Abstract
Background
Hypertrophic cardiomyopathy (HCM) is considered to be the most common cause of sudden death in young people and is associated with an elevated risk of mood disorders. Depression has emerged as a critical risk factor for development and progression of coronary artery disease; however, the association between depression and HCM outcomes is less clear. We sought to examine the impact of depression on clinical outcomes in patients with HCM.
Methods and Results
Between January 2014 and December 2017, 820 patients with HCM were recruited and followed for an average of 4.2 years. End points were defined as sudden cardiac death (SCD) events and HCM‐related heart failure events. A Chinese version of the Structured Clinical Interview followed the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition and was used to diagnose depression. During the follow‐up period, SCD events occurred in 75 individuals (21.8 per 1000 person‐years), and HCM‐related heart failure events developed in 149 individuals (43.3 per 1000 person‐years). Kaplan–Meier cumulative incidence curves showed a significant association of depression disorders with SCD events (log‐rank P=0.001) and HCM‐related heart failure events (log‐rank P=0.005). A multivariate Cox regression analysis indicated that depression was an independent predictor of SCD events and HCM‐related heart failure events (41.9 versus 21.7 per 1000 person‐years; adjusted hazard ratio [HR], 1.9; 95% CI, 1.6–2.3; P<0.001; and 69.9 versus 38.6 per 1000 person‐years; HR, 1.8; 95% CI, 1.6–2.1; P<0.001, respectively).
Conclusions
Depression is common among patients with HCM. The diagnosis of depression is significantly and independently associated with an increased risk of SCD events and heart failure events in patients with HCM.
Keywords: depression, hypertrophic cardiomyopathy, outcomes
Subject Categories: Risk Factors, Mortality/Survival, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- HCM
hypertrophic cardiomyopathy
- LVOTO
left ventricular outflow tract obstruction
- SCD
sudden cardiac death
Clinical Perspective
What Is New?
Depression is frequent in patients with hypertrophic cardiomyopathy.
Depression is associated with an increased risk of sudden cardiac death events and heart failure events in patients with hypertrophic cardiomyopathy.
What Are the Clinical Implications?
This study supports the viewpoint that depression predicts adverse clinical outcomes in patients with hypertrophic cardiomyopathy.
Further study should be encouraged to validate whether psychological intervention for depression can improve clinical outcomes in this population.
Hypertrophic cardiomyopathy (HCM) is a common autosomal dominant cardiac disease (1:500) and a major cause of sudden death in young people. It is characterized by the presence of left ventricle wall hypertrophy (for adults, thickness ≥15 mm in 1 or more left ventricular myocardial segments) that is not explained simply by abnormal loading conditions. 1 , 2 , 3 The most serious complication is HCM‐related sudden death caused by ventricular arrhythmias or death after heart failure or stroke. 4 , 5 Several clinical parameters have been recognized as major risk factors of sudden cardiac death (SCD) in HCM, including unexplained syncope, family history of SCD, ventricular tachycardia (VT) during Holter monitoring, and maximum left ventricle wall thickness >30 mm. 6 , 7 In most patients, HCM‐related heart failure deaths occurred more frequently in middle age and beyond, as a result of a lifetime process of progressive and adverse myocardial remodeling, featured by myocardial fibrosis and wall thinning. 8
Depression is the most common emotional disorder and currently affects an estimated 350 million people. 9 It is characterized by significant impairment of social and occupational functioning, and the patients may experience remissions and relapses throughout their lives. 10 Accumulating evidence has consistently shown that depression is a risk factor for mortality and adverse clinical outcomes in patients with coronary heart disease and heart failure. 11 Over the past 2 decades, numerous prospective studies have provided support for an association of depression with SCD in the elderly and patients with acute myocardial infarction or stable coronary artery disease. 12 , 13 , 14 Studies on the mental health of patients with HCM showed that these individuals are under more psychological distress than is the general population, and have impaired quality of life and sociability. 15 , 16 Despite the high prevalence and poor clinical prognosis, psychological intervention is frequently neglected in this population.
Thus far, no specific data on the prevalence of depressive disorder in patients with HCM exist. Furthermore, the relationship between depression and prognosis has not hitherto been examined in patients with HCM. Therefore, we sought to explore the prevalence of depression in patients with HCM and determine the association between depression and clinical outcomes in this population.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population and Design
We consecutively enrolled 856 patients admitted to the Jiaxing First Hospital Heart Center from January 2014 to December 2017 with a diagnosis of HCM. In accordance with the European Society of Cardiology guidelines, patients who were aged >18 years and fulfilled the diagnostic criteria of HCM were eligible for inclusion if they had an unexplained hypertrophied left ventricle with either a maximum wall thickness of ≥15 mm on 2‐dimensional echocardiography. If patients presented with a lesser degree of wall thickness (≥13 mm), the diagnosis of HCM required assessments of other information, including a family history of first‐degree relatives, clinical symptoms, and an abnormal electrocardiogram. 6 The major exclusion criteria were (1) patients with cognitive impairment or senile dementia, (2) patients with a malignant tumor whose expected survival time was <1 year, (3) patients with severe renal disease or chronic obstructive pulmonary disease, (4) patients whose age was ≤18 years old, and (5) patients who refused to participate in the study. All participants provided informed consent before recruitment. The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Jiaxing University based on the Helsinki declaration. The median follow‐up from the depression ascertainment to the latest evaluation (clinical visit, WeChat, or telephone interview) or end point event was 3.8 years (interquartile range, 1.8–4.4 years).
Clinical Characteristics
All participants completed a standardized medical history questionnaire, and baseline information included demographics, primary disease, New York Heart Association (NYHA) classification, echocardiography, electrocardiography, Holter monitor data on admission, and discharge medications with β‐blockers, renin‐angiotensin‐aldosterone system inhibitors, calcium channel blockers, amiodarone, diuretics, and antidepressant medicine. Implantable cardioverter defibrillator (ICD) implantation is recommended in patients who have had cardiac arrest because of ventricular fibrillation or with an estimated 5‐year risk of SCD of ≥6%. 17 Doppler ultrasound was performed using a multifunctional echocardiography machine (ACUSON X300PE; Siemens Co., USA) in all patients and was recorded in detail (eg, maximum left ventricular wall thickness, left ventricular ejection fraction, and instantaneous peak Doppler left ventricular outflow tract pressure gradient).
Risk Factors for Sudden Death
Each patient underwent a detailed medical history, physical examination, ambulatory ECG, and echocardiography. Then, we identified risk factors for SCD as follows: (1) unexplained syncope, (2) family history of SCD, (3) nonsustained VT on Holter, (4) maximum LV wall thickness ≥30 mm, and (5) left ventricular outflow tract obstruction (LVOTO). 6 An abnormal response of blood pressure during exercise was excluded as a prespecified risk factor since it has not been independently related to SCD in the majority of multivariate survival analyses. 18 In addition, its prognostic significance in individuals >40 years of age is unclear. 19
Assessment of Depression
All patients were assessed for depressive symptoms during their first clinic visit or hospitalization and 12 months later using the 17‐item Hamilton Depression Rating Scale (HDRS), a well‐validated instrument in the cardiac populations. The measure consists of 9 items. Patients describe how frequently they experienced symptoms over the past few months using a 4‐point scale that ranges from “0” (not at all) to “3” (nearly every day). 20 The total scores range from 0 to 27. Individuals with an HDRS score of ≥5 were given the Chinese version of the Structured Clinical Interview, for diagnosis of depression. 21 It follows the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5). In other words, the HDRS is a self‐report inventory designed to screen for depressive mood rather than to determine diagnosis of major depression. Also, the Structured Clinical Interview allows trained psychologists to provide reliable psychiatric diagnoses based on DSM‐5. The semistructured psychiatric interview was administered by psychologists who were blinded to the study design.
Primary and Secondary End Points
The prespecified primary end point in the current study was SCD. SCD was characterized as an unexpected sudden collapse occurring within 1 hour of the onset of symptoms in patients with a stable or uneventful clinical condition. In addition, potentially lethal cardiovascular events in which patients either were revived from cardiac arrest (with documented VT or ventricular fibrillation) or ICD discharge were treated as equivalent to SCD events in the current data analysis. 22 ICD therapy was regarded as an appropriate discharge triggered by ventricular fibrillation or VT (rate, ≥180 bpm). Death was confirmed by the death certificate. The secondary end points were HCM‐related heart failure events, defined as a composite of heart failure death, cardiac decompensation, heart failure hospitalization, and stroke. Heart failure death was defined as death in the presence of end‐stage heart failure or heart transplantation. Cardiac decompensation was defined as patients with NYHA functional class Ⅰ or Ⅱ that progressed to class Ⅲ or Ⅳ during the follow‐up period. 23 , 24 HCM‐related stroke was judged to be a direct consequence of embolic events related to HCM in the context of atrial fibrillation in most cases. 25 All participants were contacted by clinic visit, WeChat, and/or telephone interview quarterly after the initial assessment. For any medical records, interview reports regarding cause of death, and death certificates were retrieved from patients' first‐degree relatives. Two cardiologists who were blind to patients' depression status reviewed all pertinent clinical and death documentation and adjudicated cardiovascular events.
Statistical Analysis
Statistical analyses were carried out using SPSS 23.0 for Windows (SPSS Inc, Chicago, IL). Continuous variables were summarized as mean±SD or median (25th–75th percentile) and compared using the t test or Mann–Whitney U test. The difference among multiple groups was compared with ANOVA. Categorical variables were presented as frequency and percentage and compared using χ2 test or Fisher exact test, as appropriate. Cumulative event‐free survival curves comparing patients with or without depression were examined using Kaplan–Meier analysis, and differences between groups were tested by the log‐rank statistic. Univariate and multiple Cox proportional hazards models were used to determine the relationship between depression and clinical outcomes. The proportional hazard assumption was examined by adding an interaction term of follow‐up duration and covariates in the models for continuous variables. As for categorical variables, the proportional hazard assumption was checked graphically for Cox regression before proceeding, and no violation was found. The multivariate model was conducted to adjust for potential confounders using a stepwise selection method with an entrance and stay criteria of P<0.10, forcing the number of traditional SCD factors into all models a priori. Thus variables entered into the multivariable model for SCD events include depression (first assessment), unexplained syncope, family history of SCD, nonsustained VT on Holter, LVOTO, and maximum LV wall thickness >30 mm. After the model was completed, the remaining candidate variables (ie, β‐blockers, atrial fibrillation, and rennin‐angiotensin‐aldosterone system inhibitors) were retested separately with a sensitivity analysis to examine their influence on effect estimates. Multivariable models were built individually for SCD and heart failure events in a similar pattern. Hazard ratios (HR) and 95% CI were calculated. We also divided HDRS scores into 4 categories: <8, 8 to 13, 14 to 18, and ≥19, and assessed the HDRS predictability for SCD in patients with HCM. Two‐sided P<0.05 was considered a significant difference.
Results
Baseline Characteristics
Of 856 patients diagnosed with HCM in the Jiaxing Heart Center between January 2014 and December 2017, 36 were excluded from the current study: 2 with dementia, 4 with a malignant tumor, 8 with severe chronic obstructive pulmonary disease or renal disease, 10 who were age ≤18 years old, and 12 who refused to participate in the study. Therefore, the study cohort included 820 patients, of which 192 (23.4%) patients had a diagnosis of depression (Figure 1). Among the overall patients, 387 (47.2%) participants had HDRS scores ≥5. The clinical and demographic characteristics of the study population are shown in Table 1. No statistically significant differences existed in the clinical and demographic characteristics between the 2 groups. The mean patient age was 48.2±12.8 years (range, 18–81 years), 470 (57.3%) were male, and the initial NYHA class was 1.5±0.8. Three hundred sixty‐one patients had 1 or more conventional risk factors for SCD. Only 27 patients with depression (14.1%) agreed to take antidepressants with selective serotonin reuptake inhibitors; the remaining 165 patients (85.9%) refused to take medicine or chose to go to the psychiatric hospital for confirmation. Mean HDRS score in depressed and nondepressed patients at the first assessment were 14.9±7.9 and 4.7±3.2, and after 12 months were 14.4±7.8 and 4.5±3.1, respectively.
Figure 1. Flowchart of the screening process and dropouts of the current cohort study.
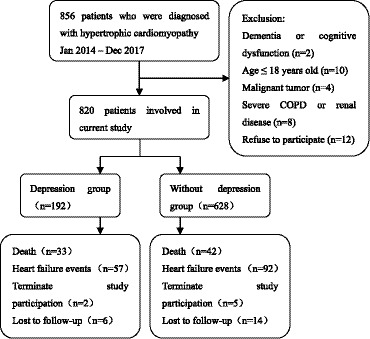
COPD indicates chronic obstructive pulmonary disease.
Table 1.
Demographic and Clinical Characteristics of the Study Population With or Without Depression
| Characteristics | Depression* (n=192) | Without Depression (n=628) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, mean±SD, y | 48.4±12.9 | 47.9±12.7 | 0.65 |
| Male, n (%) | 104 (54.3) | 366 (58.3) | 0.31 |
| BMI, mean±SD, kg/m2 | 24.4±3.7 | 24.7±3.8 | 0.62 |
| Maximum LV thickness, mean±SD, mm | 20.1±4.7 | 20.1±4.6 | 0.87 |
| NYHA class, n (%) | |||
| I/II | 171 (89.1) | 554 (88.2) | 0.75 |
| III/IV | 21 (10.9) | 74 (11.8) | 0.75 |
| Comorbid condition, n (%) | |||
| Atrial fibrillation | 18 (9.4) | 60 (9.6) | 0.94 |
| Stroke history | 3 (1.6) | 7 (1.1) | 0.91 |
| Diabetes mellitus | 25 (13.0) | 86 (13.7) | 0.81 |
| Hypertension | 34 (17.7) | 105 (16.7) | 0.75 |
| Risk factors, n (%) | |||
| Nonsustained VT on Holter | 35 (18.2) | 112 (17.8) | 0.90 |
| Unexplained syncope | 20 (10.4) | 53 (8.4) | 0.42 |
| Family history of SCD | 26 (13.5) | 79 (12.6) | 0.73 |
| LVOTO | 41 (21.3) | 142 (22.6) | 0.71 |
| Maximum LV wall thickness ≥30 mm | 10 (5.2) | 29 (4.6) | 0.74 |
| Echocardiography, mean±SD | |||
| LVEF (%) | 61.2±6.2 | 63.2±6.0 | 0.31 |
| Left atrial diameter | 40.1±9.2 | 39.8±9.0 | 0.26 |
| ICD implantation | |||
| ICD, n (%) | 20 (10.4) | 65 (10.4) | 0.98 |
| Medications at discharge, n (%) | |||
| β‐blockers | 117 (60.9) | 401 (63.8) | 0.46 |
| Calcium channel blockers | 33 (17.2) | 112 (17.8) | 0.84 |
| RAAS inhibitors | 31 (16.1) | 99 (15.8) | 0.90 |
| Diuretic | 15 (7.8) | 44 (7.0) | 0.71 |
| Amiodarone | 5 (2.6) | 17 (2.7) | 0.94 |
| Antidepressant | 27 (14.1) | 0 (0) | <0.001 |
| Laboratory parameters on admission, mean±SD | |||
| Pro‐BNP, pg/mL | 582.0±482.0 | 625.1±494.5 | 0.80 |
| Creatinine, μmol/L | 74.1±13.8 | 75.1±14.0 | 0.66 |
| HDRS score | 14.9±7.9 | 4.7±3.2 | <0.001 |
BMI indicates body mass index; HDRS, 17‐item Hamilton Depression Rating Scale; ICD, implantable cardioverter defibrillator; LV, left ventricle; LVEF, left ventricular ejection fraction; LVOTO, left ventricular outflow tract obstruction; NYHA, New York Heart Association; Pro‐BNP, pro‐brain natriuretic peptide; RAAS, rennin‐angiotensin‐aldosterone system; SCD, sudden cardiac death; and VT, ventricular tachycardia.
Depression is defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5).
Clinical Outcomes
Eight hundred patients were successfully followed up, and 20 (2.4%) individuals were lost to follow‐up. Over an average follow‐up period of 4.2 years, SCD events occurred in 75 individuals (21.8 per 1000 person‐years) and HCM‐related heart failure events developed in 149 individuals (43.3 per 1000 person‐years): 45 sudden death (109 versus 38 per 1000 person‐years; HR, 3.3; 95% CI, 1.8–5.9), 7 aborted arrests (15 versus 6 per 1000 person‐years; HR, 2.4; 95% CI, 1.3–4.8), 23 ICD discharge (47 versus 22 per 1000 person‐years; HR, 2.2; 95% CI, 1.2–4.7), 15 deaths from heart failure (26 versus 16 per 1000 person‐years; HR, 1.7; 95% CI, 1.0–3.3), 1 heart transplantation (0 versus 2 per 1000 person‐years; HR, 1.6; 95% CI, 0.2–15.2), 16 heart failure–related strokes (26 versus 18 per 1000 person‐years; HR, 1.5; 95% CI, 0.5–4.3), 28 heart failure hospitalizations (47 versus 30 per 1000 person‐years; HR, 1.7; 95% CI, 1.2–3.5), and 89 patients with progressive heart failure symptoms to NYHA class Ⅲ/Ⅳ (198 versus 81 per 1000 person‐years; HR, 2.5; 95% CI, 1.9–4.4, Table 2 and Figure S1). Clinical features of 33 patients with depression with HCM‐related SCD events are presented in Table S1. The average age was 45.9±15.3 years old (range, 21–73), and the mean NYHA class was 1.5±0.7 at initial evaluation. Of 33 individuals with depression, 10 (30.3%) had no risk factor for sudden death, and 9 (27.3%) had at least 2 risk factors. During follow‐up, 21 died suddenly, 3 survived an aborted cardiac arrest, and 9 received appropriate defibrillation shocks from an ICD.
Table 2.
Major Clinical Events of the Study Population During Follow‐Up
| Major Clinical Events, n (%) | Depression* (n=192) | Without Depression (n=628) | HR (95% CI) |
|---|---|---|---|
| HCM‐related sudden death | 21 (10.9) | 24 (3.8) | 3.3 (1.8–5.9) |
| Aborted arrest | 3 (1.6) | 4 (0.6) | 2.4 (1.3–4.8) |
| ICD discharge (VT/VF) | 9 (4.7) | 14 (2.2) | 2.2 (1.2–4.7) |
| Heart failure death | 5 (2.6) | 10 (1.6) | 1.7 (1.0–3.3) |
| Heart transplantation | 0 (0) | 1 (0.2) | 1.6 (0.2–15.2) |
| HCM‐related stroke | 5 (2.6) | 11 (1.8) | 1.5 (0.5–4.3) |
| Progression to NYHA class Ⅲ/Ⅳ | 38 (19.8) | 51 (8.1) | 2.5 (1.9–4.4) |
| Noncardiac death | 3 (1.6) | 8 (1.3) | 1.2 (0.8–2.7) |
| Heart failure hospitalization | 9 (4.7) | 19 (3.0) | 1.7 (1.2–3.5) |
HCM indicates hypertrophic cardiomyopathy; HR, hazard ratio; ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association; VF, ventricular fibrillation; and VT, ventricular tachycardia.
Depression is defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5).
Association of Depression and Clinical Outcomes
Kaplan–Meier cumulative incidence curves showed a significant association of depression disorders with SCD events (log‐rank P=0.001) and HCM‐related heart failure events (log‐rank P=0.005; Figure 2). In the univariate Cox regression model, depression had a significant relationship with increased risks of SCD events (HR, 2.1; 95% CI, 1.8–2.4; P<0.001) as well as HCM‐related heart failure events (HR, 2.0; 95% CI, 1.6–2.3; P<0.001; Table 3), and depression was retained as an independent prognostic factor for SCD events and HCM‐related heart failure events after adjustment for conventional risk factors (41.9 versus 21.7 per 1000 person‐years; HR, 1.9; 95% CI, 1.6–2.3; P<0.001; and 69.9 versus 38.6 per 1000 person‐years; HR, 1.8; 95% CI, 1.6–2.1; P<0.001, respectively) (Table 4). In addition, when the analysis was restricted to the SCD other than ICD discharge, the relative risk of SCD events remained essentially unchanged (26.7 versus 17.8 per 1000 person‐years; HR, 1.5; 95% CI, 1.2–1.9; P<0.001). Notably, unexplained syncope, a family history of SCD, LVOTO, nonsustained VT on ambulatory Holter, and maximum LV wall thickness >30 mm were significantly associated with sudden cardiac death, agreeing with the findings of previous studies.
Figure 2.
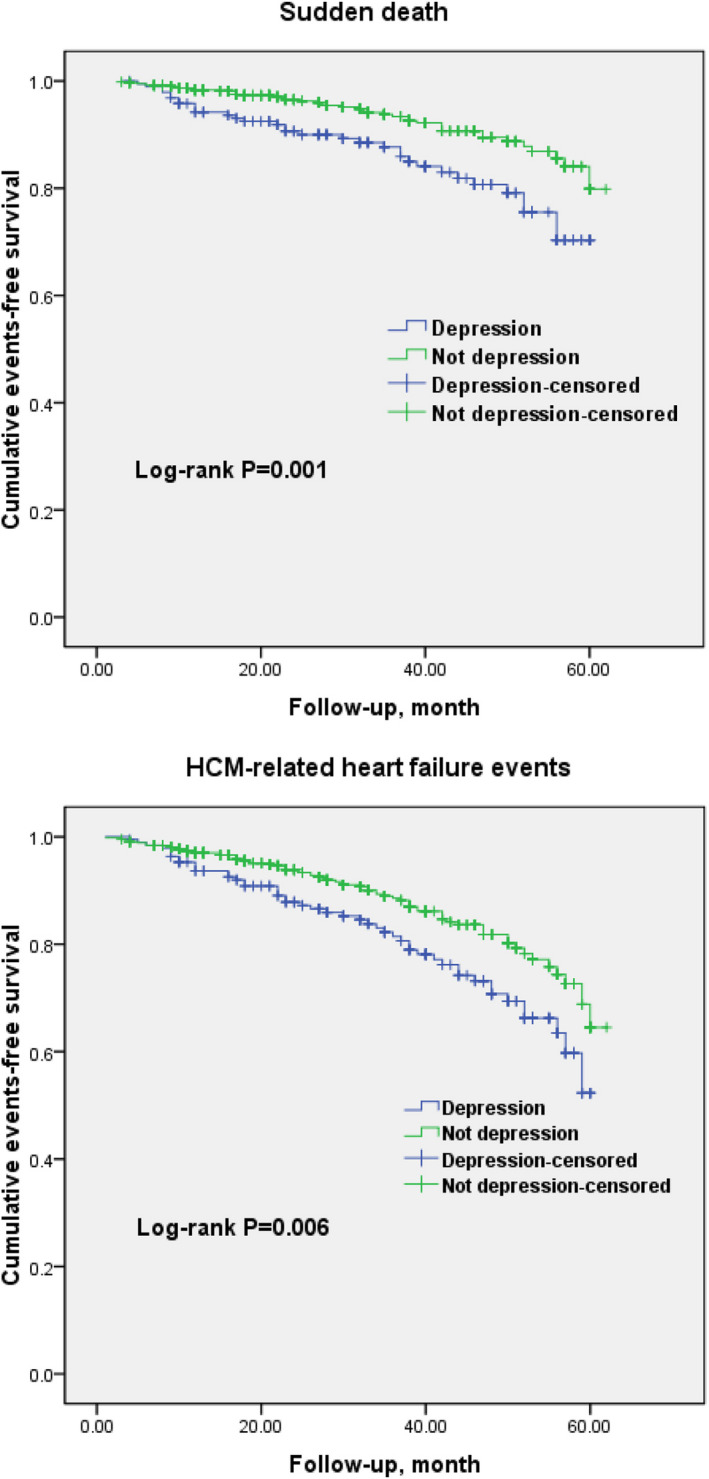
Kaplan–Meier curves for sudden cardiac death events and hypertrophic cardiomyopathy–related heart failure events in patients with and without depression.
Table 3.
Univariate Cox Regression of Variables Influencing SCD Events and HCM‐Related Heart Failure Events
| Variables | SCD Events | HCM‐Related Heart Failure Events | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age (per decade increase) | 0.98 (0.88–1.09) | 0.58 | 1.09 (0.98–1.18) | 0.10 |
| Left atrial diameter | 1.1 (0.9–1.2) | 0.21 | 1.1 (0.8–1.2) | 0.12 |
| Atrial fibrillation | 1.01 (0.84–1.11) | 0.49 | 1.3 (1.1–1.7) | 0.04 |
| β‐blockers | 0.8 (0.6–0.9) | 0.01 | 0.8 (0.7–0.9) | 0.03 |
| Calcium channel blockers | 0.93 (0.89–1.03) | 0.25 | 0.97 (0.89–1.12) | 0.65 |
| RAAS inhibitors | 0.97 (0.94–1.06) | 0.44 | 0.90 (0.79–1.08) | 0.07 |
| Diuretic | 1.01 (0.98–1.06) | 0.70 | 0.96 (0.84–1.10) | 0.34 |
| NVT on ambulatory Holter | 2.9 (2.4–3.6) | <0.001 | 1.5 (1.1–2.0) | 0.01 |
| Unexplained syncope | 2.0 (1.4–2.8) | 0.01 | 1.1 (0.9–1.2) | 0.53 |
| Family history of SCD | 1.7 (1.3–2.2) | 0.03 | 1.07 (0.95–1.12) | 0.63 |
| LVOTO | 2.3 (1.6–2.9) | <0.001 | 2.4 (2.1–3.7) | <0.001 |
| MLVWT ≥30 mm | 1.3 (1.1–1.8) | 0.04 | 1.5 (1.0–2.0) | 0.02 |
| Depression | 2.1 (1.8–2.4) | <0.001 | 2.0 (1.6–2.3) | <0.001 |
HCM indicates hypertrophic cardiomyopathy; HR, hazard ratio; LVOTO, left ventricular outflow tract obstruction; MLVWT, maximum left ventricle wall thickness; NVT, nonsustained ventricular tachycardia; RAAS, rennin‐angiotensin‐aldosterone system; and SCD, sudden cardiac death.
Table 4.
Multivariate Cox Regression of Variables Influencing SCD Events and HCM‐Related Heart Failure Events
| Variables | SCD Events | HCM‐Related Heart Failure Events | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| β‐blockers | 0.8 (0.6–1.0) | 0.03 | 0.8 (0.7–0.9) | 0.03 |
| Atrial fibrillation | NA | NA | 1.2 (1.0–1.3) | 0.09 |
| RAAS inhibitors | NA | NA | 0.9 (0.8–1.1) | 0.10 |
| NVT on ambulatory Holter | 2.7 (2.3–3.2) | <0.001 | 1.4 (1.0–1.9) | 0.03 |
| Unexplained syncope | 1.9 (1.4–2.4) | 0.01 | 1.1 (0.9–1.2) | 0.72 |
| Family history of SCD | 1.7 (1.3–2.2) | 0.02 | 1.1 (1.0–1.2) | 0.86 |
| LVOTO | 2.1 (1.6–2.6) | <0.001 | 2.2 (1.9–2.7) | <0.001 |
| MLVWT ≥30 mm | 1.21 (1.0–1.5) | 0.05 | 1.3 (1.1–2.0) | <0.001 |
| Depression | 1.9 (1.6–2.3) | <0.001 | 1.8 (1.6–2.1) | <0.001 |
HCM indicates hypertrophic cardiomyopathy; HR, hazard ratio; LVOTO, left ventricular outflow tract obstruction; MLVWT, maximum left ventricle wall thickness; NA, not applicable; NVT, nonsustained ventricular tachycardia; RAAS, rennin‐angiotensin‐aldosterone system; and SCD, sudden cardiac death.
Some 56.5% of the study sample had HDRS scores in the low normal range (<8), followed by 18.0% with scores indicative of mild depression (8–13), 14.9% with scores indicative of moderate depression (14–19), and only 10.6% who could be considered severely depressed (≥19). The clinical characteristics of the patients are shown in Table S2. Also, HDRS scores differ significantly for family history of SCD and LVOTO among groups. Results of the relationship of HDRS as categorical and continuous scores with SCD, both unadjusted and adjusted for risk factors, are shown in Table 5. Compared with the least‐depressed participants, the adjusted HRs (95% CIs) were 1.3 (1.1–1.6) for HDRS score of 8 to 13, 1.6 (1.3–1.8) for HDRS score of 14 to 19, and 2.3 (1.9–3.0) for patients in the most depressed categories (HDRS ≥19), respectively. Furthermore, there was evidence of a dose–response relationship between depression symptoms classified by HDRS scores and prognosis of SCD that began below the cutoff point of ≥8 (Figure 3).
Table 5.
Relationship of HDRS Score With SCD Events During Follow‐Up Period
| HDRS | Unadjusted | Adjusted* | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Categorical | ||||
| HDRS <7 | … | … | … | … |
| HDRS 8–13 | 1.4 (1.1–1.8) | 0.006 | 1.3 (1.1–1.6) | 0.014 |
| HDRS 14–18 | 1.8 (1.4–2.3) | <0.001 | 1.6 (1.3–1.8) | 0.003 |
| HDRS ≥19 | 2.4 (2.0–3.2) | <0.001 | 2.3 (1.9–3.0) | <0.001 |
| Continuous | 1.03 (1.02–1.05) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
HDRS indicates 17‐item Hamilton Depression Rating Scale; HR, hazard ratio; and SCD, sudden cardiac death.
Adjusted for unexplained syncope, family history of SCD, nonsustained ventricular tachycardia on Holter, maximum left ventricular wall thickness ≥30 mm, and left ventricular outflow tract obstruction.
Figure 3. Kaplan–Meier curves for sudden cardiac death events in patients classified by HDRS scores.
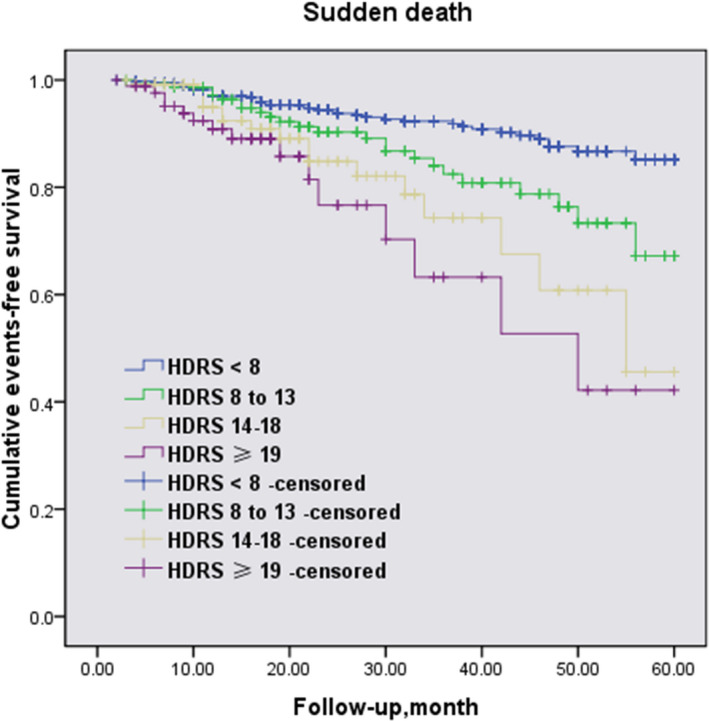
HDRS indicates Hamilton Depression Rating Scale.
Discussion
To our knowledge, this is the first prospective cohort study to evaluate the prevalence of depressive disorders in patients with HCM and investigate the potential association between depression and clinical outcomes in this population. One major methodological strength in the current study was that we diagnosed depression via formal psychological evaluation based on DSM‐5. Our results indicated that depression is common among patients with HCM with a high prevalence of 23.4%, and, in addition to traditional risk factors, depression can also predict adverse clinical outcomes for patients with HCM. In our earlier studies concerning the relation of depression on all‐cause mortality and cardiovascular events in patients with myocardial infarction and nonobstructive coronary arteries, patients with myocardial infarction and nonobstructive coronary arteries had 7.25 times the risk of mortality at a mean follow‐up of 3 years. 26 Compared with these findings, although depression in patients with HCM did not have as strong an association with these outcomes, the diagnosis of depression still had independent prognostic significance in patients with HCM.
A recent large‐scale meta‐analysis of 92 studies with 116 295 136 participants demonstrated that depression increased the risk for cardiovascular mortality by a factor of 1.63. In their study, cardiovascular disease was defined as a composite of coronary heart disease, congestive heart failure, and cerebrovascular disease. 27 Another comprehensive meta‐analysis conducted 15 years ago has shown that the relationship between depression and poorer heart failure outcomes is consistent and strong. Notably, the primary causes of heart failure in their studies were ischemic heart disease, dilated cardiomyopathy, and hypertensive heart disease. 28
However, the existing literature on the effects of depression on HCM outcomes is scarce. Two decades ago, Cox et al conducted an interesting study using the Short Form 36 Health Survey and the Hospital Anxiety and Depression questionnaire to assess health‐related quality of life and psychological well‐being of patients with HCM. The conclusion was that patients with HCM were associated with substantial restrictions on health‐related quality of life. In addition, their levels of anxiety and depression were also high when compared with the general population. 16 In an early study, Igoumenou et al reported that patients with HCM were more depressed than the general population. In contrast, no significant association was found between depressive symptoms and the risk factors for sudden death in this population. Nevertheless, their study sample was relatively small with a short follow‐up duration and the evaluation scale of depressive symptoms—the Beck Depression Inventory and the Center for Epidemiological Studies Depression Scale—was not equivalent to a diagnosis of depression. 29 Another study by Eva et al indicated that there was a significant reduction in psychological distress and an improvement in well‐being after alcohol septal ablation in patients with HCM. Their findings emphasized the importance of psychosocial outcomes and long‐term survival. 30 In the present study, we used the Chinese version of the Structured Clinical Interview based on the DSM‐5 by psychologists for the diagnosis of depression. Our results demonstrated that depression is common in patients with HCM and had a prevalence of 23.4%. This is generally consistent with the previous study by Igoumenou et al. 29 We further observed that depression is significantly and independently associated with SCD events and HCM‐related heart failure events in patients with HCM. Our findings indicated that, in addition to well‐known risk factors, depression could be of prognostic significance in patients with HCM. In other words, the present study further outlines the importance of screening for depression disorder in the HCM population before evaluation of an ICD implantation.
Numerous studies have indicated several potential mechanisms for the relationship between depression and poor prognosis but there is no consensus yet. Biologically, depression may result in impaired heart rate variability, endothelial dysfunction, chronic systemic inflammation, and hypothalamic‐pituitary‐adrenal axis dysfunction. 31 However, the exact mechanism underlying the relationship between depression and SCD events or heart failure events is less clear. Amanda et al established a genetic mouse model of HCM by knock‐in with the sarcomeric mutation gene, to investigate the influence of HCM over the development of depression and anxiety. They found that prolonged systemic stress of HCM can result in the development of mood disorders, probably by inducing structural and functional brain changes. 32 Previous studies suggest that depression was associated with chronically increased catecholamine levels, high sympathetic nervous activity, easily activated platelets, and a hypercoagulable state. 33 , 34 , 35 In addition, depression has also been related to symptomatic long QT syndrome and QT dispersion recorded by electrocardiography. 36 , 37 Finally, patients with depression are inclined to have unhealthy lifestyles, including smoking, heavy alcohol consumption, low physical activity, poor drug compliance, and disruptions of social relationships. However, our study found that both groups were comparable regarding smoking, body mass index, and comorbidities. Consequently, the poor prognosis in depressed patients is likely the result of biopsychosocial interactions. 38
Several strengths should be noted in the present study. First, the 17‐item HDRS was considered the criterion standard for assessment of depression for >50 years and defined remission of depression with a score of <7. Sawamura et al reported that a cutoff value for a 17‐item HDRS score of <5 was the best candidate for the symptomatic relief if the health‐related quality of life was considered. 21 In the current study, patients with an HDRS score of ≥5 were given the Chinese version of the Structured Clinical Interview based on DSM‐5 by psychologists, for diagnosis of depression. Second, the well‐known risk factors that have significant effects on HCM outcomes, such as unexplained syncope, family history of SCD, nonsustained VT on Holter, LVOTO, and maximum LV wall thickness >30 mm, were all used for adjustment. 2 , 6 Third, the assessment of depressive symptoms was conducted at baseline and 12 months after the patients' discharge. We supposed that hospitalization might increase negative emotions at enrollment, but there was no significant difference between baseline and 1‐year follow‐up in HDRS score. Fourth, 1 advantage of the current observational study is that patients were contacted by instant messaging (WeChat) or telephone, which promoted the level of the quality of follow‐up. In conclusion, the rate of loss to follow‐up was only 2.4% in the present study.
This study has several potential limitations. First, residual confounding may coexist with depression disorders, such as anxiety, insomnia, marital status, or economic conditions, but we did not collect relevant information on these variables. Second, an inevitable limitation is the absence of data from patients who refused to participate in this study; however, very few eligible patients were excluded from the present analysis because this was an observational study. Finally, all patients were recruited from a single Heart Center, thereby raising concerns on the generalizability of results.
Conclusions
In conclusion, the incidence of depression in Chinese patients with HCM is high. The diagnosis of depression is significantly and independently associated with an increased risk of SCD events and heart failure events in patients with HCM. Further study should be encouraged to validate whether psychological intervention for depression can improve clinical outcomes in this population.
Sources of Funding
This research was funded by Provincial‐Municipal Joint Construction of Key Medical Disciplines In Zhejiang Province (2019‐ss‐xxgbx), Jiaxing Key Innovation Team Fund (2018‐xjqxcxtd), Zhejiang Provincial Health Science and Technology Program under Grant No. 2021KY1105, Zhejiang Medical Association Clinical Research Fund Grant No. 2020ZYC‐A45, Jiaxing Science and Technology Program under Grant No. 2021AD30148 and Zhejiang Provincial Basic Public Welfare Research Program of China under Grant No. LGF21H0006.
Disclosures
None.
Supporting information
Tables S1–S2
Figure S1
Acknowledgments
The authors would like to express their gratitude to these participants and families.
(J Am Heart Assoc. 2021;10:e019071. DOI: 10.1161/JAHA.120.019071.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019071
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Chang‐Lin Zhai, Email: yesterdaygun@126.com.
Chao‐Jie He, Email: hechaojie824@163.com.
References
- 1. Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:655–668. DOI: 10.1056/NEJMra1710575. [DOI] [PubMed] [Google Scholar]
- 2. Geske JB, Ommen SR, Gersh BJ. Hypertrophic cardiomyopathy: clinical update. JACC Heart Fail. 2018;6:364–375. DOI: 10.1016/j.jchf.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 3. Alexander PMA, Nugent AW, Daubeney PEF, Lee KJ, Sleeper LA, Schuster T, Turner C, Davis AM, Semsarian C, Colan SD, et al. Long‐term outcomes of hypertrophic cardiomyopathy diagnosed during childhood: results from a national population‐based study. Circulation. 2018;138:29–36. DOI: 10.1161/CIRCULATIONAHA.117.028895. [DOI] [PubMed] [Google Scholar]
- 4. Maron BJ, Rowin EJ, Maron MS. Paradigm of sudden death prevention in hypertrophic cardiomyopathy. Circ Res. 2019;125:370–378. DOI: 10.1161/CIRCRESAHA.119.315159. [DOI] [PubMed] [Google Scholar]
- 5. Makavos G, Κairis C, Tselegkidi ME, Karamitsos T, Rigopoulos AG, Noutsias M, Ikonomidis I. Hypertrophic cardiomyopathy: an updated review on diagnosis, prognosis, and treatment. Heart Fail Rev. 2019;24:439–459. DOI: 10.1007/s10741-019-09775-4. [DOI] [PubMed] [Google Scholar]
- 6. Authors/Task Force Members , Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. DOI: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 7. Sherrid MV, Massera D. Risk stratification and hypertrophic cardiomyopathy subtypes. J Am Coll Cardiol. 2019;74:2346–2349. DOI: 10.1016/j.jacc.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 8. Raman B, Ariga R, Spartera M, Sivalokanathan S, Chan K, Dass S, Petersen SE, Daniels MJ, Francis J, Smillie R, et al. Progression of myocardial fibrosis in hypertrophic cardiomyopathy: mechanisms and clinical implications. Eur Heart J Cardiovasc Imaging. 2019;20:157–167. DOI: 10.1093/ehjci/jey135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gujral S, Aizenstein H, Reynolds CF III, Butters MA, Erickson KI. Exercise effects on depression: possible neural mechanisms. Gen Hosp Psychiatry. 2017;49:2–10. DOI: 10.1016/j.genhosppsych.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rotenstein LS, Ramos MA, Torre M, Segal JB, Peluso MJ, Guille C, Sen S, Mata DA. Prevalence of depression, depressive symptoms, and suicidal ideation among medical students: a systematic review and meta‐analysis. JAMA. 2016;316:2214–2236. DOI: 10.1001/jama.2016.17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freedland KE, Carney RM. Depression as a risk factor for adverse outcomes in coronary heart disease. BMC Med. 2013;11:131. DOI: 10.1186/1741-7015-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lahtinen M, Kiviniemi AM, Junttila MJ, Kääriäinen M, Huikuri HV, Tulppo MP. Depressive symptoms and risk for sudden cardiac death in stable coronary artery disease. Am J Cardiol. 2018;122:749–755. DOI: 10.1016/j.amjcard.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 13. Luukinen H, Laippala P, Huikuri HV. Depressive symptoms and the risk of sudden cardiac death among the elderly. Eur Heart J. 2003;24:2021–2026. DOI: 10.1016/j.ehj.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 14. Irvine J, Basinski A, Baker B, Jandciu S, Paquette M, Cairns J, Connolly S, Roberts R, Gent M, Dorian P. Depression and risk of sudden cardiac death after acute myocardial infarction: testing for the confounding effects of fatigue. Psychosom Med. 1999;61:729–737. DOI: 10.1097/00006842-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 15. Christiaans I, van Langen IM, Birnie E, Bonsel GJ, Wilde AA, Smets EM. Quality of life and psychological distress in hypertrophic cardiomyopathy mutation carriers: a cross‐sectional cohort study. Am J Med Genet A. 2009;149:602–612. DOI: 10.1002/ajmg.a.32710. [DOI] [PubMed] [Google Scholar]
- 16. Igoumenou A, Alevizopoulos G, Anastasakis A, Stavrakaki E, Toutouzas P, Stefanadis C. Depression in patients with hypertrophic cardiomyopathy: is there any relation with the risk factors for sudden death? Heart Asia. 2012;4:44–48. DOI: 10.1136/heartasia-2012-010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vamos M, Healey JS, Wang J, Connolly SJ, Mabo P, Van Erven L, Kautzner J, Glikson M, Neuzner J, O'Hara G, et al. Implantable cardioverter‐defibrillator therapy in hypertrophic cardiomyopathy: a SIMPLE substudy. Heart Rhythm. 2018;15:386–392. DOI: 10.1016/j.hrthm.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 18. O'Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, McKenna WJ, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk‐SCD). Eur Heart J. 2014;35:2010–2020. DOI: 10.1093/eurheartj/eht439. [DOI] [PubMed] [Google Scholar]
- 19. Sadoul N, Prasad K, Elliott PM, Bannerjee S, Frenneaux MP, McKenna WJ. Prospective prognostic assessment of blood pressure response during exercise in patients with hypertrophic cardiomyopathy. Circulation. 1997;96:2987–2991. DOI: 10.1161/01.CIR.96.9.2987. [DOI] [PubMed] [Google Scholar]
- 20. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. DOI: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sawamura J, Ishigooka J, Nishimura K. Re‐evaluation of the definition of remission on the 17‐item Hamilton Depression Rating Scale based on recovery in health‐related quality of life in an observational post‐marketing study. Health Qual Life Outcomes. 2018;16:14. DOI: 10.1186/s12955-018-0838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haghjoo M, Mohammadzadeh S, Taherpour M, Faghfurian B, Fazelifar AF, Alizadeh A, Rad MA, Sadr‐Ameli MA. ST‐segment depression as a risk factor in hypertrophic cardiomyopathy. Europace. 2009;11:643–649. DOI: 10.1093/europace/eun393. [DOI] [PubMed] [Google Scholar]
- 23. Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, et al. Prognostic value of quantitative contrast‐enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–495. DOI: 10.1161/CIRCULATIONAHA.113.007094. [DOI] [PubMed] [Google Scholar]
- 24. Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F. Epidemiology of hypertrophic cardiomyopathy‐related death: revisited in a large non‐referral‐based patient population. Circulation. 2000;102:858–864. DOI: 10.1161/01.CIR.102.8.858. [DOI] [PubMed] [Google Scholar]
- 25. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 26. Gu XH, He CJ, Shen L, Han B. Association between depression and outcomes in chinese patients with myocardial infarction and nonobstructive coronary arteries. J Am Heart Assoc. 2019;8:e011180. DOI: 10.1161/JAHA.118.011180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, Thapa‐Chhetri N, Fornaro M, Gallicchio D, Collantoni E, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large‐scale meta‐analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16:163–180. DOI: 10.1002/wps.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta‐analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. DOI: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 29. Igoumenou A, Alevizopoulos G, Anastasakis A, Stavrakaki E, Toutouzas P, Stefanadis C. Depression in patients with hypertrophic cardiomyopathy: is there any relation with the risk factors for sudden death?. Heart Asia. 2012;4:44–48. DOI: 10.1136/hrt.78.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic obstructive cardiomyopathy: surgical myectomy and septal ablation. Circ Res. 2017;121:771–783. DOI: 10.1161/CIRCRESAHA.116.309348. [DOI] [PubMed] [Google Scholar]
- 31. Halaris A. Inflammation‐associated co‐morbidity between depression and cardiovascular disease. Curr Top Behav Neurosci. 2017;31:45–70. DOI: 10.1007/7854_2016_28. [DOI] [PubMed] [Google Scholar]
- 32. Dossat AM, Sanchez‐Gonzalez MA, Koutnik AP, Leitner S, Ruiz EL, Griffin B, Rosenberg JT, Grant SC, Fincham FD, Pinto JR, et al. Pathogenesis of depression‐ and anxiety‐like behavior in an animal model of hypertrophic cardiomyopathy. FASEB J. 2017;31:2492–2506. DOI: 10.1096/fj.201600955RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levick JR, McDonald JN, Knight AD. Asymmetrical effects of albumin on transsynovial fluid movement. Semin Arthritis Rheum. 1991;21:184–190. DOI: 10.1016/0049-0172(91)90008-N. [DOI] [PubMed] [Google Scholar]
- 34. Ziegelstein RC, Parakh K, Sakhuja A, Bhat U. Platelet function in patients with major depression. Intern Med J. 2009;39:38–43. DOI: 10.1111/j.1445-5994.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 35. von Känel R. Changes in blood coagulation in stress and depression–from evolution to gene regulation. Ther Umsch. 2003;60:682–688. DOI: 10.1024/0040-5930.60.11.682. [DOI] [PubMed] [Google Scholar]
- 36. Wesołowska K, Elovainio M, Koponen M, Tuiskula AM, Hintsanen M, Keltikangas‐Järvinen L, Määttänen I, Swan H, Hintsa T. Is symptomatic long QT syndrome associated with depression in women and men? J Genet Couns. 2017;26:491–500. DOI: 10.1007/s10897-016-0004-4. [DOI] [PubMed] [Google Scholar]
- 37. Tolentino JC, Schmidt SL. Association between depression and cardiovascular disease: a review based on QT dispersion. Eur J Prev Cardiol. 2019;26:1568–1570. DOI: 10.1177/2047487319833509. [DOI] [PubMed] [Google Scholar]
- 38. Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. DOI: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figure S1


