Abstract
Background
Asymptomatic proximal deep vein thrombosis (DVT) is an end point frequently used to evaluate the efficacy of anticoagulant thromboprophylaxis in medical patients. Recently, the clinical relevance of asymptomatic DVT has been challenged.
Methods and Results
The objective of this study was to evaluate the relationship between asymptomatic proximal DVT and all‐cause mortality (ACM) using a cohort analysis of a randomized trial for the prevention of venous thromboembolism (VTE) in acutely ill medical patients. Patients who received at least 1 dose of study drug and had an adequate compression ultrasound examination of the legs on either day 10 or day 35 were categorized into 1 of 3 cohorts: no VTE, asymptomatic proximal DVT, or symptomatic DVT. Cox proportional hazards model, with adjustment for significant independent predictors of mortality, were used to compare the incidences of ACM. Of the 7036 patients, 6776 had no VTE, 236 had asymptomatic DVT, and 24 had symptomatic VTE. The incidence of ACM was 4.8% in patients without VTE. Both asymptomatic proximal DVT (mortality, 11.4%; hazard ratio [HR], 2.31; 95% CI, 1.52–3.51; P<0.0001) and symptomatic VTE (mortality, 29.2%; HR, 9.42; 95% CI, 4.18–21.20; P<0.0001) were independently associated with significant increases in ACM. The analysis was post hoc, and ultrasound results were not available for all patients. Adjustment for baseline variables significantly associated with ACM may not fully compensate for differences.
Conclusions
Asymptomatic proximal DVT is associated with higher ACM than no VTE and remains a relevant end point to evaluate the efficacy of anticoagulant thromboprophylaxis in medical patients.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00571649.
Keywords: medically ill, mortality, proximal DVT
Subject Categories: Embolism, Thrombosis, Vascular Disease, Ultrasound, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- ACM
all‐cause mortality
Clinical Perspective
What Is New?
Among patients hospitalized with acute medical illness, those with asymptomatic proximal deep vein thrombosis have a >2‐fold increase in mortality compared with patients without venous thromboembolism.
What Are the Clinical Implications?
Asymptomatic proximal deep vein thrombosis is an indicator of clinically important venous thromboembolic disease and remains a useful outcome for evaluating the efficacy of thromboprophylaxis in patients with acute medical illness.
Patients who are hospitalized for acute medical illness are at increased risk for venous thromboembolism (VTE), including fatal pulmonary embolism, 1 and the majority of episodes of fatal pulmonary embolism occur in patients with medical illness. 2 The need for thromboprophylaxis in these patients is predicated on the fact that the use of clinical surveillance alone to detect and treat only those patients who develop symptomatic VTE is not effective for reducing fatal pulmonary embolism. 3 Anticoagulant thromboprophylaxis reduces the risk of fatal pulmonary embolism by about 60%. 4
The source of most episodes of clinically important pulmonary embolism, including fatal pulmonary embolism, is proximal deep vein thrombosis (DVT) of the legs (ie. popliteal, femoral, or iliac vein thrombosis). 5 Such thrombi are often asymptomatic. 3 , 5 , 6 This natural history relationship is the foundation for the use of proximal DVT as an end point in clinical trials evaluating the effectiveness of anticoagulant thromboprophylaxis. Previous studies of thromboprophylaxis in patients hospitalized for acute medical illness have suggested that the presence of asymptomatic proximal DVT is associated with subsequent increased mortality. 6 , 7
The MAGELLAN (Multicenter, Randomized, Parallel Group Efficacy and Safety Study for the Prevention of Venous Thromboembolism in Hospitalized Acutely Ill Medical Patients Comparing Rivaroxaban With Enoxaparin) study was a large randomized, double‐blind clinical trial evaluating thromboprophylaxis in patients admitted to the hospital with acute medical illness. 8 The study protocol required all patients to undergo compression ultrasonography of the legs performed on day 10 and day 35 after randomization. All patients were followed to day 90. We used the data from this clinical trial to evaluate the relationship between asymptomatic proximal DVT detected on compression ultrasonography and subsequent mortality from all causes. A secondary goal was to evaluate the relationship between symptomatic VTE and all‐cause mortality (ACM).
METHODS
Data Sharing Statement
At present, the sponsor's policy is to share data after regulatory approval in accordance with the policy of its codevelopment partner. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient‐level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the criteria for listing studies and other relevant information is provided in the codevelopment partner's section of the portal.
Study Design
The results of the MAGELLAN trial (NCT00571649) have been reported in detail. 8 Briefly, this study, which included 8101 acutely ill medical patients, compared thromboprophylaxis with 10 mg of rivaroxaban daily, started in hospital and continued for a total of 35±4 days, with enoxaparin (40 mg daily) given for 10±4 days) followed by placebo. The primary end point was the composite of asymptomatic proximal DVT, symptomatic DVT, pulmonary embolism, or VTE‐related death. Eligible patients were adults at least 40 years of age hospitalized for an acute medical illness (ie, heart failure, active cancer, acute ischemic stroke, acute infectious and inflammatory disease, and acute respiratory insufficiency) who were at risk for VTE because of moderate or severe immobility and who had additional risk factors for VTE, including prolonged immobilization, age ≥75 years, history of cancer, history of VTE, history of heart failure, thrombophilia, acute infectious disease contributing to the hospitalization, and body mass index ≥35 kg/m2. The study protocol required all patients to undergo routine compression ultrasonography of both legs on day 10±4 and day 35±4 after randomization. All patients were followed to day 90. All ultrasound results, and all suspected episodes of VTE, myocardial infarction, stroke, bleeding, and all deaths were adjudicated by a central independent clinical events committee, according to prespecified criteria 9 and without knowledge of the patient's treatment group.
The trial was conducted in accordance with the Declaration of Helsinki and local regulations. The protocol was approved by the relevant local institutional review boards and ethics committees, and written informed consent was obtained from each patient.
Statistical Analysis
This analysis was defined post hoc to test the hypothesis identified from previously published literature 6 , 7 that the presence of asymptomatic proximal DVT is associated with increased mortality from all causes. The population for the analysis consisted of all patients who received at least 1 dose of the study drug and had an adequate ultrasound result at either day 10±4 or day 35±4 (modified intent‐to‐treat population). Patients were categorized into 1 of 3 mutually exclusive cohorts: (1) those without VTE; (2) those with asymptomatic proximal DVT documented by compression ultrasonography; or (3) those with symptomatic VTE (including symptomatic DVT of the lower extremity and/or symptomatic nonfatal pulmonary embolism), occurring at any time during the study and confirmed by the clinical events committee. Patients who had both asymptomatic DVT and a subsequent symptomatic VTE were counted in the asymptomatic DVT cohort.
Baseline covariates (age, sex, race, body mass index, diabetes mellitus, creatinine clearance, history of VTE, history of cancer, history of anemia, assigned treatment group) and the following reasons for hospitalization: heart failure, acute ischemic stroke, acute infectious disease, inflammatory disease, acute respiratory insufficiency, were tested for significant association with ACM (P<0.05) using a Cox proportional hazards model. The final Cox model included the time from the first thromboembolic event to death as the response variable and the 3 baseline covariates (history of cancer, body mass index, history of anemia) that were significantly (P<0.05) associated with mortality, as well as the reasons for hospitalization (heart failure, acute ischemic stroke, inflammatory disease, acute respiratory insufficiency) that were significant (P<0.05) as the predictor variables. The analysis compared the risk of mortality from all causes through the day 90 visit across the 3 cohorts. The hazard ratios (HRs) shown in the Results are adjusted HRs for the predictor variables listed above fitted by the final Cox model. A Schoenfeld residuals‐based test for the independence between residuals and time was used to check the proportional hazards assumption for each covariate included in the Cox model fitting. For both the full model and the final model, the test was not statistically significant for each of the covariates, and the global test was also not statistically significant. Therefore, the assumption of proportionality was supported. Kaplan‐Meier plots were used to display survival probabilities and cumulative mortality (without adjusting for the predictor variables) over time from the first VTE event or from the time of the first ultrasound in those without VTE.
RESULTS
Baseline Characteristics
Of the 8101 patients enrolled in the MAGELLAN study, 7036 (86.8%) were included in the combined day 10/day 35 modified intent‐to‐treat analysis set. The flow of all randomized patients through the analysis is shown in Figure 1. Of the 7036 patients included in the analysis, 6776 patients (96.3%) were included in the cohort without VTE, 236 patients (3.4%) in the cohort with asymptomatic proximal DVT, and 24 patients (0.3%) in the cohort with symptomatic VTE (Figure 1).
Figure 1. Study disposition.
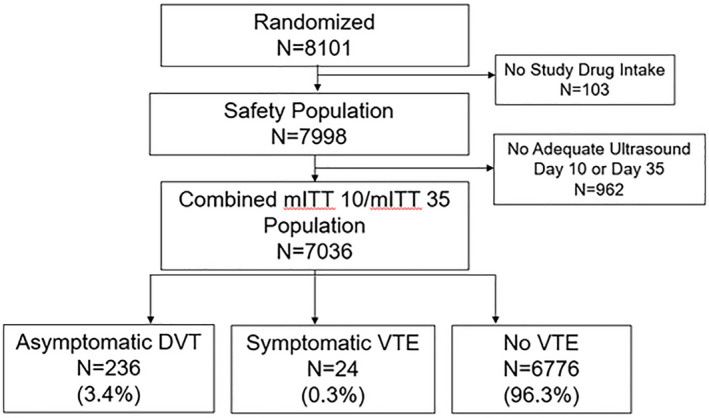
DVT indicates deep vein thrombosis; mITT 10/mITT 35, modified intent‐to‐treat day 10/day 35 (adequate ultrasound at day 10 or day 35); and VTE, venous thromboembolism.
The baseline demographic and clinical characteristics of the patients in each of the cohorts are shown in Table 1. Follow‐up through day 90 with documentation of mortality status was completed in 227 patients (96.2%) in the asymptomatic proximal DVT cohort, 24 patients (100%) in the symptomatic VTE cohort, and in 6627 patients (97.8%) in the cohort without VTE. Patients who did not complete the day 90 follow‐up were censored at the time of their last follow‐up, which documented their mortality status.
Table 1.
Baseline Characteristics of the Cohorts
| Characteristic | Asymptomatic DVT (N=236) | Symptomatic VTE (N=24) | No VTE (N=6776) | P Value* |
|---|---|---|---|---|
| Age, y, mean | 73.3 | 72.9 | 69.0 | <0.0001 |
| Male sex, % | 50.4 | 45.8 | 54.5 | 0.3214 |
| White race, % | 70.3 | 70.8 | 68.3 | 0.9715 |
| BMI, kg/m2, mean | 27.2 | 30.3 | 28.2 | 0.0345 |
| d‐dimer >2×ULN, % | 75.8 | 81.8 | 46.0 | <0.0001 |
| Creatine clearance, mL/min, mean | 67.8 | 74.6 | 79.2 | <0.0001 |
| Systolic blood pressure, mm Hg, mean | 128.5 | 132.2 | 131.4 | 0.0601 |
| Diastolic blood pressure, mm Hg, mean | 74.2 | 76.7 | 76.2 | 0.0217 |
| Pulse rate, beats/min, mean | 80.9 | 85.9 | 80.8 | 0.2173 |
| Hemoglobin, g/dL, mean | 12.4 | 13.0 | 13.2 | <0.0001 |
| Chronic venous insufficiency, % | 16.5 | 25.0 | 14.9 | 0.2999 |
| Bronchiectasis, % | 2.1 | 4.2 | 1.6 | 0.4688 |
| Diabetes mellitus, % | 28.8 | 29.2 | 30.4 | 0.8536 |
| History of ulcer, % | 1.7 | 12.5 | 2.9 | 0.0099 |
| History of cancer, % | 26.7 | 29.2 | 16.4 | <0.0001 |
| History of VTE, % | 22.0 | 12.5 | 3.9 | <0.0001 |
| History of anemia, % | 19.1 | 12.5 | 10.6 | <0.0001 |
| Reason for hospitalization, % | ||||
| Active cancer | 12.3 | 12.5 | 6.8 | 0.0030 |
| Acute infections and inflammatory disease | 46.8 | 54.2 | 46.8 | 0.7702 |
| Acute ischemic stroke | 13.6 | 8.3 | 18.2 | 0.0950 |
| Acute respiratory insufficiency | 18.3 | 20.8 | 28.0 | 0.0038 |
| Heart failure (NYHA Class III or IV) | 34.0 | 41.7 | 33.0 | 0.6306 |
| Additional VTE risk factors, % | ||||
| Acute infectious disease | 9.4 | 21.7 | 18.0 | 0.0048 |
| Age ≥75 y | 59.6 | 52.2 | 45.7 | 0.0003 |
| Chronic venous insufficiency | 18.3 | 26.1 | 18.2 | 0.6180 |
| History of venous insufficiency | 24.4 | 13.0 | 4.8 | <0.0001 |
| History of heart failure NYHA (Class III or IV) | 41.3 | 34.8 | 42.9 | 0.6574 |
| Hormone replacement therapy | 0.9 | 4.3 | 1.4 | 0.4056 |
| Morbid obesity (BMI ≥35 kg/m2) | 11.3 | 30.4 | 18.6 | 0.0083 |
| Severe varicose veins | 15.0 | 21.7 | 14.7 | 0.6364 |
| Thrombophilia (hereditary or acquired) | 0.5 | 0 | 0.4 | 0.9274 |
The P value for race is not for the comparison of “White” vs “non‐White”; it is for the comparison among all race groups. BMI indicates body mass index; DVT, deep vein thrombosis; NYHA, New York Heart Association; ULN, upper limit of normal; and VTE, venous thromboembolism.
P value tests the equivalence of variables among all three groups. For discrete variables, it tests the equivalence of frequency proportions among 3 groups; for continuous variables, it tests the equivalence of means among 3 groups.
Efficacy Outcomes
The cumulative incidences of mortality from all causes were 11.4%, 29.2%, and 4.8% in the cohorts with asymptomatic DVT, symptomatic VTE, and no VTE, respectively (P<0.0001). Compared with the 4.8% incidence of ACM in the cohort without VTE, asymptomatic proximal DVT was associated with a statistically significant increase in ACM (HR, 2.31; 95% CI, 1.52–3.51; P<0.0001). Likewise, the presence of symptomatic VTE was also associated with a statistically significant increase in ACM (HR, 9.42; 95% CI, 4.18–21.20; P<0.0001). The Kaplan‐Meier plots of the time to the occurrence of death for each of the 3 cohorts are shown in Figure 2. The number and causes of death in each of the cohorts are listed in Table 2.
Figure 2. Time to death from all causes.
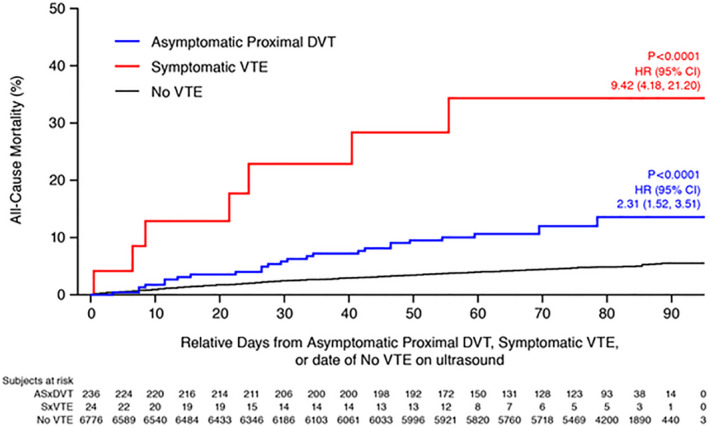
DVT indicates deep vein thrombosis; HR, hazard ratio; and VTE, venous thromboembolism.
Table 2.
Causes of Death Adjudicated by the Clinical Events Committee
|
Asymptomatic Proximal DVT N=236 n (%) |
Symptomatic VTE N=24 n (%) |
No VTE N=6776 n (%) |
|
|---|---|---|---|
| All‐cause mortality | 27 (11.44) | 7 (29.17) | 322 (4.75) |
| Cardiovascular | 4 (1.69) | 2 (8.33) | 65 (0.96) |
| Pulmonary embolism | 0 | 1 (4.17) | 2 (0.03) |
| Pulmonary embolism cannot be excluded | 4 (1.69) | 1 (4.17) | 74 (1.09) |
| Bleeding | 0 | 0 | 9 (0.13) |
| Other | 19 (8.05) | 3 (12.5) | 172 (2.54) |
| Amyloidosis | 1 (0.4) | 0 | 0 |
| Amyotrophic lateral sclerosis | 0 | 0 | 1 (0.01) |
| Cachexia | 0 | 0 | 1 (0.01) |
| Cancer | 9 (3.8) | 1 (4.2) | 65 (1.0) |
| Dehydration | 0 | 0 | 1 (0.01) |
| Infectious disease | 7 (3.0) | 1 (4.2) | 52 (0.8) |
| Multiple organ failure | 0 | 0 | 2 (0.03) |
| Not reported | 0 | 0 | 2 (0.03) |
| Respiratory failure | 2 (0.8) | 1 (4.2) | 44 (0.6) |
| Parkinson disease | 0 | 0 | 1 (0.01) |
| Renal failure | 0 | 0 | 1 (0.01) |
| Septicemia | 0 | 0 | 1 (0.01) |
| Unknown cause of death | 0 | 0 | 1 (0.01) |
DVT indicates deep vein thrombosis; and VTE, venous thromboembolism.
Of the 236 patients with asymptomatic proximal DVT, 106 patients (44.9%) received anticoagulant treatment after the diagnosis. By comparison, anticoagulant treatment after diagnosis was given to 21 of the 24 patients (87.5%) with symptomatic VTE, and to 768 of the 6776 patients (11.3%) without VTE.
A plot comparing survival after the diagnosis of asymptomatic proximal DVT with the survival in the cohort without evidence of VTE is shown in Figure 3A. The comparison of these survival curves was statistically significant (P<0.0001).
Figure 3. Survival after detection of asymptomatic proximal DVT vs negative ultrasound: Comparison of survival with and without asymptomatic proximal DVT in (A) MAGELLAN, (B) PREVENT, (C) APEX.
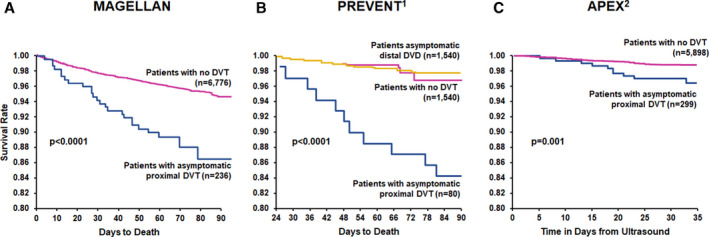
APEX indicates Acute Medically Ill VTE Prevention With Extended Duration Betrixaban study; DVT, deep vein thrombosis; MAGELLAN, Multicenter, Randomized, Parallel Group Efficacy and Safety Study for the Prevention of Venous Thromboembolism in Hospitalized Acutely Ill Medical Patients Comparing Rivaroxaban With Enoxaparin study; PREVENT, Prevention of Recurrent Venous Thromboembolism; and VTE, venous thromboembolism. B, Modified from Vaitkus et al 6 with permission. Copyright ©2005 Georg Thieme Verlag KG. C, Modified from Kalayci et al 7 with permission. Copyright ©2018 Georg Thieme Verlag KG.
DISCUSSION
The results of this analysis of patients hospitalized for acute medical illness indicate that the presence of asymptomatic proximal DVT is associated with a 2‐fold increased mortality from all causes through day 90 after randomization. The mortality among those in whom asymptomatic DVT was detected was 11.4%, compared with 4.8% among those without evidence of VTE (HR, 2.31; 95% CI, 1.52–3.51; P<0.0001). Thus, the presence of asymptomatic proximal DVT is an indicator of clinically important disease and a marker of a worse prognosis. The mortality among patients with symptomatic VTE was also significantly increased, with a nearly 10‐fold increase (29.2%; HR, 9.42; 95% CI, 4.18–21.20; P<0.0001). The time to death curves show early and continued separation for both cohorts of patients with documented thromboembolism compared with the patients without evidence of VTE (Figure 2).
The causes of the excess deaths among patients with asymptomatic proximal DVT compared with those without VTE included cancer and infectious diseases such as pneumonia (Table 2), conditions known to be associated with an increased risk of VTE. 10 , 11 Excluding pulmonary embolism as a contributing cause of death among medical patients is often difficult in the absence of autopsy examination. 12 Multiple autopsy studies have established that the diagnosis of pulmonary embolism is often unsuspected ante mortem. 12 About one‐half of the patients with asymptomatic proximal DVT in our study were not given anticoagulant therapy. Therefore, it is likely that pulmonary embolism may have been a contributing factor to death in many of these patients.
Our results are consistent with those of 2 previous studies 6 , 7 in patients hospitalized with medical illness, which provided the basis for our hypothesis of an increased mortality (decreased survival) associated with the diagnosis of asymptomatic proximal DVT. Both studies documented significantly decreased survival in patients with asymptomatic proximal DVT, as shown in the survival curves in Figure 3B and 3C. The results of our analysis (Figure 3A) are remarkably consistent with the results of these 2 studies. 6 , 7 In contrast to proximal DVT, asymptomatic distal DVT was not associated with significantly reduced survival compared with patients without DVT in the PREVENT (Prevention of Recurrent Venous Thromboembolism) study 6 (Figure 3B). This finding provides support for the conclusion that it is the proximal site of DVT that confers the poor prognosis of increased mortality. It is well established that thrombosis of the proximal deep veins (popliteal, femoral, or iliac) is much more likely to lead to clinically important pulmonary embolism than thrombosis confined to the distal deep veins (calf vein thrombosis). 5
During the past decade, evidence‐based practice guidelines have recommended against the use of asymptomatic proximal DVT as a clinically important outcome in the benefit‐risk assessment of thromboprophylaxis in medical patients. This recommendation should be reconsidered in view of the consistent data that asymptomatic proximal DVT is associated with increased mortality in this patient population. We agree with Bounameaux and Agnelli, who made the case for patients undergoing orthopedic surgery, that asymptomatic thrombosis may be clinically relevant and that asymptomatic venographically detected thrombosis is a clinically relevant outcome. 13 As the source of most episodes of fatal pulmonary embolism, 5 asymptomatic proximal DVT is not a surrogate outcome but, rather, part of the natural history continuum of VTE, and remains an appropriate outcome for evaluation of thromboprophylaxis.
Our analysis has strengths and limitations. Strengths include the relatively large sample size, the objective documentation of the presence or absence of VTE, the blinded central adjudication of all outcomes, and the low rate of loss to follow‐up. Limitations include the post hoc nature of the analysis and the fact that 962 of the 7998 patients (12%) included in the trial had inadequate ultrasound results. Although adjustment was done for baseline variables that were significantly associated with ACM, such adjustment may not fully compensate for baseline differences among the cohorts.
In conclusion, in patients hospitalized with acute medical illness, those with asymptomatic proximal DVT have a 2‐fold increase in mortality through day 90 compared with patients without VTE. Asymptomatic proximal DVT is an indicator of clinically important venous thromboembolic disease and is a useful outcome for evaluating efficacy in clinical trials of thromboprophylaxis in patients with acute medical illness.
Sources of Funding
This work was supported by Bayer U.S. LLC and Janssen R&D LLC who jointly sponsored the MAGELLAN trial.
Disclosures
Dr Raskob received consultant/honoraria fees from Janssen R&D LLC, Bayer, BMS, Daiichi Sankyo, Pfizer, and Medscape; and consultancy fees from Boehringer Ingelheim, Eli Lilly, Portola, Novartis, Anthos, Tetherex, and XaTek. Dr Spyropoulos received consultancy/research funding from Boehringer Ingelheim and consultancy fees from Daiichi Sankyo, Portola, Bayer, ATLAS (Colorado Prevention Center), and Janssen R&D, LLC. Dr Cohen received consultancy/speakers bureau fees from Aspen, Boehringer Ingelheim, and Medscape; consultancy/membership board of directors or advisory committee/speakers bureau fees from Bayer, BMS, Daiichi Sankyo, Pfizer, and Portola; and consultancy fees from AbbVie, ACI Clinical, and Boston Scientific. Dr Weitz received consultant/honoraria fees from Bayer, Boehringer Ingelheim, BMS, Daiichi‐Sankyo, Ionis, Janssen, Merck, Novartis, Pfizer, Servier, and Portola. Dr Ageno received consultant fees from Portola, Daiichi, Sankyo, Aspen, BMS, Pfizer, Boehringer Ingelheim, and Sanofi; and consultant/research funding fees from Bayer and Janssen R&D LLC. Y. De Sanctis is an employee of Bayer US LLC. Dr Lu is an employee with equity ownership of Janssen R&D LLC. Dr Xu is an employee with equity ownership of Janssen R&D LLC. J. Albanese is an employee with equity ownership of Janssen R&D LLC. C. Sugarmann is an employee with equity ownership of Janssen R&D LLC. T. Weber is an employee with equity ownership of Janssen R&D LLC. Dr Lipardi is an employee with equity ownership of Janssen R&D LLC. Dr Spiro was an employee with equity ownership of Bayer US LLC. Dr Barnathan is an employee with equity ownership of Janssen R&D LLC.
(J Am Heart Assoc. 2021;10:e019459. DOI: 10.1161/JAHA.120.019459.)
For Sources of Funding and Disclosures, see page 7.
REFERENCES
- 1. Sperry K, Key C, Anderson R. Toward a population‐based assessment of death due to pulmonary embolism in New Mexico. Hum Pathol. 1990;21:159–165. DOI: 10.1016/0046-8177(90)90124-N. [DOI] [PubMed] [Google Scholar]
- 2. Cohen AT, Edmondson RA, Phillips MJ, Ward VP, Kakkar VV. The changing pattern of venous thromboembolic disease. Haemostasis. 1996;26:65–71. [DOI] [PubMed] [Google Scholar]
- 3. Geerts WH, Bergqvist DH, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism: American College of Chest Physicians evidence‐based clinical practice guidelines (8th Edition). Chest. 2008;133:381–453. DOI: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 4. Dentali F, Douketis J, Gianni M, Lim W, Crowther MA. Meta‐analysis: anticoagulant prophylaxis to prevent symptomatic venous thromboembolism in hospitalized medical patients. Ann Intern Med. 2007;146:278–288. DOI: 10.7326/0003-4819-146-4-200702200-00007. [DOI] [PubMed] [Google Scholar]
- 5. Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:I22–I30. DOI: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 6. Vaitkus P, Leizorovicz A, Cohen A, Turpie AGG, Olsson CG, Goldhaber SZ. Mortality rates and risk factors for asymptomatic deep vein thrombosis in medical patients. Thromb Haemost. 2005;93:76–79. DOI: 10.1160/TH04-05-0323. [DOI] [PubMed] [Google Scholar]
- 7. Kalayci A, Gibson C, Chi G, Yee M, Korjian S, Datta S, Nafee T, Gurin M, Haroian N, Qamar I, et al. Asymptomatic deep vein thrombosis is associated with an increased risk of death: insights from the APEX trial. Thromb Haemost. 2018;118:2046–2052. DOI: 10.1055/s-0038-1675606. [DOI] [PubMed] [Google Scholar]
- 8. Cohen AT, Spiro TE, Büller HR, Haskell L, Hu D, Hull R, Mebazaa A, Merli G, Schellong S, Spyropoulos AC, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–523. DOI: 10.1056/NEJMoa1111096. [DOI] [PubMed] [Google Scholar]
- 9. Cohen AT, Spiro TE, Büller HR, Haskell L, Hu D, Hull R, Mebazaa A, Merli G, Schellong S, Spyropoulos A, et al. Extended‐duration rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. J Thromb Thrombolysis. 2011;31:407–416. DOI: 10.1007/s11239-011-0549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heit JA. Venous thromboembolism epidemiology: implications for prevention and management. Semin Thromb Hemost. 2002;28(suppl 2):3–13. DOI: 10.1055/s-2002-32312. [DOI] [PubMed] [Google Scholar]
- 11. Torres‐Macho J, Mancebo‐Plaza AB, Crespo‐Giménez A, Sanz de Barros MR, Bibiano‐Guillén C, Fallos‐Martí R, Calderón‐Parra J, de Miguel‐Yanes JM. Clinical features of patients inappropriately undiagnosed of pulmonary embolism. Am J Emerg Med. 2013;31:1646–1650. DOI: 10.1016/j.ajem.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 12. Goldhaber SZ, Hennekens CH, Evans DA, Newton EC, Godleski JJ. Factors associated with correct antemortem diagnosis of major pulmonary embolism. Am J Med. 1982;73:822–826. DOI: 10.1016/0002-9343(82)90764-1. [DOI] [PubMed] [Google Scholar]
- 13. Bounameaux H, Agnelli G. Symptoms and clinical relevance: a dilemma for clinical trials on prevention of venous thromboembolism. Thromb Haemost. 2013;109:585–588. DOI: 10.1160/TH12-08-0627. [DOI] [PubMed] [Google Scholar]


