Abstract
Background
Antiplatelets, anticoagulants, and statins are commonly prescribed for various indications. The associations between these medications and the risk of intracerebral hemorrhage (ICH) and cerebral microbleeds (CMBs) are unclear.
Methods and Results
We performed a retrospective study of the ARIC (Atherosclerosis Risk in Communities) study cohort, recruited from 4 US communities in 1987 to 1989 with follow‐up. In 2011 to 2013, a subset (N=1942) underwent brain magnetic resonance imaging with CMB evaluation. Time‐varying and any antiplatelet, anticoagulant, or statin use was evaluated at subsequent study visits in participants not on each medication at baseline. To determine the hazard of ICH and odds of CMB by medication use, logistic and Cox proportional hazard models were built, respectively, adjusting for the propensity to take the medication, concomitant use of other medications, and cognitive, genetic, and radiographic data. Of 15 719 individuals during up to 20 years of follow‐up, 130 participants experienced an ICH. The adjusted hazard of ICH was significantly lower among participants taking an antiplatelet at the most recent study visit before ICH versus nonusers (hazard ratio [HR], 0.53; 95% CI, 0.30–0.92). Statin users had a significantly lower hazard of an ICH compared with nonusers (adjusted HR, 0.13; 95% CI, 0.05–0.34). There was no association of CMB and antiplatelet, anticoagulant, or statin use in adjusted models.
Conclusions
In this US community‐based study, antiplatelet and statin use were associated with lower ICH hazard, whereas no association was noted between CMBs and antiplatelets, anticoagulants, and statins. Further study is needed to understand the differential roles of these medications in cerebral microhemorrhages and macrohemorrhages.
Keywords: cohort studies, intracerebral hemorrhage, medications
Subject Categories: Intracranial Hemorrhage, Epidemiology
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
- ASPREE
Aspirin in Reducing Events in the Elderly
- CMB
cerebral microbleed
- ICH
intracerebral hemorrhage
Clinical Perspective
What Is New?
Antiplatelets, anticoagulants, and statins are commonly used; however, their longitudinal impact on cerebral hemorrhagic risk is relatively unknown.
During nearly 20 years of follow‐up in the ARIC (Atherosclerosis Risk in Communities) study, we observed a lower hazard of intracerebral hemorrhage among participants who had a prior exposure to either an antiplatelet or a statin in analyses adjusted for the propensity to be prescribed each medication as well as apolipoprotein E genotype, having an ischemic stroke, and concomitant use of a medication in the other class.
In cross‐sectional analyses adjusted for the propensity of being prescribed each medication and markers of small‐vessel disease, there was no significant association between exposure to an antiplatelet, anticoagulant, or statin and the presence of cerebral microbleed on magnetic resonance imaging; long‐term use of antiplatelets and statins was linked with lower risk of intracerebral hemorrhage in this retrospective, observational study.
What Are the Clinical Implications?
There may be differential cerebral bleeding risk profiles associated with antiplatelet, anticoagulant, and statin use longitudinally.
Future studies are needed to discern the hemorrhagic effects of these medications while accounting for medication indication and markers of cerebral small‐vessel disease.
Antiplatelets, anticoagulants, and statins are commonly prescribed for a variety of indications. Although guidelines delineate when to initiate these medications, 1 , 2 , 3 it is unclear whether these medications are associated with long‐term risk of intracerebral hemorrhage (ICH) and cerebral microbleeds (CMBs) in the United States.
ICH risk in patients who take these medications has been studied, 4 , 5 , 6 , 7 but results have been conflicting and limited by short follow‐up, settings in countries other than the United States, and difficulty accounting for confounders, such as age, concomitant use of other medications, and radiographic and genetic information. There was no increased risk of ICH in older participants randomized to aspirin for primary prevention in the ASPREE (Aspirin in Reducing Events in the Elderly) trial. 8 Similarly, a meta‐analysis of 5 randomized controlled trials demonstrated no increased risk of ICH with aspirin (odds ratio [OR], 1.64; 95% CI, 0.72–3.74) over 2.5 years. 9 In contrast, another meta‐analysis of 3 years of aspirin use did identify increased risk of ICH. 4
CMBs are associated with hypertensive arteriopathy and cerebral amyloid angiopathy, 10 and their accumulation is associated with an 8‐fold increased risk of ICH. 11 Studies have demonstrated significantly elevated risk or no link between the use of statins, 12 , 13 aspirin, 14 , 15 , 16 , 17 or warfarin 15 , 18 and CMB presence, with a lack of consensus in the magnitude of these effects.
It is thus important to characterize the relationships between exposure to commonly used medications in the United States and the risks of ICH and CMB while accounting for demographic, clinical, radiographic, and genetic factors to guide clinical decision‐making. We explore in a longitudinal, US community‐based cohort (the ARIC [Atherosclerosis Risk in Communities] study), the associations of statins, antiplatelets, and anticoagulants with the development of ICH and the prevalence of CMB.
Methods
This retrospective observational study was approved by the Institutional Review Boards for each ARIC study affiliated institution. All participants provided informed consent. Requests to access the data sets from researchers trained in human subject confidentiality may be sent to ARICpub@unc.edu.
Study Population
Participants were members of the ARIC study, a prospective, population‐based cohort recruited from 4 US communities. Each ARIC study field center (Washington County, Maryland; suburbs of Minneapolis, MN; Jackson, MS; and Forsyth County, North Carolina) recruited nearly 4000 participants in 1987 to 1989, for 15 792 total participants ranging from age 45 to 64 years. Because of small numbers, as is standard in the ARIC study, all non‐White or non‐Black participants and all Black participants from Washington County and Minnesota were excluded, resulting in 15 719 participants. The design and objectives of the ARIC study are published elsewhere. 19 ARIC study participants were interviewed for demographic, social, and clinical information, provided samples for serologic tests, and underwent other tests at the baseline visit and every 3 years at visits thereafter through 1998 (visit 2: 1990–1992; visit 3: 1993–1995; and visit 4: 1996–1998), with a fifth in‐person visit in 2011 to 2013, the end point of our study. Participants were contacted by telephone annually. A follow‐up telephone call from 2004 to 2007 was used to obtain medication data given the large gap between visits 4 and 5. When analyzing the association of each medication with hemorrhage outcomes, we excluded participants who were taking the medication at visit 1 to prevent left‐truncation bias.
Hemorrhage Definition
ICH outcome was identified via surveillance and annual follow‐up telephone calls. If a hospitalization is reported, records are obtained for data abstraction. Cases in the ARIC study were defined as probable or definite ICH in accordance with the National Survey of Stroke criteria, including the following: (1) computed tomography or magnetic resonance imaging (MRI) demonstrating an intraparenchymal hematoma, (2) demonstration of ICH on autopsy or surgery, or (3) (a) at least 1 major or 2 minor neurologic deficits, (b) a bloody spinal fluid on lumbar puncture, and (c) cerebral angiography showing an avascular mass effect and no evidence of aneurysm or arteriovenous malformation. 20 A probable ICH did not have computed tomography/MRI available, but met criteria 3a, 3b, and 3c with decreased level of consciousness lasting 24 hours or until death. Of patients with strokes in the ARIC study, 98% underwent computed tomography or MRI. The criteria were implemented by a computer algorithm with additional physician review of medical records, with adjudication by a second physician in the event of discordance.
The outcome of CMB was identified by 3‐T brain MRI with T2*gradient echo sequences from 2011 to 2013 in participants who underwent this visit 5 imaging study (N=1942). 21 Participants were eligible for a brain MRI if there was no contraindication for an MRI and they met one of the following criteria: (1) had previous MRI scans from 2004 to 2006, (2) had low cognitive test scores or a decline on longitudinal testing, or (3) were from an age‐stratified sample of remaining individuals. There were sampling fractions assigned by the ARIC study for participants <80 and ≥80 years of age to approximate the age distribution for those selected with cognitive impairment to reach a goal of nearly 2000 scans. The 3‐T MRIs with 3.3‐mm slices and repetition time/echo time 200/20 ms were reviewed centrally (MRI reading center, Mayo Clinic) for CMBs. 22 CMBs were defined as homogeneous lesions of hemosiderin deposits ≤10 mm in diameter, as detected visually by trained image analysts and confirmed by a radiologist, all of whom were blinded to the exposures of the medications of interest. This analysis only used definite CMBs (85% interrater agreement on definite and not definite CMB; κ=68%). 22 , 23 The presence and location of CMBs (anywhere; lobar or cortical gray; subcortical or periventricular; deep within the basal ganglia, thalamus, corpus callosum, internal, external, and extreme capsule; or infratentorial in the brainstem and cerebellum) in the brain were recorded. 22 Topographic location was identified by transforming the T2*gradient echo into the participant's T1 image space, followed by a discrete cosine transformation of this space, obtained by statistical parametric mapping unified segmentation of the T1 image. 23 A parcellated anatomic atlas is linked to each participant's anatomic MRI. We analyzed the presence of a CMB in any location and in the lobar, subcortical, and deep regions. No subgroup analysis of brainstem CMBs was performed because of the known heterogeneity of cerebellar CMBs, as demonstrated in a radiopathologic study. 24
Medications
The study exposures were antiplatelet, anticoagulant, and statin medications. These data were obtained by extracting medication names or the generic product identification codes from medication inventory lists compiled by ARIC study staff from medication containers brought by participants at every in‐person visit (Table 1). To estimate duration of medication use, time between 2 consecutive visits was calculated if the medication was documented at both. If the medication was taken at 1 of 2 consecutive visits, then we assumed that the patient took the medication for the entirety of the intervening time period until the date of the visit when not documented. Time fragments of medication use were added to determine cumulative years of medication use. We analyzed the hazard of ICH by medication status in 2 groups to establish temporality: (1) participants who took the medication of interest during follow‐up and, if an ICH occurred, necessarily at the most recent visit before the date of ICH versus those who were never exposed; and (2) participants who took the medication at any point during follow‐up versus those who were never exposed. When analyzing the odds of the presence of a CMB on the visit 5 MRI, a participant was classified as a user of each medication class if there was any exposure to the medication of interest during the study period.
Table 1.
Codes Available to Extract Medication Use by ARIC Study Visit
| MSRA | MSRB | MSRC04 | MSRD04 | AFU | MSRF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Medication | Code | Medication | Code | Medication | Code | Medication | Code | Medication | Code | Medication | GPI No. |
| Aspirin | Strings | Aspirin | 102055 | Aspirin | 102055 | Aspirin | 102055 | Aspirin | Strings | Clopidogrel | 85158020100320 |
| Dipyridamole | Strings | Dipyridamole | 107599 | Buffered aspirin (Bufferin) | 102092 | Buffered aspirin (Bufferin) | 102092 | Warfarin | Strings | Aspirin | 64100010000315 |
| Warfarin | Strings | Warfarin | 109474 | Dipyridamole | 107599 | Dipyridamole | 107599 | Simvastatin | Strings | 64100010000605 | |
| Lovastatin | Strings | Lovastatin | 110753 | Warfarin | 119474 | Warfarin | 109915 | Dipyridamole | Strings | 64100010000307 | |
| Lovastatin | 110753 | Lovastatin | 110753 | Atorvastatin | Strings | Dipyridamole | 85159902206920 | ||||
| Pravastatin | Strings | Pravastatin | 121480 | Clopidogrel | Strings | Warfarin | 83200030200303 | ||||
| Simvastatin | Strings | Simvastatin | 102354 | Dabigatran | Strings | 83200030200305 | |||||
| Atorvastatin | 121495 | Lovastatin | Strings | 83200030200310 | |||||||
| 119740 | 83200030200313 | ||||||||||
| 83200030200315 | |||||||||||
| 83200030200317 | |||||||||||
| 83200030200320 | |||||||||||
| 83200030200325 | |||||||||||
| Edoxaban | 8337003020 | ||||||||||
| Rivaroxaban | 8337006000 | ||||||||||
| Dabigatran | 8333703020 | ||||||||||
| Apixaban | 83370010000320 | ||||||||||
| Simvastatin | 39400075000320 | ||||||||||
| 39400075000330 | |||||||||||
| 39400075000340 | |||||||||||
| 39400075000360 | |||||||||||
| Atorvastatin | 39400010100310 | ||||||||||
| 39400010100320 | |||||||||||
| 39400010100330 | |||||||||||
| 39400010100350 | |||||||||||
| Rosuvastatin | 39400060100305 | ||||||||||
| 39400060100310 | |||||||||||
| 39400060100340 | |||||||||||
| Pravastatin | 39400065100330 | ||||||||||
| 39400065100340 | |||||||||||
| Lovastatin | 39400050000305 | ||||||||||
| 39400050000310 | |||||||||||
| Pitavastatin | 39400058100330 | ||||||||||
ARIC indicates Atherosclerosis Risk in Communities; AFU, Phone call between Visits 4 and 5; GPI, Visit 5; MSRA, Visit 1; MSRB, Visit 2; MSRC, Visit 3; and MSRD, Visit 4.
Propensity Score Covariates
We created a propensity score for taking each medication class to address confounding by indication. This score was derived from variables at visit 1, or midlife, to predict use of each medication from visits 2 to 5. The variables of the propensity score were chosen a priori as they may influence medication prescription and outcomes, and are available to a community practitioner. Date of birth, sex, race, and education were self‐reported. Clinical data consisted of smoking (self‐report; defined as ever versus never), hypertension (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, based on the average of the last 2 of 3 measurements, or use of antihypertensive medication over the past 2 weeks), diabetes mellitus (fasting glucose ≥126 mg/dL or nonfasting glucose ≥200 mg/dL, physician diagnosis of diabetes mellitus, or takes a diabetes mellitus medication), coronary heart disease (myocardial infarction evident on adjudicated ECG, history of myocardial infarction, history of heart surgery, history of coronary bypass, or history of balloon angioplasty), and heart failure (taking heart failure medications or meeting a Gothenburg score of 3, based on cardiac and pulmonary symptoms as well as use of diuretics and/or digitalis 25 ). Low‐density lipoprotein was calculated as follows: total cholesterol−high‐density lipoprotein−(total triglycerides/5). Serum creatinine was measured in mg/dL. The use of either of the other medications classes at visit 1 was determined, as described in the Medications section.
Risk Factors for Adjustment
Although the propensity score includes variables readily available to clinicians before initiating medications, we also adjusted for important covariates associated with increased risk of ICH and CMB.
In models evaluating ICH, we considered ischemic stroke, 26 APOE (apolipoprotein E) genotype (ε2 and/or ε4), 27 and use of the other 2 medication classes during follow‐up. Potential ischemic strokes were identified by the same surveillance method as ICHs. 28 An ischemic stroke was included if the event occurred before or at the date of the ICH or during the time up to the end of the participant's study period if there was no ICH. APOE genotyping, performed by a TaqMan assay (Applied Biosystems, Foster City, CA), 29 was categorized by genotype into the following categories: ε3/ε3, at least one ε2 and no ε4 allele, at least one ε4 and no ε2 allele, and both an ε2 and an ε4 allele. Participants who opted out of release of genetic information for research were excluded from analyses including APOE.
When evaluating CMB presence on visit 5 MRIs, we considered these covariates: APOE genotype, 30 percentage of white matter occupied by hyperintense signal, 31 the presence of either mild cognitive impairment or dementia at visit 5, 32 and use of the other 2 medication classes from visits 2 to 5. To calculate white matter hyperintensity percentage, axial fluid‐attenuated inversion recovery images from the MRI obtained in 2011 to 2013 were centrally segmented into voxels to measure leukoaraiosis volume. 33 Percentage of white matter occupied by leukoaraiosis was dichotomized to less than or equal to versus more than the sample mean. Cognitive function at visit 5 was determined by expert review and computer algorithm, using standard criteria, including neuropsychological assessments and informant interviews, and classified as normal, mild cognitive impairment, or dementia. 34 , 35 , 36 All 1942 participants with MRI gradient echo data underwent visit 5 cognitive testing.
Statistical Analysis
Participants were compared in groups determined by use of each medication at any point during follow‐up from ARIC study visit 2 onwards. Univariate analyses were performed using Student t tests and χ2 tests, comparing individuals by medication use. Statistical analyses were performed in SAS 9.4.
Creation of Propensity Scores
We constructed separate propensity scores for taking an antiplatelet, anticoagulant, or statin at any point from visits 2 to 5 using visit 1 variables, by multivariable logistic regressions. 37 Multivariable logistic regressions were built to model the odds of taking each of the medications of interest from visits 2 to 5 as a function of visit 1 variables. The variables were entered into a propensity score estimation, including quadratic, spline, and interaction terms, and were retained in the final model if the covariate's associated P≤0.2. The predicted propensity score values were then binned into quintiles for use as adjustment covariates for the primary analyses. The balance of the propensity scores was assessed by computing the mean standardized differences of each variable in the score across the 5 score quintiles, with a prespecified threshold of balance <0.10. 38
Analysis of ICH Outcome
We determined the hazard of an ICH during visits 2 to 5 associated with each medication class by Cox proportional hazard regression models, with visit 2 as the time of origin. Participants were censored at the end of the administrative censoring date (December 31, 2013) or when lost to follow‐up.
To study the association of contemporaneous medication use and ICH, we performed Cox regression analyses to ascertain the hazard of an ICH when taking each medication class during follow‐up and at the most recent visit before the ICH if the event occurred versus no exposure to the medication of interest before ICH or censoring, excluding participants with ICH and remote medication use. To understand the impact of any medication use, remote or concurrent, we also analyzed the hazard of ICH in participants who were exposed to the medications at any time before the ICH versus those who were never exposed before an ICH or censoring. Each model was assessed for proportionality of hazards by including an interaction term of the time‐varying covariate of medication use and the log of survival time for each Cox proportional hazard model with a prespecified threshold of violation of the assumption if P<0.05.
We performed Cox proportional hazard regression analysis with a static and time‐varying variable representing medication use (any exposure from visit 2–5). The static model assigns time of nonexposure within the medication use group toward the hazard of the outcome and models the future occurrence of an outcome based on a possible future exposure. In contrast, the time‐varying variable denotes 5 possible periods of medication exposure, demarcated by the dates of each visit and the telephone call, allowing reevaluation of a participant's exposure status at each time interval. Proportionality of hazards was tested for each model.
Model 1 represents the unadjusted hazard of an ICH as a function of the medication use, model 2 adjusted for the propensity quintile of taking that medication, and model 3 adjusted for the propensity quintile of concomitant use of either of the other medication classes, occurrence of an ischemic stroke, and APOE genotype.
Sensitivity Analyses
A sensitivity analysis was performed to determine whether there was an overarching medication compliance bias among ARIC study participants who have ICH versus not. This was tested by analyzing the use of thyroid replacement medications. We chose to study the use of levothyroxine in association with the occurrence of ICH as it was a commonly prescribed medication during the ARIC study period and its use is not associated with a risk of ICH in the literature. We hypothesized that there is no difference in the proportions of participants with ICH versus participants without ICH who use levothyroxine and that there is no hazard of ICH by levothyroxine use.
To further assess the validity of the method used in this study to assess medication effects, we studied the impact of antihypertensive medication use as uncontrolled systemic blood pressure is an established risk factor for ICH. We hypothesized that patients with ICH were less likely to have taken an antihypertensive medication before the onset of ICH and that there would be a lower hazard of ICH with antihypertensive use.
Analysis of CMB Outcome
We separately analyzed the likelihood of CMB presence on MRI as a function of use of each medication of interest from visits 2 to 5. Because MRIs were only performed at visit 5, logistic regressions were used to evaluate the following: CMB anywhere, lobar CMB, subcortical CMB, and deep CMB, as a function of any use of each medication class from visits 2 to 5. The logistic regression models were constructed with survey weights accounting for the MRI sampling strategy. Model 1 was unadjusted, model 2 was adjusted for propensity quintile, and model 3 was adjusted for concomitant use of either of the other medication classes, APOE genotype, white matter hyperintensity percentage category, and mild cognitive impairment or dementia.
Data Availability
Requests for data from qualified investigators can be directed to the corresponding author.
Results
Univariate Analysis
Antiplatelet Use
The antiplatelet study cohort consisted of 14 471 participants not taking an antiplatelet at visit 1, of whom 7458 took an antiplatelet at some point from visits 2 to 5. The mean follow‐up time was 17.4 (SD, 6.8) years for antiplatelet users and 18.5 (SD, 6.4) years for antiplatelet nonusers. Among antiplatelet users, 44% took an antiplatelet at >1 visit. The median time of antiplatelet use was 11.8 (interquartile range, 6.0–15.1) years. Antiplatelet users had more comorbidities, took a statin during follow‐up, and were less likely to have both APOE ε2 and ε4 alleles (Tables 2 and 3). A total of 124 participants had an ICH during follow‐up (0.76% among antiplatelet users and 0.97% among antiplatelet nonusers); however, 23 of these ICHs occurred at a time remote to antiplatelet use among antiplatelet users. The average time from documented antiplatelet use and ICH among remote users was 6.9 (SD, 5.5) years. Among antiplatelet users with recent exposure if an ICH occurred and antiplatelet nonusers, 0.4% of antiplatelet users (N=29) and 1% of antiplatelet nonusers (N=72) developed an ICH (P<0.001). Among the 1801 participants who underwent a visit 5 MRI, 23.75% of antiplatelet users and 24.14% of antiplatelet nonusers were noted to have a CMB (P=0.857).
Table 2.
Baseline Characteristics of Participants by Use of Each Medication of Interest
| Visit 1 Characteristics | Antiplatelet Use | Anticoagulant Use | Statin Use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiplatelet Cohort (N=14 471) | Any Use From Visit 2–5 (N=7458) | No Use From Visit 2–5 (N=7013) | P Value | Anticoagulant Cohort (N=15 719) | Any Use From Visit 2–5 (N=846) | No Use From Visit 2–5 (N=14 873) | P Value | Statin Cohort (N=15 711) | Any Use From Visit 2–5 (N=4229) | No Use From Visit 2–5 (N=11 482) | P Value | |
| Age, y | 54.1 (5.8) | 54.1 (5.7) | 54.2 (5.9) | 0.040 | 54.2 (5.8) | 55.0 (5.6) | 54.1 (5.8) | <0.001 | 54. 2 (5.8) | 53.1 (5.4) | 54.5 (5.8) | <0.001 |
| Male sex | 6325 (44.0) | 3478 (46.6) | 2847 (41.2) | <0.001 | 6988 (44.8) | 470 (55.6) | 6518 (44.1) | <0.001 | 6998 (44.8) | 1909 (45.1) | 5089 (44.7) | 0.638 |
| White race | 10 264 (71.4) | 5906 (79.2) | 4358 (63.1) | <0.001 | 11 416 (73.1) | 663 (78.4) | 10 753 (72.8) | <0.001 | 11 408 (73.1) | 3361 (79.5) | 8047 (70.7) | <0.001 |
| High school education or higher | 10.795 (75.3) | 5936 (79.7) | 4859 (70.5) | <0.001 | 11 875 (76.2) | 671 (79.4) | 11 204 (76.0) | 0.023 | 11 866 (76.2) | 3455 (81.9) | 8411 (74.0) | <0.001 |
| Hypertension | 5017 (35.1) | 2495 (33.6) | 2522 (36.7) | <0.001 | 5434 (35.0) | 368 (43.8) | 5066 (34.5) | <0.001 | 5432 (35.0) | 1390 (33.0) | 4042 (35.7) | 0.002 |
| Diabetes mellitus | 1715 (12.1) | 802 (10.8) | 913 (13.4) | <0.001 | 1851 (12.0) | 97 (11.6) | 1754 (12.0) | 0.702 | 1844 (11.9) | 463 (11.0) | 1381 (12.3) | 0.037 |
| LDL, mg/dL | 137.7 (39.2) | 139.2 (38.5) | 136.0 (40.0) | <0.001 | 137.6 (39.3) | 137.8 (37.8) | 137.6 (39.4) | 0.910 | 137.6 (39.3) | 150.9 (39.1) | 132.7 (38.2) | <0.001 |
| Coronary artery disease | 590 (4.2) | 367 (5.01) | 223 (3.31) | <0.001 | 736 (4.8) | 74 (8.9) | 662 (4.6) | <0.001 | 747 (4.9) | 228 (5.5) | 519 (4.7) | 0.032 |
| Ever smoker | 8281 (57.7) | 4272 (57.3) | 4009 (58.1) | 0.345 | 9100 (58.3) | 512 (60.5) | 8588 (58.2) | 0.180 | 9105 (58.4) | 2377 (56.2) | 6728 (59.2) | 0.001 |
| Serum creatinine, mg/dL | 1.10 (0.40) | 1.10 (0.21) | 1.12 (0.53) | 0.018 | 1.11 (0.42) | 1.13 (0.19) | 1.11 (0.43) | 0.005 | 1.11 (0.42) | 1.10 (0.19) | 1.12 (0.48) | 0.002 |
| APOE genotype | 0.030 | 0.244 | <0.001 | |||||||||
| >1 ε2, no ε4 | 1973 (14.2) | 1025 (14.3) | 948 (14.2) | 2081 (13.8) | 102 (12.7) | 1979 (13.9) | 2083 (13.9) | 477 (11.8) | 1606 (14.6) | |||
| >1 ε4, no ε2 | 3839 (27.7) | 1928 (26.9) | 1911 (26.9) | 4174 (27.8) | 225 (28.0) | 3949 (27.7) | 4171 (27.8) | 1169 (28.8) | 3002 (27.4) | |||
| ε2 and ε4 alleles | 434 (3.1) | 209 (2.9) | 225 (3.4) | 470 (3.1) | 17 (2.1) | 453 (3.2) | 472 (3.1) | 106 (2.6) | 366 (3.3) | |||
Mean (SD) values are presented if continuous, and number (percentage) values are presented if categorical. APOE indicates apolipoprotein E; and LDL, low‐density lipoprotein.
Table 3.
Occurrence of ICH or Ischemic Stroke and Concomitant Medication Use Over the Duration of the Study Period From Visit 2 to 5 and Clinical and Radiographic Variables Assessed at Visit 5 by Medication Group
| Variables | Antiplatelet | Anticoagulant | Statin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiplatelet Cohort (N=14 471) | Any Use From Visit 2–5 (N=7458) | No Use From Visit 2–5 (N=7013) | P Value | Anticoagulant Cohort (N=15 719) | Any Use From Visit 2–5 (N=846) | No Use From Visit 2–5 (N=14 873) | P Value | Statin Cohort (N=15 711) | Any Use From Visit 2–5 (N=4229) | No Use From Visit 2–5 (N=11 482) | P Value | |
| Variables assessed in all participants | ||||||||||||
| ICH | 124 (0.86) | 57 (0.76) | 67 (0.97) | 0.184 | 130 (0.83) | 3 (0.35) | 127 (0.86) | 0.168 | 130 (0.83) | 16 (0.38) | 114 (0.99) | <0.001 |
| Ischemic stroke before or at time of ICH or censoring | 1065 (7.41) | 566 (7.59) | 499 (7.22) | 0.399 | 1136 (7.27) | 133 (15.72) | 1003 (6.79) | <0.001 | 1134 (7.27) | 277 (6.55) | 857 (7.53) | 0.036 |
| Concomitant antiplatelet use | … | … | … | … | 8449 (53.75) | 488 (57.68) | 7961 (53.53) | 0.0183 | 8425 (53.62) | 3195 (75.55) | 5230 (45.55) | <0.001 |
| Concomitant anticoagulant use | 789 (5.46) | 417 (5.59) | 372 (5.30) | 0.448 | … | … | … | … | 885 (5.63) | 361 (8.54) | 524 (4.56) | <0.001 |
| Concomitant statin use | 3876 (26.78) | 2866 (38.43) | 1010 (14.40) | <0.001 | 4263 (27.12) | 358 (42.32) | 3905 (26.26) | <0.001 | … | … | … | … |
| N=1801 | N=1221 | N=580 | N=1942 | N=117 | N=1825 | N=1939 | N=928 | N=1011 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables assessed in participants present at visit 5 who underwent MRI and cognitive testing | ||||||||||||
| Any CMB | 430 (23.88) | 290 (23.75) | 140 (24.14) | 0.857 | 476 (24.51) | 40 (34.19) | 436 (23.89) | 0.012 | 476 (24.55) | 247 (26.62) | 229 (22.65) | 0.043 |
| Lobar CMB | 155 (8.68) | 109 (9.02) | 46 (7.97) | 0.464 | 173 (8.98) | 21 (18.10) | 152 (8.40) | <0.001 | 173 (9.00) | 91 (9.92) | 82 (8.15) | 0.175 |
| Subcortical CMB | 348 (19.48) | 228 (18.86) | 120 (20.80) | 0.333 | 386 (20.04) | 31 (26.72) | 355 (19.61) | 0.064 | 386 (20.07) | 191 (20.83) | 195 (19.38) | 0.429 |
| Deep CMB | 48 (2.69) | 29 (2.40) | 19 (3.29) | 0.275 | 53 (2.75) | 4 (3.45) | 49 (2.71) | 0.557 | 53 (2.76) | 23 (1.20) | 30 (2.98) | 0.526 |
| White matter hyperintensity percentage | 3.99 (3.57) | 4.07 (3.51) | 3.82 (3.67) | 0.153 | 3.98 (3.56) | 4.62 (3.45) | 3.94 (3.57) | 0.047 | 3.98 (3.57) | 4.12 (3.68) | 3.86 (3.45) | 0.106 |
| Cognitive impairment | 1571 (26.74) | 1101 (27.17) | 470 (25.81) | 0.278 | 1700 (26.48) | 175 (34.45) | 1525 (25.79) | <0.001 | 1699 (26.50) | 901 (28.62) | 798 (24.46) | <0.001 |
Mean (SD) values are presented if continuous, and number (percentage) values are presented if categorical. CMB indicates cerebral microbleed; ICH, intracranial hemorrhage; and MRI, magnetic resonance imaging.
Anticoagulant Use
The anticoagulant study cohort consisted of 15 719 participants not taking an anticoagulant at visit 1, of whom 846 took an anticoagulant from visits 2 to 5. Mean follow‐up time was 17.8 (SD, 6.9) years for anticoagulant users and 18.0 (SD, 6.6) years for anticoagulant nonusers. Of anticoagulant users, 16% took an anticoagulant at >1 visit. Anticoagulant users took the medication for a median of 6.0 (interquartile range, 3.5–9.4) years. Anticoagulant users were older, had more comorbidities, and were more likely to have an ischemic stroke and cognitive impairment. There was no difference in the proportion of ICH by anticoagulant use (0.35% among anticoagulant users and 0.86% among anticoagulant nonusers; P=0.168). Among the 1942 who underwent a visit 5 MRI, anticoagulant users compared with nonusers were more likely to have any CMB (34.2% versus 23.9%, respectively; P=0.012) and lobar CMB (18.1% versus 8.4%, respectively; P<0.001).
Statin Use
The statin cohort contained 15 711 participants not on a statin at visit 1. A statin was used by 4229 participants at some point from visits 2 to 5. The mean time to ICH or censoring was 19.8 (SD, 5.6) years among statin users and 17.3 (SD, 6.9) years among statin nonusers. Nearly 28% of statin users took a statin at >1 visit for a median of 6.8 (interquartile range, 5.7–13.3) years. Statin users were younger, had higher low‐density lipoprotein levels, had ≥1 APOE ε4 allele, and used an anticoagulant or antiplatelet. Statin users were likely to be diagnosed with an ischemic stroke and cognitive impairment. Sixteen statin users developed an ICH compared with 114 statin nonusers (0.4% versus 1.0%; P<0.001). Only 7 of these ICHs occurred in context of remote statin use before the ICH. The mean time from documented statin use to ICH among remote users was 11.8 (SD, 2.7) years. Among statin users with recent exposure if an ICH occurred and statin nonusers, 0.1% of statin users (N=4) and 1% of statin nonusers (N=119) developed an ICH (P<0.0001). Among the 1939 who underwent a visit 5 MRI, there were more CMBs overall in the statin group (26.6% versus 22.7%, respectively; P=0.043).
Propensity Score
Tables 4, 5, 6 displays the visit 1 variables used to create a propensity score for each medication class. Although standardized differences were above the prespecified threshold of 0.10 for many baseline variables, after stratification by propensity quintile, the mean standardized differences across propensity strata were ≥0.10 for only 2 variables: age (mean standardized difference, 0.108) and coronary artery disease (mean standardized difference, 0.108) for anticoagulant use, indicating reasonably well‐balanced groups.
Table 4.
Propensity Score Assessment for Antiplatelet Use
| Without Propensity Score Stratification | With Propensity Score Stratification | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Proportion of Antiplatelet Users | Proportion of Antiplatelet Nonusers | Std. Diff. | Propensity Score Quintile 1 | Propensity Score Quintile 2 | Propensity Score Quintile 3 | Propensity Score Quintile 4 | Propensity Score Quintile 5 | Mean Std. Diff. | ||||||||||
| Proportion of Antiplatelet Users | Proportion of Antiplatelet Nonusers | Std. Diff. | Proportion of Antiplatelet Users | Proportion of Antiplatelet Nonusers | Std. Diff. | Proportion of Antiplatelet Users | Proportion of Antiplatelet Nonusers | Std. Diff. | Proportion of Antiplatelet Users | Proportion of Antiplatelet Nonusers | Std. Diff. | Proportion of Antiplatelet Users | Proportion of Antiplatelet Nonusers | Std. Diff. | |||||
| Aged >54 y | 0.463 | 0.473 | 0.017 | 0.482 | 0.481 | 0.001 | 0.580 | 0.569 | 0.019 | 0.515 | 0.518 | 0.006 | 0.471 | 0.461 | 0.015 | 0.316 | 0.329 | 0.022 | 0.013 |
| Men | 0.466 | 0.412 | 0.089 | 0.392 | 0.427 | 0.059 | 0.285 | 0.290 | 0.009 | 0.100 | 0.092 | 0.022 | 0.434 | 0.414 | 0.033 | 0.973 | 0.973 | 0.001 | 0.025 |
| White race | 0.792 | 0.631 | 0.304 | 0.459 | 0.378 | 0.134 | 0.611 | 0.578 | 0.054 | 0.989 | 0.987 | 0.017 | 0.998 | 0.997 | 0.012 | 0.998 | 0.999 | 0.013 | 0.046 |
| High school education or higher | 0.797 | 0.705 | 0.179 | 0.497 | 0.451 | 0.076 | 0.531 | 0.539 | 0.013 | 0.876 | 0.856 | 0.049 | 0.935 | 0.954 | 0.067 | 0.978 | 0.970 | 0.041 | 0.049 |
| CAD | 0.050 | 0.033 | 0.067 | 0.014 | 0.017 | 0.024 | 0.026 | 0.019 | 0.036 | 0.017 | 0.016 | 0.006 | 0.029 | 0.024 | 0.025 | 0.126 | 0.109 | 0.042 | 0.027 |
| Hypertension | 0.336 | 0.367 | 0.053 | 0.456 | 0.449 | 0.012 | 0.405 | 0.442 | 0.062 | 0.207 | 0.206 | 0.003 | 0.371 | 0.356 | 0.025 | 0.268 | 0.255 | 0.022 | 0.025 |
| Diabetes mellitus | 0.108 | 0.134 | 0.066 | 0.174 | 0.200 | 0.055 | 0.147 | 0.147 | 0.001 | 0.079 | 0.093 | 0.041 | 0.098 | 0.079 | 0.053 | 0.058 | 0.056 | 0.006 | 0.031 |
| LDL >160 mg/dL | 0.268 | 0.251 | 0.031 | 0.113 | 0.117 | 0.012 | 0.373 | 0.358 | 0.025 | 0.323 | 0.345 | 0.038 | 0.173 | 0.187 | 0.030 | 0.309 | 0.301 | 0.015 | 0.024 |
| Ever smoker | 0.573 | 0.581 | 0.013 | 0.589 | 0.636 | 0.078 | 0.666 | 0.651 | 0.025 | 0.505 | 0.480 | 0.041 | 0.500 | 0.504 | 0.007 | 0.622 | 0.595 | 0.045 | 0.039 |
| Creatinine>mean | 0.358 | 0.345 | 0.023 | 0.376 | 0.410 | 0.056 | 0.268 | 0.281 | 0.023 | 0.129 | 0.145 | 0.038 | 0.331 | 0.303 | 0.048 | 0.628 | 0.630 | 0.003 | 0.033 |
| Anticoagulant at visit 1 | 0.003 | 0.006 | 0.035 | 0.013 | 0.017 | 0.029 | 0.002 | 0.003 | 0.022 | 0.003 | 0.009 | 0.065 | 0.001 | 0.002 | 0.009 | 0.001 | 0.003 | 0.040 | 0.033 |
| Statin at visit 1 | 0.006 | 0.003 | 0.030 | 0.002 | 0.003 | 0.013 | 0.002 | 0.002 | 0.010 | 0.004 | 0.004 | 0.004 | 0.002 | 0.001 | 0.021 | 0.016 | 0.007 | 0.071 | 0.024 |
Std. Diff. values among antiplatelet users and nonusers for each visit 1 variable represented in the propensity score for taking the medication from visit 2 to 5 without and with propensity quintile stratification, yielding mean Std. Diff. values. CAD indicates coronary artery disease; LDL, low‐density lipoprotein; and Std. Diff., standardized difference.
Table 5.
Propensity Score Assessment for Anticoagulant Use
| Without Propensity Score Stratification | With Propensity Score Stratification | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Propensity Score Quintile 1 | Propensity Score Quintile 2 | Propensity Score Quintile 3 | Propensity Score Quintile 4 | Propensity Score Quintile 5 | Mean Std. Diff. | |||||||||||||||
| Variable | Proportion of Anticoagulant Users | Proportion of Anticoagulant Nonusers | Std. Diff. | Proportion of Anticoagulant Users | Proportion of Anticoagulant Nonusers | Std. Diff. | Proportion of Anticoagulant Users | Proportion of Anticoagulant Nonusers | Std. Diff. | Proportion of Anticoagulant Users | Proportion of Anticoagulant Nonssers | Std. Diff. | Proportion of Anticoagulant Users | Proportion of Statin Nonusers | Std. Diff. | Proportion of Anticoagulant Users | Proportion of Anticoagulant Nonusers | Std. Diff. | ||
| Aged >54 y | 0.546 | 0.464 | 0.134 | 0.084 | 0.117 | 0.091 | 0.424 | 0.576 | 0.250 | 0.458 | 0.522 | 0.105 | 0.676 | 0.625 | 0.088 | 0.715 | 0.717 | 0.004 | 0.108 | |
| Men | 0.556 | 0.441 | 0.189 | 0.105 | 0.101 | 0.011 | 0.242 | 0.271 | 0.054 | 0.477 | 0.463 | 0.023 | 0.553 | 0.580 | 0.043 | 0.875 | 0.827 | 0.113 | 0.049 | |
| White race | 0.784 | 0.728 | 0.107 | 0.590 | 0.550 | 0.065 | 0.647 | 0.674 | 0.047 | 0.745 | 0.762 | 0.033 | 0.883 | 0.838 | 0.110 | 0.878 | 0.876 | 0.006 | 0.052 | |
| High school education or higher | 0.794 | 0.760 | 0.068 | 0.695 | 0.677 | 0.032 | 0.616 | 0.624 | 0.014 | 0.837 | 0.817 | 0.043 | 0.782 | 0.810 | 0.056 | 0.890 | 0.904 | 0.037 | 0.036 | |
| CAD | 0.089 | 0.046 | 0.135 | 0.000 | 0.013 | 0.159 | 0.020 | 0.016 | 0.025 | 0.000 | 0.013 | 0.163 | 0.021 | 0.016 | 0.029 | 0.251 | 0.168 | 0.164 | 0.108 | |
| Hypertension | 0.438 | 0.345 | 0.155 | 0.074 | 0.124 | 0.143 | 0.283 | 0.220 | 0.118 | 0.288 | 0.260 | 0.051 | 0.463 | 0.449 | 0.022 | 0.662 | 0.660 | 0.003 | 0.067 | |
| Diabetes mellitus | 0.116 | 0.120 | 0.011 | 0.126 | 0.121 | 0.013 | 0.172 | 0.136 | 0.081 | 0.072 | 0.099 | 0.081 | 0.117 | 0.106 | 0.030 | 0.095 | 0.106 | 0.030 | 0.047 | |
| LDL >160 mg/dL | 0.265 | 0.260 | 0.009 | 0.137 | 0.175 | 0.088 | 0.394 | 0.356 | 0.064 | 0.190 | 0.220 | 0.063 | 0.346 | 0.319 | 0.045 | 0.240 | 0.236 | 0.007 | 0.053 | |
| Smoking | 0.605 | 0.582 | 0.039 | 0.547 | 0.504 | 0.072 | 0.515 | 0.565 | 0.081 | 0.556 | 0.575 | 0.032 | 0.606 | 0.613 | 0.010 | 0.700 | 0.666 | 0.059 | 0.051 | |
| Creatinine>mean | 0.448 | 0.349 | 0.163 | 0.105 | 0.087 | 0.049 | 0.242 | 0.240 | 0.005 | 0.340 | 0.316 | 0.042 | 0.415 | 0.437 | 0.037 | 0.745 | 0.695 | 0.092 | 0.045 | |
| Antiplatelet at visit 1 | 0.121 | 0.082 | 0.101 | 0.000 | 0.005 | 0.097 | 0.030 | 0.058 | 0.117 | 0.046 | 0.043 | 0.011 | 0.122 | 0.115 | 0.018 | 0.262 | 0.209 | 0.102 | 0.069 | |
| Anticoagulant at visit 1 | 0.007 | 0.005 | 0.023 | 0.011 | 0.000 | 0.099 | 0.010 | 0.002 | 0.080 | 0.000 | 0.003 | 0.080 | 0.000 | 0.007 | 0.119 | 0.015 | 0.012 | 0.023 | 0.080 | |
Std. Diff. values among anticoagulant users and nonusers for each visit 1 variable represented in the propensity score for taking the medication from visit 2 to 5 without and with propensity quintile stratification, yielding mean Std. Diff. values. CAD indicates coronary artery disease; LDL, low‐density lipoprotein; and Std. Diff., standardized difference.
Table 6.
Propensity Score Assessment for Statin Use
| Without Propensity Score Stratification | With Propensity Score Stratification | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Proportion of Statin Users | Proportion of Statin Nonusers | Std. Diff. | Propensity Score Quintile 1 | Propensity Score Quintile 2 | Propensity Score Quintile 3 | Propensity Score Quintile 4 | Propensity Score Quintile 5 | Mean Std. Diff. | ||||||||||
| Proportion of Statin Users | Proportion of Statin Nonusers | Std. Diff. | Proportion of Statin Users | Proportion of Statin Nonusers | Std. Diff. | Proportion of Statin Users | Proportion of Statin Nonusers | Std. Diff. | Proportion of Statin Users | Proportion of Statin Nonusers | Std. Diff. | Proportion of Statin Users | Proportion of Statin Nonusers | Std. Diff. | |||||
| Aged >54 y | 0.400 | 0.495 | 0.158 | 0.673 | 0.740 | 0.120 | 0.572 | 0.545 | 0.044 | 0.419 | 0.456 | 0.062 | 0.375 | 0.369 | 0.011 | 0.271 | 0.264 | 0.012 | 0.050 |
| Men | 0.451 | 0.447 | 0.007 | 0.472 | 0.466 | 0.010 | 0.471 | 0.422 | 0.080 | 0.423 | 0.427 | 0.006 | 0.452 | 0.459 | 0.011 | 0.467 | 0.473 | 0.009 | 0.023 |
| White race | 0.795 | 0.707 | 0.170 | 0.559 | 0.531 | 0.045 | 0.685 | 0.686 | 0.002 | 0.734 | 0.747 | 0.024 | 0.820 | 0.804 | 0.033 | 0.926 | 0.919 | 0.021 | 0.025 |
| High school education or higher | 0.819 | 0.740 | 0.159 | 0.497 | 0.530 | 0.055 | 0.725 | 0.742 | 0.032 | 0.847 | 0.811 | 0.079 | 0.866 | 0.852 | 0.031 | 0.889 | 0.887 | 0.004 | 0.040 |
| CAD | 0.055 | 0.047 | 0.031 | 0.053 | 0.036 | 0.065 | 0.043 | 0.046 | 0.012 | 0.026 | 0.038 | 0.057 | 0.051 | 0.048 | 0.011 | 0.075 | 0.064 | 0.035 | 0.036 |
| Hypertension | 0.330 | 0.357 | 0.046 | 0.454 | 0.437 | 0.027 | 0.363 | 0.326 | 0.065 | 0.295 | 0.335 | 0.071 | 0.305 | 0.317 | 0.020 | 0.301 | 0.297 | 0.008 | 0.038 |
| Diabetes mellitus | 0.110 | 0.123 | 0.032 | 0.151 | 0.145 | 0.014 | 0.122 | 0.114 | 0.022 | 0.096 | 0.117 | 0.057 | 0.106 | 0.099 | 0.019 | 0.090 | 0.089 | 0.004 | 0.023 |
| LDL >160 mg/dL | 0.372 | 0.219 | 0.271 | 0.019 | 0.022 | 0.022 | 0.077 | 0.073 | 0.013 | 0.125 | 0.153 | 0.068 | 0.352 | 0.343 | 0.015 | 0.737 | 0.691 | 0.084 | 0.040 |
| Smoking | 0.562 | 0.592 | 0.049 | 0.645 | 0.682 | 0.063 | 0.585 | 0.589 | 0.007 | 0.553 | 0.558 | 0.010 | 0.567 | 0.559 | 0.014 | 0.543 | 0.540 | 0.005 | 0.020 |
| Creatinine>mean | 0.364 | 0.352 | 0.019 | 0.365 | 0.363 | 0.005 | 0.362 | 0.345 | 0.028 | 0.368 | 0.333 | 0.058 | 0.354 | 0.365 | 0.019 | 0.365 | 0.358 | 0.010 | 0.024 |
| Antiplatelet at visit 1 | 0.094 | 0.080 | 0.041 | 0.071 | 0.064 | 0.023 | 0.101 | 0.079 | 0.060 | 0.064 | 0.079 | 0.046 | 0.073 | 0.081 | 0.025 | 0.135 | 0.120 | 0.037 | 0.038 |
| Anticoagulant at visit 1 | 0.004 | 0.005 | 0.017 | 0.006 | 0.013 | 0.058 | 0.004 | 0.003 | 0.010 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.000 | 0.005 | 0.003 | 0.026 | 0.019 |
Std. Diff. values among statin users and nonusers for each visit 1 variable represented in the propensity score for taking the medication from visit 2 to 5 without and with propensity quintile stratification, yielding mean Std. Diff. values. CAD indicates coronary artery disease; LDL, low‐density lipoprotein; and Std. Diff., standardized difference.
Multivariable Analyses
Intracerebral Hemorrhage
There were 130 participants in the ARIC study with a definite or probable ICH (Table 2). The proportionality of hazards assumption was met for every medication class analysis with interaction P≥0.05. The paucity of events in the anticoagulant group precluded meaningful survival analysis for this medication.
In the analysis of recent antiplatelet use if an ICH occurred versus antiplatelet nonuse, there was a significantly lower hazard of ICH among antiplatelet users versus nonusers maintained in all 3 models (Figure [A]; model 1 hazard ratio [HR], 0.36 [95% CI, 0.21–0.61]; model 2 HR, 0.50 [95% CI, 0.29–0.88]; model 3 HR, 0.53 [95% CI, 0.30–0.92]) with the same directionality using static and time‐varying antiplatelet exposure. There was a significantly lower hazard of ICH among antiplatelet users at any point during follow‐up versus nonusers when considering time‐varying use of antiplatelets in the unadjusted model (Figure [B]; model 1 HR, 0.56 [95% CI, 0.37–0.85]), but the association lost significance with subsequent adjustments. HRs obtained with static and time‐varying antiplatelet use maintained the same directionality. There were no significant interactions between antiplatelet use and APOE genotype, having an ischemic stroke, or concomitant anticoagulant or statin use on the ICH hazard when the interaction terms were included in model 3.
Figure 1. Medication use and the hazard of an intracerebral hemorrhage (ICH).
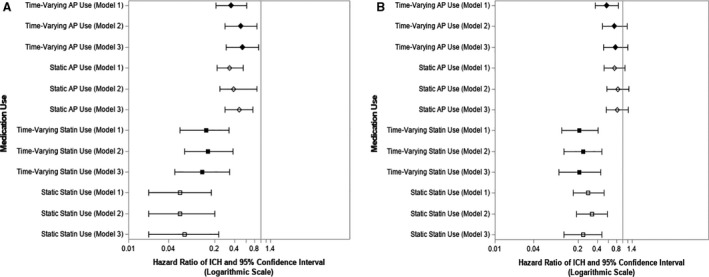
Unadjusted and adjusted hazard ratios of ICH by use of medications of interest during follow‐up and at the most recent study visit just before ICH if the event occurred vs nonuse (A); and use of medications of interest at any point during follow‐up before ICH vs nonuse (B). The hazards of ICH as function of medication use as both a static and a time‐varying covariate were modeled. Model 1 was unadjusted, model 2 was adjusted for propensity quintile to take the medication, and model 3 was adjusted for propensity quintile, concomitant use of either of the other medication classes during follow‐up, occurrence of an ischemic stroke, and apolipoprotein E genotype. AP indicates antiplatelet.
When analyzing statin users exposed to the medication class including only those with recent use if an ICH occurred versus statin nonusers, time‐varying use of statins was associated with a significantly lower hazard of ICH during follow‐up in all unadjusted and adjusted models (Figure [A]; model 1 HR, 0.15 [95% CI, 0.06–0.33]). The directionality of HRs was the same with static and time‐varying antiplatelet use. The hazard of an ICH was significantly lower with statin use at any point as a time‐varying covariate compared with nonuse, and this remained significant in sequentially adjusted models (Figure [B]; model 2 HR, 0.24 [95% CI, 0.12–0.47]; model 3 HR, 0.21 [95% CI, 0.10–0.45]). Again, there were no significant interactions between statin use and the other risk factors in model 3.
Sensitivity Analyses
We analyzed levothyroxine use as documented by study staff inspection of participants' medication containers at each study visit (Tables S1 and S2). There were 1109 participants who took levothyroxine anew after visit 1 before an ICH or, if no ICH occurred, the administrative date of censoring. Eight participants with ICH took levothyroxine before this event (6.3%), whereas 1101 (7.3%) of participants without ICH took levothyroxine before the administrative censoring date (P=0.6643). The unadjusted HR of an ICH of levothyroxine use was neither statistically significant in the static use model (HR, 0.74; 95% CI, 0.36–1.52) nor statistically significant in the time‐varying model (HR, 1.08; 95% CI, 0.56–2.07). This sensitivity analysis demonstrates the lack of a medication compliance bias in this study. Furthermore, it also lends credence to the HR estimates noted in this study because no increased hazard of ICH was noted among levothyroxine users, as expected.
We additionally analyzed antihypertensive medication use at each study visit. There were 7241 participants who took an antihypertensive anew after visit 1 and before either an ICH or the administrative censoring date. Participants who developed an ICH were significantly less likely to have taken an antihypertensive medication during the study period of interest before the ICH than those who did not develop an ICH (37.5% versus 49.1%; P=0.0117). In the unadjusted static model, participants who took an antihypertensive medication had a significantly lower hazard of ICH during follow‐up (HR, 0.56; 95% CI, 0.38–0.83). In the unadjusted time‐varying model, the ICH hazard was again lower among participants taking an antihypertensive medication (HR, 0.66; 95% CI, 0.44–0.99). This expected result further validates the method used to assess the associations between medications of interest and ICH in this study.
CMBs in Any Location
Neither antiplatelet nor statin use was associated with having a CMB in any location (Table 7). The odds of a CMB were higher among anticoagulant users (OR, 1.64; 95% CI, 1.04–2.59), but this association dissipated with adjustments. There was no significant interaction between antiplatelet use and APOE genotype, concomitant anticoagulant or statin use, white matter hyperintensity percentage, and cognitive status in predicting the odds of any CMB in model 3.
Table 7.
Medication Use and Prevalence of CMB
| Medication | Model 1* | Model 2 † | Model 3 ‡ | |
|---|---|---|---|---|
| Any CMB | ||||
| Any antiplatelet | 1.07 (0.81–1.40) | 1.05 (0.79–1.40) | 1.00 (0.74–1.35) | |
| Any anticoagulant | 1.64 (1.04–2.59) | 1.44 (0.91–2.29) | 1.21 (0.75–1.97) | |
| Any statin | 1.21 (0.95–1.56) | 1.29 (0.98–1.70) | 1.23 (0.92–1.64) | |
| Lobar CMB | ||||
| Any antiplatelet | 1.24 (0.80–1.91) | 1.17 (0.75–1.82) | 1.04 (0.66–1.64) | |
| Any anticoagulant | 1.96 (1.14–3.36) | 1.65 (0.95–2.88) | 1.34 (0.76–2.35) | |
| Any statin | 1.27 (0.86–1.87) | 1.30 (0.86–1.97) | 1.16 (0.76–1.76) | |
| Subcortical CMB | ||||
| Any antiplatelet | 1.04 (0.78–1.38) | 1.07 (0.80–1.45) | 1.01 (0.74–1.38) | |
| Any anticoagulant | 1.51 (0.94–2.43) | 1.38 (0.85–2.24) | 1.23 (0.74–2.05) | |
| Any statin | 1.12 (0.85–1.46) | 1.19 (0.88–1.61) | 1.15 (0.84–1.57) | |
| Deep CMB | ||||
| Any antiplatelet | 0.69 (0.33–1.42) | 0.64 (0.29–1.42) | 0.56 (0.25–1.23) | |
| Any anticoagulant | 1.27 (0.43–3.76) | 1.14 (0.39–3.37) | 0.94 (0.31–2.85) | |
| Any statin | 0.78 (0.48–1.25) | 0.81 (0.49–1.33) | 0.79 (0.47–1.32) | |
Data are given as odds ratio (95% CI). Unadjusted and unadjusted odds ratios for CMB, lobar CMB, subcortical CMB, and deep CMB are given as a function of any exposure to each medication of interest during the study period. CMB indicates cerebral microbleed.
Unadjusted model.
Model adjusted for propensity score quintile.
Model adjusted for propensity score quintile, apolipoprotein E genotype, white matter hyperintensity volume, cognitive status, and any concomitant use of the other 2 medication types from visit 2 to visit 5.
Lobar CMBs
Neither antiplatelet nor statin use was associated with the presence of a lobar CMB. Anticoagulant users were at significantly higher risk of having a lobar CMB in the unadjusted model (model 1 OR, 1.96; 95% CI, 1.14–3.36), but not after adjustments. There were no significant interactions between any of the medications of interest and the other risk factors in model 3.
Subcortical and Deep CMBs
Medication exposure was not associated with subcortical or deep CMBs.
DISCUSSION
This study delineates associations between medications with hemorrhagic potential (namely, antiplatelets, anticoagulants, and statins) and ICH and CMBs in the population‐based and longitudinal ARIC study cohort. Antiplatelet and statin use were associated with significantly lower risks of ICH longitudinally. There was an increased risk of CMB among anticoagulant users, but not after adjusting for the propensity for taking these medications and markers of small‐vessel disease presence and severity.
The protective effects of antiplatelets and statins against ICH in our study may be partly explained by their biochemical mechanisms at sites of action in the cerebral vasculature. These medications may be safeguards of arterial vessel wall integrity because of their antithrombotic and anti‐inflammatory properties. Antiplatelets prevent platelet aggregation and thrombosis, precluding platelet‐endothelium interaction that leads to a proinflammatory cascade and atherosclerotic wall damage. 39 In addition to their lipid‐lowering activity, statins upregulate platelet and endothelial NO synthase and platelet‐derived NO, 40 resulting in less platelet‐mediated arterial thrombosis and fragility. Thus, long‐term antiplatelet and statin use may prevent arterial wall compromise and consequent hemorrhage. 41 These medications also decrease the risk of ischemic stroke, thus indirectly preventing hemorrhagic conversion. The strength of the protective association of these medications, however, was preserved even after adjusting for ischemic stroke occurrence, suggesting that their influences extend beyond preventing an acute, macrothrombotic event. The results from our study are similar to the findings in RESTART (Restart or Stop Antithrombotics Randomized Trial). The trial demonstrated that participants randomized to an antiplatelet after an ICH were less likely to have a recurrent ICH during the 4 years of follow‐up (HR, 0.51; 95% CI, 0.25–1.03; P=0.06). 42 The authors postulated the mechanisms included prevention of ischemic stroke and thereby hemorrhagic transformation, prevention of arterial thrombosis, which can trigger hemorrhage, and mitigation of inflammation, which might be a key mechanism for the development of ICH.
Our results contrast with observational studies demonstrating no or increased ICH risk with aspirin 4 , 7 , 9 , 43 , 44 ; however, the follow‐up in these studies was at most 5 years. The protective effect of antiplatelet use in our study may reflect the longer duration of exposure to prevent vascular remodeling and longer follow‐up needed to identify ICH events as arteriopathy accrues in those not exposed. Furthermore, the results from some of these studies, which were conducted outside the United States, may not be generalizable to the US population with its specific risk factors for ICH attributable to lifestyle behaviors as well as genetic composition. Finally, there is variability by the intracranial compartment studied. For instance, the clinical trial, ASPREE, demonstrated an elevated risk of ICH with aspirin use with respect to subdural or extradural hemorrhage, but no association with ICH. Finally, observational studies are prone to confounding by indication and selection biases, which we attempted to directly address in this study.
The protective effect of statin use in our study also differs from the result of the post hoc analysis of the SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) study, which demonstrated that statin use was associated with increased risk of ICH among patients with stroke (HR, 1.68; 95% CI, 1.09–2.59). 45 This secondary prevention trial only included subjects with a prior stroke with likely already fragile vasculature, and the antithrombotic property of statins may have prevailed. In our study, we did not exclude participants with prior stroke and adjusted the effects of statin use for the occurrence of an interim ischemic stroke, after which the protective effect was maintained. A study of 345 531 individuals in Israel followed up for 9.5 years also noted a significantly protective effect of statin use against ICH (HR, 0.68; 95% CI, 0.58–0.79), suggesting that long‐term statin use in the general population may prevent the occurrence of vasculopathy that results in ICH. 46
Anticoagulant use was associated with a significantly higher risk of any and lobar CMBs, but not after adjustment for demographics and markers of the presence and severity of small‐vessel disease. Our finding is consistent with results from the Rotterdam Study, which followed up 1062 participants who ever took an antithrombotic and underwent a brain MRI in the Netherlands. 15 To our knowledge, there have not been any other large studies assessing longitudinal anticoagulant use and the prevalence of CMBs. Antiplatelet use did not confer any additional risk of CMB in our study, unlike in the Rotterdam Study, which observed an elevated risk of lobar CMB among aspirin users and no association with deep or infratentorial CMBs. One explanation may be the differences in antiplatelets taken. For instance, a low number of Rotterdam Study participants on platelet aggregation inhibitor specifically took aspirin (n=67). There is also substantial variation in APOE allelic distribution by geography, which may support the differential association of antiplatelet use and CMB. 47 Further studies are needed to help elucidate the interactions between the specific platelet aggregation medications and the neurovascular unit.
We addressed several potential sources of bias. To minimize reporting bias, we assessed medication use from lists or containers brought to each visit or read from during telephone calls. To account for nonrandom use of medications, we included commonly available variables at midlife, which may predict future use of medications by adjusting for well‐balanced propensity scores. A sensitivity analysis did not demonstrate a compliance bias, thus bolstering the validity of the primary analyses. The index time for study entry was set at visit 2, and participants on a medication of interest at visit 1 were excluded for each analysis to prevent left‐truncation bias. Time‐varying covariates were used to mitigate biasing the HR with fixed, static medication exposure, which inflates exposed time at risk. Although a more appropriate method, it relies on an assumption of participation, taking the medication from one visit to just before the next, which occurred nearly every 3 to 6 years. Once cardiovascular disease is diagnosed, rarely does the indication for one of these medications resolve. In a study of ARIC study participants, 97.8% have reported that their degree of medication adherence is either medium or high. Thus, it is within the realm of possibilities that the medication was used for a major duration of the time interval. Nevertheless, further studies are needed with more granular medication adherence data to confirm the findings in this study.
There were several limitations of our study. The propensity score method, unlike randomization, does not balance unmeasured confounders. Because all non‐White or non‐Black and all Black participants from Washington County and Minnesota were excluded, the generalizability of these results to other races/ethnicities is limited. The time intervals between follow‐up visits were lengthy. To that end, we inserted data from the intervening follow‐up telephone call for the longest interval between visits 4 and 5. Relatively few participants experienced ICH, underpowering our interaction and subgroup analyses. Only 4 patients with ICH underwent the visit 5 MRI, of whom 3 demonstrated the presence of a CMB; thus, further details about the imaging correlates of the clinical ICH events could not be determined. The interrater reliability of CMB detection was 85%; however, given that the raters were blinded to medication use, we have little reason to believe there was heterogeneity in CMB rating by treatments. Data from only 1 MRI visit were available, so it is theoretically possible that the microbleeds visualized on the visit 5 MRI preceded entry into the study. However, given the average age of participants at visit 1 was 54 years and the prevalence of CMB in participants aged 50 to 59 years has been reported at 6.5% to 11.5%, it is possible that most CMBs seen on the visit 5 MRI were accrued during the study period. 48 Although the CMB analysis of this study was cross‐sectional, future studies are needed to assess interval changes in the number of CMBs as a function of medication use, and acquisition of visit 6 MRI data may present such an opportunity.
This study presents the risks of developing ICH and having CMBs in participants from 4 US regions with use of antiplatelets, anticoagulants, and statins. Our findings suggest that there may be differential bleeding risk profiles of each medication for intracerebral microhemorrhage and macrohemorrhage. These results, derived from a large, longitudinal US cohort, are hypothesis generating and may provide justification for future studies testing the hemorrhagic effects of these medications while accounting for medication indication and small‐vessel disease markers.
Sources of Funding
Dr Sharma was supported as a StrokeNet Research Fellow by National Institutes of Health (NIH) U10 NS08672. Dr Gottesman is supported by National Institute on Aging (NIA) grant K24 AG052573. Dr Jack is funded by the NIH and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic. The ARIC (Atherosclerosis Risk in Communities) study is performed as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I). Neurocognitive data were collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, and 2U01HL096917 from the NIH (NHLBI, National Institute of Neurological Disorders and Stroke, NIA, and National Institute on Deafness and Other Communication Disorders), and with previous brain magnetic resonance imaging examinations funded by R01‐HL70825 from the NHLBI.
Disclosures
Dr Sharma was supported as a StrokeNet Research Fellow by National Institutes of Health U10 NS08672. Dr Gottesman is Associate Editor for the journal, Neurology. Dr Jack is a Lily consultant, monitors data for Roche, and speaks for Eisai, but receives no compensation from any commercial entity. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Acknowledgments
The authors thank the staff and participants of the ARIC (Atherosclerosis Risk in Communities) study for their important contributions.
Author Contributions: Dr Sharma designed and conceptualized study; analyzed and interpreted data; drafted and revised the manuscript; and conducted the statistical analysis. Drs Matsushita, Wu, Jack, Mosley, and Fornage revised the manuscript for intellectual content. Dr Griswold assisted with data analysis and interpretation; and revised the manuscript for intellectual content. Dr Gottesman designed the study; assisted with analysis and interpretation of data; and revised the manuscript.
(J Am Heart Assoc.2021;10:e014270. DOI: 10.1161/JAHA.120.014270.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.014270
For Sources of Funding and Disclosures, see page 15.
See Editorial by Gutierrez
REFERENCES
- 1. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 2. Andrus B, Lacaille D. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. 2014;63:2886. [DOI] [PubMed] [Google Scholar]
- 3. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 4. He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta‐analysis of randomized controlled trials. JAMA. 1998;280:1930–1935. [DOI] [PubMed] [Google Scholar]
- 5. Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators , Karam JG, Loney‐Hutchinson L, McFarlane SI. High‐dose atorvastatin after stroke or transient ischemic attack: the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. J Cardiometab Syndr. 2008;3:68–69. [DOI] [PubMed] [Google Scholar]
- 6. Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, Reilly PA, Ezekowitz MD, Yusuf S. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE‐LY trial. Stroke. 2012;43:1511–1517. [DOI] [PubMed] [Google Scholar]
- 7. Garcia‐Rodriguez LA, Gaist D, Morton J, Cookson C, Gonzalez‐Perez A. Antithrombotic drugs and risk of hemorrhagic stroke in the general population. Neurology. 2013;81:566–574. [DOI] [PubMed] [Google Scholar]
- 8. McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, Kirpach B, Shah RC, Ives DG, Tonkin AM, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cappelleri JC, Fiore LD, Brophy MT, Deykin D, Lau J. Efficacy and safety of combined anticoagulant and antiplatelet therapy versus anticoagulant monotherapy after mechanical heart‐valve replacement: a metaanalysis. Am Heart J. 1995;130:547–552. [DOI] [PubMed] [Google Scholar]
- 10. Martinez‐Ramirez S, Greenberg SM, Viswanathan A. Cerebral microbleeds: overview and implications in cognitive impairment. Alzheimers Res Ther. 2014;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta‐analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 2013;44:995–1001. 10.1161/STROKEAHA.111.000038 [DOI] [PubMed] [Google Scholar]
- 12. Haussen DC, Henninger N, Kumar S, Selim M. Statin use and microbleeds in patients with spontaneous intracerebral hemorrhage. Stroke. 2012;43:2677–2681. 10.1161/STROKEAHA.112.657486 [DOI] [PubMed] [Google Scholar]
- 13. Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez‐Ramirez S, Kase CS, Wolf PA, Seshadri S. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke. 2014;45:1492–1494. 10.1161/STROKEAHA.114.004130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biffi A, Halpin A, Towfighi A, Gilson A, Busl K, Rost N, Smith EE, Greenberg MS, Rosand J, Viswanathan A. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75:693–698. 10.1212/WNL.0b013e3181eee40f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vernooij MW, Haag MD, van der Lugt A, Hofman A, Krestin GP, Stricker BH, Breteler MM. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2009;66:714–720. 10.1001/archneurol.2009.42 [DOI] [PubMed] [Google Scholar]
- 16. Naka H, Nomura E, Kitamura J, Imamura E, Wakabayashi S, Matsumoto M. Antiplatelet therapy as a risk factor for microbleeds in intracerebral hemorrhage patients: analysis using specific antiplatelet agents. J Stroke Cerebrovasc Dis. 2013;22:834–840. 10.1016/j.jstrokecerebrovasdis.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 17. Ge L, Niu G, Han X, Gao Y, Wu Q, Wu H, Zhang Y, Guo D. Aspirin treatment increases the risk of cerebral microbleeds. Can J Neurol Sci. 2011;38:863–868. [DOI] [PubMed] [Google Scholar]
- 18. Song TJ, Kim J, Song D, Nam HS, Kim YD, Lee HS, Heo JH. Association of cerebral microbleeds with mortality in stroke patients having atrial fibrillation. Neurology. 2014;83:1308–1315. 10.1212/WNL.0000000000000862 [DOI] [PubMed] [Google Scholar]
- 19. The ARIC Investigators . The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 20. Robins M, Weinfeld FD. The National Survey of Stroke; study design and methodology. Stroke. 1981;12:I7–I11. [PubMed] [Google Scholar]
- 21. Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, Jack CR Jr, Graff‐Radford J, Schneider ALC, Mosley TH Jr, et al; ARIC Neurocognitive Investigators . Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: Atherosclerosis Risk in Communities‐Neurocognitive Study. Stroke. 2015;46:433–440. 10.1161/STROKEAHA.114.007847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graff‐Radford J, Simino J, Kantarci K, Mosley TH, Griswold ME, Windham BG, Sharrett AR, Albert MS, Gottesman RF, Jack CR, et al. Neuroimaging correlates of cerebral microbleeds: the ARIC study (Atherosclerosis Risk in Communities). Stroke. 2017;48:2964–2972. 10.1161/STROKEAHA.117.018336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kantarci K, Gunter JL, Tosakulwong N, Weigand SD, Senjem MS, Petersen RC, Aisen PS, Jagust WJ, Weiner MW, Jack CR Jr; Alzheimer's Disease Neuroimaging Initiative . Focal hemosiderin deposits and beta‐amyloid load in the ADNI cohort. Alzheimers Dement. 2013;9:S116–S123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pasi M, Marini S, Morotti A, Boulouis G, Xiong LI, Charidimou A, Ayres AM, Lee MJ, Biffi A, Goldstein JN, et al. Cerebellar hematoma location: implications for the underlying microangiopathy. Stroke. 2018;49:207–210. 10.1161/STROKEAHA.117.019286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, Svardsudd K. Cardiac and pulmonary causes of dyspnoea—validation of a scoring test for clinical‐epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–1014. 10.1093/oxfordjournals.eurheartj.a062365 [DOI] [PubMed] [Google Scholar]
- 26. Sussman ES, Connolly ES Jr. Hemorrhagic transformation: a review of the rate of hemorrhage in the major clinical trials of acute ischemic stroke. Front Neurol. 2013;4:69. 10.3389/fneur.2013.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biffi A, Sonni A, Anderson CD, Kissela B, Jagiella JM, Schmidt H, Jimenez‐Conde J, Hansen BM, Fernandez‐Cadenas I, Cortellini L, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68:934–943. 10.1002/ana.22134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. 10.1161/01.STR.30.4.736 [DOI] [PubMed] [Google Scholar]
- 29. Gottesman RF, Schneider ALC, Zhou Y, Chen X, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, et al. The ARIC‐PET amyloid imaging study: brain amyloid differences by age, race, sex, and apoe. Neurology. 2016;87:473–480. 10.1212/WNL.0000000000002914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim M, Bae HJ, Lee J, Kang L, Lee S, Kim S, Lee JE, Lee KM, Yoon BW, Kim BK, et al. APOE epsilon2/epsilon4 polymorphism and cerebral microbleeds on gradient‐echo MRI. Neurology. 2005;65:1474–1475. [DOI] [PubMed] [Google Scholar]
- 31. Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, Massaro JM, DeCarli C. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke. 2004;35:1831–1835. 10.1161/01.STR.0000131809.35202.1b [DOI] [PubMed] [Google Scholar]
- 32. Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, Koudstaal PJ, Ikram MA, Vernooij MW. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73:934–943. 10.1001/jamaneurol.2016.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hamedani AG, Rose KM, Peterlin BL, Mosley TH, Coker LH, Jack CR, Knopman DS, Alonso A, Gottesman RF. Migraine and white matter hyperintensities: the ARIC MRI study. Neurology. 2013;81:1308–1313. 10.1212/WNL.0b013e3182a8235b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, Schneider ALC, Hengrui S, Alonso A, Coresh J, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC‐NCS). Alzheimers Dement. 2016;2:1–11. 10.1016/j.dadm.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259. 10.1111/j.1742-7843.2006.pto_293.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. 10.1016/S0895-4356(00)00321-8 [DOI] [PubMed] [Google Scholar]
- 39. Langer HF, Gawaz M. Platelet‐vessel wall interactions in atherosclerotic disease. Thromb Haemost. 2008;99:480–486. 10.1160/TH07-11-0685 [DOI] [PubMed] [Google Scholar]
- 40. Laufs U, Gertz K, Huang P, Nickenig G, Bohm M, Dirnagl U, Endres M. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31:2442–2449. 10.1161/01.STR.31.10.2442 [DOI] [PubMed] [Google Scholar]
- 41. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 42. RESTART Collaboration . Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): a randomised, open‐label trial. Lancet. 2019;393:2613–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ. 2003;326:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S; HOT Study Group . Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. [DOI] [PubMed] [Google Scholar]
- 45. Goldstein LB, Amarenco P, Szarek M, Callahan A III, Hennerici M, Sillesen H, Zivin JA, Welch KM; SPARCL Investigators . Hemorrhagic stroke in the stroke prevention by aggressive reduction in cholesterol levels study. Neurology. 2008;70:2364–2370. [DOI] [PubMed] [Google Scholar]
- 46. Saliba W, Rennert HS, Barnett‐Griness O, Gronich N, Molad J, Rennert G, Auriel E. Association of statin use with spontaneous intracerebral hemorrhage: a cohort study. Neurology. 2018;91:e400–e409. [DOI] [PubMed] [Google Scholar]
- 47. Minihane AM, Jofre‐Monseny L, Olano‐Martin E, Rimbach G. ApoE genotype, cardiovascular risk and responsiveness to dietary fat manipulation. Proc Nutr Soc. 2007;66:183–197. [DOI] [PubMed] [Google Scholar]
- 48. Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Breteler MM. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41:S103–S106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Data Availability Statement
Requests for data from qualified investigators can be directed to the corresponding author.


