Abstract
Background
The independent prognostic value of troponin and other biomarker elevation among patients with coronavirus disease 2019 (COVID‐19) are unclear. We sought to characterize biomarker levels in patients hospitalized with COVID‐19 and develop and validate a mortality risk score.
Methods and Results
An observational cohort study of 1053 patients with COVID‐19 was conducted. Patients with all of the following biomarkers measured—troponin‐I, B‐type natriuretic peptide, C‐reactive protein, ferritin, and d‐dimer (n=446) —were identified. Maximum levels for each biomarker were recorded. The primary end point was 30‐day in‐hospital mortality. Multivariable logistic regression was used to construct a mortality risk score. Validation of the risk score was performed using an independent patient cohort (n=440). Mean age of patients was 65.0±15.2 years and 65.3% were men. Overall, 444 (99.6%) had elevation of any biomarker. Among tested biomarkers, troponin‐I ≥0.34 ng/mL was the only independent predictor of 30‐day mortality (adjusted odds ratio, 4.38; P<0.001). Patients with a mortality score using hypoxia on presentation, age, and troponin‐I elevation, age (HA2T2) ≥3 had a 30‐day mortality of 43.7% while those with a score <3 had mortality of 5.9%. Area under the receiver operating characteristic curve of the HA2T2 score was 0.834 for the derivation cohort and 0.784 for the validation cohort.
Conclusions
Elevated troponin and other biomarker levels are commonly seen in patients hospitalized with COVID‐19. High troponin levels are a potent predictor of 30‐day in‐hospital mortality. A simple risk score can stratify patients at risk for COVID‐19–associated mortality.
Keywords: biomarkers, COVID‐19, mortality, troponin
Subject Categories: Biomarkers, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- COVID‐19
coronavirus disease 2019
Clinical Perspective
What Is New?
Elevated levels of troponin, B‐type natriuretic peptide, C‐reactive protein, ferritin, and d‐dimer are common among patients hospitalized with coronavirus disease 2019 (COVID‐19).
Compared with other biomarkers, elevated peak troponin I had the greatest predictive value for mortality associated with COVID‐19.
A risk score incorporating age, hypoxia on presentation, and peak troponin I level ≥0.34 ng/mL can stratify patients at risk for COVID‐19‐associated mortality.
What Are the Clinical Implications?
Using the simple HA2T2 risk score, clinicians can identify patients at high risk for critical illness and clinical deterioration caused by COVID‐19.
Mortality risk stratification of patients hospitalized with COVID‐19 may help direct therapy and goals‐of‐care discussions.
Since its description in late 2019, coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome‐novel coronavirus 2 infection has evolved into a pandemic infecting over 29 million people and resulting in >900 000 deaths as of September 15, 2020. 1 Severe COVID‐19 is associated with acute respiratory distress syndrome as well as systemic manifestations, which include myocardial injury, cytokine storm, and coagulopathy. 2 , 3 , 4 Myocardial injury as defined by troponin elevation has been described in 7.2% to 36% of patients with COVID‐19 and has been associated with increased mortality. 5 , 6 , 7 , 8 Furthermore, elevation of inflammatory markers have been associated with severe COVID‐19. 9 However, the specificity of biomarker elevation for predicting outcomes such as arrhythmias and mortality is not well characterized. Using a multicenter registry, we sought to characterize the distribution of biomarker levels among patients hospitalized with COVID‐19, determine the independent association between biomarker elevation and outcomes of arrhythmias and mortality, and develop a novel score that incorporates biomarker levels for estimating the risk of 30‐day in‐hospital mortality.
Methods
Data Source
This observational cohort study consisted of all consecutive patients with confirmed COVID‐19 who were admitted to New York‐Presbyterian (NYP)/Weill Cornell Medicine, a quaternary referral center and 862‐bed teaching hospital; and NYP/Lower Manhattan Hospital, a 180‐bed community hospital between March 3 and April 6, 2020. The study was approved by the Weill Cornell Medicine Institutional Review Board, which waived informed consent. All cases of COVID‐19 were confirmed through real‐time reverse‐transcriptase polymerase chain reaction assays (GeneXpert, Cepheid, Sunnyvale, CA), on nasopharyngeal swabs. Using REDCap, 10 patient data were manually abstracted from NYP electronic health records to develop a COVID‐19 registry as previously described. 11 The database, statistical programs, and study materials that support the findings of this study are available from the corresponding author upon reasonable request.
Data Collection
Demographics (age, sex, and race) and pre‐existing comorbid conditions (coronary artery disease, heart failure, hypertension, diabetes mellitus, prior history of atrial fibrillation, pulmonary disease, renal disease [defined as creatinine ≥2.0 mg/dL or need for hemodialysis], and active cancer) were abstracted from the electronic health record. Hypoxia on presentation was defined as use of supplemental oxygen in the emergency department within 3 hours of presentation as abstracted from respiratory flowsheets and nursing documentation. Chest radiographic findings were abstracted from the initial and any follow‐up radiology reports and categorized based on the most abnormal findings. Transthoracic echocardiography findings on left ventricular ejection fraction and right ventricular function were abstracted. Right ventricular dysfunction was defined as tricuspid annular plane systolic excursion <17 mm or right ventricular longitudinal myocardial velocity <9.5 mm/s.
Data on laboratory values consisting of serum troponin I (TnI) (measured with chemiluminescent immunoassay; ADVIA Centaur XP, Siemens Medical Solutions, Malverne, PA), B‐type natriuretic peptide (BNP) (chemiluminescent immunoassay; ADVIA Centaur XP, Siemens Medical Solutions, Malverne, PA), CRP (C‐reactive protein) (latex‐enhanced immunoturbidimetric assay; ADVIA Chemistry XPT system, Siemens Medical Solutions, Malverne, PA), ferritin (chemiluminescent immunoassay; ADVIA Centaur XP, Siemens Medical Solutions, Malverne, PA), and d‐dimer (turbidimetric clot detection assay; ACLTM TOP CTS Coagulation System, Instrumentation Laboratory, Bedford, MA), and were reviewed and the maximum level for each biomarker was recorded. Follow‐up was through May 10, 2020, providing at least 30 days of observation for all patients; hospitalization events were determined based on review of clinical progress notes and discharge summaries. The hospital course of patients discharged but readmitted during the clinical study period was also included in the analysis. For patients who were readmitted, the duration of follow‐up was determined based on the date of first admission. Use of antiviral medications, hydroxychloroquine, steroids, and vasopressors during hospitalization was abstracted. Complications including intensive care unit admission, respiratory failure requiring mechanical ventilation, bacteremia, venous thromboembolism, arterial thromboembolism, stroke/transient ischemic attack, and acute kidney injury requiring renal replacement therapy were abstracted.
Study Outcomes
The primary outcome of the study was 30‐day all‐cause in‐hospital mortality. The secondary outcomes were atrial and ventricular arrhythmias. Atrial arrhythmias were defined as atrial fibrillation, flutter, or tachycardia. Ventricular arrhythmias were defined as frequent premature ventricular contractions, nonsustained ventricular tachycardia, sustained ventricular tachycardia, and ventricular fibrillation. Arrhythmias were identified by review of all ECGs and telemetry strips during hospitalization. ECG, telemetry findings, and complication events were reviewed and adjudicated by study investigators (KM, BP, XY, JK, and JWC). Disagreements on adjudication were resolved by consensus.
Statistical Analysis
Categorical variables are shown as frequencies, and continuous variables are presented as mean±standard deviation or median (interquartile range [IQR]) depending on normality of distribution. For comparisons of categorical variables, the χ2 or Fisher exact tests were used. For comparisons of continuous variables, the Student t test or Wilcoxon rank‐sum test were used depending on normality of distribution. For Student t test, depending on the equality of variances estimated from the F test, either the pooled variance estimator or Satterthwaite variance estimator were used for equal or unequal variances, respectively. The primary study cohort consisted of all study patients who had at least 1 measurement of all 5 biomarkers (TnI, CRP, BNP, ferritin and d‐dimer). Based on NYP‐Weill Cornell Medicine nominal reference values, abnormal levels were defined as TnI >0.04 ng/mL (limit of detection 0.03 ng/mL; maximum 500 ng/mL), BNP >100 pg/mL (limit of detection 2 pg/mL; maximum 5000 pg/mL), CRP >0.9 mg/dL (limit of detection 0.4 mg/dL; maximum 91.2 mg/dL), ferritin >322 ng/mL (limit of detection 0.5 ng/mL; maximum 16 500 ng/mL), and d‐dimer >230 ng/mL (limit of detection 150 ng/mL; maximum 55 000 ng/mL). Myocardial injury was defined as TnI level ≥0.50 ng/mL. Within this cohort, the quartiles for each biomarker level were determined. Patients were categorized as having severe biomarker level elevation on the basis of a recorded peak level ≥75th percentile for the study population. Bivariate associations between each candidate predictor and the primary outcome (30‐day in‐hospital mortality) were determined with logistic regression. Baseline demographic characteristics (age, sex, and race), comorbid conditions (including coronary artery disease, heart failure, prior history of atrial fibrillation/atrial flutter, hypertension, diabetes mellitus, pulmonary disease, renal disease, immunosuppression, smoking status, and cancer), marker of disease severity at presentation (hypoxia in the first 3 hours of admission), and severe biomarker (TnI, BNP, CRP, ferritin, and d‐dimer) elevation with bivariate significance (P<0.10) for the primary outcome were selected. Multivariable logistic regression models were then used to identify independent predictors of 30‐day mortality. Collinearity of variables was assessed with the variance inflation factor.
A risk score for 30‐day in‐hospital mortality was derived from the multivariable model by assigning weighted points based on the beta coefficients for each significant independent predictor (P<0.05). To make the score clinically interpretable, age was categorized as <65, 65 to 74, and ≥75 years (for the study population, 65 years of age was the 50th percentile cut‐off and 75 years of age was the 75th percentile cut‐off). Given that the derivation cohort consisted of patients who had all 5 biomarkers measured, a validation cohort of patients who had predictive biomarkers measured but were missing the nonpredictive biomarkers could be formed. This validation cohort was used for testing of the mortality risk score (Figure S1). Validation of the models was conducted by bootstrapping 1000 resamples of the validation cohort. The discriminative capacity and agreement between the observed and predicted probability of mortality for the risk score model was assessed with a receiver operating characteristic curve analysis with C‐statistic and calibration plot, respectively. Survival curves were then generated using the Kaplan‐Meier method and compared by using the log‐rank statistic. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and SPSS version 24 (IBM, Armonk, NY). All tests were 2‐sided with P<0.05 indicating statistical significance.
Results
Patient Characteristics and Biomarker Levels
Between March 3 and April 6, 2020, 1053 consecutive patients with COVID‐19 were admitted to NYP/Weill Cornell Medicine and NYP/Lower Manhattan Hospital. Of these, 446 (42.4%) patients had all 5 biomarkers (TnI, BNP, CRP, ferritin, and d‐dimer) checked and comprised the primary study cohort. As of May 10, 2020, 242 (54.3%) patients were discharged, 109 (24.4%) were still hospitalized, and 95 (21.3%) had died. The median length of follow‐up was 15 (IQR, 6–34; range 0–62) days. The median maximum TnI was 0.05 (IQR, 0–0.34) ng/mL, BNP was 84 (IQR, 25–300) pg/mL, CRP was 27.2 (IQR, 12.7–83.6) mg/dL, ferritin was 1219 (IQR, 598–2000) ng/mL, and d‐dimer was 2083 (IQR, 532–6106). The distribution of biomarkers levels are shown in Figure 1. Based on nominal reference biomarker values, 50.7%, 45.7%, 97.8%, 90.6%, and 94.0% had abnormal levels of TnI, BNP, CRP, ferritin, and d‐dimer, respectively. Overall, 99.6% had elevation of at least 1 of the 5 biomarkers. Overall, 93 (20.9%) patients had evidence of myocardial injury with peak TnI levels ≥0.50 ng/mL. Baseline characteristics of the primary study cohort stratified by presence or absence of severe biomarker elevation (ie, maximum levels above the 75th percentile cut‐off) are summarized in Table 1. Patients with severe elevations of TnI and BNP were older and had a significantly higher proportion of patients with coronary artery disease, heart failure, and prior stroke. Patients with severe elevations of CRP, ferritin, and d‐dimer had higher proportion of patients with hypoxia upon presentation.
Figure 1. Distribution of maximum biomarker levels among study patients.
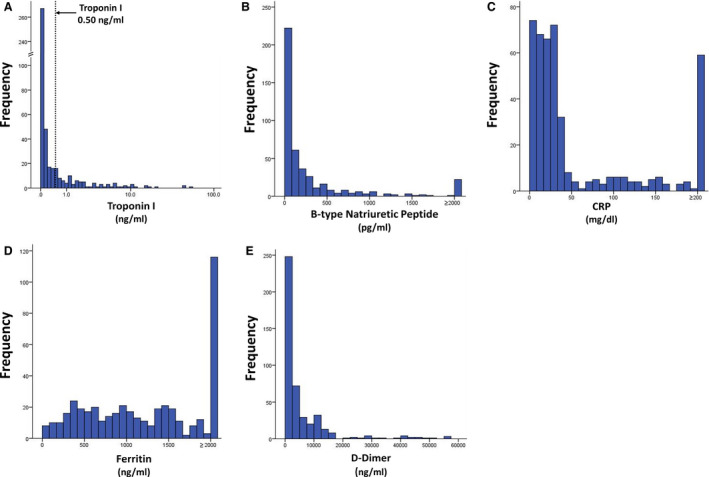
A, TnI levels shown in logarithmic scale to allow depiction of distribution of troponin levels between 0 and 1.0 ng/mL. Dotted line depicts TnI level at 0.50 ng/mL. B, BNP levels. Patients with BNP ≥2000 pg/mL are grouped together. C, CRP levels. Patients with CRP ≥200 mg/dL are grouped together. D, Ferritin levels. Patients with ferritin ≥2000 ng/mL are grouped together. E, d‐Dimer levels. BNP indicates B‐type natriuretic protein; CRP, C‐reactive protein; and TnI, troponin I.
Table 1.
Baseline Characteristics Stratified by Biomarker Levels
|
TnI ≥0.34 ng/mL (n=112) |
TnI <0.34 ng/mL (n=334) |
P Value |
BNP ≥300 pg/mL (n=112) |
BNP <300 pg/mL (n=334) |
P Value |
CRP ≥84 mg/dL (n=112) |
CRP <84 mg/dL (n=334) |
P Value |
Ferritin ≥2000 ng/mL (n=116) |
Ferritin <2000 ng/mL (n=330) |
P Value |
d‐Dimer ≥6106 ng/mL (n=111) |
d‐Dimer <6106 ng/mL (n=335) |
P Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical characteristics | |||||||||||||||
| Age, y, mean | 69.7±12.5 | 63.4±15.7 | <0.001 | 70.9±14.2 | 62.9±15.0 | <0.001 | 65.6±14.6 | 64.7±15.4 | 0.61 | 64.5±13.4 | 65.1±15.8 | 0.72 | 65.1±14.6 | 64.9±15.4 | 0.89 |
| Age category | 0.011 | <0.001 | 0.005 | 0.16 | 0.96 | ||||||||||
| <65 y | 40 (35.7) | 166 (49.7) | 48 (32.1) | 68 (50.9) | 41 (36.9) | 165 (49.3) | 53 (20.7) | 153 (46.4) | 51 (45.9) | 155 (46.3) | |||||
| 65–74 y | 32 (28.6) | 92 (27.5) | 28 (25.0) | 96 (28.7) | 44 (39.6) | 80 (23.9) | 39 (33.6) | 85 (25.8) | 32 (28.8) | 92 (27.5) | |||||
| ≥75 y | 40 (35.7) | 76 (22.8) | 36 (42.9) | 170 (20.4) | 26 (23.4) | 90 (26.9) | 24 (45.7) | 92 (27.9) | 28 (25.2) | 88 (26.3) | |||||
| Female | 30 (26.8) | 82 (37.4) | 0.041 | 31 (27.7) | 124 (37.1) | 0.07 | 36 (32.4) | 119 (35.5) | 0.55 | 19 (16.4) | 136 (41.2) | <0.001 | 30 (27.0) | 125 (37.3) | 0.049 |
| Body mass index, kg/m2 | 29.0±6.8 | 29.2±6.9 | 0.84 | 27.6±6.1 | 29.7±7.0 | 0.005 | 28.9±6.6 | 29.2±7.0 | 0.63 | 28.0±5.7 | 29.6±7.2 | 0.04 | 29.5±6.2 | 29.0±7.1 | 0.54 |
| Race | 0.08 | 0.19 | 0.12 | 0.03 | 0.03 | ||||||||||
| White | 51 (45.5) | 124 (37.1) | 51 (45.5) | 124 (37.1) | 41 (36.9) | 134 (40.9) | 43 (37.1) | 132 (40.0) | 40 (36.0) | 135 (40.3) | |||||
| Black | 14 (12.5) | 36 (10.8) | 6 (5.4) | 44 (13.2) | 11 (9.9) | 39 (11.6) | 12 (10.3) | 38 (11.5) | 21 (18.9) | 29 (8.7) | |||||
| Asian | 18 (16.1) | 38 (11.4) | 14 (12.5) | 42 (12.6) | 8 (7.2) | 48 (14.3) | 24 (20.7) | 32 (9.7) | 12 (10.8) | 44 (13.1) | |||||
| Other | 16 (14.3) | 79 (23.7) | 23 (20.5) | 72 (21.6) | 31 (27.9) | 64 (19.1) | 24 (20.7) | 71 (21.5) | 18 (16.2) | 77 (23.0) | |||||
| Not specified | 13 (11.6) | 57 (17.1) | 18 (16.1) | 52 (15.6) | 20 (18.0) | 50 (14.9) | 13 (11.2) | 57 (17.3) | 20 (18.0) | 50 (14.9) | |||||
| CAD | 41 (36.6) | 53 (15.9) | <0.001 | 44 (39.3) | 50 (15.0) | <0.001 | 25 (22.5) | 69 (20.6) | 0.67 | 30 (25.9) | 64 (19.4) | 0.14 | 19 (17.1) | 75 (22.4) | 0.24 |
| CHF | 19 (17.0) | 19 (4.3) | <0.001 | 26 (23.2) | 12 (3.6) | <0.001 | 7 (6.3) | 31 (9.3) | 0.34 | 12 (10.3) | 26 (7.9) | 0.41 | 5 (4.5) | 33 (9.9) | 0.08 |
| Prior AF | 17 (15.2) | 29 (8.7) | 0.05 | 23 (20.5) | 23 (6.9) | <0.001 | 16 (14.4) | 30 (9.0) | 0.10 | 10 (8.6) | 36 (10.9) | 0.49 | 9 (8.1) | 37 (11.0) | 0.38 |
| Prior stroke | 14 (12.5) | 17 (5.1) | 0.008 | 13 (11.6) | 18 (5.4) | 0.03 | 7 (6.3) | 24 (7.2) | 0.76 | 6 (5.2) | 25 (7.6) | 0.38 | 6 (5.4) | 25 (7.5) | 0.46 |
| Diabetes mellitus | 41 (36.6) | 102 (30.5) | 0.23 | 47 (42.0) | 96 (28.7) | 0.01 | 38 (34.2) | 105 (31.3) | 0.57 | 38 (32.8) | 105 (31.8) | 0.85 | 40 (36.0) | 103 (30.7) | 0.30 |
| Hypertension | 80 (71.4) | 194 (58.1) | 0.01 | 76 (67.9) | 198 (59.3) | 0.11 | 70 (63.1) | 204 (60.9) | 0.68 | 78 (67.2) | 196 (59.4) | 0.14 | 70 (63.1) | 204 (61.0) | 0.68 |
| Pulmonary disease | 28 (25) | 72 (21.6) | 0.40 | 33 (29.5) | 67 (20.1) | 0.04 | 24 (21.6) | 76 (22.7) | 0.82 | 21 (18.1) | 79 (23.9) | 0.20 | 25 (22.5) | 75 (22.4) | 0.98 |
| Renal disease | 19 (17.0) | 25 (7.5) | 0.004 | 29 (25.9) | 15 (4.5) | <0.001 | 10 (9.0) | 34 (10.1) | 0.73 | 20 (17.2) | 24 (7.3) | 0.002 | 8 (7.2) | 36 (10.7) | 0.28 |
| Cirrhosis | 2 (1.8) | 3 (0.9) | 0.43 | 2 (1.8) | 3 (0.9) | 0.43 | 1 (0.9) | 4 (1.2) | 0.80 | 2 (1.7) | 3 (0.9) | 0.48 | 1 (0.9) | 4 (1.2) | 0.80 |
| Active cancer | 10 (8.9) | 19 (5.7) | 0.23 | 6 (5.4) | 23 (6.9) | 0.57 | 13 (11.7) | 16 (4.8) | 0.01 | 8 (6.9) | 21 (6.4) | 0.84 | 5 (4.5) | 24 (7.2) | 0.33 |
| Prior organ transplant | 5 (4.5) | 6 (1.8) | 0.12 | 5 (4.5) | 6 (1.8) | 0.12 | 4 (3.6) | 7 (2.1) | 0.37 | 5 (4.3) | 6 (1.8) | 0.14 | 3 (2.7) | 8 (2.4) | 0.85 |
| Rheumatologic disease | 9 (8.0) | 17 (5.1) | 0.25 | 11 (9.8) | 15 (4.5) | 0.04 | 12 (10.8) | 14 (4.2) | 0.01 | 6 (5.2) | 20 (6.1) | 0.73 | 5 (4.5) | 21 (6.3) | 0.49 |
| Immunosuppressed status | 5 (4.5) | 12 (3.6) | 0.68 | 2 (1.8) | 15 (4.5) | 0.20 | 8 (7.2) | 9 (2.7) | 0.03 | 5 (4.3) | 12 (3.6) | 0.74 | 3 (2.7) | 14 (4.2) | 0.48 |
| Active smoking | 3 (2.7) | 7 (2.1) | 0.72 | 4 (3.6) | 6 (1.8) | 0.27 | 1 (0.9) | 9 (2.7) | 0.27 | 3 (2.6) | 7 (2.1) | 0.77 | 2 (1.8) | 8 (2.4) | 0.72 |
| Home medications | |||||||||||||||
| ARB/ACE use | 43 (38.4) | 100 (29.9) | 0.10 | 30 (26.8) | 113 (33.8) | 0.17 | 36 (32.4) | 107 (31.9) | 0.92 | 41 (35.3) | 102 (30.9) | 0.38 | 42 (37.8) | 101 (30.1) | 0.13 |
| Statins | 63 (56.3) | 115 (34.4) | <0.001 | 56 (50.0) | 122 (36.5) | 0.01 | 49 (44.1) | 129 (38.5) | 0.29 | 51 (44.0) | 127 (38.5) | 0.30 | 46 (41.4) | 132 (39.4) | 0.70 |
| Presenting characteristics | |||||||||||||||
| Hypoxia on presentation | 83 (74.1) | 232 (69.5) | 0.35 | 78 (69.6) | 237 (71.0) | 0.79 | 94 (84.7) | 221 (66.0) | <0.001 | 89 (76.7) | 226 (68.5) | 0.09 | 87 (78.4) | 228 (68.1) | 0.04 |
ACE indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BNP, B‐type natriuretic peptide; CAD, coronary artery disease; CHF, congestive heart failure; CRP, C‐reactive protein; and TnI, troponin.
Biomarker Elevation and Imaging Diagnostics and Treatment
The radiographic and echocardiographic findings of patients stratified by presence of severe biomarker elevation are summarized in Table 2. Compared with patients without severe ferritin level elevation, patients with ferritin level ≥200 ng/mL had increased prevalence of abnormal chest radiographs and bilateral pulmonary infiltrates. Among patients who received echocardiograms, patients with BNP ≥300 pg/nL had lower left ventricular ejection fractions and higher prevalence of right ventricular dysfunction compared with patients with BNP <300 pg/nL. There was no significant difference in left ventricular ejection fraction or right ventricular function among patients with or without severe elevation of the other biomarkers. A comparison of the antiviral and anti‐inflammatory therapy administered to patients with or without severe biomarker elevation is summarized in Table 3. Across all groups of patients with severe biomarker elevations, significantly higher proportion of patients received steroid therapy.
Table 2.
Imaging Findings and Treatment Stratified by Biomarker Levels
|
TnI ≥0.34 ng/mL (n=112) |
TnI <0.34 ng/mL (n=334) |
P Value |
BNP ≥300 pg/mL (n=112) |
BNP <300 pg/mL (n=334) |
P Value |
CRP ≥84 mg/dL (n=112) |
CRP <84 mg/dL (n=334) |
P Value |
Ferritin ≥2000 ng/mL (n=116) |
Ferritin <2000 ng/mL (n=330) |
P Value |
d‐Dimer ≥6106 ng/mL (n=111) |
d‐Dimer <6106 ng/mL (n=335) |
P Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imaging findings | |||||||||||||||
| Abnormal chest radiograph no./total no. (%) | 102 (91.1) | 301 (90.1) | 0.77 |
99 (88.4) |
304 (91.0) | 0.42 | 302 (90.1) | 101 (91.0) | 0.80 | 111 (95.7) | 292 (88.5) | 0.02 | 103 (92.8) | 300 (89.6) | 0.32 |
| Bilateral infiltrate | 87 (77.7) | 273 (81.7) | 0.35 | 87 (77.7) | 273 (81.7) | 0.35 | 96 (86.5) | 264 (78.8) | 0.08 | 102 (87.9) | 258 (78.2) | 0.02 | 96 (86.5) | 264 (78.8) | 0.08 |
| Pleural effusion | 7 (6.3) | 24 (7.2) | 0.74 | 10 (8.9) | 21 (6.3) | 0.34 | 10 (9.0) | 21 (6.3) | 0.33 | 6 (5.2) | 25 (7.6) | 0.38 | 5 (4.5) | 26 (7.8) | 0.24 |
| Lowest LVEF during hospitalization, %, median (IQR) | 60.5 (47.5–67) | 62.5 (55–67.5) | 0.30 | 57.5 (42.5–62.5) | 62.5 (57.5–69.0) | <0.001 | 59.0 (48.0–67.5) | 62.5 (55.0–66.0) | 0.51 | 58.8 (48.5–65.0) | 62.5 (57.0–67.5) | 0.23 | 62.5 (52.5–69.0) | 62.0 (48.0–65.0) | 0.26 |
| Decreased RV function, no./total no. (%) | 7 (13.7) | 6 (11.5) | 0.74 | 10 (22.2) | 3 (5.2) | 0.01 | 7 (17.5) | 6 (9.5) | 0.24 | 7 (15.6) | 6 (10.3) | 0.43 | 4 (9.5) | 9 (14.8) | 0.43 |
| Medical treatments | |||||||||||||||
| Hydroxychloroquine | 99 (88.4) | 282 (84.4) | 0.30 | 98 (87.5) | 283 (84.7) | 0.47 | 103 (92.8) | 278 (83.0) | 0.01 | 107 (92.2) | 274 (83.0) | 0.02 | 100 (90.1) | 281 (83.9) | 0.11 |
| Remdesivir | 4 (3.6) | 21 (6.3) | 0.28 | 6 (5.4) | 19 (5.7) | 0.90 | 11 (9.9) | 14 (4.2) | 0.02 | 9 (7.8) | 16 (4.8) | 0.24 | 7 (6.3) | 18 (5.4) | 0.71 |
| Steroids | 54 (48.2) | 104 (31.1) | 0.001 | 48 (42.9) | 110 (32.9) | 0.06 | 56 (50.5) | 102 (30.4) | <0.001 | 61 (52.6) | 97 (29.4) | <0.001 | 62 (55.9) | 96 (28.7) | <0.001 |
| Il‐6 inhibitor | 9 (8.0) | 31 (9.3) | 0.69 | 6 (5.4) | 34 (10.2) | 0.12 | 7 (6.3) | 33 (9.9) | 0.26 | 11 (9.5) | 29 (8.8) | 0.82 | 20 (18.0) | 20 (6.0) | <0.001 |
| Intravenous gamma globulin | 2 (1.8) | 4 (1.2) | 0.64 | 1 (0.9) | 5 (1.5) | 0.63 | 2 (1.8) | 4 (1.2) | 0.63 | 2 (1.7) | 4 (1.2) | 0.68 | 2 (1.8) | 4 (1.2) | 0.63 |
BNP indicates B‐type natriuretic peptide; CRP, C‐reactive protein; IQR, interquartile range; LVEF, left ventricular ejection fraction; RV, right ventricle; and TnI, troponin.
Table 3.
Arrhythmias and Hospital Outcomes Stratified by Biomarker Levels
|
TnI ≥0.34 ng/mL (n=112) |
TnI <0.34 ng/mL (n=334) |
P Value |
BNP ≥300 pg/mL (n=112) |
BNP <300 pg/mL (n=334) |
P Value |
CRP ≥84 mg/dL (n=112) |
CRP <84 mg/dL (n=334) |
P Value |
Ferritin ≥2000 ng/mL (n=116) |
Ferritin <2000 ng/mL (n=330) |
P Value |
d‐Dimer ≥6106 ng/mL (n=111) |
d‐Dimer <6106 ng/mL (n=335) |
P Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICU admission | 87 (77.7) | 150 (44.9) | <0.001 | 73 (65.2) | 164 (49.1) | 0.003 | 84 (75.7) | 153 (45.7) | <0.001 | 83 (71.6) | 154 (46.7) | <0.001 | 93 (83.8) | 144 (43.0) | <0.001 |
| Hypotension requiring vasopressor therapy | 83 (74.1) | 139 (41.7) | <0.001 | 70 (63.1) | 152 (45.5) | 0.001 | 82 (73.9) | 140 (41.9) | <0.001 | 78 (67.2) | 144 (43.8) | <0.001 | 92 (83.6) | 130 (38.8) | <0.001 |
| Respiratory failure requiring mechanical ventilation | 84 (75.0) | 143 (42.8) | <0.001 | 70 (62.5) | 157 (47.0) | 0.005 | 83 (74.8) | 144 (43.0) | <0.001 | 79 (68.1) | 148 (44.8) | <0.001 | 93 (83.8) | 134 (40.0) | <0.001 |
| Bacteremia | 34 (30.4) | 36 (10.8) | <0.001 | 27 (24.1) | 43 (12.9) | 0.005 | 25 (22.5) | 45 (13.4) | 0.02 | 22 (19.0) | 48 (14.5) | 0.26 | 30 (27.0) | 40 (11.9) | <0.001 |
| Venous thromboembolism | 12 (10.7) | 30 (9.0) | 0.59 | 11 (9.8) | 31 (9.3) | 0.87 | 17 (15.3) | 25 (7.5) | 0.01 | 12 (10.3) | 30 (9.1) | 0.69 | 27 (24.3) | 15 (4.5) | <0.001 |
| Stroke/TIA | 4 (3.6) | 7 (2.1) | 0.38 | 4 (3.6) | 7 (2.1) | 0.38 | 3 (2.7) | 8 (2.4) | 0.83 | 6 (5.2) | 5 (1.5) | 0.03 | 6 (5.4) | 5 (1.5) | 0.021 |
| Acute kidney injury requiring new RRT | 8 (7.1) | 7 (2.1) | 0.01 | 12 (10.7) | 3 (0.9) | <0.001 | 2 (1.8) | 13 (3.9) | 0.29 | 11 (9.5) | 4 (1.2) | <0.001 | 3 (2.7) | 12 (3.6) | 0.656 |
| Atrial arrhythmias | 41 (36.6) | 61 (18.3) | <0.001 | 41 (36.6) | 61 (18.3) | <0.001 | 34 (30.6) | 68 (20.3) | 0.03 | 37 (31.9) | 65 (19.7) | 0.007 | 28 (25.2) | 74 (22.1) | 0.500 |
| Ventricular arrhythmias | 41 (36.6) | 59 (17.7) | <0.001 | 34 (30.4) | 66 (19.8) | 0.02 | 35 (31.5) | 65 (19.4) | 0.008 | 29 (25.0) | 71 (21.5) | 0.44 | 37 (33.3) | 63 (18.8) | 0.002 |
| Death | 51 (45.5) | 44 (13.2) | <0.001 | 37 (33.0) | 58 (17.4) | 0.001 | 27 (24.3) | 68 (20.3) | 0.37 | 38 (32.8) | 57 (17.3) | 0.001 | 35 (31.5) | 60 (17.9) | 0.002 |
BNP indicates B‐type natriuretic peptide; CRP, C‐reactive protein; ICU, intensive care unit; RRT, renal replacement therapy; TIA, transient ischemic attack; and TnI, troponin.
Overall, cardiac catheterization was performed in 3 patients because of troponin elevation. One patient had ST‐segment–elevation myocardial infarction and underwent coronary angiography, which revealed a culprit right coronary artery lesion, which was successfully stented. Another patient had unstable angina and non–ST‐segment–elevation myocardial infarction and was found to have triple vessel coronary artery disease. Coronary artery bypass graft surgery was planned but the patient developed COVID‐19‐associated respiratory failure, staphylococcal aureus pneumonia, complete heart block requiring pacemaker implantation, and died of multisystem organ failure. The third patient developed new cardiomyopathy and severe eosinophilia and underwent right heart catheterization and endomyocardial biopsy, which was negative for myocarditis.
Biomarker Elevation and Outcomes
Hospital outcomes stratified by presence of severe biomarker elevation are shown in Table 4. The presence of severe elevation of all 5 biomarkers was associated with increased intensive care unit admission, hypotension requiring vasopressor support, and respiratory failure requiring mechanical support. Compared with patients without severe troponin elevation, patients with troponin level ≥0.34 ng/mL had significantly more atrial tachyarrhythmias (36.6% versus 18.3%; P<0.001), ventricular tachyarrhythmias (36.6% versus 17.7%; P<0.001), and 30‐day in‐hospital mortality (45.5% versus 13.2%; P<0.001). Severe elevation of BNP was also associated with higher prevalence of arrhythmias and death. Severe elevations of ferritin and d‐dimer were associated with increased death. The receiver operating characteristic curves for the 5 biomarker levels and their association with 30‐day in‐hospital mortality are shown in Figure 2. Of the studied biomarkers, troponin levels had the highest area under the receiver operating characteristic curve of 0.789 (95% CI, 0.739–0.838) for association with 30‐day mortality. The univariate and multivariable predictors of 30‐day in‐hospital mortality are shown in Table 4. Age and hypoxia on presentation were independently associated with mortality. Among the biomarkers, only severe elevation of troponin (adjusted odds ratio, 4.38: 95% CI, 2.32–8.28; P<0.001) was significantly associated with mortality after adjustment for comorbidities.
Table 4.
Univariate and Multivariable Predictors of 30‐Day In‐Hospital Mortality
| Variable |
Unadjusted OR (95% CI) |
P Value |
Adjusted OR (95% CI) |
P Value |
|---|---|---|---|---|
| TnI ≥0.34 ng/mL | 5.47 (3.31–9.04) | <0.001 | 4.38 (2.32–8.28) | <0.001 |
| Age | ||||
| 75 y | 13.0 (6.37–26.4) | <0.001 | 15.6 (6.20–39.2) | <0.001 |
| 65–74 y | 4.70 (2.23–9.91) | <0.001 | 4.38 (2.32–8.28) | <0.001 |
| <65 y | Reference | Reference | ||
| Hypoxia upon presentation | 2.74 (1.46–5.14) | 0.002 | 3.24 (1.49–7.02) | 0.003 |
| BNP pg/ml ≥295 | 1.94 (1.17–3.22) | 0.01 | ||
| CRP mg/dl ≥118 | 1.05 (0.61–1.80) | 0.87 | ||
| Ferritin ng/ml ≥2000 | 1.95 (1.18–3.21) | 0.009 | ||
| d‐dimer ng/ml ≥6990 | 2.11 (1.27–3.49) | 0.004 | ||
| BMI | 0.96 (0.92–1.00) | 0.03 | ||
| White race | 0.61 (0.38–0.99) | 0.04 | ||
| Coronary artery disease | 2.66 (1.58–4.46) | <0.001 | ||
| Congestive heart failure | 2.08 (1.01–4.32) | 0.049 | ||
| Prior history of AF | 2.81 (1.46–5.40) | 0.002 | ||
| Hypertension | 2.41 (1.40–4.15) | 0.002 | ||
| Renal disease | 1.89 (0.94–3.79) | 0.07 | ||
| Prior organ transplant | 3.64 (1.09–12.2) | 0.04 | ||
| Immunosuppression | 3.10 (1.15–8.40) | 0.03 | ||
| Baseline statin use | 1.67 (1.04–2.68) | 0.03 | ||
AF indicates atrial fibrillation; BMI, body mass index; BNP, B‐type natriuretic peptide; CRP, C‐reactive protein; OR, odds ratio; and TnI, troponin.
Figure 2. Receiver operating characteristic curves for biomarker levels and 30‐day in‐hospital mortality.
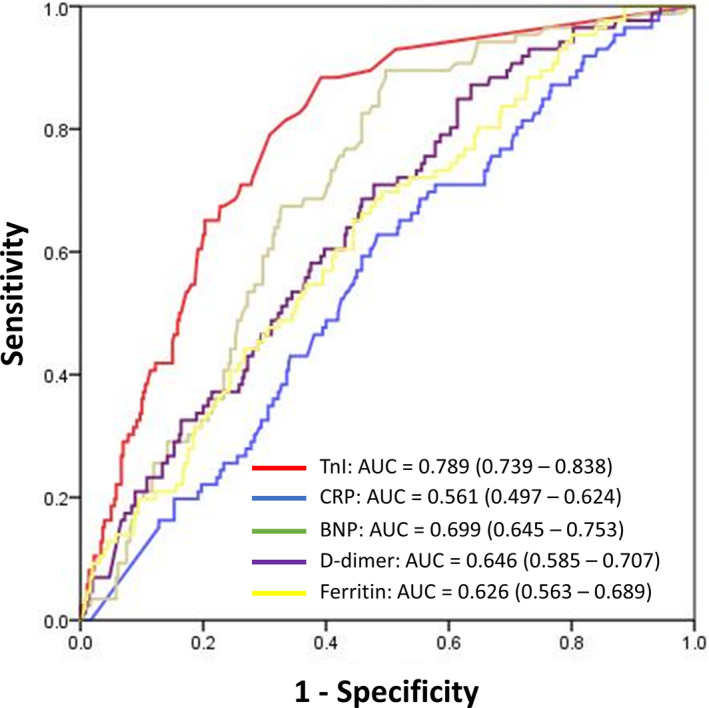
Graph lists AUC for respective curves and 95% CI. AUC indicates area under the curve; BNP, B‐type natriuretic protein; CRP, C‐reactive protein; and TnI, troponin I.
COVID‐19 Mortality Risk Score: HA2T2
A mortality risk score was developed using a multivariable logistic regression model for 30‐day mortality using the 3 independent predictors of hypoxia on presentation, age category, and troponin level ≥0.34 ng/mL. The HA2T2 mortality risk score was calculated for each patient by summing points assigned to each predictor: 2 points for severe troponin elevation, 1 point for age 65 to 74 years and 2 points for age ≥75 years, and 1 point for hypoxia upon presentation (minimum risk score 0 and maximum risk score 5). Among the derivation cohort of 446 patients, patients with a HA2T2 score of 0 (n=59) had a 30‐day mortality of 0.0% while patients with a HA2T2 score of 5 (n=29) had a 30‐day mortality of 65.5% (P‐for‐trend <0.001) (Figure 3). Overall, patients with a HA2T2 score of <3 had a 30‐day in‐hospital mortality of 5.9% while patients with a HA2T2 score of ≥3 had a 30‐day mortality of 43.7%. The survival curves of patients with HA2T2 score ≥3 and <3 are compared in Figure 4. The C‐statistic for the HA2T2 score and 30‐day mortality was 0.834 (95% CI, 0.792–0.876). Using the independent validation cohort of 440 patients who had at least 1 troponin level checked but were missing at least 1 of the other 4 biomarkers, the C‐statistic was 0.784 (95% CI, 0.729–0.838). Validation with the bootstrapping method yielded a C‐statistic of 0.792 (95% CI, 0.733–0.843). Good agreement between the observed and predicted risk was seen in both the derivation and validation cohorts (Figure 5).
Figure 3. The HA2T2 score and its association with 30‐day in‐hospital mortality.
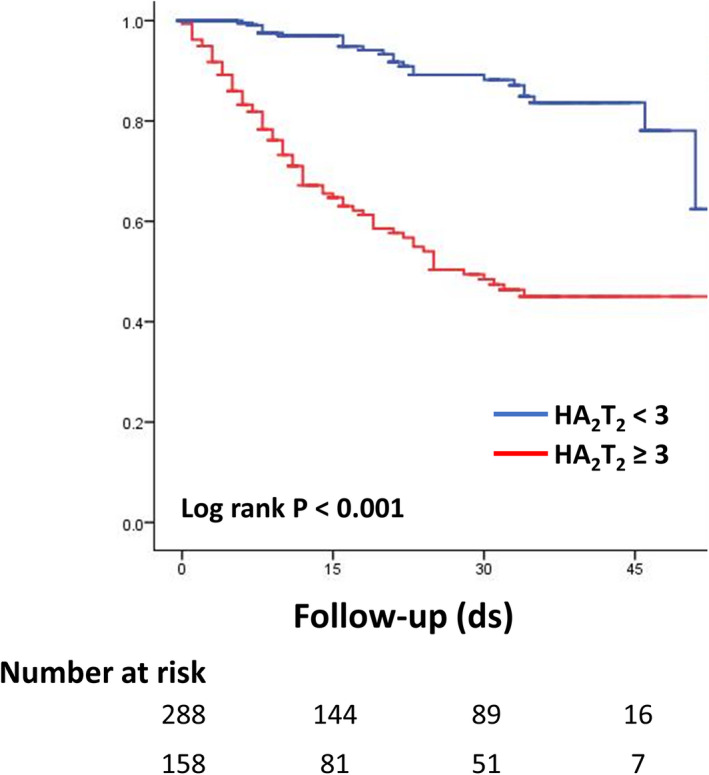
The HA2T2 score is calculated as follows: hypoxia upon presentation (defined as requiring supplemental oxygenation within 3 hours of arrival in emergency department)=1 point; age=1 point if 65 to 74 years and 2 points if ≥75 years; troponin 0.34 ng/mL ≥2 points. ds indicates days.
Figure 4. Kaplan‐Meier survival curves of patients hospitalized with coronavirus disease 2019 stratified by presence or absence of HA2T2 score ≥3.
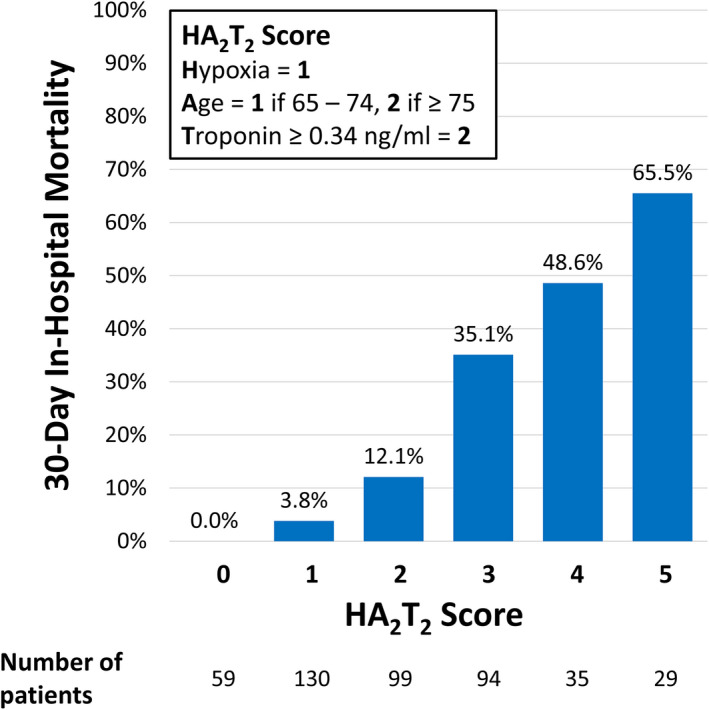
Figure 5. Calibration plots of predicted probability vs observed probability of 30‐day in‐hospital mortality for the HA2T2 risk score model.
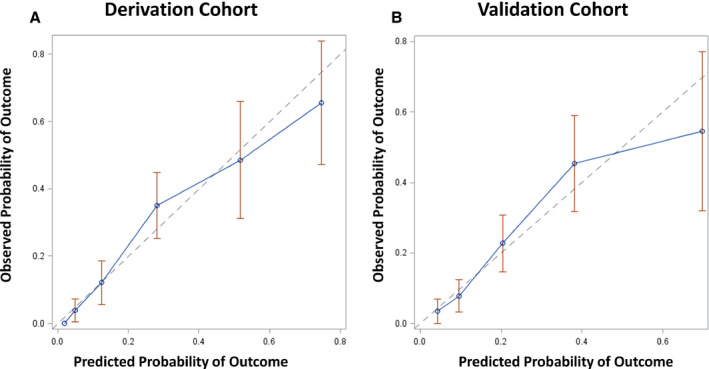
A, Calibration plot for derivation cohort (n=446). The calibration slope is 0.954 (SE 0.101) and calibration‐in‐large is 0.009 (SE 0.028). B, Calibration plot for validation cohort (n=440). The calibration slope is 0.952 (SE 0.162) and calibration‐in‐large is 0.008 (SE 0.038).
Discussion
In this study of biomarkers and mortality in a multicenter cohort of patients hospitalized with COVID‐19, we identified several important findings. First, abnormal levels of troponin, BNP, CRP, ferritin, and d‐dimer are common among patients hospitalized with COVID‐19. Second, biomarker level elevation tracked with complications such as hypotension requiring vasopressor therapy, respiratory failure requiring mechanical ventilation, arrhythmias, and death. Third, age, hypoxia on presentation, and severe troponin elevation defined as ≥0.34 ng/mL were independently associated with 30‐day in‐hospital mortality. Based on these 3 risk factors, the HA2T2 score was developed and validated to predict 30‐day mortality with acceptable discriminative capacity. Notably, patients with HA2T2 score ≥3 had >40% 30‐day mortality while those with a HA2T2 score <3 had <6% 30‐day mortality.
Biomarker Elevation and Severe COVID‐19
Elevation of multiple biomarkers has been shown to correlate with severe COVID‐19. Troponin level elevation has been reported in 7.2% to 36% of patients hospitalized with COVID‐19. 5 , 6 , 7 , 8 In addition, increased levels of inflammatory biomarkers such as procalcitonin, ferritin, erythrocyte sedimentation rate, CRP, and IL‐6 have been associated with severe COVID‐19. 8 Finally, severe d‐dimer elevation has also been reported, which is consistent with observations that COVID‐19 is associated with increased rates of thrombotic complications. 12 These findings support the notion that severe COVID‐19 is a systemic disease process that likely involves multiple mechanisms including myocardial injury, inflammation, endothelial injury, and thrombosis. 2 , 3 , 4 Indeed, we found significant elevations across 4 of the 5 studied biomarkers in the majority of patients studied in the derivation cohort. However, the relative prognostic significance of these biomarker level elevations in COVID‐19 had not been previously elucidated. In our study, although severe elevations of BNP, CRP, ferritin, and d‐dimer were associated with mortality, severe elevation of troponin was the only independent predictor of death among the biomarkers, as the presence of TnI ≥0.34 ng/mL was associated with an adjusted odds ratio >4 for 30‐day in‐hospital mortality.
To our knowledge, our study is the first to compare troponin with other biomarkers to assess its relative association with COVID‐associated mortality. Based on our findings, myocardial injury appears to be a common pathway through which COVID‐19 can lead to mortality. Whether myocardial injury is predominantly the proximate cause or simply a marker of COVID‐19‐associated mortality is unclear. The mechanisms of troponin elevation associated with COVID‐19 include microvascular injury, stress cardiomyopathy, acute coronary syndrome, hypoxia with supply–demand mismatch, systemic inflammatory response, and direct viral injury. 13 Although there have been case reports of COVID‐19‐associated myocarditis diagnosed with cardiac magnetic resonance imaging and endomyocardial biopsy, 14 , 15 its incidence is unclear. Furthermore, while some patients with COVID‐19 with troponin elevation and ST‐segment elevation have obstructive coronary disease, a substantial proportion of these patients do not undergo coronary angiography. 16 In our study, only 2 patients underwent coronary angiography, with 1 patient undergoing percutaneous coronary revascularization for ST‐segment–elevation myocardial infarction. This suggests that in the vast majority of patients in our cohort who had troponin elevation, coronary angiography was not pursued because it was not felt that revascularization would result in significant clinical benefit. In our study cohort, among the patients who underwent echocardiographic imaging, there were no significant differences in the frequency of left or right ventricular dysfunction among patients with or without severe troponin elevation.
COVID‐19 Mortality Risk Calculation
Currently, several clinical variables, laboratory findings, and chest radiographic findings have been proposed for use in prediction tools to determine the risk of severe COVID‐19 progression and mortality. Liang et al developed a risk tool using 10 variables including age, comorbidities, radiographic findings, lactic dehydrogenase levels, neutrophil levels, and direct bilirubin levels to estimate the risk of COVID‐19‐associated critical illness. 17 Similarly, Galloway et al proposed a risk tool with 12 variables that incorporated age, radiographic findings, neutrophil levels, CRP, and albumin to determine risk of intensive care unit admission or death caused by COVID‐19. 18 Using only the variables of age, hypoxia upon presentation, and troponin level, the HA2T2 score cut‐off of 3 can differentiate patients at low versus high risk of in‐hospital mortality. Although it has been argued that troponin measurement in patients with COVID‐19 has limited utility in that it often cannot be used to determine the need for coronary intervention, 19 it has become clear that troponin levels can be used as an important prognostic marker. 20 It should be noted that low‐level troponin elevation among patients hospitalized with COVID‐19 is very common, but only a relatively high cut‐off value of 0.34 ng/mL for TnI (75th percentile for our derivation cohort patients) had the greatest prognostic value.
Clinical Implications
Although elevation across a broad range of biomarkers appears to be associated with COVID‐19 severity, our study shows that troponin is the most potent predictor of mortality. Based on our receiver operating characteristic analysis, the utility of tracking a wide range of biomarkers other than troponin for prognostication purposes may be limited. With the simple HA2T2 score, clinicians can highlight patients at high risk for critical illness and clinical deterioration. Further studies can be conducted to assess whether rapid institution of antiviral therapies such as remdesivir or anti‐inflammatory agents such as dexamethasone in patients with high HA2T2 scores can lower mortality. The COVID‐19‐associated mortality score may also be used to guide prognostication. For patients with the combination of advanced age, significant comorbidities, and a high HA2T2 score, this tool can be used to aid in goals‐of‐care discussion.
Study Limitations
This is a retrospective study relying on manual chart abstraction for its data, which may be susceptible to error or misinterpretation. Next, because of its retrospective nature, we were unable to perform a comprehensive review of other biomarkers of interest such as IL‐6 and procalcitonin. Furthermore, given that biomarkers were not checked as part of a prospective protocol, there was likely selection bias because patients with multiple biomarkers checked are more likely to have greater disease severity than patients who had fewer biomarkers measured. In our study, 446 (42.3%) of the 1053 patients admitted during the study period had all 5 biomarkers measured, but 886 (84.1%) had a least 1 troponin measured, which allowed us to validate our mortality risk score on a sizeable cohort. However, a larger validation cohort using a completely independent patient sample (eg, patients from a distinct institution) would have provided a more robust assessment of the predictive value of the HA2T2 mortality risk score. We categorized patients on the basis of the maximum biomarker level measured, which can affect our results depending on when during the hospital course the level was found and how often patients had biomarkers serially measured. We did not track biomarker levels over time to analyze ranges and trends for each patient. Next, we did not assign specific causes of death in our study. Furthermore, because 24.4% of the study patients remained hospitalized at the time of closure of study data collection, we focused on a 30‐day in‐hospital mortality end point instead of an overall mortality end point over time. Our study did not examine out‐of‐hospital deaths after discharge from COVID‐19 hospitalization. Finally, this study covered a time period during which New York City was severely affected by COVID. Since then, advances and changes in treatment approaches may have reduced mortality rates among patients hospitalized with COVID‐19, which may affect the generalizability of our findings. For example, in our study, 85%, 35%, and 6% of patients were treated with hydroxychloroquine, steroids, and remdesivir, respectively. With the publication of several studies showing a mortality benefit with systemic steroid use among critically ill patients with COVID‐19, 21 current levels of steroid use in this patient population are likely much higher.
Conclusions
Elevated levels of troponin and other biomarkers are common in patients hospitalized with COVID‐19. High biomarker levels track with hospital complications such as intensive care unit admission, respiratory failure, arrhythmias, and death. High troponin levels are a particularly potent predictor of 30‐day in‐hospital mortality. Use of a simple risk score, which incorporates troponin levels, age, and presence of hypoxia on presentation, can help stratify patients at risk for in‐hospital mortality associated with COVID‐19.
Sources of Funding
Dr Goyal is supported by American Heart Association grants 18IPA34170185 and 20CDA35310455. This study received support from New York‐Presbyterian Hospital (NYPH) and Weill Cornell Medical College (WCMC), including the Clinical and Translational Science Center (CTSC) (UL1 TR000457) and Joint Clinical Trials Office (JCTO).
Disclosures
Dr Cheung has received consulting fees from Abbott, Biosense Webster, Biotronik, and Boston Scientific and fellowship grant support from Abbott, Biosense Webster, Biotronik, Boston Scientific, and Medtronic. Dr Safford has received research grant support from Amgen. The remaining authors have no disclosures to report.
Supporting information
Figure S1
Acknowledgments
We thank the following Weill Cornell Medicine medical students for their contributions to the COVID‐19 Registry through medical chart abstraction: Zara Adamou, BA, Haneen Aljayyousi, BA, Mark N. Alshak, BA (student leader), Elena Beideck, BS, Orrin S. Belden, MD/MBA, Anthony F. Blackburn, BS, Joshua W. Bliss, PharmD, Kimberly A. Bogardus, BA, Chelsea D. Boydstun, BA, Clare A. Burchenal, MPH, Eric T. Caliendo, BS, John K. Chae, BA, David L. Chang, BS, Frank R. Chen, BS, Kenny Chen, BA, Andrew Cho, PhD, Alice Chung, BA, Alisha N. Dua, MRes, Andrew Eidelberg, BS, Rahmi S. Elahjji, BA, Mahmoud Eljalby, MMSc, Emily R. Eruysal, BS, Kimberly N. Forlenza, MSc, Rana Khan Fowlkes, BA, Rachel L. Friedlander, BA, Gary George, BS, Shannon Glynn, BS, Leora Haber, BA, Janice Havasy, BS, Alex Huang, BA, Hao Huang, BS, Jennifer H. Huang, BS, Sonia Iosim, BS, Mitali Kini, BS, Rohini V. Kopparam, BS, Jerry Y. Lee, BA, Mark Lee, BS, BA, Aretina K. Leung, BA, Bethina Liu, AB, Charalambia Louka, BS, Brienne Lubor, BS, Dianne Lumaquin, BS, Matthew L. Magruder, BA, Ruth Moges, MSc, Prithvi M. Mohan, BS, Max F. Morin, BS, Sophie Mou, BA, J. J. Nario, BS, Yuna Oh, BS, Noah Rossen, BA, Emma M. Schatoff, PhD, Pooja D. Shah, BA, Sachin P. Shah, BA, Daniel Skaf, BS, Shoran Tamura, BS, Ahmed Toure, BA, Camila M. Villasante, BA, Gal Wald, BA, Graham T. Wehmeyer, BS (student leader), Samuel Williams, BA, Ashley Wu, BS, Andrew L. Yin, BA, and Lisa Zhang, BA.
(J Am Heart Assoc.2021;10:e018477. DOI: 10.1161/JAHA.120.018477.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018477
For Sources of Funding and Disclosures, see page 11.
References
- 1. Center for Systems Science and Engineering, Johns Hopkins University . COVID dashboard. https://coronavirus.jhu.edu/map.html. Accessed September 15, 2020.
- 2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS,Manson JJ; Hlh Across Speciality Collaboration UK . COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;41:2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID‐19 infection. J Am Coll Cardiol. 2020;76:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeng F, Huang Y, Guo Y, Yin M, Chen X, Xiao L, Deng G. Association of inflammatory markers with the severity of COVID‐19: a meta‐analysis. Int J Infect Dis. 2020;96:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med. 2020;382:2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atri D, Siddiqi HK, Lang J, Nauffal V, Morrow DA, Bohula EA. COVID‐19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5:518–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:819–824. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim IC, Kim JY, Kim HA, Han S. COVID‐19‐related myocarditis in a 21‐year‐old female patient. Eur Heart J. 2020;41:1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, et al. ST‐segment elevation in patients with Covid‐19—a case series. N Engl J Med. 2020;382:2478–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang W, Liang H, Ou L, Chen B, Chen A, Li C, Li Y, Guan W, Sang L, Lu J, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID‐19. JAMA Intern Med. 2020;180:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galloway JB, Norton S, Barker RD, Brookes A, Carey I, Clarke BD, Jina R, Reid C, Russell MD, Sneep R, et al. A clinical risk score to identify patients with COVID‐19 at high risk of critical care admission or death: an observational cohort study. J Infect. 2020;81:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Januzzi JL. Troponin and BNP Use in COVID‐19. 2020. https://www.acc.org/latest‐in‐cardiology/articles/2020/03/18/15/25/troponin‐and‐bnp‐use‐in‐covid19. Accessed June 28, 2020.
- 20. Chapman AR, Bularga A, Mills NL. High‐sensitivity cardiac troponin can be an ally in the fight against COVID‐19. Circulation. 2020;141:1733–1735. [DOI] [PubMed] [Google Scholar]
- 21. Group TWREAfC‐TW . Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324:1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


