Abstract
Background
Left ventricular non‐compaction remains a poorly described entity, which has led to challenges of overdiagnosis. We aimed to evaluate if the presence of a thin compacted myocardial layer portends poorer outcomes in individuals meeting cardiac magnetic resonance criteria for left ventricular non‐compaction .
Methods and Results
This was an observational, retrospective cohort study involving individuals selected from the Cleveland Clinic Foundation cardiac magnetic resonance database (N=26 531). Between 2000 and 2018, 328 individuals ≥12 years, with left ventricular non‐compaction or excessive trabeculations based on the cardiac magnetic resonance Petersen criteria were included. The cohort comprised 42% women, mean age 43 years. We assessed the predictive ability of myocardial thinning for the primary composite end point of major adverse cardiac events (composite of all‐cause mortality, heart failure hospitalization, left ventricular assist device implantation/heart transplant, ventricular tachycardia, or ischemic stroke). At mean follow‐up of 3.1 years, major adverse cardiac events occurred in 102 (31%) patients. After adjusting for comorbidities, the risk of major adverse cardiac events was nearly doubled in the presence of significant compacted myocardial thinning (hazard ratio [HR], 1.88 [95% CI, 1.18‒3.00]; P=0.016), tripled in the presence of elevated plasma B‐type natriuretic peptide (HR, 3.29 [95% CI, 1.52‒7.11]; P=0.006), and increased by 5% for every 10‐unit increase in left ventricular end‐systolic volume (HR, 1.01 [95% CI, 1.00‒1.01]; P=0.041).
Conclusions
The risk of adverse clinical events is increased in the presence of significant compacted myocardial thinning, an elevated B‐type natriuretic peptide or increased left ventricular dimensions. The combination of these markers may enhance risk assessment to minimize left ventricular non‐compaction overdiagnosis whilst facilitating appropriate diagnoses in those with true disease.
Keywords: biomarkers, cardiac magnetic resonance, cardiomyopathy, echocardiography, non‐compaction
Subject Categories: Cardiomyopathy, Magnetic Resonance Imaging (MRI)
Nonstandard Abbreviations and Acronyms
- LGE
late gadolinium enhancement
- LVESVi
left ventricular end‐systolic volume index
- LVNC
left ventricular non‐compaction
- MACE
major adverse cardiac events
Clinical Perspective
What Is New?
With the increasing use of cardiac imaging as part of cardiovascular health assessment, left ventricular non‐compaction is increasingly recognized even in those without apparent history of cardiovascular disease leading to controversy on whether this phenotype is truly an isolated disease entity, spectrum of disease, or in some cases a normal variant.
Refined imaging and biochemical criteria are thus needed to identify patients at high risk of adverse events.
The present study demonstrates that in patients with left ventricular non‐compaction, the risk of adverse events is increased in the presence of biomarkers that include significant compacted myocardial thinning, an elevated B‐type natriuretic peptide or increased left ventricle dimensions.
What Are the Clinical Implications?
The role of novel biomarkers such as the presence of significant myocardial thinning and B‐type natriuretic peptide in combination with conventional imaging biomarkers has the potential to minimize left ventricular non‐compaction overdiagnosis whilst facilitating appropriate diagnoses in those with true disease.
Left ventricular non‐compaction (LVNC) is described as a distinct myocardial disorder characterized by the presence of a bilayered myocardium: a thin, compacted myocardium on the epicardial side, and a thicker trabeculated endocardial layer. 1 , 2 The American Heart Association considers LVNC to be a genetic cardiomyopathy, 3 while the European Society of Cardiology considers LVNC an unclassified cardiomyopathy. 4 In adults, LVNC can occur as an isolated entity, which in some may relate to a genetic predisposition, though it can also occur in the presence of additional cardiac or syndromic diseases. 5
Since the initial descriptions, there are at least 6 unique criteria proposed to diagnose LVNC with varying methodologies depending on the imaging modality used and the phase of the cardiac cycle where measurements are taken. 6 , 7 , 8 With the increasing use of cardiac imaging as part of cardiovascular health assessment, LVNC is increasingly described even in those without apparent history of cardiovascular disease 9 leading to controversy on whether this phenotype is truly an isolated disease entity, spectrum of disease, or in some cases a normal variant. This difficulty relates to the lack of an established definition of LVNC as a clinical entity. Despite myriad published data, the prognostic value of LVNC in adults remains unclear as many experience a benign clinical course. 10 In others, however, the prognosis appears not as benign, being similar to dilated cardiomyopathy with regards to outcomes such as thromboembolism and ventricular arrhythmias. 11 Refined imaging and biochemical criteria may be advantageous to identify patients at high risk of adverse events.
In most of the imaging criteria, the role of the thin compacted epicardial layer, despite being part of the original description of LVNC, is subordinated to that of the trabeculated layer thickness. 6 The compacted layer can be normal in thickness, effectively negating a true diagnosis of LVNC, but still disproportionately thinner than the trabeculated layer. In these instances, the trabeculated/compacted ratio can reach hypertrabeculation cut‐off thresholds, 6 potentially contributing to overdiagnosis of LVNC. Indeed, despite many diagnostic criteria that focus on the ratio between the trabeculated to compacted myocardium, a recent meta‐analysis of 28 studies found that the degree of relative hypertrabeculation did not influence the incidence of adverse cardiovascular events. 11
We thus hypothesized that the absence of a thin compacted epicardial layer (i.e., the presence of normal thickness myocardium) would portend a better prognosis in one of the largest cohorts of individuals meeting the Petersen cardiac magnetic resonance (CMR) criteria for LVNC. We also examined the prognostic impact of plasma B‐type natriuretic peptide
(BNP), a marker of prognostic importance in individuals with or at risk of heart failure, 12 in identifying patients at high risk of long‐term complications.
Methods
Study Population
This was an observational, retrospective cohort study involving individuals selected using natural language processing from the Cleveland Clinic Foundation (OH, USA) CMR database (N=26 531). Between January 2000 and December 2018, 328 individuals ≥12 years of age, with LVNC or excessive trabeculations based on the Petersen criteria on CMR were included. Individuals meeting the above criteria were excluded if they were aged <12 years, did not have adequate clinical information or follow‐up (>3 months), did not have adequate imaging or had an established diagnosis of an alternate cardiomyopathy such as ischemic or hypertrophic cardiomyopathy (Figure S1). Individuals with a history of congenital heart disease were not excluded. Pregnancy was not an exclusion criterion though there were no pregnant women in the final cohort. This study was approved by the Cleveland Clinic Foundation institutional review board, with a waiver of individual consent. The data underlying this article cannot be shared publicly because of privacy reasons.
CMR Assessment
CMR examinations were performed on 1.5‐T or 3.0‐T MR scanners (Sonata and Avanto [Siemens Medical Solutions, Erlanga, Germany] for imaging between 2002 and 2007, and Achieva or Ingenia [Philips Healthcare, Massachusetts, USA] for imaging after 2008), using 40 to 45 mT/m maximum gradient strength, 200 T/m per second maximum slew rate with electrocardiographic gating as previously described. 13 , 14 For assessment of global cardiac function, steady‐state free precession images were acquired (slice thickness 8 mm, 2 mm gap in contiguous short‐axis images). Biventricular volumes (end‐diastolic and end‐systolic), ejection fraction and LV mass were calculated by manually tracing the endocardial and epicardial borders on steady‐state free precession images. Late gadolinium enhancement (LGE) images were obtained in 91% (n=295) of study participants in long‐ and short‐axis orientations 15 to 20 minutes after injection of 0.2 mmol/kg of Gadolinium chelate. Segmented inversion‐recovery gradient echo sequences were obtained for studies performed in 2002 to 2003 and phase‐sensitive inversion‐recovery spoiled gradient echo sequences for studies performed after 2003 (acquired spatial resolution of 1.5–2.1×1.1–1.4 mm). Late gadolinium enhancement was assessed qualitatively for visible late enhancement (areas with relatively increased signal intensity following administration of gadolinium contrast). In this patient cohort, quantification of LGE cannot be reliably performed in the setting of prominent fine LV trabeculations and relative thinning of non‐compacted myocardium, which challenges the spatial resolution of CMR. CMR images were analyzed using commercially available software (cvi42; Circle Cardiovascular Imaging, Calgary, Alberta, Canada). Normal values for ventricular size and function were determined using published data from Kawel‐Boehm et al. 15
Petersen Criteria
Assessment for LVNC was performed using the Petersen criteria as previously described. 7 , 16 , 17 In brief, using long‐axis steady‐state free precession cine images, the trabeculated and compacted layers were individually measured at the point of maximal trabecular thickness. The papillary muscles and the apical cap were excluded from the measurements. A ratio of trabeculated/compacted >2.3 in any segment during end diastole established the presence of hypertrabeculation or LVNC. Regional distribution of trabeculations were concurrently determined and the presence or absence of trabeculated/compacted ratio ≥3:1 in ≥1 of segments 1 to 3, 7 to 16 and trabeculated/compacted ratio ≥2:1 in ≥1of segments 4 to 6 were identified. 16 Other features that can be relevant in LVNC (eg, the presence of pronounced intertrabecular crypts or recesses) which were not part of the original Petersen criteria, were therefore not assessed as part of this study.
The Petersen criteria 7 (described below) was used to diagnose hypertrabeculation because of its ease of use and high, independently‐reported, intra‐ and inter‐observer variability. 17 Other mass‐based techniques to diagnose hypertrabeculation have previously been described, though are more labor intensive, which limit routine clinical application and are inherently challenged by the spatial resolution of CMR (≈1.6–2 mm in‐plane) to accurately delineate fine trabeculations (≈1 mm).
Assessment for Myocardial Thinning
Long‐axis steady‐state free precession cine images were used to assess for the presence of thinned epicardium. This was determined by the presence of abrupt thinning of compacted myocardium by 50% or greater compared with a contiguous myocardial segment (Figure 1).
Figure 1. Abrupt myocardial thinning.
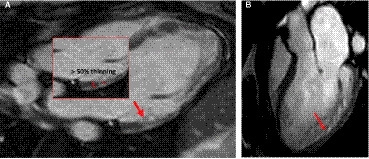
Assessment for myocardial thinning was performed using long‐axis steady‐state free precession cine images, by looking for abrupt thinning of compacted myocardium by ≥50% compared with a contiguous myocardial segment. Examples of abrupt myocardial thinning (red arrows) in 2 separate individuals are shown in these long axis 3‐chamber steady‐state free precession sequences. A, there is marked thinning with near absence of visualized compacted myocardium in the basal to mid‐inferolateral segments. Insert shows abrupt thinning is defined by thinning of compacted myocardium by ≥50% compared with a contiguous myocardial segment. B, there is significant thinning in the mid‐infero‐lateral and apico‐lateral walls.
Interobserver variability was assessed to examine agreement between the investigators to ascertain for the presence of abrupt thinning using a randomly selected group of 30 CMRs (≈10% of study sample) in a blinded fashion.
Plasma BNP/N‐Terminal Pro‐B‐Type Natriuretic Peptide Analysis and Interpretation
According to the discretion of treating physicians unaware of the present study, plasma NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) or BNP levels were measured using validated, commercially available assays (Elecsys ProBNP assay [Roche Diagnostics, Indianapolis, IN, USA] and Biosite Triage BNP assay [Biosite Diagnostics Corp, San Diego, California]).
We assessed BNP and NT‐proBNP categorically. Elevated values for NT‐proBNP were >125 pg/mL for patients aged <75 years or >450 pg/mL for those aged ≥75 years and >99 pg/mL for BNP.
Clinical Outcomes
The duration of follow‐up ranged between initial CMR visit to event/last office follow‐up.
The primary end point was a composite of major adverse cardiac events (MACE) defined as all‐cause mortality, heart failure hospitalization, left ventricular assist device implantation/heart transplant, ventricular tachycardia/appropriate implantable cardiac defibrillator therapy or ischemic stroke. We ascertained events by reviewing medical records. Heart failure (HF) hospitalization was defined as an event in which the patient was admitted to the hospital with a primary diagnosis of HF, the length of stay was at least 24 hours (or extended over a calendar date), the patient exhibited new or worsening symptoms of HF on presentation, had objective evidence of new or worsening HF, and received initiation or intensification of treatment specifically for HF. 18 Stroke was defined as an acute episode of focal cerebral, spinal, or retinal dysfunction caused by infarction of central nervous system tissue. 18 Ventricular tachycardia was defined as ≥3 consecutive beats of tachycardia with an RR interval of <600 ms (>100 beats/min) detected on device interrogation where applicable, or based on evaluation of records, history, ECGs, Holter monitoring, and telemetry performed at our institution, or locally during follow‐up. 19 The duration of follow‐up ranged from the initial CMR to October 2019.
Statistical Analysis
Baseline demographic data, risk factors, and clinical variables were descriptively summarized with continuous variables expressed as mean±SD and categorical data presented as frequencies and percentages. Agreement for the presence of abrupt compacted myocardium thinning (J.R. and D.K.) was evaluated by the Kappa statistic. Kappa values of 0.2 were interpreted as poor, 0.21 to 0.4 as fair, 0.41 to 0.6 as moderate, 0.61 to 0.80 as substantial, and 0.81 to 1.00 as almost perfect or perfect agreement. Based on prior studies on this population 7 , 9 , 11 , 16 , 17 , 20 , 21 and clinical experience, the main risk factors for adverse outcomes in this patient population include: (1) baseline demographic/medical comorbidities, and (2) risk biomarkers including CMR and biochemical parameters. We evaluated the univariate relationship of each medical risk factor (sex, age, diabetes mellitus, hypertension, atrial arrhythmias, ventricular arrhythmias, hyperlipidemia, heart failure, stroke, systemic embolization left bundle branch block, right bundle branch block, family history of LVNC) with risk of MACE using Cox proportional hazards regression models (separate model for each risk factor), and selected the significant variables to build a risk score for each patient which was equal to the sum of the products generated by multiplying the regression coefficient of each significant risk factor by the value of that variable. Following this, univariate analysis of individual CMR parameters and BNP were performed after adjusting for the medical risk score. An example of the application of the medical risk score is shown in Figure S2. Finally, multivariable Cox proportional models were constructed where the independent variables included the medical risk score and any of the biomarkers that reached a significance level <0.10 on the univariate analysis. First, all main effects were considered (full model). In successive models the non‐significant variables were removed to develop a parsimonious model. A 2‐tailed P<0.05 was interpreted as significant. The Bonferroni‒Holm method was used to control the familywise error rate. 22 Statistical analyses were performed with SAS version 9.2 (SAS Institute).
Results
A total of 328 individuals were included in the analysis (Figure S1). Baseline clinical variables, medications, biochemical and CMR parameters are listed in Table 1 (42% women, mean age 43 years). There was a high prevalence of heart failure (45% of individuals), with a history of stroke or systemic embolization in 12%, atrial arrhythmias in 35%, and ventricular arrhythmias in 31%. With regard to baseline medical therapy, heart failure related medications such as angiotensin‐converting enzyme inhibitors were used by 38% of individuals, angiotensin receptor blockers by 19%, and ß‐blockers by 59%. Approximately 10% had a documented family history of LVNC or a related phenotype such as non‐ischemic dilated cardiomyopathy. Plasma BNP or NT‐proBNP was measured in a subset of 201 individuals (61%) and levels were elevated in 140 individuals (43% of all individuals; 70% of measured individuals).
Table 1.
Baseline Characteristics
| Clinical characteristics | |
| Age, y | 43.3±17.2 |
| Women | 136 (41.5%) |
| Diabetes mellitus | 43 (13.1%) |
| Hypertension | 136 (41.5%) |
| Hyperlipidemia | 102 (31.1%) |
| Heart failure | 148 (45.1%) |
| Stroke or systemic embolization | 38 (11.6%) |
| Atrial arrythmias | 114 (34.8%) |
| Ventricular arrhythmias | 101 (30.8%) |
| Family history of LVNC or related phenotype | 33 (10.1%) |
| Medical therapy | |
| Angiotensin‐converting enzyme inhibitor, % | 123 (37.5%) |
| Angiotensin receptor blocker, % | 62 (18.9%) |
| ß‐blocker, % | 194 (59.2%) |
| Aldosterone antagonist, % | 73 (22.3%) |
| Aspirin, % | 134 (40.9%) |
| Oral anticoagulant, % | 51 (15.6%) |
| Imaging/blood biomarkers | |
| LVEDVi, mL/m2 | 113.0±44.2 |
| LVESVi, mL/m2 | 66.0±42.5 |
| LVSVi, mL/m2 | 46.3±14.3 |
| LVEF, % | 44.6±14.1 |
| LVMI, g/m2 | 67.0±22.4 |
| LAVI, mL/m2 | 43.5±19.0 |
| Maximal NC/C ratio | 3.3±1.1 |
| Mean number of trabeculated segments | 3.4±2.1 |
| Late gadolinium enhancement | 103 (31.4%) |
| RV hypertrabeculation | 36 (11.0%) |
| Abnormal RV size and/or function | 116 (35.4%) |
| Abrupt thinning | 60 (18.3%) |
| Elevated BNP | 140 (42.7%) |
Values are Mean±SD or n (%). BNP indicates brain natriuretic peptide; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LVEDVI, left ventricular end‐diastolic volume index; LVESVI, left ventricular end‐systolic volume index; LVMI, left ventricular mass index; LVSVi, Left ventricular stroke volume index; and RV, right ventricular.
Cardiac Magnetic Resonance
The study cohort was dominated by individuals with myocardial structural and/or functional abnormalities. A total of 250 individuals (76%) had an abnormal LV ejection fraction, 186 (57%) had increased LV end‐diastolic volume index, and 279 (85%) had either an abnormal LV ejection fraction or increased LV end‐diastolic volume index. With regards to CMR parameters, there was increased mean LV end‐diastolic volume, mildly depressed mean LV ejection fraction with just over one third of patients having abnormal right ventricular size and/or systolic function. Assessment for LGE using contrast‐enhanced CMR was performed in 297 individuals (91%). LGE was present in 79 individuals (24% of all individuals, 27% of measured individuals). A total of 286 (87%) had either LV enlargement, depressed LV ejection fraction, or LGE. The average number of non‐compacted segments as determined by Petersen criteria 7 was 3.4 and the average maximal ratio was 3.3. Hypertrabeculation by Petersen criteria was most common in the apical segments (segments 13–16, n=299, 91%) followed by the basal to mid‐lateral (segments 5–6 and 11–12, n=135, 41%), inferior (segments 4 and 10, n=47, 14%), anterior (segments 1 and 7, n=37, 11%) segments and least common in the basal to mid‐septum (segments 2–3 and 8–9) n=26, 8%). There was no significant relationship between LV volumes and maximal trabeculated: compacted ratio (r=0.06, P=0.281, Figure 2). Sixty individuals had abrupt myocardial thinning resulting in a thinned epicardial layer (18%). Individuals with significant compacted epicardial thinning had higher maximal trabeculated: compacted ratio compared with those with no significant compacted epicardial thinning (3.90±1.16 versus 3.19±1.02, P<0.001, Figure 2).
Figure 2. Relationship between left ventricular volume, maximal trabeculated: compacted ratio and presence of abrupt thinning.
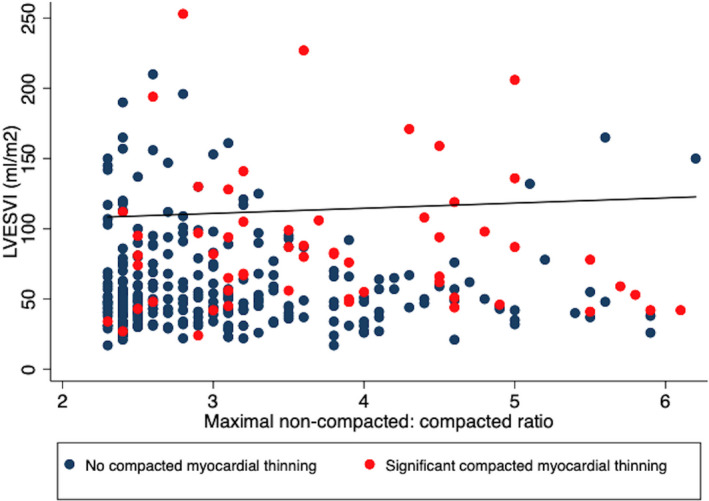
Scatterplot of left ventricular end‐systolic volume versus maximal trabeculated: compacted ratio demonstrating no significant relationship (r=0.06, P=0.281). Individuals with significant compacted myocardial thinning (red circles, 3.90±1.16) had higher maximal trabeculated: compacted ratio compared with those without significant compacted myocardial thinning (navy circles, 3.19±1.02, P<0.001). LVESVi indicates left ventricular end‐systolic volume index.
Interobserver Variability
A total of 30 patients (≈10%) were randomly selected for assessment by 2 investigators independently to determine interobserver variability for the assessment of abrupt compacted epicardial thinning (J.R. and D.K.). There was substantial interobserver agreement (Kappa, 0.80; 90% agreement).
Outcomes
MACE occurred in 102 of the 328 subjects (31%). The mean follow‐up time was 3.1 years for 226 subjects who did not have MACE during the follow‐up period and 2.2 years among the 102 subjects who did have MACE. There were 15 deaths, 41 heart failure admissions, 9 left ventricular assist device implantation/heart transplants, 70 had ventricular tachycardia/appropriate implantable cardiac defibrillator therapy, and 15 had strokes.
Medical Risk and Outcomes
A medical risk score that captured baseline demographic data and baseline comorbidities to predict MACE was constructed. Results for the univariate and multivariable models for building the medical risk score are summarized in Tables 2 and 3, respectively. History of atrial arrhythmias (HR, 1.76 [1.18‒2.62]; P=0.006), heart failure (HR, 4.13 [2.48‒6.89]; P<0.001), stroke or systemic embolization (HR, 2.11 [1.31‒3.42]; P=0.002) and the presence of a bundle branch block (2.02 [1.35‒3.04] were significant predictors of MACE. With regards to baseline medical therapy, there was an increase in MACE in those on ß‐blocker therapy, 40.2% versus 17.9% (P<0.001) and numerically increased HF hospitalization in those on ß‐blocker therapy 15.0% versus 9.0% (P=0.107).
Table 2.
Associations Between Medical Risk Factor and Risk of MACE
| HR (95% CI) | P Value | |
|---|---|---|
| Women | 1.44 (0.98‒2.13) | 0.067 |
| Age (per 1‐y increase) | 1.01 (1.00‒1.03) | 0.020 |
| Diabetes mellitus | 1.04 (0.62‒1.76) | 0.874 |
| Hypertension | 1.66 (1.12‒2.47) | 0.011 |
| Atrial arrhythmias | 2.31 (1.56‒3.44) | <0.001 |
| Ventricular arrhythmias | 2.73 (1.84‒4.05) | <0.001 |
| Hyperlipidemia | 0.82 (0.54‒1.24) | 0.347 |
| Heart failure | 4.98 (3.10‒8.0) | <0.001 |
| Stroke | 2.74 (1.71‒4.41) | <0.001 |
| Stroke or systemic embolization | 3.01 (1.91‒4.74) | <0.001 |
| Left bundle branch block | 2.00 (1.27‒3.13) | 0.003 |
| Right bundle branch block | 2.13 (1.25‒3.65) | 0.006 |
| Family history of LVNC or related phenotypes | 0.82 (0.43‒1.59) | 0.564 |
LVNC indicates left ventricular non‐compaction; HR, hazard ratio; and MACE, major adverse cardiac events.
Table 3.
Association Between Variables in Medical Risk Model and Risk of MACE
| HR (95% CI) | P Value | |
|---|---|---|
| Women | 1.12 (0.75‒1.68) | 0.567 |
| Age (per 1‐y increase) | 1.02 (0.68‒1.54) | 0.928 |
| Diabetes mellitus | 0.60 (0.35‒1.04) | 0.068 |
| Atrial arrythmias | 1.76 (1.18‒2.62) | 0.006 |
| History of heart failure | 4.13 (2.48‒6.89) | <0.001 |
| Stroke or systemic embolization | 2.11 (1.31‒3.42) | 0.002 |
| Any bundle branch block | 2.02 (1.35‒3.04) | 0.001 |
Blood and Imaging Biomarkers and Outcomes
The results of adding each imaging findings to the aforementioned medical risk score are described in Table 4. After adjusting for general medical risk, the following variables were significant univariate predictors of MACE: elevated BNP, LV end‐systolic volume index (LVESVi), left atrial volume index, presence of LGE, abrupt compacted epicardial thinning, maximal trabeculated:compacted ratio, trabeculated:compacted ratio >3:1 segments 1 to 3/7 to 16, and septal and anterior regions of hypertrabeculation.
Table 4.
Results of Univariate Analysis for Imaging Variables, After Adjusting for Medical Risk Score
| HR (95% CI) | P Value | |
|---|---|---|
| Elevated BNP | 3.54 (1.66‒7.52) | 0.001 |
| LV end‐diastolic volume index (per 1‐unit increase) | 1.004 (1.00‒1.01) | 0.081 |
| LV end‐systolic volume index (per 1‐unit increase) | 1.01 (1.00‒1.01) | 0.011 |
| LV stroke volume index (per 1‐unit increase) | 0.99 (0.97‒1.00) | 0.102 |
| Abnormal LVEF | 1.04 (0.52‒1.78) | 0.899 |
| LV mass index (per 1‐unit increase) | 1.01 (0.99‒1.01) | 0.287 |
| Left atrial volume index (per 1‐unit increase) | 1.01 (1.00‒1.03) | 0.011 |
| Presence of LGE | 1.55 (1.02‒2.35) | 0.039 |
| Abrupt thinning | 1.98 (1.29‒3.06) | 0.002 |
| RV hypertrabeculation | 0.78 (0.34‒1.82) | 0.568 |
| Abnormal RV size and/or function | 1.32 (0.88‒1.96) | 0.175 |
| Hypertrabeculation | ||
| Maximal T/C ratio (per 1‐unit increase) | 1.21 (1.03‒1.42) | 0.019 |
| No. of trabeculated segments (per 1‐unit increase) | 1.05 (0.97‒1.14) | 0.203 |
| T/C ratio >2:1 segments 4 to 6 | 0.87 (0.43‒1.77) | 0.698 |
| T/C ratio >3:1 segments 1 to 3, 7 to 16 | 1.70 (1.14‒2.53) | 0.009 |
| Basal to mid‐septal segments | 1.91 (1.11‒3.27) | 0.019 |
| Basal to mid‐anterior segments | 1.76 (1.07‒2.87) | 0.026 |
| Basal to mid‐inferior segments | 1.61 (0.99‒2.62) | 0.052 |
| Basal to mid‐lateral segments | 1.48 (0.99‒2.19) | 0.054 |
| Apical segments | 0.57 (0.32‒1.02) | 0.056 |
BNP indicates brain natriuretic peptide; LV, left ventricular; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; RV, right ventricular; and T/C, trabeculated/compacted.
The first row of Table 5 summarizes the results of the full main‐effects model, without BNP and LGE. In model 2, the non‐significant variables were removed to create a parsimonious model. The model suggests that, after adjusting for medical risk, the risk of MACE increases when LVESVi increases (HR, 1.01; adjusted‐P=0.024), there is abrupt thinning (HR, 1.77; adjusted‐P=0.022) and hypertrabeculation involving the basal to mid‐inferior walls (HR, 1.76; adjusted‐P=0.029) (Table 5). In model 3, BNP was added to the parsimonious model 2 which demonstrated that elevated BNP (HR, 3.13; P=0.016) is a significant predictor of MACE, after accounting for medical risk, LVESVi, and the presence of abrupt thinning (Table 5). In model 4, the presence of LGE was added to the parsimonious model 2 and after adjusting for medical risk, LVESVi and abrupt thinning, the presence of LGE was not a significant predictor of MACE (HR, 1.33; adjusted‐P=0.202), Table 5.
Table 5.
Results of Multivariable Model
| Independent Variables | Estimated HRs (P Value) [95% CI] | |
|---|---|---|
| Model 1: full main effects | Medical risk score (per 1‐unit increase) | 2.10 (<0.001) [1.61, 2.73] |
| LV end‐systolic volume index (per 1‐unit increase) | 1.01 (0.010) [1.00‒1.01] | |
| Left atrial volume index (per 1‐unit increase) | 1.01 (0.287) [0.99‒1.02] | |
| Abrupt thinning | 1.51 (0.118) [0.90‒2.54] | |
| Maximal T/C ratio (per 1‐unit increase) | 0.96 (0.768) [0.75‒1.23] | |
| T/C ratio >3:1 in segments 1 to 3, 7 to 16 | 1.44 (0.198) [0.83‒2.49] | |
| Basal to mid‐septal hypertrabeculation | 1.14 (0.708) [0.58‒2.23] | |
| Basal to mid‐anterior hypertrabeculation | 1.49 (0.247) [0.76‒2.92] | |
| Basal to mid‐inferior hypertrabeculation | 1.53 (0.193) [0.81‒2.90] | |
| Basal to mid‐lateral hypertrabeculation | 0.83 (0.459) [0.50‒1.37] | |
| Apical hypertrabeculation | 0.54 (0.068) [0.28‒1.05] | |
| Model 2: reduced main effects | Medical risk score (per 1‐unit increase) | 2.22 (<0.001) [1.74‒2.82] |
| LV end‐systolic volume index (per 1‐unit increase) | 1.01 (adjusted 0.024) [1.00‒1.01] | |
| Abrupt thinning | 1.77 (adjusted 0.022) [1.14‒2.74] | |
| Basal to mid inferior hypertrabeculation | 1.76 (adjusted 0.029) [1.06‒2.92] | |
| Model 3: reduced main effects with BNP | Medical risk score (per 1‐unit increase) | 1.96 (<0.001) [1.45‒2.64] |
| LV end systolic volume index (per 1‐unit increase) | 1.01 (adjusted 0.042) [1.00‒1.01] | |
| Abrupt thinning | 1.76 (adjusted 0.038) [1.10‒2.82] | |
| Basal to mid inferior hypertrabeculation | 1.69 (adjusted 0.069) [0.96‒2.98] | |
| Elevated BNP | 3.13 (adjusted 0.016) [1.44‒6.79] | |
| Model 4: reduced main effects with LGE | Medical risk score (per 1‐unit increase) | 2.17 (<0.001) [1.68‒2.80] |
| LV end‐systolic volume index (per 1‐unit increase) | 1.01 (adjusted 0.189) [1.00‒1.01] | |
| Abrupt thinning | 1.52 (adjusted 0.156) [0.96‒2.42] | |
| Basal to mid inferior hypertrabeculation | 1.77 (adjusted 0.144) [1.04‒3.01] | |
| Presence of LGE | 1.33 (adjusted 0.202) [0.86‒2.07] | |
| Model 5: final model | Medical risk score (per 1‐unit increase) | 1.96 (<0.001) [1.45‒2.65] |
| LV end‐systolic volume index (per 1‐unit increase) | 1.01 (adjusted 0.041) [1.00‒1.01] | |
| Abrupt thinning | 1.88 (adjusted 0.016) [1.18‒3.00] | |
| Elevated BNP | 3.29 (adjusted 0.006) [1.52‒7.11] |
BNP indicates brain natriuretic peptide; LGE, late gadolinium enhancement; LV, left ventricular; RV, right ventricular; and T/C, trabeculated/compacted.
Two‐way interactions between LVESVi, abrupt thinning, elevated BNP, and the presence of LGE were not significant (P values >0.104).
The final model is summarized in the last row of Table 5 (model 5). The model suggests that, after adjusting for medical risk (HR, 1.96 [95% CI, 1.45‒2.65]; P<0.001), the risk of MACE is nearly doubled in the presence of abrupt thinning (HR, 1.88 [95% CI, 1.18‒3.00]; adjusted‐P=0.016), 3 times higher in the presence of elevated BNP (HR, 3.29 [95% CI, 1.52‒7.11]; adjusted‐P=0.006), and increases by 5% for every 10‐unit increase in LVESVi (HR, 1.01 [95% CI, 1.00‒1.01]; adjusted‐P=0.041] (Figure 3). The proportionality test had a P value=0.9176 for the final model, indicating that the model assumption is reasonable.
Figure 3. Cox proportional hazards survival from major adverse cardiovascular events.
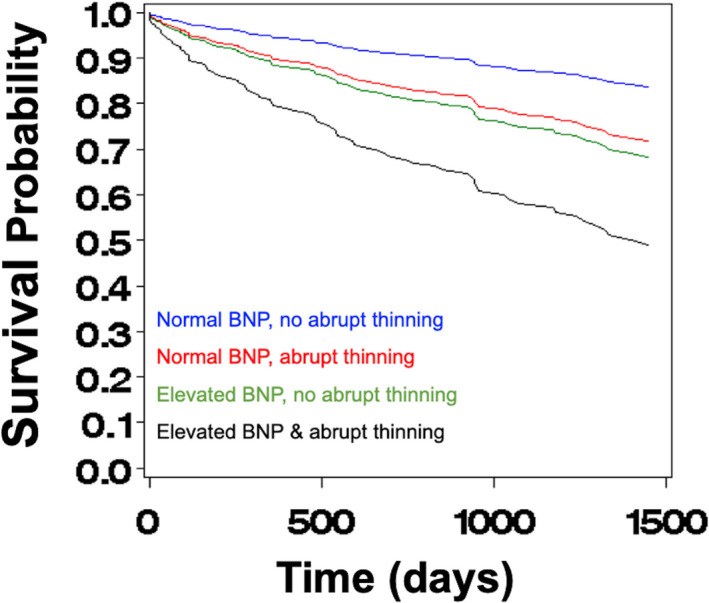
Cox proportional hazards survival plots illustrating the effect of elevated B‐type natriuretic peptide (BNP) and presence of abrupt thinning on the risk of major adverse cardiovascular events. Blue curve is based on 168 subjects with average medical risk, average left ventricular end‐systolic volume index, and neither elevated BNP nor presence of abrupt thinning. Green curve is based on 100 subjects with average medical risk, average left ventricular end‐systolic volume index, elevated BNP, but absence of abrupt thinning. Red curve is based on 20 subjects with average medical risk, average left ventricular end‐systolic volume index, presence of abrupt thinning, but normal BNP. Black curve is based on 40 subjects with average medical risk, average left ventricular end‐systolic volume index, and both elevated BNP and presence of abrupt thinning. LVESVi indicates left ventricular end‐systolic volume index; and MACE; major adverse cardiovascular events.
For the final model (Model 5 in Table 5) Uno concordance index, which is a measure of a model’s predictive accuracy, was 0.768 (SE=0.030). A model with just medical risk score yielded a concordance index of 0.729 (0.032), and a model with medical risk score and elevated BNP had a concordance index of 0.753 (0.023). The improvement in accuracy by adding elevated BNP to medical risk score did not reach statistical significance (P=0.090); however, the final model, which included elevated BNP, LVESVi, and abrupt thinning, did significantly improve the predictive accuracy over just medical risk score (P=0.048). The latter model was not significantly greater than a model with medical risk score and elevated BNP, P=0.290.
LV end‐diastolic volume index and LVESVi are highly correlated: r=0.88, P<0.001. Results of multivariable models using LV end‐diastolic volume index instead of LVESVi demonstrated that LV end‐diastolic volume index was not an independent predictor of MACE (Models summarized in Table S1).
Discussion
Despite >2000 publications on the subject, LVNC remains a poorly understood clinical entity that has led to challenges of overdiagnosis and over‐treatment. 5 , 23 There remains an unmet need for diagnostic criteria that will minimize LVNC overdiagnosis whilst facilitating appropriate LVNC diagnoses in those with true disease.
The key findings of the present study in patients with LVNC were that after adjusting for standard medical risk, the risk of adverse clinical events is (1) nearly doubled in the presence of abrupt compacted epicardial thinning, (2) over 3 times higher in the presence of elevated BNP, and (3) increases by 5% for every 10‐unit increase in LVESVi. History of atrial arrhythmias, heart failure, stroke, or systemic embolization, and bundle branch block were clinical predictors of long‐term MACE.
Several lines of evidence, including from the present study, suggest that the degree and location of even prominent trabeculations are not independent predictors of adverse outcomes. This bears emphasis considering most CMR and echocardiographic criteria principally use the degree of hypertrabeculation to assign disease status. 7 , 8 , 9 , 17 Yet such criteria clearly over‐diagnose LVNC. Indeed, in a large population‐based study (The Multi‐Ethnic Study of Atherosclerosis), a subset of patients without cardiac disease or hypertension were evaluated using the Petersen criteria, with 43% of patients having ≥1 segments satisfying this criteria. 24 While identifying hypertrabeculation is an important initial parameter to define the phenotype, it seems clear that other clinical, structural, and biochemical parameters need to be incorporated in the LVNC diagnostic paradigm. Continuing with this line of thought, in one of the few prospective studies in LVNC (n=113 patients), no adverse cardiovascular events occurred in those without LV dilation, LV dysfunction, and/or LGE. 16
The recent findings from the Copenhagen general population study have challenged prior evidence that suggest that hypertrabeculation is a benign phenomenon. 25 Using contrast enhanced cardiac tomography in 10 097 individuals, the authors report that individuals with the greatest degree of hypertrabeculation had a 1.6‐fold higher rate of MACE, 2.1‐fold higher rate of death, and 2.6‐fold higher rate of heart failure. Similarly, in the present study of individuals with LV hypertrabeculation based on Petersen criteria, there is an overall high incidence of adverse events (MACE occurred in 31% of the population). Taken together, given the high prevalence of hypertrabeculation in the general population, the findings emphasize the need for refined criteria to identify patients at high risk of adverse events.
In our study cohort, the vast majority (87%) had myocardial abnormalities evident as LV enlargement, depressed LV ejection fraction, and/or LGE, constituting what would traditionally be considered a high‐risk cohort of patients with LVNC on the basis of available evidence. 11 , 16 , 17 , 20 Given this, it is not completely surprising that already highly prevalent parameters such as LV ejection fraction and LGE were not independent predictors of MACE when considered alongside a comprehensive medical risk model (Tables 4 and 5). Our study extends knowledge in this regard as we provide further risk stratification of patients with what we believe to be predominantly true LVNC rather than simply hypertrabeculation.
To our knowledge, this is the first report in LVNC that significant epicardial thinning independently portends an adverse prognosis in MACE. Assessment for significant compacted epicardial thinning is not part of the current diagnostic criteria. This further affirms that less importance should be placed on the thickness of trabeculated segments and/or the arbitrary ratio of trabeculated to compacted myocardium. 26 Indeed the recent meta‐analysis of 28 studies, nearly all of which used the Jenni 8 or Petersen 7 criteria, or both, as diagnostic criteria, revealed that the trabeculated:compacted myocardium ratio was unrelated to adverse cardiovascular outcomes. Further, mechanistically it is the compacted myocardium that ultimately determines contractility. 26 It has previously been shown that with decreasing wall thickness, there is an exponential decrease of ejection fraction. 27 Using mathematical modeling of LV mechanics using the standard Lamé equations for a thick‐walled cylinder, it is posited that myocardial wall thinning results in sharp increases in wall stress and ultimately declining contractile function further suggesting its relevance in LVNC. 26 Indeed, in a cohort of patients classified as having infantile LVNC, posterior wall thickness Z score<−1.5 was associated with worse outcomes (death, heart transplantation and implantable cardiac defibrillator implantation, HR, 1.42 [95% CI, 1.27–41.65]). 28
With regards to baseline medical therapy, there was an increase in MACE in those on ß‐blocker therapy. Although our study was not designed to account for these differences, we postulate that this may be because of concurrent, antecedent cardiovascular conditions requiring ß‐blocker therapy such as hypertension, heart failure, or arrythmias.
In the present study, patients with elevated BNP and abrupt myocardial thinning had increased MACE compared with either marker alone. CMR criteria such as LV enlargement, systolic dysfunction, LGE, and significant epicardial thinning may reflect advanced myocardial remodeling whilst elevated BNP likely represents part of the neurohormonal adaptive response to myocardial stretch and remodeling. The use of these markers (both abnormal in only 12% of the present cohort), alongside clinical and traditional CMR risk markers may therefore be expected to enhance risk assessment in individuals with hypertrabeculation which can be present in ≈15% of asymptomatic individuals free from cardiovascular disease. 9 The additional benefit of using BNP is that it is widely available for use and can be measured with a high degree of accuracy 12 to complement imaging‐based techniques that require a higher level of expertise.
Limitations
The strengths of this study include the detailed cardiac clinical profiling, myocardial phenotyping using CMR, long‐term follow‐up, and the simultaneous assessment of BNP in the majority of patients. Some important limitations should be acknowledged. Firstly, this retrospective observational study involved patients selected from the Cleveland Clinic cardiac magnetic resonance imaging database and could have selection and referral bias. Before the findings can be incorporated into routine clinical practice, these data need to be replicated in independent cohorts. Further, the association between abrupt myocardial thinning, elevated BNP and adverse cardiovascular outcomes suggests a possible relationship, though does not determine a causal relationship. We hope our findings will stimulate further investigations into understanding disease biology and LV mechanics in LVNC. Although the total area of myocardial thinning was not measured in the present study, it is expected that more extensive myocardial thinning will be associated with worsening LV function and/or clinical outcomes. Finally, given its ubiquitous use and relative simplicity, we defined LVNC a priori using the original Petersen criteria and thus our results may not be necessarily extrapolated to LVNC diagnosed using other imaging criteria. Importantly, a recent study by Ivanov et al, found that there was no difference in the rate of clinical events between LVNC diagnosed by any current CMR criteria. 17
Conclusions
We present original observations that in patients with LVNC, the risk of adverse clinical events is increased in the presence of abrupt myocardial thinning, an elevated BNP or increased LV dimensions. Our results need confirmation in large independent cohorts before routine clinical adoption in patients with LVNC/hypertrabeculation, though our findings are expected to stimulate further research to refine diagnostic criteria in LVNC.
Sources of Funding
Drs Tang and Kwon are supported by grants from the National Institute of Health (R01HL146754).
Disclosures
None.
Supporting information
Table S1
Figures S1–S2
(J Am Heart Assoc. 2021;10:e019209. DOI: 10.1161/JAHA.120.019209.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019209
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Borges AC, Kivelitz D, Baumann G. Isolated left ventricular non‐compaction: cardiomyopathy with homogeneous transmural and heterogeneous segmental perfusion. Heart. 2003;89:e21. DOI: 10.1136/heart.89.8.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507–513. DOI: 10.1161/01.CIR.82.2.507. [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB, American Heart A, et al. Contemporary definitions and classification of the cardiomyopathies: an American heart association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–1816. DOI: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 4. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kuhl U, Maisch B, McKenna WJ, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270–276. DOI: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 5. Ross SB, Jones K, Blanch B, Puranik R, McGeechan K, Barratt A, Semsarian C. A systematic review and meta‐analysis of the prevalence of left ventricular non‐compaction in adults. Eur Heart J. 2020;41:1428–1436. DOI: 10.1093/eurheartj/ehz317. [DOI] [PubMed] [Google Scholar]
- 6. Arbustini E, Favalli V, Narula N, Serio A, Grasso M. Left ventricular noncompaction: a distinct genetic cardiomyopathy? J Am Coll Cardiol. 2016;68:949–966. DOI: 10.1016/j.jacc.2016.05.096. [DOI] [PubMed] [Google Scholar]
- 7. Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, Watkins H, Neubauer S. Left ventricular non‐compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101–105. DOI: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 8. Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non‐compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666–671. DOI: 10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weir‐McCall JR, Yeap PM, Papagiorcopulo C, Fitzgerald K, Gandy SJ, Lambert M, Belch JJF, Cavin I, Littleford R, Macfarlane JA, et al. Left ventricular noncompaction: anatomical phenotype or distinct cardiomyopathy? J Am Coll Cardiol. 2016;68:2157–2165. DOI: 10.1016/j.jacc.2016.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zemrak F, Ahlman MA, Captur G, Mohiddin SA, Kawel‐Boehm N, Prince MR, Moon JC, Hundley WG, Lima JAC, Bluemke DA, et al. The relationship of left ventricular trabeculation to ventricular function and structure over a 9.5‐year follow‐up: the mesa study. J Am Coll Cardiol. 2014;64:1971–1980. DOI: 10.1016/j.jacc.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aung N, Doimo S, Ricci F, Sanghvi MM, Pedrosa C, Woodbridge SP, Al‐Balah A, Zemrak F, Khanji MY, Munroe PB, et al. Prognostic significance of left ventricular noncompaction: systematic review and meta‐analysis of observational studies. Circ Cardiovasc Imaging. 2020;13:e009712. DOI: 10.1161/CIRCIMAGING.119.009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang WHW, Girod JP, Lee MJ, Starling RC, Young JB, Lente FV, Francis GS. Plasma b‐type natriuretic peptide levels in ambulatory patients with established chronic symptomatic systolic heart failure. Circulation. 2003;108:2964–2966. DOI: 10.1161/01.CIR.0000106903.98196.B6 [DOI] [PubMed] [Google Scholar]
- 13. Kwon DH, Hachamovitch R, Popovic ZB, Starling RC, Desai MY, Flamm SD, Lytle BW, Marwick TH. Survival in patients with severe ischemic cardiomyopathy undergoing revascularization versus medical therapy: association with end‐systolic volume and viability. Circulation. 2012;126:S3–S8. DOI: 10.1161/CIRCULATIONAHA.111.084434. [DOI] [PubMed] [Google Scholar]
- 14. Cavalcante JL, Kusunose K, Obuchowski NA, Jellis C, Griffin BP, Flamm SD, Kwon DH. Prognostic impact of ischemic mitral regurgitation severity and myocardial infarct quantification by cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2020;13:1489–1501. DOI: 10.1016/j.jcmg.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 15. Kawel‐Boehm N, Maceira A, Valsangiacomo‐Buechel ER, Vogel‐Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. DOI: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andreini D, Pontone G, Bogaert J, Roghi A, Barison A, Schwitter J, Mushtaq S, Vovas G, Sormani P, Aquaro GD, et al. Long‐term prognostic value of cardiac magnetic resonance in left ventricle noncompaction: a prospective multicenter study. J Am Coll Cardiol. 2016;68:2166–2181. DOI: 10.1016/j.jacc.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 17. Ivanov A, Dabiesingh DS, Bhumireddy GP, Mohamed A, Asfour A, Briggs WM, Ho J, Khan SA, Grossman A, Klem I, et al. Prevalence and prognostic significance of left ventricular noncompaction in patients referred for cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2017;10:e006174. DOI: 10.1161/CIRCIMAGING.117.006174. [DOI] [PubMed] [Google Scholar]
- 18. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation. 2015;132:302–361. DOI: 10.1016/j.jacc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 19. Bencardino G, Spera FR, Pinnacchio G, Perna F, Narducci ML, Comerci G, Pelargonio G, Gabrielli FA, La Rosa G, Lanza GA, et al. Prognostic significance of non‐sustained ventricular tachycardia on stored electrograms in pacemaker recipients. PLoS One. 2019;14:e0225059. DOI: 10.1371/journal.pone.0225059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grigoratos C, Barison A, Ivanov A, Andreini D, Amzulescu MS, Mazurkiewicz L, De Luca A, Grzybowski J, Masci PG, Marczak M, et al. Meta‐analysis of the prognostic role of late gadolinium enhancement and global systolic impairment in left ventricular noncompaction. JACC Cardiovasc Imaging. 2019;12:2141–2151. DOI: 10.1016/j.jcmg.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 21. van Waning JI, Caliskan K, Hoedemaekers YM, van Spaendonck‐Zwarts KY, Baas AF, Boekholdt SM, van Melle JP, Teske AJ, Asselbergs FW, Backx A, et al. Genetics, clinical features, and long‐term outcome of noncompaction cardiomyopathy. J Am Coll Cardiol. 2018;71:711–722. DOI: 10.1016/j.jacc.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 22. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 23. Sharain K, Anavekar NS. Outcomes in left ventricular noncompaction: heterogeneity in results or heterogeneity in diagnosing a heterogeneous disease? Circ Cardiovasc Imaging. 2020;13:e010268. DOI: 10.1161/CIRCIMAGING.119.010268. [DOI] [PubMed] [Google Scholar]
- 24. Kawel N, Nacif M, Arai AE, Gomes AS, Hundley WG, Johnson WC, Prince MR, Stacey RB, Lima JA, Bluemke DA. Trabeculated (noncompacted) and compact myocardium in adults: the multi‐ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2012;5:357–366. DOI: 10.1161/CIRCIMAGING.111.971713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sigvardsen PE, Fuchs A, Kuhl JT, Afzal S, Kober L, Nordestgaard BG, Kofoed KF. Left ventricular trabeculation and major adverse cardiovascular events: the copenhagen general population study. Eur Heart J Cardiovasc Imaging. 2021;22:67–74. DOI: 10.1093/ehjci/jeaa110. [DOI] [PubMed] [Google Scholar]
- 26. MacIver DH. A new understanding and definition of non‐compaction cardiomyopathy using analysis of left ventricular wall mechanics and stresses. Int J Cardiol. 2014;174:819–821. DOI: 10.1016/j.ijcard.2014.04.141. [DOI] [PubMed] [Google Scholar]
- 27. Maciver DH. A new method for quantification of left ventricular systolic function using a corrected ejection fraction. Eur J Echocardiogr. 2011;12:228–234. DOI: 10.1093/ejechocard/jeq185. [DOI] [PubMed] [Google Scholar]
- 28. Wang C, Takasaki A, Watanabe Ozawa S, Nakaoka H, Okabe M, Miyao N, Saito K, Ibuki K, Hirono K, Yoshimura N, et al. Long‐term prognosis of patients with left ventricular noncompaction‐ comparison between infantile and juvenile types. Circ J. 2017;81:694–700. DOI: 10.1253/circj.CJ-16-1114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S2


