Abstract
Background
Edge‐to‐edge transcatheter mitral valve repair as salvage therapy in high surgical risk patients with severe mitral regurgitation presenting with cardiogenic shock (CS) has been described in small case series, but large clinical results have not been reported. This study aimed to evaluate outcomes of transcatheter mitral valve repair with MitraClip in patients with mitral regurgitation and CS using a large national database.
Methods and Results
From January 2014 to March 2019, we identified hospitalizations for CS in patients with mitral valve disease using data from Centers for Medicare and Medicaid Services. Those with a prior surgical or percutaneous mitral valve intervention were excluded. We compared survival between patients who underwent MitraClip during the index hospitalization and those who did not using propensity‐matched analysis. The analysis included 38 166 patients (mean age, 71±11 years, 41.6% women) of whom 622 (1.6%) underwent MitraClip. MitraClip was increasingly used during CS hospitalizations over the study period (P<0.001). After matching, patients receiving MitraClip had significantly lower in‐hospital mortality (odds ratio, 0.6; 95% CI, 0.47–0.77; P<0.001) and 1‐year mortality (hazard ratio, 0.76; 95% CI, 0.65–0.88; P<0.001) compared with those without MitraClip. The survival benefit associated with MitraClip was consistent across subgroups of interest, with the exception of patients requiring acute mechanical circulatory support or hemodialysis at index.
Conclusions
In patients with mitral regurgitation presenting with CS, use of MitraClip is increasing and associated with greater in‐hospital and 1‐year survival. Further studies are warranted to optimize patient selection and procedure timing for those receiving MitraClip as a treatment option in CS.
Keywords: all‐cause mortality, cardiogenic shock, MitraClip, mitral regurgitation, transcatheter mitral valve repair
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Mortality/Survival, Cardiopulmonary Resuscitation and Emergency Cardiac Care, Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- CCS
Clinical Classification Software
- CS
cardiogenic shock
- MCS
mechanical circulatory support
- MR
mitral regurgitation
- TMVr
transcatheter mitral valve repair
Clinical Perspective
What Is New?
This study reports clinical outcomes for a large nationwide cohort of patients who underwent transcatheter mitral valve repair with MitraClip in the setting of cardiogenic shock.
Over the study period from 2014 through early 2019, the use of transcatheter mitral valve repair during cardiogenic shock hospitalizations increased significantly.
In a matched‐cohort analysis of 1192 patients, MitraClip therapy was associated with higher in‐hospital and 1‐year survival in patients with mitral regurgitation and cardiogenic shock.
What Are the Clinical Implications?
MitraClip may be considered as a therapeutic option for some critically ill patients.
Clinical studies on the patient and procedural characteristics associated with MitraClip in the setting of cardiogenic shock could further improve outcomes by optimizing procedural timing and patient selection.
Management of severe mitral regurgitation (MR) in patients presenting with cardiogenic shock (CS) remains challenging because of complications including respiratory failure from acute pulmonary edema and multiorgan dysfunction. 1 , 2 Inotropic and mechanical circulatory support may be lifesaving, but MR and shock may persist without definitive treatment. Surgical mitral valve repair or replacement in these critically ill patients carries significant mortality and morbidities. 3 The typical pathology of severe MR in CS consists of either a flail leaflet segment secondary to ruptured chordae or papillary muscle because of acute myocardial infarction, or progressive decompensation with chronic MR from leaflet restriction because of ischemic or non‐ischemic cardiomyopathy. Given the high surgical risk in this patient population, edge‐to‐edge transcatheter mitral valve repair (TMVr) has been attempted as salvage therapy in isolated case reports or small case series. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Currently, the only US Food and Drug Administration‐approved edge‐to‐edge TMVr device is the MitraClip system (Abbott, Santa Clara, CA). Large clinical series on the safety and efficacy of MitraClip in critically ill patients with CS have not been reported. Our study aims to evaluate the short‐ and mid‐term outcomes associated with MitraClip therapy in patients with CS derived from a large national database.
Methods
Data Source
This retrospective study used data from the Centers for Medicare and Medicaid Services, consisting of Part A and Part B institutional claims for US Fee‐for‐Service Medicare beneficiaries, as well as deidentified patient demographics (age, sex, geographic location, race, or ethnicity), date of death if applicable, and Medicare insurance enrollment information. Our study used a deidentified database and thus was exempt from institutional review board approval. Deidentified health information can be used as specified in the Health Insurance Portability and Accountability Act Privacy Rule, and informed consent was therefore exempt. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
Patients were eligible for study inclusion if they had a primary or secondary diagnosis of CS (International Classification of Diseases, Ninth Edition, [ICD‐9] 785.51, International Classification of Diseases, Tenth Revision [ICD‐10] R57.0) during an inpatient encounter occurring January 1, 2014 through March 31, 2019. To account for hospital transfers within a single episode of care, the index event included all inpatient encounters following the initial CS diagnosis if a subsequent hospital admission occurred within 24 hours of hospital discharge (“consecutive claims”). Only index events that occurred at, or were transferred to, hospitals where MitraClip was performed were included in the analysis. Patients with a diagnosis of mitral valve disease occurring during or before the index event were included, while those with a prior surgical or percutaneous mitral valve intervention were excluded. The study cohort was further limited to patients with continuous, Fee‐for‐Service (non‐health maintenance organization) Medicare enrollment for at least 1 year before hospital admission and at least 30 days after hospital discharge, retaining those who died at any time following the index event. To evaluate the impact of MitraClip on clinical outcomes in the setting of CS, the final study cohort was dichotomized into patients who underwent a MitraClip procedure (ICD‐9 35.97, ICD‐10 02UG3JZ) during the index event (MitraClip group) versus those who did not (non‐MitraClip group).
As a proxy for CS etiology, Clinical Classification Software (CCS) developed through the Healthcare Cost and Utilization Project was used to classify each CS index event (Table S1). The CCS is a tool for clustering patient diagnoses and procedures into clinically meaningful categories and has been used previously to study CS. 19 , 20 Baseline comorbidities were identified using primary and secondary diagnosis codes captured during inpatient and outpatient encounters occurring before the index event (Table S2). 21 , 22 Propensity scores for undergoing MitraClip were computed for every patient in the study cohort on the basis of a multivariable logistic regression model. Patient characteristics incorporated into the propensity score were quintiles for age, sex, race, and pre‐selected baseline comorbidities including hypertension, chronic heart failure, pulmonary disease, renal disease, peripheral vascular disease, atrial fibrillation, cerebrovascular disease, prior ventricular tachycardia or fibrillation, prior myocardial infarction, concomitant tricuspid regurgitation, and aortic stenosis. Hospitalization characteristics included in the propensity score were year of index admission, the presence of consecutive claims during the index event (ie, hospital transfers), and the use of acute mechanical circulatory support (intra‐aortic balloon pump, extracorporeal membrane oxygenation, or Impella), mechanical ventilation, hemodialysis, and coronary interventions (percutaneous coronary intervention or coronary artery bypass grafting) during the index event. Propensity score matching was performed using a nearest neighbor approach with a caliper of 0.2, followed by exact matching on CCS categories for the index event.
Index Event and Long‐Term Outcomes
For patients undergoing MitraClip during the index event, the timing of the MitraClip procedure relative to other pre‐selected hospital procedures (eg, percutaneous coronary intervention, intra‐aortic balloon pump, mechanical ventilation) was reported based on procedure dates available in the Medicare inpatient claims data. The median number of days from admission or discharge relative to the MitraClip procedure was computed based on admission date and discharge date, respectively. Total length‐of‐stay, number of days spent in an intensive or coronary care unit, and discharge status are reported for each matched group. In‐hospital mortality is defined based on date of death relative to admission and discharge dates for the index event.
To assess long‐term outcomes, follow‐up through June 30, 2019 was available in the Medicare claims data. All‐cause mortality through 1‐year post index hospitalization included deaths that occurred during the index hospitalization. A landmark analysis evaluated all‐cause mortality from the time of live discharge, excluding those patients who died during the index event. For the main analysis, patients were censored at the time of heart transplant, implant of a left ventricular assist device (LVAD), surgical or transcatheter mitral valve intervention occurring after the index event, or at the end of Medicare enrollment. A sensitivity analysis was performed using a composite end point of death, heart transplant, or LVAD implant.
Given the acute critical nature of CS and that patients in the MitraClip group had to survive the index hospitalization until the time of the MitraClip procedure, a second sensitivity analysis was performed to mitigate potential immortal time bias. An additional person‐time variable of “treatment time” was incorporated into the propensity score matching algorithm: for the MitraClip arm, treatment time was the number of days from hospital admission to MitraClip procedure; for the control group, a random time between the admission date and the date of discharge or death was assigned to each patient in order to calculate the treatment time, as described previously. 23 For the second sensitivity analysis, patients were matched with treatment time incorporated into the propensity score matching algorithm and the outcomes of in‐hospital and 1‐year all‐cause mortality were re‐assessed.
Predictors of Mortality After MitraClip
To identify independent predictors of in‐hospital and 1‐year mortality, a multivariable analysis was performed. The predictors of interest were age, sex, race and patient comorbidities including concomitant aortic or tricuspid valve disease, hypertension, chronic heart failure, pulmonary disease, renal failure, peripheral vascular disorder, prior ventricular tachycardia or fibrillation, atrial fibrillation or flutter, prior acute myocardial infarction, and cerebral vascular disease. For in‐hospital mortality, logistic regression was used to perform a multivariable analysis on the adjusted effect of each predictor by including all covariates in the model. Similarly, Cox regression was used to assess 1‐year mortality.
Statistical Analysis
Continuous variables were compared using Student t‐test or Mann‐Whitney test if the distribution was not normal and categorical variables were compared using a Chi‐square (χ2) test. The standardized mean differences were computed for all variables used for matching, and standardized mean differences values >0.1 were considered meaningful. Event‐free survival was estimated by the Kaplan‐Meier method from the index hospitalization until 1 year post discharge, and differences between matched cohorts were compared using a univariate Cox proportional hazards model. The proportional hazards assumption was tested using Schoenfeld residuals and was met. Results in relevant subgroups defined by age, sex, race, and characteristics of the index hospitalization are reported on the basis of crude mortality rates using the log‐rank test. All analyses were performed on R version 3.6.0 and matching was performed using the MatchIt package.
Results
Study Population
Of 320 204 US Medicare beneficiaries with a diagnosis of CS during an inpatient encounter occurring from January 2014 through March 2019, there were 38 166 patients who met study inclusion and exclusion criteria. Within the final study cohort, there were 622 patients with a MitraClip procedure during the index inpatient event and 37 544 patients without. After 1:1 propensity and exact matching, each arm included 596 patients, resulting in a total 1192 patients for analysis (Figure 1).
Figure 1. Cohort diagram for MitraClip in cardiogenic shock.
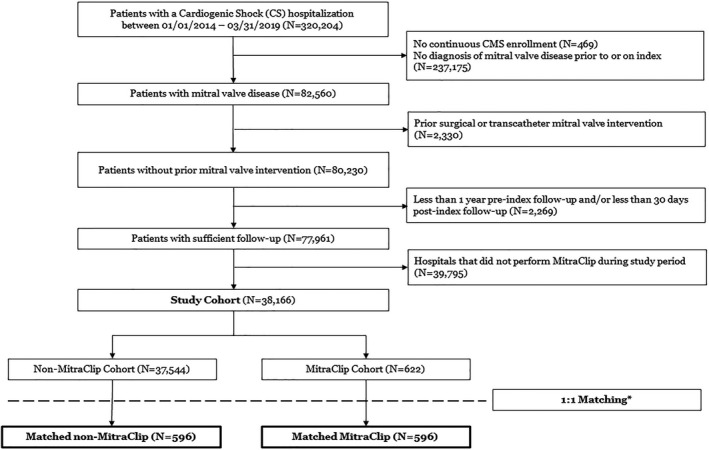
CS indicates cardiogenic shock. *Exact matching on Clinical Classification Software categories for index cardiogenic shock hospitalization; propensity score matching on age, sex, race, presence of consecutive claims, index year, selected comorbidities, and selected interventions during index cardiogenic shock hospitalization. CMS indicates Centers for Medicare and Medicaid Services.
Patient characteristics for the 2 groups before and after matching are reported in Table 1. Before matching, patients receiving MitraClip during a CS hospitalization were younger, more likely to be White, and more likely to have consecutive hospitalizations (ie, inter‐hospital transfers) during the index event compared with those without MitraClip. The CCS categories and interventions performed during the index hospitalization were significantly different before matching, as was the year of admission, with MitraClip hospitalizations falling later during the study period. The proportion of male patients and the prevalence of most comorbidities was similar between groups with the exception of hypertension and atrial fibrillation, both of which were higher for the non‐MitraClip group before matching. After matching, the 2 groups were well balanced with no significant difference across all variables of interest (Table 1). The standardized mean differences estimates were <0.1 for all matched parameters. Propensity score density plots and love plots for matched covariates demonstrated close matching between the 2 groups (Figure S1).
Table 1.
Baseline Characteristics for Study Cohort Before and After Matching
| All Variables Expressed as N (%) | Before Matching | P Value | After Matching | P Value | ||
|---|---|---|---|---|---|---|
| Non‐MitraClip (N=37 544) | MitraClip (N=622) | Non‐MitraClip (N=596) | MitraClip (N=596) | |||
| Patient demographics | ||||||
| Age, y | <0.001 | 0.322 | ||||
| ≤63 | 7180 (19.1) | 44 (7.1) | 55 (9.2) | 44 (7.4) | ||
| 64–69 | 7456 (19.9) | 93 (15.0) | 78 (13.1) | 92 (15.4) | ||
| 70–74 | 7402 (19.7) | 112 (18.0) | 109 (18.3) | 110 (18.5) | ||
| 75–81 | 8516 (22.7) | 170 (27.3) | 185 (31.0) | 163 (27.3) | ||
| ≥82 | 6990 (18.6) | 203 (32.6) | 169 (28.4) | 187 (31.4) | ||
| Female | 15 624 (41.6) | 269 (43.2) | 0.437 | 247 (41.4) | 257 (43.1) | 0.598 |
| Race † , White | 29 028 (77.3) | 515 (82.8) | 0.001 | 481 (80.7) | 492 (82.6) | 0.455 |
| Index hospitalization | ||||||
| Consecutive hospitalizations ‡ | 4641 (12.4) | 131 (21.1) | <0.001 | 108 (18.1) | 114 (19.1) | 0.710 |
| Index year | <0.001 | 0.894 | ||||
| 2014–2015 | 12 817 (34.1) | 118 (19.0) | 112 (18.8) | 114 (19.1) | ||
| 2016–2017 | 14 712 (39.2) | 250 (40.2) | 249 (41.8) | 241 (40.4) | ||
| 2018–2019 Q1 | 10 015 (26.7) | 254 (40.8) | 235 (39.4) | 241 (40.4) | ||
| Pericarditis, endocarditis, and myocarditis; cardiomyopathy | 13 536 (36.1) | 217 (34.9) | 0.576 | 205 (34.4) | 205 (34.4) | >0.999 |
| Acute myocardial infarction | 10 559 (28.1) | 116 (18.6) | <0.001 | 108 (18.1) | 108 (18.1) | >0.999 |
| Coronary atherosclerosis and other heart disease | 26 307 (70.1) | 421 (67.7) | 0.214 | 406 (68.1) | 406 (68.1) | >0.999 |
| Pulmonary heart disease | 12 830 (34.2) | 292 (46.9) | <0.001 | 278 (46.6) | 278 (46.6) | >0.999 |
| Cardiac dysrhythmias | 28 338 (75.5) | 513 (82.5) | <0.001 | 497 (83.4) | 497 (83.4) | >0.999 |
| Cardiac arrest and ventricular fibrillation | 12 867 (34.3) | 127 (20.4) | <0.001 | 121 (20.3) | 121 (20.3) | >0.999 |
| Congestive heart failure; non‐hypertensive | 25 323 (67.4) | 507 (81.5) | <0.001 | 489 (82.0) | 489 (82.0) | >0.999 |
| Index circulatory support (IABP/Impella/ECMO) | 8847 (23.6) | 216 (34.7) | <0.001 | 178 (29.9) | 195 (32.7) | 0.318 |
| Index mechanical ventilation | 16 344 (43.5) | 292 (46.9) | 0.097 | 250 (41.9) | 271 (45.5) | 0.243 |
| Index coronary interventions (PCI/CABG) | 8287 (22.1) | 95 (15.3) | <0.001 | 75 (12.6) | 94 (15.8) | 0.135 |
| Index hemodialysis | 6782 (18.1) | 110 (17.7) | 0.848 | 112 (18.8) | 101 (16.9) | 0.450 |
| Patient comorbidities | ||||||
| Hypertension | 33 760 (89.9) | 542 (87.1) | 0.027 | 530 (88.9) | 520 (87.2) | 0.421 |
| Chronic heart failure | 29 423 (78.4) | 494 (79.4) | 0.560 | 496 (83.2) | 475 (79.7) | 0.136 |
| Atrial fibrillation/flutter | 29 207 (77.8) | 396 (63.7) | <0.001 | 407 (68.3) | 389 (65.3) | 0.296 |
| Renal failure | 20 336 (54.2) | 340 (54.7) | 0.837 | 354 (59.4) | 330 (55.4) | 0.178 |
| Pulmonary disease | 21 120 (56.3) | 331 (53.2) | 0.140 | 338 (56.7) | 318 (53.4) | 0.269 |
| Prior myocardial infarction | 18 560 (49.4) | 288 (46.3) | 0.131 | 269 (45.1) | 277 (46.5) | 0.684 |
| Peripheral vascular disorder | 14 442 (38.5) | 235 (37.8) | 0.759 | 198 (33.2) | 225 (37.8) | 0.116 |
| Concomitant valvular disease (tricuspid and/or aortic) | 12 581 (33.5) | 209 (33.6) | 0.996 | 215 (36.1) | 204 (34.2) | 0.544 |
| Cerebral vascular disease | 10 591 (28.2) | 175 (28.1) | >0.999 | 178 (29.9) | 168 (28.2) | 0.566 |
| Prior ventricular tachycardia/fibrillation | 10 164 (27.1) | 152 (24.4) | 0.155 | 150 (25.2) | 149 (25.0) | >0.999 |
The reference group for race is “Non‐White”, which combined all the other Centers for Medicare and Medicaid Services race categories including Black, Asian, Hispanic, North American native, and others because of the cell size suppression policy.
Consecutive hospitalization defined as a subsequent inpatient admission that occurred within 24 hours of prior inpatient discharge.
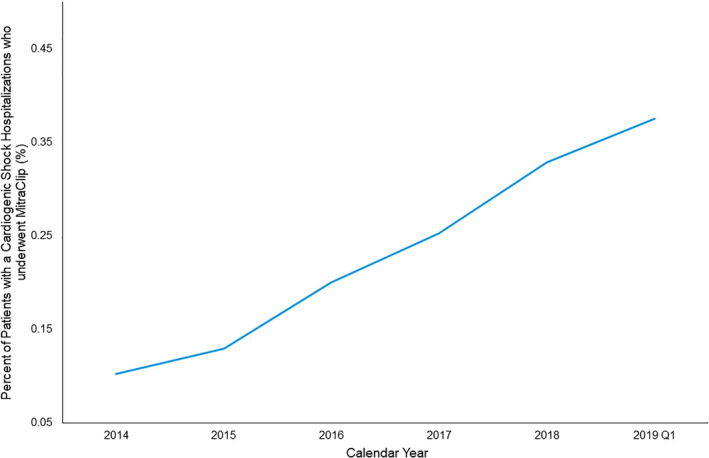
MitraClip Use in CS Over Time
Figure 2 illustrates a steady increase in the use of MitraClip during CS hospitalizations from 2014 through 2019. While the total proportion of CS hospitalizations that include a MitraClip procedure remains exceedingly low at <1%, there has been a >3‐fold increase in use over the 5 years of the study period, from 0.1% in 2014 to >0.3% in 2018 and the first quarter of 2019 (P<0.001).
Figure 2. MitraClip usage in cardiogenic shock hospitalizations over time.
Index Hospitalization of Patients With CS
Among those patients who underwent MitraClip during the index CS hospitalization, the timing of the MitraClip procedure relative to hospital admission, discharge or death, and other cardiovascular interventions are illustrated in Figure 3. The MitraClip procedure occurred a median 6.0 days (interquartile range [IQR], 2.0–130) after hospital admission and 7 days (IQR, 4.0–14) before discharge or death. Coronary interventions including percutaneous coronary intervention or coronary artery bypass grafting were performed in 94 patients (15.8%) and occurred a median of 5.5 days (IQR, 0.0–14) before the MitraClip procedure. Acute mechanical circulatory support including intra‐aortic balloon pump, Impella, or extracorporeal membrane oxygenation was required in 195 patients (32.7%) and was initiated at a median of 1 day (IQR, 0.0–6.0) before MitraClip. Mechanical ventilation was used in 271 patients (45.5%) and hemodialysis in 101 patients (16.9%), both commonly occurring on the same day as MitraClip. Mechanical ventilation was initiated at a median of 0.0 days (IQR, 0.0–7.0) before MitraClip. Hemodialysis was used at a median of 0.0 days relative to MitraClip (IQR, 7.0 before 5.0 post‐MitraClip).
Figure 3. Within the MitraClip arm (n=596), timing of the MitraClip procedure relative to other events during index hospitalization.
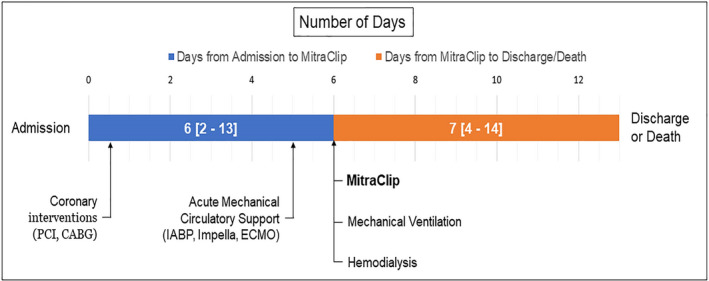
CABG indicates coronary artery bypass grafting; ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; and PCI, percutaneous coronary intervention. Days reported as median (interquartile range).
Among the matched cohort, patients who received MitraClip spent more days in an intensive or coronary care unit than the non‐MitraClip group (9.0 days [IQR, 3.0–17] versus 7.0 days [IQR, 2.0–14]; P=0.017) during the index hospitalization. When considering only those patients who survived the index hospitalization, combined intensive care unit or coronary care unit stay was similar between groups (MitraClip, 9.0 days [IQR, 3.0–16]; non‐MitraClip, 8.0 days [IQR, 3.0–16]; P=0.725). Total length‐of‐stay was longer in the MitraClip group for the overall cohort (MitraClip, 16 days [IQR, 10–25]; non‐MitraClip, 12 days [IQR, 7.8–20]; P<0.001), as well as within those who survived the index hospitalization (MitraClip, 17 days [IQR, 11–26]; non‐MitraClip, 14 days [IQR, 9–22]; P=0.002). The discharge status, including in‐hospital mortality, was significantly different for the 2 groups within the matched cohort (Table 2). In particular, patients who received MitraClip had significantly lower in‐hospital mortality compared with matched patients who did not undergo MitraClip during the index hospitalization (24.8% versus 35.4%, odds ratio [OR], 0.6 [95% CI, 0.47–0.77]; P<0.001).
Table 2.
Discharge Status for Matched Population
| Discharge Status for Index Event, n (%) | Non‐MitraClip (n=596) | MitraClip (n=596) | P Value |
|---|---|---|---|
| In‐hospital mortality | 211 (35.4) | 148 (24.8) | <0.001 |
| Discharged to home/self‐care/HHA | 179 (30.0) | 208 (34.9) | |
| Transferred to short‐term hospital/SNF/IPT/LTC/Others | 174 (29.2) | 220 (36.9) | |
| Hospice—medical facility/home | 32 (5.4) | 20 (3.4) |
HHA indicates home health agency; IPT, integrated physical therapy; LTC, long‐term care; and SNF, skilled nurse facility.
Mid‐Term Outcomes
Patients who underwent MitraClip had significantly higher 1‐year survival following the CS episode compared with matched patients without MitraClip, driven largely by mortality events in the non‐MitraClip cohort during the index hospitalization (hazard ratio [HR], 0.76 [95% CI, 0.65–0.88], P<0.001) (Figure 4). When considering the composite end point of death, LVAD implant, or heart transplant, MitraClip was also associated with a survival benefit (HR, 0.66 [95% CI, 0.57–0.77], P<0.001) (Figure S2). In a landmark analysis, after excluding patients who died during the index hospitalization, patients who received MitraClip and were discharged alive had similar mid‐term survival compared with those without MitraClip (HR, 0.85 [95% CI, 0.68–1.1], P=0.131) (Figure S3).
Figure 4. Kaplan‐Meier survival curve in hospitalized patients with mitral regurgitation and cardiogenic shock.
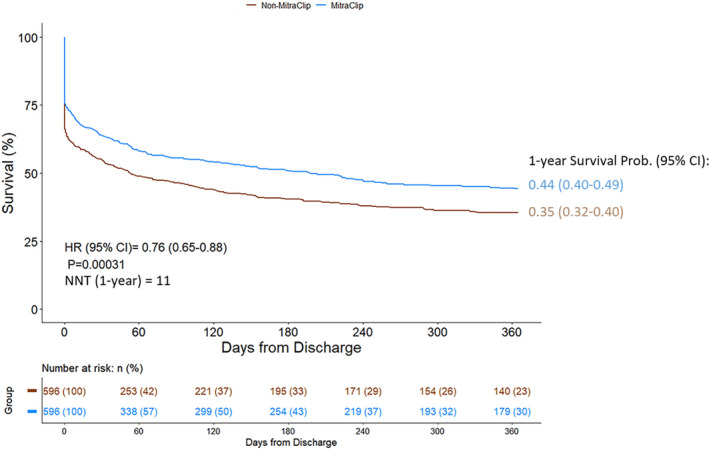
NNT indicates numbers needed to treat.
In a sensitivity analysis to control for potential immortal time bias, the treatment time was well balanced between matched cohorts (MitraClip: 9.1±10.3 days, non‐MitraClip: 8.4±9.6 days, P=0.230, standardized mean differences=0.049). After controlling for the period of time leading up to the MitraClip procedure during which death cannot occur, the results were consistent wherein MitraClip was associated with lower in‐hospital mortality (24.8% versus 31.8%; OR, 0.71 [95% CI, 0.55–0.91], P=0.0069) and higher 1‐year survival (HR, 0.78 [95% CI, 0.67–0.90]; P=0.001).
The improved 1‐year survival associated with MitraClip was consistent across various subgroups of interest, including age ≥75 years, sex, race, and diagnoses during index hospitalization including acute MI, coronary atherosclerosis, pulmonary heart disease, and cardiac dysrhythmias (Figure 5). For the subgroups of acute mechanical circulatory support (MCS) and hemodialysis, however, the survival benefit associated with MitraClip was observed only among patients who did not require these advanced interventions, and not for those who did (acute MCS, P interaction=0.004; hemodialysis, P interaction=0.011). A CCS classification of pericarditis, endocarditis, myocarditis, or cardiomyopathy during the index hospitalization also significantly impacted the survival benefit associated with MitraClip (P interaction=0.031). It is noted that the subgroups with fewer patients may lack of power to detect a significant effect. Furthermore, since the subgroups were not balanced on the patient characteristics, these results should be interpreted with caution.
Figure 5. Subgroup analysis for unadjusted 1‐year mortality with MitraClip therapy according to subgroups of interest.
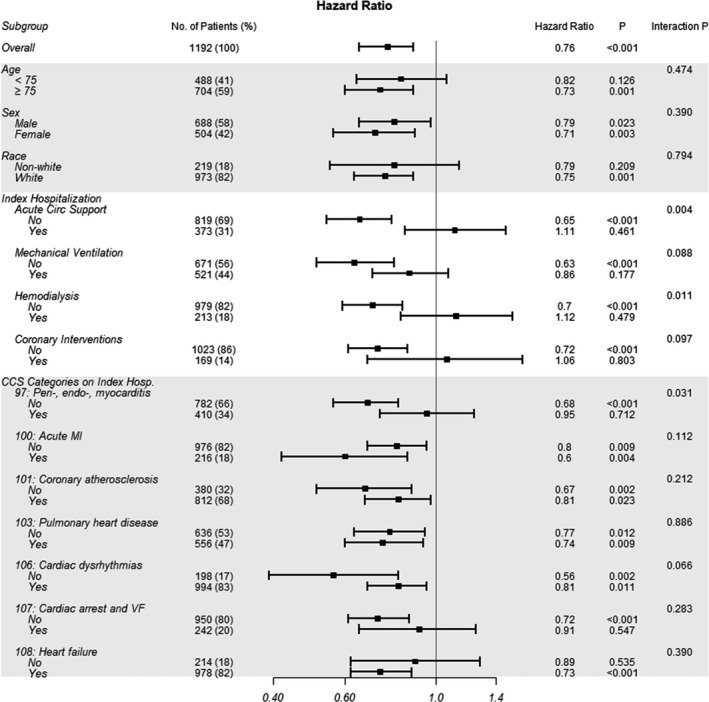
CCS indicates Clinical Classification Software; MI, myocardial infarction; and VF, ventricular fibrillation.
Predictors of In‐Hospital and 1‐Year Mortality After MitraClip
Independent predictors of in‐hospital and 1‐year mortality after MitraClip are listed in Table 3. Peripheral vascular disease was an independent predictor of in‐hospital mortality, while consecutive hospitalization during index event, more recent hospitalization, and prior history of ventricular arrhythmias were protective. Independent predictors of 1‐year mortality were pulmonary disease, renal failure, and peripheral vascular disease, while hypertension and chronic heart failure were protective.
Table 3.
Multivariate Predictors of In‐Hospital and 1‐Year Mortality following MitraClip in Cardiogenic Shock (n=596)
| Covariate | In‐Hospital All‐Cause Mortality | 1‐y All‐Cause Mortality | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age at index, y | ||||
| ≤63 | 1 (ref) | 1 (ref) | ||
| 64–69 | 1.48 (0.56–4.25) | 0.442 | 1.14 (0.67–1.93) | 0.629 |
| 70–74 | 2.36 (0.94–6.59) | 0.081 | 1.23 (0.73–2.07) | 0.431 |
| 75–81 | 2.14 (0.87–5.9) | 0.116 | 1.32 (0.8–2.17) | 0.276 |
| ≥82 | 1.7 (0.69–4.71) | 0.272 | 1.39 (0.85–2.29) | 0.187 |
| Sex (female) | 1.17 (0.78–1.77) | 0.450 | 1.06 (0.84–1.35) | 0.612 |
| Race (White) | 0.78 (0.45–1.37) | 0.375 | 1.08 (0.78–1.5) | 0.624 |
| Consecutive hospitalization during index event | 0.54 (0.3–0.93) | 0.030 | 0.87 (0.65–1.16) | 0.336 |
| Index, y | ||||
| 2014–2015 | 1 (ref) | 1 (ref) | ||
| 2016–2017 | 0.53 (0.32–0.89) | 0.017 | 0.76 (0.56–1.02) | 0.071 |
| 2018–2019 Q1 | 0.45 (0.26–0.77) | 0.003 | 0.76 (0.56–1.03) | 0.079 |
| Concomitant valvular disease (tricuspid and/or aortic) | 1.33 (0.86–2.05) | 0.195 | 1.03 (0.8–1.31) | 0.833 |
| Hypertension | 0.75 (0.37–1.55) | 0.426 | 0.66 (0.44–0.99) | 0.046 |
| Chronic heart failure | 0.63 (0.32–1.23) | 0.172 | 0.68 (0.46–0.99) | 0.042 |
| Pulmonary disease | 1.45 (0.92–2.28) | 0.108 | 1.45 (1.13–1.87) | 0.004 |
| Renal failure | 1.47 (0.93–2.35) | 0.105 | 1.77 (1.35–2.32) | <0.001 |
| Peripheral vascular disorder | 2.11 (1.36–3.29) | <0.001 | 1.44 (1.12–1.84) | 0.004 |
| Prior ventricular tachycardia/fibrillation | 0.52 (0.3–0.87) | 0.015 | 0.9 (0.68–1.19) | 0.445 |
| Atrial fibrillation/flutter | 0.99 (0.61–1.63) | 0.964 | 1.14 (0.86–1.51) | 0.357 |
| Prior myocardial infarction | 1 (0.65–1.56) | 0.991 | 0.89 (0.69–1.15) | 0.360 |
| Prior cerebral vascular disease | 0.86 (0.54–1.37) | 0.541 | 1 (0.77–1.3) | 0.996 |
HR indicates hazard ratio; and OR, odds ratio.
In‐hospital mortality is multivariable logistic regression; 1‐year mortality is multivariable Cox regression.
Discussion
Our matched cohort analysis, reporting the largest MitraClip series in hospitalized patients with a diagnosis of CS, supports the following key findings: (1) MitraClip, though used rarely in patients with CS, is increasingly being considered as a potential therapeutic option. (2) Among patients with a CS hospitalization during our study period, those receiving MitraClip were more likely to be older, White, have consecutive hospitalizations (ie, inter‐hospital transfers), and require MCS during index hospitalization. (3) After propensity score matching, percutaneous edge‐to‐edge repair with MitraClip in patients with MR and CS was associated with reduced in‐hospital mortality and greater 1‐year survival, compared with patients who did not receive MitraClip, that was largely driven by survival during the index hospitalization. (4) On subgroup analysis, the survival benefit associated with MitraClip was consistent across most subgroups of interest with the exception of those requiring acute MCS and hemodialysis during the index hospitalization.
CS remains a potentially fatal condition and is commonly attributable to acute myocardial infarction or decompensated heart failure. 1 , 2 Severe MR may result from mechanical complications of acute myocardial infarction leading to papillary muscle dysfunction or rupture, acute chordal rupture in patients with chronic primary MR, or progressive decompensation of cardiomyopathy with chronic MR. The goal of care in CS is to hemodynamically stabilize patients and to reverse multiorgan dysfunction, followed by bridge to recovery or more definitive therapies. Although inotropic and mechanical circulatory support are the first‐line therapy in these patients, in the presence of severe MR, they do not address the underlying etiology and many patients may require mitral valve intervention. Although mitral valve surgery may provide a definitive treatment, given the acuity of their conditions and presence of multiorgan dysfunction, a majority of these patients are not suitable surgical candidates. TMVr may be an emerging therapeutic option to address MR and stabilize patients with CS sufficiently to enable recovery or bridge to more advanced therapies. The mechanism of clinical improvement after TMVr may be because of the rapid decrease in left ventricle, left atrium, and pulmonary artery pressures, and the corresponding increase of cardiac output observed after a successful correction of the MR. 24 As shown in our study, MitraClip use in this critically ill patient population has increased over time. Therefore, evaluating the clinical impact of this therapy in patients with MR and CS is timely.
Our study represents the largest known description of patients with a diagnosis of CS who underwent MitraClip procedure. The association of MitraClip with improved in‐hospital and 1‐year survival aligns with results reported in prior single‐center and small multicenter studies (Table 4). 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Together, these findings suggest the use of MitraClip as a potential therapy in critically ill patients with MR. After controlling for patient demographics and comorbidities, as well as the treating hospital and the presence of inter‐hospital transfers, MitraClip was associated with a 10.6% absolute reduction of in‐hospital mortality in our US nationwide study of all‐comer patients with CS. When considering only patients who survived the index hospitalization in a landmark analysis, patients with and without MitraClip had similar 1‐year survival, suggesting that the effect was driven by in‐hospital survival. One potential explanation would be by reducing the MR and improving forward flow, MitraClip in the presence of CS may allow sufficient stabilization of the patient to enable successful hospital discharge. Notably, only 32.5% of patients in the analytic cohort were discharged home from the CS hospitalization, either to self‐care or with the support of a home health agency. This finding suggests that despite hospital discharge, patients who underwent MitraClip in the context of CS still need significant time and resources to recover.
Table 4.
Prior Case Reports and Case Series Reporting MitraClip Use in Cardiogenic Shock
| Study | No. | Age (y) | Women | STS PROM (%) | Prior MI | On MCS | Primary MR | DOA to MitraClip (d) | LOS (d) | In‐Hospital Mortality | Follow‐Up (mo) | Follow‐Up Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zuern CS et al (2011) 4 | 1 | 51 | 0 | … | 1 | 1 | 0 | 10 | 17 | 0 | 3 | 0 |
| Pleger ST et al (2013) 5 | 6 | 68.7 [7.6] | 1 (17%) | 27.3 [21.4] | 5 (83%) | 0 |
2 (33%) Mixed |
41.2 [41.6] {10–113} |
53.7 [41.1] {13–119} |
0 | … | … |
| Couture P et al (2014) 6 | 1 | 78 | 0 | 83 | 1 | 1 | 0 | 60 | 81 | 0 | 12 | 0 |
| Wolff R et al (2014) 7 | 1 | 68 | 0 | 64 | 1 | 1 | 1 | 1 | 14 | 0 | 6 | 0 |
| Bahlmann E et al (2015) 8 | 1 | 77 | 0 | 78* | 1 | 1 | 1 | … | 16 | 0 | … | … |
| Tang GHL et al (2018) 9 | 1 | 77 | 1 | … | 0 | 1 | 1 | … | 15 | 0 | 9 | 0 |
| Buckert D et al (2017) 10 | 1 | 78 | 0 | 18.4 | 1 | 1 | 1 | … | 14 | 0 | 4 | 0 |
| Hernandez M et al (2018) 11 | 1 | 70 | 0 | 7.1 | 1 | 1 |
1 Mixed |
3 | 33 | 0 | 6 | 0 |
| Haberman D et al (2019) 12 | 20 | 68.1 [9.9] | 14 (70%) | … | 20 (100%) | 7 | 0 |
32 [25] {7–90} |
… | 1 (5%) |
Median, 15 {3–88} |
2 |
| Rizik DG et al (2019) 13 | 3 | 72, 92, 81 | 0 | … | 1 (33%) | 2 | 2 (67%) | … |
7, 3, 4 Post‐op |
0 | 1 | 0 |
| Chan V et al (2019) 14 | 27 | 71 [13] | 10 (37%) | 18.5 | 5 (19%) | 5 | 2 |
Median, 23 (9–37) |
Median, 40 {22–70} Post‐op |
8 (30%) | 6.7 [8.9] | 17 (63%) |
| Cheng R et al (2019) 15 | 29 | 65.5 [17.0] | 5 (17%) | … | 10 (35%) | 8 (28%) | … | … | … | 5 (17%) | 14.8 [15.7] | 7 (24%) |
| Flint K et al (2019) 16 | 12 | 71.7 [12.8] | 3 (25%) | 23.9 [18.2] | 4 (33%) | 5 (42%) |
10 (83%) 6 Mixed |
… | … | 1 (8%) |
Median, 6.6 {1.4–12.6} |
5 (42%) |
| Garcia S et al (2020) 17 | 11 | 74.5 [13.3] | 5 (45%) |
15.5 [IQR, 6.9–39.1] |
5 (45%) | 5 (45%) | 7 (64%) | … | … | 3/11 (27%) | 12 | 6/9 (66%) |
| Estevez‐Loureiro R et al (2020) 18 | 44 | 70.0 [10.8] | 16 (36%) | 15.1 {6.2–23.2} | 44 (100%) | 16 (36%) | 44 (10%) | Median, 18 {13–36.8} |
Median, 16 {8–27} Post‐op |
4 (9%) | 6 | 8 (18%) |
All variables expressed as n (%), mean (SD), (range) , and interquartile range. DOA indicates date of admission; IQR, interquartile range; LOS, length of stay; MCS, mechanical circulatory support; MI, myocardial infarction; MR, mitral regurgitation; PCI, percutaneous coronary intervention; and STS PROM, Society of Thoracic Surgeons predicted risk of operative mortality.
EuroScore listed, as STS PROM score was not available.
Two sensitivity analyses were performed as part of the current study. In the first, a composite end point of death, heart transplant, or LVAD implant reinforced the robustness of the survival benefit associated with MitraClip by treating heart transplant and LVAD as equivalent end points to mortality rather than censoring for these clinically relevant events. The second sensitivity analysis controlled for potential survivor bias given that patients receiving MitraClip had to survive the index hospitalization long enough to undergo the procedure while those in the control group had no such requirement. While this marginally impacted in‐hospital survival in the control group, the significant in‐hospital survival effect associated with MitraClip was maintained, suggesting that immortal time bias does not drive the results observed in this observational study.
Within the MitraClip group, pulmonary disease, renal failure, and peripheral vascular disorder were independent predictors of reduced 1‐year survival following CS. This speaks to the high burden of pre‐existing comorbidities in patients with CS that may increase their mortality risk. Interestingly, chronic heart failure was protective, which might be because of the potential ability of a compromised left ventricle over a normal one to withstand severe MR. Further analysis would be necessary to confirm this hypothesis.
Subgroup analyses showed that the survival benefit associated with MitraClip was consistent irrespective of age, sex, race, and other cardiac risk factors. However, this effect was significantly impacted by certain high‐risk features in this CS cohort. For example, MitraClip therapy was not associated with improved survival among patients requiring MCS or hemodialysis during the index hospitalization. In our study, patients who received MitraClip often needed mechanical ventilation or hemodialysis on the same day as the procedure. In the unmatched study cohort, there was a higher prevalence of MCS use in patients receiving MitraClip. These temporally associated events and need for MCS may be surrogate markers of the critically ill nature of patients with CS who received MitraClip, and suggests that patient selection is important to ensure improved outcomes with MitraClip in the setting of CS. The use of hemodialysis is particularly relevant given that renal failure was associated with greater 1‐year mortality for patients who received MitraClip.
Irrespective of MitraClip therapy, 1‐year mortality among patients who were successfully discharged in our study remained high at ≈40%. This is reflective of the extremely high‐risk nature of CS. However, we note this is comparable with the 31.2% 1‐year mortality of patients with functional MR who underwent MitraClip in the Society of Thoracic Surgeons/American College of Cardiology TVT (Transcatheter Valve Therapy) Registry, 25 highlighting the complexity of treating MR in patients with advanced cardiovascular disease. Importantly, when considering the composite end point of all‐cause mortality, heart transplant, or LVAD, the benefit associated with MitraClip in our study was maintained, reinforcing the potential impact on key clinical end points.
Study Limitations
Our study has several limitations. First, our data are derived from an administrative claims database, which lacks granular clinical details such as MR etiology and severity of CS, as well as MitraClip procedural characteristics such as amount of residual MR. However, given the challenges of informed consent and randomization in critically ill patients, administrative claims data provide a viable approach to study the real‐world use of MitraClip in a large nationwide cohort of patients with CS. Second, outcomes were not centrally adjudicated in this retrospective observational cohort study. However, the primary end point of all‐cause mortality has been widely validated in Centers for Medicare and Medicaid Services claims data and has been shown to demonstrate close concordance with trial‐adjudicated mortality. Third, although we attempted to control for all available variables in the propensity matching, as the study is observational residual confounding may be present. Importantly, the clinical decision to perform a MitraClip procedure on a patient in CS cannot be elucidated in a retrospective observational cohort. While our second sensitivity analysis aimed to account for potential immortal time bias in the matched cohort, it is plausible that patients selected to receive MitraClip were those more likely to survive long‐term. Finally, the reported P‐values are at a nominal alpha level without controlling for the potential inflation of the Type I error rate.
Conclusions
In patients with MR presenting with CS, use of TMVr with MitraClip is increasing and is associated with greater in‐hospital and 1‐year survival. MitraClip may be a treatment option for critically ill patients with CS, and further study is warranted to optimize procedural timing and patient selection associated with the improved outcomes, particularly in those patients requiring acute MCS and hemodialysis.
Sources of Funding
Funding for this study was provided by Abbott. The funder actively participated in the design and conduct of the study; acquisition, management, analysis, and interpretation of the data; and preparation and review of the article. The funder had no role in the approval of the article and decision to submit the article for publication.
Disclosures
Dr Tang is a consultant and receives speaker honoraria for Abbott Structural Heart and Medtronic, and is a consultant for W. L. Gore and Associates. Dr Estevez‐Loureiro is a consultant for Abbott and Boston Scientific. Dr Yu and Dr Prillinger are salaried employees of Abbott. Dr Psotka has received grant funding from the US Food and Drug Administration, as well as consulting fees from Amgen, Cytokinetics, and Windtree. Dr Zaid has no disclosures to report.
Supporting information
Tables S1–S2
Figures S1–S3
Acknowledgments
Author contributions: Co‐authors Tang, Estevez‐Loureiro, Yu, Prillinger, Psotka were responsible for study concept and design. Co‐authors Tang, Estevez‐Loureiro, Yu, Prillinger, Zaid, Psotka were responsible for acquisition, analysis, or interpretation of data. Co‐authors Tang, Yu, Prillinger were responsible for drafting of the article. Critical revision of the article for important intellectual content were done by co‐authors Tang, Estevez‐Loureiro, Yu, Prillinger, Zaid, Psotka. Statistical analysis was performed by coauthors Yu, Prillinger.
(J Am Heart Assoc. 2021;10:e019882. DOI: 10.1161/JAHA.120.019882.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019882
This study was presented at the EuroPCR eCourse, June 25, 2020.
For Sources of Funding and Disclosures, see page 12.
References
- 1. Tcheng JE, Jackman JD Jr, Nelson CL, Gardner LH, Smith LR, Rankin JS, Califf RM, Stack RS. Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann Intern Med. 1992;117:18–24. DOI: 10.7326/0003-4819-117-1-18. [DOI] [PubMed] [Google Scholar]
- 2. Akodad M, Schurtz G, Adda J, Leclercq F, Roubille F. Management of valvulopathies with acute severe heart failure and cardiogenic shock. Arch Cardiovasc Dis. 2019;112:773–780. DOI: 10.1016/j.acvd.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 3. Schroeter T, Lehmann S, Misfeld M, Borger M, Subramanian S, Mohr FW, Bakthiary F. Clinical outcome after mitral valve surgery due to ischemic papillary muscle rupture. Ann Thorac Surg. 2013;95:820–824. DOI: 10.1016/j.athoracsur.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 4. Zuern CS, Schreieck J, Weig HJ, Gawaz M, May AE. Percutaneous mitral valve repair using the MitraClip in acute cardiogenic shock. Clin Res Cardiol. 2011;100:719–721. DOI: 10.1007/s00392-011-0324-1. [DOI] [PubMed] [Google Scholar]
- 5. Pleger ST, Chorianopoulos E, Krumsdorf U, Katus HA, Bekeredjian R. Percutaneous edge‐to‐edge repair of mitral regurgitation as a bail‐out strategy in critically ill patients. J Invasive Cardiol. 2013;25:69–72. [PubMed] [Google Scholar]
- 6. Couture P, Cloutier‐Gill LA, Ducharme A, Bonan R, Asgar AW. MitraClip intervention as rescue therapy in cardiogenic shock: one‐year follow‐up. Can J Cardiol. 2014;30:1108.e15–1108.e16. DOI: 10.1016/j.cjca.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 7. Wolff R, Cohen G, Peterson C, Wong S, Hockman E, Lo J, Strauss BH, Cohen EA. MitraClip for papillary muscle rupture in patient with cardiogenic shock. Can J Cardiol. 2014;30:1461.e13–1461.e14. DOI: 10.1016/j.cjca.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 8. Bahlmann E, Frerker C, Kreidel F, Thielsen T, Ghanem A, van der Schalk H, Grahn H, Kuck KH. MitraClip implantation after acute ischemic papillary muscle rupture in a patient with prolonged cardiogenic shock. Ann Thorac Surg. 2015;99:e41–e42. DOI: 10.1016/j.athoracsur.2014.09.075. [DOI] [PubMed] [Google Scholar]
- 9. Tang GHL, Cohen M, Dutta T, Undemir C. Afterload mismatch after transcatheter mitral valve repair with MitraClip for degenerative mitral regurgitation in acute cardiogenic shock. Catheter Cardiovasc Interv. 2018;92:e168–e171. DOI: 10.1002/ccd.27019. [DOI] [PubMed] [Google Scholar]
- 10. Buckert D, Markovic S, Kunze M, Wohrle J, Rottbauer W, Walcher D. Percutaneous mitral valve repair with the MitraClip NT system in a patient presenting with prolonged cardiogenic shock. Clin Case Rep. 2017;5:1807–1810. DOI: 10.1002/ccr3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernández‐Enríquez M, Freixa X, Sanchis L, Regueiro A, Burgos F, Navarro R, Masotti M, Sitges M, Sabaté M. MitraClip® repair in cardiogenic shock due to acute mitral regurgitation: from near‐death to walking. J Heart Valve Dis. 2018;27:114–116. [PubMed] [Google Scholar]
- 12. Haberman D, Taramasso M, Czarnecki A, Kerner A, Chrissoheris M, Spargias K, Poles L, Agmon Y, Scianna S, Beeri R, et al. Salvage MitraClip in severe secondary mitral regurgitation complicating acute myocardial infarction: data from a multicentre international study. Eur J Heart Fail. 2019;21:1161–1164. DOI: 10.1002/ejhf.1565. [DOI] [PubMed] [Google Scholar]
- 13. Rizik DG, Burke RF, Goldstein JA. Urgent mechanical circulatory support and transcatheter mitral valve repair for refractory hemodynamic compromise. Catheter Cardiovasc Interv. 2019;94:886–892. DOI: 10.1002/ccd.28439. [DOI] [PubMed] [Google Scholar]
- 14. Chan V, Messika‐Zeitoun D, Labinaz M, Hynes M, Nicholson D, Dryden A, Mesana T, Hibbert B. Percutaneous mitral repair as salvage therapy in patients with mitral regurgitation and refractory cardiogenic shock. Circ Cardiovasc Interv. 2019;12:e008435. DOI: 10.1161/CIRCINTERVENTIONS.119.008435. [DOI] [PubMed] [Google Scholar]
- 15. Cheng R, Dawkins S, Hamilton MA, Makar M, Hussaini A, Azarbal B, Patel JK, Kobashigawa JA, Trento A, Makkar RR, et al. Percutaneous mitral repair for patients in cardiogenic shock requiring inotropes and temporary mechanical circulatory support. JACC Cardiovasc Interv. 2019;12:2440–2441. DOI: 10.1016/j.jcin.2019.05.042. [DOI] [PubMed] [Google Scholar]
- 16. Flint K, Brieke A, Wiktor D, Carroll J. Percutaneous edge‐to‐edge mitral valve repair may rescue select patients in cardiogenic shock: findings from a single center case series. Catheter Cardiovasc Interv. 2019;94:e82–e87. DOI: 10.1002/ccd.28089. [DOI] [PubMed] [Google Scholar]
- 17. Garcia S, Alsidawi S, Bae R, Cavalcante J, Eckman P, Gössl M, Steffen R, Sun B, Schmidt CW, Sorajja P. Percutaneous mitral valve repair with MitraClip in inoperable patients with severe mitral regurgitation complicated by cardiogenic shock. J Invasive Cardiol. 2020;32:228–231. [DOI] [PubMed] [Google Scholar]
- 18. Estevez‐Loureiro R, Adamo M, Arzamendi D, Denti P, Freixa X, Nombela‐Franco L, Pascual I, Melica B, Attias D, Serrador A, et al. Transcatheter mitral valve repair in patients with acute myocardial infarction: insights from the European Registry of MitraClip in Acute Mitral Regurgitation following an acute myocardial infarction (EREMMI). EuroIntervention. 2020;15:1248–1250. DOI: 10.4244/EIJ-D-19-00653. [DOI] [PubMed] [Google Scholar]
- 19. El Sibai R, Bachir R, El Sayed M. Outcomes in cardiogenic shock patients with extracorporeal membrane oxygenation use: a matched cohort study in hospitals across the United States. Biomed Res Int. 2018;2018:2428648. DOI: 10.1155/2018/2428648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maxwell BG, Powers AJ, Sheikh AY, Lee PH, Lobato RL, Wong JK. Resource use trends in extracorporeal membrane oxygenation in adults: an analysis of the Nationwide Inpatient Sample 1998–2009. J Thorac Cardiovasc Surg. 2014;148:416–421.e1. DOI: 10.1016/j.jtcvs.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 21. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in‐hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55:698–705. DOI: 10.1097/MLR.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 22. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. DOI: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 23. Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time‐to‐treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol. 2005;162:1016–1023. DOI: 10.1093/aje/kwi307. [DOI] [PubMed] [Google Scholar]
- 24. Siegel RJ, Biner S, Rafique AM, Rinaldi M, Lim S, Fail P, Hermiller J, Smalling R, Whitlow PL, Herrmann HC, et al. The acute hemodynamic effects of MitraClip therapy. J Am Coll Cardiol. 2011;57:1658–1665. DOI: 10.1016/j.jacc.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 25. Sorajja P, Vemulapalli S, Feldman T, Mack M, Holmes DR Jr, Stebbins A, Kar S, Thourani V, Ailawadi G. Outcomes with transcatheter mitral valve repair in the United States: an STS/ACC TVT registry report. J Am Coll Cardiol. 2017;70:2315–2327. DOI: 10.1016/j.jacc.2017.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S3


