Abstract
Background
Recent studies have increasingly shown that sodium‐glucose cotransporter 2 (SGLT2) inhibitors may have beneficial cardiovascular and metabolic effects in patients without diabetes mellitus. Hence, we conducted a systematic review and meta‐analysis to determine the effect of SGLT2 inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus.
Methods and Results
Four electronic databases (PubMed, Embase, Cochrane, and SCOPUS) were searched on August 30, 2020 for articles published from January 1, 2000 to August 30, 2020, for studies that examined the effect of SGLT2 inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus. A random‐effects pairwise meta‐analysis model was used to summarize the studies. A total of 8 randomized‐controlled trials were included with a combined cohort of 5233 patients. In patients without diabetes mellitus, those with heart failure treated with SGLT2 inhibitors had a 20% relative risk reduction in cardiovascular deaths and heart failure hospitalizations, compared with those who were not treated (risk ratio, 0.78; P<0.001). We additionally found that treatment with SGLT2 inhibitors improved multiple metabolic indices. Patients on SGLT2 inhibitors had a reduction in body weight of −1.21 kg (P<0.001), body mass index of −0.47 kg/m2 (P<0.001), systolic blood pressure of −1.90 mm Hg (P=0.04), and fasting plasma glucose of −0.38 mmol/L (P=0.05), compared with those without. There were no between‐group differences in NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels, waist circumference, diastolic blood pressure, glycated hemoglobin, low‐density lipoprotein cholesterol levels, and estimated glomerular filtration rates. Across our combined cohort of 5233 patients, hypoglycemia was reported in 22 patients.
Conclusions
SGLT2 inhibitors improve cardiovascular outcomes in patients without diabetes mellitus with heart failure. In patients without diabetes mellitus, SGLT2 inhibitors showed positive metabolic outcomes in weight and blood pressure control.
Keywords: nondiabetics, sodium/glucose cotransporter 2 inhibitors
Subject Categories: Risk Factors, Heart Failure, Metabolic Syndrome
Nonstandard Abbreviations and Acronyms
- SGLT2
sodium‐glucose cotransporter 2
Clinical Perspective
What Is New?
Among patients without diabetes mellitus, those with heart failure treated with sodium‐glucose cotransporter 2 inhibitors had a relative risk reduction in cardiovascular deaths and heart failure hospitalizations, compared with those who received placebo.
Additionally, treatment with sodium‐glucose cotransporter 2 inhibitors in patients without diabetes mellitus improved their metabolic parameters including body weight and blood pressure.
Across a combined cohort of 5233 patients without diabetes mellitus, hypoglycemia was reported in 22 patients.
What Are the Clinical Implications?
Future research of sodium‐glucose cotransporter 2 inhibitors in other subgroups of patients without diabetes mellitus, particularly those without heart failure, is warranted to determine its overall role in the management of patients without diabetes mellitus.
Sodium‐glucose cotransporter 2 (SGLT2) inhibitor is a class of antihyperglycemic drugs increasingly used for patients with diabetes mellitus. 1 By blocking glucose reabsorption at the proximal renal tubule, SGLT2 inhibitors increase urinary glucose excretion and hence lower blood glucose in patients with diabetes mellitus.
Beyond and independent of glycemic control, clinical trials have demonstrated an improvement in cardiovascular morbidity and mortality in patients with diabetes mellitus treated with SGLT2 inhibitors compared with placebo. 2 , 3 , 4 Furthermore, treatment with SGLT2 inhibitors in patients with diabetes mellitus was shown to be associated with metabolic benefits such as weight loss, 5 , 6 blood pressure reduction, 7 and improvement in renal function. 4 , 8 The efficacy of SGLT2 inhibitors is reflected in the 2019 European Society of Cardiology guideline as a first‐line therapy for patients with type 2 diabetes mellitus and established cardiovascular disease. 9
Despite its initial indication as a diabetic drug with cardiovascular outcome benefits, recent clinical trials have demonstrated similar cardiovascular and metabolic benefits in patients without diabetes mellitus treated with SGLT2 inhibitors. In patients with heart failure (HF), the EMPEROR‐Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure With Reduced Ejection Fraction) study 10 and the DAPA‐HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) trial 11 demonstrated that SGLT2 inhibitors improve cardiovascular outcomes in patients with HF with reduced ejection fraction, regardless of diabetic status. Beyond cardiovascular benefits, recent clinical trials showed that the metabolic benefits of SGLT2 inhibitors extended beyond patients with diabetes mellitus to those without. 12 , 13 Given the increasing burden of metabolic syndrome and the subsequent morbidity and mortality, there is an urgent need to identify agents that may attenuate the deleterious effects of this condition in patients without diabetes mellitus. 14 , 15
To date, there has been no meta‐analysis examining whether SGLT2 inhibitor improves cardiovascular or metabolic outcomes in patients without diabetes mellitus. Hence, we conducted a systematic review and meta‐analysis to determine the effect of SGLT2 inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus. We hypothesized that in patients without diabetes mellitus, treatment with SGLT2 inhibitors, compared with those without, was associated with improvement in cardiovascular and metabolic outcomes.
METHODS
Ethics approval and consent to participate were not applicable. The data that support the findings of this study are available from the corresponding author upon reasonable request. The meta‐analysis was reported according to the Preferred Reporting Items of Systematic Reviews and Meta‐Analyses guidelines. 16 Searches of 4 databases (PubMed, Embase, Cochrane, and SCOPUS) were conducted on August 30, 2020 for articles published from January 1, 2000 to August 30, 2020. Literature search was performed using the following terms in combination: (“empagliflozin” OR “canagliflozin” OR “dapagliflozin” OR “Ertugliflozin”) AND ("trial”).
Studies evaluating the cardiovascular and metabolic outcomes of SGLT2 inhibitors in patients without diabetes mellitus were included. Cardiovascular outcomes included all‐cause mortality, nonfatal myocardial infarction, nonfatal stroke, HF hospitalization, and unplanned revascularizations. NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels were also used as a surrogate outcome of HF. Metabolic outcomes included systolic blood pressure, diastolic blood pressure, weight, body mass index, waist circumference, hemoglobin A1c (HbA1c), fasting plasma glucose, low‐density lipoprotein cholesterol, and estimated glomerular filtration rate. We included all randomized‐controlled trials, according to the population, intervention, comparison, outcome, and study design inclusion and exclusion criteria (Table 1). We excluded all studies that did not report on cardiovascular or metabolic outcomes in patients without diabetes mellitus.
Table 1.
PICOS, Inclusion Criteria, and Exclusion Criteria Applied to Database Search
| PICOS | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population |
|
|
| Intervention |
|
|
| Comparison |
|
|
| Outcome |
|
|
| Study design |
|
|
PICOS indicates population, intervention, comparison, outcome, and study design; and SGLT2, sodium‐glucose cotransporter 2.
Four reviewers independently performed the literature search and data extraction, and all disagreements were resolved by mutual consensus. During the title and abstract review stage, an "inclusive" approach was adopted, where only studies that clearly fit the exclusion criteria, such as the study not being a clinical trial, not involving SGLT2 inhibitor use, and/or were focused only on patients with diabetes mellitus, were excluded; the rest of the studies were included. Clinical trials involving SGLT2 inhibitors were selected for full text review to identify if subgroup analysis of patients without diabetes mellitus was performed.
Reviews in SGLT2 inhibitor were identified from the title and abstract review, and separate hand searches of their bibliographies were conducted, with no additional trials identified. Additionally, as the European Society of Cardiology Virtual Congress 2020 took place after the initial database search, hand search was conducted for conference abstracts and press releases from the Virtual Congress 2020, which may not have been included at the time of the initial search. Through this, the EMPEROR‐reduced trial 10 was identified, which was jointly published in the New England Journal of Medicine. Although the DAPA‐CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) trial was recently presented at the European Society of Cardiology Virtual Congress 2020, subgroup analyses for patients without diabetes mellitus were not available, hence the study was excluded. 17 Hence through hand searches, EMPEROR‐reduced trial 10 was additionally included into the study.
Apart from cardiovascular and metabolic outcomes, baseline information of patients without diabetes mellitus were collected for age, sex, body weight, body mass index, systolic blood pressure, diastolic blood pressure, HbA1c, and low‐density lipoprotein cholesterol. For the SGLT2 inhibitor regimes, we collected data on the drug name, drug dosage, drug frequency, control group, length of intervention, and mean length of follow‐up. Data relating to blinding and withdrawals were extracted to assess risk of bias. Quality control was performed by 2 independent reviewers using the Cochrane Risk of Bias tool, 18 which assesses 7 domains (random sequence generation, allocation concealment, masking of participants and personnel, blinding of outcome assessment; incomplete outcome data, selective outcome reporting, and other sources of bias), as shown in Figure S1. The quality of pooled evidence was evaluated using the Grading of Recommendations Assessment, Development and Evaluation system, 19 which accounts for statistical heterogeneity, publication bias, risk of bias, indirectness, and statistical imprecision, as shown in Table S1. A Preferred Reporting Items of Systematic Reviews and Meta‐Analyses checklist 20 is included in Figure S2.
Statistical Analysis
The results were quantitatively pooled and analyzed using Review Manager (RevMan) Version 5.4, 21 using general approaches laid out by the Cochrane Handbook. 22 In studies without SDs, P values or CIs were converted to SDs. 22 In studies without SDs, P values, andCIs, the square‐root of weighted mean variance of all other studies was used to estimate the SD. 23 In the Bays 2014 24 study where different dosages of SGLT2 inhibitor are used in subgroups of patients, the mean change of the SGLT2 inhibitor arm is calculated from the weighted mean change of the individual subgroups. For panel data or longitudinal outcomes, preintervention baseline imbalances were corrected using the simple analysis of change scores method. 22 In studies reporting the outcome in different scales, a simple unit conversion was performed. Inverse variance was used in deriving the pooled outcomes. The random‐effects model was used to account for between‐study variance. Between‐study heterogeneity was presented using I2 and τ2 statistics. We considered I2 of <30% to indicate low heterogeneity between studies, 30% to 60% to indicate moderate heterogeneity, and >60% to indicate substantial heterogeneity. Two‐sided P values of <0.05 were regarded to indicate nominal statistical significance.
RESULTS
The Preferred Reporting Items of Systematic Reviews and Meta‐Analyses flowchart is presented in Figure 1. Literature search of the 4 databases (PubMed, Embase, Cochrane, SCOPUS) retrieved 6522 results, and hand search uncovered 1 additional relevant study; 2414 duplicates were removed. Title and abstract screening excluded a further 4055 articles as they did not include patients without diabetes mellitus, did not evaluate cardiovascular outcomes or metabolic parameters, or were of an inappropriate study type. Full text screening excluded 46 articles. Eight articles were included for the meta‐analysis. 10 , 12 , 13 , 24 , 25 , 26 , 27 , 28
Figure 1. PRISMA flow diagram of study selection.
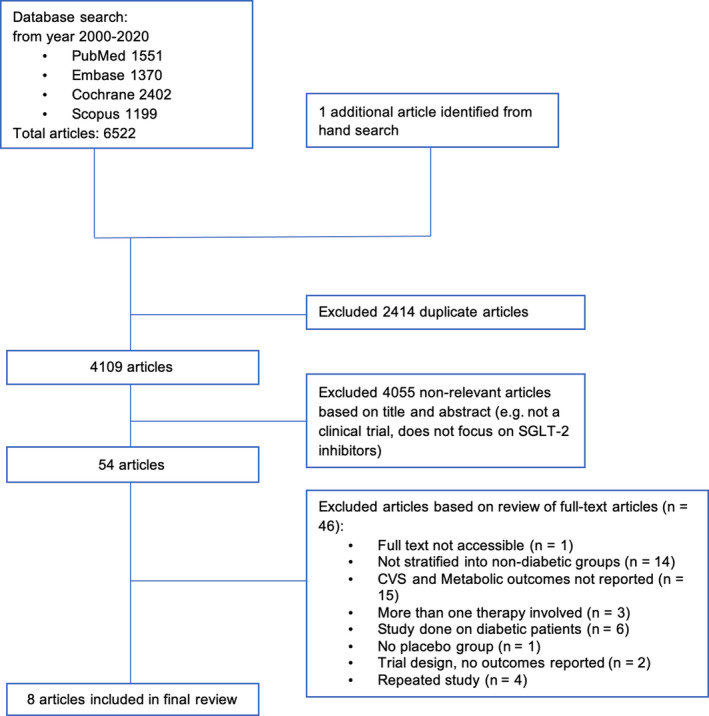
PRISMA indicates Preferred Reporting Items of Systematic Reviews and Meta‐Analyses; and SLGT2, sodium‐glucose cotransporter 2.
Baseline Characteristics
The 8 studies comprised a combined cohort of 5233 patients. In Nassif 2019, 26 Petrie 2020, 27 and Packer 2020, 10 patients without diabetes mellitus had HF. The participant baseline characteristics of the included studies are shown in Table 2.
Table 2.
Participant Baseline Characteristics
| Study | Sample Size of Patients Without DM | Mean Age, y | Men | Body Weight, kg | Body Mass Index, kg/m2 | Systolic Blood Pressure, mm Hg | Diastolic Blood Pressure, mm Hg | HbA1c/% | LDL‐C | Heart Failure |
|---|---|---|---|---|---|---|---|---|---|---|
| Bays 2014 24 | 376 | 44.8 | 53 | 101.3 | 37 | NR | NR | NR | NR | NR |
| González‐Ortiz 2016 25 | 32 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Hollander 2017 13 | 166 | 45.0 | 31 | 103.8 | 37.6 | 123.5 | NR | 5.6 | NR | NR |
| Nassif 2019 26 | 97 | NR | NR | NR | NR | NR | NR | NR | NR | 97 |
| Petrie 2020 27 | 2605 | 66.2 | 1973 | NR | 27.2 | 120.6 | NR | 5.75 | NR | 2605 |
| Cherney 2020 12 | 53 | 51 | 36 | 83 | 28 | 126 | 76.2 | 5.6 | 2.8 | NR |
| Díaz‐Cruz 2020 28 | 30 | NR | NR | 77 | 30.5 | 120 | 73.5 | 5.85 | NR | NR |
| Packer 2020 10 | 1874 | NR | NR | NR | NR | NR | NR | NR | NR | 1874 |
DM indicates diabetes mellitus; HbA1c, glycated hemoglobin, hemoglobin A1c; LDL‐C, low‐density lipoprotein cholesterol; and NR, not reported.
Across the 8 studies, the SGLT2 inhibitor drug name, dosage, frequency, control group, length of intervention, and length of follow‐up were summarized and are included in Table S2. Dapagliflozin, canagliflozin, and empagliflozin were the SGLT2 inhibitors used in 5, 2, and 1 studies respectively. Dapagliflozin and empagliflozin were administered at a dosage of 10 mg throughout the randomized controlled trials. All regimes were given once daily and compared with a control group receiving placebo. The length of follow‐up ranged from 12 weeks to 18 months.
Pooled Cardiovascular Outcomes
The pooled cardiovascular outcomes are presented in Figure 2. The 3 studies analyzed focused on patients with HF. Comparing patients receiving SGLT2 inhibitors with patients without, the random effects model demonstrated that the risk ratio for the composite of cardiovascular deaths and HF hospitalization was 0.78 (95% CI, 0.69–0.89; P<0.001) (Figure 2A). There was a statistically insignificant change in NT‐proBNP.
Figure 2. Forest plot of a composite of cardiovascular death and heart failure hospitalization (A) and Forest plot of mean change in NT‐proBNP in pg/mL (B).
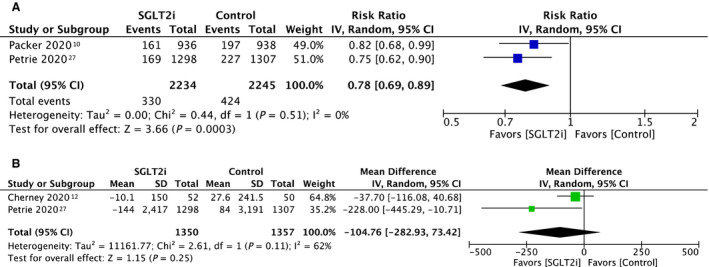
NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide; and SLGT2i, sodium‐glucose cotransporter 2 inhibitor.
Pooled Metabolic Outcomes
The pooled metabolic outcomes are presented in Figure 3. In patients without diabetes mellitus, the random effects model demonstrated that patients receiving SGLT2 inhibitors had a mean reduction in body weight of −1.21 kg (95% CI, −1.82 to −0.61; P<0.001) (Figure 3A), body mass index of −0.47 kg/m2 (95% CI, −0.73 to −0.21; P<0.001) (Figure 3B), systolic blood pressure of −1.90 mm Hg (95% CI, −3.69 to −0.11; P=0.04) (Figure 3D), and fasting plasma glucose of −0.38 mmol/L (95% CI, −0.77 to 0.01; P=0.05) (Figure 3G), compared with those without. There were no significant changes in waist circumference, diastolic blood pressure, HbA1c, low‐density lipoprotein cholesterol, and estimated glomerular filtration rate. In the combined cohort of 5233 patients, hypoglycemia occurred in 22 patients.
Figure 3. Forest plot of mean change in (A) body weight in kg, (B) BMI in kg/m2, (C) waist circumference in cm, (D) systolic blood pressure in mm Hg, (E) diastolic blood pressure in mm Hg, (F) HbA1c in %, (G) fasting plasma glucose in mmol/L, (H) LDL‐C in mmol/L, and (I) eGFR in mL/min per 1.73 m2.

BMI indicates body mass index; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; and LDL, low‐density lipoprotein.
Characteristics of Included Studies
The individual breakdown of the risk of bias and study characteristics are summarized in Table 3. Among the studies included, all studies were randomized‐controlled trials. Among the 8 studies, 3 studies included patients with HF, 3 studies included patients with obesity, 1 study included patients with chronic kidney disease, and 1 study included patients with prediabetes mellitus and prehypertension. All studies were assessed to have a low risk of selection bias (owing to random sequence generation), reporting bias, and other bias. A minority of studies (≤2 trials) were assessed to have an unclear risk of selection bias (owing to allocation concealment) and performance bias. Half of the studies had an unclear risk in detection bias. Two studies 13 , 24 experienced high dropout rates, contributing to a potential attrition bias; Hollander 2017 13 and Bays 2014 24 reported that 31% and 25% of study participants did not complete the study, respectively.
Table 3.
Characteristics of Included Studies
| Study | Study Population | Location | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias | Mean Length of Follow‐Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Bays 2014 24 | Overweight and obese without diabetes mellitus | Multiple sites in the United States and Puerto Rico | Low risk | Low risk | Low risk | Unclear risk | High risk | Low risk | Low risk | 12 wk |
| González‐Ortiz 2016 25 | Overweight and obese without diabetes mellitus | NR | Low risk | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | 3 mo |
| Hollander 2017 13 | Overweight and obese without diabetes mellitus | 18 sites in the United States | Low risk | Unclear risk | Low risk | Low risk | High risk | Low risk | Low risk | 26 wk |
| Nassif 2019 26 | Heart failure with reduced ejection fraction | 26 sites in the United States | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | 13 wk |
| Petrie 2020 27 | Heart failure with and without diabetes mellitus | 410 centers in 20 countries | Low risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | 18 mo (median) |
| Cherney 2020 12 | Chronic kidney disease without diabetes mellitus | 6 hospitals in Canada, Malaysia, and the Netherlands | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | 12 wk |
| Díaz‐Cruz 2020 28 | Prediabetes mellitus and prehypertension without pharmacological treatment | NR | Low risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | 12 wk |
| Packer 2020 10 | Heart failure with reduced ejection fraction | 520 centers in 20 countries | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | 16 mo (median) |
NR indicates not reported.
DISCUSSION
In this pairwise meta‐analysis of randomized‐controlled trials of patients without diabetes mellitus, we demonstrated that patients with HF treated with SGLT2 inhibitors had a 20% relative risk reduction in cardiovascular deaths and HF hospitalizations, compared with placebo. In addition, we found that treatment with SGLT2 inhibitors was associated with a reduction in body weight, body mass index, systolic blood pressure, and fasting plasma glucose, compared with placebo. There were no differences in serum NT‐proBNP level, waist circumference, diastolic blood pressure, serum HbA1c, low‐density lipoprotein cholesterol, and estimated glomerular filtration rate.
Patients with HF are at an increased risk of recurrent hospitalizations and mortality. 29 In 2014, in the United States, HF accounted for over 900 000 hospitalizations and totaled an estimated $11 billion in healthcare costs. 30 Hence, to reduce the huge healthcare burden attributed to HF, effective pharmacological therapy is highly sought after. In this meta‐analysis, we showed that treatment with SGLT2 inhibitors results in a significant risk reduction in cardiovascular mortality and HF hospitalization in patients without diabetes mellitus. Current guidelines recommends SGLT2 inhibitors for use in the treatment of diabetes mellitus to reduce HF risk. 9 We propose that SGLT2 inhibitors may additionally confer a benefit in the treatment of patients with HF on top of current recommended therapy, even in patients without diabetes mellitus. This might be of consideration to clinical practice while awaiting updates to HF guidelines.
However, there is a lack of studies evaluating cardiovascular outcomes in patients without diabetes mellitus and HF. Furthermore, the effect of SGLT2 inhibitors on the end points of myocardial infarction or stroke have not been explored. In the EMPA‐REG OUTCOME (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes) trial in patients with diabetes mellitus, although statistically insignificant, patients receiving empagliflozin had a lower rate of myocardial infarction and higher rate of stroke. 2 Future studies of SGLT2 inhibitors in patients without diabetes mellitus should additionally focus on capturing these cardiovascular end points to further ascertain the efficacy of SGLT2 inhibitors as patients with HF who require concomitant risk reduction for future MI and stroke.
In patients without diabetes mellitus, we showed that treatment with SGLT2 inhibitors improved metabolic parameters, compared with those who were not treated. Although the absolute changes were modest, this benefit is seen across body weight and blood pressure parameters and indicates a potential utility of SGLT2 inhibitors in optimizing the cardiovascular risk profile of patients without diabetes mellitus. Although there was a borderline significant reduction in fasting plasma glucose, there was no significant change in HbA1c in patients without diabetes mellitus. Furthermore, across our combined cohort of 5233 patients, hypoglycemia was reported in 22 patients. This suggests a relatively good safety profile of SGLT2 inhibitors in the population without diabetes mellitus. As there are limited studies evaluating the lipid and glucose profile of patients, future research will be needed to fully elucidate the effects of SGLT2 inhibitors in patients without diabetes mellitus.
The pathophysiological mechanisms by which SGLT2 inhibitors lead to improvement in metabolic parameters are proposed to include the natriuretic effects 31 or complementary pathways to those of commonly used antihypertensives, 32 which results in blood pressure lowering and a negative caloric balance from the inhibition of renal glucose reabsorption, hence achieving weight loss. 24 However, the modest improvement in metabolic parameters does not appear to fully account for the significant risk reduction in cardiovascular outcomes in patients without diabetes mellitus. Hence, there is a need to further explore other mechanisms of action of SGLT2 inhibitors on cardiovascular health in patients without diabetes mellitus.
Limitations
Our study should be interpreted in due consideration of the limitations. First, given that many studies did not report baseline characteristics of patients without diabetes mellitus, we do not know if between‐study differences may account for differences in study outcomes across patients without diabetes mellitus.
Second, we are unable to comment on the differences in drug efficacy across SGLT2 inhibitors, such as between dapagliflozin and empagliflozin. Further clinical trials or network meta‐analysis will be important to derive the efficacy of individual SGLT2 inhibitors.
Third, across the outcomes analyzed, although the quality of evidence assessed using Grading of Recommendations Assessment, Development and Evaluation was high for most outcomes, the quality of evidence for change in NT‐proBNP, HbA1c, and fasting plasma glucose was moderate, low, and low, respectively. There was substantial heterogeneity observed for mean change in NT‐proBNP (I2=62%) and mean percentage change in HbA1c (I2=86%), and moderate heterogeneity observed for mean change in fasting plasma glucose (I2=54%). The substantial heterogeneity may be attributed to the notable difference in background medical conditions of the 8 included study populations. In the 2 studies analyzed for mean change in NT‐proBNP, Cherney 2020 12 studied 102 patients who had chronic kidney disease with proteinuria, whereas Petrie 2020 27 was a comparatively larger trial that studied 2605 patients with HF.
Fourth, there were high dropout rates observed in Hollander 2017 13 and Bays 2014. 24 This may have contributed to a high risk of attrition bias in these trials.
CONCLUSIONS
Among patients without diabetes mellitus, we demonstrated that patients with HF treated with SGLT2 inhibitors had a relative risk reduction in cardiovascular deaths and HF hospitalizations, compared with those who received placebo. Additionally, treatment with SGLT2 inhibitors in patients without diabetes mellitus improved their metabolic parameters including body weight and blood pressure. Future research of SGLT2 inhibitors in other subgroups of patients without diabetes mellitus, particularly those without HF, is warranted to determine its overall role in the management of patients without diabetes mellitus.
Sources of Funding
Sia was supported by the National University of Singapore Yong Loo Lin School of Medicine's Junior Academic Faculty Scheme.
Disclosures
None.
Supporting information
Tables S1–S2
Figures S1–S2
Acknowledgments
Author contributions: Y. H. Teo, Y. N. Teo, Syn, and Sia designed the study and developed the study protocol and tools. Y. H. Teo, Y. N. Teo, Kow, and Yoong were responsible for data collection. Y. H. Teo, Y. N. Teo, Syn, and Sia analyzed data and wrote the article. All authors contributed to the conceptualization of the research questions, interpretation of the results, and article writing. All authors read and approved the final article.
(J Am Heart Assoc. 2021;10:e019463. DOI: 10.1161/JAHA.120.019463.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. Fattah H, Vallon V. The potential role of SGLT2 inhibitors in the treatment of type 1 diabetes mellitus. Drugs. 2018;78:717–726. DOI: 10.1007/s40265-018-0901-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. DOI: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 3. Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME® trial. Eur Heart J. 2016;37:1526–1534. DOI: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. DOI: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 5. Yoshida A, Matsubayashi Y, Nojima T, Suganami H, Abe T, Ishizawa M, Fujihara K, Tanaka S, Kaku K, Sone H. Attenuation of weight loss through improved antilipolytic effect in adipose tissue via the SGLT2 inhibitor tofogliflozin. J Clin Endocrinol Metab. 2019;104:3647–3660. DOI: 10.1210/jc.2018-02254. [DOI] [PubMed] [Google Scholar]
- 6. Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add‐on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double‐blind, placebo‐controlled 102‐week trial. BMC Med. 2013;11:43. DOI: 10.1186/1741-7015-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211–220. DOI: 10.1016/S2213-8587(15)00417-9. [DOI] [PubMed] [Google Scholar]
- 8. Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Heerspink HJL, Zelniker TA, Dwyer JP, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE‐TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–617. DOI: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 9. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al. 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre‐diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J. 2020;41:255–323. DOI: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 10. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. DOI: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 11. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Lohlavek JB, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. DOI: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 12. Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Abdul Gafor AH, Greasley PJ, Laverman GD, Lim SK, Di Tanna GL, Reich HN, et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non‐diabetic patients with chronic kidney disease (DIAMOND): a randomised, double‐blind, crossover trial. Lancet Diabetes Endocrinol. 2020;8:582–593. DOI: 10.1016/S2213-8587(20)30162-5. [DOI] [PubMed] [Google Scholar]
- 13. Hollander P, Bays HE, Rosenstock J, Frustaci ME, Fung A, Vercruysse F, Erondu N. Coadministration of canagliflozin and phentermine for weight management in overweight and obese individuals without diabetes: a randomized clinical trial. Diabetes Care. 2017;40:632–639. DOI: 10.2337/dc16-2427. [DOI] [PubMed] [Google Scholar]
- 14. Ford ES. Risks for all‐cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. DOI: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 15. Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA. 2020;323:2526–2528. DOI: 10.1001/jama.2020.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. DOI: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Office EP . DAPA‐CKD trial meets primary endpoint in patients with chronic kidney disease. ESC Congress. 2020.
- 18. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. DOI: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. 2013.
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. DOI: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Review Manager (RevMan). Version 5.4. The Cochrane Collaboration. 2020.
- 22. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester, UK: John Wiley & Sons; 2019. DOI: 10.1002/9781119536604. [DOI] [Google Scholar]
- 23. Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10. DOI: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 24. Bays HE, Weinstein R, Law G, Canovatchel W. Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity (Silver Spring). 2014;22:1042–1049. DOI: 10.1002/oby.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. González‐Ortiz M, Díaz‐Cruz C, Patiño‐Laguna AJ, López‐Murillo LD, Martínez‐Abundis E. Effect of dapagliflozin on visceral adiposity and blood pressure in patients with overweight or obesity without diabetes mellitus. Diabetes. 2016;65:A513. [Google Scholar]
- 26. Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Austin B, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE‐HF trial. Circulation. 2019;140:1463–1476. DOI: 10.1161/CIRCULATIONAHA.119.042929. [DOI] [PubMed] [Google Scholar]
- 27. Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, De Boer RA, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353–1368. DOI: 10.1001/jama.2020.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Díaz‐Cruz C, González‐Ortiz M, Rosales‐Rivera LY, Patiño‐Laguna AJ, Ramírez‐Rodríguez ZG, Díaz‐Cruz K, Martínez‐Abundis E. Effects of dapagliflozin on blood pressure variability in patients with prediabetes and prehypertension without pharmacological treatment: a randomized trial. Blood Press Monit. 2020;25:346–350. DOI: 10.1097/MBP.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 29. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. DOI: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11:e004873. DOI: 10.1161/CIRCHEARTFAILURE.117.004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Javed Z, Papageorgiou M, Deshmukh H, Rigby AS, Qamar U, Abbas J, Khan AY, Kilpatrick ES, Atkin SL, Sathyapalan T. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: a randomized controlled study. Clin Endocrinol (Oxf). 2019;90:805–813. DOI: 10.1111/cen.13968. [DOI] [PubMed] [Google Scholar]
- 32. Mancia G, Cannon Christopher P, Tikkanen I, Zeller C, Ley L, Woerle Hans J, Broedl Uli C, Johansen OddE. Impact of empagliflozin on blood pressure in patients with type 2 diabetes mellitus and hypertension by background antihypertensive medication. Hypertension. 2016;68:1355–1364. DOI: 10.1161/HYPERTENSIONAHA.116.07703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S2


