Abstract
Background
Psychosocial factors predict heart disease risk, but our understanding of underlying mechanisms is limited. We sought to evaluate the physiologic correlates of psychosocial factors by measuring their relationships with heart rate variability (HRV), a measure of autonomic health, in the ARIC (Atherosclerosis Risk in Communities) study. We hypothesize that increased psychosocial stress associates with lower HRV.
Methods and Results
We studied 9331 participants in ARIC with short‐term HRV data at visits 2 and 4. The mean (SD) age was 54.4 (5.7) years, 55% were women, and 25% were Black. Psychosocial factors included: (1) vital exhaustion (VE), (2) anger proneness, a personality trait, and (3) perceived social support. Linear models adjusted for sociodemographic and cardiovascular risk factors. Low frequency HRV (ln ms2) was significantly lower in the highest versus lowest quartiles of VE (B=−0.14, 95% CI, −0.24 to −0.05). When comparing this effect to age (B=−0.04, 95% CI, −0.05 to −0.04), the difference was equivalent to 3.8 years of accelerated aging. Perceived social support associated with lower time‐domain HRV. High VE (versus low VE) also associated with greater decreases in low frequency over time, and both anger and VE associated with greater increases in resting heart rate over time. Survival analyses were performed with Cox models, and no evidence was found that HRV explains the excess risk found with high VE and low perceived social support.
Conclusions
Vital exhaustion, and to a lesser extent anger and social support, were associated with worse autonomic function and greater adverse changes over time.
Keywords: autonomic dysfunction, depressive symptoms, heart rate variability, psychological stress, somatic symptoms
Subject Categories: Epidemiology, Cardiovascular Disease, Aging, Risk Factors, Mental Health
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
- HF
high frequency
- HR
heart rate
- HRV
heart rate variability
- ISEL
Interpersonal Support Evaluation List
- LF
low frequency
- LSNS
Lubben Social Network Scale
- MVEQ
Maastricht Vital Exhaustion Questionnaire
- RMSSD
root mean square of successive differences in normally conducted RR intervals
- RR
time interval between conducted heart beats
- SDNN
SD of normally conducted RR intervals
- STAS
Speilberger State‐Trait Anger Scale
- VE
vital exhaustion
Clinical Perspective
What Is New?
Vital exhaustion and social isolation are associated with electrocardiographic markers of reduced parasympathetic function, higher sympathovagal balance, and reduced baroreflex sensitivity.
The highest quartile of vital exhaustion was associated with the same autonomic effect as ≈4 years of accelerated aging compared with the lowest quartile.
What Are the Clinical Implications?
These findings support future research to evaluate whether markers of autonomic function, if modifiable and easily measured in clinical settings, may be useful in the evaluation and management of certain psychosocial conditions.
Psychological stress is associated with an increased risk of cardiovascular mortality beyond what can be explained by maladaptive health behaviors. 1 , 2 Both depression and post‐traumatic stress disorder have been linked to a higher risk of cardiovascular disease and worse prognosis, 3 , 4 however the mechanisms, likely multifactorial, are not yet known. 5 Stress is associated with autonomic dysfunction, as measured by decreased heart rate variability (HRV). 6 Decreased HRV has been identified as an independent predictor of cardiovascular mortality. 7 , 8 HRV measures sympathetic and parasympathetic activity that fluctuates in response to activities such as respiration, body position, and physical activity. 9 HRV has been proposed as an index of the physiological impacts of stress on the body, 10 as it captures sympathetic arousal and parasympathetic withdrawal, which are key in the neurobiological stress response. 11 Although HRV shows promise as a physiologic metric of stress, more research is needed to understand its potential as a clinical marker of stress.
One scientific challenge is the heterogeneous nature of psychosocial stress. Stress can arise from the environment, such as perceived social support, 12 and also from internal situational responses, such as personality traits, 13 both of which lead to physiological changes. 14 Chronic stress can also manifest as somatic symptoms. 15 Stress, both physical and psychosocial, requires a new homeostatic point, which is the responsibility of the autonomic nervous system to mediate. The autonomic system provides a mechanism to “reset” the body after stressful events, and may provide a method of resilience to chronic stressors. 16 This allows for recovery from stressful events and protection from maladaptive responses to future stresses, such as how normal HRV can reduce the risk of future depression. 17 Each of these components of psychosocial stress may thus contribute to autonomic dysfunction. 18 Vital exhaustion, defined by excessive fatigue, feelings of demoralization, and increased irritability, is considered a manifestation of chronic psychological distress and depressive somatic symptoms. 19 Vital exhaustion, independent of traditional cardiovascular risk factors, predicts coronary artery disease and increased mortality. 20 The mechanisms of how these psychological factors lead to worse outcomes are unknown, but may include direct autonomic and indirect behavioral effects. For example, social isolation increases the risk of incident heart failure or anger episodes and temperament precede coronary disease and myocardial ischemia. 21 , 22 , 23 If associations with abnormal HRV are found, this may help to partially explain the excess cardiovascular mortality risk seen with various psychosocial factors.
To gain a better understanding of the relationship between psychological factors and changes in autonomic function, we evaluated the association of vital exhaustion, anger proneness, and social isolation with HRV in the ARIC (Atherosclerosis Risk in Communities) study cohort. These analyses are based on the hypothesis that psychosocial stress leads to autonomic dysregulation, both acutely and chronically. We hypothesize that each of these psychosocial factors associate with decreased HRV. We hypothesize that, over time, HRV will decrease at a faster pace in those with greater psychosocial burden. We hypothesize that abnormal HRV will mediate the increased cardiovascular mortality seen with increased psychosocial stress.
Methods
Study Population
The data used for this analysis are available upon request from the ARIC investigators. The ARIC study is a community‐based, prospective cohort study designed to investigate risk factors for atherosclerosis and cardiovascular disease in the general population. Details of the ARIC study have been described elsewhere. 24 Briefly, 15 792 adults aged 46 to 64 years were recruited in 1987 to 1989 (visit 1) from 4 communities in the United States: Forsyth Co, NC; Jackson, MS; Minneapolis suburbs, MN; and Washington Co, MD. Follow‐up among participants was conducted annually via telephone interviews (semi‐annually since 2012), surveillance of hospitalizations, death certificates, and subsequent visits approximately every 3 years until 1998. Subsequent visits occurred in 2011 to 2013, 2016 to 2017, and 2018 to 2019. The study collected information on medical, social, and demographic variables through examinations and questionnaires. Psychological variables (vital exhaustion, anger proneness, and social isolation) were collected at visit 2 (1990–1992). Anger proneness was repeated at visit 4 (1996–1998). Participants taking medications with a potential moderating effect on HRV were excluded, included beta‐blockers, anti‐arrhythmics, calcium channel blocks, and cardiotonics as is routinely done in studies involving HRV. Participants racially identified as Asian, American Indian, unknown, or as Black from predominantly White ARIC field sites of Washington Co., MD and Minneapolis, MN were excluded because of small numbers, similar to other ARIC studies. 24 Participants with incomplete or missing HRV data were also excluded, as well as those with missing follow‐up information. Institutional review boards at each of the participating institutions approved the study, and participants provided written informed consent.
Assessment of Heart Rate Variability
During visit 1, HRV was assessed by a 2‐minute ECG using standardized methods during spontaneous respirations. Participants were asked to remain comfortable in a supine position for 20 minutes beforehand and were asked to refrain from smoking or caffeine ingestion for at least 1 hour before the recording. Beats were identified by computer algorithm. Beat‐to‐beat (NN) intervals were extracted and the time of each beat was recorded. A computer algorithm was used to identify all intervals outside of a >25% deviation from a 5‐beat moving average. 25 These beats considered to be potential ectopic beats or artifact and were removed, and the interval data were generated using linear interpolation. During visit 4, HRV was measured again using a 6‐minute ECG using a similar protocol. Resting measures of HRV have been found to play an important role in psychological conditions, such as a post‐traumatic stress disorder and depression prediction, 17 , 26 and short‐term recordings have had utility in assessing mental stress. 6 HRV is intrinsically dependent on the recording duration, and different recording lengths cannot be directly compared. 27 When comparing across different recording lengths, HRV was log‐transformed and normalized within visit 1 and 4, and the SD change between timepoints within individuals was treated as the dependent variable to allow for modeling between time points. HRV is intrinsically dependent on the absolute heart rate, and adjustments for heart rate have not yet been standardized, 28 so averaged RR (time interval between conducted heart beats) intervals were included as an additional outcome that may signify sympathovagal balance. 29 Sacha et al showed that significant sex differences in HRV exist in their prognostic and clinical value, 30 and we elected a priori to perform analyses separately by sex.
Variations in heart rate can be assessed by a number of mathematical measures, usually divided into the time and frequency domains. 9 Time domain measures we used include the RR interval duration (converted to heart rate in beats‐per‐minute), the SD of normally conducted RR intervals (SDNN), the root mean square of successive differences in normally conducted RR intervals (RMSSD), and the proportion of normally conducted RR intervals that differ by >50 ms divided by the total number of normally conducted RR intervals. Frequency‐domain measures computed through power spectral analysis categorize variability as very low frequency (0.0033 to <0.04 Hz), low frequency (LF, 0.04 to <0.15 Hz) or high frequency (HF, 0.15–0.40 Hz). 31 These frequency categories reflect autonomically mediated heart rate responses to physiologic stimuli, including influences of the renin‐angiotensin‐aldosterone system, baroreceptor activity, and respiration. 31 The sympathetic and parasympathetic nervous systems influence them to different degrees. We analyzed total frequency, high frequency (HF), and low frequency power (LF) HRV, which are frequency domain measures. HF reflects primarily parasympathetic nervous system activity, while LF reflects both sympathetic and parasympathetic activity. 32 Total power HRV is a non‐specific global measure. RMSSD is an approximate correlate of HF, and SDNN is an approximate correlate of total frequency power, supporting the physiological basis of these markers. 33 These metrics are well‐known as physiologic markers of acute and chronic stress, and measure slightly different aspects of autonomic nervous system function. We include multiple measures, given the lack of consensus on the most relevant metric for this analysis. Very low frequency was not estimated from the visit 1, 2‐minute ECG because it requires at least 5 minutes of data for analysis. 9 LF/HF ratio was included as a measure that, although no longer regarded as a reflection of sympathovagal balance, adjusts for the effect of absolute heart rate. Before analysis, the frequency domain measures (HF, LF, total frequency power) and PNN50 were log‐transformed for normality and to reduce skewness. 34
Vital Exhaustion Measurement
We assessed vital exhaustion during visit 2 using the 21‐item Maastricht Vital Exhaustion Questionnaire (MVEQ), 35 which has been shown to prospectively increase the risk of future coronary heart disease and mortality in the ARIC study. 36 Each response was scored 0 for “No”, 1 for “Don't Know”, and 2 for “Yes”, with reverse scoring for questions 9 and 14. The scores range from 0 to 21, with higher scores representing higher exhaustion. The Cronbach alpha for internal consistency has been reported as 0.89 by Appels et al. 35 To simplify data presentation and interpretation, the exhaustion scores were also categorized in quartiles: Q1 (0–1), Q2 (2–4), Q3 (5–7), and Q4 (8–21).
Anger Trait Measurement
Anger proneness was evaluated using the 10‐item Spielberger State‐Trait Anger Scale (STAS). This scale highly correlates with the 15‐item STAS, which has a high level of internal consistency (Cronbach alpha of 0.87). 37 The anger score ranges from 10 to 40, with a higher score suggesting higher anger trait. A trait score of 10 to 14 is defined as low anger, 15 to 21 as moderate anger, and 22 to 40 as high anger. 37 High anger is associated with increased irritation and annoyance, increased perception of anger‐provoking situations, and increased frequency of response to such situations with rage or fury. 37 This scale was collected at visit 2 and at visit 4.
Perceived Social Support Measurement
We measured social isolation using the Interpersonal Support Evaluation List (ISEL) and the Lubben Social Network Scale (LSNS) at visit 2. The ISEL version used was the 16‐item questionnaire version designed to assess the perceived social support and is composed of 4 subscales: tangible, appraisal, belonging, and self‐esteem. It is validated against the 40‐item full questionnaire, and it is highly correlated with other perceived support‐instruments. It has an internal consistency in the range of 0.88 to 0.90. 38 , 39 The LSNS is a well‐validated 10‐item questionnaire that assesses the size of an individual's active social network and perceived support. The scores range from 0 to 50, with higher scores indicating greater social support. A score ≤20 is considered “socially isolated”, 21 to 25 is “high risk”, 26 to 30 is “moderate risk”, and ≥31 is “low risk” for social isolation. 40
Other Covariates
Additional potentially confounding variables were incorporated into the adjusted regression models, including demographic and behavioral characteristics and medical comorbidities. Information on race, sex, alcohol use, and smoking status were self‐reported. Blood pressure was measured using standardized protocols and hypertension was defined as a diastolic blood pressure of ≥90 mm Hg, systolic blood pressure of ≥140 mm Hg, or antihypertensive medication use. Diabetes mellitus was defined as a self‐reported history of diagnosis of diabetes mellitus by a physician, antihyperglycemic medication use, a fasting serum glucose ≥126 mg/dL, or a non‐fasting serum glucose of ≥200 mg/dL. We calculated body mass index as weight (kg) divided by height (m) squared. Coronary heart disease was defined as a self‐reported history of myocardial infarction, coronary revascularization, coronary artery bypass surgery, or electrocardiographic signs of prior myocardial infarction and adjudicated events thereafter. These comorbid conditions were collected at all study visits. Outcome events were also collected during follow‐up by obtaining available death certificates, coroner reports, informant interviews, hospital records, and autopsy reports. The cause of death was adjudicated by the ARIC Morbidity and Mortality Classification Committee following a standard protocol. Myocardial infarction was adjudicated by physician review based on chest pain, cardiac biomarkers/enzymes from hospitalization, ECG evidence including a new pathological Q wave, coronary heart disease history, and other associated information. Cardiovascular mortality was defined as mortality events in patients with coronary heart disease, prior myocardial infarction, and by International Classification of Diseases, Ninth Revision (ICD‐9) and Tenth Revision (ICD‐10) codes at time of death when available.
Statistical Analysis
The overall analytical strategy is summarized in Figure 1, which describes the cross‐sectional analyses done at visit 2 (carrying forward data from visit 1 to visit 2), and the change analyses done between visits 1 or 2 and visit 4. As HRV data were not collected at visit 2, the HRV at visit 1 was carried forward to study association with baseline psychosocial factors, as has been done in prior studies. 27 The term cross‐sectional and change are used to better identify the analytical approach. All analyses were assessed for interaction by sex. The psychological assessments (MVEQ, STAS, ISEL, LSNS) were tested for normality. The variables were compared using Spearman rank‐order correlation. The psychological variables were also compared with HRV variables using Pearson correlation. The association between psychosocial factors (main independent variables) and HRV (dependent variable) was estimated using linear regression models, adjusting for demographic and comorbid factors (age, body mass index, race, hypertension, coronary heart disease, diabetes mellitus). Both continuous and categorical representations of psychosocial factors were used to facilitate interpretation. Additional analyses were performed to assess the interaction of race and sex. Except when specified, the psychosocial factors were measured as 10‐point differences in scale score. Additional adjusted models were used comparing the changes in HRV over time, as described above. As anger proneness was collected at visit 2 and 4, a regression model was used to test the change in anger and the change in HRV over time. We further investigated any further associations between HRV and psychosocial stress metrics with sequential survival models. We used Cox proportional hazard models to test the relationship of increased psychosocial stress (top quartile) with all‐cause mortality, myocardial infarction, and cardiovascular mortality. We first evaluated the relationship of the stress metric alone, and then sequentially adjusted for selected HRV metrics that were associated with it, as a way to examine for autonomic mechanisms behind the increased CVD risk commonly found in those with increased chronic psychological stress. 41 All models adjusted for sex, age, body mass index, race, hypertension, coronary heart disease, and diabetes mellitus. The follow‐up time was from enrollment in 1987 until 2018. Adjustment for multiple hypothesis testing were not performed because of the correlation between HRV measures. Significant relationships between exposure variables and HRV were also measured as units of HRV‐age, which were measured by dividing the coefficient of the exposure variable by the coefficient for age. This was calculated to help understand the significance of the effect sizes. Statistical analysis was performed using R 3.6.1 (R Core Team 2018, Vienna, Austria).
Figure 1. Flow chart of study participants showing when heart rate variability and psychosocial measures were collected, and what time points are analyzed.
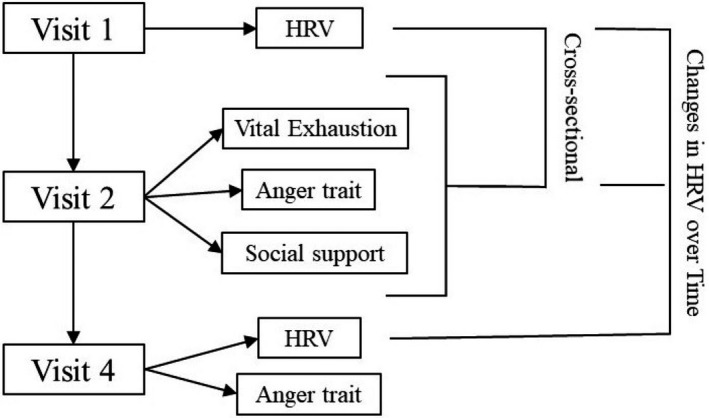
Visit 1 was between 1987 to 1989, visit 2 was between 1990 to 1992, and visit 4 was between 1996 to 1998. Psychosocial measures were collected initially in visit 2, and anger trait was repeated in visit 4. HRV indicates heart rate variability.
Results
Study Sample
From the initial sample, after exclusion by race (n=88), HRV‐modifying medications (n=2892), incomplete HRV data (n=2382), and missing follow‐up information (n=1187), there were 9331 participants available at the time of visit 2. The mean age (SD) was 56.7 (5.7) years, 43.2% were men, 78% were White, 28.7% had hypertension, 4.5% had coronary heart disease, and 12.8% had diabetes mellitus, as seen in Table 1. At visit 2, the median score (interquartile interval) for MVEQ was 4 (1–7), for STAS was 16 (13–18), for ISEL 38 (33–42), and for LSNS was 33 (29–36). All correlations between the psychosocial measures were mild (range, −0.32 to 0.50, Table S1). There were 6574 participants available at time of visit 4 for change analyses.
Table 1.
Sample Characteristics: ARIC Visits 1, 2, and 4
| Visit | Visit 1 | Visit 2 | Visit 4 |
|---|---|---|---|
| n | 12 821 | 9331 | 6574 |
| Age, y | 54.4 (5.7) | 56.7 (5.7) | 62.1 (5.5) |
| Men | 5760 (44.9%) | 4031 (43.2%) | 2753 (41.9%) |
| Race | |||
| Black | 3212 (25.1%) | 2055 (22.0%) | 1308 (19.9%) |
| White | 9609 (74.9%) | 7276 (78.0%) | 5266 (80.1%) |
| Hypertension | 3479 (27.1%) | 2677 (28.7%) | 1980 (30.1%) |
| CHD | 398 (3.1%) | 423 (4.5%) | 264 (4.0%) |
| DM | 1284 (10.1%) | 1202 (12.9%) | 818 (12.4%) |
| MEDS | |||
| Hypertension | 2095 (16.3%) | 1784 (19.1%) | 1044 (15.9%) |
| Cholesterol | 315 (2.5%) | 509 (5.5%) | 669 (10.2%) |
| MVEQ | … | 4.0 [1;7] | … |
| STAS | … | 16.0 [13;18] | 15.0 [13;17] |
| ISEL | … | 38.0 [33;42] | … |
| LSNS | … | 33.0 [29;36] | … |
| HR, beats/min | 68.0 (10.3) | … | 64.1 (9.4) |
| Ln HF | 2.1 (1.3) | … | 4.4 (1.2) |
| Ln LF | 2.7 (1.4) | … | 5.0 (1.2) |
| Ln TP | 3.3 (1.3) | … | 5.7 (1.1) |
| LF/HF | 2.8 (2.8) | … | 2.7 (2.8) |
| SDNN, ms | 37.5 (19.3) | … | 37.0 (19.3) |
| RMSSD, ms | 28.9 (22.6) | … | 27.6 (24.0) |
| Ln PNN50 | 1.5 (1.3) | … | 7.7 (12.4) |
Values correspond to mean (SD), n (%), or median [interquartile interval]. ARIC indicates Atherosclerosis Risk in Communities; CHD, coronary heart disease; DM, diabetes mellitus; HF, high frequency; HR, heart rate; LF, low frequency; MEDS, medications; MVEQ, Maastricht Vital Exhaustion Questionnaire; PNN50, proportion of normally conducted RR intervals that differ by >50 ms divided by the total number of normally conducted RR intervals; RMSSD, the root mean square of successive differences in normally conducted RR intervals; SDNN, the SD of normally conducted RR intervals; and TP, total frequency power.
Vital Exhaustion
Of the participants who met inclusion criteria and participated in visit 2, 9316 individuals had complete information on MVEQ. Table 2 shows the breakdown of demographic characteristics, comorbid conditions, and HRV measures in quartiles based on vital exhaustion score. Those with higher vital exhaustion (VE) were more likely to be women, Black race, and have more comorbidities. Differences in HRV measurements for every 10‐point increase in MVEQ are shown in Table 3. There was a correlation between MVEQ score and LF HRV (−0.063; 95% CI, −0.083 to −0.043), and no correlation with HF HRV. Each 10‐point increased MVEQ was associated with a −0.11 (95% CI, −0.18 to −0.04) change in LF (ln ms2), −0.07 (95% CI, −0.13 to 0.0) change in HF (ln ms2), and a 0.62 (95% CI, 0.12 to 1.12) change in heart rate (beats/minute) in visit 1. Every 10‐point increased MVEQ was associated with a −0.16 (95% CI, −0.24 to −0.09) change in LF, −0.09 (95% CI, −0.17 to −0.02) change in HF, and 1.23 (95% CI, 0.65 to 1.81) change in heart rate in visit 4. LF HRV was significantly lower in the highest versus lowest quartiles of VE (B=−0.14, 95% CI, −0.24 to −0.05). There was no interaction between vital exhaustion and age (Figure S1), sex, or race. When assessing the interaction of both race and sex together, we found no significant interactions. In comparison, each additional year of age associated with −0.04 (95% CI, −0.04 to −0.03) change in LF HRV. 42 Therefore, each 10‐point increase in VE score associated with 2.9 years of higher HRV‐age. The top quartile of VE score, versus bottom quartile, associated with 3.8 (95% CI, 1.4‒5.6) years of HRV‐age, as seen in Figure 2. The relationships of VE with HRV changes from visit 2 to visit 4 were also examined. The most prominent findings were an association of VE with larger declines in LF HRV (standardized units) over time from visit 1 to visit 4, as well as greater increases in heart rate and decreases in SDNN, similar in magnitude to the effect on LF (Table 4).
Table 2.
Cross‐Sectional Sample Characteristics by Vital Exhaustion Quartiles: ARIC Visit 2
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Value | |
|---|---|---|---|---|---|
| MVEQ score, range | 0–1 | 2–4 | 5–7 | 8–21 | |
| n | 2419 | 3029 | 1884 | 1992 | |
| Age, y | 56.4 (5.6) | 56.8 (5.6) | 56.7 (5.7) | 56.9 (5.8) | 0.102 |
| Men (%) | 1418 (58.6%) | 1393 (46.0%) | 648 (34.4%) | 569 (28.6%) | <0.001 |
| Race | <0.001 | ||||
| Black | 392 (16.2%) | 605 (20.0%) | 479 (25.4%) | 578 (29.0%) | |
| White | 2027 (83.8%) | 2424 (80.0%) | 1405 (74.6%) | 1414 (71.0%) | |
| Hypertension, n (%) | 584 (24.1%) | 863 (28.5%) | 548 (29.1%) | 680 (34.1%) | <0.001 |
| CHD (%) | 75 (3.10%) | 123 (4.06%) | 84 (4.46%) | 140 (7.03%) | <0.001 |
| DM (%) | 260 (10.7%) | 337 (11.1%) | 241 (12.8%) | 360 (18.1%) | <0.001 |
| HR, beats/min | 66.8 (10.2) | 67.6 (9.81) | 68.0 (9.98) | 69.1 (10.5) | <0.001 |
| Ln HF | 2.09 (1.28) | 2.07 (1.31) | 2.15 (1.30) | 2.08 (1.34) | 0.358 |
| Ln LF | 2.84 (1.31) | 2.74 (1.33) | 2.73 (1.34) | 2.58 (1.41) | <0.001 |
| Ln TP | 3.39 (1.22) | 3.33 (1.25) | 3.36 (1.24) | 3.26 (1.29) | 0.013 |
| LF/HF | 3.08 (3.04) | 2.86 (2.85) | 2.64 (2.67) | 2.48 (2.69) | <0.001 |
| SDNN, ms | 38.6 (18.7) | 37.7 (19.4) | 37.4 (19.7) | 36.1 (18.9) | 0.001 |
| RMSSD, ms | 28.9 (21.9) | 28.6 (22.9) | 29.5 (23.3) | 28.7 (21.3) | 0.99 |
| Ln PNN50 | 1.54 (1.23) | 1.53 (1.25) | 1.57 (1.27) | 1.51 (1.30) | 0.346 |
Vital exhaustion from visit 2 was broken into quartiles. Demographic measures from visit 2 and heart rate variability measures from visit 1 were separated by quartile. Values correspond to mean (SD) or n (%). ARIC indicates Atherosclerosis Risk in Communities; CHD, coronary heart disease; DM, diabetes mellitus; HF, high frequency; HR, heart rate; LF, low frequency; MVEQ, Maastricht Vital Exhaustion Questionnaire; PNN50, proportion of normally conducted RR intervals that differ by more than 50 ms divided by the total number of normally conducted RR intervals; RMSSD, the root mean square of successive differences in normally conducted RR intervals; SDNN, the standard deviation of normally conducted RR intervals; and TP, total frequency power.
Table 3.
Association of Psychosocial Factors at Visit 2 With Heart Rate Variability Measurements Carried Forward From Visit 1 in the ARIC Study
| Psychosocial Factors With Heart Rate Variability and Mean HR at Visit 1 | ||||||
|---|---|---|---|---|---|---|
| Frequency Domain | Time Domain | |||||
| Ln HF | Ln LF | LF/HF | SDNN | RMSSD | HR | |
| MVEQ | −0.07* (−0.13 to 0.0) | −0.11 † (−0.18 to −0.04) | −0.06 (−0.20 to 0.08) | −0.94 (−1.91 to 0.03) | −0.38 (−1.51 to 0.76) | 0.62* (0.12‒1.12) |
| STAS | −0.03 (−0.10 to 0.03) | −0.02 (−0.08 to 0.05) | 0.10 (−0.02 to 0.30) | 0.10 (−0.90 to 1.00) | 0.20 (−0.90 to 1.00) | −0.20 (−0.70 to 0.30) |
| ISEL | 0.01 (−0.03 to 0.04) | 0.02 (−0.02 to 0.06) | −0.003 (−0.08 to 0.08) | −0.31 (−0.86 to 0.24) | −0.61 (−1.26 to 0.03) | −0.10 (−0.39 to 0.19) |
| LSNS | 0.0 (−0.04 to 0.04) | −0.01 (−0.06 to 0.03) | −0.07 (−0.16 to 0.02) | −0.61* (−1.23 to 0.0) | −0.82* (−1.53 to −0.10) | 0.08 (−0.24 to 0.40) |
Psychosocial factors were analyzed in adjusted linear regression models with heart rate variability from visit 1. The estimates are for the amount of change in heart rate variability for every 10‐point change in Maastricht Vital Exhaustion Questionnaire (n=9316), Spielberger Trait Anger Scale (n=9324), Interpersonal Support Evaluation List (n=9317), and Lubben Social Network Scale (n=9317), respectively. Models were adjusted for age, race, sex, body mass index, hypertension, coronary heart disease, and diabetes mellitus. ARIC indicates Atherosclerosis Risk in Communities; HF, high frequency (ln ms2); HR, heart rate (beats/minute); ISEL, Interpersonal Support Evaluation List; LF, low frequency (ln ms2); LSNS, Lubben Social Network Scale; MVEQ, Maastricht Vital Exhaustion Questionnaire; RMSSD, the root mean square of successive differences in normally conducted RR intervals (ms); SDNN, the SD of normally conducted RR intervals (ms); STAS, Spielberger Trait Anger Scale; and TP, total frequency power (ln ms2).
P<0.05.
P<0.01.
Figure 2. Comparison of the relationship of low frequency heart rate variability and vital exhaustion quartile by heart rate variability‐age units.
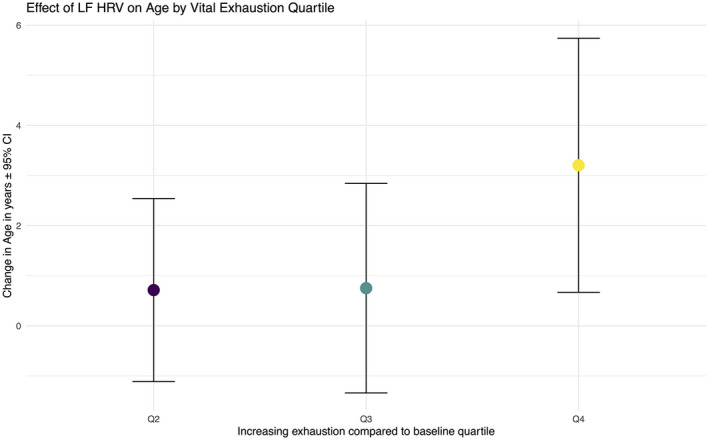
Heart rate variability‐age units were calculated by the ratio of the coefficient for vital exhaustion by the coefficient for age in models predicting low frequency heart rate variability. The effect of having the highest quartile of exhaustion was equivalent to an increase of ≈ 4 years of heart rate variability‐age (as measured by low frequency heart rate variability). HRV indicates heart rate variability, and LF, low frequency.
Table 4.
Association of Psychosocial Factors at Visit 2 With Changes in z‐Normalized Heart Rate Variability Between Visit 1 and Visit 4
| Psychosocial Factors With Standardized Heart Rate Variability and HR Changes in Time | ||||||
|---|---|---|---|---|---|---|
| Frequency Domain | Time Domain | |||||
| Ln HF | Ln LF | LF/HF | SDNN | RMSSD | HR | |
| MVEQ | −0.04 (−0.12 to 0.04) | −0.09* (−0.17 to −0.01) | −0.08 (−0.16 to 0.0) | −0.09* (−0.17 to −0.01) | –0.05 (−0.12 to 0.03) | 0.15 † (0.09‒0.21) |
| STAS | 0.01 (−0.07 to 0.09) | 0.01 (−0.08 to 0.09) | −0.05 (−0.10 to 0.03) | −0.02 (−0.10 to 0.06) | –0.03 (−0.10 to 0.04) | 0.10 ‡ (0.05‒0.20) |
| ISEL | 0.0 (−0.01 to 0.0) | 0.0 (−0.01 to 0.0) | 0.0 (0.0 to 0.01) | 0.0 (0.0 to 0.01) | 0.0 (0.0 to 0.01) | −0.01 † (−0.01 to 0.0) |
| LSNS | −0.01 (−0.06 to 0.04) | 0.0 (−0.06 to 0.05) | 0.03 (−0.03 to 0.08) | 0.02 (−0.03 to 0.07) | 0.02 (−0.03 to 0.07) | −0.06 † (−0.10 to −0.02) |
| ∆STAS | 0.0 (−0.09 to 0.08) | 0.04 (−0.05 to 0.10) | 0.07 (−0.02 to 0.20) | 0.01 (−0.08 to 0.10) | 0.01 (−0.07 to 0.10) | −0.10 † (−0.20 to −0.03) |
Psychosocial factors were analyzed in adjusted linear regression models with the change in z‐normalized heart rate variability from visit 1 to visit 4 as the outcome, showing both directionality and magnitude of change. The estimates are for the amount of change in heart rate variability for every 10‐point change in Maastricht Vital Exhaustion Questionnaire (n=5371), Spielberger Trait Anger Scale (n=5356), Interpersonal Support Evaluation List (n=5330), and Lubben Social Network Scale (n=5330) respectively. An additional model included the effect of a 10‐point change in Spielberger Trait Anger Scale (n=5343) from visit 2 to visit 4. Models were adjusted for age, race, sex, body mass index, hypertension, coronary heart disease, and diabetes mellitus. HF indicates high frequency (ln ms2); HR, heart rate (beats/minute); ISEL, Interpersonal Support Evaluation List; LF, low frequency (ln ms2); LSNS, Lubben Social Network Scale; MVEQ, Maastricht Vital Exhaustion Questionnaire; STAS, Spielberger Trait Anger Scale; TP, total frequency power (ln ms2); SDNN, the standard deviation of normally conducted RR intervals (ms); and RMSSD, the root mean square of successive differences in normally conducted RR intervals (ms).
P<0.05.
P<0.01.
P<0.001.
In the outcome analysis, from 1987 to 2018, there were 4277 overall deaths and 1107 myocardial infarction events, with 1232 deaths attributable to cardiovascular disease. As seen in Figure 3, the median survival of those with the top quartile of VE and the bottom quartile of LF HRV was 25.4 years, compared with those with normal VE and HRV who had a median survival of 31.2 years. The top quartile of VE had a hazard ratio (HR) for all‐cause mortality 1.29 (95% CI, 1.20‒1.39), an HR for myocardial infarction of 1.20 (95% CI, 1.04‒1.39), and an HR for cardiovascular mortality of 1.31 (95% CI, 1.15‒1.50). After adjusting for LF and HF HRV (Table 5), the HR for all‐cause mortality, myocardial infarction, and cardiovascular mortality was unchanged. LF HRV showed a reduced HR for all outcomes, however HF was associated with an increased HR for death when included as an additional covariate.
Figure 3. Survival curve for groups of high vs low exhaustion with normal vs low low frequency heart rate variability, with shaded intervals indicating 95% CIs, with time in years.
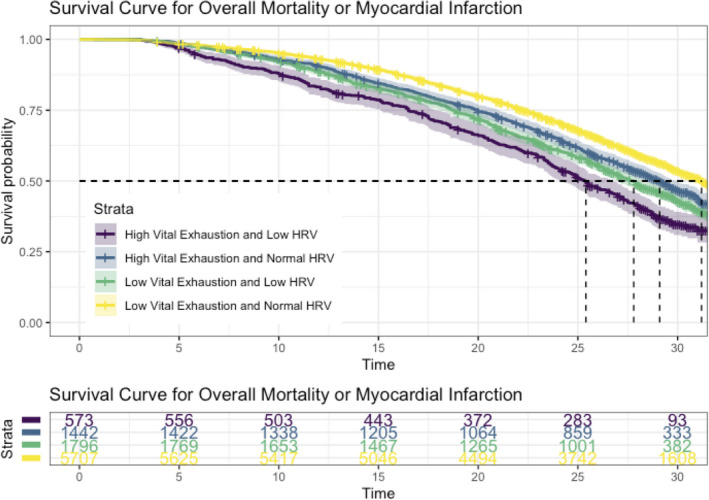
HRV indicates heart rate variability, and LF, low frequency.
Table 5.
Cox Proportional Hazard Models for Death, Myocardial Infarction, and Cardiovascular Mortality
| Death | MI | CVD Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MVEQ | 1.29* (1.20, 1.39) | 1.29* (1.20‒1.38) | 1.29* (1.20‒1.38) | 1.20 † (1.04, 1.39) | 1.20 † (1.04‒1.39) | 1.20 † (1.03‒1.39) | 1.31* (1.15‒1.50) | 1.30* (1.14‒1.49) | 1.30* (1.14‒1.49) |
| Ln LF | 0.94* (0.92‒0.96) | 0.89* (0.86‒0.92) | 0.95 † (0.91‒0.99) | 0.92 † (0.85‒0.99) | 0.92* (0.88‒0.96) | 0.89* (0.84‒0.95) | |||
| Ln HF | 1.07* (1.03‒1.11) | 1.05 (0.97‒1.13) | 1.04 (0.97‒1.12) | ||||||
The initial models used vital exhaustion as the exposure, with subsequent models adjusted for LF and HF heart rate variability. All models included adjustment for age, sex, BMI, race, hypertension, CHD, and diabetes mellitus. CVD indicates cardiovascular disease; HF, high frequency; LF, low frequency; MI, myocardial infarction; and MVEQ, Maastricht Vital Exhaustion Questionnaire.
P<0.001.
P<0.01.
Anger Proneness
There were 9324 participants at visit 2 with data on anger trait and HRV (Table S2). There were no significant associations of anger tertile with HRV. After adjustment for covariates, anger proneness was not significantly associated with any HRV measures from visit 1 (Table 3). For every 10‐point increase in anger trait, there was a greater increase in heart rate from visit 1 to visit 4. As anger trait was repeated at visit 4, those with an increase in anger trait from visit 2 to visit 4 had a larger decline in heart rate over time from visit 1 to visit 4, as seen in Table 4. There was no interaction between anger proneness and age, sex, or race.
Social Isolation
There were 9317 participants at visit 2 with data on social support and HRV. Tables S3 and S4 show the breakdown of demographic characteristics, comorbid conditions, and HRV measures with both ISEL and LSNS categories of social support. Changes in HRV for every 10‐point increase in the ISEL and LSNS are shown in Table 3, and changes in HRV over time from visit 1 to visit 4 are shown in Table 4. Social isolation as measured by LSNS showed a reduction in SDNN (−0.61, 95% CI, −1.23 to −0.00) and RMSSD (−0.82, 95% CI, −1.53 to −0.10). Higher social support was associated with a larger decline in heart rate over time. There was no interaction between social support and age, sex or race. As there was a relationship between LSNS and SDNN and RMSSD, outcome analyses were performed similarly to that of vital exhaustion. The bottom quartile of LSNS, which represents the least social support, associated with all‐cause mortality, with HR, 1.46; 95% CI, 1.28‒1.65; The relationships with myocardial infarction (HR, 1.10; 95% CI, 0.84‒1.44) and cardiovascular mortality (HR, 1.23; 95% CI, 0.96‒1.58) were lower and not significant. After sequentially adjusting for RMSSD and SDNN (Table 6), the associations were unchanged, however both RMSSD and SDNN had small associations with overall and cardiovascular mortality.
Table 6.
Cox Proportional Hazard Models for Death, Myocardial Infarction, and Cardiovascular Disease Mortality
| Death | MI | CVD Mortality | |||||||
| LSNS | 1.46* (1.28, 1.65) | 1.46* (1.28, 1.65) | 1.46* (1.29, 1.66) | 1.10 (0.84, 1.44) | 1.10 (0.84, 1.45) | 1.10 (0.84, 1.44) | 1.23 † (0.96, 1.58) | 1.23 † (0.96, 1.58) | 1.24 † (0.97, 1.59) |
| RMSSD | 1.00 (1.00, 1.00) | 1.01* (1.00, 1.01) | 1.00 (1.00, 1.00) | 1.00 † (1.00, 1.01) | 1.00 (1.00, 1.00) | 1.01* (1.00, 1.01) | |||
| SDNN | 0.99* (0.99, 0.99) | 0.99* (0.99, 1.00) | 0.99* (0.98, 1.00) | ||||||
The initial models used social isolation as the exposure, with subsequent models adjusted for RMSSD and SDNN. All models included adjustment for age, sex, body mass index, race, hypertension, CHD, and diabetes mellitus. CVD indicates cardiovascular disease; LSNS, Lubben Social Network Scale; MI, myocardial infarction; RMSSD, the root mean square of successive differences in normally conducted RR intervals; and SDNN, the SD of normally conducted RR intervals.
P<0.001.
P<0.05.
Discussion
Main Finding
In this community‐based sample of 9331 participants, we found several associations with HRV and HR with psychosocial factors. Most notably, we found that only vital exhaustion associated with low HRV; in addition, we found fewer associations of HRV and heart rate with anger and social support. Specifically, when comparing the highest versus lowest quartile of VE, the effects on LF HRV were the same as the effects of nearly 4 added years of age (HRV‐age). 42 Vital exhaustion also associated with decreased LF HRV over time. Both anger and social isolation were associated with higher heart rates, suggesting that lower heart rates were protective. This is similar to work showing a protective effect of normal HRV at the development of depression and post‐traumatic stress disorder, 17 , 43 and highlights the importance of the autonomic nervous system as a potential system that provides resilience to psychological stressors. Our findings were robust after rigorous adjustment for covariates and support the hypothesis that vital exhaustion exerts a persistent and independent influence on autonomic function. Our findings may have important implications about the cardiotoxicity of somatic symptoms of stress specifically in contrast with other measures of stress (anger) and situational factors (social support), which may have less biological impact.
Relationship Between Psychosocial Stress on the Autonomic Nervous System
Our findings are supported by the epidemiological literature in studies evaluating somatic symptoms and outcomes. 44 , 45 , 46 , 47 Somatic, but not cognitive symptoms of depression, were associated with inflammatory markers and overall mortality in post‐myocardial infarction participants from the TRIUMPH registry (Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status). 48 , 49 Vital exhaustion is both a marker of chronic psychological distress and somatic depressive symptoms, 19 which may explain its potential role in cardiovascular mortality. Appels and Mulder showed that vital exhaustion was predictive of future myocardial infarction, independently of past medical risk factors, 20 and validated these findings within the ARIC cohort as well. 36 , 50 The association of low HRV and vital exhaustion was primarily seen with HF and LF HRV. This suggests decreased parasympathetic nervous system modulation (HF) and also dysfunctional pathways involving both autonomic branches and/or baroreflex sensitivity (LF). 31 We examined these potential autonomic mechanisms underlying excess cardiovascular disease that has previously been reported in those with high vital exhaustion. As seen in Figure 3, both vital exhaustion and low HRV show an increased risk of overall mortality. The results of our survival analysis suggest that short‐term HF and LF HRV do not explain the excess risk of vital exhaustion. 51 Research into other HRV measures (such as long‐term measures) and/or other mechanisms, such as inflammation and hormonal mechanisms, are warranted. 52 , 53
Our finding of the association between decreased social support decreased SDNN and RMSSD support the concept that these psychosocial factors can lead to physiological changes. The association of social support with RMSSD and SDNN suggest decreased resting parasympathetic activity in those with low social support. In previous studies, social isolation and anger proneness were both associated with elevated risk of cardiovascular mortality. 1 , 41 However, social isolation measured by LSNS did not show mediation by HRV in outcomes analysis (Table 6). There was a relationship between increased social isolation, by LSNS, in both cardiovascular and overall mortality. The reasons for the weaker positive findings with anger and social isolation are not clear. One possibility is that the mechanisms involved are through other pathways than autonomic dysfunction or are not effectively measured by the HRV metrics we used in this study, and that longer recording times are needed, for example. 54 In general, few studies have studied the relationship between psychosocial stress and autonomic dysfunction, and no studies, to our knowledge, have ever studied the relationship of anger or social support with HRV. One study found that the relationship between social isolation and incident heart failure was fully mediated by vital exhaustion. 21 In contrast with vital exhaustion, anger may be considered a cognitive stressor 55 ; this further underscores the hypothesis that somatic symptoms are specifically related to (and perhaps driven by) autonomic dysfunction. Another important consideration is that social support is not a continuous phenomenon—the effect of physical touch on vagus activation versus perceived support can be quite different. 56 , 57 The lack of social support can also be attributed to different underlying etiologies, from current situational or environmental factors to early childhood experiences. 58
Limitations and Future Directions
The current study has several limitations. Our main analysis required carrying forward the HRV data from visit 1 to visit 2, when the psychosocial data were collected. Because of potential changes over time, this may lead to misclassification. In most cases, such misclassification would cause bias to the null and type II error. However, during visit 4, we were able to evaluate anger and HRV that were both acquired simultaneously. Although we presume that vital exhaustion leads to changes in HRV, as seen in the relationship between visit 2 and visit 4, the relationship can also be bi‐directional. Those that have lower HRV may be more susceptible to developing vital exhaustion. These findings cannot conclude evidence of temporality or causality in this relationship. In addition, there are several limitations based on the measurement of HRV. Most importantly, additional HRV data including power spectral plots, Poincaré plots, or tachograms to assess the quality of the ECG data were not available for analysis. HRV was only collected at visit 1 and visit 4, and both times had different recording lengths which make direct comparisons difficult. We were able to partially compensate for this by standardizing the HRV measurement. The reliability of HRV is decreased in shorter duration recording, while only 2‐minute recordings were available from visit 1. Compared with higher frequency and time domain measures, LF is a less reliable measure in the 2‐minute recordings (versus the 6‐minute recordings). This limits our ability to distinguish true, between‐visit changes in LF from measurement error, but would bias our findings towards the null. Very low frequency needs a minimum of 5 minutes of recording, which was not done except at visit 4, and thus was not interpretable. The finding of increased mortality with increased HF HRV is unexpected in light of prior literature which has found a protective effect. 59 In some cases, HF HRV can be inflated because of ectopic beats or increased respiratory effort because of underlying lung disease. There are also no consensus guidelines on comparing HRV between different length recordings, particularly with power spectral analysis. 54 Our methods to use normalized values are not validated, and therefore the change analysis results should be interpreted with caution. However, our study is strengthened by careful inclusion/exclusion criteria in regards to medication use and high‐quality ECG data, rigorous statistical analyses, and focused hypothesis testing in a large, well‐characterized community sample. Future work is needed to understand a wider array of mechanisms alongside HRV that may explain the increased mortality from psychosocial stress. One candidate study possible within ARIC includes the evaluation of available inflammatory markers, which associate with depression, 60 as mediating mechanisms between stress and mortality. 61
Conclusions
We found that vital exhaustion, and to a lesser extent anger proneness or social isolation, were associated with autonomic dysfunction as measured via short duration measures of HRV and heart rate. The finding may reflect an important physiologic mechanism that explains the relationship of vital exhaustion, a somatic symptom commonly found in depression, and cardiovascular disease. This elucidation of mechanism may be particularly useful when considering not only the clinical importance of vital exhaustion as a marker of autonomic dysfunction, but also the evaluation of future therapies that target autonomic dysfunction to confer cardiovascular benefits.
Sources of Funding
The ARIC study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Dr Anish Shah was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award number UL1TR002378 and the TL1TR002383 (Blumberg, PI). Additional support was provided by American Heart Association grant 16EIA26410001 and National Heart, Lung, Blood Institute grant K24HL148521 (Alonso), as well as K23HL127251 (Amit Shah). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Supporting information
Tables S1–S4
Figure S1
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
(J Am Heart Assoc.2021;10:e017172. DOI: 10.1161/JAHA.120.017172.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017172
For Sources of Funding and Disclosures, see page 11.
References
- 1. Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all‐cause mortality in older men and women. Proc Natl Acad Sci USA. 2013;110:5797–5801. DOI: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9:360–370. DOI: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 3. Smolderen KG, Buchanan DM, Gosch K, Whooley M, Chan PS, Vaccarino V, Parashar S, Shah AJ, Ho PM, Spertus JA. Depression treatment and 1‐year mortality after acute myocardial infarction: insights from the TRIUMPH registry (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status). Circulation. 2017;135:1681–1689. DOI: 10.1161/CIRCULATIONAHA.116.025140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post‐traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108:29–33. DOI: 10.1016/j.amjcard.2011.02.340. [DOI] [PubMed] [Google Scholar]
- 5. Scherrer JF, Salas J, Cohen BE, Schnurr PP, Schneider FD, Chard KM, Tuerk P, Friedman MJ, Norman SB, van den Berk‐Clark C, et al. Comorbid conditions explain the association between posttraumatic stress disorder and incident cardiovascular disease. J Am Heart Assoc. 2019;8:e011133. DOI: 10.1161/JAHA.118.011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salahuddin L, Cho J, Jeong MG, Kim D. Ultra short term analysis of heart rate variability for monitoring mental stress in mobile settings. Annu Int Conf IEEE Eng Med Biol Soc. 2007;2007:4656–4659. [DOI] [PubMed] [Google Scholar]
- 7. Carney RM, Howells WB, Blumenthal JA, Freedland KE, Stein PK, Berkman LF, Watkins LL, Czajkowski SM, Steinmeyer B, Hayano J, et al. Heart rate turbulence, depression, and survival after acute myocardial infarction. Psychosom Med. 2007;69:4–9. DOI: 10.1097/01.psy.0000249733.33811.00. [DOI] [PubMed] [Google Scholar]
- 8. Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. J Cardiovasc Electrophysiol. 2005;16:13–20. DOI: 10.1046/j.1540-8167.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- 9. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 10. Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. DOI: 10.1016/S0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 11. Taggart P, Boyett MR, Logantha SJRJ, Lambiase PD. Anger, emotion, and arrhythmias: from brain to heart. Front Physiol. 2011;2:67. DOI: 10.3389/fphys.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ozbay F, Johnson DC, Dimoulas E, Morgan CA, Charney D, Southwick S. Social support and resilience to stress: from neurobiology to clinical practice. Psychiatry (Edgmont). 2007;4:35–40. [PMC free article] [PubMed] [Google Scholar]
- 13. Childs E, White TL, De Wit H. Personality traits modulate emotional and physiological responses to stress. Behav Pharmacol. 2014;25:493–502. DOI: 10.1097/FBP.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kogler L, Müller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, Derntl B. Psychosocial versus physiological stress—meta‐analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage. 2015;119:235–251. DOI: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lipowski ZJ. Somatization: the concept and its clinical application. Am J Psychiatry. 1988;145:1358–1368. [DOI] [PubMed] [Google Scholar]
- 16. Carnevali L, Koenig J, Sgoifo A, Ottaviani C. Autonomic and brain morphological predictors of stress resilience. Front Neurosci. 2018;12:228. DOI: 10.3389/fnins.2018.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang M, Shah A, Su S, Goldberg J, Lampert RJ, Levantsevych OM, Shallenberger L, Pimple P, Bremner JD, Vaccarino V. Association of depressive symptoms and heart rate variability in Vietnam war‐era twins. JAMA Psychiatry. 2018;75:705. DOI: 10.1001/jamapsychiatry.2018.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Cai L, Qian J, Peng J. Social support moderates stress effects on depression. Int J Ment Health Syst. 2014;8:41. DOI: 10.1186/1752-4458-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vroege EM, Zuidersma M, De Jonge P. Vital exhaustion and somatic depression: the same underlying construct in patients with myocardial infarction? Psychosom Med. 2012;74:446–451. DOI: 10.1097/PSY.0b013e31825a7194. [DOI] [PubMed] [Google Scholar]
- 20. Appels A, Mulder P. Excess fatigue as a precursor of myocardial infarction. Eur Heart J. 1988;9:758–764. DOI: 10.1093/eurheartj/9.7.758. [DOI] [PubMed] [Google Scholar]
- 21. Cen CW, Loehr L, Lin FC, Hammond WP, Foraker RE, Rose K, Mosley T, Corbie‐Smith G. Social isolation, vital exhaustion, and incident heart failure: findings from the Atherosclerosis Risk in Communities Study. Eur J Heart Fail. 2012;14:748–753. DOI: 10.1093/eurjhf/hfs064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE. Triggering of acute myocardial infarction onset by episodes of anger. Circulation. 1995;92:1720–1725. DOI: 10.1161/01.CIR.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 23. Williams JE, Nieto FJ, Sanford CP, Tyroler HA. Effects of an angry temperament on coronary heart disease risk: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2001;154:230–235. DOI: 10.1093/aje/154.3.230. [DOI] [PubMed] [Google Scholar]
- 24. Anon . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 25. Liao D, Barnes RW, Chambless LE, Simpson RJ, Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability—the ARIC study. Atherosclerosis Risk in Communities. Am J Cardiol. 1995;76:906–912. DOI: 10.1016/S0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- 26. Shah A, Vaccarino V. Heart rate variability in the prediction of risk for posttraumatic stress disorder. JAMA Psychiatry. 2015;72:964–965. DOI: 10.1001/jamapsychiatry.2015.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability. Hypertension. 2003;42:1106–1111. DOI: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 28. Sacha J. Interaction between heart rate and heart rate variability. Ann Noninvasive Electrocardiol. 2014;19:207–216. DOI: 10.1111/anec.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldberger JJ. Sympathovagal balance: how should we measure it? Am J Physiol. 1999;276:H1273–H1280. [DOI] [PubMed] [Google Scholar]
- 30. Sacha J, Barabach S, Statkiewicz‐Barabach G, Sacha K, Müller A, Piskorski J, Barthel P, Schmidt G. Gender differences in the interaction between heart rate and its variability—how to use it to improve the prognostic power of heart rate variability. Int J Cardiol. 2014;171:42–45. DOI: 10.1016/j.ijcard.2013.11.116. [DOI] [PubMed] [Google Scholar]
- 31. Akselrod S, Gordon D, Ubel A, Shannon D, Barger C, Cohen R. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat‐to‐beat cardiovascular control. Science. 1981;213:220–222. DOI: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 32. Reyes del Paso GA, Langewitz W, Mulder LJM, van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50:477–487. DOI: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- 33. Malik M. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. DOI: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 34. Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biol Psychiatry. 2013;73:1103–1110. DOI: 10.1016/j.biopsych.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Appels A, Höppener P, Mulder P. A questionnaire to assess premonitory symptoms of myocardial infarction. Int J Cardiol. 1987;17:15–24. DOI: 10.1016/0167-5273(87)90029-5. [DOI] [PubMed] [Google Scholar]
- 36. Williams JE, Mosley TH, Kop WJ, Couper DJ, Welch VL, Rosamond WD. Vital exhaustion as a risk factor for adverse cardiac events (from the Atherosclerosis Risk In Communities [ARIC] Study). Am J Cardiol. 2010;105:1661–1665. DOI: 10.1016/j.amjcard.2010.01.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spielberger CD. State‐Trait Anger Expression Inventory. In: Weiner IB, Craighead W eds. The Corsini Encyclopedia of Psychology. Hoboken, NJ: John Wiley & Sons, Inc.; 2010:1. [Google Scholar]
- 38. Heitzmann CA, Kaplan RM. Assessment of methods for measuring social support. Health Psychol. 1988;7:75–109. DOI: 10.1037/0278-6133.7.1.75. [DOI] [PubMed] [Google Scholar]
- 39. Sarason IG, Sarason BR, Shearin EN, Pierce GR. A brief measure of social support: practical and theoretical implications. J Soc Pers Relat. 1987;4:497–510. DOI: 10.1177/0265407587044007. [DOI] [Google Scholar]
- 40. Payne TJ, Andrew M, Butler KR, Wyatt SB, Dubbert PM, Mosley TH. Psychometric evaluation of the interpersonal support evaluation list‐short form in the ARIC Study Cohort. SAGE Open. 2012;2:1–8. DOI: 10.1177/2158244012461923. [DOI] [Google Scholar]
- 41. Denollet J, Gidron Y, Vrints CJ, Conraads VM. Anger, suppressed anger, and risk of adverse events in patients with coronary artery disease. Am J Cardiol. 2010;105:1555–1560. DOI: 10.1016/j.amjcard.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 42. Umetani K, Singer DH, McCraty R, Atkinson M. Twenty‐four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593–601. DOI: 10.1016/S0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 43. Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB, Hauger RL, Huang M, Murphy JA, Naviaux RK, et al. Association of predeployment heart rate variability with risk of postdeployment posttraumatic stress disorder in active‐duty marines. JAMA Psychiatry. 2015;72:979–986. DOI: 10.1001/jamapsychiatry.2015.0922. [DOI] [PubMed] [Google Scholar]
- 44. Winzeler K, Voellmin A, Hug E, Kirmse U, Helmig S, Princip M, Cajochen C, Bader K, Wilhelm FH. Adverse childhood experiences and autonomic regulation in response to acute stress: the role of the sympathetic and parasympathetic nervous systems. Anxiety Stress Coping. 2017;30:145–154. DOI: 10.1080/10615806.2016.1238076. [DOI] [PubMed] [Google Scholar]
- 45. Penninx BWJH. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2017;74:277–286. DOI: 10.1016/j.neubiorev.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 46. Sgoifo A, Carnevali L, Alfonso Mde L, Amore M. Autonomic dysfunction and heart rate variability in depression. Stress. 2015;18:343–352. DOI: 10.3109/10253890.2015.1045868. [DOI] [PubMed] [Google Scholar]
- 47. Ohira T, Roux AVD, Prineas RJ, Kizilbash MA, Carnethon MR, Folsom AR. Associations of psychosocial factors with heart rate and its short‐term variability: Multi‐Ethnic Study of Atherosclerosis. Psychosom Med. 2008;70:141–146. DOI: 10.1097/PSY.0b013e318160686a. [DOI] [PubMed] [Google Scholar]
- 48. Smolderen KG, Spertus JA, Reid KJ, Buchanan DM, Vaccarino V, Lichtman JH, Bekelman DB, Chan PS. Association of somatic and cognitive depressive symptoms and biomarkers in acute myocardial infarction: insights from the translational research investigating underlying disparities in acute myocardial infarction patients’ health status registry. Biol Psychiatry. 2012;71:22–29. DOI: 10.1016/j.biopsych.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smolderen KG, Spertus JA, Reid KJ, Buchanan DM, Krumholz HM, Denollet J, Vaccarino V, Chan PS. The association of cognitive and somatic depressive symptoms with depression recognition and outcomes after myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Garg PK, Claxton JS, Soliman EZ, Chen LY, Lewis TT, Mosley T, Alonso A. Associations of anger, vital exhaustion, anti‐depressant use, and poor social ties with incident atrial fibrillation: the Atherosclerosis Risk in Communities Study. Eur J Prev Cardiol. 2020;Epub: 204748731989716. DOI: 10.1177/2047487319897163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ripplinger CM, Noujaim SF, Linz D. The nervous heart. Prog Biophys Mol Biol. 2016;120:199–209. DOI: 10.1016/j.pbiomolbio.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shah ASAJ, Lampert R, Goldberg J, Bremner JD, Li L, Thames MD, Vaccarino V, Shah ASAJ. Alterations in heart rate variability are associated with abnormal myocardial perfusion. Int J Cardiol. 2020;305:99–105. DOI: 10.1016/j.ijcard.2020.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stein PK, Lundequam EJ, Clauw D, Freedland KE, Carney RM, Domitrovich PP. Circadian and ultradian rhythms in cardiac autonomic modulation. Conf Proc IEEE Eng Med Biol Soc. 2006;2006:429–432. [DOI] [PubMed] [Google Scholar]
- 54. Schroeder EB, Whitsel EA, Evans GW, Prineas RJ, Chambless LE, Heiss G. Repeatability of heart rate variability measures. J Electrocardiol. 2004;37:163–172. DOI: 10.1016/j.jelectrocard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 55. Cox DE, DeVore BB, Harrison PK, Harrison DW. The effect of anger expression style on cardiovascular responses to lateralized cognitive stressors. Brain Inform. 2017;4:231–239. DOI: 10.1007/s40708-017-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Feldman R, Singer M, Zagoory O. Touch attenuates infants’ physiological reactivity to stress. Dev Sci. 2010;13:271–278. DOI: 10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 57. Hopp H, Shallcross AJ, Ford BQ, Troy AS, Wilhelm FH, Mauss IB. High cardiac vagal control protects against future depressive symptoms under conditions of high social support. Biol Psychol. 2013;93:143–149. DOI: 10.1016/j.biopsycho.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brooks KP, Gruenewald T, Karlamangla A, Hu P, Koretz B, Seeman TE. Social relationships and allostatic load in the MIDUS study. Health Psychol. 2014;33:1373–1381. DOI: 10.1037/a0034528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lampert R, Ickovics JR, Viscoli CJ, Horwitz RI, Lee FA. Effects of Propranolol on recovery of heart rate variability following acute myocardial infarction and relation to outcome in the Beta‐Blocker Heart Attack Trial. Am J Cardiol. 2003;91:137–142. DOI: 10.1016/S0002-9149(02)03098-9. [DOI] [PubMed] [Google Scholar]
- 60. Dooley LN, Kuhlman KR, Robles TF, Eisenberger NI, Craske MG, Bower JE. The role of inflammation in core features of depression: insights from paradigms using exogenously‐induced inflammation. Neurosci Biobehav Rev. 2018;94:219–237. DOI: 10.1016/j.neubiorev.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pollitt RA, Kaufman JS, Rose KM, Diez‐Roux AV, Zeng D, Heiss G. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. J Epidemiol Community Health. 2008;62:484–491. DOI: 10.1136/jech.2006.054106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figure S1


