Abstract
Background
The mechanism through which high‐density lipoprotein (HDL) induces cardioprotection is not completely understood. We evaluated the correlation between cholesterol efflux capacity (CEC), a functional parameter of HDL, and coronary collateral circulation (CCC). We additionally investigated whether A1BP (apoA1‐binding protein) concentration correlates with CEC and CCC.
Methods and Results
In this case‐control study, clinical and angiographic data were collected from 226 patients (mean age, 58 years; male, 72%) with chronic total coronary occlusion. CEC was assessed using a radioisotope and J774 cells, and human A1BP concentration was measured using enzyme‐linked immunosorbent assay. Differences between the good and poor CCC groups were compared, and associations between CEC, A1BP, and other variables were evaluated. Predictors of CCC were identified by multivariable logistic regression analysis. The CEC was higher in the good than in the poor CCC group (22.0±4.6% versus 20.2±4.7%; P=0.009). In multivariable analyses including age, sex, HDL‐cholesterol levels, age (odds ratio [OR], 0.96; P=0.003), and CEC (OR, 1.10; P=0.004) were identified as the independent predictors of good CCC. These relationships remained significant after additional adjustment for diabetes mellitus, acute coronary syndrome, and Gensini score. The A1BP levels were not significantly correlated with CCC (300 pg/mL and 283 pg/mL in the good CCC and poor CCC groups, respectively, P=0.25) or CEC.
Conclusions
The relationship between higher CEC and good CCC indicates that well‐functioning HDL may contribute to CCC and may be cardioprotective; this suggests that a specific function of HDL can have biological and clinical consequences.
Keywords: apolipoprotein A‐1, coronary artery disease, lipoproteins, macrophages
Subject Categories: Coronary Artery Disease, Lipids and Cholesterol
Nonstandard Abbreviations and Acronyms
- A1BP
apoA1‐binding protein
- CCC
coronary collateral circulation
- CEC
cholesterol efflux capacity
Clinical Perspective
What Is New?
Cholesterol efflux capacity was significantly higher in patients with good coronary collateral circulation (CCC) than in those with poor CCC.
Younger age and higher cholesterol efflux capacity were identified as independent predictors of good CCC after adjusting confounders such as high‐density lipoprotein cholesterol.
What Are the Clinical Implications?
Higher cholesterol efflux capacity indicating well‐functioning high‐density lipoprotein cholesterol may be considered as a marker for good CCC.
Cholesterol efflux capacity value predictive of good CCC can be a candidate of therapeutic target for patients with ischemic vascular diseases.
Coronary collateral circulation (CCC) is defined as arteriole‐to‐arteriole anastomoses that undergo expansion and remodeling in the setting of coronary artery disease. 1 Recurrent and severe myocardial ischemia is assumed to stimulate the development of coronary collaterals. 2 It has been reported that clinical factors, including degree of coronary stenosis, longer duration of lesion occlusion, and heart rate can influence CCC. 3 Several types of vascular cells, such as endothelial cells, monocytes, and smooth muscle cells, are known to participate in the process. 4 Well‐developed coronary collaterals may protect the at‐risk myocardium and have been shown to lower the mortality rate by 36%. 5 Stimulation for the development of CCC is even considered a new therapeutic approach for ischemic heart disease.
High‐density lipoprotein‐cholesterol (HDL‐C) levels have long been considered as a negative predictor of cardiovascular risk. In this regard, pharmacological therapies elevating HDL‐C levels were attempted, but most studies did not obtain the expected benefit. 6 Lately, an individual’s cholesterol efflux capacity (CEC) beyond HDL‐C levels has emerged as a negative predictor of cardiovascular outcomes. 7
The mechanisms through which HDL induces cardioprotection include cholesterol efflux, inhibition of low‐density lipoprotein oxidation, inhibition of vascular inflammation or thrombosis, and promotion of angiogenesis. 8 The effect of HDL on endothelial and other cells and eventually on angiogenesis has been investigated but not completely clarified. 9 Although some animal studies demonstrated the angiogenic effect of HDL, 10 the association between HDL‐C levels and CCC was shown to be inconsistent. 11 , 12 Conversely, a recent study showed that A1BP (apoA1‐binding protein) accelerates cholesterol efflux from endothelial cells and thereby regulates angiogenesis. 13 However, the role of CEC in development of CCC is poorly understood.
With the aforementioned gap in research, the aim of this study was to examine the association between CEC, a functional parameter of HDL, and CCC in patients with chronic total coronary occlusion, following evaluation of the significance of the association by adjusting possible confounders. In addition, we investigated whether A1BP, an HDL‐related protein known to influence cholesterol efflux, correlates with CEC and CCC.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
All consecutive patients who visited Severance Hospital and underwent coronary angiography from January 2001 to August 2009 were included in the database of the Cardiovascular Genome Center at Yonsei University College of Medicine, Seoul, Korea. The patients underwent coronary angiography for chest discomfort or pain. A total of 226 patients with chronic total occlusion of coronary arteries were in the database, and the study population consisted of these patients. The angiographic diagnosis was made based on findings showing total occlusion in at least 1 epicardial coronary artery. This study complies with the Declaration of Helsinki and received proper ethical oversight. The Institutional Review Board of Severance Hospital, Seoul, Korea approved the research protocol (2020‐1453‐001). Written informed consent was obtained from all study subjects.
Clinical and Angiographic Data Collection
Trained nurses obtained the clinical data, including demographic variables and medical history. Blood samples were collected from all study subjects at enrollment and stored at −80°C. In patients with acute coronary syndrome, coronary angiography was performed without delay after hospital visit. Blood samples were collected immediately before coronary angiography if the patient was fasting for more than 12 hours. In patients with a nonfasting state, blood samples were collected within 24 hours after angiography with 12‐hour fasting. All patients received oral aspirin before angiography, and 5000 U of intravenous heparin was used at the beginning of the procedure. The characteristics of coronary artery disease and collateral circulation were evaluated by 2 interventional cardiologists who were blinded to the other data of the patients. CCC was assessed according to the Rentrop classification as follows: grade 0, no filling; grade 1, filling of side branches via collateral channels without epicardial filling; grade 2, partial filling of the epicardial coronary artery via collateral channels; and grade 3, complete filling of the epicardial coronary artery. 14 If a patient had more than 1 collateral vessel, the highest collateral grade was selected. Then, the patients were classified according to their collateral grades as having poor (grade 0 or 1) or good (grade 2 or 3) CCC. Although analysis of collaterals with the Rentrop classification can be less accurate than analysis with the invasive pressure‐ or velocity‐based collateral flow index, 15 this index was not available in our study subjects. However, because the Rentrop classification has often been used in several well‐designed studies analyzing collaterals, 14 we used this in the present study. The severity of coronary artery disease was assessed using the Gensini score, which quantifies disease severity by a point system based on luminal narrowing with a multiplier for specific lesion locations. 16
Assessment of CEC and A1BP
The cholesterol efflux assay was performed using the following method: J774A1 cells (RRID: CVDL_0358; passage number 20–29) were plated and radiolabeled with 2 μCi of 3H‐cholesterol/mL for 24 hours. The cells were provided courtesy of Yury I Miller at University of California, San Diego, CA, USA. For the upregulation of adenosine triphosphate‐binding cassette transporter subfamily member A1, cells were incubated with medium containing 0.2% bovine serum albumin and 0.3 mmol/L 8‐(4‐chlorophenylthio)‐cyclic adenosine monophosphate for 6 hours, as described previously. 17 The medium was then replaced with another medium containing 0.2% bovine serum albumin and the patients’ apolipoprotein‐depleted serum for 4 hours. 18 The experiment was conducted by treating the cells with 2 mg/mL of an acyl‐coenzyme A:cholesterol acyltransferase inhibitor. The cholesterol efflux proportion was calculated using the following formula: CEC (%)={3H‐cholesterol (μCi) in medium containing HDL/[3H‐cholesterol (μCi) in medium containing HDL+3H‐cholesterol (μCi) in cells]}×100. We subtracted the background value from all sample values. The values were adjusted based on the CEC of the pooled serum that was run on each plate. Each sample was run in duplicate.
An enzyme‐linked immunosorbent assay kit (MyBioSource, San Diego, CA, USA) was used to assay human A1BP in serum samples of study subjects. Assays were performed according to the manufacturer’s specification. Briefly, samples were incubated at 37°C for 90 minutes on capture antibody‐coated plates. Detection antibody and horseradish peroxidase‐conjugated antibody were then used to recognize retained A1BP. Chromogenic 3,3′,5,5′‐tetramethylbenzidine substrate was then added. The plates were read at an absorbance of 450 nm. Appropriate specificity controls were included, and all samples were run in duplicates.
Statistical Analysis
Continuous variables with normal distribution are presented as mean±SD, whereas those not meeting normality are presented as median (interquartile range). Categorical data are presented as frequencies and percentages. Differences between the 2 groups were compared using the chi‐square test for categorical variables. Continuous variables with normality were compared by Student’s t test, whereas those without normality were compared by Mann–Whiney U test. Pearson correlation analysis was used to evaluate the relationship between 2 continuous variables. For the relationship between continuous and categorical variables, we used point‐biserial correlation analysis. Predictors of CCC were identified by univariable logistic regression analysis. Those univariable factors with P values<0.05 and major relevant clinical factors (sex, diabetes mellitus, acute coronary syndrome, HDL‐C, and Gensini score) were then entered into a multivariable analysis. We checked interactions between variables in multivariable model by using interaction terms. All analyses used 2‐tailed tests with a significance level of 0.05. Statistical Package for the Social Sciences version 17.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
Clinical Characteristics
The clinical and laboratory parameters of the 226 study subjects are shown in Table 1. The patients’ mean age was 58 years, and male patients accounted for 72% of the total population. Moreover, 24% of the study population had diabetes mellitus, and 73% presented with acute coronary syndrome. Coronary angiography showed multivessel disease in 70% of study subjects. Patients with good CCC were likely to be younger (median age of 56 years and 61 years in the good and poor CCC groups, respectively; P=0.009). The CEC was higher in the good CCC group compared with the poor CCC group (22.0±4.6% and 20.2±4.7%, respectively; P=0.008). Values of other parameters including HDL‐C and A1BP were comparable between the 2 groups (Figure).
Table 1.
Clinical Characteristics of the Study Population
| Good CCC (n=155) | Poor CCC (n=71) | P Value | |
|---|---|---|---|
| Age, y | 56 (48, 63) | 61 (54, 65) | 0.009 |
| Male sex | 109 (70.3) | 54 (76.1) | 0.37 |
| Medical history | |||
| Diabetes mellitus | 33 (21.3) | 22 (31.0) | 0.11 |
| Hypertension | 66 (42.6) | 32 (45.1) | 0.73 |
| Smoking | 28 (18.1) | 16 (22.5) | 0.43 |
| Acute coronary syndrome | 108 (71.1) | 57 (82.6) | 0.067 |
| Body mass index, kg/m2 | 24.4 (22.7, 26.8) | 24.1 (21.7, 26.5) | 0.19 |
| Laboratory values, mg/dL | |||
| Total cholesterol | 181 (153, 207) | 185 (150, 211) | 0.89 |
| Triglyceride | 136 (94, 178) | 131 (87, 195) | 0.99 |
| High‐density lipoprotein‐cholesterol | 39 (33, 46) | 40 (32, 47) | 0.99 |
| Low‐density lipoprotein‐cholesterol | 110 (78, 135) | 113 (76, 140) | 0.88 |
| Angiographic findings | |||
| Left main disease | 7 (4.5) | 4 (5.6) | 0.98 |
| 1‐vessel disease | 46 (29.7) | 21 (29.6) | |
| 2‐vessel disease | 46 (29.7) | 18 (25.3) | 0.46 |
| 3‐vessel disease | 63 (40.6) | 31 (43.7) | |
| Gensini score | 68 (52, 87) | 68 (48, 84) | 0.46 |
| Cholesterol efflux capacity, % | 22.0±4.6 | 20.2±4.7 | 0.008 |
| ApoA1‐binding protein, pg/mL | 266 (146, 399) | 217 (124, 331) | 0.25 |
Data are presented as means±SDs, numbers (%), or medians (interquartile ranges).
CCC indicates coronary collateral circulation.
Figure 1. CEC and A1BP in patients with good or poor CCC.
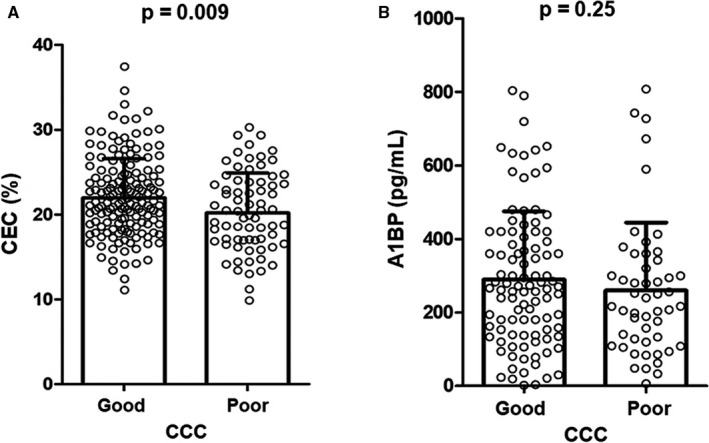
The CEC was significantly higher in the good CCC group compared with the poor CCC group (A), whereas the A1BP levels did not differ between the 2 groups (B). A1BP indicates apoA1‐binding protein; CCC, coronary collateral circulation; and CEC, cholesterol efflux capacity.
Correlation Between CEC, A1BP, and Other Variables
When analyzed in all subjects, CEC value showed a positive correlation with good CCC (r=0.18; P=0.008). Although CEC tended to be positively correlated with age and A1BP, the correlation was not statistically significant. It was not correlated with HDL‐C levels or acute coronary syndrome (Table 2). Although A1BP levels showed correlation tendency with diabetes mellitus, smoking, and CEC, it was not statistically significant (Table 2).
Table 2.
Correlations Between CEC, A1BP, and Other Variables
| CEC | A1BP | |||
|---|---|---|---|---|
| r (95% CI) | P Value | r (95% CI) | P Value | |
| Age | 0.12 (−0.01 to 0.25) | 0.069 | −0.10 (−0.26 to 0.06) | 0.22 |
| Male sex | −0.04 (−0.17 to 0.09) | 0.55 | 0.02 (−0.14 to 0.18) | 0.78 |
| Diabetes mellitus | −0.06 (−0.19 to 0.07) | 0.34 | −0.15 (−0.30 to 0.01) | 0.073 |
| Hypertension | −0.09 (−0.21 to 0.05) | 0.20 | −0.07 (−0.23 to 0.09) | 0.37 |
| Smoking | −0.02 (−0.15 to 0.11) | 0.76 | 0.15 (−0.01 to 0.30) | 0.074 |
| Acute coronary syndrome | −0.04 (−0.18 to 0.09) | 0.52 | −0.04 (−0.20 to 0.13) | 0.68 |
| Body mass index | 0.09 (−0.04 to 0.22) | 0.16 | −0.08 (−0.24 to 0.08) | 0.33 |
| Triglyceride | 0.02 (−0.11 to 0.15) | 0.79 | 0.04 (−0.12 to 0.20) | 0.62 |
| High‐density lipoprotein‐cholesterol | 0.05 (−0.08 to 0.18) | 0.42 | 0.05 (−0.11 to 0.21) | 0.52 |
| Low‐density lipoprotein‐cholesterol | 0.04 (−0.10 to 0.16) | 0.61 | 0.05 (−0.11 to 0.21) | 0.53 |
| Gensini score | 0.57 (−0.07 to 0.190 | 0.39 | 0.01 (−0.15 to 0.17) | 0.94 |
| Good coronary collateral circulation | 0.18 (0.05 to 0.30) | 0.008 | 0.08 (−0.08 to 0.23) | 0.35 |
| CEC | … | … | −0.15 (−0.31 to 0.01) | 0.062 |
| A1BP | −0.15 (−0.31 to 0.01) | 0.062 | … | … |
A1BP indicates apoA1‐binding protein; and CEC, cholesterol efflux capacity.
Predictors of Collateral Circulation
In the univariable logistic regression, age (odds ratio [OR], 0.97; P=0.017) and CEC (OR, 1.09; P=0.009) were significantly associated with good CCC. In a multivariable analysis model including age, male sex, HDL‐C levels, and CEC, age (OR, 0.96; P=0.003), and CEC (OR, 1.10; P=0.004) were identified as independent predictors of good CCC. In particular, this significant relationship was obtained after adjusting HDL‐C levels. In a model additionally including diabetes mellitus, acute coronary syndrome, and the number of diseased vessels, the relationship remained significant (Table 3). We tested if there was interaction between CEC and acute coronary syndrome status using a multivariable model for good CCC. When we examined the interaction term, acute coronary syndrome x CEC, in a model, we found no significant interaction between the 2 variables (OR, 0.98; 95% CI, 0.83, 1.16; P=0.82). In addition, we checked for potential interaction between key variables such as age, male sex, diabetes mellitus, acute coronary syndrome, HDL‐C, and CEC. In the meantime, we found significant interaction between age and sex (P for interaction=0.015), and between age and acute coronary syndrome (P for interaction=0.018). However, the predictive value of CEC remained significant (OR, 1.10; P=0.009) after taking into account these 2 interaction terms. Age and sex showed different predictive values in subgroups classified by sex and acute coronary syndrome (Table S1).
Table 3.
Predictors of Good Coronary Collateral Circulation Identified by Multiple Logistic Regression
| Univariable Analysis | Multivariable Analysis 1 | Multivariable Analysis 2 | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Age | 0.97 (0.94–0.99) | 0.017 | 0.96 (0.93–0.98) | 0.003 | 0.96 (0.93–0.99) | 0.009 |
| Male sex | 0.75 (0.38–1.40) | 0.37 | 0.63 (0.31–1.22) | 0.18 | 0.64 (0.31–1.30) | 0.21 |
| Diabetes mellitus | 0.60 (0.32–1.14) | 0.12 | 0.72 (0.36–1.42) | 0.34 | ||
| Hypertension | 0.90 (0.51–1.60) | 0.73 | ||||
| Smoking | 0.76 (0.38–1.54) | 0.43 | ||||
| Acute coronary syndrome | 0.52 (0.24–1.03) | 0.070 | 0.54 (0.26–1.15) | 0.11 | ||
| Body mass index | 1.02 (0.97–1.08) | 0.39 | ||||
| Triglyceride | 1.00 (0.99–1.00) | 0.99 | ||||
| High‐density lipoprotein‐cholesterol | 1.01 (0.99–1.03) | 0.55 | 1.00 (0.99–1.02) | 0.81 | 1.00 (0.98–1.02) | 0.82 |
| Low‐density lipoprotein‐cholesterol | 1.00 (0.99–1.01) | 0.60 | ||||
| Gensini score | 1.00 (0.99–1.01) | 0.51 | 1.01 (1.00–1.02) | 0.25 | ||
| Cholesterol efflux capacity | 1.09 (1.02–1.16) | 0.009 | 1.10 (1.03–1.18) | 0.004 | 1.09 (1.02–1.17) | 0.011 |
| ApoA1‐binding protein | 1.00 (0.99–1.00) | 0.35 | ||||
Discussion
The major findings of this study are as follows: CEC was significantly higher in patients with good CCC than in those with poor CCC. Younger age and higher CEC were identified as independent predictors of good CCC in the multivariable analysis. The value of CEC was significant after adjustment of HDL‐C levels. Individuals’ A1BP level did not show correlations with CCC or CEC. To the best of our knowledge, our study is the first to demonstrate the link between CEC, a functional parameter of HDL, and CCC, the clinical presentation of angiogenesis in humans.
Previous studies have reported that CEC has a predictive value for cardiovascular outcomes. A positive association between CEC and cardiovascular risk has been suggested in high‐risk populations. 19 On the contrary, most other studies have shown an inverse relationship between CEC and vascular risk or mortality in individuals with 20 or without 21 coronary artery disease. Moreover, additional biological functions including angiogenesis, anti‐inflammation, and antithrombosis were identified for HDL. 8 However, evidence regarding the effect of HDL function on clinical results has been highly limited. Notably, our study showed the association between CEC and CCC in patients with chronic total coronary occlusion. Although we further examined whether A1BP, a possible contributor to HDL activity, is associated with CCC, we could not find a significant association between A1BP and CCC.
HDL itself has angiogenic components. Vascular endothelial growth factor receptor‐2 is activated by HDL, and this is mediated by sphingosine‐1‐phosphate 22 or scavenger receptor B‐1. 23 Nitric oxide and adenosine triphosphate‐binding cassette subfamily G member 1 are known to participate in this process in vascular cells. 9 We identified that CEC was a predictor for CCC, independent of HDL‐C levels. In our study, the value of CEC was significant, whereas that of HDL‐C was not. These findings can be possibly explained as follows. First, our study population had atherosclerotic cardiovascular disease, and the functionality of their HDL may not be intact. Therefore, CEC, which represents HDL functionality, rather than HDL‐C levels, might have better shown the effects of HDL on CCC. Second, a prior study exhibited that HDL, in low levels, protected endothelial progenitor cells and promoted related angiogenesis. However, when the levels are high, HDL paradoxically impaired cell functioning and inhibited angiogenesis. 24 Accordingly, HDL might not become an independent predictor in our study. Third, we could not rule out possible changes in cellular cholesterol concentration, or signal transduction by cholesterol efflux might have affected CCC. Higher CEC is known to reduce cholesterol levels in the cell membrane.
When cholesterol is abundant in the endothelial cell membrane, membrane receptors are easily activated, and inflammation, cell proliferation, or angiogenesis can be promoted. In contrast, A1BP‐mediated cholesterol efflux decreases membrane cholesterol and inhibits angiogenesis. 13 A1BP is known to enhance the overall binding of HDL to endothelial cells and simultaneously accelerate cholesterol efflux by lowering the binding affinity of HDL. 25 A1BP was reported to reduce lipid rafts by cholesterol efflux, 13 upregulate Notch signaling, and impair vascular endothelial growth factor receptor 2 dimerization. 26 To check whether A1BP, a protein related to both CEC and angiogenesis, could explain the findings, we performed A1BP assay in all samples from the study population. Nevertheless, no significant associations were identified between A1BP and CEC or CCC. A1BP secreted by surrounding tissues worked as a negative regulator of angiogenesis. 13 However, the circulating A1BP levels analyzed in our study might not have sufficiently represented local A1BP activity and this needs to be clarified by further investigation. Interestingly, A1BP influences immune cells to inhibit the downstream signaling of toll‐like receptor 4 27 and reduce vascular inflammation in mice. 28 Studying the influence of the anti‐inflammatory effect of A1BP on the development of CCC may help reveal the possible role of A1BP.
Severity of coronary artery stenosis showed positive association with good CCC. 29 Although we included the Gensini score as a parameter of disease severity, its association with CCC was not significant. Meanwhile, we analyzed associations between diabetes mellitus and cholesterol levels that may affect angiogenesis but did not find significant correlations in the current study. Gensini score did not show a significant correlation with CEC in our results. A previous study has shown a possible negative correlation between the value of CEC multiplied by HDL‐C and number of diseased vessels. However, the conventional CEC value was not associated with disease severity. 30 More studies are needed to provide a clear explanation on this relationship.
Our study has some limitations. First, it is difficult to make clear conclusions regarding cause‐effect relationship between CEC and CCC based on our data. To our knowledge, however, the current results are the best available evidence on this study topic. Second, in study patients with acute coronary syndrome, CEC might be altered from that in patients with stable condition. In a previous study, patients with acute coronary syndrome showed lower levels of CEC compared with healthy controls. 31 However, as shown in our analysis, there was no significant interaction between acute coronary syndrome and CEC. Therefore, we can assume that the presence of acute coronary syndrome did not alter the relationship between CEC and CCC considerably. In addition, a larger study population might have helped draw firmer conclusions regarding our main findings. Nevertheless, the inclusion criteria of the present study were quite difficult to meet, and the size of our study population was the largest among similar studies. As mentioned earlier, our study is valuable because it is the first to show the association between CEC, a representative functional parameter of HDL, and CCC in clinical setting. Finally, the difference of CEC between patients with good and poor CCC was small (mean values of 22.0% and 20.2%; P=0.009). The differences of CEC (expressed as percentage) between cases and control groups in prior studies were similar in size: 11.8% and 10.4% in the report of Shao et al 32 and 12.9% and 11.5% in that of Norimatsu et al. 30 However, the predictive value of CEC (based on 1 SD change) on cardiovascular risk was robust (OR, 0.30; P=0.003) in the study by Shao et al. Likewise, when we assessed OR per 1‐SD increase in CEC, we found that higher CEC was associated with higher probability of good CCC (OR, 1.57; P=0.004). This association remained significant after adjusting other confounders (OR, 1.51; P=0.011). Therefore, even though our intergroup difference in mean CEC was not large, this index may have potential of clinical utility.
In conclusion, the relationship between higher CEC and CCC suggests that well‐functioning HDL may contribute to the development of CCC and reduce cardiovascular risk. This important evidence shows that a specific function of HDL affects biological and clinical consequences. Although additional validation is required, higher CEC may be considered as a marker for good CCC, based on our results. Conversely, CEC value predictive of good CCC can be a candidate of therapeutic target for patients with ischemic vascular diseases. Further studies are needed to develop optimal ways to enhance this specific HDL function.
Sources of Funding
This work was supported by the National Research Foundation of Korea grant funded by the Korean government (grant No. 2019R1F1A1057952).
Disclosures
None.
Supporting information
Table S1
(J Am Heart Assoc. 2021;10:e019060. DOI: 10.1161/JAHA.120.019060.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019060
For Sources of Funding and Disclosures, see page 7.
References
- 1. Jamaiyar A, Juguilon C, Dong F, Cumpston D, Enrick M, Chilian WM, Yin L. Cardioprotection during ischemia by coronary collateral growth. Am J Physiol Heart Circ Physiol. 2019;316:H1–H9.DOI: 10.1152/ajpheart.00145.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koerselman J, van der Graaf Y, de Jaegere PP, Grobbee DE. Coronary collaterals: an important and underexposed aspect of coronary artery disease. Circulation. 2003;107:2507–2511.DOI: 10.1161/01.CIR.0000065118.99409.5F. [DOI] [PubMed] [Google Scholar]
- 3. Meier P, Schirmer SH, Lansky AJ, Timmis A, Pitt B, Seiler C. The collateral circulation of the heart. BMC Med. 2013;11:143. DOI: 10.1186/1741-7015-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zimarino M, D'Andreamatteo M, Waksman R, Epstein SE, De Caterina R. The dynamics of the coronary collateral circulation. Nat Rev Cardiol. 2014;11:191–197.DOI: 10.1038/nrcardio.2013.207. [DOI] [PubMed] [Google Scholar]
- 5. Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta‐analysis. Eur Heart J. 2012;33:614–621.DOI: 10.1093/eurheartj/ehr308. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz GG, Olsson AG, Abt M, Ballantyre CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter A, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099.DOI: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 7. Ebtehaj S, Gruppen EG, Bakker SJL, Dullaart RPF, Tietge UJF. HDL (high‐density lipoprotein) cholesterol efflux capacity is associated with incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol. 2019;39:1874–1883.DOI: 10.1161/ATVBAHA.119.312645. [DOI] [PubMed] [Google Scholar]
- 8. Rye KA, Barter PJ. Cardioprotective functions of HDLs. J Lipid Res. 2014;55:168–179.DOI: 10.1194/jlr.R039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan JTM, Ng MKC, Bursill CA. The role of high‐density lipoproteins in the regulation of angiogenesis. Cardiovasc Res. 2015;106:184–193.DOI: 10.1093/cvr/cvv104. [DOI] [PubMed] [Google Scholar]
- 10. Wu BJ, Shrestha S, Ong KL, Johns D, Dunn LL, Hou L, Barter PJ, Rye KA. Increasing HDL levels by inhibiting cholesteryl ester transfer protein activity in rabbits with hindlimb ischemia is associated with increased angiogenesis. Int J Cardiol. 2015;199:204–212.DOI: 10.1016/j.ijcard.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 11. Kadi H, Ozyurt H, Ceyhan K, Koc F, Celik A, Burucu T. The relationship between high‐density lipoprotein cholesterol and coronary collateral circulation in patients with coronary artery disease. J Investig Med. 2012;60:808–812.DOI: 10.2310/JIM.0b013e31824e980c. [DOI] [PubMed] [Google Scholar]
- 12. Hsu PC, Su HM, Juo SH, Yen HW, Voon WC, Lai WT, Sheu SH, Lin TH. Influence of high‐density lipoprotein cholesterol on coronary collateral formation in a population with significant coronary artery disease. BMC Res Notes. 2013;6:105. DOI: 10.1186/1756-0500-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fang L, Choi SH, Baek JS, Liu C, Almazan F, Ulrich F, Wiesner P, Taleb A, Deer A, Pattison J, et al. Control of angiogenesis by AIBP‐mediated cholesterol efflux. Nature. 2013;498:118–122.DOI: 10.1038/nature12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi JH, Chang SA, Choi JO, Song B, Hahn JH, Choi SH, Lee SC, Lee SH, Oh JK, Choe Y, et al. Frequency of myocardial infarction and its relationship to angiographic collateral flow in territories supplied by chronically occluded coronary arteries. Circulation. 2013;127:703–709.DOI: 10.1161/CIRCULATIONAHA.112.092353. [DOI] [PubMed] [Google Scholar]
- 15. Billinger M, Kloos P, Eberli FR, Windbecker S, Meier B, Seiler C. Physiologically assessed coronary collateral flow and adverse cardiac ischemic events: a follow‐up study in 403 patients with coronary artery disease. J Am Coll Cardiol. 2002;40:1545–1550.DOI: 10.1016/S0735-1097(02)02378-1. [DOI] [PubMed] [Google Scholar]
- 16. Rampidis GP, Benetos G, Benz DC, Giannopoulos AA, Buechel RR. A guide for Gensini score calculation. Atherosclerosis. 2019;287:181–183.DOI: 10.1016/j.atherosclerosis.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 17. Khera AV, Cuchel M, de la Llera‐Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Philips JA, Mucksavage ML, Wilensky RL, et al. Cholesterol efflux capacity, high‐density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135.DOI: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borja MS, Ng KF, Irwin A, Hong J, Wu X, Isquith D, Zhao XQ, Prazen B, Gildengorin V, Oda MN, et al. HDL‐apolipoprotein A‐I exchange is independently associated with cholesterol efflux capacity. J Lipid Res. 2015;56:2002–2009.DOI: 10.1194/jlr.M059865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li XM, Tang WH, Mosior MK, Huang Y, Yping WU, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol. 2013;33:1696–1705.DOI: 10.1161/ATVBAHA.113.301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu C, Zhang Y, Ding D, Li X, Yang Y, Li Q, Zheng Y, Wang D, Ling W. Cholesterol efflux capacity is an independent predictor of all‐cause and cardiovascular mortality in patients with coronary artery disease: a prospective cohort study. Atherosclerosis. 2016;249:116–124.DOI: 10.1016/j.atherosclerosis.2015.10.111. [DOI] [PubMed] [Google Scholar]
- 21. Rohatgi A, Khera A, Berry JD, Eg G, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393.DOI: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miura S, Fujino M, Matsuo Y, Kawamura A, Tanigawa H, Nishikawa H, Saku K. High density lipoprotein‐induced angiogenesis requires the activation of Ras/MAP kinase in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:802–808.DOI: 10.1161/01.ATV.0000066134.79956.58. [DOI] [PubMed] [Google Scholar]
- 23. Tan JTM, Prosser HCG, Vanags LZ, Monger SA, Ng MKC, Bursill CA. High‐density lipoproteins augment hypoxia‐induced angiogenesis via regulation of post‐translational modulation of hypoxia‐inducible factor 1α. FASEB J. 2014;28:206–217. [DOI] [PubMed] [Google Scholar]
- 24. Huang CY, Lin FY, Shih CM, Au HK, Chang YJ, Nakagami H, Morishita R, Chang NC, Shyu KG, Chen JW. Moderate to high concentrations of high‐density lipoprotein from healthy subjects paradoxically impair human endothelial progenitor cells and related angiogenesis by activating Rho‐associated kinase pathways. Arterioscler Thromb Vasc Biol. 2012;32:2405–2417.DOI: 10.1161/ATVBAHA.112.248617. [DOI] [PubMed] [Google Scholar]
- 25. Fang L, Miller YI. Targeted cholesterol efflux. Cell Cycle. 2013;12:3345–3346.DOI: 10.4161/cc.26401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mao R, Meng S, Gu Q, Araujo‐Gutierrez R, Kumar S, Yan Q, Almazan F, Youker KA, Fu Y, Pownall HJ, et al. AIBP limits angiogenesis through γ‐secretase‐mediated upregulation of notch signaling. Circ Res. 2017;120:1727–1739.DOI: 10.1161/CIRCRESAHA.116.309754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choi SH, Wallace AM, Schneider DA, Burg E, Kim J, Alekseeva E, Ubags ND, Cool CD, Fang L, Suratt BJ, et al. AIBP augments cholesterol efflux from alveolar macrophages to surfactant and reduces acute lung inflammation. JCI Insight. 2018;3:e120519. DOI: 10.1172/jci.insight.120519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schneider DA, Choi SH, Agatisa‐Boyle C, Zhu L, Kim J, Pattison J, Sears DD, Gordts PLSM, Fang L, Miller YI. AIBP protects against metabolic abnormalities and atherosclerosis. J Lipid Res. 2018;59:854–863.DOI: 10.1194/jlr.M083618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pohl T, Seiler C, Billinger M, Herren E, Wustmann K, Mehta H, Windecker S, Eberli FR, Meier B. Frequency distribution of collateral flow and factors influencing collateral channel development. Functional collateral channel measurement in 450 patients with coronary artery disease. J Am Coll Cardiol. 2001;38:1872–1878.DOI: 10.1016/S0735-1097(01)01675-8. [DOI] [PubMed] [Google Scholar]
- 30. Norimatsu K, Kuwano T, Miura SI, Shimizu T, Shiga Y, Suematsu Y, Miyase Y, Adachi S, Nakamura A, Imaizumi S, et al. Significance of the percentage of cholesterol efflux capacity and total cholesterol efflux capacity in patients with or without coronary artery disease. Heart Vessels. 2017;32:30–38.DOI: 10.1007/s00380-016-0837-7. [DOI] [PubMed] [Google Scholar]
- 31. Hafiane A, Jabor B, Ruel I, Ling J, Genest J. High‐density lipoprotein mediated cellular cholesterol efflux in acute coronary syndromes. Am J Cardiol. 2014;113:249–255.DOI: 10.1016/j.amjcard.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 32. Shao B, Tang C, Sinha A, Mayer PS, Davenport GD, Brot N, Oda MN, Zhao XQ, Heinecke JW. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high‐density lipoprotein oxidation by myeloperoxidase. Circ Res. 2014;114:1733–1742.DOI: 10.1161/CIRCRESAHA.114.303454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


