Abstract
Background
Hospitalization for heart failure (HF) is very common in patients with atrial fibrillation (AF). We hypothesized that biomarkers of inflammation can identify patients with AF at increased risk of this important complication.
Methods and Results
Patients with established AF were prospectively enrolled. Levels of hs‐CRP (high‐sensitivity C‐reactive protein) and interleukin‐6 were measured from plasma samples obtained at baseline. We calculated an inflammation score ranging from 0 to 4 (1 point for each biomarker between the 50th and 75th percentile, 2 points for each biomarker above the 75th percentile). Individual associations of biomarkers and the inflammation score with HF hospitalization were obtained from multivariable Cox proportional hazards models. A total of 3784 patients with AF (median age 72 years, 24% prior HF) were followed for a median of 4.0 years. The median (interquartile range) plasma levels of hs‐CRP and interleukin‐6 were 1.64 (0.81–3.69) mg/L and 3.42 (2.14–5.60) pg/mL, respectively. The overall incidence of HF hospitalization was 3.04 per 100 person‐years and increased from 1.34 to 7.31 per 100 person‐years across inflammation score categories. After multivariable adjustment, both biomarkers were significantly associated with the risk of HF hospitalization (per increase in 1 SD, adjusted hazard ratio [HR], 1.22; 95% CI, 1.11–1.34 for log‐transformed hs‐CRP; adjusted HR, 1.48; 95% CI, 1.35–1.62 for log‐transformed interleukin‐6). Similar results were obtained for the inflammation score (highest versus lowest score, adjusted HR, 2.43; 95% CI, 1.80–3.30; P value for trend <0.001).
Conclusions
Biomarkers of inflammation strongly predicted HF hospitalization in a large, contemporary sample of patients with AF.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02105844.
Keywords: atrial fibrillation, C‐reactive protein, heart failure, hospitalization, inflammation, interleukin‐6
Subject Categories: Arrhythmias, Atrial Fibrillation, Heart Failure, Inflammation
Clinical Perspective
What Is New?
In a large, contemporary cohort of patients with atrial fibrillation, biomarkers of inflammation (hs‐CRP [high‐sensitivity C‐reactive protein], interleukin‐6) were strongly associated with hospitalization for heart failure and other adverse outcomes during a median follow‐up duration of 4.0 years.
Large absolute risk differences for a first hospitalization for heart failure were observed across different categories of inflammation (eg, both hs‐CRP and interleukin‐6 above the 75th percentile versus both biomarkers up to or below the 50th percentile, incidence 7.31 versus 1.34 per 100 person‐years) and a strong relationship persisted after multivariable adjustment (adjusted hazard ratio, 2.43; 95% CI, 1.80–3.30).
What Are the Clinical Implications?
Our study suggests that inflammation is an important predictor of hospitalization for heart failure and other adverse outcomes in patients with atrial fibrillation.
Whether treatments targeted at inflammatory pathways can improve outcomes in patients with atrial fibrillation needs to be evaluated in randomized clinical trials.
Atrial fibrillation (AF) and its associated complications are a major burden for healthcare systems around the world. 1 The global incidence and prevalence of AF are expected to rise further in light of an ever‐increasing life expectancy. 2 Although the risk of thromboembolic complications such as ischemic stroke can be effectively mitigated by long‐term oral anticoagulation, 3 event rates for other important outcomes, such as hospitalization for heart failure and death, remain high. 4 Unlike the risk of stroke, the incidence of heart failure among patients with AF has not changed over at least 2 decades. 5 In fact, heart failure accounts for a substantial proportion of deaths in adequately anticoagulated, contemporary patients with AF. 6
Heart failure and AF are closely linked, and both conditions often occur concurrently, predisposing affected patients to poor outcomes. 7 , 8 In addition, established therapies providing a survival benefit in most patients with heart failure, such as beta blockers, may be less effective in patients with heart failure and concomitant AF. 9 Finally, AF‐related treatment modalities that may improve outcomes in patients with heart failure, such as catheter ablation, are restricted to a small group of selected patients in high‐income countries. 10
Thus, there is an unmet clinical need to identify potentially modifiable risk factors for heart failure hospitalization in patients with AF. Biomarkers of inflammation have been associated with incident atherothrombotic events in multiple populations, 11 , 12 , 13 , 14 and an association with heart failure hospitalization has been shown in patients with prior myocardial infarction. 15 However, there are no such studies in patients with AF, where underlying pathophysiologic patterns are mostly unrelated to ischemia and atherosclerosis. More data are therefore needed to assess whether biomarkers of inflammation are associated with heart failure hospitalization and other adverse outcomes in patients with AF.
Methods
Data used to conduct this research may be made available for other researchers upon reasonable request, if the request is in line with the informed consent signed by all patients. For all analyses, we used a combined data set of patients with documented AF enrolled in 2 prospective, multicenter cohort studies conducted in Switzerland. Between January 2010 and August 2017, a total of 1553 and 2415 patients were enrolled in the BEAT‐AF (Basel Atrial Fibrillation) and Swiss‐AF (Swiss Atrial Fibrillation) studies, respectively. 16 , 17 Patients with AF due to reversible, secondary causes (eg, perioperative AF or AF due to severe electrolyte abnormalities) were excluded. Patients with any acute illness or who were hospitalized for any reason could be enrolled only at least 4 weeks after the acute episode or hospital discharge. Baseline visits were done in person in both cohorts. During this visit, an ECG was obtained in all patients. Cardiac rhythm at baseline was adjudicated based on these ECGs. Follow‐up visits occurred at 12‐month intervals and were done either by mail and telephone (BEAT‐AF), or in person (Swiss‐AF). Co‐enrollment in both cohorts was prohibited. A central study team located in Basel, Switzerland, coordinated the conduct of both studies. Case report forms to collect information on clinical characteristics, comorbidities and medications, and general methodology were very similar in the 2 cohorts.
In this study, we included all patients for whom at least 1 baseline measurement of either hs‐CRP (high‐sensitivity C‐reactive protein) or interleukin‐6 (IL‐6) was available. We excluded 177 patients for whom information on both biomarkers, prespecified covariates needed for the multivariable analyses, or follow‐up was missing. After accounting for accidental co‐enrollment of 7 patients in both cohorts, the final sample for this study comprised 3784 (95.4%) patients. Approval by the local research ethics boards at all participating sites was obtained before study initiation, and all study participants provided written informed consent. Swiss‐AF is registered on ClinicalTrials.gov (identifier NCT02105844).
Biomarkers of Inflammation
At baseline, an EDTA plasma sample was obtained from each patient enrolled in either of the 2 cohort studies. Samples were immediately processed, aliquoted, and stored at −80°C. Commercially available assays (cobas c 311 and cobas Elecsys®, Roche Diagnostics, Mannheim, Germany) were used to measure plasma levels of hs‐CRP and IL‐6. All analyses were centrally performed by Roche Diagnostics (Rotkreuz, Switzerland). A total of 6 and 3 patients had plasma levels below the detection limit for hs‐CRP and IL‐6, respectively. The values for these patients were set to 0.01 mg/L for hs‐CRP (lowest value observed in the remaining patients 0.02 mg/L) and 0.05 pg/mL for IL‐6 (lowest value observed in the remaining patients 0.06 pg/mL).
Outcomes
All outcomes had standardized definitions and were independently adjudicated by at least 2 reviewers. The prespecified primary outcome for this analysis was time to first hospitalization for heart failure, defined as any unplanned hospitalization for signs and symptoms of heart failure associated with at least 1 overnight stay. Prespecified secondary outcomes were all‐cause mortality, cardiovascular death, a composite end point of ischemic stroke, myocardial infarction or cardiovascular death, and a composite end point of ischemic stroke or systemic embolism. The study definitions for each of these outcomes are provided in Table S1 and were identical in both cohorts.
Statistical Analysis
Continuous variables were visually inspected to determine the normality of distribution and were presented as mean± SD or median (interquartile range), as appropriate. Plasma levels of both hs‐CRP and IL‐6 were used to calculate a simple, prespecified inflammation score ranging from 0 to 4 (1 point for each biomarker between the 50th and 75th percentile, 2 points for each biomarker above the 75th percentile). The linear correlation between hs‐CRP and IL‐6 was quantified using the Pearson correlation coefficient. Because biomarker distributions were strongly skewed, we log‐transformed plasma levels of hs‐CRP and IL‐6. Incidence rates for all outcomes were presented as number of events per 100 person‐years.
For the primary analysis, multivariable Cox proportional‐hazards models were built to quantify the individual associations of hs‐CRP, IL‐6, and the inflammation score with the outcomes of interest. Separate Cox models were built for the 2 biomarkers. We selected model covariates a priori on the basis of clinical considerations. Covariates included were age, sex, history of heart failure, hypertension, diabetes mellitus, prior stroke or transient ischemic attack, history of coronary artery disease, estimated glomerular filtration rate, body mass index, smoking status, use of oral anticoagulation, and study cohort (BEAT‐AF or Swiss‐AF). Patients who died during the time of follow‐up were censored. Results were presented as adjusted hazard ratios (HR) with 95% CIs per increase in 1 SD of log‐transformed plasma levels of hs‐CRP or IL‐6, and per 1‐point increase of the inflammation score. Post hoc sensitivity analyses adding baseline statin use and cardiac rhythm at baseline to the models were performed to further investigate the associations between the inflammation score and adverse outcome events. We performed competing risk analyses using the method reported by Fine and Gray, 18 accounting for the competing risks of death (first hospitalization for heart failure; composite of ischemic stroke or systemic embolism) and noncardiovascular death (cardiovascular death; composite of ischemic stroke, myocardial infarction or cardiovascular death), respectively. Unadjusted Kaplan‐Meier curves were used to visualize cumulative incidence rates for the primary outcome first hospitalization for heart failure and the secondary outcomes across inflammation score categories, censoring patients who died during the time of follow‐up. The log‐rank test was used to assess between‐group differences. Cumulative incidence function plots accounting for competing risks were used for an alternative visualization of the incidences of the primary and secondary outcomes, and Gray’s test of equality was used to assess between‐group differences.
For the primary outcome hospitalization for heart failure, we evaluated the possibility of nonlinear associations by entering a quadratic term for either log‐transformed hs‐CRP or log‐transformed IL‐6 in the respective multivariable models. Subgroup analyses for the primary outcome according to sex, history of heart failure, AF type, history of coronary artery disease, and history of stroke or transient ischemic attack were specified a priori. Tests for interaction were performed in the nonstratified multivariable models using multiplicative interaction terms. A 2‐sided P<0.05 was considered statistically significant. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient Population
Baseline characteristics of the 3784 patients are shown in Table 1. The median (interquartile range) age was 72 (66–78) years and 27.9% of patients were female. The proportion of patients with permanent AF was 23.0%. A total of 51.8% of patients were in sinus rhythm and 43.6% were in AF or atrial flutter at baseline, respectively. A total of 23.8% had a history of heart failure and 26.6% had coronary artery disease. A total of 84.2% of patients received oral anticoagulation at baseline, and 43.9% received a statin. A minority (7.9%) reported active tobacco use, and 48.0% had smoked in the past. The median (interquartile range) plasma levels of hs‐CRP and IL‐6 at baseline were 1.64 (0.81–3.69) mg/L and 3.42 (2.14–5.60) pg/mL, respectively. The Pearson correlation coefficient between log‐transformed plasma levels of hs‐CRP and IL‐6 was 0.57 (P<0.001).
Table 1.
Baseline Characteristics
| Baseline Variable | n=3784 |
|---|---|
| Age, y, median (IQR) | 72.4 (66.2–78.2) |
| Female sex, % | 1054 (27.9) |
| Permanent atrial fibrillation, % | 869 (23.0) |
| Cardiac rhythm at baseline | |
| Sinus rhythm, % | 1960 (51.8) |
| Atrial fibrillation or flutter, % | 1649 (43.6) |
| Other, %* | 175 (4.6) |
| CHA2DS2‐VASc score, mean (SD) † | 3.2 (1.7) |
| Heart failure, % | 899 (23.8) |
| Arterial hypertension, % | 2603 (68.8) |
| Diabetes mellitus, % | 597 (15.8) |
| Prior stroke or transient ischemic attack, % | 646 (17.1) |
| Peripheral artery disease, % | 277 (7.3) |
| Coronary artery disease, % | 1008 (26.6) |
| Prior myocardial infarction, % | 557 (14.7) |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 (Modification of Diet in Renal Disease), mean (SD) | 65.7 (20.2) |
| Body mass index, kg/m2, median (IQR) | 26.7 (24.2–29.9) |
| Oral anticoagulation, % | 3186 (84.2) |
| Direct oral anticoagulant, % | 1336 (35.3) |
| Vitamin K antagonist, % | 1850 (48.9) |
| Statin use, % | 1660 (43.9) |
| Tobacco use | |
| Never, % | 1668 (44.1) |
| Past, % | 1818 (48.0) |
| Active, % | 298 (7.9) |
| Plasma levels of biomarkers | |
| hs‐CRP mg/L, median (IQR) ‡ | 1.64 (0.81–3.69) |
| IL‐6, pg/mL, median (IQR) § | 3.42 (2.14–5.60) |
CHA2DS2‐VASc indicates congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack, vascular disease, age 65 to 74 years, sex category; hs‐CRP, high‐sensitivity C‐reactive protein; IL‐6, interleukin‐6; and IQR, interquartile range.
Includes n=26 for whom baseline rhythm was not available.
The CHA2DS2‐VASc score ranges from 0 to 9 (1 point for each history of heart failure, hypertension, age 65 to 74 years, diabetes mellitus, vascular disease, and female sex; 2 points for each age >75 years and prior stroke or transient ischemic attack).
hs‐CRP available for 3767 (99.6%) patients, range (min‐max) 0.01 to 198.04 mg/L.
IL‐6 available for 3667 (96.9%) patients, range (min‐max) 0.05 to 335.63 pg/mL.
Outcomes
During a median (interquartile range) follow‐up of 4.0 (2.9–5.1) years, at least 1 hospitalization for heart failure occurred in 447 (11.8%) of patients, corresponding to an incidence rate of 3.04 per 100 person‐years (Table 2). After multivariable adjustment, both biomarkers were significantly associated with heart failure hospitalization (per increase in 1 SD, adjusted HR, 1.22; 95% CI, 1.11–1.34; P<0.001 for log‐transformed hs‐CRP; adjusted HR, 1.48; 95% CI, 1.35–1.62; P<0.001 for log‐transformed IL‐6). Although there was no evidence for a nonlinear association for hs‐CRP (P=0.999 for quadratic log‐transformed hs‐CRP), there was evidence of a nonlinear relationship for IL‐6 (β=−0.086, SE, 0.031, P=0.006 for quadratic log‐transformed IL‐6), suggesting less steep increases in risk at higher IL‐6 concentrations.
Table 2.
Associations of Biomarkers of Inflammation With Adverse Outcome Events
| Outcome | Events | Rate Per 100 Person‐Years | Marker | Univariable HR (95% CI) | P Value | Adjusted HR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
| Hospitalization for heart failure | 447 | 3.04 | hs‐CRP | 1.46 (1.33–1.60) | <0.001 | 1.22 (1.11–1.34) | <0.001 |
| IL‐6 | 1.80 (1.67–1.93) | <0.001 | 1.48 (1.35–1.62) | <0.001 | |||
| All‐cause mortality | 432 | 2.80 | hs‐CRP | 1.62 (1.48–1.78) | <0.001 | 1.40 (1.27–1.54) | <0.001 |
| IL‐6 | 1.97 (1.84–2.11) | <0.001 | 1.67 (1.53–1.81) | <0.001 | |||
| Cardiovascular death | 264 | 1.71 | hs‐CRP | 1.54 (1.37–1.73) | <0.001 | 1.31 (1.16–1.49) | <0.001 |
| IL‐6 | 1.97 (1.81–2.15) | <0.001 | 1.65 (1.49–1.84) | <0.001 | |||
| Ischemic stroke, myocardial infarction or cardiovascular death | 440 | 2.93 | hs‐CRP | 1.42 (1.29–1.55) | <0.001 | 1.27 (1.15–1.39) | <0.001 |
| IL‐6 | 1.71 (1.59–1.85) | <0.001 | 1.46 (1.34–1.60) | <0.001 | |||
| Ischemic stroke or systemic embolism | 121 | 0.80 | hs‐CRP | 1.22 (1.03–1.46) | 0.026 | 1.21 (1.01–1.45) | 0.04 |
| IL‐6 | 1.33 (1.13–1.57) | 0.001 | 1.19 (0.99–1.44) | 0.07 |
Because of positively skewed distribution of plasma levels of both hs‐CRP and IL‐6, a logarithmic transformation was performed. Hazard ratios and 95% CIs are shown as per increase in 1SD of log‐transformed concentrations of hs‐CRP and IL‐6, respectively. Multivariable analyses were adjusted for age, sex, history of heart failure, hypertension, diabetes mellitus, prior stroke or transient ischemic attack, history of coronary artery disease, estimated glomerular filtration rate as per Modification of Diet in Renal Disease formula, body mass index, smoking status, anticoagulation use, and study cohort (BEAT‐AF [Basel Atrial Fibrillation] or Swiss‐AF [Swiss Atrial Fibrillation]). Observations in model: 3767 (99.6% complete cases) for hs‐CRP and 3667 (96.9% complete cases) for IL‐6. For patients excluded from the analyses, plasma levels of the respective biomarkers were missing. HR indicates hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein; and IL‐6, interleukin‐6.
The incidence of heart failure hospitalization increased across inflammation score categories, from 1.34 (inflammation score 0) to 7.31 (score 4) per 100 person‐years (P<0.001; Figure). The inflammation score remained strongly associated with the risk of heart failure hospitalization after multivariable adjustment (highest score [score of 4] versus reference category [score of 0], adjusted HR, 2.43; 95% CI, 1.80–3.30; P value for trend<0.001) (Table 3).
Figure 1. Cumulative incidence of heart failure hospitalization across inflammation score categories (Kaplan‐Meier method).
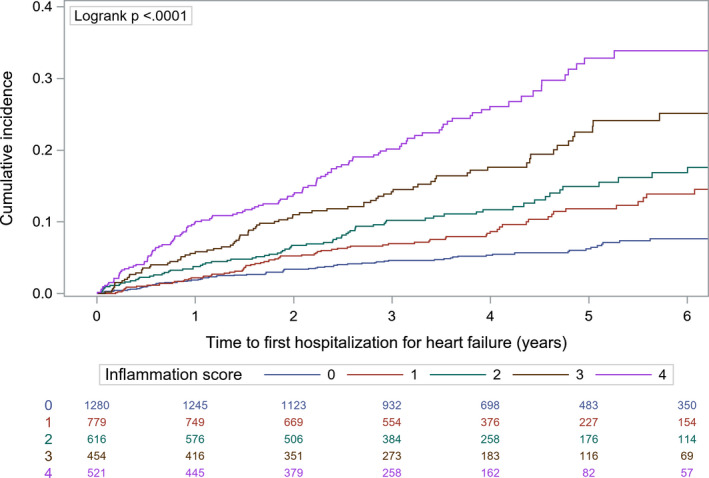
Table 3.
Association of the Inflammation Score With Adverse Outcome Events
| Outcome | Inflammation Score | Patients | Events | Rate Per 100 Person‐Years | Adjusted HR (95% CI) | P Value for Trend |
|---|---|---|---|---|---|---|
| Hospitalization for heart failure | 0 | 1280 | 75 | 1.34 | Reference category | |
| 1 | 779 | 75 | 2.40 | 1.32 (0.96–1.82) | <0.001 | |
| 2 | 616 | 76 | 3.22 | 1.45 (1.04–2.00) | ||
| 3 | 454 | 79 | 4.80 | 2.04 (1.48–2.83) | ||
| 4 | 521 | 120 | 7.31 | 2.43 (1.80–3.30) | ||
| All‐cause mortality | 0 | 1280 | 43 | 0.75 | Reference category | |
| 1 | 779 | 73 | 2.24 | 2.20 (1.50–3.21) | <0.001 | |
| 2 | 616 | 82 | 3.33 | 2.85 (1.95–4.15) | ||
| 3 | 454 | 84 | 4.74 | 3.85 (2.64–5.60) | ||
| 4 | 521 | 137 | 7.51 | 4.96 (3.47–7.09) | ||
| Cardiovascular death | 0 | 1280 | 28 | 0.49 | Reference category | |
| 1 | 779 | 51 | 1.57 | 2.36 (1.49–3.76) | <0.001 | |
| 2 | 616 | 43 | 1.75 | 2.24 (1.38–3.63) | ||
| 3 | 454 | 53 | 2.99 | 3.61 (2.26–5.77) | ||
| 4 | 521 | 81 | 4.44 | 4.26 (2.72–6.65) | ||
| Ischemic stroke, myocardial infarction or cardiovascular death | 0 | 1280 | 74 | 1.31 | Reference category | |
| 1 | 779 | 92 | 2.91 | 1.80 (1.32–2.45) | <0.001 | |
| 2 | 616 | 68 | 2.84 | 1.55 (1.11–2.17) | ||
| 3 | 454 | 85 | 5.01 | 2.65 (1.92–3.65) | ||
| 4 | 521 | 112 | 6.42 | 2.86 (2.10–3.90) | ||
| Ischemic stroke or systemic embolism | 0 | 1280 | 34 | 0.60 | Reference category | |
| 1 | 779 | 27 | 0.84 | 1.23 (0.74–2.06) | 0.03 | |
| 2 | 616 | 14 | 0.58 | 0.81 (0.43–1.53) | ||
| 3 | 454 | 22 | 1.28 | 1.84 (1.05–3.20) | ||
| 4 | 521 | 23 | 1.29 | 1.76 (1.00–3.07) | ||
Multivariable analyses were adjusted for age, sex, history of heart failure, hypertension, diabetes mellitus, prior stroke or transient ischemic attack, history of coronary artery disease, estimated glomerular filtration rate as per Modification of Diet in Renal Disease formula, body mass index, smoking status, anticoagulation use, and study cohort (BEAT‐AF [Basel Atrial Fibrillation] or Swiss‐AF [Swiss Atrial Fibrillation]). Observations in model: 3650 (96.5% complete cases due to missing information on plasma levels of high‐sensitivity C‐reactive protein or interleukin‐6). HR indicates hazard ratio.
As shown in Table 2, both hs‐CRP and IL‐6 were significantly associated with all‐cause mortality, cardiovascular death and the composite of ischemic stroke, myocardial infarction, or cardiovascular death after multivariable adjustment (all P<0.001). The association of hs‐CRP and IL‐6 with the composite end point of ischemic stroke or systemic embolism was weak. Consistent results were observed with the inflammation score (Table 3). Kaplan‐Meier curves for the secondary outcomes stratified by inflammation score categories are shown in Figures S1 through S4, and cumulative incidence function plots accounting for competing risks are shown in Figures S5 through S8.
For all outcomes, post hoc sensitivity analyses that additionally adjusted for baseline statin use and cardiac rhythm at baseline yielded consistent results (Table S2). Analyses that accounted for the competing risks of death and noncardiovascular death, respectively, showed consistent results for the primary and secondary outcomes (Table S2).
Subgroup Analyses
The associations of hs‐CRP and IL‐6 with the primary outcome across several subsets of patients are shown in Table 4. There were stronger relative associations with heart failure hospitalization in patients without a history of heart failure or coronary artery disease for both hs‐CRP and IL‐6 (each P value for interaction<0.05). There was no significant interaction with either biomarker according to sex, AF type, or history of stroke or transient ischemic attack.
Table 4.
Subgroup Analyses for the End Point Hospitalization for Heart Failure
| Subgroup | Group | n/N | Rate Per 100 Person‐Years | Adjusted HR (95% CI) | P Value for Interaction |
|---|---|---|---|---|---|
| hs‐CRP | |||||
| All | 3784/3784 | 3.04 | 1.22 (1.11–1.34) | ||
| Sex | Male | 2730/3784 | 3.00 | 1.22 (1.09–1.36) | 0.95 |
| Female | 1054/3784 | 3.14 | 1.22 (1.00–1.48) | ||
| History of heart failure | Yes | 899/3784 | 7.57 | 1.09 (0.95–1.24) | 0.04 |
| No | 2885/3784 | 1.92 | 1.34 (1.17–1.53) | ||
| Type of atrial fibrillation | Permanent | 869/3784 | 5.49 | 1.28 (1.08–1.52) | 0.81 |
| Nonpermanent | 2915/3784 | 2.41 | 1.18 (1.05–1.33) | ||
| History of coronary artery disease | Yes | 1008/3784 | 5.66 | 1.04 (0.90–1.20) | 0.001 |
| No | 2776/3784 | 2.25 | 1.38 (1.21–1.57) | ||
| History of stroke or transient ischemic attack | Yes | 646/3784 | 4.11 | 1.13 (0.92–1.40) | 0.69 |
| No | 3138/3784 | 2.84 | 1.24 (1.11–1.39) | ||
| IL‐6 | |||||
| All | 3784/3784 | 3.04 | 1.48 (1.35–1.62) | ||
| Sex | Male | 2730/3784 | 3.00 | 1.47 (1.32–1.63) | 0.94 |
| Female | 1054/3784 | 3.14 | 1.54 (1.27–1.87) | ||
| History of heart failure | Yes | 899/3784 | 7.57 | 1.35 (1.18–1.54) | 0.04 |
| No | 2885/3784 | 1.92 | 1.59 (1.40–1.80) | ||
| Type of atrial fibrillation | Permanent | 869/3784 | 5.49 | 1.70 (1.44–2.00) | 0.13 |
| Nonpermanent | 2915/3784 | 2.41 | 1.39 (1.24–1.56) | ||
| History of coronary artery disease | Yes | 1008/3784 | 5.66 | 1.26 (1.09–1.46) | 0.001 |
| No | 2776/3784 | 2.25 | 1.63 (1.44–1.84) | ||
| History of stroke or transient ischemic attack | Yes | 646/3784 | 4.11 | 1.35 (1.08–1.69) | 0.46 |
| No | 3138/3784 | 2.84 | 1.50 (1.36–1.66) | ||
Results are shown as adjusted HR per increase in 1 SD of log‐transformed biomarker levels. Multivariable analyses were adjusted for age, sex, history of heart failure, hypertension, diabetes mellitus, prior stroke or transient ischemic attack, history of coronary artery disease, estimated glomerular filtration rate as per Modification of Diet in Renal Disease formula, body mass index, smoking status, anticoagulation use, and study cohort (BEAT‐AF [Basel Atrial Fibrillation] or Swiss‐AF [Swiss Atrial Fibrillation]), excluding the covariate of interest for each subgroup. Observations in model: 3767 (99.6% complete cases) for hs‐CRP and 3667 (96.9% complete cases) for IL‐6. For patients excluded from the analyses, plasma levels of the respective biomarkers were missing. HR indicates hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein; IL‐6, interleukin‐6; n, number of patients in subgroup; and N, total number of patients.
Discussion
In this large, contemporary cohort of patients with AF, hs‐CRP, and IL‐6 were strongly and consistently associated with the risk of heart failure hospitalization and other adverse clinical outcomes. These results persisted after extensive adjustment for other risk factors and comorbidities. Using a simple inflammation score, we found that patients with higher levels of both inflammatory biomarkers had a more than 5‐fold higher absolute risk of heart failure hospitalization compared with those with lower levels of both biomarkers.
There has been an ongoing controversy around the inflammatory hypothesis in cardiovascular disease. Longitudinal studies in apparently healthy individuals have previously shown an association between elevated biomarkers of inflammation and future cardiovascular events. 13 , 14 It is possible that inflammation may be a marker of underlying subclinical disease and established clinical risk factors, such as hypertension, diabetes mellitus, or obesity. Indeed, even the most rigorously conducted epidemiological studies, including this one, are ultimately unable to prove a causal relationship between inflammation and outcomes. However, encouraging evidence from different populations is emerging and shows that inflammation may play a causal role. Three large randomized clinical trials in patients with ischemic heart disease and/or a history of myocardial infarction consistently demonstrated that anti‐inflammatory drugs counteracting interleukin‐1β‐mediated inflammation reduce the risk of ischemic events. 19 , 20 , 21 Of note, a post hoc analysis from 1 of these trials showed a dose‐dependent reduction in heart failure hospitalization related to ischemic heart disease in patients randomized to canakinumab. 15
As previous studies have focused on patients with atherosclerosis, it is unclear whether these findings can be extrapolated to patients with AF, a heterogeneous population in whom the mechanisms for heart failure occurrence are mostly independent of overt ischemic heart disease. For instance, the great majority of patients in our cohort did not have coronary artery disease. Our results suggest that even in this patient population, inflammation may play a role in the occurrence of adverse outcomes, such as heart failure hospitalization or death. Subgroup analyses in our study suggested stronger associations of hs‐CRP and IL‐6 with heart failure hospitalization in individuals without a history of heart failure or coronary artery disease. The clinical significance of these findings is currently uncertain, but they support the hypothesis that inflammatory processes do play a role in patients without established heart failure or coronary artery disease.
Even in a broader context, inflammation seems to play an important role in AF. Animal studies have highlighted the significance of NLRP3 inflammasome activation, by regulating cytokine‐dependent pathways involved in the pathogenesis of AF and atrial remodeling. 22 Further, elevated biomarkers indicating systemic inflammation have been linked to atrial remodeling, 23 incident AF 24 as well as arrhythmia burden in patients with established AF. 25 Previous studies in patients with AF have shown that elevated levels of CRP or IL‐6 are associated with mortality. 26 , 27 , 28 , 29 Our study adds to these findings and suggests that inflammation is a strong predictor of adverse outcomes in patients with AF. Although our results should not prompt physicians to measure biomarkers of inflammation or use our score as part of routine clinical care, we do think that they provide support for the initiation of clinical trials investigating the efficacy and safety of anti‐inflammatory treatments in this population. Whether or not biomarkers of inflammation should be repeatedly measured over time should be addressed in future studies.
Our simple inflammation score effectively stratified heart failure risk among patients with AF included in this study, suggesting an additive effect of the 2 evaluated biomarkers. There was a large absolute risk difference of about 6 heart failure hospitalizations per 100 person‐years between patients in the highest versus the lowest inflammation score category. Thus, although the risk of ischemic stroke or systemic embolism was low in this population with a high proportion of anticoagulation use, there is a significant number of patients with AF at high risk of other outcomes, and 2 readily available inflammatory biomarkers can efficiently risk stratify these patients. Randomized clinical trials are warranted to determine whether anti‐inflammatory treatments can reduce the risk of heart failure hospitalization and other outcomes in this patient population.
Strengths and Limitations
Key strengths of our study are the prospective design, the large sample size with a large number of outcome events, the high proportion of patients with complete data and the availability of 2 biomarkers of inflammation. The magnitude of increased risk of adverse outcomes associated with high concentrations of hs‐CRP and IL‐6, especially for heart failure hospitalization and death, is striking. However, the associations do not prove causality, and our results may be subject to residual confounding, as discussed previously. In this cohort, women represented only about 28% of participants, which may limit the generalizability of our findings to women with AF. However, subgroup analyses for the primary outcome hospitalization for heart failure did suggest consistent results according to sex. Because biomarkers of inflammation were measured only at the time of enrollment, we were unable to assess the significance of changes in biomarker levels over time.
Conclusions
In this large, contemporary cohort of patients with AF, we found that biomarkers of inflammation were strongly associated with heart failure hospitalization and other adverse outcomes. Large absolute risk differences were observed across different levels of inflammation. Whether treatments targeted at inflammatory pathways can improve outcomes in patients with AF needs to be evaluated in randomized clinical trials.
Sources of Funding
BEAT‐AF has been supported by the Swiss National Science Foundation (PP00P3_159322), the Swiss Heart Foundation, the University of Basel, Roche Diagnostics, Boehringer Ingelheim, Sanofi‐Aventis, Merck Sharp & Dome, Bayer, Daiichi‐Sankyo, Pfizer/Bristol‐Myers Squibb, and the Foundation for Cardiovascular Research Basel. The Swiss‐AF cohort study is supported by grants from the Swiss National Science Foundation (Grant numbers 33CS30_148474 and 33CS30_177520), the University of Basel, Roche Diagnostics, and the Foundation for Cardiovascular Research Basel (FCVR Basel). Measurements of hs‐CRP and IL‐6 levels were performed by Roche Diagnostics at no charge.
Benz reports a personal research grant from the German Heart Foundation (Deutsche Herzstiftung e.V.). Krisai is supported by the University of Basel, the Mach‐Gaensslen Foundation and the Bangerter‐Rhyner Foundation. Blum is supported by the Mach‐Gaensslen Foundation. Conen holds a McMaster University Department of Medicine Mid‐Career Research Award.
Disclosures
Kobza reports institutional grants from Abbott, Biosense‐Webster, Biotronik, Boston Scientific, Medtronic, and Sis Medical. Bonati reports consultancy or advisory board fees or speaker’s honoraria from Amgen, Bayer, Bristol‐Myers Squibb, Claret Medical, and InnovHeart, and travel grants from AstraZeneca and Bayer. Beer reports institutional grants from the Swiss National Foundation of Science and The Swiss Heart Foundation. Kühne reports personal fees from Bayer, Boehringer Ingelheim, Pfizer BMS, Daiichi Sankyo, Medtronic, Biotronik, Boston Scientific, and Johnson & Johnson; and grants from Bayer, Pfizer BMS, and Boston Scientific. Conen reports speaker honoraria from Servier Canada, outside of the current work. The remaining authors have no disclosures to report.
Supporting information
Appendix S1
Tables S1–S2
Figures S1–S8
(J Am Heart Assoc. 2021;10:e019168. DOI: 10.1161/JAHA.120.019168.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019168
For Sources of Funding and Disclosures, see page 8.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y‐H, McAnulty JH Jr, Zheng Z‐J, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. DOI: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. DOI: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. DOI: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 4. Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J, Commerford P, Jansky P, Avezum A, Sigamani A, et al. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet. 2016;388:1161–1169. DOI: 10.1016/S0140-6736(16)30968-0. [DOI] [PubMed] [Google Scholar]
- 5. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna W, Seward JB, Iwasaka T, Tsang TS. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community‐based study over two decades. Eur Heart J. 2006;27:936–941. DOI: 10.1093/eurheartj/ehi694. [DOI] [PubMed] [Google Scholar]
- 6. Marijon E, Le Heuzey J‐Y, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom J, Themeles E, Ezekowitz M, Wallentin L, et al. Causes of death and influencing factors in patients with atrial fibrillation: a competing‐risk analysis from the randomized evaluation of long‐term anticoagulant therapy study. Circulation. 2013;128:2192–2201. DOI: 10.1161/CIRCULATIONAHA.112.000491. [DOI] [PubMed] [Google Scholar]
- 7. Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? Eur Heart J. 2015;36:3250–3257. DOI: 10.1093/eurheartj/ehv513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conen D, Chae CU, Glynn RJ, Tedrow UB, Everett BM, Buring JE, Albert CM. Risk of death and cardiovascular events in initially healthy women with new‐onset atrial fibrillation. JAMA. 2011;305:2080–2087. DOI: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, Lip GY, Coats AJ, Andersson B, Kirchhof P, et al. Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: an individual‐patient data meta‐analysis. Lancet. 2014;384:2235–2243. DOI: 10.1016/S0140-6736(14)61373-8. [DOI] [PubMed] [Google Scholar]
- 10. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. DOI: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 11. Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, Hsia J, Gersh BJ, Rifai N, Ridker PM, et al. Prognostic significance of the Centers for Disease Control/American Heart Association high‐sensitivity C‐reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. DOI: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 12. Ridker PM, MacFadyen JG, Glynn RJ, Bradwin G, Hasan AA, Rifai N. Comparison of interleukin‐6, C‐reactive protein, and low‐density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: secondary analyses from the Cardiovascular Inflammation Reduction Trial. Eur Heart J. 2020;14:2952–2961. DOI: 10.1093/eurheartj/ehaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin‐6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. DOI: 10.1161/01.CIR.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 14. Ridker PM, Hennekens CH, Buring JE, Rifai N. C‐reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. DOI: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 15. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti‐inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–1299. DOI: 10.1161/CIRCULATIONAHA.118.038010. [DOI] [PubMed] [Google Scholar]
- 16. Conen D, Rodondi N, Mueller A, Beer J, Auricchio A, Ammann P, Hayoz D, Kobza R, Moschovitis G, Shah D, et al. Design of the Swiss Atrial Fibrillation Cohort Study (Swiss‐AF): structural brain damage and cognitive decline among patients with atrial fibrillation. Swiss Med Wkly. 2017;147:w14467. DOI: 10.4414/smw.2017.14467. [DOI] [PubMed] [Google Scholar]
- 17. Conen D, Rodondi N, Müller A, Beer JH, Ammann P, Moschovitis G, Auricchio A, Hayoz D, Kobza R, Shah D, et al. Relationships of overt and silent brain lesions with cognitive function in patients with atrial fibrillation. J Am Coll Cardiol. 2019;73:989–999. DOI: 10.1016/j.jacc.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 18. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. DOI: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 19. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. DOI: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 20. Tardif J‐C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and safety of low‐dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. DOI: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 21. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu X‐F, Ireland MA, Lenderink T, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. DOI: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 22. Yao C, Veleva T, Scott L Jr, Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsina KM, Abu‐Taha I, et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–2242. DOI: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lazzerini PE, Laghi‐Pasini F, Acampa M, Srivastava U, Bertolozzi I, Giabbani B, Finizola F, Vanni F, Dokollari A, Natale M, et al. Systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin‐6‐mediated changes in connexin expression. J Am Heart Assoc. 2019;8:e011006. DOI: 10.1161/JAHA.118.011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conen D, Ridker PM, Everett BM, Tedrow UB, Rose L, Cook NR, Buring JE, Albert CM. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J. 2010;31:1730–1736. DOI: 10.1093/eurheartj/ehq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, et al. C‐reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. DOI: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 26. Roldán V, Marín F, Díaz J, Gallego P, Jover E, Romera M, Manzano‐fernández S, Casas T, Valdés M, Vicente V, et al. High sensitivity cardiac troponin T and interleukin‐6 predict adverse cardiovascular events and mortality in anticoagulated patients with atrial fibrillation. J Thromb Haemost. 2012;10:1500–1507. DOI: 10.1111/j.1538-7836.2012.04812.x. [DOI] [PubMed] [Google Scholar]
- 27. Hermida J, Lopez FL, Montes R, Matsushita K, Astor BC, Alonso A. Usefulness of high‐sensitivity C‐reactive protein to predict mortality in patients with atrial fibrillation (from the Atherosclerosis Risk In Communities [ARIC] Study). Am J Cardiol. 2012;109:95–99. DOI: 10.1016/j.amjcard.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aulin J, Siegbahn A, Hijazi Z, Ezekowitz MD, Andersson U, Connolly SJ, Huber K, Reilly PA, Wallentin L, Oldgren J. Interleukin‐6 and C‐reactive protein and risk for death and cardiovascular events in patients with atrial fibrillation. Am Heart J. 2015;170:1151–1160. DOI: 10.1016/j.ahj.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 29. Hijazi Z, Aulin J, Andersson U, Alexander JH, Gersh B, Granger CB, Hanna M, Horowitz J, Hylek EM, Lopes RD, et al. Biomarkers of inflammation and risk of cardiovascular events in anticoagulated patients with atrial fibrillation. Heart. 2016;102:508–517. DOI: 10.1136/heartjnl-2015-308887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Tables S1–S2
Figures S1–S8


