Abstract
Background
Patients with recurring heart failure (HF) following cardiac resynchronization therapy fare poorly. Their management is undecided. We tested remote hemodynamic‐guided pharmacotherapy.
Methods and Results
We evaluated cardiac resynchronization therapy subjects included in the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients) trial, which randomized patients with persistent New York Heart Association Class III symptoms and ≥1 HF hospitalization in the previous 12 months to remotely managed pulmonary artery (PA) pressure‐guided management (treatment) or usual HF care (control). Diuretics and/or vasodilators were adjusted conventionally in control and included remote PA pressure information in treatment. Annualized HF hospitalization rates, changes in PA pressures over time (analyzed by area under the curve), changes in medications, and quality of life (Minnesota Living with Heart Failure Questionnaire scores) were assessed. Patients who had cardiac resynchronization therapy (n=190, median implant duration 755 days) at enrollment had poor hemodynamic function (cardiac index 2.00±0.59 L/min per m2), high comorbidity burden (67% had secondary pulmonary hypertension, 61% had estimated glomerular filtration rate <60 mL/min per 1.73 m2), and poor Minnesota Living with Heart Failure Questionnaire scores (57±24). During 18 months randomized follow‐up, HF hospitalizations were 30% lower in treatment (n=91, 62 events, 0.46 events/patient‐year) versus control patients (n=99, 93 events, 0.68 events/patient‐year) (hazard ratio, 0.70; 95% CI, 0.51–0.96; P=0.028). Treatment patients had more medication up‐/down‐titrations (847 versus 346 in control, P<0.001), mean PA pressure reduction (area under the curve −413.2±123.5 versus 60.1±88.0 in control, P=0.002), and quality of life improvement (Minnesota Living with Heart Failure Questionnaire decreased −13.5±23 versus −4.9±24.8 in control, P=0.006).
Conclusions
Remote hemodynamic‐guided adjustment of medical therapies decreased PA pressures and the burden of HF symptoms and hospitalizations in patients with recurring Class III HF and hospitalizations, beyond the effect of cardiac resynchronization therapy.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00531661.
Keywords: cardiac resynchronization therapy, heart failure, hemodynamic, hemodynamic monitoring, pulmonary artery pressure, remote monitoring
Subject Categories: Catheter Ablation and Implantable Cardioverter-Defibrillator, Heart Failure
Nonstandard Abbreviations and Acronyms
- CRT
cardiac resynchronization therapy
- GDMT
guideline‐directed medical therapy
- PAP
pulmonary artery pressure
Clinical Perspective
What Is New?
Standard management strategies for heart failure recurring among patients treated with cardiac resynchronization therapy had no impact on chronically elevated cardiac filling pressures but medical therapy when guided by remote assessment of pulmonary artery pressure was effective and reduced heart failure events.
What Are the Clinical Implications?
Patients persisting with heart failure who had cardiac resynchronization therapy gain significant symptom improvement and suffer fewer hospitalizations when medical therapy is individualized and adjusted preemptively during remote monitoring of hemodynamic function with the goal of reducing pulmonary artery pressure.
Cardiac resynchronization therapy (CRT) is an important therapy in patients with heart failure (HF). 1 Patients responding favorably typically manifest suppression of HF within weeks of implant. 2 However, some of these may decompensate 2 to 3 years later. 3 Others present with HF soon after implant and continue with frequent hospitalizations. 4 Accompanying comorbidities may aggravate the frequency and severity of HF events (“comorbid HF”). 5 Generally, the recurrence and/or persistence of clinical HF signals poorer prognosis. 6 , 7 Prediction and prevention of HF by information gathered by remote monitoring have produced indifferent results. 8 There are no trial data or recommendations to guide management of patients who have had CRT. In practice they receive little care. 4 , 9
To address this deficit, the current study examined the clinical and hemodynamic characteristics of patients with CRT enrolled in the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association [NYHA] Class III Heart Failure Patients) trial as they were a closely studied group with persistent symptoms despite application of guideline‐directed medical therapies (GDMT) coupled with a history of hospitalization following CRT. 10 , 11 , 12 Based on these findings we then examined clinical and hemodynamic outcomes in the CRT group specifically addressing the question of whether pulmonary artery pressure (PAP)‐guided HF management was more effective in reducing decompensation events and lowering PAPs over time compared with standard management strategies in the control CRT group.
Methods
Study Design
The data, analytic methods, and study materials may be made available to other researchers for purposes of reproducing the results or replicating the procedure, following submission and review of a project proposal.
This was a post hoc analysis of 190 patients included in the CHAMPION trial who had received CRT implants an average of 874±684 (median 755) days before enrollment. Qualification for trial enrollment required persistent NYHA Class III symptoms and at least 1 HF hospitalization (HFH) in the prior 12 months despite maximally tolerated GDMT. Characteristics of enrolled patients with CRT are contrasted to others in Table 1. The design, primary results, and randomized access period results of the prospective, multicenter CHAMPION trial (Food and Drug Administration‐approved investigational device exemption trial, Clinicaltrials.gov NCT00531661) have been published previously. 10 , 11 , 12 , 13 , 14 The study complied with the Declaration of Helsinki, the locally appointed ethics committee approved the research protocol, and informed consent was obtained from subjects (or their legally authorized representatives). Patients with glomerular filtration rate <25 mL/min per 1.73 m2 and diuretic unresponsiveness were excluded from the CHAMPION trial.
Table 1.
Baseline Demographics of Patients With Versus Without CRT at Enrollment in CHAMPION
| CRT/CRT‐D (n=190) | No CRT/CRT‐D (n=360) | P Value* | |
|---|---|---|---|
| Demographics | |||
| Age, y | 63.8±12.3 (190) | 60.3±13.0 (360) | 0.0039 |
| Sex (% men) | 167 (87.9%) | 232 (64.4%) | <0.0001 |
| Race (% White) | 157 (82.6%) | 244 (67.8%) | 0.0002 |
| Laboratory assessments | |||
| Systolic blood pressure, mm Hg | 117±20 (190) | 125±22 (360) | 0.0001 |
| Heart rate, bpm | 73±11 (189) | 73±13 (360) | 0.2971 |
| Body mass index, kg/m2 | 29.7±6.1 (190) | 31.2±7.3 (360) | 0.0324 |
| Serum urea nitrogen, mg/dL | 31.9±19.0 (178) | 27.2±15.7 (337) | 0.0064 |
| Creatinine, mg/dL | 1.5±0.5 (190) | 1.3±0.4 (360) | <0.0001 |
| Glomerular filtration rate, mL/min per 1.73 m2 | 57.2±21.3 (190) | 63.1±23.4 (360) | 0.0047 |
| Hemodynamics | |||
| Ejection fraction (%) | 25±10 (189) | 31±15 (359) | <0.0001 |
| Cardiac output, L/min | 4.2±1.4 (189) | 4.6±1.5 (359) | 0.0011 |
| Cardiac index, L/min per m2 | 2.0±0.6 (189) | 2.2±0.6 (359) | <0.0001 |
| Pulmonary vascular resistance, Wood units | 2.8±1.9 (189) | 2.7±1.9 (359) | 0.5124 |
| PA mean pressure, mm Hg | 29.8±9.2 (190) | 29.1±10.4 (360) | 0.2136 |
| PA wedge pressure, mm Hg | 19.2±7.7 (190) | 17.7±8.2 (360) | 0.0165 |
| Medical history | |||
| Ischemic cardiomyopathy (%) | 134 (70.5%) | 198 (55.0%) | 0.0005 |
| Hypertension (%) | 138 (72.6%) | 289 (80.3%) | 0.0524 |
| Hyperlipidemia (%) | 153 (80.5%) | 269 (74.7%) | 0.1380 |
| Coronary artery disease (%) | 141 (74.2%) | 243 (67.5%) | 0.1181 |
| Myocardial infarction (%) | 101 (53.2%) | 170 (47.2%) | 0.2093 |
| Diabetes mellitus (%) | 82 (43.2%) | 187 (51.9%) | 0.0595 |
| Atrial tachycardia flutter/fibrillation (%) | 111 (58.4%) | 144 (40.0%) | <0.0001 |
| Chronic obstructive pulmonary disease (%) | 53 (27.9%) | 106 (29.4%) | 0.7668 |
| Treatment history | |||
| CRT‐D/iplantable cardioverter‐defibrillator implant (%) | 190 (100.0%) | 186 (51.7%) | <0.0001 |
| Angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker use (%) | 153 (80.5%) | 274 (76.1%) | 0.2819 |
| Beta blocker use (%) | 173 (91.1%) | 326 (90.6%) | 1.0000 |
CHAMPION indicates CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; CRT, cardiac resynchronization therapy; CRT‐D, cardiac resynchronization therapy with implantable cardioverter‐defibrillator; and PA, pulmonary artery.
P value testing patients with CRT/CRT‐D vs patients without CRT/CRT‐D obtained from exact Wilcoxon rank‐sum test for continuous measures and Fisher's exact test for categorical measures.
All patients initially underwent right heart catheterization evaluation with hemodynamic assessment and implantation of a PAP sensor. 10 , 11 , 12 Hemodynamic information from the implant procedure could be used in management of all patients enrolled. Following successful sensor implantation, study subjects were taught how to use the patient electronic unit to remotely interrogate the implanted sensor and upload PAP information daily. Patients were randomized either to a control group, whose HF syndromes were treated based on traditional clinical signs and symptoms as daily uploaded pressures were unavailable to investigators, or to a treatment group, for whom daily uploaded pressures were available to investigators to guide disease management. Medical management in both groups consisted of standard GDMT and traditional signs and symptoms, but long‐term management included remotely obtained PAP information only in the treatment group. In the treatment group, daily uploaded information was reviewed weekly by the investigator team, with general recommendation to adjust medical therapies with the goal of lowering PAPs to a target range (diastolic 8–20 mm Hg or PA mean 10–25 mm Hg). 10 , 11 , 12 and maintaining this level by adjusting diuretics or vasodilators. 15 During times of hemodynamic stability, investigators were instructed to ensure that all GDMT was delivered at recommended doses. All patients provided informed consent for the CHAMPION trial, and the protocol was reviewed and approved by the appropriate institutional review board at the 64 participating US clinical sites. The trial met all its primary safety and efficacy end points along with all secondary end points. 11 , 12 and the system received Food and Drug Administration approval in May 2014.
CRT Subgroup Characterization
Patients with CRT were compared with patients without CRT enrolled in CHAMPION using baseline hemodynamic and demographic information, modified Charlson Comorbidity Index and mortality rates over the course of the study. The Charlson Comorbidity Index was modified to include a history of ischemic cardiomyopathy and atrial arrhythmias. Each of these cardiovascular comorbidities was assigned a weight of 1 in the Charlson calculation methodology.
Hemodynamic Monitoring System
The CardioMEMS HF System (Abbott, Atlanta, GA) consists of a small permanently implanted microelectromechanical sensor disc fitted with nitinol loops at the polar ends of the sensor as described previously. 10 , 11 , 12 The sensor is active and empowered only during external interrogation using radio frequency energy. This system does not require a lead or battery for long‐term function. The Patient Electronics Unit consists of the interrogation antenna embedded in a pad that encourages patients to consistently acquire daily pressures in a supine body position. PAP and heart rate data were encrypted, transmitted to a secured study website, and displayed graphically for investigator review.
End Points
The primary end point of HFH rates was assessed after all patients completed 6 months of follow‐up. 10 However, subjects remained in their randomized study group until the last‐enrolled patient completed at least 6 months of study follow‐up. This “randomized access period” encompasses a significantly longer clinical trial experience, with an average of 18 months follow‐up equivalent to ≈797 patient‐years. 12 To assess the impact of PAP‐guided medical therapy in recipients of CRT, patients assigned to treatment (n=91) and control (n=99) groups were compared for (1) HFH rates over the entire randomized follow‐up period (average 18 months), (2) documented HF medication changes during the 6‐month primary follow‐up portion of the trial (according to the clinical protocol), (3) measurement of hemodynamic status by area under the curve of PAP profile, and (4) quality of life assessment using the Minnesota Living with Heart Failure Questionnaire (MLHFQ) at baseline, 6 and 12 months of randomized follow‐up. Decreasing values of MLHFQ scores over time represent an improved quality of life.
Area under the curve analyses established a baseline PAP defined as the 7‐day average of PAPs uploaded from the patients' homes during the first week following implant. Each subsequent uploaded daily PAP pressure was compared with the baseline and the difference over time was quantified as a cumulative difference from baseline expressed using the trapezoidal rule in mm Hg‐days. Negative area under the curve measures indicated that patients spent more time with PAPs lower than the baseline.
Differences in baseline characteristics between treatment and control groups were evaluated using the Andersen‐Gill model for analysis of recurrent HFH and a backwards elimination approach in which covariates associated with P<0.15 were included in the modeling. The randomization variable was also included in the model. Further covariate analysis included days from CRT implantation.
Medication Change Analyses
Investigators in the CHAMPION trial reported all HF‐related medication changes, including the motivation for changing medications (ie, PAP‐directed or clinical assessment) for treatment and control group patients. 10 , 15 All medication changes in the control group were determined by clinical assessment. PAP‐guided medication changes were communicated to patients remotely using a script to protect the blinded nature of the trial. Also, each contact with a treatment group patient was matched to a randomly selected control patient to equalize study contact. Using methods previously published, 15 all HF medications were normalized using dose‐equivalency formulae. Angiotensin interventions (angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers) were converted to lisinopril equivalents, beta blockers to carvedilol equivalents, and mineralocorticoid receptor antagonists to spironolactone equivalents. Loop diuretics were converted to furosemide equivalents and thiazides to metolazone equivalent. Sacubitril/valsartan was not available at the time of the CHAMPION trial. These medication analyses discovered that a small number of documented changes in medications did not result in a different bioavailable dose of the drug category; therefore, these were excluded from the present analysis. All effective HF medication changes during the first 6 months of follow‐up are reported as dosage increase or decrease and were compared between control and treatment group patients with CRT.
Statistical Analysis
Prespecified supplementary efficacy analyses over the completed randomized access period (average of 18 months) included both recurrent‐event and time‐to‐first‐event analyses consistent with the primary efficacy evaluation. The Andersen–Gill extension of the Cox proportional hazards model was implemented to analyze recurrent events, which included HFH rates, as well as recurrent HFHs plus death, and the Cox proportional hazards model with log‐rank test was implemented to analyze mortality. The cumulative HFH rate was plotted over time using the Nelson‐Aalen cumulative hazard rate function. Prespecified supplementary safety analyses included freedom from device/system related complications and freedom from pressure sensor failure, consistent with the primary safety evaluation.
A clinical event classification committee provided independent expert end point adjudication. The committee included an independent, blinded group of experts in HF clinical trials. All adverse events, hospitalizations, and mortality events from the randomized access period were adjudicated by the clinical event classification committee.
Results
Patient Profile and Study Disposition
Among 550 patients randomized between 2007 and 2009, a total of 190 patients had CRT devices. These differed significantly from the non‐CRT cohort. Patients with CRT were older with the greater proportion male and had lower systemic blood pressure, poorer left ventricular ejection fraction, more comorbidities (eg, ischemic disease), and history of atrial fibrillation (Table 1). They were marked by worse renal function (61% of patients with CRT had comorbid chronic kidney disease [estimated glomerular filtration rates <60 mL/min per 1.73 m2] with a group average estimated glomerular filtration rate of 57±21 mL/min per 1.73 m2). The modified Charlson comorbidity was higher in the group with CRT compared with the population without CRT (CRT 4.8±2.0 versus non‐CRT 4.4±2.2, P=0.0135). Importantly, hemodynamic function was more compromised in patients with CRT: lower cardiac indices (2.00±0.59 L/min per m2 versus 2.23±0.62 L/min per m2 in non‐CRT, P<0.001) and higher PA wedge pressures, and 67% had secondary pulmonary hypertension (mean PAP >25 mm Hg) with an average mean PAP of 29.8±9.2 mm Hg, that is, characteristics of a patient group with severe HF despite chronic CRT therapy. However, 8 baseline clinical variables were identified as having P<0.15 indicating possible imbalances between treatment and control groups: systolic blood pressure, creatinine, glomerular filtration rate, ejection fraction, PA diastolic pressure, PA wedge pressure, coronary artery disease, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, and beta blockers (GDMT). PA systolic pressure and PA mean pressure also had P<0.15 but these variables are highly correlated with PA diastolic pressure. Likewise, angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers also had a P<0.15 but were highly correlated with GDMT (Table 2). After controlling for the variables that were potentially imbalanced, the randomization variable (treatment effect) remained significant (hazard ratio [HR], 0.71; 95% CI, 0.50–0.99; P<0.04).
Table 2.
Baseline Demographics of Patients with CRT at Enrollment in CHAMPION Trial
| Variable | Treatment Group (n=91) | Control Group (n=99) | P Value* |
|---|---|---|---|
| Demographics | |||
| Age, y | 64±13 | 63.7±11.6 | 0.8069 |
| Male, n (%) | 79 (87) | 88 (89) | 0.8243 |
| White, n (%) | 77 (85) | 80 (81) | 0.5670 |
| Laboratory finding | |||
| Body mass index, kg/m2 | 29.2±4.8 | 30.3±7.1 | 0.4661 |
| Systolic blood pressure, mm Hg | 113.9±18.1 | 120.2±20.9 | 0.0391 |
| Heart rate, bpm | 72.1±10.4 | 73.6±10.7 | 0.2319 |
| Creatinine, mg/dL | 1.5±0.5 | 1.4±0.4 | 0.1139 |
| Glomerular filtration rate, mL/min per 1.73 m2 | 55.2±22.2 | 59.1±20.3 | 0.0813 |
| Serum urea nitrogen, mg/dL | 34.2±20.8 | 29.9±17.2 | 0.2565 |
| Ejection fraction, % | 26.0±9.8 | 24.4±9.4 | 0.1019 |
| Hemodynamics | |||
| PA systolic pressure, mm Hg | 43.8±14.4 | 46.9±13.5 | 0.1412 |
| PA diastolic pressure, mm Hg | 18.1±8.4 | 20.8±7.4 | 0.0146 |
| PA mean pressure, mm Hg | 28.4±9.5 | 31.1±8.8 | 0.0626 |
| PA wedge pressure, mm Hg | 18.0±7.7 | 20.4±7.5 | 0.0520 |
| Cardiac output, L/min | 4.2±1.4 | 4.3±1.5 | 0.9671 |
| Cardiac index, L/min per m2 | 2.0±0.6 | 2.0±0.6 | 0.8373 |
| Pulmonary vascular resistance, Wood units | 2.8±1.8 | 2.9±2.0 | 0.5403 |
| Medical history | |||
| Ischemic cardiomyopathy, n (%) | 61 (67) | 73 (74) | 0.3418 |
| Chronic obstructive pulmonary disease, n (%) | 26 (29) | 27 (27) | 0.8724 |
| Coronary artery disease, n (%) | 63 (69) | 78 (79) | 0.1394 |
| Diabetes mellitus, n (%) | 40 (44) | 42 (42) | 0.8839 |
| History of myocardial infarction, n (%) | 50 (55) | 51 (52) | 0.6644 |
| Hyperlipidemia, n (%) | 76 (84) | 77 (78) | 0.3621 |
| Hypertension, n (%) | 62 (68) | 76 (77) | 0.1961 |
| History of atrial fibrillation, n (%) | 51 (56) | 60 (61) | 0.5577 |
| Treatment history | |||
| CRT with implantable cardioverter‐defibrillator, n (%) | 91 (100) | 99 (100) | 1.0000 |
| ACE/ARB, n (%) | 69 (76) | 84 (85) | 0.1430 |
| BB, n (%) | 83 (91) | 90 (91) | 1.0000 |
| ACE/ARB‐ and BB‐guideline‐directed medical therapy, n (%) | 63 (69) | 79 (80) | 0.0985 |
| Aldosterone antagonist, n (%) | 35 (38) | 43 (43) | 0.5554 |
| Loop diuretic, n (%) | 84 (92) | 96 (97) | 0.1988 |
| Thiazide diuretic, n (%) | 12 (13) | 7 (7) | 0.2260 |
| Thiazide diuretic as needed, n (%) | 8 (9) | 10 (10) | 0.8085 |
| Nitrate, n (%) | 22 (24) | 18 (18) | 0.3740 |
| Hydralazine, n (%) | 7 (8) | 9 (9) | 0.7979 |
ACE/ARB indicates angiotensin‐converting enzyme/angiotensin receptor blocker; BB, beta blocker; CHAMPION, CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; CRT, cardiac resynchronization therapy; and PA, pulmonary artery.
P value testing treatment vs control is from Wilcoxon rank‐sum test or Fisher's exact test.
HFH Rates
During an average of 18 months follow‐up, control group patients had 93 HFH events (0.68 events/patient‐year) compared with the treatment group with 62 HFH events (0.46 events/patient‐year), representing a 30% relative risk reduction (HR, 0.70; 95% CI, 0.51–0.96; P=0.028) (Table 3, Figure 1). (This difference was not weighted by more numerous recurrences among some “sicker” patients in the treatment group [Table S1]). There was a 15% between‐groups difference in the risk of a first HFH (HR, 0.85; 95% CI, 0.56–1.3) in favor of the treatment group, which is in agreement with the hypothesized direction of association, though possibly explainable by chance (P=0.45). The combined end point of all‐cause death and recurrent HFHs also was lower in the treatment group by 28% (81 events, 0.61 events/patient‐year) compared with control subjects (118 events, 0.87 events/patient‐year) associated with a HR of 0.72 (95% CI, 0.54–0.95; P=0.022). There was a nonstatistically significant 23% between‐groups difference in all‐cause mortality favoring the treatment group (P=0.38). Reduction in HFHs remained significant when days from CRT implant to CardioMEMS implant were included in covariant modeling measured as a continuous variable or binary (≤755 versus >755 days) variable. This indicates that the favorable effects of remote hemodynamic guided therapy occurred irrespectively of duration of CRT implant, in this series.
Table 3.
Clinical Outcomes in Patients With CRT at Time of Enrollment in CHAMPION Trial*
| Clinical End Point | Treatment Group (n=91) | Control Group (n=99) | Absolute Reduction | Number Needed to Treat |
Relative Risk Reduction (RRR) Hazard Ratio (HR) (95% CI) P Value † |
|---|---|---|---|---|---|
| Heart failure hospitalizations, No. (events/patient‐year) | 62 (0.46) | 93 (0.68) | 31 (0.22) | 5 |
RRR=0.30 HR=0.70 (0.51–0.96) P=0.0280 † |
| Deaths and heart failure hospitalizations, No. (events/patient‐year) | 81 (0.61) | 118 (0.87) | 37 (0.26) | 4 |
RRR=0.28 HR=0.72 (0.54–0.95) P=0.0223 † |
| Mortality, No. (%) | 19 (20.1) | 25 (25.3) | 6 (4.4) | N/A |
RRR=0.23 HR=0.77 (0.42–1.39) P=0.3813 ‡ |
CHAMPION indicates CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; and CRT, cardiac resynchronization therapy.
Events are annualized and include an average of 18 months of follow‐up.
HR, 95% CI, and P value are from the Andersen‐Gill model.
HR and 95% CI are from the Cox proportional hazards model; P value is from log‐rank test.
Figure 1. Hospitalization rates over time using a Nelson‐Aalen cumulative hazard rate.
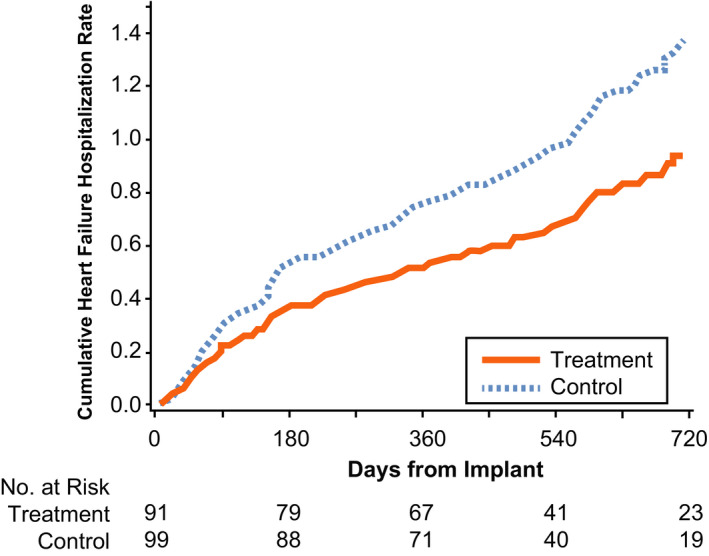
Heart failure hospitalization rates were 30% lower in the CRT‐D treatment group (hazard ratio, 0.70; 95% CI, 0.51–0.96; P=0.028) compared with the control group managed by standard clinical methods. CRT‐D indicates cardiac resynchronization therapy with implantable cardioverter‐defibrillator.
Medical Management
Maximally tolerated GDMT was required for patients with HF with reduced ejection fraction before enrollment in the CHAMPION trial, 10 as detailed in Table S2. There were no baseline differences in medical therapies between groups. Approximately 2.5 times more medication changes were made in the treatment group guided by knowledge of PAP in the first 6 months following sensor implantation (847 medication changes in treatment versus 346 in control, P<0.001) as shown in Figure 2 (Table S3). These changes represent ≈1½ medications changes per patient/month for the treatment group and ≈½ change per patient/month for the control group. Increases and decreases in diuretic therapies were more frequent in the treatment group compared with control and, in general, diuretics were the most commonly adjusted medication in both groups. Importantly, vasodilator dosing was increased more frequently in the treatment group (Table S3). Significant up‐titration of GDMT was seen only in the treatment group. The frequency of increases and decreases in medical therapies during the first 6 months follow‐up is illustrated in Figure 2.
Figure 2. Frequency of increases and decreases in medical management of CRT patients involved in the CHAMPION Trial.
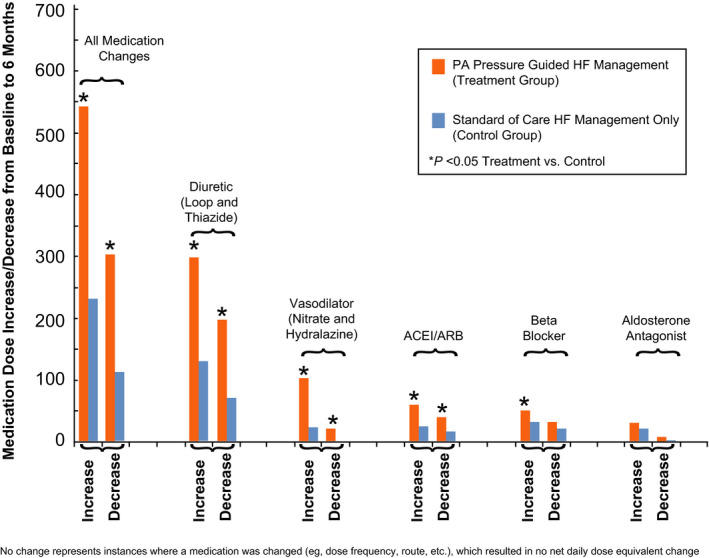
Medication changes in the PA pressure guided heart failure group (treatment group, red bars, n=91) are compared with the standard of care heart failure management only group (control group, blue bars, n=99). ACEI indicates angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CHAMPION, CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; CRT, cardiac resynchronization therapy; HF, heart failure; and PA, pulmonary artery.
Hemodynamic Outcomes
Sensor‐based mean PAPs, averaged from the first 7 days of pressures uploaded from home, were similar between groups. Subsequently, more medication changes in the treatment group were associated with significant lowering of PAPs over time (without affecting renal function: serum creatinine change compared with baseline 0.087 in treatment versus control 0.12 mg/L, P=0.62). Thus, mean PAPs were lower following hemodynamic‐guided care as quantified by an area under the curve analysis (–413.2±123.5 versus 60.1±88.0 in control, P=0.0023) after 6‐month follow‐up (Figure 3).
Figure 3. Pulmonary artery pressure changes over time compared with each patient's baseline (BL) pressure defined as the average pressure from the first week postsensor implantation through 6 months of follow‐up (x‐axis, time in months).
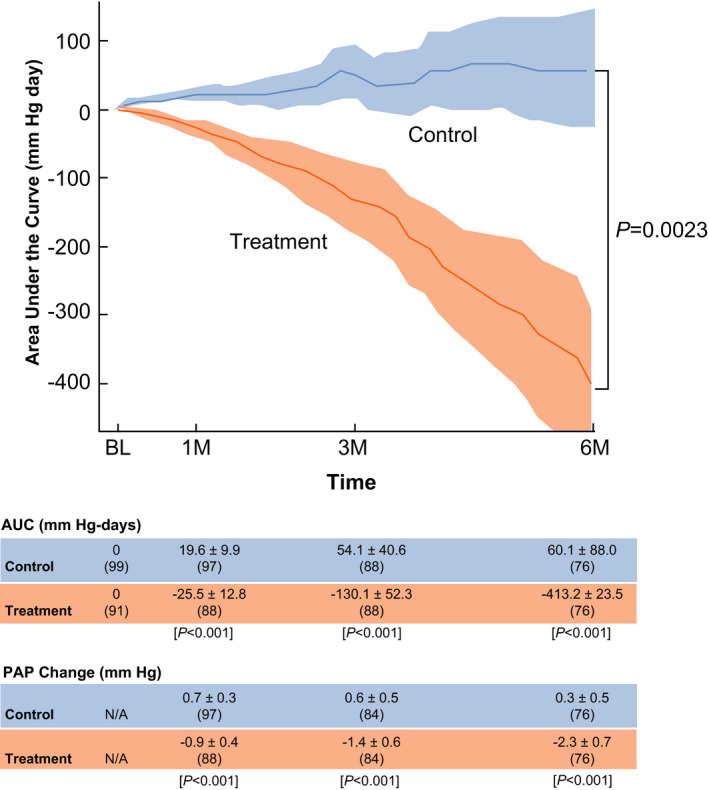
An area under the curve analysis was performed to quantify the time pressures were below the patient's baseline with units of mm Hg‐day (y‐axis). The treatment group experienced significant reductions in pulmonary artery pressures over time (P=0.0023). AUC indicates area under the curve; and PAP, pulmonary artery pressure.
Quality of Life
Total MLHFQ scores were similar between groups at baseline but changed significantly in the treatment group compared with the control group (–13.5±23 versus –4.9±24.8; P=0.0064) (Table 4). Improvement in the physical component of the MLHFQ score accounted for much of the overall improvement in quality of life in treatment patients.
Table 4.
Baseline and 12‐Month Quality of Life Score and Change, Measured by the Minnesota Living With Heart Failure Questionnaire
| Total Score | Emotional Score | Physical Score | |
|---|---|---|---|
| Baseline | |||
| Treatment (n=91) | 57.3±23.0 | 11.9±7.5 | 25.0±10.1 |
| Control (n=99) | 57.5±23.8 | 12.0±7.5 | 24.7±10.3 |
| 6 mo | |||
| Treatment (n=78) | 42.5±21.4* | 8.4±7.0* | 18.9±9.1* |
| Control (n=79) | 51.5±23.4 | 10.7±7.9 | 23.0±9.9 |
| Change from baseline | |||
| Treatment (n=78) | −13.5±23.3 † | −3.2±6.4 † | −5.5±11.1 † |
| Control (n=79) | −4.9±24.8 | −1.1±7.6 | −1.3±1.2 |
Lower scores represent an improved quality of life.
Differences between treatment and control at 6 months (P=0.013 total score, P=0.06 emotional score, P=0.009 physical score).
Changes between baseline and 6 months significantly favored the treatment group (P=0.0064 total score, P=0.03 emotional score, P=0.005 physical score).
Discussion
Recurrence of HF following CRT is a disappointing result, portends poor prognosis, and lacks proven therapy. Here, we show a successful clinical management strategy by basing HF medication changes on frequent remote assessment of PAPs. This allowed investigators to individualize medical interventions with the goal of decreasing PAPs. This strategy reduced HFHs and was accompanied by a moderate to large improvement in quality of life (MLWHF score decreased by 13.5 points. 10 ) compared with traditional clinical management. The results underscore the synergy between electrical resynchronization with CRT therapy and enhanced HF disease management.
Current expert consensus documents offer little guidance for postimplant management of recipients of CRT, beyond device troubleshooting and CRT delivery (% biventricular pacing). 9 , 16 , 17 However, CRT exerts a range of effects 18 and may not be a durable solution for every patient with HF with electrical dyssynchrony. The most efficacious clinical result of CRT is normalization of left ventricular function soon after implant, which imparts a normal long‐term survival. These “complete responders” likely have a pure electropathy but constitute a minority. 19 The remainder—“partial” responders—demonstrate reverse structural remodeling, but after 2 to 3 years may begin to decompensate. 3 Those poorly responsive within months of implant manifest recurrent HF and high risk of death. 4 The first occurrence of worsening HF in patients with CRT, irrespective of timing, is a sentinel event heralding progressive deterioration in clinical condition. 6 One CRT trial reported that the occurrences of first and second HF events were associated with 7‐ and nearly 19‐fold respective increases in the risk of subsequent mortality. 7 The authors stressed the need to identify measures for the prevention of repeated HF episodes.
This retrospective analysis provides a comprehensive hemodynamic and disease state characterization of patients receiving CRT versus patients with HF without a CRT indication but sharing similar clinical assessment, that is, Class III and prior HFH (this is unique among CRT studies. 1 ). Patients with CRT had a higher comorbidity index, higher prevalence of renal dysfunction, and severe secondary pulmonary hypertension (Table 1). Importantly, the secondary pulmonary hypertension found in patients with CRT was not “fixed” but responded to medical management guided by knowledge of PAPs. The severity of hemodynamic compromise of our CRT subgroup versus the remainder of the CHAMPION study population is significant. These incomplete responders to CRT had higher PA wedge pressures and lower cardiac output (Table 1). In fact, many of the clinical characteristics of these patients fit the American Heart Association/American College of Cardiology guideline definition of “advanced HF,” which include NYHA Class III symptoms, episodes of fluid retention at rest, objective evidence of severe cardiac dysfunction, severe impairment of functional capacity, history of ≥1 HFH in the past 6 months, and presence of all these despite attempts to optimize therapy including CRT when indicated. 20 The CRT group had cardiac indexes similar to Intermacs 2 to 4 patients enrolled in the MOMENTUM 3 (Multicenter Study of MAGLEV Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3) trial (mean CI was also 2.0±0.5 in MOMENTUM 3 21 ). These discordances between clinical assessment and disease progression illustrates the need to recognize heterogeneity (or “phenogroups”) among enrollees tested in clinical trials. 22 , 23
Previous CRT trials have not characterized hemodynamic function as required in CHAMPION. 1 However, comparison of commonly assessed indices confirm that the patients with CRT enrolled in CHAMPION were markedly sicker. Thus, death and HF hospitalization rates (0.87 events/patient‐year) in the CHAMPION CRT control group was twice that of the Class III patients enrolled in the COMPANION (Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure) trial (0.43 events/patient‐year), 24 and 30% higher than nonresponders to CRT who were prospectively identified in a recent registry (0.67 events/patient‐year). 4 Quality of life was profoundly depressed as indicated by MLHFQ scores in excess of 57, which were 10+ points poorer than reported for incomplete responders at time of diagnosis. 4 Moreover, prior CRT trials have selected patients with lighter comorbidity burden and generally excluded renal dysfunction, both known to modulate HF events (“comorbid HF”). 5 , 24 , 25 However, these are highly prevalent among nonresponders in real‐world practice. 4 , 5 , 25 , 26 Hence, the CRT subgroup in CHAMPION illustrates the need to investigate the underlying HF disease state in all patients with less than optimal response to CRT. Heart failure management beyond correction of interventricular dyssynchrony, in the case of the current study, should include consideration of hemodynamic guided control of congestion and secondary pulmonary hypertension.
The inefficacy of traditional HF management strategies is highlighted in the control arm. Patients underwent a right heart catheterization and received GDMT in expert HF centers, including increased dosing of diuretics over 6 months directed by traditional clinical tools (signs, symptoms, and daily weight assessments). This usual clinical care strategy had no impact on filling pressures, which were unaffected and remained chronically elevated (Figure 3). Consequently hemodynamic stability was not achieved during follow‐up. This may explain results from a recent large international study of prospectively identified nonresponders showing that empiric adjustments to drug therapy did not affect progressive clinical deterioration. 4 Similarly, patients with CRT did not improve with sacubtril‐valsartan in PARADIGM‐HF (Prospective Comparison of ARNI [Angiotensin Receptor–Neprilysin Inhibitor] With ACEI [Angiotensin‐Converting Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure). Our results contrast sharply, revealing success of pharmacotherapy if hemodynamically guided.
The current study delivers on the promise of remote management to influence disease progression in patients with CRT, by shifting from reactive treatment for congestive symptoms to preemptive and individualized medical intervention using an actionable signal that reflects the underlying disease. Although remote monitoring of patients with cardiac implantable electronic devices to evaluate device performance and monitor various diagnostic parameters is common clinical practice. 27 , 28 (and was not prohibited in CHAMPION), generally it has been unsuccessful in reducing HFH rates. 8 , 29 , 30 Studies of device‐based intrathoracic impedance specifically as a surrogate for hemodynamic measures showed poor sensitivity and specificity and failed to reduce HFHs. 31 , 32 This is in striking contrast to our findings, that is, direct measurements of hemodynamic information led to informed and individualized medication adjustments and significantly improved outcomes. Thus, a remotely obtained signal must directly reflect the underlying pathophysiology and respond appropriately to medical intervention in order to be useful. Intracardiac pressure changes presage clinical signs of congestive HF by several weeks. 33 Nonhemodynamic signals may only provide a trigger of concern about individual patients but cannot provide useful information to actually manage the patient's disease. 34 , 35 Most suggested algorithms focus on device troubleshooting and ensuring adequate CRT, but few recommend assessment and treatment of the underlying HF disease syndrome. 36 PAP monitoring provided a thorough and efficient means to provide improved long‐term disease management for patients with CRT persisting with recurrent HF.
Implications
There are no trial data or guidelines to direct therapy in the years following CRT implant 9 , 16 , 36 , 37 because the procedure is regarded widely as the “final stop” for this group of patients with HF, considered already “fully” optimized (or even resistant) to medical management. 4 , 38 Thus GDMT is seldom adjusted afterwards 4 and indeed was not advocated among recent solutions proposed for patients poorly responsive to CRT. 36 The relapse of clinical HF is considered to be a sign of progression of underlying disease and not salvageable (in keeping with this common understanding, best empiric therapy (control patients) did not affect high PA pressure and HF hospitalization continued). The diversion of the natural history of such a group of patients (with a prognosis similar to many forms of cancer 3 , 39 ) by GDMT when hemodynamically guided is revealing, pointing to a state of persistent and treatable neurohormonal activation despite chronic CRT. Furthermore, interventions had no adverse effects among patients with compromised renal function with low cardiac index and permitted further up‐titration of neurohormonal antagonists. This is an important finding given deficiency of data supporting medical management in patients with HF with reduced ejection fraction with advanced renal disease and resolves a prevalent concern among caregivers. 26 Unsurprisingly, these step‐by‐step adjustments took months to affect clinical outcomes in this sick patient group and may be anticipated to lead to long‐term survival benefit. 6 The improvement in individual health status in the present study was significant (reflected by MLHFQ scores). This has been found to be a strong, independent predictor of mortality, cardiovascular events, hospitalization, and costs of care. 40 Enabling our treatment strategy requires a multidisciplinary, protocol‐driven CRT clinic incorporating hemodynamic monitoring including EP and HF (currently HF specialists are involved in the management of only 15% of patients with CRT) and a remote monitoring workflow. 41 , 42
Limitations
The CHAMPION protocol did not provide standardized management strategies for the control group but relied on local standards, which may have been variable as reported in the ADVANCE CRT Registry. 4 In addition, the protocol did not document motivation for medication changes in the control group but assumed the changes were made on the basis of close monitoring of signs and symptoms as consistent with the conventional standard of care. Because outcomes are unlikely to change if PAPs are not acted upon, enrollment in the CHAMPION trial required diuretic responsiveness and patients' adherence to PAP measurements and medical regimen. Without these components, PAP monitoring strategies would not be effective.
We aimed to assess treatment of patients with CRT defined by recurrent HF and NYHA Class III within 3 years after implant, 3 , 4 , 7 in distinction to “nonresponders,” which is a term applied usually within a few months of implant and which lacks consensus definition. 43 Preenrollment history or clinical course following CRT implantation was not recorded in the CHAMPION trial. QRS width and morphology are not known and although they may provide information on the likelihood of short‐term response, are modest predictors of durable CRT effect in NYHA Class III patients. 3 , 44 Other typical assessments of CRT optimization were not captured in the CHAMPION trial, for example, history of AF, lead placement, and percentage of biventricular pacing. However, randomization should have minimized the impact of these limitations. The study also did not assess markers of structural remodeling because occurrence of clinical HF is clinically more meaningful, 7 , 9 used most often in practice, 4 drove the primary end point of pivotal CRT trials, 1 and was found to be a stronger marker of poor prognosis. 45
Conclusions
In patients persisting or recurring with severe HF despite CRT, remote PAP‐guided medical management should be considered. This strategy significantly improved clinical well‐being and reduced HFH rates. The current exploratory analysis strongly supports the hypothesis that hemodynamic and CRT interventions may have significant clinical synergy and should be tested in prospective clinical trials. It seems reasonable that successful longitudinal management of patients with CRT should include thorough electrophysiologic and HF disease evaluations. 41 , 42
Sources of Funding
This work was supported by CardioMEMS Inc, subsequently acquired by St Jude Medical, and then Abbott.
Disclosures
Niraj Varma reports consulting Fees/Honoraria from St. Jude Medical, Boston Scientific, Biotronik, Medtronic. Robert Bourge reports grant support and consulting fees from Abbott (CardioMEMS). Lynne Stevenson is an unpaid consultant for Abbott and Biotronik and Chair of the DSMB for LivaNova. Mariarosa Rosa Costanzo reports consulting Fees/Honoraria from Abbott, Medtronic, Boston Scientific, and grant support from Abbott to the Advocate Heart Institute for the CardioMEMS postapproval study and GUIDE‐HF clinical trial. Philip B. Adamson reports salary support from Abbott. Greg Ginn reports salary support from Abbott. John Henderson reports salary support from Abbott. David Shavelle reports grant support from Abbott for CardioMEMS clinical trials.
Supporting information
Appendix S1
Tables S1–S3
(J Am Heart Assoc. 2021;10:e017619. DOI: 10.1161/JAHA.120.017619.)
For Sources of Funding and Disclosures, see page 11.
See Editorial by Briasoulis and Alvarez
References
- 1. Wells G, Parkash R, Healey JS, Talajic M, Arnold JM, Sullivan S, Peterson J, Yetisir E, Theoret‐Patrick P, Luce M, et al. Cardiac resynchronization therapy: a meta‐analysis of randomized controlled trials. CMAJ. 2011;183:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anand IS, Carson P, Galle E, Song R, Boehmer J, Ghali JK, Jaski B, Lindenfeld J, O'Connor C, Steinberg JS, et al. Cardiac resynchronization therapy reduces the risk of hospitalizations in patients with advanced heart failure: results from the comparison of medical therapy, pacing and defibrillation in heart failure (COMPANION) trial. Circulation. 2009;119:969–977. [DOI] [PubMed] [Google Scholar]
- 3. Rickard J, Cheng A, Spragg D, Bansal S, Niebauer M, Baranowski B, Cantillon DJ, Tchou PJ, Grimm RA, Tang WH, et al. Durability of the survival effect of cardiac resynchronization therapy by level of left ventricular functional improvement: fate of "nonresponders". Heart Rhythm. 2014;11:412–416. [DOI] [PubMed] [Google Scholar]
- 4. Varma N, Boehmer J, Bhargava K, Yoo D, Leonelli F, Costanzo M, Saxena A, Sun L, Gold MR, Singh J, et al. Evaluation, management, and outcomes of patients poorly responsive to cardiac resynchronization device therapy. J Am Coll Cardiol. 2019;74:2588–2603. [DOI] [PubMed] [Google Scholar]
- 5. Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11:e004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bello NA, Claggett B, Desai AS, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Pfeffer MA, Solomon SD. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail. 2014;7:590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldenberg I, Hall WJ, Beck CA, Moss AJ, Barsheshet A, McNitt S, Polonsky S, Brown MW, Zareba W. Reduction of the risk of recurring heart failure events with cardiac resynchronization therapy: MADIT‐CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy). J Am Coll Cardiol. 2011;58:729–737. [DOI] [PubMed] [Google Scholar]
- 8. Ono M, Varma N. Remote monitoring to improve long‐term prognosis in heart failure patients with implantable cardioverter‐defibrillators. Expert Rev Med Devices. 2017;14:335–342. [DOI] [PubMed] [Google Scholar]
- 9. Mullens W, Auricchio A, Martens P, Witte K, Cowie MR, Delgado V, Dickstein K, Linde C, Vernooy K, Leyva F, et al. Optimized implementation of cardiac resynchronization therapy—a call for action for referral and optimization of care. Eur J Heart Fail. 2020. Nov 2; online ahead of print. DOI: 10.1002/ejhf.2046. [DOI] [PubMed] [Google Scholar]
- 10. Adamson PB, Abraham WT, Aaron M, Aranda JM Jr, Bourge RC, Smith A, Stevenson LW, Bauman JG, Yadav JS. Champion trial rationale and design: the long‐term safety and clinical efficacy of a wireless pulmonary artery pressure monitoring system. J Card Fail. 2011;17:3–10. [DOI] [PubMed] [Google Scholar]
- 11. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. DOI: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 12. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow‐up results from the CHAMPION randomised trial. Lancet. 2016;387:453–461. DOI: 10.1016/S0140-6736(15)00723-0. [DOI] [PubMed] [Google Scholar]
- 13. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–944. DOI: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]
- 14. Givertz MM, Stevenson LW, Costanzo MR, Bourge RC, Bauman JG, Ginn G, Abraham WT. Pulmonary artery pressure‐guided management of patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2017;70:1875–1886. DOI: 10.1016/j.jacc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 15. Costanzo MR, Stevenson LW, Adamson PB, Desai AS, Heywood JT, Bourge RC, Bauman J, Abraham WT. Interventions linked to decreased heart failure hospitalizations during ambulatory pulmonary artery pressure monitoring. JACC Heart Fail. 2016;4:333–344. [DOI] [PubMed] [Google Scholar]
- 16. Brignole M, Auricchio A, Baron‐Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace. 2013;15:1070–1118. [DOI] [PubMed] [Google Scholar]
- 17. Daubert JC, Saxon L, Adamson PB, Auricchio A, Berger RD, Beshai JF, Breithard O, Brignole M, Cleland J, DeLurgio DB, et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow‐up recommendations and management. Europace. 2012;14:1236–1286. [DOI] [PubMed] [Google Scholar]
- 18. Steffel J, Ruschitzka F. Superresponse to cardiac resynchronization therapy. Circulation. 2014;130:87–90. DOI: 10.1161/CIRCULATIONAHA.113.006124. [DOI] [PubMed] [Google Scholar]
- 19. Manne M, Rickard J, Varma N, Chung MK, Tchou P. Normalization of left ventricular ejection fraction after cardiac resynchronization therapy also normalizes survival. Pacing Clin Electrophysiol. 2013;36:970–977. DOI: 10.1111/pace.12174. [DOI] [PubMed] [Google Scholar]
- 20. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 21. Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC, Colombo PC, Walsh MN, Milano CA, Patel CB, Jorde UP, et al. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med. 2017;376:440–450. DOI: 10.1056/NEJMoa1610426. [DOI] [PubMed] [Google Scholar]
- 22. Goldstein BA, Rigdon J. Using machine learning to identify heterogeneous effects in randomized clinical trials‐moving beyond the forest plot and into the forest. JAMA Netw Open. 2019;2:e190004. DOI: 10.1001/jamanetworkopen.2019.0004. [DOI] [PubMed] [Google Scholar]
- 23. Blackstone EH. Precision medicine versus evidence‐based medicine: individual treatment effect versus average treatment effect. Circulation. 2019;140:1236–1238. DOI: 10.1161/CIRCULATIONAHA.119.043014. [DOI] [PubMed] [Google Scholar]
- 24. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, et al. Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. DOI: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 25. Zeitler EP, Friedman DJ, Daubert JP, Al‐Khatib SM, Solomon SD, Biton Y, McNitt S, Zareba W, Moss AJ, Kutyifa V. Multiple comorbidities and response to cardiac resynchronization therapy: MADIT‐CRT long‐term follow‐up. J Am Coll Cardiol. 2017;69:2369–2379. DOI: 10.1016/j.jacc.2017.03.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hein AM, Scialla JJ, Edmonston D, Cooper LB, DeVore AD, Mentz RJ. Medical management of heart failure with reduced ejection fraction in patients with advanced renal disease. JACC Heart Fail. 2019;7:371–382. DOI: 10.1016/j.jchf.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varma N, Epstein AE, Irimpen A, Schweikert R, Love C. Efficacy and safety of automatic remote monitoring for implantable cardioverter‐defibrillator follow‐up: the Lumos‐T safely reduces routine office device follow‐up (TRUST) trial. Circulation. 2010;122:325–332. DOI: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 28. Slotwiner D, Varma N, Akar JG, Annas G, Beardsall M, Fogel RI, Galizio NO, Glotzer TV, Leahy RA, Love CJ, et al. HRS expert consensus statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm. 2015;12:e69–e100. DOI: 10.1016/j.hrthm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 29. Hindricks G, Varma N, Kacet S, Lewalter T, Søgaard P, Guédon‐Moreau L, Proff J, Gerds T, Anker S, Torp‐Pedersen C, et al. Daily remote monitoring of implantable cardioverter‐defibrillators: insights from the pooled patient‐level data from three randomised controlled trials (IN‐TIME, ECOST, TRUST). Eur Heart J. 2016;37:3164–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morgan JM, Kitt S, Gill J, McComb JM, Ng GA, Raftery J, Roderick P, Seed A, Williams SG, Witte KK, et al. Remote management of heart failure using implantable electronic devices. Eur Heart J. 2017;38:2352–2360. DOI: 10.1093/eurheartj/ehx227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Muñoz Aguilera R, Lunati M, Yu CM, et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–1726. DOI: 10.1161/CIRCULATIONAHA.111.043042. [DOI] [PubMed] [Google Scholar]
- 32. Böhm M, Drexler H, Oswald H, Rybak K, Bosch R, Butter C, Klein G, Gerritse B, Monteiro J, Israel C, et al. Fluid status telemedicine alerts for heart failure: a randomized controlled trial. Eur Heart J. 2016;37:3154–3163. DOI: 10.1093/eurheartj/ehw099. [DOI] [PubMed] [Google Scholar]
- 33. Adamson PB, Zile MR, Cho YK, Bennett TD, Bourge RC, Aaron MF, Aranda JM Jr, Abraham WT, Kueffer FJ, Taepke RT. Hemodynamic factors associated with acute decompensated heart failure: part 2—use in automated detection. J Card Fail. 2011;17:366–373. DOI: 10.1016/j.cardfail.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 34. Varma N, Ricci RP. Telemedicine and cardiac implants: what is the benefit? Eur Heart J. 2012;34:1885–1895. DOI: 10.1093/eurheartj/ehs388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Desai AS, Stevenson LW. Connecting the circle from home to heart‐failure disease management. N Engl J Med. 2010;363:2364–2367. DOI: 10.1056/NEJMe1011769. [DOI] [PubMed] [Google Scholar]
- 36. Curtis AB, Poole JE. The right response to nonresponse to cardiac resynchronization therapy. J Am Coll Cardiol. 2019;74:2604–2606. DOI: 10.1016/j.jacc.2019.08.1063. [DOI] [PubMed] [Google Scholar]
- 37. Yancy CW, Januzzi JL Jr, Allen LA, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Jessup M, Lindenfeld J, Maddox TM, et al. 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol. 2018;71:201–230. [DOI] [PubMed] [Google Scholar]
- 38. Sze E, Samad Z, Dunning A, Campbell KB, Loring Z, Atwater BD, Chiswell K, Kisslo JA, Velazquez EJ, Daubert JP. Impaired recovery of left ventricular function in patients with cardiomyopathy and left bundle branch block. J Am Coll Cardiol. 2018;71:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greene SJ, Felker GM. The urgency of doing: addressing gaps in use of evidence‐based medical therapy for heart failure. JACC Heart Fail. 2019;7:22–24. DOI: 10.1016/j.jchf.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 40. Rumsfeld JS, Alexander KP, Goff DC, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, et al. Cardiovascular health: the importance of measuring patient‐reported health status: a scientific statement from the American Heart Association. Circulation. 2013;127:2233–2249. DOI: 10.1161/CIR.0b013e3182949a2e. [DOI] [PubMed] [Google Scholar]
- 41. Altman RK, Parks KA, Schlett CL, Orencole M, Park M‐Y, Truong QA, Deeprasertkul P, Moore SA, Barrett CD, Lewis GD, et al. Multidisciplinary care of patients receiving cardiac resynchronization therapy is associated with improved clinical outcomes. Eur Heart J. 2012;33:2181–2188. DOI: 10.1093/eurheartj/ehs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gorodeski EZ, Magnelli‐Reyes C, Moennich LA, Grimaldi A, Rickard J. Cardiac resynchronization therapy‐heart failure (CRT‐HF) clinic: a novel model of care. PLoS One. 2019;14:e0222610. DOI: 10.1371/journal.pone.0222610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fornwalt BK, Sprague WW, BeDell P, Suever JD, Gerritse B, Merlino JD, Fyfe DA, Leon AR, Oshinski JN. Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation. 2010;121:1985–1991. DOI: 10.1161/CIRCULATIONAHA.109.910778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feeny AK, Rickard J, Patel D, Toro S, Trulock KM, Park CJ, LaBarbera MA, Varma N, Niebauer MJ, Sinha S, et al. Machine learning prediction of response to cardiac resynchronization therapy. Circ Arrhythm Electrophysiol. 2019;12:e007316. DOI: 10.1161/CIRCEP.119.007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vidula H, Kutyifa V, McNitt S, Goldenberg I, Solomon SD, Moss AJ, Zareba W. Long‐term survival of patients with left bundle branch block who are hypo‐responders to cardiac resynchronization therapy. Am J Cardiol. 2017;120:825–830. DOI: 10.1016/j.amjcard.2017.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Tables S1–S3


