart failure independent of ventricular hypertrophy. J Am Coll Cardiol. 2004;43:55–60. DOI: 10.1016/j.jacc.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 21. Choi E‐Y, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, Almeida ALC, Yoneyama K, Opdahl A, Jain A, et al. N‐terminal pro‐B‐type natriuretic peptide, left ventricular mass, and incident heart failure: Multi‐Ethnic Study of Atherosclerosis. Circ Heart Fail. 2012;5:727–734. DOI: 10.1161/CIRCHEARTFAILURE.112.968701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890–896. DOI: 10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharma RK, Volpe G, Rosen BD, Ambale‐Venkatesh B, Donekal S, Fernandes V, Wu CO, Carr J, Bluemke DA, Lima JA. Prognostic implications of left ventricular dyssynchrony for major adverse cardiovascular events in asymptomatic women and men: the Multi‐Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2014;3:e000975. DOI: 10.1161/JAHA.114.000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. AbouEzzeddine OF, McKie PM, Scott CG, Rodeheffer RJ, Chen HH, Michael Felker G, Jaffe AS, Burnett JC, Redfield MM. Biomarker‐based risk prediction in the community. Eur J Heart Fail. 2016;18:1342–1350. DOI: 10.1002/ejhf.663. [DOI] [PubMed] [Google Scholar]
- 25. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. DOI: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 26. Kerkela R, Ulvila J, Magga J. Natriuretic peptides in the regulation of cardiovascular physiology and metabolic events. J Am Heart Assoc. 2015;4:e002423. DOI: 10.1161/JAHA.115.002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henkel DM, Glockner J, Miller WL. Association of myocardial fibrosis, B‐type natriuretic peptide, and cardiac magnetic resonance parameters of remodeling in chronic ischemic cardiomyopathy. Am J Cardiol. 2012;109:390–394. DOI: 10.1016/j.amjcard.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 28. Reinstadler SJ, Feistritzer HJ, Reindl M, Klug G, Metzler B. Utility of NT‐proBNP in predicting infarct scar and left ventricular dysfunction at a chronic stage after myocardial infarction. Eur J Intern Med. 2016;29:e16–e18. DOI: 10.1016/j.ejim.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 29. Rosen BD, Lima JA, Nasir K, Edvardsen T, Folsom AR, Lai S, Bluemke DA, Jerosch‐Herold M. Lower myocardial perfusion reserve is associated with decreased regional left ventricular function in asymptomatic participants of the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2006;114:289–297. DOI: 10.1161/CIRCULATIONAHA.105.588525. [DOI] [PubMed] [Google Scholar]
- 30. Leong DP, Hoogslag GE, Piers SR, Hoke U, Thijssen J, Marsan NA, Schalij MJ, Zeppenfeld K, Bax JJ, Delgado V. The relationship between time from myocardial infarction, left ventricular dyssynchrony, and the risk for ventricular arrhythmia: speckle‐tracking echocardiographic analysis. J Am Soc Echocardiogr. 2015;28:470–477. DOI: 10.1016/j.echo.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 31. Carr JC, Simonetti O, Bundy J, Li D, Pereles S, Finn JP. Cine MR angiography of the heart with segmented true fast imaging with steady‐state precession. Radiology. 2001;219:828–834. DOI: 10.1148/radiology.219.3.r01jn44828. [DOI] [PubMed] [Google Scholar]
- 32. Malayeri AA, Johnson WC, Macedo R, Bathon J, Lima JA, Bluemke DA. Cardiac cine MRI: quantification of the relationship between fast gradient echo and steady‐state free precession for determination of myocardial mass and volumes. J Magn Reson Imaging. 2008;28:60–66. DOI: 10.1002/jmri.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MESA cohort participates in the National Heart, Lung, and Blood Institute's Biologic Specimen and Data Repository. The MESA data are available upon request, including data from examinations 1 to 5 used in this analysis. Requests for data can be made through the following Web site: https://biolincc.nhlbi.nih.gov/studies/mesa/.


