Abstract
Background
Pulmonary arterial hypertension (PAH) manifests with progressive right ventricular (RV) dysfunction, which eventually impairs the left ventricular function. We hypothesized that 4‐dimensional–flow magnetic resonance imaging can detect flow hemodynamic changes associated with efficient intracardiac flow during noninvasive inhaled nitric oxide (iNO) challenge in children with PAH.
Methods and Results
Children with PAH (n=10) underwent 2 same‐day separate iNO challenge tests using: (1) 4‐dimensional–flow magnetic resonance imaging and (2) standard catheterization hemodynamics. Intracardiac flow was evaluated using the particle tracking 4‐flow component analysis technique evaluating the direct flow, retained inflow, delayed ejection flow, and residual volume. Respective flow hemodynamic changes were compared with the corresponding catheterization iNO challenge results. The RV analysis revealed decreased direct flow in patients with PAH when compared with controls (P<0.001) and increase in residual volume (P<0.001). Similarly, the left ventricular analysis revealed decreased direct flow in patients with PAH when compared with controls (P=0.004) and increased proportion of the residual volume (P=0.014). There was an increase in the RV direct flow during iNO delivery (P=0.009), with parallel decrease in the residual volume (P=0.008).
Conclusions
Children with PAH have abnormal biventricular flow associated with impaired diastolic filling. The flow efficiency is significantly improved in the RV on iNO administration with no change in the left ventricle. The changes in the RV flow have occurred despite the minimal change in catheterization hemodynamics, suggesting that flow hemodynamic evaluation might provide more quantitative insights into vasoreactivity testing in PAH.
Keywords: intracardiac flow, nitric oxide, pulmonary hypertension
Subject Categories: Heart Failure, Endothelium/Vascular Type/Nitric Oxide, Pulmonary Hypertension, Congenital Heart Disease
Nonstandard Abbreviations and Acronyms
- 4D
4‐dimensional
- iNO
inhaled nitric oxide
- mPAP
mean pulmonary arterial pressure
- PAH
pulmonary arterial hypertension
- RHC
right heart catheterization
Clinical Perspective
What Is New?
Children with pulmonary arterial hypertension have abnormal flow through both ventricles, associated with impaired diastolic filling.
The flow efficiency is significantly improved in the right ventricle on inhaled nitric oxide administration with no change in the left ventricle.
What Are the Clinical Implications?
The changes in the right ventricular flow have occurred despite the minimal change in catheterization hemodynamics, suggesting that flow hemodynamic evaluation might provide more quantitative insights into vasoreactivity testing in pulmonary arterial hypertension.
Four‐dimensional–flow magnetic resonance imaging–based evaluation may aid with the therapy guidance and clinical decision making in pediatric patients with pulmonary arterial hypertension.
Pulmonary arterial hypertension (PAH) is characterized by the progressive pulmonary vascular remodeling, leading to chronically elevated right ventricular (RV) afterload, which culminates into systolic and diastolic RV failure. 1 , 2 , 3 Progressive RV dysfunction further impairs the left ventricular (LV) function via interventricular interactions and by reduced LV preload. 4 , 5 Indeed, large studies have identified biventricular function and hemodynamics as the strong predictors of short‐ and long‐term survival in PAH. 6 , 7 Similar pathophysiologic processes and biventricular function with strong clinical prognostic importance are seen in pediatric PAH. 8 , 9 , 10 However, the amount of pediatric studies investigating the biventricular function and its change with respect to the therapeutic intervention remain limited.
Intracardiac flow analysis using 4‐dimensional (4D)–flow magnetic resonance imaging (MRI) has emerged as a novel imaging modality to assess comprehensively biventricular systolic and diastolic function. 11 Simultaneously, qualitative flow evaluation of ventricular filling patterns has been recently hypothesized to be an early predictor of heart failure before a noticeable tissue deformation or echocardiographic abnormalities. 12 , 13 Our previous investigations in adult patients with PAH of mixed cause showed that intracardiac quantitative flow hemodynamic indexes, such as vorticity and helicity, have been associated with the disease severity, standard catheterization hemodynamics, and echocardiographic markers of diastolic dysfunction. 14 , 15 , 16 In the pediatric setting, intracardiac flow analysis is yet to be explored, but earlier studies suggest that the flow hemodynamic patterns might be different between children with PAH and adult patients with PAH with similar pulmonary vascular resistance and pressures. 17 Last, the short‐term effect of the inhaled nitric oxide (iNO) challenge, the clinically cardinal test assessing the pulmonary vasoreactivity, on flow hemodynamics has never been explored.
Consequently, in this study we sought to (1) compare the biventricular intracardiac flow between children with PAH and their healthy normotensive peers using 4D‐flow MRI particle tracking 4‐flow component analysis technique, 18 (2) assess the flow hemodynamic changes using the same technique on iNO challenge, hence simulating noninvasive acute vasoreactivity testing, and (3) compare the respective flow hemodynamic changes with the corresponding same‐day standard catheterization iNO testing. We specifically hypothesized that the intracardiac flow will be abnormal in in both ventricles in patients with PAH in comparison to controls, and we further hypothesized that the iNO will improve the intracardiac flow hemodynamics.
Methods
As a part of prospective investigation MatCH‐uPP (MRI and Protocol Catheterization Hemodynamics in Pediatric Pulmonary Hypertension), approved by Colorado Multi‐institutional Review Board, we enrolled 10 patients with PAH with consent/assent appropriate for age. A cardiac MRI study, including 4D‐flow MRI acquisition, was performed without anesthesia within 48 hours of a clinically indicated right heart catheterization (RHC). Our inclusion criteria included any with previously diagnosed PAH by catheterization and echocardiogram, per previously diagnosed guidelines, 19 age 7 to 21 years with a mean pulmonary arterial pressure (mPAP) >25 mm Hg by RHC, or RV pressure >50% of systemic arterial pressure, established by echocardiogram before the age of 18 years. We excluded any patient with previous pulmonary vascular intervention and anatomic pulmonary vascular stenosis because the additional elements of the RV afterload would have additional effects on flow hemodynamics. We also excluded significant intracardiac shunts, defined as a pulmonary flow (Qp):systemic flow(Qs) >1.2, pulmonary thromboembolic disease, pulmonary hypertension attributable to left heart disease, veno‐occlusive disease, and pulmonary capillary hemangiomatosis. In addition, 10 healthy volunteers were recruited to undergo 4D‐flow MRI to establish the pediatric normative values. The data that support the findings of this study are available from the corresponding author on reasonable request.
Nitric Oxide Challenge and Determination of Acute Vascular Reactivity
Right Heart Catheterization
Part of the PAH RHC at our institution is acute vasoreactivity testing (AVT) challenge, with pulmonary vascular resistance (PVR), mPAP, and cardiac output obtained at room air and under vasodilator challenge. The latter involves FiO2 1.0+40 ppm iNO. Such challenges are routine components of the clinical evaluation of reactivity. Criteria for acute vascular reactivity were based on the criterial of Sitbon et al 20 and were used for data derived by RHC and by MRI: (1) decrease in mPAP of ≥10 mm Hg to absolute value of mPAP <40 mm Hg and (2) unchanged or increased cardiac index.
Four‐D‐Flow MRI
The acquisition protocol and data analysis are described in the later section. 4D‐flow MRI and standard volume data sets were acquired at baseline FiO2. 4D‐flow MRI was repeated after 5 minutes of administration of FiO2 1.0+40 ppm iNO. iNO was delivered by the INOmax DSIR Plus MRI delivery system. A proportional amount of drug was added to the respiratory device circuit (noninvasive application). As the drug transitioned through the inspiratory limb, it was diluted to the dose set on the delivery system. A respiratory therapist was with the child during the 10 minutes of iNO delivery. The child was monitored at all times by heart rate, blood pressure, and oxygen saturation measurements, and was able to communicate with the team immediately outside of the scanner. All subjects receiving iNO were monitored for at least 15 minutes after iNO was stopped.
Cardiovascular MRI and 4D‐Flow MRI Protocol
All subjects underwent comprehensive cardiac MRI evaluation for volumetric, functional, and flow evaluation using a previously described MRI protocol with a 3‐T magnet system (Ingenia; Philips Medical Systems, Best, the Netherlands). 17 Standard 2‐dimensional cine balanced steady‐state free precession long‐axis, short‐axis, and 4‐chamber–axis images were obtained during end‐expiratory breath holds for volumetric and functional analysis. 4D‐flow MRI was acquired in a sagittal plane covering the midthorax with retrospective ECG gating and a diaphragmatic navigator for assessment of blood flow hemodynamics. Typical sequence parameters were as follows: echo times, 2.4 to 2.6 ms; repetition times, 4.2 to 5.0 ms; flip angle, 10°; temporal resolution, 38 to 48 ms; field of view, 250×320 or 200×250 mm2; 14 to 18 cardiac phases; voxel size, 2.0×2.0×2.0 to 2.8 mm3; Velocity encoding (VENC), 100 to 200 cm/s; and acquisition time, 10 to 15 minutes, depending on respiratory gating efficiency.
Intracardiac Flow Analysis
4D‐flow MRI visualization and analysis was performed using Circle CVI42 platform (Version 5.9.3; Circle Cardiovascular Imaging, Calgary, AB, Canada). Blood flow organization analysis was performed using a particle tracking approach developed by Eriksson et al, enabling parcellation of the LV end‐diastolic volume into 4 virtual components based on their anatomic residency with respect to the cardiac cycle. 18 , 21 The detailed schematic of the 4D‐flow postprocessing and particle tracking analysis has been described elsewhere. 21 , 22 Briefly, the initial step consists from the segmentation of individual ventricular cavities, defined by time‐resolved magnetic resonance angiography reconstructed from the 4D‐flow MRI data sets. The LV cavity was basally delineated by the aortic and mitral valve annuli and apically by the endocardial extent of the LV. An identical approach is applied for the RV analysis, where the anatomical borders are defined basally by the tricuspid and pulmonary valves and apically by the endocardial extent of the RV. Next, ventricular inflow and outflow orifices were defined by respectful atrioventricular and semilunar valves. The mismatch between inflow and outflow <5% was considered acceptable. On the basis of combination of path‐line trajectories and temporal‐geometric conditions, 4 ventricular flow components are defined per original description by Eriksson et al 18 : (1) direct flow: blood that crosses the atrioventricular valve to enter the ventricle and is ejected within the same cardiac cycle, (2) retained inflow: blood that crosses the atrioventricular valve to enter the ventricle but does not get ejected during the same cardiac cycle, (3) delayed ejection flow: blood present inside the ventricle as part of the end‐systolic volume that exits the ventricle during the subsequent systole, and (4) residual volume: blood that recirculates inside the ventricle for at least 2 cardiac cycles. Every considered component represents the portion of the overall ventricular end‐diastolic volume and is reported as percentage (Figure 1). The direct flow represents the most efficient form of flow propagation through the ventricle, and its decreasing proportion is associated with systolic and diastolic dysfunction. 23 , 24 On the other hand, residual volume represents the least effective route of interventricular flow, and its increasing proportion is typically associated with impaired myocardial function and functional worsening.
Figure 1. Representation of the intracardiac particle tracking visualization in the right ventricle (RV) and left ventricle (LV) throughout the cardiac cycle.
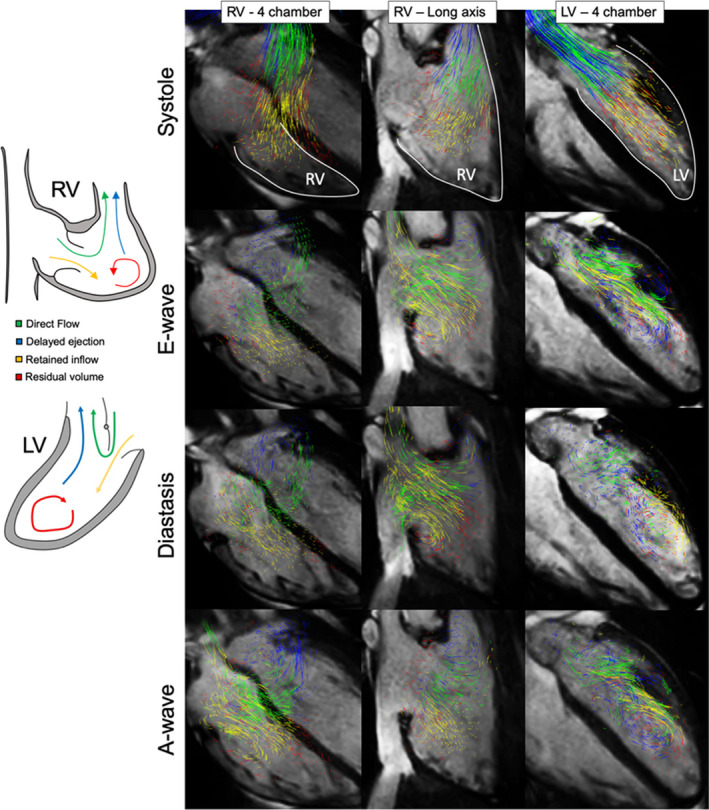
Four components of the end‐diastolic volume are color coded as follows: green, direct flow; blue, retained inflow; yellow, delayed ejection flow; and red, residual volume.
Last, qualitative analysis of the intracardiac inflow patterns was performed to appreciate secondary flow, such as vortical formations along the atrioventricular leaflets known to be impaired in the setting of diastolic dysfunction. 12 Every study was evaluated for the presence of vortex formation along specific mitral and tricuspid leaflets. A fully formed vortex was defined as the complete concentric set of rings detected by path‐line projection at any phase of diastolic filling.
Statistical Analysis
Analyses were performed using freely available software Estimation Statistics Beta 25 and Prism (version 7.0; GraphPad Software Inc, La Jolla, CA). Variables were checked for the distributional assumption of normality using normal plots, in addition to Kolmogorov‐Smirnov and Shapiro‐Wilk tests. Comparison of flow hemodynamic data was done using unpaired (PAH versus control) and paired (pre‐iNO versus post‐iNO) Student t test. The comparison of 4D‐flow MRI characteristics before and after nitric oxide challenge and among PAH versus control were analyzed using paired and unpaired 2‐group Gardner‐Altman plots, respectively. This approach is analogous to Student t test but presents the effect size as a bootstrap 95% CI. The relationship between the blood inflow indexes and catheterization hemodynamics was analyzed using the Pearson method. Significance was based on α value 0.05.
Results
Patient demographics and standard PAH‐related hemodynamic data are presented in the Table. There were no differences between PAH and control groups in age, size, and sex distribution. The median time from PAH diagnosis was 5.5 years (range, 1–13 years). Eight patients with PAH had idiopathic PAH, and 2 patients had heritable PAH. Three patients were classified by World Health Organization functional class category I, 4 as category II, and 3 as category III. All 10 patients were taking phosphodiesterase‐5 inhibitors, 6 patients were receiving endothelin‐receptor antagonists, and 2 patients were on intravenous prostanoid therapy. At the time of initial diagnosis, only 2 patients met acute vasoreactivity criteria. There were no significant differences in RV or LV function or size parameters, but patients with PAH had increased RV volumes and reduced ejection fraction, nearly approaching significance. Baseline mPAP was 36±12 mm Hg (range, 15–62 mm Hg), PVR index was 8.1±3.6 Wood units (range, 3.2–13.6 Wood units), RV systolic pressure was 56±16 mm Hg (range, 30–86 mm Hg), and RV diastolic pressure was 6±2 mm Hg (range, 3–8 mm Hg). Graphical representation of the baseline catheterization hemodynamic indexes and their corresponding values on iNO challenge is portrayed in Figure 2. Paired mean difference in mPAP was −4.7 mm Hg, −1.6 Wood units in PVR index, −5.6 mm Hg in RV systolic pressure, and −0.6 mm Hg in RV diastolic pressure. Per criteria proposed by Sitbon et al, 20 2 patients have met acute vasoreactivity criteria.
Table 1.
Demographics and Baseline CMR Hemodynamics
| Variable | PAH Group (n=10) | Control Group (n=10) | P Value |
|---|---|---|---|
| Age, y | 14.2±3.5 | 12.1±4.3 | 0.149 |
| BSA, m2 | 1.40±0.23 | 1.19±0.20 | 0.079 |
| Sex (women), n (%) | 7 (70) | 6 (60) | 0.639 |
| RV EDVi, mL/m2 | 104±19 | 86±16 | 0.034* |
| RV ESVi, mL/m2 | 51±14 | 35±6 | 0.024* |
| RV SVi, mL/m2 | 53±8 | 51±14 | 0.592 |
| RV EF, % | 50±5 | 60±5 | <0.001* |
| RV CI, L/min per m2 | 3.8±1.0 | 3.4±0.7 | 0.130 |
| LV EDVi, mL/m2 | 90±16 | 83±16 | 0.364 |
| LV ESVi, mL/m2 | 41±8 | 35±8 | 0.251 |
| LV SVi, mL/m2 | 49±9 | 47±9 | 0.316 |
| LV EF, % | 56±2 | 60±5 | 0.127 |
Data reported as mean±SD, unless otherwise indicated. BSA indicates body surface area; CI, cardiac index; CMR, cardiovascular magnetic resonance imaging; EDVi, end‐diastolic volume index; EF, ejection fraction; ESVi, end‐systolic volume index; LV, left ventricular; PAH, pulmonary arterial hypertension; RV, right ventricular; and SVi, stroke volume index.
P<0.05.
Figure 2. Graphical comparison of the catheterization hemodynamics at baseline and during inhaled nitric oxide (iNO) challenge.
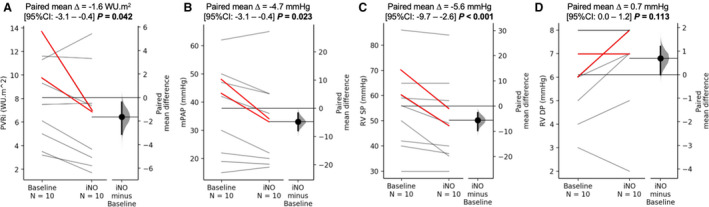
There was a significant reduction in pulmonary vascular resistance index (PVRi) (A), mean pulmonary arterial pressure (mPAP) (B), and the right ventricular (RV) systolic pressure (SP) (C) on iNO administration. There was no difference in the RV diastolic pressure (DP) (D). Red lines represent the cases that met the Sitbon criteria for positive acute vasoreactivity test. WU indicates Wood unit.
Four‐D‐Flow MRI: PAH Versus Control
Particle tracking postprocessing was successfully completed in the RV in all subjects with PAH and control subjects. Two subjects with PAH were excluded from the LV analysis because of incomplete coverage of the LV, limiting accurate segmentation and flow hemodynamic analysis. Graphical representation of the 4D‐flow MRI comparison between PAH and control groups is depicted in Figure 3. The RV analysis revealed significantly decreased proportion of the direct flow in patients with PAH when compared with controls (19.3±7.2% versus 37.0±12.4%; P<0.001). Inversely, the patients with PAH demonstrated increased proportion of the residual volume (40.7±12.4% versus 18.3±4.7%; P<0.001). There were no significant differences in the RV delayed ejection and retained inflow. Similarly to the RV findings, LV direct flow was decreased in the patients with PAH (23.2±8.0% versus 35.5±6.3%; P=0.004). The LV residual flow was increased in the patients with PAH (27.1±10.0% versus 16.6±3.7%; P=0.014). There were no differences in the LV delayed ejection and retained inflow.
Figure 3. Comparison of 4‐dimensional–flow magnetic resonance imaging particle tracking parameters between patients with pulmonary arterial hypertension (PAH) and controls.
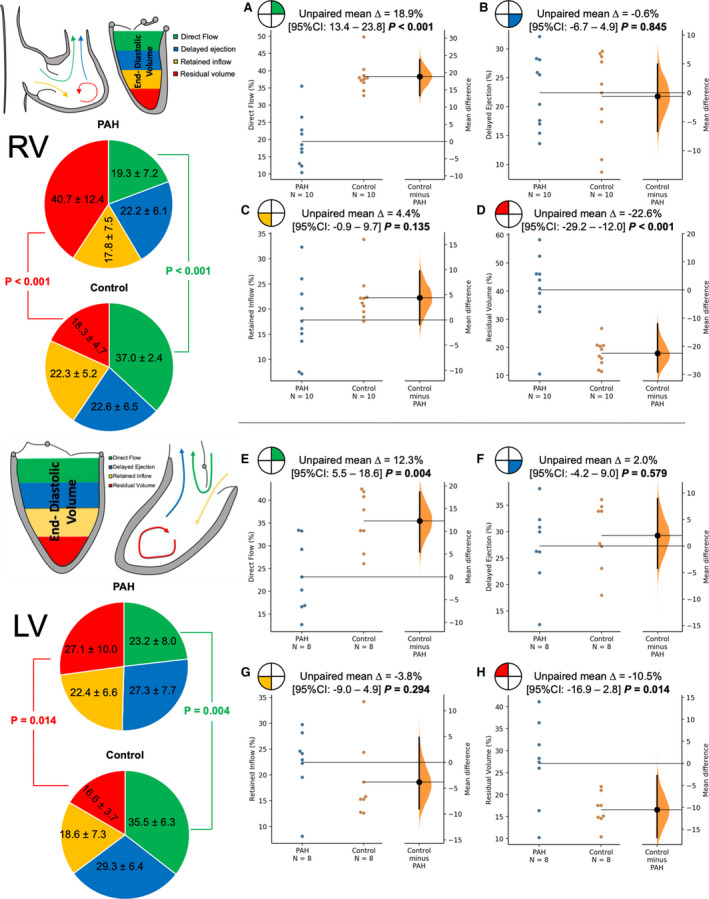
Top panels (A through D) show the comparison of the individual flow/end‐diastolic components in the right ventricle (RV). Patients with PAH had significantly decreased direct flow (A) when compared with controls and significantly increased residual volume (D) when compared with controls. There were no differences in the RV delayed ejection (B) and retained inflow (C). Bottom panels (E through H) show the identical analysis for the left ventricle (LV). Patients with PAH had significantly decreased direct volume (E) and increased residual volume (H), with no differences in the LV delayed volume (F) and retained inflow (G).
Qualitative analysis of the RV inflow demonstrated similar results between the patients with PAH and controls (Figure 4). All considered subjects demonstrated prominent vortex formation along each leaflet of the tricuspid valve. The LV diastolic flow demonstrated mild qualitative differences. All control subjects presented physiologic large‐scale vortex formed along the anterior mitral valve leaflet, which fused with the smaller vortex formed along the posterior leaflet. This fully formed vortex prevails during diastole and moved toward the LV apex. On the other hand, 4 subjects with PAH demonstrated the vortex formation solely along the anterior mitral valve leaflet, which did not fully develop during diastasis and dissipated at the end of diastole.
Figure 4. Comparison of qualitative flow analysis between representative patient with pulmonary arterial hypertension (PAH) and control subject.
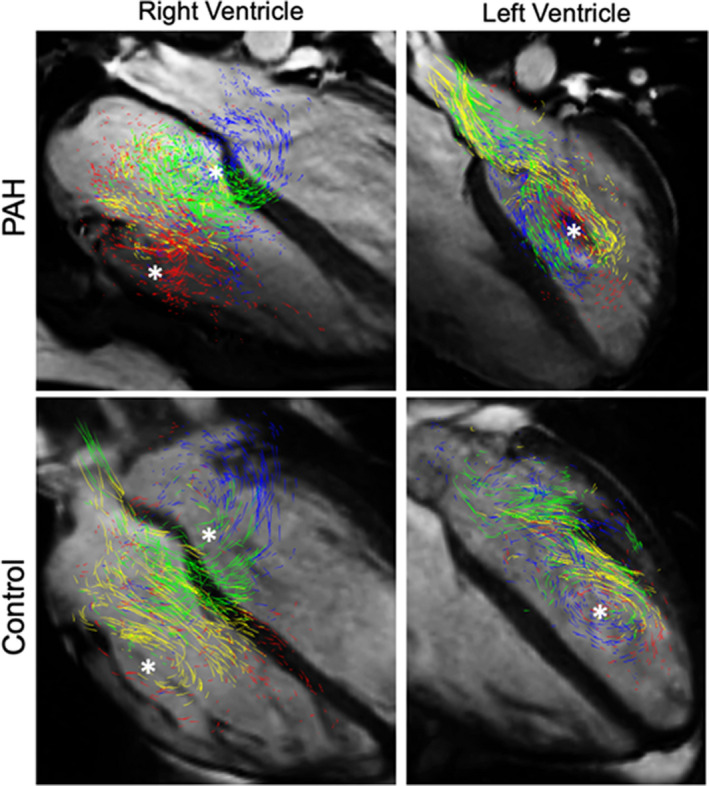
The right ventricular inflow patterns were similar between subjects with PAH and control subjects, with prominent vortices formed along each tricuspid leaflet (* marking vortex formed along the anterior and septal leaflets; posterior vortex is hindered in the 4‐chamber view). The left ventricular vortex was not fully developed in 4 patients with PAH but was present in all control subjects.
Four‐D‐Flow MRI iNO Challenge
RV flow hemodynamic differences with respect to the iNO challenge are summarized in Figure 5. There was a significant increase in direct flow during iNO delivery (19.3%±7.2% versus 25.4%±8.2%; P=0.009), with parallel decrease in the residual volume (40.7%±12.4% versus 32.1%±13.7%; P=0.008). There were no changes in delayed ejection (22.2%±6.1% versus 22.5%±9.9%; P=0.986) and retained inflow (17.8%±8.5% versus 20.0%±14.4%; P=0.547). LV flow hemodynamic changes are displayed in Figure 6. There were no differences in direct flow (23.2%±8.0% versus 28.0%±8.0%; P=0.115), delayed ejection (27.3%±7.7% versus 27.9%±11.1%; P=0.850), retained inflow (22.4%±6.6% versus 20.3%±5.8%; P=0.259), and residual. (27.1%±10.0% versus 23.8%±11.6%; P=0.450).
Figure 5. Changes in the right ventricular (RV) flow hemodynamic profile associated with nitric oxide challenge.
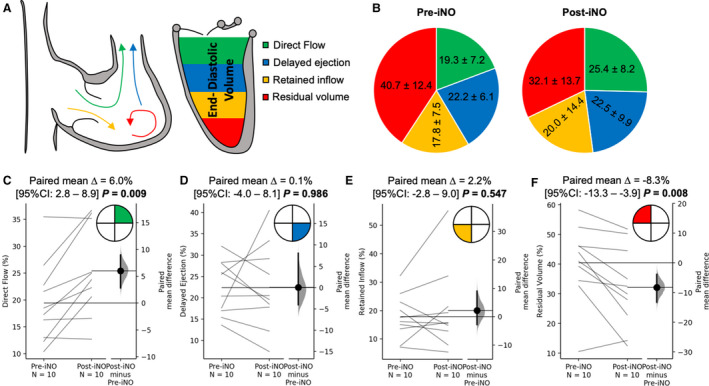
A, Graphical representation of the effect of inhaled nitric oxide (iNO) challenge on the flow components derived by 4‐dimensional–flow magnetic resonance imaging tracking, measured in the RV. B, Pie chart representation of average values of the 4 flow components at baseline and after iNO challenge. C through F, Pairwise comparison of individual flow components. There was a significant increase in direct flow (C) from baseline on iNO administration, but the opposite effect was observed in the residual volume. There were no changes in delayed ejection and retained volume.
Figure 6. Changes in the left ventricular (LV) flow hemodynamic profile associated with nitric oxide challenge.
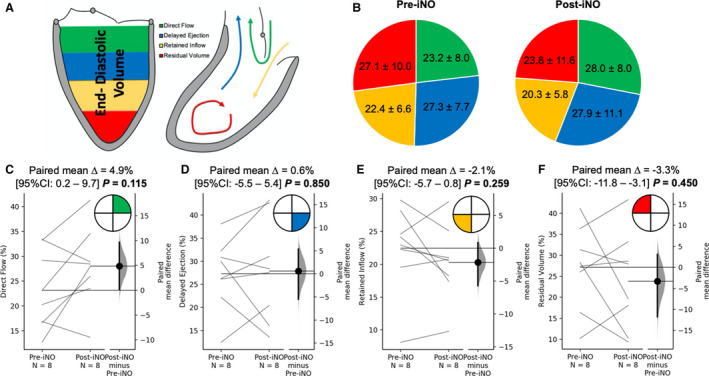
A, Graphical representation of the inhaled nitric oxide (iNO) challenge on the flow components derived in the LV. B, Pie chart representation of average values of the 4 flow components at baseline and after iNO challenge. C through F, Pairwise comparison of individual flow components did not reveal any changes in the LV on iNO administration.
Changes in Catheterization Versus 4D‐Flow MRI
To assess whether the determinant acute vasoreactivity is associated with 4D‐flow MRI, we performed linear regression analysis between the relative catheterization indexes and 4D‐flow MRI hemodynamics. Given that direct flow and residual volume in the RV were the only 4D‐flow MRI markers that showed difference on iNO challenge, we specifically correlated mPAP and PVR index separately with these flow components. The summary of correlative analysis can be viewed in Figure 7. There were no significant correlations between catheterization and 4D‐flow MRI changes in any of the considered indexes.
Figure 7. Linear regression analysis between the changes in primary catheterization hemodynamics of 4‐dimensional–flow magnetic resonance imaging derived individual flow components in the right ventricle.
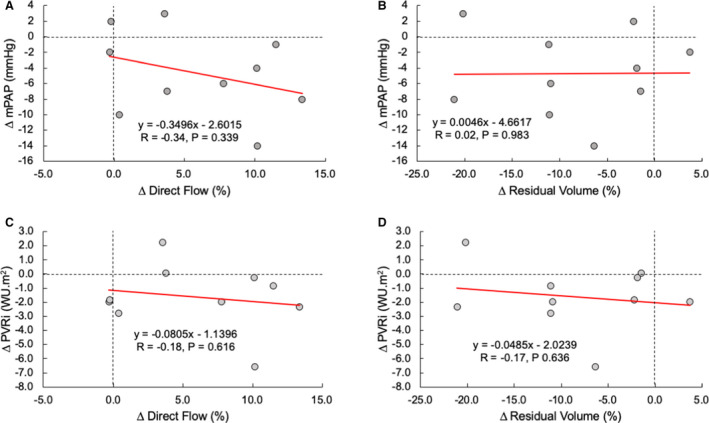
Changes in mean pulmonary arterial pressure (mPAP) were not associated with the change in direct flow (A) and residual volume (B). Similarly, change in the pulmonary vascular resistance index (PVRi) was not associated with direct flow (A) and residual volume (B). WU indicates Wood unit.
Discussion
Compromised biventricular function in pediatric and adult PAH is a recognized predictor of long‐term clinical outcomes. 6 , 8 , 10 , 26 Specifically in pediatric PAH, biventricular dysfunction has been previously described in terms of compromised systolic and diastolic performance, 26 , 27 electromechanical dyssynchrony 9 /discoordination, 10 and ventricular vascular coupling. 28 , 29 In this study, we have shown that (1) biventricular flow hemodynamic patterns are impaired in children with PAH in comparison to their healthy peers, (2) iNO improves the RV flow efficiency with no significant change in the LV, and (3) catheterization indexes dictating the acute vasoreactivity are not associated with the improved RV flow hemodynamic function. Prior studies investigating the intracardiac flow hemodynamics have been mainly focused on adult patients and observed a strong association with the biventricular diastolic dysfunction and standard catheterization indexes. 14 , 15 We extend the impact of these studies by showing that the RV flow efficiency is acutely and significantly improved by iNO therapy without corresponding change in the LV. The particle tracking approach has been previously shown to be sensitive to detect inefficient intracardiac flow in dilated cardiomyopathy, 21 ischemic heart disease, 23 and congenital heart disease. 22 Our results suggests that the right‐to‐left ventricular interdependency in pediatric PAH is also manifested by abnormal biventricular flow and could be a contributing factor to the right heart failure.
RV Flow and Diastolic Dysfunction in PAH
Previous adult PAH studies investigating the intracardiac flow hemodynamics in PAH demonstrated abnormal RV filling patterns in the presence of diastolic dysfunction. Fenster et al demonstrated that early RV diastolic filling produces significantly reduced vorticity, which was significantly associated with the isovolumic relaxation time. 14 Similarly, Han et al described significantly reduced RV inflow kinetic energy, suggestive of compromised RV diastolic recoil. 30 Our results suggest that RV flow is also significantly impaired in children with PAH, as demonstrated by decreased direct flow and increased residual volume. It is believed that myocardial elastic recoil is one of the primary forces providing the kinetic energy to the ventricular filling. 12 Prior studies have demonstrated that before isovolumetric contraction, direct flow possesses the highest and residual volume possesses the least amount of kinetic energy. 21 Consequently, the presystolic blood flow organization and momentum of individual flow components is influenced by the myocardial relaxation properties. Interestingly, the qualitative inspection of the RV inflow patterns was similar between patients with PAH and control subjects. This observation further supports the need for quantitative flow analyses, such as flow component–based particle tracking or other previously applied flow biomarkers represented by viscous energy loss, inflow kinetic energy, or vorticity.
In normal healthy RV, the proportions of the flow components have been shown to be more favorable in comparison to the LV. 31 Specifically, the ratio of direct flow/delayed ejection (flow components leaving the ventricle) is higher in the RV, suggesting that the diastolic filling creates more favorable loading conditions for energetically effective ejection than LV. This is most likely attributable to inherently higher RV compliance. In the setting of PAH, the chronically increased afterload leads to RV hypertrophy and consequently decreased compliance and diastolic dysfunction. Diastolic dysfunction in PAH has been thoroughly described in terms of increased RV stiffness attributable to increased collagen deposition in the myocardial tissue and impaired relaxation attributable to increased tensions within sarcomere apparatus. 1 More important, catheterization studies in adults 32 demonstrated the clinically prognostic value of diastolic dysfunction in PAH. Similar results were achieved using noninvasive imaging in pediatric PAH studies. 33 , 34 We speculate that comprehensive and quantitative evaluation of the RV intracardiac flow might provide additional insights into PAH‐mediated diastolic dysfunction and aid with the prognosis of clinical outcomes.
Flow Imaging and Ventricular‐Ventricular Interdependency
Previous studies investigating the presystolic blood flow organization inside the LV showed that the fate of the end‐diastolic volume flow components presented in this study is dependent on 3 factors: position within the ventricle, flow orientation with respect to the outflow tract, and diastolic momentum of the individual components. Presystolic momentum is a major determinant of the blood flow ejecting components direct flow and delayed ejection flow, which together make up the total forward LV stroke volume during a given cardiac cycle. 21 The pioneering work applying this method was conducted in patients with dilated cardiomyopathy, revealing energetically inefficient diastolic filling despite clinical compensation, suggesting that particle flow analysis might be sensitive to detect subclinical ventricular distress. 21 , 24 Stoll et al reported that the kinetic energy of the blood flow represented by the direct flow component (the most efficient form) is a better predictor of the functional capacity than conventional cardiac volumetric and functional MRI measurements in patients with ischemic and dilated cardiomyopathies. 23 All the aforementioned studies demonstrated reduction in the direct flow with parallel increase in recirculating residual volume. In this study, we showed that pressure overloaded RV in children with PAH is associated with similar changes. Interestingly, the LV flow components showed similar, yet less prominent, changes in the LV without corresponding changes in conventional MRI indexes.
In addition to changes in LV quantitative flow indexes, our observations suggest that normally formed LV vortex can be maldeveloped in PAH. Similar results were observed in adult PAH studies observing reduced LV vorticity, which correlated with tissue Doppler indexes of LV diastolic dysfunction. 15 Furthermore, dilated RV and compromised septal compliance have been suggested to compromise the vortex formation along the anterior mitral valve leaflet. 13 , 15 These and other findings suggest that right‐to‐left mediated ventricular interdependency might be better monitored by biventricular flow imaging, enabling more comprehensive quantitative analysis.
The sensitivity of flow imaging to detect subtle changes in the ventricular function in the setting of pathologic ventricular interdependency has been further demonstrated in additional studies. 4D‐flow MRI investigation by Fredriksson et al reported significant alteration in the RV flow hemodynamics, suggestive of early diastolic dysfunction in patients with LV chronic ischemic disease with no changes in conventional MRI or echocardiography. 35 Our previous investigations in patients with tetralogy of Fallot showed reduced direct flow and increased residual volume, thereby demonstrating right‐to‐left ventricular interdependency driven by chronic RV volume overload. 22 In summary, ventricular interdependency in pediatric PAH is also evident by impaired LV flow and might be considered as a part of routine imaging follow‐up in this patient population.
Effect of iNO on Cardiac Flow
Nitric oxide deficiency and endothelial dysfunction are well‐recognized features of PAH. 36 In the context of PAH, the result of acute vasoreactivity testing by means of iNO challenge represents a crucial step in the clinical management to test for vasodilator responsiveness. 19 In this study, we applied for the first time cardiac MRI biomarkers to assess the iNO response. Our results directly showed that the RV filling patterns and overall flow efficiency acutely improve on iNO administration. Specifically, we observed immediate improvement in the RV direct flow and reduction in residual volume. Recent study by Rahaghi et al demonstrated in healthy subjects that iNO challenge can be quantified using noncontrast computed tomographic angiography by fine analysis of cross‐sectional area of pulmonary arterial and venous vessels. 37 The change of vessel caliber in our study can be estimated by reduction in PVR index from catheterization. It is important to acknowledge that only 2 of 10 patients have met hemodynamic criteria for positive acute vasoreactivity proposed by Sitbon et al. 20 Furthermore, we failed to observe any correlation between respective changes in catheterization hemodynamics and 4D‐flow MRI derived flow components. This might be partially explained by different loading conditions when exposed to anesthesia, to which pediatric patients are inherently exposed during catheterization but not during MRI evaluation.
Aside of the direct effect on smooth muscle tone, nitric oxide has been shown to possess a regulatory inotropic effect, modulates myocardial oxygen consumption, and enhances diastolic function by promoting myocyte relaxation. 38 , 39 , 40 Early studies have demonstrated that iNO reduces the RV end‐diastolic pressure but has no effect on RV contractility, as measured by unchanged dP/dt. 41 , 42 Interestingly, the iNO challenge in our study did not lead to reduced RV end‐diastolic pressure despite the significant change in RV flow components. The lack of correlation between changes in catheterization hemodynamics and flow components along with no observed change in the RV end‐diastolic pressure suggests that 4D‐flow MRI might provide additional and more quantitative approach for the evaluation of acute vasoreactivity tests. The iNO might have direct positive inotropic effect in the RV, but the role of exogenous nitric oxide on the RV function has not been completely understood, mostly because most iNO is consumed before reaching the coronary circulation. 40 , 41 , 42 Given the altered coronary flow in patients with PAH, 43 additional studies investigating the effect of iNO and prostanoid therapy on myocardial/coronary perfusion might be warranted.
Limitations
We would like to acknowledge several limitations related to this study. First, this was an initial investigation with smaller sample size inherently attributable to the nature of the study with pediatric patient population. Pediatric MRI evaluations in children aged <8 years require, per institutional protocol, general anesthesia, which limited the recruitment of younger children. Similarly, given the pediatric nature of this investigation, we were not able to execute a case‐control study with age‐, size‐, and sex‐matched controls who would also undergo iNO testing. In general, we consider our results preliminary, and further validation in larger patient cohort is necessary. Second, the RHC evaluation is being performed under anesthesia in all considered patients per institutional protocol. Consequently, the loading conditions were different during MRI acquisition and catheterization procedures. However, comparative testing of physiologic response to iNO under anesthesia versus under normal conditions is not ethically sound in the clinical setting. Further studies in adult patients with PAH, allowing for a larger sample size and identical testing conditions during MRI and catheterization testing, are necessary to further validate our findings. Third, this type of flow component analysis has not been previously applied in the pediatric setting for the RV analysis and in patients with PAH per se. Consequently, we lacked external comparative values, which further signifies the importance of the external validation of our results. Last, participants in this study represented predominantly patients with mild to moderate PAH severity, who were not therapy naïve, and did not include patients with severe PAH or patients with congenital heart disease. Our next steps plan to include flow analysis in patients with more heterogeneous PAH diagnoses and those with worse RV function.
Conclusions
Children with PAH have abnormal flow through both ventricles associated with impaired diastolic filling. The flow efficiency is significantly improved in the RV on iNO administration with no change in the LV. The changes in the RV flow have occurred despite the minimal change in catheterization hemodynamics, suggesting that flow hemodynamic evaluation might provide more quantitative insights into vasoreactivity testing in PAH. Furthermore, 4D‐flow MRI‐based evaluation may aid with the therapy guidance and clinical decision making in pediatric patients with PAH.
Sources of Funding
This work was supported by Rady Family and Jayden DeLuca Foundations.
Disclosures
None.
(J Am Heart Assoc. 2021;10:e020548. DOI: 10.1161/JAHA.120.020548.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Rain S, Handoko ML, Trip P, Gan CT‐J, Westerhof N, Stienen GJ, Paulus WJ, Ottenheijm CAC, Marcus JT, Dorfmüller P, et al. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation. 2013;128:2016–2025, 1–10. DOI: 10.1161/CIRCULATIONAHA.113.001873. [DOI] [PubMed] [Google Scholar]
- 2. Vonk‐Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62:D22–D33. DOI: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 3. Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69:236–243. DOI: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 4. Hardegree EL, Sachdev A, Fenstad ER, Villarraga HR, Frantz RP, McGoon MD, Oh JK, Ammash NM, Connolly HM, Eidem BW, et al. Impaired left ventricular mechanics in pulmonary arterial hypertension identification of a cohort at high risk. Circ Heart Fail. 2013;6:748–755. DOI: 10.1161/CIRCHEARTFAILURE.112.000098. [DOI] [PubMed] [Google Scholar]
- 5. Kasner M, Westermann D, Steendijk P, Dröse S, Poller W, Schultheiss H‐P, Tschöpe C. Left ventricular dysfunction induced by nonsevere idiopathic pulmonary arterial hypertension: a pressure‐volume relationship study. Am J Respir Crit Care Med. 2012;186:181–189. DOI: 10.1164/rccm.201110-1860OC. [DOI] [PubMed] [Google Scholar]
- 6. Swift AJ, Capener D, Johns C, Hamilton N, Rothman A, Elliot C, Condliffe R, Charalampopoulos A, Rajaram S, Lawrie A, et al. Magnetic resonance imaging in the prognostic evaluation of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;196:228–239. DOI: 10.1164/rccm.201611-2365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis RA, Johns CS, Cogliano M, Capener D, Tubman E, Elliot CA, Charalampopoulos A, Sabroe I, Thompson AAR, Billings CG, et al. Identification of cardiac magnetic resonance imaging thresholds for risk stratification in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2020;201:458–466. DOI: 10.1164/rccm.201909-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moledina S, Pandya B, Bartsota M, Mortensen KH, McMillan M, Quyam S, Taylor AM, Haworth SG, Schulze‐Neick I, Muthurangu V. Prognostic significance of cardiac magnetic resonance imaging in children with pulmonary hypertension. Circ Cardiovasc Imaging. 2013;6:407–414. DOI: 10.1161/CIRCIMAGING.112.000082. [DOI] [PubMed] [Google Scholar]
- 9. Schäfer M, Collins KK, Browne LP, Ivy DD, Abman S, Friesen R, Frank B, Fonseca B, DiMaria M, Hunter KS, et al. Effect of electrical dyssynchrony on left and right ventricular mechanics in children with pulmonary arterial hypertension. J Heart Lung Transplant. 2018;37:870–878. DOI: 10.1016/j.healun.2018.01.1308. [DOI] [PubMed] [Google Scholar]
- 10. Frank BS, Schäfer M, Douwes JM, Ivy DD, Abman SH, Davidson JA, Burzlaff S, Mitchell MB, Morgan GJ, Browne LP, et al. Novel measures of left ventricular electromechanical discoordination predict clinical outcomes in children with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2020;318:H401–H412. DOI: 10.1152/ajpheart.00355.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodriguez Muñoz D, Markl M, Moya Mur JL, Barker A, Fernández‐Golfín C, Lancellotti P, Zamorano Gómez JL. Intracardiac flow visualization: current status and future directions. Eur Heart J Cardiovasc Imaging. 2013;14:1029–1038. DOI: 10.1093/ehjci/jet086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pedrizzetti G, La Canna G, Alfieri O, Tonti G. The vortex–an early predictor of cardiovascular outcome? Nat Rev Cardiol. 2014;11:545–553. DOI: 10.1038/nrcardio.2014.75. [DOI] [PubMed] [Google Scholar]
- 13. Schafer M, Humphries S, Stenmark KR, Kheyfets VO, Buckner JK, Hunter KS, Fenster BE. 4D‐flow cardiac magnetic resonance‐derived vorticity is sensitive marker of left ventricular diastolic dysfunction in patients with mild‐to‐ moderate chronic obstructive pulmonary disease. Eur Heart J Cardiovasc Imaging. 2018;19:415–424. DOI: 10.1093/ehjci/jex069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fenster BE, Browning J, Schroeder JD, Schafer M, Podgorski CA, Smyser J, Silveira LJ, Buckner JK, Hertzberg JR. Vorticity is a marker of right ventricular diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2015;309:H1087–H1093. DOI: 10.1152/ajpheart.00278.2015. [DOI] [PubMed] [Google Scholar]
- 15. Schäfer M, Browning J, Schroeder JD, Shandas R, Kheyfets VO, Buckner JK, Hunter KS, Hertzberg JR, Fenster BE. Vorticity is a marker of diastolic ventricular interdependency in pulmonary hypertension. Pulm Circ. 2016;6:46–54. DOI: 10.1086/685052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schäfer M, Barker AJ, Kheyfets V, Stenmark KR, Crapo J, Yeager ME, Truong U, Buckner JK, Fenster BE, Hunter KS. Helicity and vorticity of pulmonary arterial flow in patients with pulmonary hypertension: quantitative analysis of flow formations. J Am Heart Assoc. 2017;6:e007010. DOI: 10.1161/JAHA.117.007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schäfer M, Ivy DD, Abman SH, Stenmark K, Browne LP, Barker AJ, Mitchell MB, Morgan GJ, Wilson N, Shah A, et al. Differences in pulmonary arterial flow hemodynamics between children and adults with pulmonary arterial hypertension as assessed by 4D‐flow CMR studies. Am J Physiol Heart Circ Physiol. 2019;316:H1091–H1104. DOI: 10.1152/ajpheart.00802.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eriksson J, Carlhäll C, Dyverfeldt P, Engvall J, Bolger A, Ebbers T. Semi‐automatic quantification of 4D left ventricular blood flow. J Cardiovasc Magn Reson. 2010;12:1–10. DOI: 10.1186/1532-429X-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, et al. Pediatric pulmonary hypertension. Circulation. 2015;132:2037–2099. [DOI] [PubMed] [Google Scholar]
- 20. Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Hervé P, Simonneau G. Long‐term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–3111. DOI: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 21. Eriksson J, Dyverfeldt P, Engvall J, Bolger AF, Ebbers T, Carlhäll CJ. Quantification of presystolic blood flow organization and energetics in the human left ventricle. Am J Physiol Heart Circ Physiol. 2011;300:H2135–H2141. DOI: 10.1152/ajpheart.00993.2010. [DOI] [PubMed] [Google Scholar]
- 22. Schäfer M, Browne LP, Jaggers J, Barker AJ, Morgan GJ, Ivy DD, Mitchell MB. Abnormal left ventricular flow organization following repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2020;160:1008–1015. DOI: 10.1016/j.jtcvs.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 23. Stoll VM, Hess AT, Rodgers CT, Bissell MM, Dyverfeldt P, Ebbers T, Myerson SG, Carlhäll C‐J, Neubauer S. Left ventricular flow analysis. Circ Cardiovasc Imaging. 2019;12:1–12. DOI: 10.1161/CIRCIMAGING.118.008130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eriksson J, Bolger AF, Ebbers T, Carlhäll C‐J. Four‐dimensional blood flow‐specific markers of LV dysfunction in dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2013;14:417–424. DOI: 10.1093/ehjci/jes159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho J, Tumkaya T, Aryal S, Choi H, Claridge‐Chang A. Moving beyond P values: data analysis with estimation graphics. Nat Methods. 2019;16:565–566. DOI: 10.1038/s41592-019-0470-3. [DOI] [PubMed] [Google Scholar]
- 26. Burkett DA, Slorach C, Patel SS, Redington AN, Ivy DD, Mertens L, Younoszai AK, Friedberg MK. Impact of pulmonary hemodynamics and ventricular interdependence on left ventricular diastolic function in children with pulmonary hypertension. Circ Cardiovasc Imaging. 2016;9:1–12. DOI: 10.1161/CIRCIMAGING.116.004612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burkett DA, Slorach C, Patel SS, Redington AN, Ivy DD, Mertens L, Younoszai AK, Friedberg MK. Left ventricular myocardial function in children with pulmonary hypertension: relation to right ventricular performance and hemodynamics. Circ Cardiovasc Imaging. 2015;8:1–10. DOI: 10.1161/CIRCIMAGING.115.003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Truong U, Patel S, Kheyfets V, Dunning J, Fonseca B, Barker AJ, Ivy D, Shandas R, Hunter K. Non‐invasive determination by cardiovascular magnetic resonance of right ventricular‐vascular coupling in children and adolescents with pulmonary hypertension. J Cardiovasc Magn Reson. 2015;17:81. DOI: 10.1186/s12968-015-0186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schäfer M, Ivy DD, Abman SH, Barker AJ, Browne LP, Fonseca B, Kheyfets V, Hunter KS, Truong U. Apparent aortic stiffness in children with pulmonary arterial hypertension. Circ Cardiovasc Imaging. 2017;10:e005817. DOI: 10.1161/CIRCIMAGING.116.005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han QJ, Witschey WRT, Fang‐Yen CM, Arkles JS, Barker AJ, Forfia PR, Han Y. Altered right ventricular kinetic energy work density and viscous energy dissipation in patients with pulmonary arterial hypertension: a pilot study using 4D flow MRI. PLoS One. 2015;10:e0138365. DOI: 10.1371/journal.pone.0138365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fredriksson AG, Zajac J, Eriksson J, Dyverfeldt P, Bolger AF, Ebbers T, Carlhäll C. 4‐D blood flow in the human right ventricle. Am J Physiol Heart Circ Physiol. 2011;301:2344–2350. DOI: 10.1152/ajpheart.00622.2011. [DOI] [PubMed] [Google Scholar]
- 32. Trip P, Rain S, Handoko ML, Van DerBruggen C, Bogaard HJ, Marcus JT, Boonstra A, Westerhof N, Vonk‐Noordegraaf A, De Man S, et al. Clinical relevance of right ventricular diastolic stiffness in pulmonary hypertension. Eur Respir J. 2015;45:1603–1612. DOI: 10.1183/09031936.00156714. [DOI] [PubMed] [Google Scholar]
- 33. Jone P‐N, Schäfer M, Li L, Craft M, Ivy DD, Kutty S. Right atrial deformation in predicting outcomes in pediatric pulmonary hypertension. Circ Cardiovasc Imaging. 2017;10:e006250. DOI: 10.1161/CIRCIMAGING.117.006250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schäfer M, Wilson N, Ivy DD, Ing R, Abman S, Browne LP, Morgan G, Ross M, McLennan D, Barker AJ, et al. Noninvasive wave intensity analysis predicts functional worsening in children with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2018;315:H968–H977. DOI: 10.1152/ajpheart.00227.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fredriksson AG, Svalbring E, Eriksson J, Dyverfeldt P, Alehagen U, Engvall J, Ebbers T, Carlhäll C‐J. 4D flow MRI can detect subtle right ventricular dysfunction in primary left ventricular disease. J Magn Reson Imaging. 2016;43:558–565. DOI: 10.1002/jmri.25015. [DOI] [PubMed] [Google Scholar]
- 36. Klinger JR, Abman SH, Gladwin MT. Pulmonary perspective nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:639–646. DOI: 10.1164/rccm.201304-0686PP. [DOI] [PubMed] [Google Scholar]
- 37. Rahaghi FN, Winkler T, Kohli P, Nardelli P, Martí‐Fuster B, Ross JC, Radhakrishnan R, Blackwater T, Ash SY, de La Bruere I, et al. Quantification of the pulmonary vascular response to inhaled nitric oxide using noncontrast computed tomography imaging. Circ Cardiovasc Imaging. 2019;12:e008338. DOI: 10.1161/CIRCIMAGING.118.008338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Massion PB, Feron O, Dessy C, Balligand J‐L. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res. 2003;93:388–398. DOI: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 39. Rastaldo R, Pagliaro P, Cappello S, Penna C, Mancardi D, Westerhof N, Losano G. Nitric oxide and cardiac function. Life Sci. 2007;81:779–793. DOI: 10.1016/j.lfs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 40. Cotton JM, Kearney MT, Shah AM. Nitric oxide and myocardial function in heart failure: friend or foe? Heart. 2002;88:564–566. DOI: 10.1136/heart.88.6.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Semigran MJ, Cockrill BA, Kacmarek R, Thompson BT, Zapol WM, Dec GW, Fifer MA. Hemodynamic effects of inhaled nitric oxide in heart failure. J Am Coll Cardiol. 1994;24:982–988. DOI: 10.1016/0735-1097(94)90859-1. [DOI] [PubMed] [Google Scholar]
- 42. Cockrill BA, Kacmarek RM, Fifer MA, Bigatello LM, Ginns LC, Zapol WM, Semigran MJ. Comparison of the effects of nitric oxide, nitroprusside, and nifedipine on hemodynamics and right ventricular contractility in patients with chronic pulmonary hypertension. Chest. 2001;119:128–136. DOI: 10.1378/chest.119.1.128. [DOI] [PubMed] [Google Scholar]
- 43. Van Wolferen SA, Marcus JT, Westerhof N, Spreeuwenberg MD, Marques KM, Bronzwaer JG, Henkens IR, Gan CTJ, Boonstra A, Postmus PE, et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J. 2008;29:120–127. DOI: 10.1093/eurheartj/ehm567. [DOI] [PubMed] [Google Scholar]


