Abstract
Background
Stenosis has historically been the major factor used to determine carotid stroke sources. Recent evidence suggests that specific plaque features detected on imaging may be more highly associated with ischemic stroke than stenosis. We sought to determine computed tomography angiography (CTA) imaging features of carotid plaque that optimally discriminate ipsilateral stroke sources.
Methods and Results
In this institutional review board–approved retrospective cross‐sectional study, 494 ipsilateral carotid CTA‐brain magnetic resonance imaging pairs were available for analysis after excluding patients with alternative stroke sources. Carotid CTA and clinical markers were recorded, a multivariable Poisson regression model was fitted, and backward elimination was performed with a 2‐sided threshold of P<0.10. Discriminatory value was determined using receiver operating characteristic analysis, area under the curve, and bootstrap validation. The final CTA carotid‐source stroke prediction model included intraluminal thrombus (prevalence ratio, 2.8 [P<0.001]; 95% CI, 1.6–4.9), maximum soft plaque thickness (prevalence ratio, 1.2 [P<0.001]; 95% CI, 1.1–1.4), and the rim sign (prevalence ratio, 2.0 [P=0.007]; 95% CI, 1.2–3.3). The final discriminatory value (area under the curve=78.3%) was higher than intraluminal thrombus (56.4%, P<0.001), maximum soft plaque thickness (76.4%, P=0.007), or rim sign alone (69.9%, P=0.001). Furthermore, NASCET (North American Symptomatic Carotid Endarterectomy Trial) stenosis categories (cutoffs of 50% and 70%) had lower stroke discrimination (area under the curve=67.4%, P<0.001).
Conclusions
Optimal discrimination of ipsilateral carotid sources of stroke requires information on intraluminal thrombus, maximum soft plaque thickness, and the rim sign. These results argue against the sole use of carotid stenosis to determine stroke sources on CTA, and instead suggest these alternative markers may better diagnose vulnerable carotid plaque and guide treatment decisions.
Keywords: atherosclerosis, carotid artery, computed tomography angiography, stroke
Subject Categories: Atherosclerosis, Vascular Disease, Cerebrovascular Disease/Stroke
Nonstandard Abbreviations and Acronyms
- CTA
computed tomography angiography
- DWI
diffusion‐weighted imaging
- IPH
intraplaque hemorrhage
- MPRAGE
magnetization‐prepared rapid acquisition gradient echo
- NASCET
North American Symptomatic Carotid Endarterectomy Trial
- TOAST
Trial of Org 10172 in Acute Stroke Treatment
Clinical Perspective
What Is New?
Certain markers of vulnerable plaque on computed tomography angiography including intraluminal thrombus, soft plaque thickness, and the rim sign are strongly associated with downstream stroke.
In this study, specific markers of vulnerable plaque on computed tomography angiography were more predictive of stroke than degree of carotid stenosis.
What Are the Clinical Implications?
Alternative markers of plaque may be used to improve diagnosis of vulnerable plaque and prevent future stroke.
Computed tomography (CT) and CT angiography (CTA) are often used in acute stroke decision‐making given their rapid evaluation for acute intracranial hemorrhage and arterial occlusion. Simultaneously performed neck CTA can detect large vessel atherosclerotic stroke sources. Currently, the only CTA variable used to detect a carotid plaque source is percent diameter stenosis based on stroke cause classification systems including TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria and extrapolated from NASCET (North American Symptomatic Carotid Endarterectomy Trial) and other trials. 1 , 2
More recently, carotid stroke sources were found to be more highly associated with vulnerable plaque components including magnetic resonance imaging (MRI)–detected carotid intraplaque hemorrhage (IPH). 3 IPH has long been known to be important in stroke risk, 4 , 5 but MRI detection only became possible 10 years ago. 6 Newer heavily T1‐weighted sequences including the magnetization‐prepared rapid acquisition gradient echo (MPRAGE) sequence can be used to accurately detect IPH separate from lipid and necrosis, and outperforms other T1‐weighted sequences and 3‐dimensional time‐of‐flight. 7 , 8 Three separate meta‐analyses have found that patients with symptomatic carotid IPH have a high annual stroke risk of 15% to 45% despite medical therapy. 9 , 10 , 11
Because carotid MRI has inherent limitations of lengthy acquisition times and patient contraindications, there is a critical need to optimize CTA prediction models of carotid plaque features associated with ipsilateral stroke. Initial attempts at linking plaque density with IPH were limited by overlapping Hounsfield units for IPH with other plaque components, including lipid‐rich and necrotic elements. 12 CTA markers linked to IPH using univariable analysis include stenosis, maximum plaque thickness (specifically soft plaque), 13 ulceration 12 and calcification. 14 These are in addition to clinical factors including age, male sex, and hypertension. 15 , 16 , 17 , 18 Of these, IPH is most highly linked to maximum soft plaque thickness. 13 Along with soft plaque, the addition of thin adventitial calcification (a “rim sign”) on CTA is highly predictive of IPH. 17
Given that CTA can predict IPH with some likelihood, our goal was to determine the optimal combination of CTA markers associated with ipsilateral stroke. Our hypothesis was that CTA markers of carotid IPH, specifically soft plaque thickness and a rim sign, would be required for optimal discrimination. In this retrospective study, we evaluated patients undergoing potential stroke workup with CTA neck and MRI brain within 1 month of each other from 2009 to 2016 and used multivariable Poisson regression to determine essential CTA markers of carotid‐source stroke, and compared these methods with traditional lumen stenosis.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Clinical Study Design
Institutional review board approval was obtained before this retrospective cohort study from 2009 to 2016 in patients undergoing acute stroke workup at 2 facilities, the University Medical Center and the VA Medical Center. Informed consent was waived by the institutional review board. The only inclusion criteria were patients undergoing stroke workup who had both carotid CTA and brain MRI within a 1‐month period of each other. A total of 950 patients met inclusion criteria.
Exclusions were made to eliminate alternate stroke sources after review of electronic medical records. A total of 696 patients were excluded for the following reasons: cardioembolic sources (235; eg, atrial fibrillation), vasculopathy (98; eg, dissection and fibromuscular dysplasia), posterior circulation strokes (88), intracranial large and small vessel disease (96; eg, intracranial atherosclerosis with >50% stenosis, isolated perforator distribution stroke, and vasculitis), hypercoagulable states (76; eg, antiphospholipid syndrome), ischemic stroke mimics (41; eg, multiple sclerosis and complex migraine headache), proximal vascular disease (3; eg, aortic arch thrombus), iatrogenic stroke (17; eg, postcardiothoracic surgery), drugs of abuse (10; eg, methamphetamine use), head/neck tumor (21), and intracranial hemorrhage (11). A total of 254 patients remained in the analysis, contributing 508 carotid arteries (Figure 1). Unilateral carotid occlusions (11), near‐occlusions (2), and previously stented carotid arteries (1) were excluded because CTA markers could not easily be assessed. In addition, no patients with recent carotid endarterectomy were included in this study. A total of 494 carotid artery and ipsilateral brain pairs (508–14=494) remained for final analysis.
Figure 1. Flow diagram detailing the selection process for the final included analysis sample.
CTA indicates computed tomography angiography; and MRI, magnetic resonance imaging.
CTA Imaging, Markers, and Reviewers
CTA was performed per institutional protocol as previously described. 17 CTA markers included the time between MRI and CTA, rim sign present or absent, NASCET percent diameter stenosis, mm‐stenosis, maximum plaque thickness (soft and hard), ulceration, and intraluminal thrombus as previously defined. 17 CTA markers were determined by reviewers blinded to stroke presence and distribution, as well as stroke status and clinical covariates. These reviewers had experience with neurovascular imaging interpretation and included 2 radiology residents (L.B.E. and P.J.H.) and a board‐certified neuroradiology attending physician (J.S.M.). Carotid measurements were obtained using a picture archiving and communication system workstation, multiplanar reformats, and a sub‐mm measurement tool. Briefly, both resident reviewers underwent specific training in detecting CTA features and had experience with neurovascular imaging interpretation. On study initiation, the carotid imaging markers were determined by the 2 residents. In cases of disagreement, consensus was obtained with the neuroradiology attending. Kappa analysis was previously performed to determine the rim sign interrater reliability (0.85) and intrarater reliability (0.86). 17
Ipsilateral Ischemic Stroke Determination
Ischemic stroke was determined using the American Heart Association definition of infarction as previously described. 19 Briefly, ipsilateral ischemic stroke was defined by brain infarction or retinal cell death based on: (1) imaging evidence of cerebral or retinal ischemia in the carotid distribution; or (2) clinical symptoms persisting ≥24 hours, with other causes excluded. 20 Stroke imaging used the diffusion‐weighted imaging (DWI) technique derived from diffusion tensor imaging trace images from our standard clinical imaging protocol, as previously described. 21 Diffusion tensor imaging trace images have been shown to be superior to conventional diffusion‐weighted sequences in detecting recent infarcts. 22 Briefly, diffusion tensor imaging parameters were 2‐dimensional, 128×128 matrix, 3‐mm slice thickness, B value=2000, and 20 directions. Although the referring clinician suspected a possible recent infarct, only objective DWI images were used to determine whether a recent infarct was present. DWI positivity was defined as hyperintense signal on diffusion tensor imaging trace corresponding to a recent infarct at the time of the scan. 23 , 24 Only DWI‐positive embolic infarcts in the ipsilateral internal carotid artery territory were placed in the DWI‐positive category for carotid‐source strokes after excluding other stroke sources under our clinical study design. Embolic infarcts were defined as those involving the cortex or subcortical white matter, whereas microvascular infarcts were defined as those involving only the basal ganglia or adjacent white matter. DWI images were interpreted by a subspecialty trained, certificate of added qualification–certified neuroradiologist blinded to carotid imaging.
Clinical Demographics
Clinical demographics and carotid atherosclerosis risk factors were determined by chart review. These included age, male sex, diabetes mellitus, hypertension, hyperlipidemia, and smoking status, and were determined by standard clinical definitions as previously described. 17 For hypertension, the diagnosis was made when the average of ≥2 diastolic blood pressure measurements on at least 2 subsequent visits was ≥90 mm Hg or when the average of multiple systolic blood pressure readings on ≥2 subsequent visits was ≥140 mm Hg. For hyperlipidemia, the diagnosis was made when low‐density lipoprotein was >100 mg/dL. Cerebrovascular medications were also recorded, including antiplatelet, anticoagulant, statin, and antihypertensive medications.
Statistical Analysis
To account for up to 2 carotid arteries per patient, generalized estimating equations were used. Carotid arteries were treated as separate units grouped within each patient. This is because stroke may be associated with local markers of carotid plaque vulnerability (eg, stenosis), as well as systemic clinical factors affecting both carotid arteries (eg, age). Given that >1 marker for stroke was being studied, confounding was investigated on the outcome variable (ipsilateral stroke), therefore only 1 data table was required, with P values from univariable generalized estimating equation Poisson regression models. The Poisson regression approach directly estimates the prevalence ratio, which is more intuitive than the odds ratio obtained from logistic regression. 25 Next, all potential confounding variables with P<0.20 from the univariable model were placed in the initial multivariable generalized estimating equation Poisson regression model for stroke. This was followed by backwards elimination until all remaining variables met the threshold P<0.10. The significance criteria of P<0.10 was used to protect against residual confounding. 25
For hypothesis testing of markers predictive of ipsilateral ischemic stroke, we used the traditional P<0.05. It is widely accepted that in binary models, 10 outcome events for every predictor variable are sufficient to avoid overfitting. With 108 ipsilateral stroke events and 384 nonstroke events, 108/10 or up to 11 predictor variables could be included in the model without overfitting, which is more than remaining variables in our final model. To assess the discriminatory potential of the final model and each marker, we reported clustered data area under the receiver operating characteristic curve (AUC), with bootstrapped 95% CIs. 26 To guard against overfitting and optimism of the AUCs, in which an AUC could be higher in the present sample of patients than it would in future patients, we performed a bootstrap validation for each clustered data AUC calculation on the fixed list of predictors in the model. 27 In all cases, the optimism was <0.2%, and the original AUCs and bootstrapped‐validated AUCs were identical to the precision reported. Therefore, there was no need to report both. All statistical analyses were performed with STATA 14 statistical software (StataCorp LLC).
Results
Clinical Characteristics
Two‐hundred fifty‐four patients were included in the final analysis. Patients were predominantly older men (mean age, 63.5 years; 61.8% men) with carotid atherosclerosis risk factors (47.7% were current or prior smokers, 61.0% had hypertension, 48.4% had hyperlipidemia, and 26.8% had diabetes mellitus), and many were on medical treatment (50.0% taking antihypertensives, 41.7% taking statins, and 42.5% taking antiplatelets) (Table 1).
Table 1.
Clinical Characteristics
| Clinical Characteristics |
Patients n=254 |
|---|---|
| Age, mean (SD), y | 63.5 (15.0) |
| Male sex, n (%) | 157 (61.8) |
| Non‐White, n (%) | 75 (15.2) |
| Smoking, n (%) | |
| Current smoker | 52 (21.7) |
| Prior smoker | 66 (26.0) |
| Hypertension, n (%) | 155 (61.0) |
| Hyperlipidemia, n (%) | 123 (48.4) |
| Diabetes mellitus, n (%) | 68 (26.8) |
| Antihypertension, n (%) | 127 (50.0) |
| Statin, n (%) | 106 (41.7) |
| Antiplatelet, n (%) | 108 (42.5) |
| Anticoagulation, n (%) | 9 (3.5) |
| Time between MRI and CTA in d, n (SD) | −0.1 (7.9) |
CTA indicates computed tomography angiography.
Imaging and Clinical Characteristics by Vessel
We evaluated imaging and clinical characteristics by vessel, in groups positive and negative for stroke in Table 2. There were 108 ipsilateral strokes. An example of a patient with a positive rim sign and measurements of stenosis and plaque thickness is shown in Figure 2. Each patient contributed up to 2 ipsilateral carotid‐brain pairs (494 total). The degree of luminal stenosis was higher in the stroke versus nonstroke group (mean NASCET stenosis of 38.0% versus 11.6% and mm‐stenosis of 2.94 versus 4.08 mm, P<0.001). A slight majority of strokes occurred in the mild stenosis group, with 57.4% of strokes in the <50% group compared with 42.6% of strokes in the ≥50% stenosis group. Maximum total plaque thickness was higher in the stroke versus the nonstroke group (4.37 versus 2.32 mm, P<0.001), as was maximum soft‐plaque thickness (3.94 versus 2.06 mm, P<0.001) and maximum hard‐plaque thickness (2.28 versus 1.47 mm, P<0.001). There was a higher prevalence of plaque ulceration (47.2% versus 17.9%, P<0.001) and intraluminal thrombus (13.0% versus 0.3%, P<0.001), although intraluminal thrombus was rare. The carotid rim sign was also highly prevalent in the stroke versus the nonstroke groups (50.9% versus 11.1%, P<0.001). Many clinical confounders were also more prevalent in stroke, including male sex, age, hypertension, hyperlipidemia, diabetes mellitus, and cardiovascular medications. Factors with P<0.20 were considered potential confounders between groups positive and negative for stroke, requiring multivariable regression.
Table 2.
Imaging and Clinical Characteristics by Vessel
| Imaging and Clinical Characteristics by ipsilateral Carotid‐Brain Pair |
Stroke (−) n=386 |
Stroke (+) n=108 |
PR | P Value |
|---|---|---|---|---|
| Carotid NASCET percent stenosis, mean (SD) | 11.6 (21.0) | 38.0 (31.7) | 9.3 | <0.001 |
| Carotid NASCET stenosis category | ||||
| Mild (0%–49.9%), n (%) | 354 (91.7) | 62 (57.4) | 9.5 | <0.001 |
| Moderate (50%–69.9%), n (%) | 21 (5.5) | 23 (21.3) | ||
| Severe (70%–99.9%), n (%) | 11 (2.9) | 23 (21.3) | ||
| Carotid mm stenosis, mean (SD) | 4.08 (1.05) | 2.94 (1.55) | 0.6 | <0.001 |
| Carotid maximum total plaque thickness, mean (SD), mm | 2.32 (1.89) | 4.37 (2.26) | 1.4 | <0.001 |
| Maximum soft plaque thickness, mean (SD), mm | 2.06 (1.69) | 3.94 (2.03) | 1.4 | <0.001 |
| Maximum hard plaque thickness, mean (SD), mm | 1.47 (1.43) | 2.28 (1.69) | 1.3 | <0.001 |
| Carotid plaque ulceration, n (%) | 69 (17.9) | 51 (47.2) | 2.8 | <0.001 |
| Carotid intraluminal thrombus, n (%) | 1 (0.3) | 14 (13.0) | 4.7 | <0.001 |
| Carotid rim sign, n (%) | 43 (11.1) | 55 (50.9) | 4.1 | <0.001 |
| Time between MRI and CTA in d, n (SD) | −0.1 (8.5) | 0.0 (5.0) | 1.0 | 0.972 |
| Male sex, n (%) | 224 (58.0) | 80 (74.1) | 1.8 | 0.005 |
| Age, mean (SD), y | 62.4 (15.6) | 66.8 (13.0) | 1.0 | 0.013 |
| Non‐White, n (%) | 59 (15.3) | 16 (14.8) | 1.0 | 0.915 |
| Smoking, n (%) | ||||
| Current smoker | 78 (20.2) | 27 (25.0) | 1.2 | 0.321 |
| Prior smoker | 101 (26.2) | 25 (23.2) | 0.9 | 0.560 |
| Hypertension, n (%) | 222 (57.5) | 76 (70.4) | 1.6 | 0.025 |
| Hyperlipidemia, n (%) | 169 (43.8) | 69 (63.9) | 1.9 | 0.001 |
| Diabetes mellitus, n (%) | 91 (23.6) | 40 (37.0) | 1.6 | 0.010 |
| Antihypertensive medications, n (%) | 182 (47.2) | 61 (56.5) | 1.3 | 0.112 |
| Statin, n (%) | 142 (36.8) | 57 (52.8) | 1.7 | 0.005 |
| Antiplatelet, n (%) | 154 (39.9) | 51 (47.2) | 1.3 | 0.211 |
| Anticoagulation, n (%) | 13 (3.4) | 4 (3.7) | 1.1 | 0.868 |
From the 254 patients, 494 carotid arteries were analyzed after excluding occlusions (11), near occlusions (2) and stented carotid arteries (1). Mean/SDs were calculated using ordinary formulas. Significance tests and P values were based on univariable GEE Poisson regression taking into account the correlation of up to 2 carotids per person. Factors with P<0.20 were included in the initial multivariable Poisson regression analysis prior to backwards elimination.
CTA, computed tomography angiography; NASCET, North American Symptomatic Carotid Endarterectomy Trial; and PR, prevalence ratio.
Figure 2. Computed tomography (CT) angiography carotid imaging markers and stroke workup.
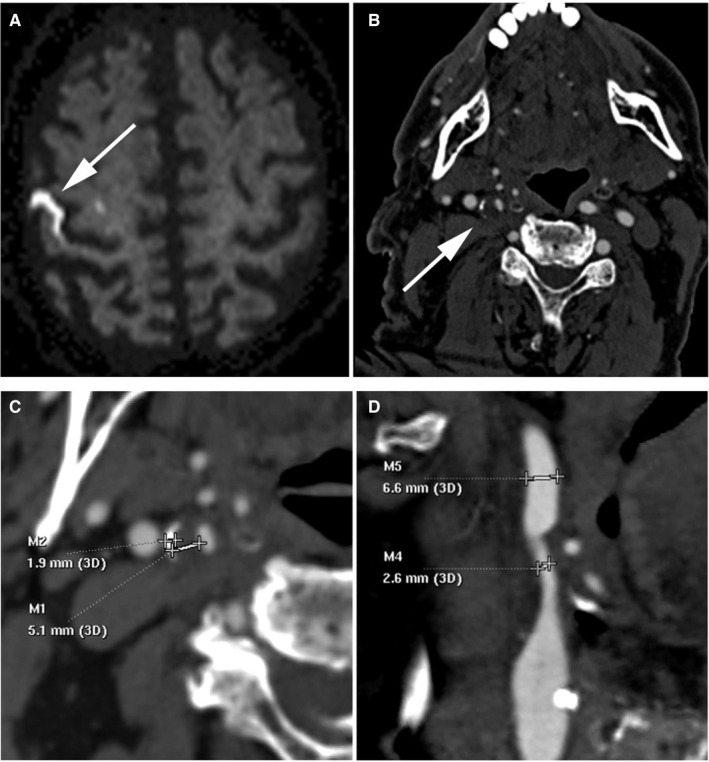
This 83‐year‐old man presented with abrupt onset left‐sided weakness and numbness with an acute infarct in the right middle cerebral artery distribution on diffusion‐weighted imaging (A). CT angiography (CTA) of the carotid arteries demonstrated a thick right carotid bifurcation/proximal internal carotid artery plaque with a positive rim sign (B), consisting of thin peripheral adventitial calcification (<2 mm) and internal soft plaque (≥2 mm). Maximum soft plaque thickness measured 5.1 mm (M1) and maximum hard plaque thickness measured 1.9 mm (M2) using the picture archiving and communication system sub‐mm measurement tool (C). NASCET (North American Symptomatic Carotid Endarterectomy Trial) percent diameter stenosis measured [(6.6–2.6)/6.6]×100%=61%, and mm‐stenosis measured 2.6 mm on multiplanar reformats (D). No other CTA markers were present (eg, no ulceration or intraluminal thrombus).
Multivariable Generalized Estimating Equation Poisson Regression Analysis
Multivariable regression analysis was performed with the outcome of ipsilateral stroke. Potential confounders were eliminated in a backward fashion with a threshold of P<0.10. The final model for ipsilateral stroke prediction included the following CTA markers: the presence of intraluminal thrombus (prevalence ratio=2.8, P<0.001; 95% CI, 1.6–4.9), maximum soft plaque thickness (for each mm increase in thickness, prevalence ratio=1.2, P<0.001; 95% CI, 1.1–1.4), and rim sign (prevalence ratio=2.0, P=0.007; 95% CI, 1.2–3.3) (Table 3). NASCET stenosis, ulceration, and all other markers were eliminated with P>0.10. In addition, each measurement of stenosis (mm‐stenosis, NASCET categories, NASCET continuous) was eliminated from the final multivariable model after backwards elimination to the P<0.10 level, if either placed together or separately in the initial model.
Table 3.
Final Stroke Prediction Model
| Vulnerable Carotid Plaque (ipsilateral Stroke) Predictor | PR | P Value | 95% CI | |
|---|---|---|---|---|
| Intraluminal thrombus | 2.8 | <0.001 | 1.6 | 4.9 |
| Maximal soft plaque thickness (per each mm) | 1.2 | <0.001 | 1.1 | 1.4 |
| Positive rim sign | 2.0 | 0.007 | 1.2 | 3.3 |
The final stroke prediction model depended on 3 factors with P<0.10: intraluminal thrombus, maximum soft plaque thickness and a positive rim sign.
PR indicates prevalence ratio.
Ipsilateral Stroke Receiver Operating Characteristic Comparison Analysis
Receiver operating characteristic comparison analysis for ipsilateral stroke is shown in Figure 3. The discriminatory value of our final model for stroke was an AUC of 78.3%. This was significantly higher than each marker alone, including intraluminal thrombus (56.4%, P<0.001), maximum soft plaque thickness (76.4%, P=0.007), and rim sign alone (69.9%, P=0.001). NASCET stenosis categories (cutoffs of 50% and 70%) had significantly lower discrimination for stroke (AUC=67.4%, P<0.001) compared with our final model, and the rim sign added significant discrimination to these categories (AUC=74.3%, P=0.003). Furthermore, the continuous NASCET measurement had significantly lower discrimination for stroke (AUC=73.8%, P=0.03) compared with our final model.
Figure 3. Receiver operating characteristic comparison analysis demonstrates the superiority of the final model and the rim sign in predicting ipsilateral stroke.
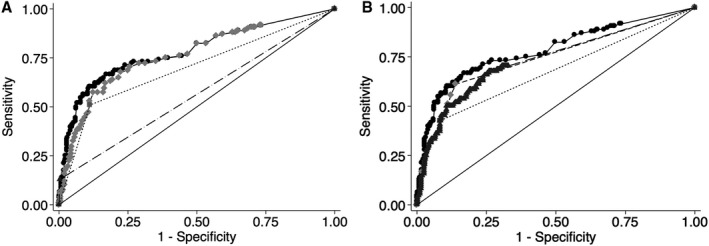
A, Final model versus singular components: the discriminatory value of our final model for stroke was an area under the receiver operating characteristic curve (AUC) of 78.3 %, significantly higher than each plaque component alone: intraluminal thrombus (56.4%, P<0.001), maximum soft plaque thickness (76.4%, P=0.007), and rim sign alone (69.9%, P=0.001). Final model: solid circles, solid line; intraluminal thrombus: black triangle, dashed line; maximum soft plaque thickness: light gray diamonds, dashed line; rim sign: gray circle, dotted line. B, Final model, rim sign, NASCET (North American Symptomatic Carotid Endarterectomy Trial) categories, and continuous measurement: our final model (AUC=78.3%) has significantly higher stroke source discrimination compared with traditional NASCET stenosis cutoffs of 50% and 70% (AUC=67.4%, P<0.001), and the rim sign added significant discrimination to these categories (AUC=74.3%, P=0.003). Furthermore, the continuous NASCET measurement had significantly lower discrimination for stroke (AUC=73.8%, P=0.03) compared with our final model. Final model: solid circles, solid line; rim sign + NASCET categories: light gray diamonds, dashed line; NASCET categories: dark gray square, dotted line. Continuous NASCET: dark gray triangles, dashed line.
Discussion
Carotid plaque features are critical determinants of stroke. For more than 30 years, stenosis has been the primary determinant of carotid stroke potential based on data from NASCET and other trials and stratification criteria including TOAST. 1 , 2 Increasing evidence suggests that plaque features, many of which can be detected on MRI, ultrasonography, and CTA are more strongly associated with stroke than luminal stenosis. 3 In our cross‐sectional evaluation of patients undergoing stroke workup, we found that specific imaging markers on CTA, including soft plaque thickness, an adventitial rim sign, and intraluminal thrombus, were significantly associated with downstream acute infarction and that considering these imaging markers were more predictive of stroke than degree of stenosis.
The inclusion of soft plaque thickness in the prediction of carotid sources of stroke corroborates prior research. Studies have shown that plaque thickness indicates a more vulnerable plaque phenotype, with increased plaque thickness associated with MPRAGE‐detected IPH. 16 , 17 Importantly, plaque thickness and plaque area have been associated with IPH, and this association is enhanced in the setting of low vitamin D. 19 This may be related to a larger lipid core, higher microvessel density, and higher potential for intraplaque hemorrhage. Alternatively, increased plaque thickness may be secondary to bouts of microvessel leakage, since IPH accelerates plaque growth and stenosis. 28
The importance of the rim sign in our final stroke‐prediction model fits with its high association with carotid IPH. 17 Thin peripheral calcification may be a marker of chronic adventitial inflammation, and adventitial microvessel leakage has been implicated in carotid IPH. 29 Adventitial inflammation has also been linked to endothelial bone morphogenic protein‐4 production, which may stimulate calcification. 30 In addition, activation of the angiotensin system in animal models results in IPH, 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 and low vitamin D levels correspond to increased IPH in humans. 19 Both the angiotensin system and vitamin D play a central role in calcium homeostasis, and aberrations in these pathways may lead to carotid IPH and adventitial calcification. 39
Intraluminal thrombus, although rare, was highly associated with acute ipsilateral stroke in the current study, affirming previous data. 3 The rarity of intraluminal thrombus in the setting of carotid‐source stroke is somewhat puzzling. Intraluminal thrombi may still exist below CTA detection limits or the entire clot may have embolized during the stroke. Catheter‐directed optical coherence tomography has detected small adherent thrombi in patients with stroke, and similar higher‐resolution techniques may increase sensitivity for culprit plaques. 40
Overall, these findings highlight the ability of CTA to identify plaque features that are strongly associated with cerebrovascular ischemia. Although the AUC of the CTA final model (78.3%) is slightly lower than that obtained with MRI‐IPH (86.2%), 3 our findings suggest that routine CTA can still provide robust carotid plaque assessment, which is highly correlated with cerebrovascular ischemia. Although much attention is focused on MRI‐based plaque assessment, CTA appears to be a powerful tool in evaluating carotid stroke sources. 41
Furthermore, these data question the sole use of stenosis to identify stroke sources and suggest that stroke causes criteria should be revised. A threshold of ≥50% stenosis has been used for years as part of the TOAST criteria in identifying potential large artery stroke sources. 1 Our current data suggest that a slight majority (57.4%) of carotid stroke sources have <50% stenosis by NASCET criteria. Furthermore, the AUC using NASCET stenosis cutoffs was significantly lower (67.4%) than our final model (78.3%), suggesting that CTA‐identified plaque characteristics have improved discrimination compared with stenosis alone. This highlights the importance of detailed carotid plaque evaluation, even in cases of nonstenosing plaque.
While this study lacks information on future stroke risk, the cross‐sectional nature has the important advantage of evaluating plaque features in close proximity to stroke onset when determination of stroke sources is paramount. A key next step is to prospectively examine these CTA markers and their associated recurrent stroke risk and determine which markers better stratify patients for endarterectomy versus specific medical treatment regimens. Another potential limitation is that recruitment was limited to patients undergoing stroke workup at 1 of 2 local centers. Further confirmation of these results is warranted by other centers with regional differences.
Determining stroke cause is critically important in future stroke prevention by directing appropriate treatment. In the setting of ipsilateral embolic stroke, CTA identification of carotid intraluminal thrombus, high soft plaque thickness, or a rim sign suggests a carotid plaque stroke source is likely and further imaging tests may not add further information. The majority of carotid‐source strokes occur in patients with <50% carotid stenosis, arguing against stroke source identification using stenosis alone. Instead, these alternative markers may allow clinicians to better diagnose vulnerable carotid plaque and guide treatment decisions, including stratification to medical therapy or surgery.
Conclusions
Vulnerable carotid plaque stroke sources can be identified with CTA features including intraluminal thrombus, maximum soft plaque thickness, and a rim of adventitial calcification. These markers can identify vulnerable plaque and potential stroke sources otherwise ignored by stenosis.
Sources of Funding
This work was supported by a Radiological Society of North America Research Scholar Grant, General Electric Radiology Research Academic Fellowship, and a grant for the Study Design and Biostatistics Center, with funding, in part, from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, grant 8UL1TR000105 (formerly UL1RR025764). American Heart Association grant number 17SDG33460420.
Disclosures
None.
(J Am Heart Assoc. 2021;10:e019462. DOI: 10.1161/JAHA.120.019462.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. DOI: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 2. North American Symptomatic Carotid Endarterectomy Trial C . Beneficial effect of carotid endarterectomy in symptomatic patients with high‐grade carotid stenosis. New Engl J Med. 1991;325:445–453. [DOI] [PubMed] [Google Scholar]
- 3. McNally JS, McLaughlin MS, Hinckley PJ, Treiman SM, Stoddard GJ, Parker DL, Treiman GS. Intraluminal thrombus, intraplaque hemorrhage, plaque thickness, and current smoking optimally predict carotid stroke. Stroke; a journal of cerebral circulation. 2015;46:84–90. DOI: 10.1161/STROKEAHA.114.006286. [DOI] [PubMed] [Google Scholar]
- 4. Sadoshima S, Fukushima T, Tanaka K. Cerebral artery thrombosis and intramural hemorrhage. Stroke. 1979;10:411–414. DOI: 10.1161/01.STR.10.4.411. [DOI] [PubMed] [Google Scholar]
- 5. Lusby RJ, Ferrell LD, Ehrenfeld WK, Stoney RJ, Wylie EJ. Carotid plaque hemorrhage. Its role in production of cerebral ischemia. Arch Surg. 1982;117:1479–1488. DOI: 10.1001/archsurg.1982.01380350069010. [DOI] [PubMed] [Google Scholar]
- 6. Moody AR, Murphy RE, Morgan PS, Martel AL, Delay GS, Allder S, MacSweeney ST, Tennant WG, Gladman J, Lowe J, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation. 2003;107:3047–3052. DOI: 10.1161/01.CIR.0000074222.61572.44. [DOI] [PubMed] [Google Scholar]
- 7. Ota H, Yarnykh VL, Ferguson MS, Underhill HR, DeMarco JK, Zhu DC, Oikawa M, Dong LI, Zhao X, Collar A, et al. Carotid intraplaque hemorrhage imaging at 3.0‐t mr imaging: comparison of the diagnostic performance of three t1‐weighted sequences. Radiology. 2010;254:551–563. DOI: 10.1148/radiol.09090535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McNally JS, Yoon HC, Kim SE, Narra KK, McLaughlin MS, Parker DL, Treiman GS. Carotid MRI detection of intraplaque hemorrhage at 3t and 1.5t. J Neuro. 2015;25:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, Dunning A, Mushlin AI, Sanelli PC. Carotid plaque MRI and stroke risk: a systematic review and meta‐analysis. Stroke. 2013;44:3071–3077. DOI: 10.1161/STROKEAHA.113.002551. [DOI] [PubMed] [Google Scholar]
- 10. Hosseini AA, Kandiyil N, Macsweeney ST, Altaf N, Auer DP. Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke. Ann Neurol. 2013;73:774–784. DOI: 10.1002/ana.23876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saam T, Hetterich H, Hoffmann V, Yuan C, Dichgans M, Poppert H, Koeppel T, Hoffmann U, Reiser MF, Bamberg F. Meta‐analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62:1081–1091. DOI: 10.1016/j.jacc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 12. Uk‐I JM, Fox AJ, Aviv RI, Howard P, Yeung R, Moody AR, Symons SP. Characterization of carotid plaque hemorrhage: a CT angiography and mr intraplaque hemorrhage study. Stroke. 2010;41:1623–1629. DOI: 10.1161/STROKEAHA.110.579474. [DOI] [PubMed] [Google Scholar]
- 13. Gupta A, Baradaran H, Mtui EE, Kamel H, Pandya A, Giambrone A, Iadecola C, Sanelli PC. Detection of symptomatic carotid plaque using source data from MR and CT angiography: a correlative study. Cerebrovasc Dis. 2015;39:151–161. DOI: 10.1159/000373918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van den Bouwhuijsen QJ, Bos D, Ikram MA, Hofman A, Krestin GP, Franco OH, van der Lugt A, Vernooij MW. Coexistence of calcification, intraplaque hemorrhage and lipid core within the asymptomatic atherosclerotic carotid plaque: the rotterdam study. Cerebrovasc Dis. 2015;39:319–324. DOI: 10.1159/000381138. [DOI] [PubMed] [Google Scholar]
- 15. Cheung HM, Moody AR, Singh N, Bitar R, Zhan J, Leung G. Late stage complicated atheroma in low‐grade stenotic carotid disease: Mr imaging depiction–prevalence and risk factors. Radiology. 2011;260:841–847. DOI: 10.1148/radiol.11101652. [DOI] [PubMed] [Google Scholar]
- 16. McLaughlin A, Hinckley PJ, Treiman SM, Kim SE, Stoddard GJ, Parker DL, Treiman GS, McNally JS. Optimal prediction of carotid intraplaque hemorrhage using clinical and lumen imaging markers. AJNR Am J Neuroradiol. 2015;36:2360–2366. DOI: 10.3174/ajnr.A4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eisenmenger LB, Aldred BW, Kim SE, Stoddard GJ, de Havenon A, Treiman GS, Parker DL, McNally JS. Prediction of carotid intraplaque hemorrhage using adventitial calcification and plaque thickness on cta. AJNR Am J Neuroradiol. 2016;37:1496–1503. DOI: 10.3174/ajnr.A4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun J, Canton G, Balu N, Hippe DS, Xu D, Liu J, Hatsukami TS, Yuan C. Blood pressure is a major modifiable risk factor implicated in pathogenesis of intraplaque hemorrhage: an in vivo magnetic resonance imaging study. Arterioscler Thromb Vasc Biol. 2016;36:743–749. DOI: 10.1161/ATVBAHA.115.307043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McNally JS, Burton TM, Aldred BW, Kim S‐E, McLaughlin MS, Eisenmenger LB, Stoddard GJ, Majersik JJ, Miller DV, Treiman GS, et al. Vitamin d and vulnerable carotid plaque. AJNR Am J Neuroradiol. 2016;37:2092–2099. DOI: 10.3174/ajnr.A4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ(, Culebras A, Elkind MSV, George MG, Hamdan AD, Higashida RT, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2013;44:2064–2089. DOI: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McNally JS, Kim SE, Yoon HC, Findeiss LK, Roberts JA, Nightingale DR, Narra KK, Parker DL, Treiman GS. Carotid magnetization‐prepared rapid acquisition with gradient‐echo signal is associated with acute territorial cerebral ischemic events detected by diffusion‐weighted mri. Circ Cardiovasc Imaging. 2012;5:376–382. DOI: 10.1161/CIRCIMAGING.111.967398. [DOI] [PubMed] [Google Scholar]
- 22. Chou MC, Tzeng WS, Chung HW, Wang CY, Liu HS, Juan CJ, Lo CP, Hsueh CJ, Chen CY. T2‐enhanced tensor diffusion trace‐weighted image in the detection of hyper‐acute cerebral infarction: comparison with isotropic diffusion‐weighted image. Eur J Radiol. 2010;74:e89–94. DOI: 10.1016/j.ejrad.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 23. Lansberg MG, Thijs VN, O'Brien MW, Ali JO, de Crespigny AJ, Tong DC, Moseley ME, Albers GW. Evolution of apparent diffusion coefficient, diffusion‐weighted, and t2‐weighted signal intensity of acute stroke. AJNR Am J Neuroradiol. 2001;22:637–644. [PMC free article] [PubMed] [Google Scholar]
- 24. Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion‐weighted magnetic resonance imaging. Ann Neurol. 1995;37:231–241. [DOI] [PubMed] [Google Scholar]
- 25. Zou GY, Donner A. Extension of the modified poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22:661–670. DOI: 10.1177/0962280211427759. [DOI] [PubMed] [Google Scholar]
- 26. Pepe M, Longton G, Janes H. Estimation and comparison of receiver operating characteristic curves. Stata J. 2009;9:1. DOI: 10.1177/1536867X0900900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. DOI: . [DOI] [PubMed] [Google Scholar]
- 28. Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, Isaac C, McDonough J, Natiello C, Small R, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high‐resolution magnetic resonance imaging study. Circulation. 2005;111:2768–2775. DOI: 10.1161/CIRCULATIONAHA.104.504167. [DOI] [PubMed] [Google Scholar]
- 29. Sun J, Song Y, Chen H, Kerwin WS, Hippe DS, Dong L, Chen M, Zhou C, Hatsukami TS, Yuan C. Adventitial perfusion and intraplaque hemorrhage: a dynamic contrast‐enhanced mri study in the carotid artery. Stroke. 2013;44:1031–1036. DOI: 10.1161/STROKEAHA.111.000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chemistry. 2003;278:31128–31135. DOI: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 31. Skultetyova D, Filipova S, Riecansky I, Skultety J. The role of angiotensin type 1 receptor in inflammation and endothelial dysfunction. Recent Pat Cardiovasc Drug Discov. 2007;2:23–27. DOI: 10.2174/157489007779606130. [DOI] [PubMed] [Google Scholar]
- 32. da Cunha V, Martin‐McNulty B, Vincelette J, Choy DF, Li W‐W, Schroeder M, Mahmoudi M, Halks‐Miller M, Wilson DW, Vergona R, et al. Angiotensin ii induces histomorphologic features of unstable plaque in a murine model of accelerated atherosclerosis. J Vasc Surg. 2006;44:364–371. 10.1016/j.jvs.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 33. Lee BS, Choi JY, Kim JY, Han SH, Park JE. Simvastatin and losartan differentially and synergistically inhibit atherosclerosis in apolipoprotein e(‐/‐) mice. Korean Circ J. 2012;42:543–550. DOI: 10.4070/kcj.2012.42.8.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Negro R. Endothelial effects of antihypertensive treatment: focus on irbesartan. Vasc Health Risk Manag. 2008;4:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Regoli D, Plante GE, Gobeil F Jr. Impact of kinins in the treatment of cardiovascular diseases. Pharmacol Ther. 2012;135:94–111. DOI: 10.1016/j.pharmthera.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 36. Aghamohammadzadeh R, Heagerty AM. Obesity‐related hypertension: epidemiology, pathophysiology, treatments, and the contribution of perivascular adipose tissue. Ann Med. 2012;44(suppl 1):S74–84. DOI: 10.3109/07853890.2012.663928. [DOI] [PubMed] [Google Scholar]
- 37. Borghi C, Cicero AF. The role of irbesartan in the treatment of patients with hypertension: a comprehensive and practical review. High Blood Press Cardiovasc Prev. 2012;19:19–31. DOI: 10.2165/11632100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38. Ribatti D, Levi‐Schaffer F, Kovanen PT. Inflammatory angiogenesis in atherogenesis–a double‐edged sword. Ann Med. 2008;40:606–621. DOI: 10.1080/07853890802186913. [DOI] [PubMed] [Google Scholar]
- 39. Norman PE, Powell JT. Vitamin d and cardiovascular disease. Circ Res. 2014;114:379–393. DOI: 10.1161/CIRCRESAHA.113.301241. [DOI] [PubMed] [Google Scholar]
- 40. Yoshimura S, Kawasaki M, Yamada K, Enomoto Y, Egashira Y, Hattori A, Nishigaki K, Minatoguchi S, Iwama T. Visualization of internal carotid artery atherosclerotic plaques in symptomatic and asymptomatic patients: a comparison of optical coherence tomography and intravascular ultrasound. AJNR Am J Neuroradiol. 2012;33:308–313. DOI: 10.3174/ajnr.A2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baradaran H, Al‐Dasuqi K, Knight‐Greenfield A, Giambrone A, Delgado D, Ebani E, Kamel H, Gupta A. Association between carotid plaque features on cta and cerebrovascular ischemia: a systematic review and meta‐analysis. American Journal of Neuroradiology. 2017;38:2321–2326. DOI: 10.3174/ajnr.A5436. [DOI] [PMC free article] [PubMed] [Google Scholar]


