Abstract
Background
As an initial treatment strategy, percutaneous coronary intervention (PCI) for coronary chronic total occlusion (CTO) did not show midterm survival benefits compared with optimal medical therapy (OMT). We sought to evaluate the benefit of PCI compared with OMT in patients with CTO over extended long‐term follow‐up.
Methods and Results
Between March 2003 and February 2012, 2024 patients with CTO were enrolled in a single‐center registry and followed for ≈10 years. We excluded patients with CTO who underwent coronary artery bypass graft (n=477) and classified patients into the CTO‐PCI group (n=883) or OMT group (n=664) according to initial treatment strategy. Patients with multivessel disease received PCI for obstructive non‐CTO lesions in both groups. In the CTO‐PCI group, 699 patients (79.2%) underwent successful revascularization. The CTO‐PCI group had a lower 10‐year rate of cardiac death (10.4% versus 22.3%; hazard ratio [HR], 0.44 [95% CI, 0.32–0.59]; P<0.001) than the OMT group. After propensity score matching analyses, the CTO‐PCI group had a lower 10‐year rate of cardiac death (13.6% versus 20.8%; HR, 0.64 [95% CI, 0.45–0.91]; P=0.01) than the OMT group. The relative reduction in cardiac death at 10 years was mainly driven by a relative reduction between 3 and 10 years (8.3% versus 16.6%; HR, 0.43 [95% CI, 0.27–0.71]; P<0.001) but not at 3 years (5.7% versus 5.0%; HR, 1.12 [95% CI, 0.63–2.00]; P=0.71). The beneficial effects of CTO‐PCI were consistent among subgroups.
Conclusions
As an initial treatment strategy, CTO‐PCI might reduce late cardiac death compared with OMT in patients with CTO. Extended follow‐up of randomized trials may confirm the findings of the present study.
Keywords: chronic total occlusion, medical therapy, percutaneous coronary intervention
Subject Categories: Percutaneous Coronary Intervention
Nonstandard Abbreviations and Acronyms
- CTO
chronic total occlusion
- DECISION‐CTO
Drug‐Eluting Stent Implantation Versus Optimal Medical Treatment in Patients With Chronic Total Occlusion
- EUROCTO
Randomized Multicentre Trial to Evaluate the Utilization of Revascularization or Optimal Medical Therapy for the Treatment of Chronic Total Occlusions
- OMT
optimal medical therapy
Clinical Perspective
What Is New?
We observed clinical outcomes of patients treated with chronic total occlusion (CTO) percutaneous coronary intervention (PCI) and optimal medical therapy through extended long‐term follow‐up of a dedicated CTO registry.
CTO‐PCI might reduce a 10‐year rate of cardiac death compared with optimal medical therapy, as well as that of all‐cause mortality, acute myocardial infarction, and any revascularization.
The relative reduction in risk of adverse outcome was mainly driven by a relative reduction between 3 and 10 years, not at 3 years.
What Are the Clinical Implications?
CTO‐PCI might offer patients long‐term survival benefits compared with optimal medical therapy only.
Survival benefits of CTO‐PCI might stand out later rather than earlier.
Late survival benefit of CTO‐PCI should be confirmed through extended follow‐up of randomized controlled trials.
Coronary chronic total occlusion (CTO) is frequent during coronary angiography. 1 However, CTO percutaneous coronary intervention (PCI) has been performed infrequently and is technically challenging despite recent advancements in interventional equipment and innovative approaches to cross CTO lesions. 2 The key barrier limiting transition of CTO‐PCI to conventional procedure is lack of randomized trial–derived evidence to support its use. 3 Two randomized trials involving CTO‐PCI versus optimal medical therapy (OMT) were recently performed: the DECISION‐CTO (Drug‐Eluting Stent Implantation Versus Optimal Medical Treatment in Patients With Chronic Total Occlusion) trial and EUROCTO (A Randomized Multicentre Trial to Evaluate the Utilization of Revascularization or Optimal Medical Therapy for the Treatment of Chronic Total Occlusions). However, the DECISION‐CTO trial and EURO‐CTO did not demonstrate an improvement in major cardiac cerebrovascular events up to 3 years. 4 , 5 Both trials had several limitations including underpowered sample size, midterm follow‐up, and selection of low‐risk population. Therefore, a study utilizing a large sample size, long‐term follow‐up, and enrollment of consecutive patients is needed to explore the difference between CTO‐PCI and OMT. We sought to evaluate the benefit of CTO‐PCI compared with OMT through extended long‐term follow‐up of a dedicated CTO registry.
METHODS
Study Population
The detailed characteristics of the CTO registry have been previously published. 6 , 7 , 8 Briefly, the present registry comprised 2024 consecutive patients with CTO who had symptomatic angina and/or a positive functional ischemia study and who underwent revascularization or OMT as an initial approach between March 2003 and February 2012 from a dedicated catheterization registry in the Samsung Medical Center. The institutional review board at Samsung Medical Center approved the study protocol and waived the requirement for informed consent related to additional analysis of data collected as part of a prior research study in which individuals provided informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request. Patients with prior coronary artery bypass graft, ST‐segment–elevation myocardial infarction, or cardiogenic shock or cardiopulmonary resuscitation as initial presentation were excluded. According to the initial treatment strategy, patients were classified into coronary artery bypass grafting group, CTO‐PCI group, or OMT group. The treatment strategy was determined by the discretion of the treating physicians and/or patients after diagnostic coronary angiography. After excluding initial coronary artery bypass graft group (n=477), 1547 patients were finally included in this analysis. Patients with failed CTO‐PCI were considered the CTO‐PCI group. The patient flow of the study is shown in Figure 1. Patients with multivessel disease received PCI for obstructive non‐CTO lesions in both groups.
Figure 1. Patient flow.
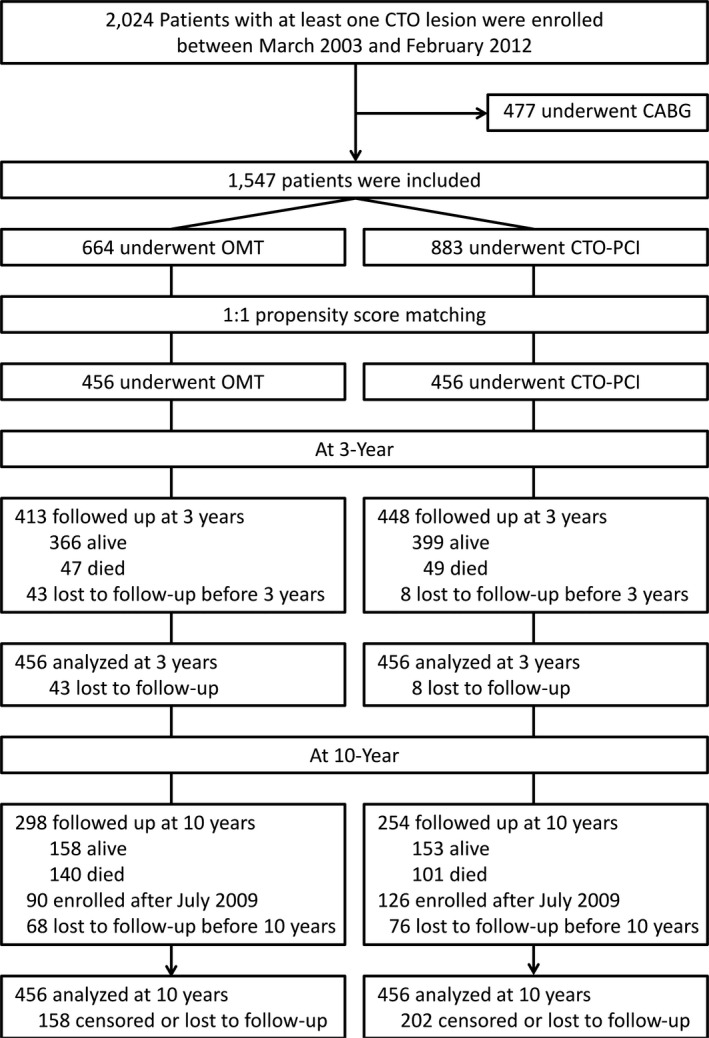
CABG indicates coronary arterial bypass graft; CTO, chronic total occlusion; OMT, optimal medical therapy; and PCI, percutaneous coronary intervention.
Management
PCI was performed using a standard technique. Antiplatelet therapy and periprocedural anticoagulation followed a standard regimen. Decisions to perform bilateral injection and a retrograde approach, in addition to the type of wire, microcatheter, use of intravascular ultrasound, and type of drug‐eluting stents, were left to the physician. During the follow‐up period, OMT for secondary prevention was performed in accordance with guidelines. 9 Duration of dual antiplatelet therapy in patients receiving drug‐eluting stents was determined by the treating physician.
Study Outcomes and Follow‐Up
The primary outcome was a 10‐year rate of cardiac death. Secondary outcomes were 10‐year rates of all‐cause death, acute myocardial infarction, and any revascularization. Cause of death was considered cardiac unless a definite noncardiac cause could be established. Acute myocardial infarction was defined as an increase in creatine kinase MB fraction or a value of troponin greater than the upper limit of the normal range with ischemic symptoms or signs. Periprocedural enzyme increase was not included in this definition of acute myocardial infarction. Any revascularization was defined as any subsequent PCI procedure or coronary artery bypass graft surgery, as determined by the patient's physician to be clinically indicated.
Clinical follow‐up assessment was performed at 1 month, 6 months, 1 year, and annually thereafter by independent research personnel in our dedicated catheterization registry. 10 The follow‐up period was extended through July 31, 2019, to achieve a 10‐year follow‐up evaluation as best as possible. If a patient was unable to visit the hospital, information was collected by telephone contact or review of medical records.
Statistical Analysis
Continuous variables are presented as mean±SD and categorical variables as number and percentage. Group comparisons were performed using t test or Wilcoxon rank‐sum test for continuous variables and chi‐square test or Fisher exact test for categorical data as appropriate. Cumulative incidence curves were constructed using Kaplan–Meier estimates. The Cox proportional hazards model was used to compare the risks of clinical outcome between the CTO‐PCI and OMT groups. As previous reports suggested no difference of hard end points between CTO‐PCI and OMT for at least 3 years, a landmark analysis was performed according to time point at 3 years after decision of initial treatment strategy. 4 , 5 Hazard ratios (HRs) were calculated separately for events that occurred within 3 years after initial treatment strategy and those that occurred between 3 years and the end of follow‐up. Multivariable models for each end point were determined using backward stepwise elimination procedures where the least significant variable was removed one at a time from the full model including variables from Table 1. Furthermore, we used propensity score matching to balance intergroup differences. The propensity score matching method was determined to be adequate when overall balance was achieved, indicated by standardized mean difference <10%. All of the clinical variables in Table 1 were applied in this analysis. When balance was achieved, the matched data set was analyzed using a Cox regression model for each clinical outcome. We also conducted subgroup analyses on the basis of clinically relevant variables: age (<75 years versus ≥75 years), presentation of acute coronary syndrome, diabetes mellitus, chronic kidney disease, left ventricular ejection fraction (<40% versus ≥40%), CTO vessel (left anterior descending versus left circumflex or right coronary artery), location of CTO (proximal or mid versus distal), and collateral flow (Rentrop grade 0–2 versus Rentrop grade 3). Tests for interaction were performed to assess heterogeneity of treatment effect among subgroups. All tests were 2‐tailed, and P<0.05 was considered statistically significant. All analyses were performed using R 3.6.1 (R Foundation for Statistical Computing).
Table 1.
Baseline Characteristics
| Total Population | Propensity Score–Matched Population | |||||||
|---|---|---|---|---|---|---|---|---|
| OMT (n=664) | CTO‐PCI (n=883) | P Value | SMD, % | OMT (n=456) | CTO‐PCI (n=456) | P Value | SMD, % | |
| Age, y | 65.9±11.3 | 61.5±10.8 | <0.001 | −40.8 | 64.6±11.7 | 64.6±10.1 | 0.98 | 0.2 |
| Men | 509 (76.7) | 713 (80.7) | 0.05 | 10.4 | 354 (77.6) | 370 (81.1) | 0.22 | 8.7 |
| Diabetes mellitus | 318 (47.9) | 384 (43.5) | 0.09 | −8.9 | 211 (46.3) | 218 (47.8) | 0.69 | 3.1 |
| Hypertension | 439 (66.1) | 547 (61.9) | 0.09 | −8.6 | 301 (66.0) | 302 (66.2) | >0.99 | 0.5 |
| Dyslipidemia | 158 (23.8) | 289 (32.7) | <0.001 | 19.0 | 115 (25.2) | 113 (24.8) | 0.94 | −1.0 |
| Current smoker | 182 (27.4) | 285 (32.3) | 0.04 | 10.4 | 131 (28.7) | 141 (30.9) | 0.52 | 4.8 |
| Renal failure | 61 (9.2) | 68 (7.7) | 0.30 | −5.6 | 39 (8.6) | 39 (8.6) | >0.99 | 0 |
| Family history of CAD | 73 (11.0) | 135 (15.3) | 0.01 | 11.9 | 56 (12.3) | 55 (12.1) | >0.99 | 0 |
| Previous MI | 211 (31.8) | 180 (20.4) | <0.001 | −28.3 | 126 (27.6) | 120 (26.3) | 0.71 | −3.0 |
| Previous PCI | 208 (31.3) | 178 (20.2) | <0.001 | −27.8 | 129 (28.3) | 122 (26.8) | 0.66 | −3.4 |
| Previous stroke | 67 (10.1) | 66 (7.5) | 0.07 | −9.9 | 44 (9.6) | 33 (7.2) | 0.23 | −8.7 |
| Peripheral artery disease | 40 (6.0) | 23 (2.6) | 0.001 | −21.5 | 22 (4.8) | 18 (3.9) | 0.63 | −4.3 |
| Left ventricular ejection fraction, %* | 53.6±12.9 | 57.6±11.3 | <0.001 | 35.7 | 55.4±11.7 | 55.5±11.7 | 0.85 | 1.2 |
| Acute coronary syndrome | 98 (14.8) | 215 (24.3) | <0.001 | 22.3 | 85 (18.6) | 89 (19.5) | 0.80 | 2.2 |
| Multivessel disease | 517 (77.9) | 595 (67.4) | <0.001 | −22.3 | 360 (78.9) | 360 (78.9) | >0.99 | 0 |
| CTO vessel | ||||||||
| Left anterior descending | 168 (25.3) | 373 (42.2) | <0.001 | 34.3 | 132 (28.9) | 132 (28.9) | >0.99 | 0 |
| Left circumflex | 228 (34.3) | 261 (29.6) | 0.05 | −10.5 | 158 (34.6) | 157 (34.4) | >0.99 | 0 |
| Right coronary | 370 (55.7) | 383 (43.4) | <0.001 | −24.9 | 239 (52.4) | 239 (52.4) | >0.99 | 0 |
| Multi‐CTO | 94 (14.2) | 130 (14.7) | 0.71 | 1.9 | 67 (14.7) | 70 (15.4) | 0.85 | 1.8 |
| Blunt stump | 335 (50.5) | 385 (43.6) | 0.01 | −13.8 | 219 (48.0) | 218 (47.8) | >0.99 | −0.4 |
| Bridging collateral | 236 (35.5) | 265 (30.0) | 0.02 | −12.1 | 146 (32.0) | 152 (33.3) | 0.72 | 2.8 |
| Calcification | 116 (17.5) | 140 (15.9) | 0.40 | −4.4 | 77 (16.9) | 79 (17.3) | 0.93 | 1.2 |
| Collateral flow | 0.46 | 0.98 | ||||||
| 0 | 21 (3.2) | 19 (2.2) | −7.0 | 13 (2.9) | 10 (2.2) | −4.4 | ||
| 1 | 138 (20.8) | 168 (19.0) | −4.5 | 98 (21.5) | 89 (19.5) | −5.0 | ||
| 2 | 269 (40.5) | 364 (41.2) | 1.4 | 181 (39.7) | 190 (41.7) | 4.1 | ||
| 3 | 236 (35.5) | 332 (37.6) | 4.2 | 164 (36.0) | 167 (36.6) | 1.2 | ||
| Proximal or mid, CTO location | 424 (63.9) | 643 (72.8) | <0.001 | 20.3 | 291 (63.8) | 300 (65.8) | 0.58 | 4.1 |
| SYNTAX score | 19.8±9.7 | 19.6±8.8 | 0.65 | −2.4 | 19.9±9.3 | 19.8±9.0 | 0.91 | −0.8 |
| Medication | ||||||||
| Aspirin | 560 (84.3) | 818 (92.6) | <0.001 | 26.2 | 409 (89.7) | 413 (90.6) | 0.74 | 2.9 |
| Clopidogrel | 405 (61.0) | 804 (91.1) | <0.001 | 75.2 | 371 (81.4) | 380 (83.3) | 0.49 | 5.2 |
| Statin | 446 (67.2) | 645 (73.0) | 0.01 | 12.9 | 317 (69.5) | 321 (70.4) | 0.83 | 1.9 |
| β‐Blocker | 368 (55.4) | 505 (57.2) | 0.52 | 3.6 | 248 (54.4) | 258 (56.6) | 0.55 | 4.4 |
| Renin‐angiotensin system blockade | 413 (62.2) | 520 (58.9) | 0.21 | −6.8 | 275 (60.3) | 282 (61.8) | 0.68 | 3.1 |
Data are presented as mean±average or number of patients (percentage). CAD indicates coronary artery disease; MI, myocardial infarction; SMD, standardized mean difference; and SYNTAX, Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery.
Left ventricular ejection fraction was available in 593 patients treated with optimal medical therapy (OMT) and 773 patients with chronic total occlusion (CTO) percutaneous coronary intervention (PCI) among the total population and all patients among the propensity score–matched population.
RESULTS
Baseline Characteristics
The population consisted of 1547 patients with CTO who were treated with OMT (n=664) or CTO‐PCI (n=883) as an initial treatment strategy. In the CTO‐PCI group, 699 patients (79.2%) underwent successful revascularization. Baseline patient characteristics are listed in Table 1. Compared with OMT, patients in the CTO‐PCI group were younger and more likely to be men. In addition, patients in the CTO‐PCI group had a higher prevalence of dyslipidemia, current smoking, and presentation of acute coronary syndrome. However, patients in the CTO‐PCI group had a lower prevalence of prior myocardial infarction, prior PCI, reduced left ventricular ejection fraction, and multivessel diseases. These patients were also more likely to receive aspirin, clopidogrel, or statin after discharge. After propensity score matching, there were no significant differences in baseline characteristics between the OMT and CTO‐PCI groups of the propensity score–matched population (Table 1).
Clinical Outcomes in the Total Population
The median duration of follow‐up was 7.9 years (interquartile range:, 5.0–11.2 years). Table 2 displays Kaplan‐Meier estimates of the clinical outcomes at 3 years, between 3 and 10 years, and at 10 years in the total population. The 10‐year rate of cardiac death was lower in the CTO‐PCI group (10.4%) than in the OMT group (22.3%, P<0.001). After adjustment using multivariable Cox regression model, the CTO‐PCI group had a significantly lower 10‐year rate of cardiac death (HR, 0.59; 95% CI, 0.43–0.80 [P<0.001]) than the OMT group, as well as that of all‐cause death, acute myocardial infarction, and any revascularization. At 3‐year follow‐up, the rate of cardiac death was not significantly different between the CTO‐PCI and OMT groups (4.0% versus 6.5%; HR, 0.88 [95% CI, 0.54–1.43]; P=0.61). Between 3‐ and 10‐year follow‐up, the rate of cardiac death was significantly lower in the CTO‐PCI group than the OMT group (6.7% versus 16.9%; HR, 0.47 [95% CI, 0.31–0.70]; P<0.001).
Table 2.
Clinical Outcomes in the Total Population
| OMT (n=664) | CTO‐PCI (n=883) | Univariable Analysis | Multivariable‐Adjusted | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| Cardiac death | ||||||
| At 3 y | 38 (6.5) | 34 (4.0) | 0.60 (0.38–0.95) | 0.03 | 0.88 (0.54–1.43) | 0.61 |
| Between 3 and 10 y | 68 (16.9) | 39 (6.7) | 0.35 (0.24–0.52) | <0.001 | 0.47 (0.31–0.70) | <0.001 |
| At 10 y | 106 (22.3) | 73 (10.4) | 0.44 (0.32–0.59) | <0.001 | 0.59 (0.43–0.80) | <0.001 |
| All‐cause death | ||||||
| At 3 y | 91 (14.8) | 73 (8.4) | 0.54 (0.40–0.73) | <0.001 | 0.78 (0.57–1.08) | 0.13 |
| Between 3 and 10 y | 135 (30.1) | 82 (12.8) | 0.37 (0.28–0.49) | <0.001 | 0.45 (0.34–0.60) | <0.001 |
| At 10 y | 226 (40.4) | 155 (20.1) | 0.43 (0.35–0.53) | <0.001 | 0.57 (0.46–0.71) | <0.001 |
| Acute myocardial infarction | ||||||
| At 3 y | 32 (5.5) | 26 (3.1) | 0.55 (0.33–0.92) | 0.02 | 0.63 (0.37–1.08) | 0.09 |
| Between 3 and 10 y | 25 (6.9) | 19 (2.9) | 0.46 (0.25–0.83) | 0.01 | 0.47 (0.26–0.86) | 0.01 |
| At 10 y | 57 (12.0) | 45 (5.8) | 0.51 (0.34–0.75) | <0.001 | 0.61 (0.40–0.92) | 0.02 |
| PCI at CTO lesions | ||||||
| At 3 y | 19 (3.3) | 41 (4.9) | 1.47 (0.86–2.54) | 0.16 | 1.65 (0.95–2.86) | 0.07 |
| Between 3 and 10 y | 12 (2.8) | 45 (8.0) | 2.33 (1.23–4.41) | 0.009 | 2.06 (1.06–3.98) | 0.03 |
| At 10 y | 31 (6.1) | 86 (12.4) | 1.81 (1.20–2.73) | 0.005 | 1.67 (1.10–2.53) | 0.02 |
| PCI at any lesions | ||||||
| At 3 y | 57 (9.9) | 79 (9.4) | 0.92 (0.66–1.30) | 0.65 | 1.03 (0.73–1.46) | 0.87 |
| Between 3 and 10 y | 51 (14.8) | 84 (14.8) | 0.96 (0.68–1.37) | 0.84 | 1.03 (0.72–1.48) | 0.87 |
| At 10 y | 108 (23.2) | 163 (22.8) | 0.94 (0.74–1.20) | 0.64 | 1.04 (0.80–1.34) | 0.77 |
| CABG | ||||||
| At 3 y | 25 (4.5) | 11 (1.3) | 0.29 (0.14–0.59) | <0.001 | 0.25 (0.12–0.53) | <0.001 |
| Between 3 and 10 y | 27 (7.5) | 11 (1.9) | 0.23 (0.12–0.47) | <0.001 | 0.25 (0.12–0.51) | <0.001 |
| At 10 y | 52 (11.6) | 22 (3.2) | 0.26 (0.16–0.42) | <0.001 | 0.23 (0.14–0.39) | <0.001 |
| Any revascularization | ||||||
| At 3 y | 81 (14.2) | 86 (10.3) | 0.70 (0.51–0.94) | 0.02 | 0.74 (0.54–1.01) | 0.06 |
| Between 3 and 10 y | 71 (21.6) | 92 (16.6) | 0.70 (0.51–0.95) | 0.02 | 0.71 (0.51–0.98) | 0.04 |
| At 10 y | 152 (32.7) | 178 (25.2) | 0.70 (0.56–0.87) | 0.001 | 0.72 (0.57–0.90) | 0.005 |
Percentages are presented as Kaplan‐Meier estimates. Hazard ratios (HRs) are for patients with chronic total occlusion (CTO) percutaneous coronary intervention (PCI) strategy relative to those with optimal medical therapy (OMT) strategy. Multivariable models for each end point were determined using backward stepwise elimination procedures where the least significant variable was removed one at a time from the full model including variables from Table 1. CABG indicates coronary artery bypass graft.
Clinical Outcomes in the Propensity Score–Matched Population
Table 3 summarizes the clinical outcomes at 3 years, between 3 and 10 years, and at 10 years in the propensity score–matched population. The CTO‐PCI group also had a significantly lower 10‐year rate of cardiac death (13.6% versus 20.8%; HR, 0.64 [95% CI, 0.45–0.91]; P=0.01) than the OMT group. The relative reduction in cardiac death at 10 years was mainly driven by a relative reduction between 3 and 10 years, not at 3 years. At 3‐year follow‐up, the rate of cardiac death was not significantly different between the CTO‐PCI and OMT groups (5.7% versus 5.0%; HR, 1.12 [95% CI, 0.63–2.00]; P=0.71). Between 3‐ and 10‐year follow‐up, the rate of cardiac death was significantly lower in the CTO‐PCI group than the OMT group (8.3% versus 16.6%; HR, 0.43 [95% CI, 0.27–0.71]; P<0.001) (Figure 2). The late‐diverging pattern of cardiac death was also observed in survival curves of all‐cause death, acute myocardial infarction, and any revascularization (Figure S1).
Table 3.
Clinical Outcomes in the Propensity Score–Matched Population
| OMT (n=456) | CTO‐PCI (n=456) | HR (95% CI) | P Value | |
|---|---|---|---|---|
| Cardiac death | ||||
| At 3 y | 21 (5.0) | 25 (5.7) | 1.12 (0.63–2.00) | 0.71 |
| Between 3 and 10 y | 50 (16.6) | 23 (8.3) | 0.43 (0.27–0.71) | <0.001 |
| At 10 y | 71 (20.8) | 48 (13.6) | 0.64 (0.45–0.91) | 0.01 |
| All‐cause death | ||||
| At 3 y | 47 (11.0) | 49 (10.9) | 0.98 (0.65–1.47) | 0.91 |
| Between 3 and 10 y | 93 (27.9) | 52 (16.2) | 0.52 (0.38–0.73) | <0.001 |
| At 10 y | 140 (35.9) | 101 (25.3) | 0.68 (0.53–0.87) | 0.002 |
| Acute myocardial infarction | ||||
| At 3 y | 23 (5.6) | 16 (3.7) | 0.65 (0.34–1.25) | 0.20 |
| Between 3 and 10 y | 17 (6.0) | 8 (2.7) | 0.43 (0.19–0.97) | 0.04 |
| At 10 y | 40 (11.2) | 24 (6.3) | 0.56 (0.34–0.92) | 0.02 |
| PCI at CTO lesions | ||||
| At 3 y | 15 (3.7) | 23 (5.4) | 1.45 (0.75–2.82) | 0.27 |
| Between 3 and 10 y | 10 (3.2) | 13 (5.1) | 1.23 (0.53–2.85) | 0.64 |
| At 10 y | 25 (6.8) | 36 (10.2) | 1.36 (0.82–2.25) | 0.23 |
| PCI at any lesions | ||||
| At 3 y | 47 (11.6) | 44 (10.3) | 0.86 (0.57–1.31) | 0.48 |
| Between 3 and 10 y | 39 (14.9) | 35 (12.9) | 0.79 (0.50–1.23) | 0.30 |
| At 10 y | 86 (24.7) | 79 (21.9) | 0.83 (0.61–1.12) | 0.22 |
| CABG | ||||
| At 3 y | 17 (4.3) | 7 (1.6) | 0.38 (0.16–0.93) | 0.03 |
| Between 3 and 10 y | 16 (6.2) | 3 (0.9) | 0.17 (0.05–0.57) | 0.004 |
| At 10 y | 33 (10.2) | 10 (2.5) | 0.28 (0.13–0.57) | <0.001 |
| Any revascularization | ||||
| At 3 y | 63 (15.6) | 50 (11.8) | 0.72 (0.49–1.06) | 0.09 |
| Between 3 and 10 y | 49 (19.7) | 37 (13.8) | 0.62 (0.41–0.94) | 0.03 |
| At 10 y | 112 (32.2) | 87 (23.9) | 0.68 (0.51–0.90) | 0.007 |
Percentages are presented as Kaplan‐Meier estimates. Hazard ratios (HRs) are for patients with chronic total occlusion (CTO) percutaneous coronary intervention (PCI) strategy relative to those with optimal medical therapy (OMT) strategy. CABG indicates coronary artery bypass graft.
Figure 2. Kaplan‐Meier event curves at 10 years and 3‐year landmark analysis for cardiac death.
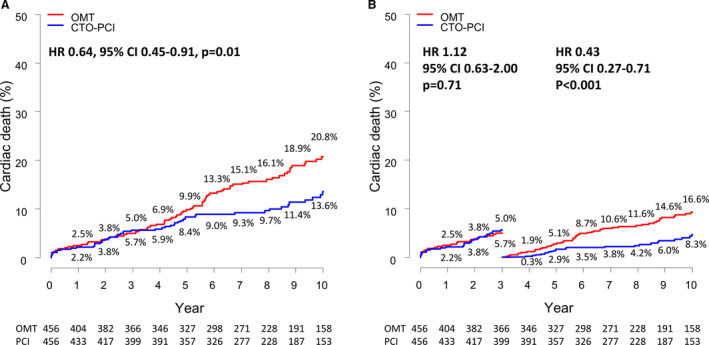
A, 10‐year cumulative event curves for cardiac death. B, Time‐to‐event curves with landmark analysis from 0 to 3 and 3 to 10 years for cardiac death. CTO indicates chronic total occlusion; HR, hazard ratio; OMT, optimal medical therapy; and PCI, percutaneous coronary intervention.
Subgroup Analysis
Cox regression analysis was performed to assess whether the reduction in cardiac death in the CTO‐PCI group compared with the OMT group was consistent among subgroups. Survival benefit of CTO‐PCI was seen in all subgroups tested for cardiac death (Figure 3). A trend for interaction between treatment strategy and CTO vessel on cardiac death was noted (P for interaction=0.06). Among the patients with CTO of the left anterior descending artery, the 10‐year rate of cardiac death was lower in the CTO‐PCI group than in the OMT group (9.9% versus 21.2%; HR, 0.39 [95% CI, 0.21–0.75]; P=0.004), whereas the rate was not significantly different in those without CTO of the left anterior descending artery (10.8% versus 13.3%; HR, 0.80 [95% CI, 0.52–1.23]; P=0.31).
Figure 3. Ten‐year rates of cardiac death among subgroup.
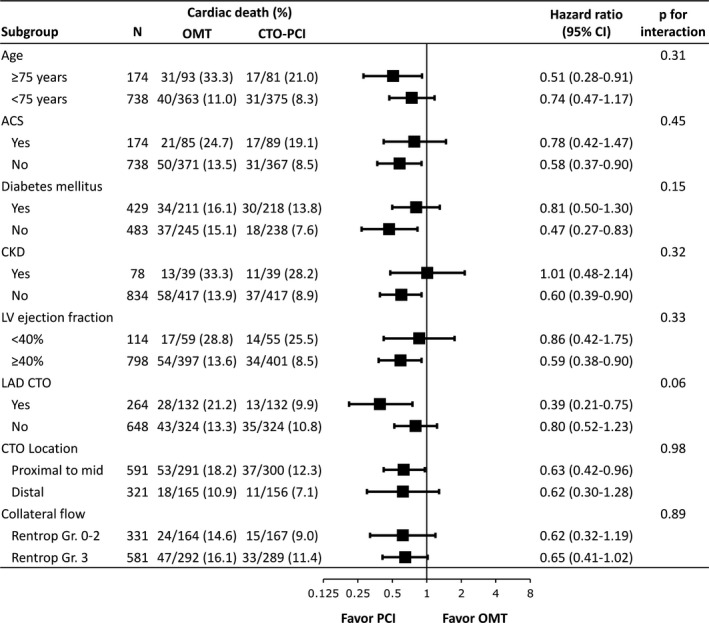
Hazard risks for 10‐year rate of cardiac death were estimated using the Cox regression analysis in subgroups of patients treated with chronic total occlusion (CTO) percutaneous coronary intervention (PCI) and optimal medical therapy (OMT). ACS indicates acute coronary syndrome; CKD; chronic kidney disease; LAD, left anterior descending; and LV, left ventricular.
Comparison of Clinical Outcomes Among the OMT, Failed CTO‐PCI, and Successful CTO‐PCI Groups
Table S1 summarizes the baseline characteristics in the OMT, failed CTO‐PCI, and successful CTO‐PCI groups. After adjustment using multivariable Cox regression model, the successful CTO‐PCI group had significantly lower risk of cardiac death than the failed CTO‐PCI group, as well as that of all‐cause death (Table S2). However, the OMT and failed CTO‐PCI groups had comparable risks of cardiac death and all‐cause death (Table S3).
DISCUSSION
In this large‐scale cohort of consecutive patients with CTO lesions, we compared extended long‐term clinical outcomes between CTO‐PCI and OMT as the initial treatment strategy. The major findings of our study were as follows: (1) patients in the CTO‐PCI group had a lower risk of cardiac mortality than patients in the OMT group, as well as that of all‐cause mortality, acute myocardial infarction, and any revascularization; (2) the differences diverged 3 years after initial treatment strategy; and (3) there were no significant interactions between the initial approach and cardiac death among the subgroups.
Several observational studies comparing CTO‐PCI with OMT have reported a lower risk of major adverse cardiac events with CTO‐PCI. 11 , 12 Methodological limitation prevents any definitive conclusions on the effects of CTO‐PCI. The DECISION‐CTO trial and EUROCTO are randomized controlled trials for systematically comparing the CTO‐PCI and OMT. In the DECISION‐CTO trial, 834 patients were randomly assigned to the CTO‐PCI (n=417) or OMT (n=398) group as an initial treatment strategy for CTO lesions. During a median follow‐up of 4 years, the incidence of the composite of death, myocardial infarction, stroke, and target vessel revascularization was similar between the PCI and OMT groups. 5 The study was underpowered because of early termination before achievement of target enrollment, and the high number of crossovers in the OMT group may have led to underestimation of the effect of the CTO‐PCI strategy. 13 EUROCTO also ended prematurely after randomizing 407 instead of the planned 1200 patients because of slow enrollment. At 12 months, the cardiovascular event rate was comparable between the CTO‐PCI and OMT groups. 4 Despite randomized controlled trials as a high level of evidence, both the DECISION‐CTO trial and EUROCTO were limited by low enrollment, leading to underpowered sample size and midterm follow‐up duration. In addition, both trials included a minimally symptomatic population (baseline angina frequency score >75). 14 It could be speculated that the patients who may see most advantage from CTO‐PCI were not included in the randomized controlled trials because of severe signs of ischemia, leading to enrollment of low‐risk patients and consequently limiting the inferential aim regarding risk reduction of hard outcomes. The rates of cardiac death in the DECISION‐CTO trial (2.7% during median follow‐up of 4 years) and EUROCTO (0.5% at 12 months) were lower than the 3‐year rate of cardiac death in the present study (4.7%). As a result of these limitations, extended long‐term follow‐up might help us to better understand the impact of CTO‐PCI in the general population.
An overall reduction in the 10‐year rate of cardiac death was observed in the present study, mainly driven by a reduction between 3 and 10 years, not at 3 years. The 3‐year rate of cardiac death was similar between the OMT and PCI groups, as with previous results of the DECISION‐CTO trial and EUROCTO. After 3 years, the event curve of cardiac death showed a significant late divergence in favor of CTO‐PCI, which can be partly explained through a greater increase of acute myocardial infarction in the OMT group (OMT versus CTO‐PCI between 3 and 10 years; 6.0% versus 2.7%, respectively). There are plausible explanations for increased myocardial infarction and cardiac mortality in patients with OMT. First, CTO‐PCI is associated with an increase in donor vessel fractional flow reserve, 15 and the risk of a clinical event including myocardial infarction is inversely proportional to the value of the fractional flow reserve. 16 It is possible that patients with CTO are vulnerable to myocardial infarction secondary to ischemic imbalance because of lower fractional flow reserve of collateral donor vessel. In addition, coronary collateral flow does not regress completely after CTO‐PCI. 17 It is possible that collateral recruitment or persistent collateral channels act as a protective role in case of reocclusion after CTO‐PCI. Moreover, patients with CTO present with a higher risk of cardiogenic shock or mortality, probably because of the larger myocardium area at risk if a donor vessel of CTO is the culprit artery. 18 Finally, meta‐analysis showed the association between presence of CTO lesions and increased risk of ventricular arrhythmia. 19 A recent study also reported that the CTO lesion was an independent predictor of ventricular tachycardia recurrence after successful ablation. 20 Association with ventricular arrhythmia could be another explanation for the increased cardiac mortality in patients with CTO. 21
Limitations
Our study was not randomized, and selection of treatment strategy was based on patient and physician preferences. The CTO‐PCI group had a lower prevalence of prior myocardial infarction, reduced left ventricular ejection fraction, and multivessel disease, and these patients were more likely to receive antiplatelets and statin. Such confounders induced a spurious association with clinical outcomes. Although we performed propensity score–matched analysis to adjust for potential confounding factors, the propensity score–matched analysis not only does not correct for unmeasured variables but also does not overcome initial selection bias. Second, we have no information regarding alteration of medical therapy during follow‐up and the health status assessment, including angina frequency, physical limitation, and quality of life because of the retrospective nature of our registry. Third, although the vital status and cause of death of all patients, including patients lost to follow‐up, were confirmed with Statistics Korea, we cannot exclude the possibility of under‐reporting of acute myocardial infarction and any revascularization. Finally, although the strength of this study is its large sample size, long‐term follow‐up, and enrollment of consecutive patients, it is still an observational study and could not be a substitute for a randomized controlled trial.
Conclusions
As the initial treatment strategy, PCI might reduce late cardiac death compared with OMT in patients with CTO. Extended follow‐up of the DECISION‐CTO trial and EUROCTO may confirm the findings of the present study.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S3
Figure S1
(J Am Heart Assoc.2021;10:e019022. DOI: 10.1161/JAHA.120.019022.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019022
For Sources of Funding and Disclosures, see page 10.
See Editorial by Walker and Kumbhani
References
- 1. Jeroudi OM, Alomar ME, Michael TT, El Sabbagh A, Patel VG, Mogabgab O, Fuh E, Sherbet D, Lo N, Roesle M, et al. Prevalence and management of coronary chronic total occlusions in a tertiary Veterans Affairs hospital. Catheter Cardiovasc Interv. 2014;84:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brilakis ES, Banerjee S, Karmpaliotis D, Lombardi WL, Tsai TT, Shunk KA, Kennedy KF, Spertus JA, Holmes DR Jr, Grantham JA. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2015;8:245–253. [DOI] [PubMed] [Google Scholar]
- 3. Patel MR, Gutierrez JA. Chronic total occlusion trials: a step in the right direction. JACC Cardiovasc Interv. 2017;10:2171–2173. [DOI] [PubMed] [Google Scholar]
- 4. Werner GS, Martin‐Yuste V, Hildick‐Smith D, Boudou N, Sianos G, Gelev V, Rumoroso JR, Erglis A, Christiansen EH, Escaned J, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39:2484–2493. [DOI] [PubMed] [Google Scholar]
- 5. Lee SW, Lee PH, Ahn JM, Park DW, Yun SC, Han S, Kang H, Kang SJ, Kim YH, Lee CW, et al. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion. Circulation. 2019;139:1674–1683. [DOI] [PubMed] [Google Scholar]
- 6. Kim BS, Yang JH, Jang WJ, Song YB, Hahn JY, Choi JH, Kim WS, Lee YT, Gwon HC, Lee SH, et al. Clinical outcomes of multiple chronic total occlusions in coronary arteries according to three therapeutic strategies: bypass surgery, percutaneous intervention and medication. Int J Cardiol. 2015;197:2–7. [DOI] [PubMed] [Google Scholar]
- 7. Yang JH, Kim BS, Jang WJ, Ahn J, Park TK, Song YB, Hahn JY, Choi JH, Lee SH, Gwon HC, et al. Optimal medical therapy vs. percutaneous coronary intervention for patients with coronary chronic total occlusion‐ a propensity‐matched analysis. Circ J. 2015;80:211–217. [DOI] [PubMed] [Google Scholar]
- 8. Jang WJ, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH, Kim WS, Lee YT, Gwon HC. Long‐term survival benefit of revascularization compared with medical therapy in patients with coronary chronic total occlusion and well‐developed collateral circulation. JACC Cardiovasc Interv. 2015;8:271–279. [DOI] [PubMed] [Google Scholar]
- 9. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–651. [DOI] [PubMed] [Google Scholar]
- 10. Park TK, Song YB, Ahn J, Carriere KC, Hahn JY, Yang JH, Choi SH, Choi JH, Lee SH, Gwon HC. Clopidogrel versus aspirin as an antiplatelet monotherapy after 12‐month dual‐antiplatelet therapy in the era of drug‐eluting stents. Circ Cardiovasc Interv. 2016;9:e002816. DOI: 10.1161/CIRCINTERVENTIONS.115.002816. [DOI] [PubMed] [Google Scholar]
- 11. Tomasello SD, Boukhris M, Giubilato S, Marza F, Garbo R, Contegiacomo G, Marzocchi A, Niccoli G, Gagnor A, Varbella F, et al. Management strategies in patients affected by chronic total occlusions: results from the Italian Registry of Chronic Total Occlusions. Eur Heart J. 2015;36:3189–3198. [DOI] [PubMed] [Google Scholar]
- 12. Iannaccone M, D'Ascenzo F, Piazza F, De Benedictis M, Doronzo B, Behnes M, Garbo R, Mashayekhi K. Optimal medical therapy vs. coronary revascularization for patients presenting with chronic total occlusion: a meta‐analysis of randomized controlled trials and propensity score adjusted studies. Catheter Cardiovasc Interv. 2019;93:E320–E325. [DOI] [PubMed] [Google Scholar]
- 13. Brilakis ES, Mashayekhi K, Burke MN. How DECISION‐CTO can help guide the decision to perform chronic total occlusion percutaneous coronary intervention. Circulation. 2019;139:1684–1687. [DOI] [PubMed] [Google Scholar]
- 14. Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long‐term outcome in outpatients with coronary disease. Circulation. 2002;106:43–49. [DOI] [PubMed] [Google Scholar]
- 15. Ladwiniec A, Cunnington MS, Rossington J, Mather AN, Alahmar A, Oliver RM, Nijjer SS, Davies JE, Thackray S, Alamgir F, et al. Collateral donor artery physiology and the influence of a chronic total occlusion on fractional flow reserve. Circ Cardiovasc Interv. 2015;8:e002219. DOI: 10.1161/CIRCINTERVENTIONS.114.002219. [DOI] [PubMed] [Google Scholar]
- 16. Ahn JM, Park DW, Shin ES, Koo BK, Nam CW, Doh JH, Kim JH, Chae IH, Yoon JH, Her SH, et al. Fractional flow reserve and cardiac events in coronary artery disease: data from a prospective IRIS‐FFR Registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve). Circulation. 2017;135:2241–2251. [DOI] [PubMed] [Google Scholar]
- 17. Perera D, Kanaganayagam GS, Saha M, Rashid R, Marber MS, Redwood SR. Coronary collaterals remain recruitable after percutaneous intervention. Circulation. 2007;115:2015–2021. [DOI] [PubMed] [Google Scholar]
- 18. Allahwala UK, Jolly SS, Dzavik V, Cairns JA, Kedev S, Balasubramanian K, Stankovic G, Moreno R, Valettas N, Bertrand O, et al. The presence of a CTO in a non‐infarct‐related artery during a STEMI treated with contemporary primary PCI is associated with increased rates of early and late cardiovascular morbidity and mortality: the CTO‐TOTAL Substudy. JACC Cardiovasc Interv. 2018;11:709–711. [DOI] [PubMed] [Google Scholar]
- 19. Shimokawahara H, Ogawa A, Mizoguchi H, Yagi H, Ikemiyagi H, Matsubara H. Vessel stretching is a cause of lumen enlargement immediately after balloon pulmonary angioplasty: intravascular ultrasound analysis in patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2018;11:e006010. DOI: 10.1161/CIRCINTERVENTIONS.117.006010. [DOI] [PubMed] [Google Scholar]
- 20. Di Marco A, Paglino G, Oloriz T, Maccabelli G, Baratto F, Vergara P, Bisceglia C, Anguera I, Sala S, Sora N, et al. Impact of a chronic total occlusion in an infarct‐related artery on the long‐term outcome of ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2015;26:532–539. [DOI] [PubMed] [Google Scholar]
- 21. Behnes M, Akin I, Kuche P, Schupp T, Reiser L, Bollow A, Taton G, Reichelt T, Ellguth D, Engelke N, et al. Coronary chronic total occlusions and mortality in patients with ventricular tachyarrhythmias. EuroIntervention. 2020;15:1278–1285. DOI: 10.4244/EIJ-D-18-00496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


