Abstract
Bioprosthetic mitral structural valve degeneration and failed mitral valve repair (MVr) have traditionally been treated with reoperative mitral valve surgery. Transcatheter mitral valve‐in‐valve (MVIV) and valve‐in‐ring (MVIR) replacement are now feasible, but data comparing these approaches are lacking. We sought to compare the outcomes of (1) reoperative mitral valve replacement (redo‐MVR) and MVIV for structural valve degeneration, and (2) reoperative mitral valve repair (redo‐MVr) or MVR and MVIR for failed MVr. A literature search of PubMed, Embase, and the Cochrane Library was conducted up to July 31, 2020. Thirty‐two studies involving 25 832 patients were included. Redo‐MVR was required in ≈35% of patients after index surgery at 10 years, with 5% to 15% 30‐day mortality. MVIV resulted in >95% procedural success with 30‐day and 1‐year mortality of 0% to 8% and 11% to 16%, respectively. Recognized complications included left ventricular outflow tract obstruction (0%–6%), valve migration (0%–9%), and residual regurgitation (0%–6%). Comparisons of redo‐MVR and MVIV showed no statistically significant differences in mortality (11.3% versus 11.9% at 1 year, P=0.92), albeit higher rates of major bleeding and arrhythmias with redo‐MVR. MVIR resulted in 0% to 34% mortality at 1 year, whereas both redo‐MVr and MVR for failed repairs were performed with minimal mortality and durable long‐term results. MVIV is therefore a viable alternative to redo‐MVR for structural valve degeneration, whereas redo‐MVr or redo‐MVR is preferred for failed MVr given the suboptimal results with MVIR. However, not all patients will be candidates for MVIV/MVIR because anatomical restrictions may preclude transcatheter options from adequately addressing the underlying pathology.
Keywords: redo mitral valve repair, reoperative mitral valve replacement, transcatheter mitral valve replacement, valve‐in‐ring, valve‐in‐valve
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- LVOTO
left ventricular outflow tract obstruction
- MVIR
transcatheter mitral valve‐in‐ring replacement
- MVIV
transcatheter mitral valve‐in‐valve replacement
- MVr
mitral valve repair
- MVR
mitral valve replacement
- redo‐MVr
reoperative mitral valve repair
- redo‐MVR
reoperative surgical mitral valve replacement
- SVD
structural valve degeneration
- TMVR
transcatheter mitral valve replacement
Structural valve degeneration (SVD) of bioprosthetic mitral valves is common and frequently occurs after 5 or more years after initial valve replacement. 1 , 2 , 3 , 4 , 5 Reoperative surgical mitral valve replacement (redo‐MVR) has been the gold standard treatment for bioprosthetic SVD for many years and is required in up to 35% of patients at 10 years after index surgery. 6 , 7 Redo‐MVR is, however, associated with significant mortality and morbidities, particularly among elderly patients. 8 Similarly, mitral valve replacement (MVR) and reoperative mitral valve repair (redo‐MVr) are options for patients with failed MVr, but with significant attendant risks in inexperienced centers. At the time of SVD or repair failure, patients tend to be older and hence are at a higher risk for reoperation. To meet the needs of these high‐risk populations, transcatheter MVR (TMVR) using valve‐in‐valve (MVIV) and valve‐in‐ring (MVIR) techniques have emerged in the past few years. However, there have been no clinical trials comparing the aforementioned approaches, with very few studies offering direct comparisons. Here, we critically review and assess the available data comparing the outcomes of redo‐MVR and MVIV for SVD, as well as redo‐MVr, MVR, and MVIR for those with failed MVrs.
METHODS
The authors declare that all supporting data, analytic methods, and study materials are available within this article (and its online supplementary files). A comprehensive search of PubMed, Embase, and the Cochrane Library was performed without any limitations to identify all studies up to July 31, 2020. The keywords “reoperative mitral valve replacement,” “mitral valve replacement,” “mitral valve repair,” “transcatheter mitral valve replacement”, “valve‐in‐valve”, “valve‐in‐ring”, “reoperative mitral valve repair,” and “re‐repair mitral valve” were indexed in all combinations for original reports and clinical work, including cross‐sectional studies, observational studies, clinical trials, and reviews.
Studies evaluating (1) bioprosthetic mitral SVD, (2) reoperative mitral valve surgery, be it re‐repair, MVR for a failed surgical annuloplasty, or redo‐MVR, or (3) MVIV or MVIR for previous mitral valve surgery were included. Studies were excluded if they assessed outcomes not related to patients with previous mitral repairs or replacements or case reports. Throughout this process, the Cochrane methodology and Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines were followed. The Newcastle‐Ottawa Scale Quality Assessment scores were used to rate all included studies (Table S1). Two independent investigators (A.S., F.Y.) assessed quality ratings, and disagreements were resolved via mutual discussion.
Primary variables of interest were short‐ and midterm mortality, along with the rates of commonly recurring adverse events such as left ventricular outflow tract obstruction (LVOTO), valve thrombosis, valve migration or embolization, major vascular complication, life‐threatening or major bleeding, and postprocedural mitral regurgitation.
Note that a meta‐analysis was not performed for a variety of reasons. First, most of the transcatheter series included patients with relatively small sample sizes. Although small samples can still be combined with caution, we felt that this would detract from the generalizability of our results. Second, among the surgical series compiled, short‐term outcomes were consistently reported, but mid‐ and long‐term outcomes were reported on a case‐by‐case basis given the wide heterogeneity among these studies. Moreover, we do not have long‐term data following MVIV and MVIR. Finally, although the conduct of surgical MV replacement and repair is generally well established, the technical aspects of MVIV and MVIR are evolving. Thus, earlier transcatheter series differ considerably from later series, especially with regard to the delivery technique used.
RESULTS
Our initial search identified 2123 studies, 708 of which remained after de‐duplication. 79 full‐text articles were assessed for eligibility and 32 were included in our qualitative synthesis (Figure 1). The vast majority of these studies were retrospective cohort and case‐control studies, with 1 prospective comparative cohort trial.
Figure 1. PRISMA diagram for study selection and identification.
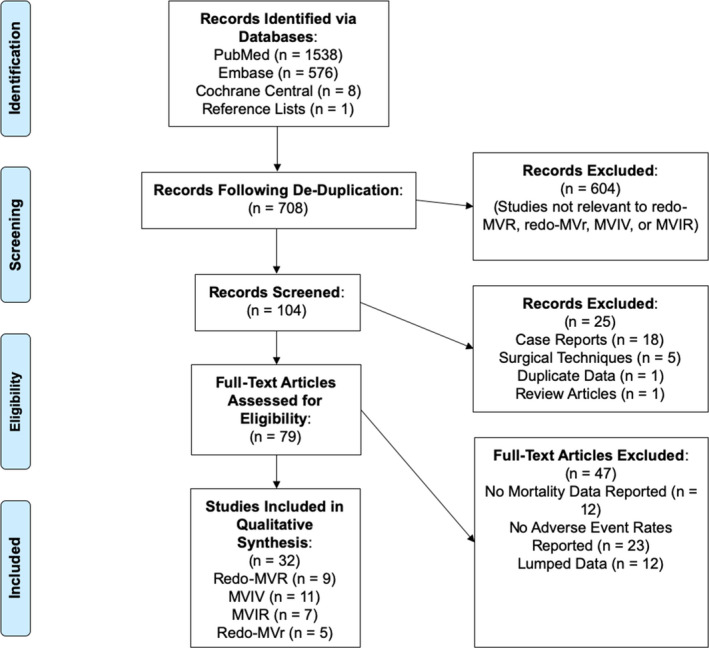
PRISMA flowchart for selection of studies for eventual inclusion in the systematic review. MVIR indicates transcatheter mitral valve‐in‐ring replacement; MVIV, transcatheter mitral valve‐in‐valve replacement; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses; redo‐MVr, reoperative mitral valve repair; and redo‐MVR, reoperative mitral valve replacement.
Surgical and Transcatheter Options in Degenerated Bioprosthetic Mitral Valves
Reoperative Surgical Mitral Valve Replacement
Bioprosthetic mitral valves are subject to SVD because of prosthesis degeneration, thrombosis, and paravalvular leak and may lead to significant stenosis, regurgitation, or both. Rates of SVD vary widely in the literature but have been reported at ≈25% to 30% at 10 years and ≈50% to 70% at 15 years. 9 Similarly, freedom from reoperation is estimated at 96.6%, 86.6%, and 75.3% at 5, 10, and 15 years, respectively. 10
Short‐ and long‐term outcomes
The volume of redo‐MVR has increased steadily over the past few decades, with a concomitant improvement in outcomes. 8 , 11 , 12 Mortality at 30 days following redo‐MVR ranges from 5% to 15% (Figure 2). 7 , 21 Age, sex, preoperative New York Heart Association class, indication for reoperation, type of prosthetic valve, number of previous operations, hepatic and renal failure, and timing of reoperation have all been associated with mortality and postoperative complications after redo‐MVR. 15 Preoperative diagnosis is a key driver of mortality in most series, with prosthetic valve endocarditis high in the rank list. 11 Note that because prosthetic valve endocarditis is virtually always tackled with surgery as opposed to TMVR, comparisons of the 2 techniques should exclude patients with endocarditis.
Figure 2. Thirty‐day mortality following reoperative mitral valve replacement (MVR) for bioprosthetic mitral structural valve degeneration (SVD).
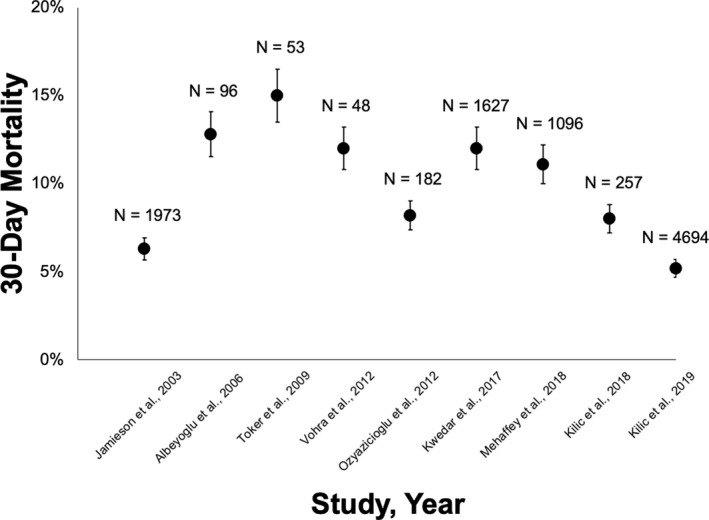
Shown here are the 30‐day mortality rates, in ascending chronological order of year of publication, following redo‐MVR for bioprosthetic mitral SVD among various surgical series. The error bars indicate the 95% CIs. 7 , 8 , 14 , 17 , 18 , 20 , 22 , 23
Long‐term outcomes are highly variable in the literature and are not consistently reported. As an example, Vohra et al retrospectively looked at outcomes in 49 adults with bioprosthetic or mechanical valves who underwent redo‐MVR between 2000 and 2010 with a mean follow‐up of 47.5±37 months. Median time to reoperation was 8.2±6.6 years for first‐time redo‐MVR and 6.4±5.6 years for second‐time redo‐MVR. In‐hospital mortality was 12%, and mortality rates at 1 and 5 years were 18.4% and 27.2%, respectively. 7 In another report of 347 reoperations on mitral prostheses, Bortolotti et al reported actuarial survival rates of 63±3% at 5 years, 38±4% at 10 years, and 24±5% at 15 years. 16 Note that the choice of prosthesis at the index operation has implications for potential reintervention because transcatheter approaches cannot be performed in patients with prior mechanical valves.
Transcatheter Mitral Valve‐in‐Valve Replacement
In the United States, the SAPIEN 3 valve (Edwards Lifesciences LLC, Irvine, CA) was approved in 2017 for MVIV by the U.S. Food and Drug Administration in high surgical‐risk patients and is the only transcatheter valve that is approved for MVIV. 24 , 25
Short‐ and midterm outcomes
Patient characteristics from the various MVIV studies are shown in Table 1. Reported outcomes of MVIV are limited to 30 days to 1 year given that this is a relatively new procedure. Mortality at 30 days and 1 year following MVIV has been reported at 0% to 8% and 11% to 16%, respectively (Figure 3), with >95% procedural success. 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36
Table 1.
Patient Characteristics in Studies of Transcatheter Mitral Valve‐in‐Valve Replacement
| Study, YearREF | Mean Age (y) | Female (%) | NYHA Class (%) | STS‐PROM (%) | DM (%) | Cr (mmol/L) | HTN (%) | PVD (%) | CVA (%) | COPD (%) | LVEF (%) | Failure Mechanism (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yoon et al, 2019 (N=322) 35 | 72.6±12.9 | 58.7 |
III: 87.8 IV: 32.3 |
9.2±7.2 | 23.3 | 130±113 | 69.6 | 11.1 | 17.7 | 28.6 | 55.3±11.5 |
MR: 36.6 MS: 40.7 B: 22.7 |
| Guerrero et al, 2019 (N=1326) 37 , * | 73.4±11.9 | 59.2 | ≥III: 86.5 | 11.0±8.6 | NA | na (5.3% on dialysis) | NA | NA | 17.5 | 46.2 | 54.9±12.1 |
MR: 55.6 MS: 25.0 B: 19.4 |
| Hu et al, 2018 (N=172) 30 | 74.5±12.5 | 53.5 | ≥III: 97.3 | 16.8±15.2 | 17.2 | NA (35.2% chronic renal failure) | NA | NA | NA | NA | 51.2±11.5 |
MR: 49.3 MS: 31.9 B: 18.8 |
| Urena et al, 2018 (N=34) 32 | 73.0 | 70.6 |
≥III: 91.2 |
NA (Euro‐SCORE II: 10.9 | 17.6 | NA (67.6% eGFR <60 mL/min) | NA | NA | NA | 20.6 | 58.0±10.0 | NA |
| Kamioka et al, 2018 (N=62) 31 | 74.9±9.4 | 61.3 | IV: 30.7 | 8.7±10.1 | 24.2 | 133±133 | 38.7 | 6.5 | 35.5 | 33.9 | 54.6±11.9 |
MR: 50.0 MS: 71.0 B: 8.1 |
| Yoon et al, 2017 (N=176) 36 | 72.9±12.8 | 63.1 |
≥III: 88.1 |
9.3±7.0 | 26.1 | 124±98 | 61.9 | 6.3 | 21.0 | 24.4 | 55.3±11.1 |
MR: 36.4 MS: 35.8 B: 27.8 |
| Eleid et al, 2017 (N=60) 29 | 75.0±11.0 | 57.0 |
III: 45.0 IV: 55.0 |
12.5±7.2 | 22.0 | 115±44 | 85.0 | 17.0 | 8.0 | 35.0 | 57.0±11.0 |
MR: 63.0 MS: 32.0 B: 5.0 |
| Eleid et al, 2016 (N=33) 28 | 76.0±11.0 | 60.0 |
III: 48.0 IV: 52.0 |
13.2±7.4 | 23.0 | 133±80 | 85.0 | 17.0 | 10.0 | 48.0 | 56.0±12.0 |
MR: 60.6 MS: 33.3 B: 6.1 |
| Ye et al, 2015 (N=31) 34 | 78.7±8.8 | 58.0 |
≥III: 96.8 |
9.7 (5–16.6) | 22.6 | >150: 6.5% | NA | 12.9 | 32.3 | 22.6 | 60.0 (40.0–65.0) |
MR: 54.2 MS: 38.7 B: 16.1 |
| Cheung et al, 2013 (N=23) 27 | 81.1±5.8 | 60.9 |
≥III: 95.6 |
12.6±6.9 | 17.4 | NA (56.5% chronic renal failure) | NA | 30.4 | 34.8 | 26.1 | 54.5±12.3 |
MR: 39.1 MS: 30.4 B: 30.4 |
| Wilbring et al, 2013 (N=7) 33 | 79 (Q1–Q3: 75–81) | 71.4 | III: 100% | 12.3±2.1 | 42.9 | NA (42.9% chronic renal failure) | NA | NA | 71.4 | NA | 49.3±11.8 |
MR: 100% MS: 0% B: 28.6% |
Patient characteristics in 11 studies of transcatheter mitral valve‐in‐valve replacement are shown here in descending order of year of publication. B indicates both; COPD, chronic obstructive pulmonary disease; Cr, creatinine clearance; CVA, cerebrovascular accident; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; Euro‐SCORE, European System for Cardiac Operative Risk Evaluation; HTN, hypertension; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MS, mitral stenosis; NA, not available; NYHA, New York Heart Association; PVD, peripheral vascular disease; Q1, quartile 1; Q3, quartile 3; and STS‐PROM, Society of Thoracic Surgeons‐Predicted Risk of Mortality.
Transseptal access only.
Figure 3. Thirty‐day and 1‐year mortality following mitral valve‐in‐valve (MVIV) replacement.
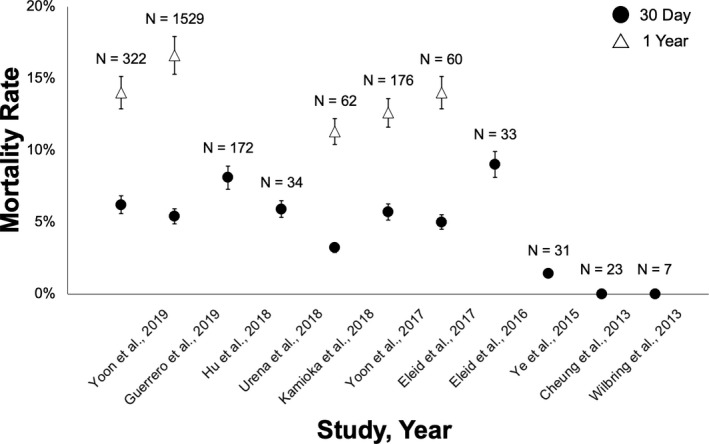
Mortality rates at 30 days and 1 year are shown among the various MVIV series evaluated, in descending chronological order of year of publication. The error bars indicate the 95% CIs. 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36
Of note, Guerrero and colleagues recently reported the 1‐year outcomes of 1576 patients undergoing MVIV, with either a transapical or transseptal approach, from the STS/ACC TVT (Society for Thoracic Surgeons/ American College of Cardiology Transcatheter Valve) Registry. 1‐year mortality was reported at 21.7% and 15.8% for the transapical and transseptal groups, respectively. Furthermore, higher complication rates (device embolization, LVOTO, and conversion to open surgery) and worse outcomes (all‐cause mortality and cardiovascular death at 30 days and 1 year) were noted with the transapical approach. 37
Direct comparison with reoperative mitral valve replacement
Few data directly comparing redo‐MVR to MVIV exist in the literature. 38 Kamioka et al compared the clinical and echocardiographic outcomes of 59 patients who underwent redo‐MVR with 62 who underwent MVIV (22.6% transapical; 77.4% transseptal) up to August 2017. Patients who had active endocarditis, required concomitant procedures for coronary artery disease or aortic disease, or underwent additional valve replacement were excluded. There was no statistical difference in mortality at 30 days (MVIV 3.2% versus redo‐MVR 3.4%, P=1.00) and at 1 year between the 2 groups (MVIV 11.3% versus redo‐MVR 11.9%, P=0.92). MVIV was associated with a much lower rate of major bleeding and atrial arrhythmias, as well as a shorter hospital stay. 31
Complications
Reported complications of MVIV include LVOTO, valve migration or embolization, elevated postprocedural gradients, residual mitral regurgitation, and valve thrombosis (Table 2).
Table 2.
Short‐Term Outcomes of Transcatheter Mitral Valve‐in‐Valve Replacement
| Study, YearREF | 30‐d Mortality | Stroke | Bleeding* | MVC | Device Thrombosis | Technical Success | LVOTO | Valve Embolization | ≥3+Residual MR |
|---|---|---|---|---|---|---|---|---|---|
| Yoon et al, 2019 (N=322) 35 | 6.2 | 2.3 | 4.6 | 1.6 | NA | 94.4 | 2.2 | 1.7 | 3.3 |
| Guerrero et al, 2019 (N=1326) 37 , * |
TS: 5.0 TA: 8.1 |
TS: 1.1 TA: 1.0 |
NA |
TS: 1.2 TA: 2.5 |
TS: 0.2 TA: 0.5 |
TS: 97.3 TA: 94.6 |
TS: 0.8 TA: 2.0 |
TS: 0.2 TA: 0.5 |
NA |
| Hu et al, 2018 (N=172) 30 | 7.5 | 3.2 | 8.7 | 0.0 | 3.2 | 97.1 | 0.0 | 5.3 | 5.5 |
| Urena et al, 2018 (N=34) 32 | 5.9 | 5.9 | 5.9 | 5.9 | 8.8 | 94.1 | 5.9 | 2.9 | 0.0 |
| Kamioka et al, 2018 (N=62) 31 | 3.2 | 0.0 | 6.5 | 1.6 | 1.9 | NA | 3.2 | NA | 3.8 |
| Yoon et al, 2017 (N=176) 36 | 5.7 | 2.3 | 2.3 | 1.7 | NA | 96.0 | 3.2 | 1.1 | 3.6 |
| Eleid et al, 2017 (N=60) 29 | 6.0 | 0.0 | 7.0 | 3.3 | 2.0 | 97.0 | 5.0 | 0.0 | 0.0 |
| Eleid et al, 2016 (N=33) 28 | 8.0 | 0.0 | 8.0 | 0.0 | 2.0 | 93.9 | 4.0 | 6.0 | 0.0 |
| Ye et al, 2015 (N=31) 34 | 1.0 | 3.2 | 3.2 | 0.0 | 0.0 | 98.6 | NA | 0.0 | 5.0 |
| Cheung et al, 2013 (N=23) 27 | 0.0 | 4.4 | 0.0 | NA | 0.0 | 100.0 | NA | 0.0 | NA |
| Wilbring et al, 2013 (N=7) 33 | 0.0 | NA | 0.0 | NA | NA | 100.0 | NA | NA | NA |
Procedural and 30‐day outcomes of transcatheter mitral valve‐in‐valve replacement are shown. LVOTO indicates left ventricular outflow tract obstruction; MR, mitral regurgitation; MVC, major vascular complication; NA, not available; TA, transapical; and TS, transseptal.
Life‐threatening bleeding.
Surgical and Transcatheter Options in Failed Mitral Valve Repair
Patients with failed surgical MVr have historically undergone rerepair or MVR. Given the risks associated with reoperation, MVIR, although not approved by the Food and Drug Administration, has emerged as an alternative option, but robust data comparing these approaches are lacking. 39
Mitral Valve Rerepair Following Failed Surgical Annuloplasty
Short‐ and long‐term outcomes following redo‐MVr are generally favorable in experienced mitral centers. 40 , 41 For instance, Kilic et al reported on 305 patients with previous repairs, 48 of whom underwent redo‐MVr. There was 0% operative mortality with 96% long‐term freedom from mortality at 5 years. 22
Surgical Mitral Valve Replacement Following Failed Annuloplasty
MVR is also an option for patients with failed mitral bands or rings. Evidence suggests that, although in‐hospital mortality and rates of postoperative complications may favor MVR, long‐term mortality is likely similar to that of surgical rerepair. 18 In the study by Kilic et al, 257 of 305 patients underwent MVR following a previous repair. 8% operative mortality was noted, along with an increase in blood transfusion rates and duration of mechanical ventilation compared with the redo‐MVr cohort. However, long‐term freedom from mortality was comparable to the 48 patients who underwent rerepair (P=0.29). 22
Multiple groups have reported on the outcomes of reoperative mitral valve surgery without making the distinction between rerepair and reoperative replacement. 8 , 23 In an early series, specific to reoperation in failed MVr, Cerfolio et al reported an operative mortality of 4.0%, with 87.0% of patients in New York Heart Association class I or II at 5‐year follow‐up. 42 A contemporary cohort of reoperation for failed MVr was recently analyzed, with a reported mortality of 6.5%. A propensity‐matched cohort from that series indicated that the failed MVr itself did not affect survival compared with other reoperative procedures with normal mitral valves. 43 Other groups have reported mortality rates between 0% and 9.0%. 40 , 41 , 44 , 45
Transcatheter Mitral Valve‐in‐Ring Replacement
In contrast to surgical reoperation, MVIR is much less invasive and avoids issues related to redo sternotomies and cardiopulmonary bypass. Several articles have reported preliminary outcomes. Patient characteristics are shown in Table 3. Thirty‐day and 1‐year mortality are estimated at 0% to 18% and 0% to 34%, respectively (Figure 4). Studies of MVIR have also noted several recurrent complications (Table 4). 30 , 57 Of note, only 3 of these studies reported the characteristics of the rings that had been implanted. 29 , 36 , 49
Table 3.
Patient Characteristics in Studies of Transcatheter Mitral Valve‐in‐Ring Replacement
| Study, YearREF | Mean Age (y) | Female (%) | NYHA Class (%) | STS‐PROM (%) | DM (%) | Cr (mmol/L) | HTN (%) | PVD (%) | CVA (%) | COPD (%) | LVEF (%) | Failure Mechanism (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yoon et al, 2019 (N=141) 35 | 71.7±9.7 | 36.9 |
III: 89.4 IV: 25.5 |
8.1±46.3 | 21.3 | 145±104 | 68.6 | 10.6 | 12.1 | 27.0 | 44.3±15.7 |
MR: 77.3 MS: 6.4 B: 16.3 |
| Hu et al, 2018 (N=73) 30 | 70.0±10.8 | 40.4 | ≥III: 100.0 | 13.4±9.0 | 14.0 | NA (28.1% Chronic Renal Failure) | NA | NA | NA | NA | 36.7±14.5 |
MR: 68.2 MS: 24.2 B: 7.6 |
| Urena et al, 2018 (N=30) 32 | 70.0 | 70.0 | ≥III: 80.0 | NA (Euro‐SCORE II: 9.6%) | 13.3 | NA (56.7% eGFR <60 mL/min) | NA | NA | NA | 30.0 | 57.0±10.0 | NA |
| Long et al, 2018 (N=8) 53 | 68.7±14.2 | 50.0 |
III: 12.5 IV: 4.2 |
8.9±5.2 | 20.8 | NA | NA | NA | NA | 66.5 | 42.5±14.1 |
MR: 16.6 MS: 70.8 B: 12.5 |
| Yoon et al, 2017 (N=72) 36 | 71.4±10.2 | 41.7 | ≥III: 91.7 | 8.1±6.2 | 16.7 | 150±124 | 56.9 | 9.7 | 5.6 | 27.8 | 45.6±17.4 |
MR: 77.8 MS: 4.2 B: 18.1 |
| Eleid et al, 2017 (N=15) 29 | 72±8 | 60.0 |
III: 47.0 IV: 53.0 |
11.4±7.3 | 13.0 | 150±141 | 73.0 | 20.0 | 7.0 | 40.0 | 50.0±19.0 |
MR: 73.0 MS: 20.0 B: 7.0 |
| Descoutures et al, 2013 (N=17) 49 | 70.0±16.0 | NA | ≥III: 100.0 | 13.0±9.0 | NA | NA | NA | NA | NA | NA | NA |
MR: 70.6 MS: 29.4 |
Patient characteristics in 7 studies of transcatheter mitral valve‐in‐ring replacement are shown here in descending order of year of publication. B indicates both; COPD, chronic obstructive pulmonary disease; Cr, creatinine clearance; CVA, cerebrovascular accident; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; Euro‐SCORE, European System for Cardiac Operative Risk Evaluation; HTN, hypertension; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MS, mitral stenosis; NA, not available; NYHA, New York Heart Association; PVD, peripheral vascular disease; and STS‐PROM, Society of Thoracic Surgeons‐Predicted Risk of Mortality.
Figure 4. Thirty‐day and 1‐year mortality following mitral valve‐in‐ring (MVIR) replacement.

Mortality rates at 30 days and 1 year are shown among the various MVIR series, in descending chronological order of year of publication. The error bars indicate the 95% CIs. 29 , 30 , 32 , 35 , 36 , 49 , 53
Table 4.
Short‐Term Outcomes of Transcatheter Mitral Valve‐in‐Ring Replacement
| Study, YearREF | 30‐d Mortality | Stroke | Bleeding* | MVC | Device Thrombosis | Technical Success | LVOTO | Valve Embolization | ≥3+Residual MR |
|---|---|---|---|---|---|---|---|---|---|
| Yoon et al, 2019 (N=141) 35 | 9.9 | 0.0 | 6.7 | 3.8 | NA | 80.9 | 5.0 | 1.4 | 12.6 |
| Hu et al, 2018 (N=73) 30 | 6.8 | 5.4 | 0.0 | 1.0 | 0.0 |
TA: 89.7 TS: 86.7 |
6.0 | 5.0 | 12.0 |
| Urena et al, 2018 (N=30) 32 | 6.7 | 0.0 | 3.3 | 6.7 | 6.7 | 80.0 | 0.0 | 3.4 | NA |
| Long et al, 2018 (N=8) 53 | 0.0 | NA | NA | NA | 0.0 | 100.0 | 8.3 | 0.0 | NA |
| Yoon et al, 2017 (N=72) 36 | 8.3 | 0.0 | 8.3 | 1.4 | NA | 83.3 | 2.3 | 2.8 | 13.6 |
| Eleid et al, 2017 (N=15) 29 | 0.0 | NA | 13.0 | 0.0 | 7.0 | 73.0 | 20.0 | 13.0 | <10 |
| Descoutures et al, 2013 (N=17) 49 | 18.0 | NA | NA | NA | NA | 89.0 | NA | NA | NA |
Procedural and 30‐day outcomes of transcatheter mitral valve‐in‐ring replacement are shown. LVOTO indicates left ventricular outflow tract obstruction; MR, mitral regurgitation; MVC, major vascular complication; NA, not available; TA, transapical; and TS, transseptal.
Life‐threatening bleeding.
Studies with long‐term outcomes following MVIR are limited. The largest series of TMVR cases published to date by Yoon et al included 141 MVIR cases from an international registry. Procedural success was achieved in 80.9% of these cases with a 30‐day mortality of 9.9% and 1‐year mortality of 30.6%. 35
DISCUSSION
Two key conclusions are evident from this systematic review: (1) given its safety profile, MVIV is a viable alternative to redo‐MVR in high surgical‐risk patients with bioprosthetic mitral SVD, with certain caveats; and (2) for those with failed surgical mitral annuloplasty, reoperative MVr or MVR is preferred to MVIR in suitable candidates. For certain high‐ or extreme‐surgical risk patients, however, MVIR may be appropriate after a thorough assessment of all procedural risks and evaluation of the type of ring that has been implanted.
Any discussion of bioprosthetic mitral SVD and failed MVr begins with a basic understanding of the surgical principles of MVR and MVr and their relation to the technical aspects of MVIV and MVIR, respectively. In contemporary practice, when repair is not feasible, a valve replacement operation that leaves leaflets and chords intact is preferred for optimal preservation of cardiac function. During chordal‐sparing valve replacement, the posterior leaflet is usually preserved, and the anterior leaflet may be divided centrally and then plicated to avoid prosthetic leaflet impingement and LVOTO. 58 , 59 In contrast, the anterior leaflet is almost always left intact during MVr. Thus, the anatomy after MVr is significantly different from that following MVR. As is discussed next, this has significant implications for subsequent transcatheter therapies, especially with regard to the risk of postprocedural LVOTO, and may partially explain why we observe better outcomes with MVIV than with MVIR.
Reoperative Mitral Valve Replacement and Transcatheter Mitral Valve‐in‐Valve Replacement
Prosthetic valve reoperations carry significant risks. Thirty‐day mortality is estimated at 5% to 15% with significantly higher rates of adverse events, including major bleeding and atrial arrhythmias, when compared with MVIV. In contrast, short‐term mortality for MVIV ranges from 0% to 9%, with comparable valve hemodynamics and improvements in functional status at 1 year.
There are several common themes that emerge from the literature when assessing the merits and risks of redo‐MVR and MVIV. First, patients who develop SVD or require redo‐MVR are generally very sick and have a high competing risk of death. Second, those who present in a higher New York Heart Association class and/or with a lower ejection fraction for redo‐MVR have worse outcomes, and earlier intervention may be warranted in select cases. Third, MVIV via a transseptal approach, given its safety profile, is a reasonable alternative to redo‐MVR in select high surgical‐risk patients. However, redo‐MVR should be performed in patients who are not high risk for surgery, those with paravalvular leak not amenable to percutaneous closure, prosthetic valve endocarditis, prosthetic valve thrombosis, patients with a high risk of LVOTO, or when concomitant cardiac surgical procedures (eg, coronary artery bypass grafting, tricuspid valve repair, Maze procedure, etc) are needed. The risk of LVOTO, which can be assessed by preoperative computed tomography imaging, is a particularly important anatomical reason that precludes use of MVIV (discussed later). Finally, transapical MVIV is associated with higher mortality and morbidity as compared with transseptal MVIV, and the latter approach should be pursued whenever possible.
Comparisons of transcatheter and surgical options for patients with bioprosthetic mitral SVD have to be evaluated carefully, however, for a number of reasons. First, patients assigned to these 2 treatment modalities are generally preselected based on surgical risk, and it is difficult to directly compare outcomes with wide applicability. Given the risk of selection bias, a randomized trial comparing the safety, effectiveness, and durability of redo‐MVR and MVIV is eagerly awaited. Furthermore, when comparing redo‐MVR and MVIV, it is important to note whether patients in the transcatheter group underwent transapical or transseptal MVIV. Studies have shown that transapical MVIV has inferior outcomes compared with its transseptal counterpart. These 2 delivery techniques differ in numerous aspects. Transapical implantation may enable better control over the implant position given that the cardiac apex is considerably closer to the mitral valve than the peripheral veins. 60 However, the transseptal approach eliminates the need for either thoracotomy or trauma to the left ventricle and is associated with a mortality benefit and greater improvements in postprocedural functional performance. 61 Thus, although transapical MVIV may not be a viable alternative to redo‐MVR, transseptal MVIV should be considered for high‐risk patients with suitable anatomy, with the caveat that the transseptal approach may be more technically challenging requiring operator expertise at high‐volume centers. 62
Failed Surgical Repairs and Transcatheter Mitral Valve‐in‐Ring Replacement
For patients with a prior ring or band annuloplasty, MVIR seems to be inferior to both redo‐MVr and MVR. As with redo‐MVR/MVIV, selection bias may be at play given that patients being considered for MVIR tend to be at high or extreme risk for reoperative surgery. Mortality with MVIR at 30 days is ≈0% to 18%, and ≈0% to 34% at 1 year. In contrast, reoperative MVr can be performed with <5% mortality at most large institutions with excellent long‐term outcomes. Similarly, although short‐term mortality for reoperative MVR may be comparable to that of MVIR, the long‐term benefits with regard to valve durability and freedom from reoperation are undeniable with the former. Thus, redo‐MVr and MVR should both be preferred to MVIR in appropriately selected patients.
A crucial consideration in preoperative planning for MVIR is assessment of the annuloplasty ring currently in place. The 3 most important characteristics to consider are ring rigidity, shape, and radio‐opacity. 63
Valves currently used for TMVR are designed to anchor and fully expand within a circular geometry. The mitral annulus, however, takes on a D‐shape, and many annuloplasty rings have been designed to accommodate this. The rigidity of a ring determines its ability to conform to the circular shape upon implantation and thereby optimize valve function. Generally, rings are classified as rigid, semirigid, or flexible. Rigid rings provide the most anchoring capacity for a transcatheter valve but are the least able to conform and thus pose the greatest risk of para‐ and intravalvular regurgitation at the 2 corners. Semirigid rings provide an optimal balance between a solid anchor and the ability to adopt a circular shape in response to MVIR. However, smaller‐sized semirigid rings tend to be more rigid and are thus less likely to fully circularize. 64
Rings may also be incomplete or complete in shape. Flexible‐incomplete rings are generally implanted in an effort to preserve the 3‐dimensional saddle‐geometry of the annulus. Incomplete rings have an opening at the anterior leaflet and do not provide a solid anchoring surface during MVIR procedures. Furthermore, the discontinuous portions of incomplete rings may result in significant paravalvular leak. 65 Thus, the ideal ring for MVIR appears to be a semirigid, complete annuloplasty ring with adequate radio‐opacity. The “Mitral Valve‐in‐Valve” mobile application, developed by Dr. Vinayak Bapat, serves as a valuable reference for preprocedural planning and optimal valve positioning during the procedure. 66
Complications of Transcatheter Mitral Valve Replacement
Left Ventricular Outflow Tract Obstruction
Previously underappreciated, LVOTO is a serious complication of TMVR that results from displacement of the native anterior mitral leaflet into the LVOT upon expansion of the transcatheter valve. 67 This occurs in ≈0% to 6% of patients following MVIV and ≈0% to 20% following MVIR. Furthermore, procedural mortality with TMVR is significantly higher among patients with LVOTO. Anatomical factors that increase the risk of LVOTO include protrusion of the device into the left ventricle, device flaring, and a shallow aortomitral angle between the plane of the aortic and mitral annuli. Device‐specific preoperative assessment of the “Neo‐LVOT” is useful to identify those at risk of this complication, with a sensitivity of 96.2% and specificity of 92.3%. 35 , 68 , 69
Several risk‐reduction strategies have been studied for patients at risk of LVOTO. 70 , 71 Laceration of the anterior mitral leaflet to prevent LVOTO is one such technique that involves splitting the anterior leaflet with an electrified wire. 72 , 73 , 74 In patients at risk of LVOTO owing to a prominent septum, preoperative alcohol septal ablation has also been evaluated with promising results. 75
Other Limitations
Recognized other adverse events associated with MVIV and MVIR include (1) procedural and delayed device migration or embolization, (2) elevated postprocedural gradients, (3) residual mitral regurgitation, and (4) valve thrombosis. These complications appear to be more common in MVIR than MVIV, with small valve size (<25 mm) appearing as a consistent predictor of significantly elevated postprocedural gradients. Improvements in TMVR, including imaging and valve technology, will undoubtedly address these issues in the near future.
Sources of Funding
None.
Disclosures
Dr Tang is a physician proctor for Medtronic and a consultant for Abbott Structural Heart, Medtronic and W.L. Gore & Associates. Dr Kaneko is a speaker for Abbott Structural Heart and Baylis Medical, a consultant for 4C Medical, and has served as a proctor and educator for Edwards Lifesciences and Medtronic. Dr Bapat has served as a consultant for Medtronic, Edwards Lifesciences, 4C Medical, and Boston Scientific. Dr Bhatt discloses the following relationships—Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II executive committee funded by CSL Behring), Belvoir Publications (Editor‐in‐Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor‐in‐Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co‐leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. The remaining authors have no disclosures to report.
Supporting information
Table S1
(J Am Heart Assoc. 2021;10:e019854. DOI: 10.1161/JAHA.120.019854.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019854
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Foster AH, Greenberg GJ, Underhill DJ, McIntosh CL, Clark RE. Intrinsic failure of hancock mitral bioprostheses: 10‐ to 15‐year experience. Ann Thorac Surg. 1987;44:568–577. DOI: 10.1016/S0003-4975(10)62137-6. [DOI] [PubMed] [Google Scholar]
- 2. Gallo I, Nistal F, Blasquez R, Arbe E, Artinano E. Incidence of primary tissue valve failure in porcine bioprosthetic heart valves. Ann Thorac Surg. 1988;45:66–70. DOI: 10.1016/S0003-4975(10)62400-9. [DOI] [PubMed] [Google Scholar]
- 3. Gallucci V, Bortolotti U, Milano A, Valfre C, Mazzucco A, Thiene G. Isolated mitral valve replacement with the hancock bioprosthesis: a 13‐year appraisal. Ann Thorac Surg. 1984;38:571–578. DOI: 10.1016/S0003-4975(10)62313-2. [DOI] [PubMed] [Google Scholar]
- 4. Magilligan DJ Jr, Lewis JW Jr, Tilley B, Peterson E. The porcine bioprosthetic valve. Twelve years later. J Thorac Cardiovasc Surg. 1985;89:499–507. DOI: 10.1016/S0022-5223(19)38753-7. [DOI] [PubMed] [Google Scholar]
- 5. Rahimtoola SH. Perspective on valvular heart disease: an update. J Am Coll Cardiol. 1989;14:1–23. DOI: 10.1016/0735-1097(89)90047-8. [DOI] [PubMed] [Google Scholar]
- 6. Bourguignon T, Bouquiaux‐Stablo AL, Loardi C, Mirza A, Candolfi P, Marchand M, Aupart MR. Very late outcomes for mitral valve replacement with the carpentier‐edwards pericardial bioprosthesis: 25‐year follow‐up of 450 implantations. J Thorac Cardiovasc Surg. 2014;148:2004–2011.e2001. DOI: 10.1016/j.jtcvs.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 7. Vohra HA, Whistance RN, Roubelakis A, Burton A, Barlow CW, Tsang GM, Livesey SA, Ohri SK. Outcome after redo‐mitral valve replacement in adult patients: a 10‐year single‐centre experience. Interact Cardiovasc Thorac Surg. 2012;14:575–579. DOI: 10.1093/icvts/ivs005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehaffey HJ, Hawkins RB, Schubert S, Fonner C, Yarboro LT, Quader M, Speir A, Rich J, Kron IL, Ailawadi G. Contemporary outcomes in reoperative mitral valve surgery. Heart. 2018;104:652–656. DOI: 10.1136/heartjnl-2017-312047. [DOI] [PubMed] [Google Scholar]
- 9. Magilligan DJ Jr, Lewis JW Jr, Stein P, Alam M. The porcine bioprosthetic heart valve: experience at 15 years. Ann Thorac Surg. 1989;48:324–329; discussion 330. DOI: 10.1016/S0003-4975(10)62850-0. [DOI] [PubMed] [Google Scholar]
- 10. Kaneko T, Aranki S, Javed Q, McGurk S, Shekar P, Davidson M, Cohn L. Mechanical versus bioprosthetic mitral valve replacement in patients <65 years old. J Thorac Cardiovasc Surg. 2014;147:117–126. [DOI] [PubMed] [Google Scholar]
- 11. Bosch X, Pomar JL, Pelletier LC. Early and late prognosis after reoperation for prosthetic valve replacement. J Thorac Cardiovasc Surg. 1984;88:567–572. DOI: 10.1016/S0022-5223(19)38294-7. [DOI] [PubMed] [Google Scholar]
- 12. Cohn LH, Aranki SF, Rizzo RJ, Adams DH, Cogswell KA, Kinchla NM, Couper GS, Collins JJ Jr. Decrease in operative risk of reoperative valve surgery. Ann Thorac Surg. 1993;56:15–20; discussion 20–11. DOI: 10.1016/0003-4975(93)90397-Z. [DOI] [PubMed] [Google Scholar]
- 13. Akins CW, Buckley MJ, Daggett WM, Hilgenberg AD, Vlahakes GJ, Torchiana DF, Madsen JC. Risk of reoperative valve replacement for failed mitral and aortic bioprostheses. Ann Thorac Surg. 1998;65:1545–1551; discussion 1551–1542. DOI: 10.1016/S0003-4975(98)00301-4. [DOI] [PubMed] [Google Scholar]
- 14. Albeyoglu SC, Filizcan U, Sargin M, Cakmak M, Goksel O, Bayserke O, Cinar B, Eren E. Determinants of hospital mortality after repeat mitral valve surgery for rheumatic mitral valve disease. Thorac Cardiovasc Surg. 2006;54:244–249. DOI: 10.1055/s-2006-923946. [DOI] [PubMed] [Google Scholar]
- 15. Antunes MJ, Magalhaes MP. Isolated replacement of a prosthesis or a bioprosthesis in the mitral valve position. Am J Cardiol. 1987;59:346–349. DOI: 10.1016/0002-9149(87)90811-3. [DOI] [PubMed] [Google Scholar]
- 16. Bortolotti U, Milano A, Mossuto E, Mazzaro E, Thiene G, Casarotto D. Early and late outcome after reoperation for prosthetic valve dysfunction: analysis of 549 patients during a 26‐year period. J Heart Valve Dis. 1994;3:81–87. [PubMed] [Google Scholar]
- 17. Jamieson WR, Burr LH, Miyagishima RT, Janusz MT, Fradet GJ, Lichtenstein SV, Ling H. Reoperation for bioprosthetic mitral structural failure: risk assessment. Circulation. 2003;108(suppl 1):II98–II102. DOI: 10.1161/01.cir.0000089184.46999.f4. [DOI] [PubMed] [Google Scholar]
- 18. Kwedar K, McNeely C, Zajarias A, Markwell S, Vassileva CM. Outcomes of early mitral valve reoperation in the medicare population. Ann Thorac Surg. 2017;104:1516–1521. DOI: 10.1016/j.athoracsur.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 19. McGrath LB, Fernandez J, Laub GW, Anderson WA, Bailey BM, Chen C. Perioperative events in patients with failed mechanical and bioprosthetic valves. Ann Thorac Surg. 1995;60:S475–478. DOI: 10.1016/0003-4975(95)00444-P. [DOI] [PubMed] [Google Scholar]
- 20. Toker ME, Eren E, Guler M, Kirali K, Yanartas M, Balkanay M, Yakut C. Second and third cardiac valve reoperations: factors influencing death and long‐term survival. Tex Heart Inst J. 2009;36:557–562. [PMC free article] [PubMed] [Google Scholar]
- 21. Frank G, Tyers O, Eric Jamieson WR, Ian Munro A, Germann E, Burr LH, Miyagishima RT, Ling H. Reoperation in biological and mechanical valve populations: fate of the reoperative patient. Ann Thorac Surg. 1995;60:S464–S468; discussion S468–S469. DOI: 10.1016/0003-4975(95)00302-2. [DOI] [PubMed] [Google Scholar]
- 22. Kilic A, Helmers MR, Han JJ, Kanade R, Acker MA, Hargrove WC, Atluri P. Redo mitral valve surgery following prior mitral valve repair. J Card Surg. 2018;33:772–777. DOI: 10.1111/jocs.13944. [DOI] [PubMed] [Google Scholar]
- 23. Kilic A, Acker MA, Gleason TG, Sultan I, Vemulapalli S, Thibault D, Ailawadi G, Badhwar V, Thourani V, Kilic A. Clinical outcomes of mitral valve reoperations in the United States: an analysis of the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2019;107:754–759. DOI: 10.1016/j.athoracsur.2018.08.083. [DOI] [PubMed] [Google Scholar]
- 24. Dvir D, Webb J. Mitral valve‐in‐valve and valve‐in‐ring: technical aspects and procedural outcomes. EuroIntervention. 2016;12:Y93–Y96. DOI: 10.4244/EIJV12SYA25. [DOI] [PubMed] [Google Scholar]
- 25. Gallo M, Dvir D, Demertzis S, Pedrazzini G, Berdajs D, Ferrari E. Transcatheter valve‐in‐valve implantation for degenerated bioprosthetic aortic and mitral valves. Expert Rev Med Devices. 2016;13:749–758. DOI: 10.1080/17434440.2016.1207521. [DOI] [PubMed] [Google Scholar]
- 26. Bouleti C, Fassa AA, Himbert D, Brochet E, Ducrocq G, Nejjari M, Ghodbane W, Depoix JP, Nataf P, Vahanian A. Transfemoral implantation of transcatheter heart valves after deterioration of mitral bioprosthesis or previous ring annuloplasty. JACC Cardiovasc Interv. 2015;8:83–91. DOI: 10.1016/j.jcin.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 27. Cheung A, Webb JG, Barbanti M, Freeman M, Binder RK, Thompson C, Wood DA, Ye J. 5‐year experience with transcatheter transapical mitral valve‐in‐valve implantation for bioprosthetic valve dysfunction. J Am Coll Cardiol. 2013;61:1759–1766. DOI: 10.1016/j.jacc.2013.01.058. [DOI] [PubMed] [Google Scholar]
- 28. Eleid MF, Cabalka AK, Williams MR, Whisenant BK, Alli OO, Fam N, Pollak PM, Barrow F, Malouf JF, Nishimura RA, et al. Percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv. 2016;9:1161–1174. DOI: 10.1016/j.jcin.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 29. Eleid MF, Whisenant BK, Cabalka AK, Williams MR, Nejjari M, Attias D, Fam N, Amoroso N, Foley TA, Pollak PM, et al. Early outcomes of percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv. 2017;10:1932–1942. DOI: 10.1016/j.jcin.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 30. Hu J, Chen Y, Cheng S, Zhang S, Wu K, Wang W, Zhou Y. Transcatheter mitral valve implantation for degenerated mitral bioprostheses or failed surgical annuloplasty rings: a systematic review and meta‐analysis. J Card Surg. 2018;33:508–519. DOI: 10.1111/jocs.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kamioka N, Babaliaros V, Morse MA, Frisoli T, Lerakis S, Iturbe JM, Binongo J, Corrigan F, Yousef A, Gleason P, et al. Comparison of clinical and echocardiographic outcomes after surgical redo mitral valve replacement and transcatheter mitral valve‐in‐valve therapy. JACC Cardiovasc Interv. 2018;11:1131–1138. DOI: 10.1016/j.jcin.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 32. Urena M, Brochet E, Lecomte M, Kerneis C, Carrasco JL, Ghodbane W, Abtan J, Alkhoder S, Raffoul R, Iung B, et al. Clinical and haemodynamic outcomes of balloon‐expandable transcatheter mitral valve implantation: a 7‐year experience. Eur Heart J. 2018;39:2679–2689. DOI: 10.1093/eurheartj/ehy271. [DOI] [PubMed] [Google Scholar]
- 33. Wilbring M, Alexiou K, Tugtekin SM, Sill B, Hammer P, Schmidt T, Simonis G, Matschke K, Kappert U. Transapical transcatheter valve‐in‐valve implantation for deteriorated mitral valve bioprostheses. Ann Thorac Surg. 2013;95:111–117. DOI: 10.1016/j.athoracsur.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 34. Ye J, Cheung A, Yamashita M, Wood D, Peng D, Gao M, Thompson CR, Munt B, Moss RR, Blanke P, et al. Transcatheter aortic and mitral valve‐in‐valve implantation for failed surgical bioprosthetic valves: an 8‐year single‐center experience. JACC Cardiovasc Interv. 2015;8:1735–1744. [DOI] [PubMed] [Google Scholar]
- 35. Yoon S‐H, Whisenant BK, Bleiziffer S, Delgado V, Dhoble A, Schofer N, Eschenbach L, Bansal E, Murdoch DJ, Ancona M, et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J. 2019;40:441–451. DOI: 10.1093/eurheartj/ehy590. [DOI] [PubMed] [Google Scholar]
- 36. Yoon SH, Whisenant BK, Bleiziffer S, Delgado V, Schofer N, Eschenbach L, Fujita B, Sharma R, Ancona M, Yzeiraj E, et al. Transcatheter mitral valve replacement for degenerated bioprosthetic valves and failed annuloplasty rings. J Am Coll Cardiol. 2017;70:1121–1131. [DOI] [PubMed] [Google Scholar]
- 37. Whisenant B, Kapadia SR, Eleid MF, Kodali SK, McCabe JM, Krishnaswamy A, Morse M, Smalling RW, Reisman M, Mack M, et al. 1‐year outcomes of mitral valve‐in‐valve using the sapien 3 aortic transcatheter heart valve. JAMA Cardiol. 2020;5:1245–1252. DOI: 10.1001/jamacardio.2020.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murzi M, Berti S, Gasbarri T, Trianni G, Maffei S, Solinas M, Dvir D, Cerillo AG. Transapical transcatheter mitral valve‐in‐valve implantation versus minimally invasive surgery for failed mitral bioprostheses. Interact Cardiovasc Thorac Surg. 2017;25:57–61. DOI: 10.1093/icvts/ivx067. [DOI] [PubMed] [Google Scholar]
- 39. de Weger A, Ewe SH, Delgado V, Bax JJ. First‐in‐man implantation of a trans‐catheter aortic valve in a mitral annuloplasty ring: novel treatment modality for failed mitral valve repair. Eur J Cardiothorac Surg. 2011;39:1054–1056. DOI: 10.1016/j.ejcts.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 40. Anyanwu AC, Itagaki S, Varghese R, Castillo J, Chikwe J, Adams DH. Re‐repair of the mitral valve as a primary strategy for early and late failures of mitral valve repair. Eur J Cardiothorac Surg. 2014;45:352–357; discussion 357–358. DOI: 10.1093/ejcts/ezt256. [DOI] [PubMed] [Google Scholar]
- 41. Aphram G, De Kerchove L, Mastrobuoni S, Navarra E, Solari S, Tamer S, Baert J, Poncelet A, Rubay J, Astarci P, et al. Re‐repair of the failed mitral valve: insights into aetiology and surgical management. Eur J Cardiothorac Surg. 2018;54:774–780. DOI: 10.1093/ejcts/ezy111. [DOI] [PubMed] [Google Scholar]
- 42. Cerfolio RJ, Orzulak TA, Pluth JR, Harmsen WS, Schaff HV. Reoperation after valve repair for mitral regurgitation: early and intermediate results. J Thorac Cardiovasc Surg. 1996;111:1177–1183; 1183–1174. DOI: 10.1016/S0022-5223(96)70219-2. [DOI] [PubMed] [Google Scholar]
- 43. Onorati F, Gatti G, Perrotti A, Mariscalco G, Reichart D, Milano A, Della Ratta E, Rubino A, Santarpino G, Salsano A, et al. Impact of failed mitral valve repair on hospital outcome of redo mitral valve procedures. Eur J Cardiothorac Surg. 2017;51:906–912. DOI: 10.1093/ejcts/ezw436. [DOI] [PubMed] [Google Scholar]
- 44. Dumont E, Gillinov AM, Blackstone EH, Sabik JF III, Svensson LG, Mihaljevic T, Houghtaling PL, Lytle BW. Reoperation after mitral valve repair for degenerative disease. Ann Thorac Surg. 2007;84:444–450; discussion 450. DOI: 10.1016/j.athoracsur.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 45. Gillinov AM, Cosgrove DM, Lytle BW, Taylor PC, Stewart RW, McCarthy PM, Smedira NG, Muehrcke DD, Apperson‐Hansen C, Loop FD. Reoperation for failure of mitral valve repair. J Thorac Cardiovasc Surg. 1997;113:467–473; discussion 473–465. DOI: 10.1016/S0022-5223(97)70359-3. [DOI] [PubMed] [Google Scholar]
- 46. Allende R, Doyle D, Urena M, Ribeiro HB, Amat‐Santos IJ, Bernier M, Pasian S, DeLarochelliere R, Dumont E, Rodes‐Cabau J. Transcatheter mitral "valve‐in‐ring" implantation: a word of caution. Ann Thorac Surg. 2015;99:1439–1442. DOI: 10.1016/j.athoracsur.2014.06.083. [DOI] [PubMed] [Google Scholar]
- 47. Attizzani GF, Cheung Tam C, Markowitz A. Transcatheter mitral valve‐in‐ring implantation in prohibitive surgical risk patients: single center initial experience in the United States. Catheter Cardiovasc Interv. 2016;88:E233–E238. DOI: 10.1002/ccd.26134. [DOI] [PubMed] [Google Scholar]
- 48. Cheung A, Denti P, Kiaii B, Bagur R, Webb J, Latib A, Alfieri O. Mitral valve‐in‐ring implantation with a dedicated transcatheter mitral valve replacement system. JACC Cardiovasc Interv. 2017;10:2012–2014. DOI: 10.1016/j.jcin.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 49. Descoutures F, Himbert D, Maisano F, Casselman F, de Weger A, Bodea O, Van der Kley F, Colombo A, Giannini C, Rein KA, et al. Transcatheter valve‐in‐ring implantation after failure of surgical mitral repair. Eur J Cardiothorac Surg. 2013;44:e8–e15. DOI: 10.1093/ejcts/ezt155. [DOI] [PubMed] [Google Scholar]
- 50. Eleid MF, Foley TA, Said SM, Pislaru SV, Rihal CS. Severe mitral annular calcification: multimodality imaging for therapeutic strategies and interventions. JACC Cardiovasc Imaging. 2016;9:1318–1337. DOI: 10.1016/j.jcmg.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 51. Gualis J, Estevez‐Loureiro R, Alonso D, Castano M. Transapical mitral valve‐in‐valve ring implantation with the edwards sapien 3 prosthesis. J Card Surg. 2017;32:791–793. DOI: 10.1111/jocs.13506. [DOI] [PubMed] [Google Scholar]
- 52. Kliger C, Al‐Badri A, Wilson S, Weiss D, Jelnin V, Kronzon I, Perk G, Fontana GP, Ruiz CE. Successful first‐in‐man percutaneous transapical‐transseptal melody mitral valve‐in‐ring implantation after complicated closure of a para‐annular ring leak. EuroIntervention. 2014;10:968–974. DOI: 10.4244/EIJV10I8A164. [DOI] [PubMed] [Google Scholar]
- 53. Long A, Mahoney P. Transcatheter mitral valve‐in‐valve and valve‐in‐ring replacement in high‐risk surgical patients: feasibility, safety, and longitudinal outcomes in a single‐center experience. J Invasive Cardiol. 2018;30:324–328. [PubMed] [Google Scholar]
- 54. Neves PC, Paulo NS, Gama V, Vouga L. Transapical aortic valve and mitral valve in ring prosthesis implantation—a new advance in transcatheter procedures. Interact Cardiovasc Thorac Surg. 2014;19:344–346. DOI: 10.1093/icvts/ivu137. [DOI] [PubMed] [Google Scholar]
- 55. Regazzoli D, Stella S, De Pinto S, Montorfano M, Ancona MB, Mangieri A, Buzzatti N, Giannini F, Agricola E, Monaco F, et al. Transfemoral implantation of a balloon‐expandable transcatheter valve in a rigid mitral annuloplasty ring optimized by post‐dilatation. JACC Cardiovasc Interv. 2017;10:e177–e179. DOI: 10.1016/j.jcin.2017.07.043. [DOI] [PubMed] [Google Scholar]
- 56. Wilbring M, Kappert U, Matschke K. Transapical transcatheter valve‐in‐ring implantation for failed mitral valve repair in the absence of radiopaque markers. J Thorac Cardiovasc Surg. 2015;149:e92–e94. DOI: 10.1016/j.jtcvs.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 57. Frerker C, Schmidt T, Schlüter M, Bader R, Schewel J, Schewel D, Thielsen T, Kreidel F, Alessandrini H, Schlingloff F, et al. Transcatheter implantation of aortic valve prostheses into degenerated mitral valve bioprostheses and failed annuloplasty rings: outcomes according to access route and Mitral Valve Academic Research Consortium (MVARC) criteria. EuroIntervention. 2016;12:1520–1526. DOI: 10.4244/EIJ-D-16-00209. [DOI] [PubMed] [Google Scholar]
- 58. Sasaki H, Ihashi K. Chordal‐sparing mitral valve replacement: pitfalls and techniques to prevent complications. Eur J Cardiothorac Surg. 2003;24:650–652. DOI: 10.1016/S1010-7940(03)00457-3. [DOI] [PubMed] [Google Scholar]
- 59. Morimoto N, Aoki M, Murakami H, Nakagiri K, Yoshida M, Mukohara N. Mid‐term echocardiographic comparison of chordal preservation method of mitral valve replacement in patients with mitral stenosis. J Heart Valve Dis. 2013;22:326–332. [PubMed] [Google Scholar]
- 60. Dvir D. Transseptal instead of transapical valve implantation: making mitral great again? JACC Cardiovasc Interv. 2016;9:1175–1177. DOI: 10.1016/j.jcin.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 61. Whisenant B, Kapadia SR, Eleid MF, Kodali SK, McCabe JM, Krishnaswamy A, Morse M, Smalling RW, Reisman M, Mack M, et al. One‐year outcomes of mitral valve‐in‐valve using the sapien 3 transcatheter heart valve. JAMA Cardiol. 2020;5:1245–1252. DOI: 10.1001/jamacardio.2020.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guerrero M, Salinger M, Pursnani A, Pearson P, Lampert M, Levisay J, Russell H, Feldman T. Transseptal transcatheter mitral valve‐in‐valve: a step by step guide from preprocedural planning to postprocedural care. Catheter Cardiovasc Interv. 2018;92:E185–E196. DOI: 10.1002/ccd.27128. [DOI] [PubMed] [Google Scholar]
- 63. Bapat V. Mitral valve‐in‐ring: the good, the bad, and the ugly. EuroIntervention. 2016;11:1092–1094. DOI: 10.4244/EIJV11I10A221. [DOI] [PubMed] [Google Scholar]
- 64. Ostovar R, Kuehnel RU, Erb M, Hartrumpf M, Claus T, Haase R, Albes JM. How do transcatheter heart valves fit in mitral annuloplasty rings and which combination can be recommended? Thorac Cardiovasc Surg. 2019;67:257–265. DOI: 10.1055/s-0038-1645867. [DOI] [PubMed] [Google Scholar]
- 65. Hachinohe D, Latib A, Montorfano M, Colombo A. Transcatheter mitral valve implantation in rigid mitral annuloplasty rings: potential differences between complete and incomplete rings. Catheter Cardiovasc Interv. 2019;93:E71–E74. DOI: 10.1002/ccd.27658. [DOI] [PubMed] [Google Scholar]
- 66. Bapat V. Valve‐in‐valve apps: why and how they were developed and how to use them. EuroIntervention. 2014;10(suppl U):U44–U51. DOI: 10.4244/EIJV10SUA7. [DOI] [PubMed] [Google Scholar]
- 67. Regueiro A, Granada JF, Dagenais F, Rodes‐Cabau J. Transcatheter mitral valve replacement: insights from early clinical experience and future challenges. J Am Coll Cardiol. 2017;69:2175–2192. DOI: 10.1016/j.jacc.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 68. Blanke P, Naoum C, Dvir D, Bapat V, Ong K, Muller D, Cheung A, Ye J, Min JK, Piazza N, et al. Predicting lvot obstruction in transcatheter mitral valve implantation: concept of the Neo‐LVOT. JACC Cardiovasc Imaging. 2017;10:482–485. DOI: 10.1016/j.jcmg.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 69. Murphy DJ, Ge Y, Don CW, Keraliya A, Aghayev A, Morgan R, Galper B, Bhatt DL, Kaneko T, Di Carli M, et al. Use of cardiac computerized tomography to predict neo‐left ventricular outflow tract obstruction before transcatheter mitral valve replacement. J Am Heart Assoc. 2017;6:e007353. DOI: 10.1161/JAHA.117.007353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chhatriwalla AK, Allen KB, Saxon JT, Cohen DJ, Aggarwal S, Hart AJ, Baron SJ, Dvir D, Borkon AM. Bioprosthetic valve fracture improves the hemodynamic results of valve‐in‐valve transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2017;10:e005216. DOI: 10.1161/CIRCINTERVENTIONS.117.005216. [DOI] [PubMed] [Google Scholar]
- 71. Kamioka N, Corrigan F, Iturbe JM, Caughron H, Lerakis S, Thourani V, Block P, Guyton R, Babaliaros V. Mitral bioprosthetic valve fracture: bailout procedure for undersized bioprosthesis during mitral valve‐in‐valve procedure with paravalvular leak closure. JACC Cardiovasc Interv. 2018;11:e21–e22. DOI: 10.1016/j.jcin.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 72. Kamioka N, Khan JM, Lederman RJ, Block P, Babaliaros VC, Greenbaum AB. Lampoon transseptal mitral valve in ring. Ann Cardiothorac Surg. 2018;7:834–836. DOI: 10.21037/acs.2018.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Khan JM, Babaliaros VC, Greenbaum AB, Foerst JR, Yazdani S, McCabe JM, Paone G, Eng MH, Leshnower BG, Gleason PT, et al. Anterior leaflet laceration to prevent ventricular outflow tract obstruction during transcatheter mitral valve replacement. J Am Coll Cardiol. 2019;73:2521–2534. DOI: 10.1016/j.jacc.2019.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Khan JM, Rogers T, Schenke WH, Mazal JR, Faranesh AZ, Greenbaum AB, Babaliaros VC, Chen MY, Lederman RJ. Intentional laceration of the anterior mitral valve leaflet to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: pre‐clinical findings. JACC Cardiovasc Interv. 2016;9:1835–1843. DOI: 10.1016/j.jcin.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guerrero M, Wang DD, Himbert D, Urena M, Pursnani A, Kaddissi G, Iyer V, Salinger M, Chakravarty T, Greenbaum A, et al. Short‐term results of alcohol septal ablation as a bail‐out strategy to treat severe left ventricular outflow tract obstruction after transcatheter mitral valve replacement in patients with severe mitral annular calcification. Catheter Cardiovasc Interv. 2017;90:1220–1226. DOI: 10.1002/ccd.26975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


