Abstract
Background
Several randomized trials have compared the patency of coronary artery bypass conduits. All of the published studies, however, have performed pairwise comparisons and a comprehensive evaluation of the patency rates of all conduits has yet to be published. We set out to investigate the angiographic patency rates of all conduits used in coronary bypass surgery by performing a network meta‐analysis of the current available randomized evidence.
Methods and Results
A systematic literature search was conducted for randomized controlled trials comparing the angiographic patency rate of the conventionally harvested saphenous vein, the no‐touch saphenous vein, the radial artery (RA), the right internal thoracic artery, or the gastroepiploic artery. The primary outcome was graft occlusion. A total of 4160 studies were retrieved of which 14 were included with 3651 grafts analyzed. The weighted mean angiographic follow‐up was 5.1 years. Compared with the conventionally harvested saphenous vein, both the RA (incidence rate ratio [IRR] 0.54; 95% CI, 0.35–0.82) and the no‐touch saphenous vein (IRR 0.55; 95% CI, 0.39–0.78) were associated with lower graft occlusion. The RA ranked as the best conduit (rank score for RA 0.87 versus 0.85 for no‐touch saphenous vein, 0.23 for right internal thoracic artery, 0.29 for gastroepiploic artery, and 0.25 for the conventionally harvested saphenous vein).
Conclusions
Compared with the conventionally harvested saphenous vein, only the RA and no‐touch saphenous vein grafts are associated with significantly lower graft occlusion rates. The RA ranks as the best conduit.
Registration
URL: https://www.crd.york.ac.uk/prospero; Unique identifier: CRD42020164492.
Keywords: coronary artery bypass, coronary artery bypass graft, coronary artery disease
Subject Categories: Cardiovascular Surgery, Clinical Studies, Meta Analysis, Quality and Outcomes, Revascularization
Nonstandard Abbreviations and Acronyms
- CON‐SV
conventionally harvested saphenous vein
- GEA
gastroepiploic artery
- IRR
incidence rate ratio
- NT‐SV
no‐touch saphenous vein
- RA
radial artery
- RITA
right internal thoracic artery
Clinical Perspective
What Is New?
The radial artery and the no‐touch saphenous vein have significantly better patency rate compared with the conventional saphenous vein.
The radial artery ranks as the best conduit.
What Are the Clinical Implications?
As no other large angiographic randomized trial is currently underway and owing to the contradicting results of the trials evaluating the clinical outcomes of patients with coronary artery bypass grafting based on graft type, our results will inform surgeons' decisions and guidelines on grafting strategy and conduit selection.
Coronary artery bypass grafting (CABG) remains the most commonly performed cardiac operation. 1 Several arterial and venous conduits can be used to complement the gold standard internal thoracic artery to left anterior descending anastomosis during CABG.
In the past, multiple observational studies have compared the patency rates of the various conduits. 2 , 3 , 4 , 5 The inherent bias of the observational series relies on the fact that angiography is limited to symptomatic patients who typically represent only a small proportion of the populations and the patency results obtained cannot be extrapolated to the majority of the patients.
Several randomized trials (RCTs) have compared the patency of different conduits. RCTs overcome treatment allocation biases through the use of randomization and have per‐protocol angiographic follow‐up, so that results are more generalizable. However, all published studies to date have performed pairwise comparisons and a comprehensive evaluation of the patency rates of all conduits used for CABG has not been published.
We have performed a network meta‐analysis of the RCTs comparing all the conduits currently used for CABG surgery in order to inform evidence‐based decision on grafting strategy.
METHODS
Ethical approval of this analysis was not required as no human subjects were involved. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Search Strategy
A medical librarian (M.D.) performed a comprehensive search to identify RCTs that compared the conventionally‐harvested saphenous vein (CON‐SV), the no‐touch saphenous vein (NT‐SV), the radial artery (RA), the right internal thoracic artery (RITA), or the gastroepiploic artery (GEA). Searches were done on November 11, 2019 in the following databases: Ovid MEDLINE, Ovid EMBASE, and the Cochrane Library. The search strategy included the terms "radial artery," “internal mammary artery,” “internal thoracic artery,” “gastroepiploic artery,” and "saphenous vein." The full search strategy is available in Table S1. This review was registered with the PROSPERO register of systematic reviews (CRD42020164492).
Study Selection and Quality Assessment
Searches across the chosen databases retrieved 6723 studies. After results were de‐duplicated, 2 independent reviewers (N.B.R and Y.R.) screened a total of 4160 citations. Discrepancies were resolved by consensus and opinion of a third author (M.G.). Titles and abstracts were reviewed against predefined inclusion and exclusion criteria. Articles were considered for inclusion if they were written in English and were RCTs comparing angiographic patency for at least 2 of the 5 conduits in patients with CABG. Animal studies, case reports, conference presentations, editorials, expert opinions, observational studies, and studies not defining or reporting the outcomes of interest were excluded.
The full text was pulled for the selected studies for a second round of eligibility screening. Reference lists of articles selected for inclusion were also searched for relevant articles. The full preferred reporting items for systematic reviews and meta‐analyses flow diagram outlining the study selection process are available in Figure S1. For overlapping studies, the study with the longest angiographic follow‐up was included. Two investigators performed data extraction independently (N.B.R. and Y.R.), and a third investigator verified the extracted data for accuracy (M.G). The following variables were included: study demographics (sample size, publication year, institution, and country), patient demographics (age, sex, and comorbidities), imaging and procedure‐related variables (trial definition of graft occlusion, completeness of angiographic follow‐up, method of imaging used, graft configuration, details of proximal and distal anastomoses, use of off‐pump CABG, and severity of the target vessel stenosis). The quality of the included trials was assessed using the Cochrane Collaboration's tool for assessing risk of bias (Table S2).
The primary outcome was graft occlusion at protocol‐defined angiographic follow‐up. An additional analysis was performed for late mortality.
Statistical Analysis
For the outcomes, the incidence rate with underlying Poisson process was used to account for different follow‐up times among the studies with the total number of events observed within a treatment group calculated out of the total person‐time follow‐up for that treatment group. Random effect network meta‐analysis was performed using the generic inverse variance method with the “netmeta” statistical package in R with CON‐SV as reference. The Cochran's Q statistic was used to assess inconsistency using the decomposition approach. Rank scores with probability ranks of different treatment groups were calculated for the primary outcome. Ranks closer to 1 indicate the probability that the treatment group leads to the greatest reduction in graft occlusion.
Subgroup analyses were performed for studies with duration of follow‐up ≥5 years versus <5 years, target vessel stenosis ≥70% versus <70%, and completeness of angiographic follow‐up ≥50% versus <50% of patients, for studies that used computed tomography angiography as imaging technique, and for studies that used a similar definition for graft occlusion (ie, occlusion defined as lack of visual opacification of the graft).
Meta‐regression was used to explore the effect on the primary outcome of age, sex, hypertension, diabetes mellitus, dyslipidemia, target vessel stenosis, duration of follow‐up, completeness of angiographic follow‐up, percentage of proximal anastomoses on the ascending aorta, percentage of grafts to the circumflex coronary system, and use of off‐pump CABG. Leave‐one‐out analysis was performed to assess robustness of the main analysis. Net heat plot was used to evaluate for inconsistency in the network model (Figure S2).
Heterogeneity was reported as low (I2=0–25%), moderate (I2=26–50%), or high (I2 >50%).
For hypothesis testing purposes, we built 95% CIs without multiplicity adjustment. Although this approach clearly leads to increased risk of type I error, multiplicity adjustment is not routinely recommended in meta‐analytical research. 6 All statistical analyses were performed using R (version 3.3.3, R Project for Statistical Computing).
RESULTS
A total of 4160 studies were retrieved of which 14 met inclusion criteria and were included in the final analysis (Table 1). 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 The detailed inclusion and exclusion criteria of the individual trials are summarized in Table S3. Three trials were multicenter, 2 originated from Italy, 2 from Sweden, 2 from Korea, and 1 each from the United Kingdom, Belgium, Australia, Norway, and Brazil. Two trials used within‐patient randomization. 8 , 10 For the SAVE‐RITA (Saphenous Vein versus Right Internal Thoracic Artery as a Y‐Composite Graft) trial, only the RITA arm was analyzed as this was the only trial that used the CON‐SV as a Y‐composite graft based on the in situ left internal thoracic artery and showed different results from when the CON‐SV was anastomosed to the aorta. For the RAPCO (Radial Artery Patency and Clinical Outcomes) trial, the unpublished 10‐year results were obtained by the senior author.
Table 1.
Characteristics of the Included Randomized Trials
| Author, y | Institution | Country | Study Period | Number of Patients |
|---|---|---|---|---|
| Collins, 2008 7 | Royal Brompton Hospital | United Kingdom | 1998–2000 | 142 |
| Deb, 2012 8 | Multicenter | Canada | 1996–2001 | 510 |
| Deb, 2019 9 | Multicenter | Canada | 2011–2013 | 250 |
| Dreifaldt, 2019 10 | University Hospital | Sweden | 2004–2009 | 108 |
| Gaudino, 2005 11 | Catholic University | Italy | 1994–1997 | 120 |
| Glineur, 2011 12 | Cliniques Universitaire St Luc. | Belgium | 2003–2006 | 210 |
| Goldman, 2011 13 | Multicenter | United States | 2003–2009 | 757 |
| Buxton, 2020 14 | University of Melbourne | Australia | 1996–2004 | 619 |
| Kim, 2018 15 | Seoul National University Hospital | Korea | 2008–2011 | 224 |
| Muneretto, 2004 16 | University of Brescia Medical School | Italy | 2000–2002 | 160 |
| Pettersen, 2017 17 | St. Olavs University Hospital | Norway | 2013–2014 | 100 |
| Samano, 2015 18 | Orebro University | Sweden | 1993–1997 | 156 |
| Santos, 2002 19 | University of Sã o Paulo | Brazil | 1998–1999 | 60 |
| Song, 2012 20 | Yonsei University College of Medicine | Korea | 2008–2009 | 60 |
A total of 3396 randomized patients were included in the final analysis. Demographics of the included patients are presented in Table S4. The number of patients in the trials ranged from 60 to 757. The mean age range was 55.7 to 77.3 years in the RA group, 58.0 to 76.9 years in the CON‐SV group, 59.1 to 63.5 years in the RITA group, 63.4 to 77.6 years in the NT‐SV group, and 56.1 to 61.9 years in the GEA group. Female patients ranged from 0% to 44% in the RA group, 1% to 46% in the CON‐SV group, 5% to 19% in the RITA group, 7% to 17% in the NT‐SV group, and 12% to 13% in the GEA group. The prevalence of hypertension ranged from 45% to 79% in the RA group, 45% to 84% in the CON‐SV group, 28% to 67% in the RITA group, 56% to 84% in the NT‐SV group, and 80% to 82% in the GEA group. The prevalence of diabetes mellitus ranged from 9% to 43% in the RA group, 4% to 45% in the CON‐SV group, 7% to 46% in the NT‐SV group, and 20% to 27% in the GEA group.
The details of angiography and procedure‐related variables are summarized in Tables S5 and S6.
A total of 3651 grafts were analyzed across the 14 included trials: 1178 RA grafts, 1362 CON‐SV grafts, 399 RITA grafts, 576 NT‐SV grafts, and 136 GEA grafts. The weighted mean angiographic follow‐up was 5.1 years. The crude patency rates of the analyzed conduits were as follows: CON‐SV, 81.8% (95% CI ,74.8–87.3); GEA, 61.2% (95% CI, 52.2–69.4); NT‐SV, 89.3% (95% CI, 85.4–92.3); RA, 93.2% (95% CI, 87.4–96.4); and RITA, 90.9% (95% CI, 72.1–97.5). Details of patency rates are given in Table 2.
Table 2.
Pooled Patency of the Different Grafts
| Conduit | Number of Studies | Pooled Patency Rate (95% CI) | Pooled Angiographic Follow‐Up in Years |
|---|---|---|---|
| Radial artery | 11 | 93.2 (87.4 – 96.4) | 5.5 |
| Conventionally harvested saphenous vein | 11 | 81.8 (74.8 – 87.3) | 4.5 |
| Right internal thoracic artery | 5 | 90.9 (72.1 – 97.5) | 6.9 |
| No‐touch saphenous vein | 5 | 89.3 (85.4 – 92.3) | 4.7 |
| Gastroepiploic artery | 2 | 61.2 (52.2 – 69.4) | 2.8 |
At network meta‐analysis, compared with the CON‐SV, only the RA (incidence rate ratio [IRR], 0.54; 95% CI, 0.35–0.82) and the NT‐SV (IRR, 0.55; 95% CI, 0.39–0.78) were associated with significantly lower rate of graft occlusion, whereas the RITA (IRR, 1.02, 95% CI, 0.63–1.65) and GEA (IRR, 0.98; 95% CI, 0.57–1.68) were not (Figures 1 and 2; Table 3 and Table S7). Width of the CI supports a clinically meaningful benefit of RA and NT‐SV in comparison to the CON‐SV. The RA ranked as the best conduit (rank score for RA 0.87 versus 0.85 for NT‐SV, 0.23 for RITA, 0.29 for GEA, and 0.25 for CON‐SV).
Figure 1. Forest plot for graft occlusion for the different conduits.
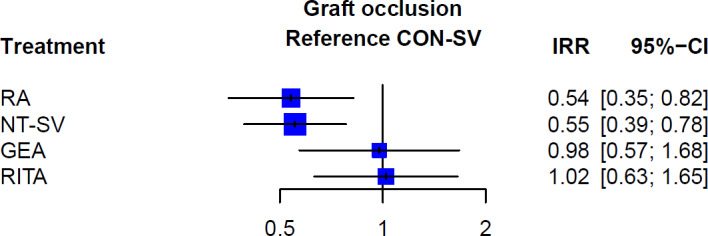
CON‐SV indicates conventionally harvested saphenous vein; GEA, gastroepiploic artery; IRR, incidence rate ratio; NT‐SV, no‐touch saphenous vein; RA, radial artery; and RITA, right internal thoracic artery.
Figure 2. Netgraph of the different comparisons for the primary outcome of graft occlusion.
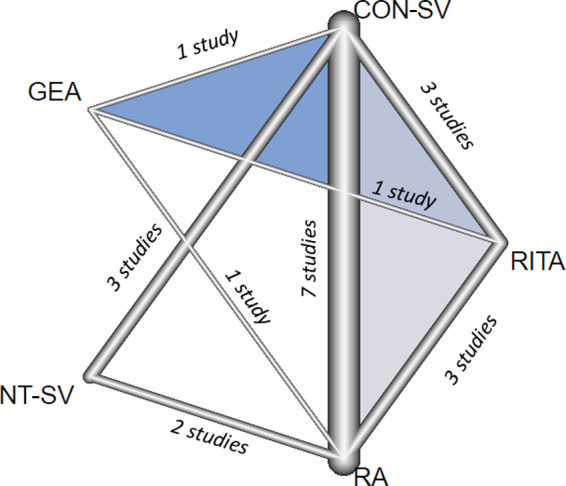
Lines represent direct comparisons and the thickness of the lines correspond to the number of studies comparing treatment pairs. CON‐SV indicates conventionally harvested saphenous vein; GEA, gastroepiploic artery; NT‐SV, no‐touch saphenous vein; RA, radial artery; and RITA, right internal thoracic artery.
Table 3.
League Tables Summarizing the Results of the Network Meta‐Analysis (Expressed as Incidence Rate Ratio With 95% CI) for Graft Occlusion Using Random Effects Model
| Graft Occlusion | ||||
|---|---|---|---|---|
| Radial Artery | ||||
| 0.54 [0.33 – 0.90] | Right Internal Thoracic Artery | |||
| 1.03 [0.64 – 1.64] | 1.90 [1.02 – 3.51] | No‐Touch Saphenous Vein | ||
| 0.57 [0.32 – 1.01] | 1.04 [0.59 – 1.84] | 0.55 [0.28 – 1.07] | Gastroepiploic Artery | |
| 0.54 [0.35 – 0.82] | 1.02 [0.63 – 1.65] | 0.55 [0.39 – 0.78] | 0.98 [0.57 – 1.68] | Conventionally Harvested Saphenous Vein |
These results were confirmed in the individual pairwise meta‐analyses (Figure S3 and Table S8).
The results of all the sensitivity analyses were consistent with the primary analysis (Data S1, Figures S4 through S8).
The mean clinical follow‐up was 5.1 years. Point estimates favored the RA and NT, consistent with the patency findings, although the aggregate outcomes did not reach statistical significance (RA: IRR, 0.82; 95% CI, 0.58–1.16, NT‐SV: IRR, 0.91; 95% CI, 0.49–1.70; RITA: IRR, 1.47; 95% CI, 0.77–2.80; GEA: IRR, 0.41; 95% CI, 0.04–4.38). Notably, given the width of the CI no conclusive statement can be made on the comparative effectiveness of the different grafts (Figures S9 and S10, Table S7).
Heterogeneity/inconsistency estimates and net split are shown in Tables S9 and S10. Heterogeneity was low to moderate (<30%) and level of evidence was high in all comparisons.
Leave‐one‐out analysis and funnel plot did not find strong evidence of invalidity of the main analysis (Figures S11 and S12).
Meta‐Regression
At meta‐regression, the percentage of patients with hypertension and the percentage of use of the off‐pump technique were associated with the IRR for the primary outcome in the RA versus CON‐SV comparison and the percentage of female patients was inversely associated with it. There was no association between the other variables including age, diabetes mellitus, dyslipidemia, target vessel stenosis, duration of follow‐up, completeness of angiographic follow‐up, percentage of proximal anastomoses on the ascending aorta, and percentage of grafts to the circumflex coronary system with the IRR for the primary outcome (Table S11).
DISCUSSION
In this network meta‐analysis of 14 RCTs (3651 grafts), we found that compared with the CON‐SV, the RA and NT‐SV have significantly lower occlusion rate at a mean follow‐up of 5 years. The RA ranked as the best conduit, whereas the randomized evidence supporting a higher patency rate for the RITA and right GEA was limited. As no other comparative angiographic RCT is currently underway, these results are likely to represent the basis for evidence‐based decisions on grafting strategy for many years.
Observational studies comparing different grafting strategies have known bias and limitations, so that often treatment allocation bias, and not true biological effect, may explain their results. 21 On the other hand, the randomized comparisons of the clinical outcomes of patients receiving different type of grafts are very limited and have provided conflicting results. 22 Although the analysis of the Radial Artery Database International Alliance (RADIAL) databases has suggested better outcomes for patients who received the RA rather than the CON‐SV to graft the second most important target vessel, 23 the large ART (Arterial Revascularization Trial) did not find a difference in survival and event‐free survival at 10 years among patients randomized to receive the RITA or the CON‐SV. 24 The high crossover and cointervention rates in ART may have diluted any potential treatment effect and do not allow definitive conclusion on the clinical effect of the use of the RITA. 22 Currently, the ROMA (Randomized Comparison of the Outcome of Single versus Multiple Arterial Grafts) trial (ClinicalTrials.gov registration number: 1703018094) is testing the hypothesis that the use of multiple arterial grafting improves freedom from cardiovascular events and death in patients with CABG, but results are expected only after 2025. 25
In the absence of definitive clinical results, the analysis of the published angiographic RCTs allows a solid estimate of the patency rate of the different conduits, minimizing the risk of bias and hidden confounders. The association between graft patency and clinical outcomes, although debated, is biologically plausible and is supported by the 5‐year results of the RADIAL database, where the patency and clinical data were highly concordant. 23 In addition, the use of the network meta‐analysis further reduces the risk of spurious associations and is generally accepted to be more effective than the use of pairwise meta‐analysis in reducing bias and confounders. 26
A previous network meta‐analysis published by Benedetto and colleagues in 2015 compared the angiographic outcomes of the CON‐SV, RITA, RA, and GEA and found significantly higher patency for the RITA and RA when compared with the CON‐SV. 27 The GEA was associated with the highest rate of functional and complete graft occlusion on angiography, whereas the NT‐SV was not included in the analysis. Compared with the Benedetto analysis, we have included 5 more trials, 3 of which reported on the RITA and 2 on the NT‐SV.
Our results contradict the common beliefs that the RITA is the natural second graft of choice. The reasons for this finding are likely multifactorial: the randomized evidence comparing the RITA with the CON‐SV is much less solid that the evidence comparing the RA to the CON‐SV (3 trials and 198 patients for the RITA, 7 trials and 1671 patients for the RA). In addition, the RITA is more fragile and less surgeon friendly than the RA and its use in any configuration is technically more complex than the use of the RA. It has been shown that the results of RITA, but not of RA grafting, are significantly influenced by surgeon's experience 28 , 29 and it is likely that a difference in deliverability, rather than in biology, between the 2 conduits may explain the difference in patency. Issues in the deliverability of the RITA have been seen in ART, where the crossover from the bilateral internal thoracic artery to the single internal thoracic artery was as high as 14% and have been suggested as a possible explanation for the neutral results of the trial. 22 It is also possible that a more strict attention to the degree of competitive flow related to the concerns for postoperative spasm may have advantaged the RA.
The results of the clinical analysis were consistent with the outcomes of the angiographic analysis, with point estimates favoring the RA and NT‐SV. The difference did not reach statistical significance, a finding that is likely owing to the limited follow‐up time (5 years) and is consistent with the results of a pooled analysis of individual patients' data from 5 RCTs comparing the RA and the CON‐SV at the same follow‐up. 23
The use of the RA is a class I indication in the most recent myocardial revascularization guidelines. 30 The RA is a versatile conduit that can be safely and easily harvested via either open or endoscopic techniques and can be used to graft any coronary target. 31 Because of its tendency to develop string sign in case of competitive flow, the RA should be used only to graft targets with severe stenosis. The use of calcium channel blockers or other antispasmodic medications seems to be associated with better outcomes in patients with RA grafts. 32 , 33
The NT‐SV has a promising patency rate, but its use is associated with a higher risk of harvest‐site complications and should be restricted to patients without risk factors for surgical site infections. 34
This study must be interpreted in light of its limitations. There may be variability in surgeon and center expertise, technical variables, patency definition, and postoperative protocols among the included RCTs. However, the level of heterogeneity in the main analysis was low and the results were solid at all the sensitivity analyses, so, even if present, this variability had limited impact on the outcomes. Given the risk of type I error given multiple tests, we did not report P values, but relied instead on 95% CI. Although this approach does not address multiplicity, it is in compliance with leading recommendations. Irrespectively, the reader should exercise analytical caution and clinical judgment in reading intervals, which are only nominally representing 95% probability statements on theoretical future experiments. Despite using studies with protocol‐driven angiography, we could not account for incomplete follow‐up, and the results of this analysis reflect outcomes of grafts that underwent evaluation. Of note, some comparisons are based on a small number of studies and may be underpowered. Finally, we had only limited information on secondary prevention and antispasmodic therapy, which are known to affect graft patency.
In conclusion, in network meta‐analysis of 14 angiographic RCTs, we found that based on the current randomized evidence, only the RA and the NT‐SV have significantly better patency rates compared with the CON‐SV. The RA ranks as the best conduit. These results should inform surgeons' decisions and guidelines on grafting strategy and conduit selection.
Sources of Funding
Prof. Fremes is supported in part by the Bernard S Goldman Chair in Cardiovascular Surgery. Derrick Y Tam is supported by Canadian Institutes of Health Research (CIHR) Fellowship.
Disclosures
None.
Supporting information
Data S1
Tables S1–S11
Figures S1–S12
(J Am Heart Assoc. 2021;10:e019206. DOI: 10.1161/JAHA.120.019206).
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019206
For Sources of Funding and Disclosures, see page 6.
References
- 1. D’Agostino RS, Jacobs JP, Badhwar V, Fernandez FG, Paone G, Wormuth DW, Shahian DM. The Society of Thoracic Surgeons adult cardiac surgery database: 2019 update on outcomes and quality. Ann Thorac Surg. 2019;107:24–32. DOI: 10.1016/j.athoracsur.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 2. Fukui T, Tabata M, Manabe S, Shimokawa T, Takanashi S. Graft selection and one‐year patency rates in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2010;89:1901–1905. DOI: 10.1016/j.athoracsur.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 3. Cao C, Manganas C, Horton M, Bannon P, Munkholm‐Larsen S, Ang SC, Yan TD. Angiographic outcomes of radial artery versus saphenous vein in coronary artery bypass graft surgery: a meta‐analysis of randomized controlled trials. J Thorac Cardiovasc Surg. 2013;146:255–261. DOI: 10.1016/j.jtcvs.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 4. Suma H, Tanabe H, Yamada J, Mikuriya A, Horii T, Isomura T. Midterm results for use of the skeletonized gastroepiploic artery graft in coronary artery bypass. Circ J. 2007;71:1503–1505. DOI: 10.1253/circj.71.1503. [DOI] [PubMed] [Google Scholar]
- 5. Tatoulis J, Buxton BF, Fuller JA, Meswani M, Theodore S, Powar N, Wynne R. Long‐term patency of 1108 radial arterial‐coronary angiograms over 10 years. Ann Thorac Surg. 2009;88:23–29; discussion 29–30. DOI: 10.1016/j.athoracsur.2009.03.086. [DOI] [PubMed] [Google Scholar]
- 6. 16.7.2 Multiplicity in systematic reviews. Available at: https://handbook‐5‐1.cochrane.org/chapter_16/16_7_2_multiplicity_in_systematic_reviews.htm Accessed January 11, 2021..
- 7. Collins P, Webb CM, Chong CF, Moat NE; Radial Artery Versus Saphenous Vein Patency (RSVP) Trial Investigators . Radial artery versus saphenous vein patency randomized trial: five‐year angiographic follow‐up. Circulation. 2008;117:2859–2864. DOI: 10.1161/CIRCULATIONAHA.107.736215. [DOI] [PubMed] [Google Scholar]
- 8. Deb S, Cohen EA, Singh SK, Une D, Laupacis A, Fremes SE; RAPS Investigators . Radial artery and saphenous vein patency more than 5 years after coronary artery bypass surgery: results from RAPS (Radial Artery Patency Study). J Am Coll Cardiol. 2012;60:28–35. DOI: 10.1016/j.jacc.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 9. Deb S, Singh SK, de Souza D, Chu MWA, Whitlock R, Meyer SR, Verma S, Jeppsson A, Al‐Saleh A, Brady K, et al.; SUPERIOR SVG Study Investigators . SUPERIOR SVG: no touch saphenous harvesting to improve patency following coronary bypass grafting (a multi‐centre randomized control trial, NCT01047449). J Cardiothorac Surg. 2019;14:85. DOI: 10.1186/s13019-019-0887-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dreifaldt M, Mannion JD, Geijer H, Lidén M, Bodin L, Souza D. The no‐touch saphenous vein is an excellent alternative conduit to the radial artery 8 years after coronary artery bypass grafting: a randomized trial. J Thorac Cardiovasc Surg. 2021;161:624–630. DOI: 10.1016/j.jtcvs.2019.09.177. [DOI] [PubMed] [Google Scholar]
- 11. Gaudino M, Cellini C, Pragliola C, Trani C, Burzotta F, Schiavoni G, Nasso G, Possati G. Arterial versus venous bypass grafts in patients with in‐stent restenosis. Circulation. 2005;112:I265–I269. DOI: 10.1161/CIRCULATIONAHA.104.512905. [DOI] [PubMed] [Google Scholar]
- 12. Glineur D, D’hoore W, de Kerchove L, Noirhomme P, Price J, Hanet C, El Khoury G. Angiographic predictors of 3‐year patency of bypass grafts implanted on the right coronary artery system: a prospective randomized comparison of gastroepiploic artery, saphenous vein, and right internal thoracic artery grafts. J Thorac Cardiovasc Surg. 2011;142:980–988. DOI: 10.1016/j.jtcvs.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 13. Goldman S, Sethi GK, Holman W, Thai H, McFalls E, Ward HB, Kelly RF, Rhenman B, Bakaeen FG, et al. Radial artery grafts vs saphenous vein grafts in coronary artery bypass surgery: a randomized trial. JAMA. 2011;305:167–174. DOI: 10.1001/jama.2010.1976. [DOI] [PubMed] [Google Scholar]
- 14. Buxton BF, Hayward PA, Raman J, Moten SC, Rosalion A, Gordon I, Seevanayagam S, Matalanis G, Benedetto U, Gaudino M, et al. Long‐term results of the RAPCO trials. Circulation. 2020;142:1330–1338. DOI: 10.1161/CIRCULATIONAHA.119.045427. [DOI] [PubMed] [Google Scholar]
- 15. Kim M‐S, Hwang HY, Kim JS, Oh SJ, Jang M‐J, Kim K‐B. Saphenous vein versus right internal thoracic artery as a Y‐composite graft: five‐year angiographic and clinical results of a randomized trial. J Thorac Cardiovasc Surg. 2018;156:1424–1433.e1. DOI: 10.1016/j.jtcvs.2018.04.123. [DOI] [PubMed] [Google Scholar]
- 16. Muneretto C, Bisleri G, Negri A, Manfredi J, Carone E, Morgan JA, Metra M, Dei CL. Left internal thoracic artery‐radial artery composite grafts as the technique of choice for myocardial revascularization in elderly patients: a prospective randomized evaluation. J Thorac Cardiovasc Surg. 2004;127:179–184. DOI: 10.1016/j.jtcvs.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 17. Pettersen Ø, Haram PM, Winnerkvist A, Karevold A, Wahba A, Stenvik M, Wiseth R, Hegbom K, Nordhaug DO. Pedicled vein grafts in coronary surgery: perioperative data from a randomized trial. Ann Thorac Surg. 2017;104:1313–1317. DOI: 10.1016/j.athoracsur.2017.03.076. [DOI] [PubMed] [Google Scholar]
- 18. Samano N, Geijer H, Liden M, Fremes S, Bodin L, Souza D. The no‐touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: A randomized trial. J Thorac Cardiovasc Surg. 2015;150:880–888. DOI: 10.1016/j.jtcvs.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 19. Santos GG, Stolf NAG, Moreira LFP, Haddad VLS, Simões RMC, Carvalho SRV, Salgado AA, Avelar SF. Randomized comparative study of radial artery and right gastroepiploic artery in composite arterial graft for CABG. Eur J Cardiothorac Surg. 2002;21:1009–1014. DOI: 10.1016/s1010-7940(02)00180-x. [DOI] [PubMed] [Google Scholar]
- 20. Song S‐W, Sul S‐Y, Lee H‐J, Yoo K‐J. Comparison of the radial artery and saphenous vein as composite grafts in off‐pump coronary artery bypass grafting in elderly patients: a randomized controlled trial. Korean Circ J. 2012;42:107–112. DOI: 10.4070/kcj.2012.42.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaudino M, Di Franco A, Rahouma M, Tam DY, Iannaccone M, Deb S, D’Ascenzo F, Abouarab AA, Girardi LN, Taggart DP, et al. Unmeasured confounders in observational studies comparing bilateral versus single internal thoracic artery for coronary artery bypass grafting: a meta‐analysis. J Am Heart Assoc. 2018;7:e008010. DOI: 10.1161/JAHA.117.008010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaudino M, Bakaeen FG, Benedetto U, Di Franco A, Fremes S, Glineur D, Girardi LN, Grau J, Puskas JD, Ruel M, et al.; ATLANTIC (Arterial Grafting International Consortium) Alliance members . Arterial grafts for coronary bypass: a critical review after the publication of ART and RADIAL. Circulation. 2019;140:1273–1284. DOI: 10.1161/CIRCULATIONAHA.119.041096. [DOI] [PubMed] [Google Scholar]
- 23. Gaudino M, Benedetto U, Fremes S, Biondi‐Zoccai G, Sedrakyan A, Puskas JD, Angelini GD, Buxton B, Frati G, Hare DL, et al. Radial‐artery or saphenous‐vein grafts in coronary‐artery bypass surgery. N Engl J Med. 2018;378:2069–2077. DOI: 10.1056/NEJMoa1716026. [DOI] [PubMed] [Google Scholar]
- 24. Taggart DP, Benedetto U, Gerry S, Altman DG, Gray AM, Lees B, Gaudino M, Zamvar V, Bochenek A, Buxton B, et al.; Arterial Revascularization Trial Investigators . Bilateral versus single internal‐thoracic‐artery grafts at 10 years. N Engl J Med. 2019;380:437–446. DOI: 10.1056/NEJMoa1808783. [DOI] [PubMed] [Google Scholar]
- 25. Gaudino M, Benedetto U, Fremes S, Ballman K, Biondi‐Zoccai G, Sedrakyan A, Nasso G, Raman J, Buxton B, Hayward PA, et al. The RADial artery International ALliance (RADIAL) extended follow‐up study: rationale and study protocol. Eur J Cardiothorac Surg. 2019;56:1025–1030. DOI: 10.1093/ejcts/ezz247. [DOI] [PubMed] [Google Scholar]
- 26. Song F, Xiong T, Parekh‐Bhurke S, Loke YK, Sutton AJ, Eastwood AJ, Holland R, Chen Y‐F, Glenny A‐M, Deeks JJ, et al. Inconsistency between direct and indirect comparisons of competing interventions: meta‐epidemiological study. BMJ. 2011;343:d4909. DOI: 10.1136/bmj.d4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benedetto U, Raja SG, Albanese A, Amrani M, Biondi‐Zoccai G, Frati G. Searching for the second best graft for coronary artery bypass surgery: a network meta‐analysis of randomized controlled trials. Eur J Cardiothorac Surg. 2015;47:59–65. DOI: 10.1093/ejcts/ezu111. [DOI] [PubMed] [Google Scholar]
- 28. Schwann TA, Habib RH, Wallace A, Shahian D, Gaudino M, Kurlansky P, Engoren MC, Tranbaugh RF, Schwann AN, Jacobs JP. Bilateral internal thoracic artery versus radial artery multi‐arterial bypass grafting: a report from the STS database†. Eur J Cardiothorac Surg. 2019;56:926–934. DOI: 10.1093/ejcts/ezz106. [DOI] [PubMed] [Google Scholar]
- 29. Gaudino M, Bakaeen F, Benedetto U, Rahouma M, Di Franco A, Tam DY, Iannaccone M, Schwann TA, Habib R, Ruel M, et al. Use rate and outcome in bilateral internal thoracic artery grafting: insights from a systematic review and meta‐analysis. J Am Heart Assoc. 2018;7:e009361. DOI: 10.1161/JAHA.118.009361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neumann F‐J, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J‐P, Falk V, Head SJ, et al.; ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. DOI: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 31. Verma S, Goodman SG, Mehta SR, Latter DA, Ruel M, Gupta M, Yanagawa B, Al‐Omran M, Gupta N, Teoh H, et al. Should dual antiplatelet therapy be used in patients following coronary artery bypass surgery? A meta‐analysis of randomized controlled trials. BMC Surg. 2015;15:112. DOI: 10.1186/s12893-015-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He G‐W, Taggart DP. Spasm in arterial grafts in coronary artery bypass grafting surgery. Ann Thorac Surg. 2016;101:1222–1229. DOI: 10.1016/j.athoracsur.2015.09.071. [DOI] [PubMed] [Google Scholar]
- 33. Gaudino M, Benedetto U, Fremes SE, Hare DL, Hayward P, Moat N, Moscarelli M, Di Franco A, Nasso G, Peric M, et al. Effect of calcium‐channel blocker therapy on radial artery grafts after coronary bypass surgery. J Am Coll Cardiol. 2019;73:2299–2306. DOI: 10.1016/j.jacc.2019.02.054. [DOI] [PubMed] [Google Scholar]
- 34. Papakonstantinou NA, Baikoussis NG, Goudevenos J, Papadopoulos G, Apostolakis E. Novel no touch technique of saphenous vein harvesting: is great graft patency rate provided? Ann Card Anaesth. 2016;19:481–488. DOI: 10.4103/0971-9784.185537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S11
Figures S1–S12


