Abstract
Background
Although many hospitals have resuscitation champions, it is unknown if hospitals with very active physician or nonphysician champions have higher survival rates for in‐hospital cardiac arrest (IHCA).
Methods and Results
We surveyed adult hospitals in Get With The Guidelines‐Resuscitation about resuscitation practices, including about their resuscitation champion. Hospitals were categorized as having a very active physician champion, a very active nonphysician champion, or other (no champion or not very active champion). For each hospital, we calculated risk‐standardized survival rates for IHCA during the period of 2016 to 2018 and categorized them into quintiles of risk‐standardized survival rates. The association between a hospital's resuscitation champion type and their quintile of survival was evaluated using multivariable hierarchical proportional odds logistic regression. Overall, 192 hospitals (total of 44 477 IHCAs) comprised the study cohort. Risk‐standardized survival rates for IHCA varied widely between hospitals (median: 24.7%; range: 9.2%–37.5%). Very active physician champions were present in 29 (15.1%) hospitals, 64 (33.3%) had very active nonphysician champions, and 99 (51.6%) did not have a very active champion. Compared with sites without a very active resuscitation champion, hospitals with a very active physician champion were 4 times more likely to be in a higher survival quintile, even after adjusting for resuscitation practices across hospital groups (adjusted odds ratio [OR], 3.90; 95% CI, 1.39–10.95). In contrast, there was no difference in survival between sites without very active champions and those with very active non‐physician champions (adjusted OR, 1.28; 95% CI, 0.62–2.65).
Conclusions
The background and engagement level of a resuscitation champion is a critical factor in a hospital's survival outcomes for IHCA.
Keywords: cardiac arrest, survival, outcomes
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care, Cardiopulmonary Arrest
Nonstandard Abbreviations and Acronyms
- GWTG
Get With The Guidelines
Clinical Perspective
What Is New?
Hospitals vary widely in survival rates for in‐hospital cardiac arrest. Although many hospitals have resuscitation champions, it is unknown whether the type of resuscitation champion is associated with better survival outcomes for in ‐hospital cardiac arrest.
In a large national registry, we found that hospitals with a very active physician resuscitation champion had higher survival rates for in‐hospital cardiac arrest and were nearly 4 times as likely to be in the top hospital quintile for cardiac arrest survival.
In contrast, hospitals with a very active nonphysician champions did not have higher survival rates for in‐hospital cardiac arrest.
What Are the Clinical Implications?
Hospitals with a very active physician champion for resuscitation care may have better survival outcomes.
Identifying strong physician champions and providing adequate time and resources for these champions may be a priority in resuscitation quality improvement across hospitals.
In‐hospital cardiac arrest is a common event in hospitals, affecting ≈300 000 patients annually in the United States. 1 Survival rates to discharge are low at 20% to 25% but vary widely between hospitals by as much as 3‐fold. 2 Identifying practices at hospitals with the highest survival rates for in‐hospital cardiac arrest is important so that best practices can be disseminated to improve survival rates for hospitals nationwide. 3 One proposed strategy to increase survival rates for in‐hospital cardiac arrest is to have a dynamic and active resuscitation champion to oversee, manage, and improve hospital resuscitation practices.
In theory, a resuscitation champion is a dynamic and charismatic individual who is an active leader in implementing programs to improve resuscitation response and quality at their hospital. This may include introducing or improving performance of mock codes, debriefing after a cardiac arrest among resuscitation team members, and fostering better communication and team dynamics among those who respond to a cardiac arrest. An active resuscitation champion would also ensure frequent review of cardiac arrest survival outcomes and process‐of‐care measures (eg, time to defibrillation and time to epinephrine) to identify gaps in resuscitation care for further improvement. Although most hospitals may have a resuscitation champion, their background and engagement level are likely to differ widely. To date, no study has examined if having a very active physician or nonphysician champion is associated with higher survival rates for in‐hospital cardiac arrest.
Accordingly, we conducted a survey of hospitals participating in a national registry of in‐hospital cardiac arrest about their resuscitation practices. Based on responses, we categorized hospitals as having a very active physician champion, a very active nonphysician champion, or other (not very active champion or no champion) and evaluated whether having a very active champion is associated with higher hospital survival rates for in‐hospital cardiac arrest. If hospitals with very active champions have higher survival rates, we examined whether this was because they were more likely to achieve timely defibrillation and epinephrine administration, perform recommended resuscitation practices (eg, mock codes, immediate debriefing after an acute resuscitation), or have mechanisms in place to clearly identify team leaders, monitor chest compression quality, or use mechanical devices during an acute resuscitation.
Methods
The data that support the findings of this study are available from the corresponding author upon request.
Study Population
Get With The Guidelines (GWTG) ‐ Resuscitation is a large, prospective, national quality‐improvement registry of in‐hospital cardiac arrest and is sponsored by the American Heart Association. Its design has been described in detail previously. 4 In brief, trained quality‐improvement hospital personnel identify all patients without do‐not‐resuscitate orders with a cardiac arrest (defined as absence of a palpable central pulse, apnea, and unresponsiveness) who undergo cardiopulmonary resuscitation (CPR). Cases are identified by multiple methods, including centralized collection of cardiac arrest flow sheets, reviews of hospital paging system logs, and routine checks of code carts, pharmacy tracer drug records, and hospital billing charges for resuscitation medications. 4 The registry uses standardized Utstein‐style definitions for all patient variables and outcomes to facilitate uniform reporting across hospitals. 5 , 6 In addition, data accuracy is ensured by rigorous certification of hospital staff and use of standardized software with data checks for completeness and accuracy.
Because in‐hospital cardiac arrest survival has improved over the past decade, 7 we restricted our study population to the 234 hospitals within GWTG‐Resuscitation that entered cases throughout the period from January 1, 2016 to December 31, 2018 (Figure 1). This study was based on hospital responses to a survey on resuscitation practices; therefore, we restricted our cohort to the 208 hospitals (88.9%) that completed this resuscitation survey (see section below entitled Measures and Data Collection). Our focus was on adult in‐hospital cardiac arrest; consequently we excluded 12 pediatric hospitals, as well as pediatric cases in hospitals with both pediatric and adult patients. Finally, we excluded 4 sites with fewer than 20 cases of in‐hospital cardiac arrest events during the study period because our analyses are on the hospital level. Our final study cohort comprised 44 477 adult patients at 192 hospitals.
Figure 1. Definition of the study cohort.
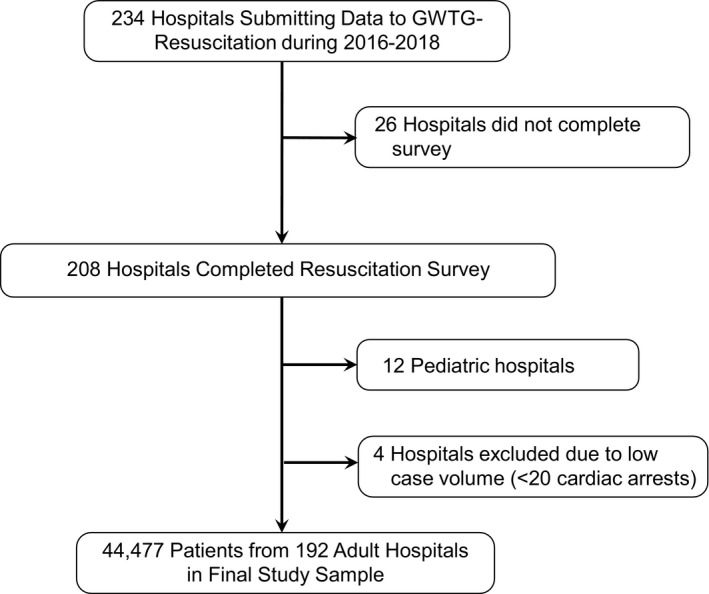
GWTG indicates Get With The Guidelines.
Measures and Data Collection
From April to June of 2018, we conducted a detailed survey of hospital resuscitation practices among actively participating hospitals within GWTG‐Resuscitation. At each site, the director of the hospital's resuscitation committee (eg, “Code Blue” committee) was asked to provide survey responses. This current survey was developed based on clinical expertise in our team, results from our prior resuscitation survey in 2014, 8 and outside experts. Resuscitation practices in this survey focused on the type of resuscitation champion at each hospital, the composition of a hospital's rapid response and resuscitation teams, the prevention and treatment of in‐hospital cardiac arrest (eg, use of simulation training, intra‐arrest monitoring devices of CPR quality, post‐event debriefing), and hospital culture. The latter included question items about administrative leadership, quality improvement, safety, and perceived barriers at one's hospital.
Independent Variable and Study Outcomes
The independent variable for this study was the type of resuscitation champion a hospital had and was categorized based on our prior qualitative research study 9 as a very active physician champion, a very active nonphysician champion, and other (no champion or not very active champion). This was based on our survey question on a resuscitation champion's engagement level as very active, somewhat active, or not active as a driving force in improving cardiac arrest care. These categories were defined a priori before conducting any analyses. If a hospital had both a physician and a nonphysician resuscitation champion (which was typically a nurse), that hospital would be categorized as having a physician champion. The primary study outcome was a hospital's proportion of patients with in‐hospital cardiac arrest who survived to hospital discharge. The secondary outcome was the proportion of patients with favorable neurological survival, which was defined as survival to discharge with a cerebral performance category score of 1 (no to mild neurological disability) or 2 (moderate neurological disability). 10
Statistical Analysis
Baseline differences in hospital characteristics and resuscitation practices were compared across the 3 hospital categories of resuscitation champion using chi‐square statistics. The primary outcome for this study was hospital rates of survival to discharge for in‐hospital cardiac arrest. For each facility, we first computed risk‐standardized survival rates to discharge for in‐hospital cardiac arrest using previously validated methodology. 2 Briefly, this validated model considered a total of 26 variables to predict survival to discharge after in‐hospital cardiac arrest. Using multivariable hierarchical logistic regression, an initial model of 18 predictors was derived with a c‐statistic of 0.738. Further model reduction yielded a final parsimonious model (c‐statistic of 0.734) of 9 predictors (age; initial cardiac arrest rhythm; hospital location of arrest; hypotension, sepsis, metastatic or hematologic malignancy, and hepatic insufficiency within 24 hours of cardiac arrest; and treatment with mechanical ventilation or continuous intravenous vasopressors at the time of cardiac arrest). For this study, we re‐constructed a hierarchical logistic regression model with our study cohort and confirmed that the final model comprised these 9 final predictors to predict survival to hospital discharge (c‐statistic of 0.718). Using the hospital‐specific random intercept estimates derived from this hierarchical model, a risk‐standardized survival rate for each hospital was determined by multiplying the registry's unadjusted survival rate by the ratio of a hospital's predicted to expected survival rate. 2
To facilitate clinical interpretability of study findings, hospitals were then divided into quintiles of risk‐standardized survival rates and categorized into 3 groups to simplify reporting: top quintile, middle three quintiles, and bottom quintile. To evaluate the association between a hospital's resuscitation champion type and hospital quintile of risk‐standardized survival for in‐hospital cardiac arrest, we constructed a hierarchical proportional odds logistic regression model, which quantified the odds of a hospital being in the top hospital survival quintile as compared with hospitals in the middle quintiles and of being in the middle quintiles as compared with the bottom quintile. This model then adjusted for resuscitation practices that had a bivariate association (P<0.10) across hospital champion groups to determine if survival differences by resuscitation champion type could be explained by differences in these resuscitation practices. In this adjusted model, we also included hospital teaching status and the number of in‐hospital cardiac arrest events at each hospital (categorized as <150, 150–250, or >250), regardless of their bivariate association.
Similarly, for the secondary outcome of favorable neurological survival, we computed hospital rates of risk‐adjusted favorable neurological survival using multivariable hierarchical logistic regression. Because validated risk‐standardization methodologies for this outcome have not been developed, this model considered for inclusion the following variables: age, sex, location of arrest (categorized as intensive care, monitored unit, non‐monitored unit, emergency room, procedural/surgical area, and other), initial cardiac arrest rhythm (ventricular fibrillation, pulseless ventricular tachycardia, asystole, and pulseless electrical activity), comorbidities or medical conditions present before cardiac arrest (heart failure, myocardial infarction, or diabetes mellitus; renal, hepatic, or respiratory insufficiency; baseline evidence of motor, cognitive, or functional deficits; acute stroke; acute nonstroke neurologic disorder; pneumonia; hypotension; sepsis; major trauma; metabolic or electrolyte abnormality; and metastatic or hematologic malignancy), and interventions present at the time of cardiac arrest (mechanical ventilation, intravenous vasopressor support). As with survival to discharge, hospitals were categorized into quintiles and a hierarchical proportional odds logistic regression model assessed whether a hospital's resuscitation champion type was associated with rates of favorable neurological survival.
All study analyses were performed with SAS 9.2 (SAS Institute, Cary, NC) and R version 2.10.0. 11 The hierarchical models were fitted with the use of the GLIMMIX macro in SAS and evaluated at a 2‐sided significance level of 0.05. The authors ensured the manuscript adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting in observational studies. 12 Dr. Paul Chan had full access to the data and takes responsibility for their integrity. All authors have read and agree to the article as written. The Mid America Heart Institute Institutional Review Board approved the study protocol.
Results
Of 192 hospitals in the study cohort comprising 44 477 IHCAs, 29 (15.1%) had a very active physician champion, 64 (33.3%) had a very active nonphysician champion (of which 38 were critical care nurses; 20 were medical‐surgical nurses; and 6 were nurse practitioners, physician assistants, or nurse anesthetists), and 99 (51.6%) did not have an active champion or had no champion. Hospitals with a very active physician champion were more likely to be major teaching hospitals, whereas hospitals with a very active nonphysician champion or without a very active champion were more likely to be nonteaching hospitals (Table 1). Compared with the other two hospital champion groups, hospitals with a very active physician champion were more likely to use a lanyard or hat to denote their code leader, report always communicating well, monitor diastolic pressures, and have designated staff members assigned to perform chest compressions during an acute resuscitation. Hospitals with a very active physician champion were also more likely to conduct code debriefings immediately following a resuscitation event and to not cite direct feedback as a barrier to resuscitation care. Notably there were no differences in the three hospital champion groups in the number of in‐hospital cardiac arrest events, credentials of who typically led their acute resuscitations, use of devices to measure chest compression quality, use of mechanical devices for delivering CPR, use of an individual to monitor CPR quality during an acute resuscitation, allowing nurses to deploy a manual defibrillator without a physician, and frequency of resuscitation simulations.
Table 1.
Characteristics of Study Hospitals, Stratified by Resuscitation Champion Type at Hospitals
| Very Active MD Champion (n=29) | Very Active Non‐MD Champion (n=64) | No Champion or Not Active Champion (n=99) | P Value | |
|---|---|---|---|---|
| Hospital academic status | ||||
| Major teaching | 14 (56.0%) | 16 (28.1%) | 21 (26.9%) | |
| Minor teaching | 7 (28.0%) | 18 (31.6%) | 20 (25.6%) | |
| Nonteaching | 4 (16.0%) | 23 (40.4%) | 37 (47.4%) | |
| Missing | 4 | 7 | 21 | 0.03 |
| US census region | ||||
| Northeast and Mid‐Atlantic | 3 (12.0%) | 6 (10.5%) | 16 (20.3%) | |
| South Atlantic | 3 (12.0%) | 18 (31.6%) | 21 (26.6%) | |
| North Central | 6 (24.0%) | 13 (22.8%) | 19 (24.1%) | |
| South Central | 5 (20.0%) | 10 (17.5%) | 12 (15.2%) | |
| Mountain/Pacific | 8 (32.0%) | 10 (17.5%) | 11 (13.9%) | |
| Missing | 4 | 7 | 20 | 0.38 |
| No. IHCA events | ||||
| <150 | 9 (31.0%) | 22 (34.4%) | 36 (36.4%) | 0.54 |
| 150–250 | 5 (17.2%) | 10 (15.6%) | 24 (24.2%) | |
| >250 | 15 (51.7%) | 32 (50.0%) | 39 (39.4%) | |
| Code leader uses lanyards or hat | ||||
| Yes | 10 (34.5%) | 12 (18.8%) | 8 (8.1%) | |
| No | 19 (65.5%) | 52 (81.3%) | 91 (91.9%) | 0.002 |
| Who typically leads codes | ||||
| Attending‐level physicians | 15 (51.7%) | 42 (65.6%) | 63 (63.6%) | |
| Critical care nurses | 0 (0.0%) | 4 (6.3%) | 7 (7.1%) | |
| Nurse‐practitioner or nurse | 0 (0.0%) | 0 (0.0%) | 1 (1.0%) | |
| Physician trainees—residents | 10 (34.5%) | 13 (20.3%) | 22 (22.2%) | |
| Physician trainees—fellows | 4 (13.8%) | 3 (4.7%) | 6 (6.1%) | |
| Other | 0 (0.0%) | 2 (3.1%) | 0 (0.0%) | 0.39 |
| Code team members communicate well during resuscitations | ||||
| Always (80%–100%) | 13 (44.8%) | 18 (28.1%) | 21 (21.2%) | |
| Most of the time (60%–80%) | 8 (27.6%) | 41 (64.1%) | 58 (58.6%) | |
| About half the time (40%–60%) | 6 (20.7%) | 5 (7.8%) | 16 (16.2%) | |
| Sometimes (20%–40%) | 2 (6.9%) | 0 (0.0%) | 4 (4.0%) | 0.005 |
| Code team members comfortable making their voices during resuscitations | ||||
| Always (80%–100%) | 8 (27.6%) | 19 (29.7%) | 30 (30.3%) | |
| Most of the time (60%–80%) | 13 (44.8%) | 37 (57.8%) | 51 (51.5%) | |
| About half the time (40%–60%) | 8 (27.6%) | 6 (9.4%) | 10 (10.1%) | |
| Sometimes (20%–40%) | 0 (0.0%) | 1 (1.6%) | 7 (7.1%) | |
| Never or rarely (0%–20%) | 0 (0.0%) | 1 (1.6%) | 1 (1.0%) | 0.22 |
| Devices used to assist in resuscitation | ||||
| CPR process measure device | 11 (37.9%) | 18 (28.1%) | 24 (24.2%) | 0.35 |
| Capnography | 18 (62.1%) | 45 (70.3%) | 54 (54.5%) | 0.13 |
| Mechanical CPR device | 4 (13.8%) | 3 (4.7%) | 8 (8.1%) | 0.34 |
| Monitoring of diastolic pressures | 7 (24.1%) | 8 (12.5%) | 7 (7.1%) | 0.046 |
| Number of devices routinely used | ||||
| 1 | 15 (51.7%) | 31 (48.4%) | 63 (63.6%) | |
| 2 | 9 (31.0%) | 28 (43.8%) | 29 (29.3%) | |
| 3 | 5 (17.2%) | 5 (7.8%) | 7 (7.1%) | 0.15 |
| Staff member usually assigned performing chest compressions | ||||
| No staff member usually assigned | 13 (44.8%) | 37 (57.8%) | 55 (55.6%) | |
| Critical care nurses | 1 (3.4%) | 3 (4.7%) | 7 (7.1%) | |
| Medical‐surgical floor nurses | 1 (3.4%) | 9 (14.1%) | 12 (12.1%) | |
| Physician trainees | 3 (10.3%) | 4 (6.3%) | 4 (4.0%) | |
| Nursing student or paramedic | 1 (3.4%) | 0 (0.0%) | 1 (1.0%) | |
| Respiratory therapist | 6 (20.7%) | 7 (10.9%) | 6 (6.1%) | |
| Clinical technician | 2 (6.9%) | 3 (4.7%) | 14 (14.1%) | |
| Other | 2 (6.9%) | 1 (1.6%) | 0 (0.0%) | 0.04 |
| An individual outside of leader monitors CPR quality | ||||
| Yes | 7 (24.1%) | 18 (28.1%) | 15 (15.2%) | |
| No | 22 (75.9%) | 46 (71.9%) | 84 (84.8%) | 0.12 |
| Code debriefing performed immediately | ||||
| Always or almost always (80%–100%) | 5 (17.2%) | 6 (9.4%) | 16 (16.2%) | |
| Frequently (60%–80%) | 7 (24.1%) | 15 (23.4%) | 9 (9.1%) | |
| Occasionally (20%–60%) | 6 (20.7%) | 21 (32.8%) | 24 (24.2%) | |
| Rarely (1%–20%) | 11 (37.9%) | 17 (26.6%) | 34 (34.3%) | |
| Never (0%) | 0 (0.0%) | 5 (7.8%) | 16 (16.2%) | 0.03 |
| Nursing staff can use manual defibrillator | 5 (17.2%) | 14 (21.9%) | 29 (29.3%) | 0.33 |
| Mock codes | ||||
| Yes | 25 (86.2%) | 56 (87.5%) | 85 (85.9%) | |
| No | 4 (13.8%) | 8 (12.5%) | 14 (14.1%) | 0.96 |
| Frequency of mock codes | ||||
| Not done | 4 (13.8%) | 8 (12.5%) | 14 (14.1%) | 0.34 |
| Less than once quarterly | 13 (44.8%) | 38 (59.4%) | 63 (63.6%) | |
| At least quarterly | 12 (41.4%) | 18 (28.1%) | 22 (22.2%) | |
| Barriers to resuscitation care | ||||
| Lack of direct feedback | ||||
| Yes | 12 (41.4%) | 24 (37.5%) | 63 (63.6%) | |
| No | 17 (58.6%) | 40 (62.5%) | 36 (36.4%) | 0.002 |
| Inadequate training | ||||
| Yes | 5 (17.2%) | 12 (18.8%) | 28 (28.3%) | |
| No | 24 (82.8%) | 52 (81.3%) | 71 (71.7%) | 0.26 |
| Lack of support from administration | ||||
| Yes | 3 (10.3%) | 5 (7.9%) | 17 (17.3%) | |
| No | 26 (89.7%) | 58 (92.1%) | 81 (82.7%) | |
| Missing | 0 | 1 | 1 | 0.23 |
| Lack of financial resources | ||||
| Yes | 10 (34.5%) | 13 (20.6%) | 25 (25.3%) | |
| No | 19 (65.5%) | 50 (79.4%) | 74 (74.7%) | |
| Missing | 0 | 1 | 0 | 0.36 |
| Are cardiac arrest data routinely reviewed | ||||
| Yes | 29 (100.0%) | 61 (95.3%) | 88 (88.9%) | |
| No | 0 (0.0%) | 3 (4.7%) | 11 (11.1%) | 0.09 |
| Rank the purpose of routine cardiac arrest data review | ||||
| Review IHCA metrics | ||||
| Strongly agree | 25 (86.2%) | 49 (76.6%) | 63 (63.6%) | |
| Somewhat agree | 4 (13.8%) | 10 (15.6%) | 20 (20.2%) | |
| Neither agree nor disagree | 0 (0.0%) | 2 (3.1%) | 3 (3.0%) | |
| Strongly disagree | 0 (0.0%) | 0 (0.0%) | 3 (3.0%) | |
| No routine data review | 0 (0.0%) | 3 (4.7%) | 10 (10.1%) | 0.34 |
| Identify areas for improvement | ||||
| Strongly agree | 23 (79.3%) | 48 (75.0%) | 55 (55.6%) | |
| Somewhat agree | 5 (17.2%) | 11 (17.2%) | 22 (22.2%) | |
| Neither agree nor disagree | 1 (3.4%) | 2 (3.1%) | 6 (6.1%) | |
| Somewhat disagree | 0 (0.0%) | 0 (0.0%) | 3 (3.0%) | |
| Strongly disagree | 0 (0.0%) | 0 (0.0%) | 3 (3.0%) | |
| No routine data review | 0 (0.0%) | 3 (4.7%) | 10 (10.1%) | 0.25 |
| Identify errors in resuscitation Care | ||||
| Strongly agree | 20 (69.0%) | 43 (67.2%) | 42 (42.4%) | |
| Somewhat agree | 7 (24.1%) | 14 (21.9%) | 26 (26.3%) | |
| Neither agree nor disagree | 1 (3.4%) | 2 (3.1%) | 11 (11.1%) | |
| Somewhat disagree | 1 (3.4%) | 1 (1.6%) | 6 (6.1%) | |
| Strongly disagree | 0 (0.0%) | 1 (1.6%) | 4 (4.0%) | |
| No routine data review | 0 (0.0%) | 3 (4.7%) | 10 (10.1%) | 0.059 |
| Track success of QI initiative | ||||
| Strongly agree | 20 (69.0%) | 41 (64.1%) | 46 (46.9%) | |
| Somewhat agree | 5 (17.2%) | 15 (23.4%) | 20 (20.4%) | |
| Neither agree nor disagree | 3 (10.3%) | 5 (7.8%) | 16 (16.3%) | |
| Somewhat disagree | 1 (3.4%) | 0 (0.0%) | 3 (3.1%) | |
| Strongly disagree | 0 (0.0%) | 0 (0.0%) | 3 (3.1%) | |
| No routine data review | 0 (0.0%) | 3 (4.7%) | 10 (10.2%) | |
| Missing | 0 | 0 | 1 | 0.14 |
IHCA indicates in‐hospital cardiac arrest; MD, physician; and QI, quality improvement.
Hospital processes of care during acute resuscitations did not differ between the 3 resuscitation champion hospital groups. Rate of prompt defibrillation within the first 2 minutes of a shockable cardiac arrest rhythm of ventricular fibrillation or pulseless ventricular tachycardia were similar, with a hospital mean rate of 74% to 75% in each group. Similarly, rates of prompt epinephrine administration within the first 5 minutes of a nonshockable cardiac arrest rhythm of asystole or pulseless electrical activity were not different between the 3 hospital champion groups (Table 2).
Table 2.
Mean Hospital Rates for Prompt Defibrillation and Epinephrine Administration by Hospital Resuscitation Champion Type
| Process of Care Measure | Very Active MD Champion | Very Active Non‐MD Champion | No Champion or Not Active Champion | P Value |
|---|---|---|---|---|
| Prompt defibrillation ≤2 minutes | 0.98 | |||
| Mean±SD | 74.5±6.9% | 74.4±6.4% | 74.3±5.9% | |
| Median (IQR) | 74.4% (70.4%, 79.1%) | 75.2% (70.4%, 79.1%) | 74.6% (70.6%, 78.4%) | |
| Prompt epinephrine ≤5 minutes | 0.62 | |||
| Mean±SD | 92.4±1.9% | 92.4±2.0% | 92.1±2.5% | |
| Median (IQR) | 92.7% (91.7%, 93.3%) | 92.6% (91.2%, 93.7%) | 92.2% (90.7%, 93.8%) | |
IQR indicates interquartile range; MD, physician; and SD, standard deviation.
Overall, risk‐standardized survival rates to discharge for in‐hospital cardiac arrest varied widely across study hospitals (median: 24.7%; range: 9.2%–37.5%) (Figure 2). Hospitals with a very active physician champion had a mean risk‐standardized survival rate to discharge of 29.5±4.3%, whereas rates were lower at 26.7±5.3% in hospitals with a very active nonphysician champion and 26.3±5.2% in hospitals without very active champions (P=0.01). Hospitals with a very active physician champion also had higher rates of favorable neurological survival than the other 2 hospital champion groups (Table 3).
Figure 2. Distribution of risk‐standardized survival rates for in‐hospital cardiac arrest among study hospitals.
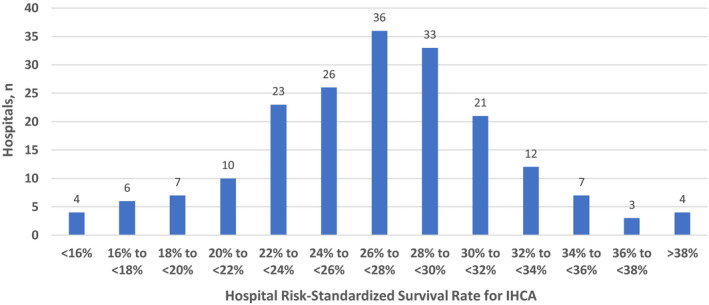
IHCA indicates in‐hospital cardiac arrest.
Table 3.
Mean Hospital Rates for Survival Outcomes by Hospital Resuscitation Champion Type
| Survival Outcomes | Very Active MD Champion | Very Active Non‐MD Champion | No Champion or Not Active Champion | P Value |
|---|---|---|---|---|
| Risk standardized rate of survival to discharge | 0.01 | |||
| Mean±SD | 29.5±4.3% | 26.7±5.3% | 26.3±5.2% | |
| Median (IQR) | 29.7% (26.6%, 32.5%) | 27.4% (23.6%, 29.7%) | 26.5% (23.0%, 29.6%) | |
| Risk‐adjusted rate of favorable neurological survival | ||||
| Mean±SD | 26.7±5.7% | 22.5±7.0% | 22.3±7.2% | 0.009 |
| Median (IQR) | 24.4% (21.8%, 31.7%) | 23.2% (18.5%, 26.6%) | 22.5% (17.7%, 26.5%) | |
IQR indicates interquartile range; MD, physician; and SD, standard deviation.
Compared with hospitals without a very active resuscitation champion, hospitals with a very active physician champion were more than 4 times as likely to be in the top quintile of risk‐standardized survival (odds ratio [OR], 4.39; 95% CI, 1.89–10.23; P<0.001). Hospitals with a very active nonphysician champion, however, were not more likely to be in a higher quintile of survival than hospitals without a very active champion (Table 4). After adjusting for differences in resuscitation practices across the 3 hospital champion groups, hospitals with a very active physician champion were still almost 4 times as likely to be in the top survival quintile as compared with hospitals without a very active champion (adjusted OR, 3.90; 95% CI, 1.39– 10.95; P=0.01). Similarly, hospitals with a very active physician champion were almost 4 times as likely to be in the top quintile of favorable neurological survival as compared with hospitals without a very active champion (OR, 3.91; 95% CI, 1.69–9.04; P=0.001). These differences in rates of favorable neurological survival were only modestly attenuated after adjusting for differences in resuscitation practices between the hospital groups (adjusted OR, 3.11; 95% CI, 1.08–8.90; P=0.036) (see Table 4).
Table 4.
Unadjusted and Adjusted Associations Between Hospital Resuscitation Champion Type and Survival Outcomes for In‐Hospital Cardiac Arrest
| Hospital Resuscitation Champion Type | |||||
|---|---|---|---|---|---|
| Not Active Champion | Very Active MD Champion | Very Active Non‐MD Champion | |||
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Risk‐standardized survival to discharge* | |||||
| Unadjusted for hospital practices | Reference | 4.39 (1.89, 10.23) | <0.001 | 1.30 (0.69, 2.45) | 0.45 |
| Adjusted for hospital practices | Reference | 3.90 (1.39, 10.95) | 0.01 | 1.28 (0.62, 2.65) | 0.51 |
| Risk‐adjusted favorable neurological survival* | |||||
| Unadjusted for hospital practices | Reference | 3.91 (1.69, 9.04) | 0.001 | 0.96 (0.51, 1.80) | 0.90 |
| Adjusted for hospital practices | Reference | 3.11 (1.08, 8.90) | 0.036 | 0.83 (0.39, 1.74) | 0.62 |
Hospitals without a very active resuscitation champion were the reference group for these comparisons. MD indicates physician; and OR indicates odds ratio.
Both outcomes are adjusted for differences in patient case‐mix severity across hospitals (see Methods for variables used for risk‐standardized survival rate to discharge and risk‐adjusted favorable neurological discharge). Adjusted models included as covariates hospital teaching status, in‐hospital cardiac arrest volume (<100, 100–250, >250), and resuscitation practices that had a bivariate association (P<0.10) across hospital champion groups.
Discussion
Using data from acute care hospitals in the United States that were participating in a national registry, we found that approximately half of hospitals did not have a very active resuscitation champion, whereas 15% of hospitals had a very active physician resuscitation champion, and 33% had a very active nonphysician champion. Compared with hospitals without a very active resuscitation champion, hospitals with a very active physician champion had higher rates of risk‐standardized survival for in‐hospital cardiac arrest and were 4 times as likely to be in the top hospital quintile of cardiac arrest survival. In contrast, there were no differences in survival between hospitals with a very active nonphysician champion and those without a very active resuscitation champion. A similar pattern was seen across the 3 hospital groups for the outcome of survival with favorable neurological status. Collectively, our study provides important insights into which type of resuscitation champion at a hospital is associated with higher survival rates for in‐hospital cardiac arrest.
To date, few studies have evaluated resuscitation practices across hospitals, as such studies require site‐ and patient‐level data from many hospitals in a large registry. In a recent survey of GWTG‐Resuscitation hospitals, we conducted one of the first such studies and identified several key hospital practices associated with higher survival for in‐hospital cardiac arrest. These included frequent review of cardiac arrest data to identify gaps in care and implement quality improvement programs, adequate resuscitation education for hospital staff, and monitoring for interruptions in chest compressions during CPR. 8 In our subsequent qualitative study in which we interviewed multiple stakeholders in resuscitation care at hospitals that were either top or bottom performers for in‐hospital cardiac arrest survival, we found that a dynamic and active resuscitation champion was a critical attribute for top‐performing sites to achieve high rates of cardiac arrest survival. 9 A recurrent theme from these site interviews was that a very active resuscitation champion helped hospital staff prioritize cardiac arrest outcomes as a measure of hospital quality and a cornerstone of patient safety. Moreover, the presence of a very active resuscitation champion was often a sign of support from a hospital's administration to commit resources for their time and quality improvement initiatives. However, the results from our qualitative study required validation because they were based on site visits at only 9 hospitals.
This study extends the findings from our recent qualitative work and found that hospitals were indeed more likely to excel in in‐hospital cardiac arrest survival only if they had a very active physician resuscitation champion. Higher survival rates for in‐hospital cardiac arrest at hospitals with a very active physician champion were not explained by better compliance with processes‐of‐care measures such as prompt defibrillation and epinephrine, and were only modestly explained by resuscitation practices such as lanyards or hats to denote code team leaders during an acute resuscitation, frequency of resuscitation simulations, use of devices to monitor or deliver high‐quality CPR, and immediate debriefing after resuscitation events. This suggests that the higher survival rates at sites with a very active physician champion are likely mediated by other quality measures (eg, improved post‐resuscitation care in the intensive care unit and delivery of more consistent and effective chest compressions during an acute resuscitation) and leadership activities (eg, building a culture of teamwork and communication during acute resuscitations) not captured in our study survey. Identification of what these other programs or activities are warrants further study in order to disseminate best practices in resuscitation care.
Contrary to our expectations, hospitals with a very active nonphysician champion did not have higher survival rates for in‐hospital cardiac arrest than hospitals without a very active champion. The reasons for this were not explicitly captured in our survey. We do know from our prior qualitative study that hospitals with a very active physician champion frequently also had a very active nonphysician (typically nursing) champion, suggesting that they were successful because these hospitals had leaders in both their medical and nursing staff. Moreover, our prior qualitative interviews suggested that a physician champion typically wielded more clout in enacting quality improvement initiatives and obtaining support from hospital administration. These explanations may help explain why hospitals with and without a very active physician champion had different survival outcomes. Alternatively, unmeasured confounding may help explain why hospitals with a very active nonphysician champion did not have better survival outcomes. Hospitals with a very active nonphysician champion may not have had as much administrative support or dedicated resources (time, staff, and money) for quality improvement efforts as compared with those with a very active physician champion, which could explain the former's lack of effect on hospital survival rates for in‐hospital cardiac arrest. These factors were not measured in our survey and deserve further study.
Our study should be interpreted in the context of the following limitations. First, the survey data were reported by a single respondent in collaboration with other staff at the hospital, and the reported policies and practices were not independently confirmed. However, survey respondents were typically the director of each hospital's Code Blue committee and were therefore among the most knowledgeable individuals to evaluate their institution's resuscitations practices. Moreover, inaccurate responses would be expected to be nondifferential and bias findings toward the null, thereby reinforcing the validity of our positive associations. Second, our study population was limited to hospitals participating in GWTG‐Resuscitation and our findings may not apply to nonparticipating hospitals. Specifically, the prevalence of some resuscitation strategies may be lower in nonparticipating hospitals and the prevalence of perceived resuscitation barriers may be higher, although GWTG‐Resuscitation does represent a diverse set of US hospitals. Finally, although we found that hospitals with a very active physician champion generally achieved higher rates of in‐hospital cardiac arrest survival, we could only explain part of this survival difference. Therefore, the specific programs and initiatives that underlie the higher survival rates for in‐hospital cardiac arrest at hospitals with a very active physician champion remain poorly defined and deserve further study.
In conclusion, although many hospitals have a resuscitation champion, the background and engagement level of a resuscitation champion is a critical factor in a hospital's survival outcomes for in‐hospital cardiac arrest. Using survey information from acute care hospitals participating in a national quality improvement registry, we found that fewer than half of hospitals reported having a very active physician or nonphysician resuscitation champion. Hospitals with a very active physician resuscitation champion were 4 times more likely to be in the top quintile of in‐hospital cardiac arrest survival, whereas there was no difference between hospitals with a very active nonphysician champion and those without a very active champion.
Sources of Funding
Drs P. Chan and Nallamothu are supported by grants from National Heart, Lung, and Blood Institute (1R01HL123980). GWTG‐Resuscitation is sponsored by the American Heart Association.
Disclosures
None.
Supporting information
Appendix S1
(J Am Heart Assoc.2021;10:e017509. DOI: 10.1161/JAHA.120.017509.)
For Sources of Funding and Disclosures, see page 10.
See Editorial by O'Halloran et al.
References
- 1. Holmberg MJ, Ross CE, Fitzmaurice GM, Chan PS, Duval‐Arnould J, Grossestreuer AV, Yankama T, Donnino MW, Andersen LW; American Heart Association's Get With The Guidelines‐Resuscitation Investigators . Annual incidence of adult and pediatric in‐hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019;12:e005580. [PMC free article] [PubMed] [Google Scholar]
- 2. Chan PS, Berg RA, Spertus JA, Schwamm LH, Bhatt DL, Fonarow GC, Heidenreich PA, Nallamothu BK, Tang F, Merchant RM. Risk‐standardizing survival for in‐hospital cardiac arrest to facilitate hospital comparisons. J Am Coll Cardiol. 2013;62:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan PS, Nallamothu BK. Improving outcomes following in‐hospital cardiac arrest: life after death. JAMA. 2012;307:1917–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, Berg RA, Nichol G, Lane‐Trultt T. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297–308. [DOI] [PubMed] [Google Scholar]
- 5. Cummins RO, Chamberlain D, Hazinski MF, Nadkarni V, Kloeck W, Kramer E, Becker L, Robertson C, Koster R, Zaritsky A, et al. Recommended guidelines for reviewing, reporting, and conducting research on in‐hospital resuscitation: the in‐hospital 'Utstein style'. American Heart Association. Circulation. 1997;95:2213–2239. [DOI] [PubMed] [Google Scholar]
- 6. Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, Cassan P, Coovadia A, D'Este K, Finn J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation. 2004;110:3385–3397. [DOI] [PubMed] [Google Scholar]
- 7. Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS. Trends in survival after in‐hospital cardiac arrest. N Engl J Med. 2012;367:1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan PS, Krein SL, Tang F, Iwashyna TJ, Harrod M, Kennedy M, Lehrich J, Kronick S, Nallamothu BK; American Heart Association's Get With the Guidelines‐Resuscitation I . Resuscitation practices associated with survival after in‐hospital cardiac arrest: a nationwide survey. JAMA Cardiol. 2016;1:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nallamothu BK, Guetterman TC, Harrod M, Kellenberg JE, Lehrich JL, Kronick SL, Krein SL, Iwashyna TJ, Saint S, Chan PS. How do resuscitation teams at top‐performing hospitals for in‐hospital cardiac arrest succeed? A qualitative study. Circulation. 2018;138:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piscator E, Goransson K, Bruchfeld S, Hammar U, El Gharbi S, Ebell M, Herlitz J, Djarv T. Predicting neurologically intact survival after in‐hospital cardiac arrest‐external validation of the Good Outcome Following Attempted Resuscitation score. Resuscitation. 2018;128:63–69. [DOI] [PubMed] [Google Scholar]
- 11. R Development Core Team . R: A Language and Environment for Statistical Computing. Austria: R Foundation for Statistical Computing V; 2008. Available at: http://www.R‐project.org. ISBN 3‐900051‐07‐0. Accessed February 3, 2020. [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; Initiative S . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. DOI: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


