Abstract
Background
Acute decompensated heart failure (ADHF) and respiratory tract infections (RTIs) are potentially life‐threatening complications in patients experiencing stroke during hospitalization. We aimed to test whether blood biomarker panels might predict these complications early after admission.
Methods and Results
Nine hundred thirty‐eight patients experiencing ischemic stroke were prospectively recruited in the Stroke‐Chip study. Post‐stroke complications during hospitalization were retrospectively evaluated. Blood samples were drawn within 6 hours after stroke onset, and 14 biomarkers were analyzed by immunoassays. Biomarker values were normalized using log‐transformation and Z score. PanelomiX algorithm was used to select panels with the best accuracy for predicting ADHF and RTI. Logistic regression models were constructed with the clinical variables and the biomarker panels. The additional predictive value of the panels compared with the clinical model alone was evaluated by receiver operating characteristic curves. An internal validation through a 10‐fold cross‐validation with 3 repeats was performed. ADHF and RTI occurred in 19 (2%) and 86 (9.1%) cases, respectively. Three‐biomarker panels were developed as predictors: vascular adhesion protein‐1 >5.67, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) >4.98 and d‐dimer >5.38 (sensitivity, 89.5%; specificity, 71.7%) for ADHF; and interleukin‐6 >3.97, von Willebrand factor >3.67, and d‐dimer >4.58 (sensitivity, 82.6%; specificity, 59.8%) for RTI. Both panels independently predicted stroke complications (panel for ADHF: odds ratio [OR] [95% CI], 10.1 [3–52.2]; panel for RTI: OR, 3.73 [1.95–7.14]) after adjustment by clinical confounders. The addition of the panel to clinical predictors significantly improved areas under the curve of the receiver operating characteristic curves in both cases.
Conclusions
Blood biomarkers could be useful for the early prediction of ADHF and RTI. Future studies should assess the usefulness of these panels in front of patients experiencing stroke with respiratory symptoms such as dyspnea.
Keywords: ADHF, biomarkers, stroke, stroke‐associated infection
Subject Categories: Ischemic Stroke, Prognosis
Nonstandard Abbreviations and Acronyms
- ADHF
acute decompensated heart failure
- RTI
respiratory tract infections
- vWF
Von Willebrand factor
Clinical Perspective
What Is New?
This is a retrospective study of a multicenter cohort with 938 patients experiencing ischemic stroke in which the measurement of 14 biomarkers was performed with early blood samples, extracted in the first 6 hours after the episode.
Two biomarker combinations add predictive value to the clinical models for the prediction of 2 major complications after stroke: acute decompensated heart failure and respiratory tract infections.
What Are the Clinical Implications?
These 2 biomarker panels, jointly with clinical variables, would be able to predict acute decompensated heart failure and respiratory tract infection in the first hours after stroke.
This would allow clinicians to monitor the patients at the highest risk to experience these pathologies and treat them accordingly, reducing the length of stay and the mortality of these patients.
Mortality of patients experiencing stroke in Europe ranges from 13% to 35%. 1 Acute post‐stroke complications, such as respiratory infections and cardiac complications, are major causes of death in patients experiencing acute stroke. 2 Early detection and treatment of these medical complications might represent an opportunity to further improve the outcome of these patients. 3
Acute decompensated heart failure (ADHF) is defined as a new onset or rapidly or gradual worsening of heart failure (HF) symptoms that require urgent therapy. 4 A frequency of 17% of ADHF in acute stroke patients has been reported, and this cardiac complication is a strong independent predictor of poor functional outcome in these patients. 5
Respiratory tract infection (RTI) represents a well‐known complication in patients experiencing stroke, and its frequency is around 12%. 6 RTIs are also linked with poor functional outcome of the patients, a prolonged length of stay in the hospital, and increased mortality up to 1 year. 7
Both RTI and ADHF may have common symptoms such as dyspnea, and early chest radiograph may not show typical changes, so differentiating between the 2 clinical conditions can be difficult in daily clinical practice. In this context, blood biomarkers might serve as a useful tool to identify those patients at the highest risk of developing these complications, as well as for the early differential diagnosis of post‐stroke RTI or ADHF.
In this study, we aimed to test whether blood biomarkers measured at admission might identify patients at the highest risk of RTI or ADHF.
Methods
The present study represents a retrospective analysis of the patients experiencing ischemic stroke prospectively recruited at the multicenter Stroke‐Chip study. 8 Certified neurologists, blinded to the biomarker analytic results, retrospectively evaluated clinical records to determine the occurrence of post‐stroke complications during hospitalization. The Stroke‐Chip study protocol was approved by each recruiting center’s ethics committee, and all patients or relatives gave written informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Diagnosis of Post‐Stroke Complications
ADHF was defined, according to American Heart Association Guidelines, 9 as new onset of dyspnea and/or fatigue, together with fluid retention, which may lead to pulmonary, or splanchnic congestion or peripheral edema, in the absence of other causes of dyspnea.
RTIs were diagnosed according to the Centers for Disease Control and Prevention criteria. 10 In summary, the diagnosis of RTI required the presence of fever (body temperature >38°C) or elevated white blood cell count (>1.2×1010/L) and evidence of organ‐specific involvement (purulent sputum, pulmonary infiltrate, cough, etc) or a positive blood culture. RTIs were classified as upper RTI, lower RTI, and pneumonia. An abnormal chest radiograph was required for the diagnosis of stroke‐associated pneumonia.
Blood Sample Collection and Biomarker Measurement
Samples were drawn at admission, within 6 hours after stroke onset, and before the administration of acute‐phase therapies such as reperfusion. Blood was collected into EDTA tubes, centrifuged at 1500g for 15 minutes at 4°C, and plasma aliquots were frozen at −80°C until biomarker measurement.
A 14‐biomarker panel was measured. This panel included insulin‐like growth factor–binding protein‐3, tumor necrosis factor receptor‐1, growth‐related oncogene‐α, Fas ligand, heat shock 70 kDa protein‐8, NT‐proBNP (N‐terminal pro‐B‐type natriuretic eptide), d‐dimer, interleukin‐6, von Willebrand factor (vWF), vascular adhesion protein‐1, endostatin, S100 calcium‐binding protein B, apolipoprotein CIII, and neuron cell adhesion molecule. The selection of these biomarkers and the measurement methods were described previously. 8
Biomarker values were log‐transformed with a base of 10 and divided by the value of the control sample of each plate. Because of the high intra‐assay variability for some of the molecules, all the values were standardized by means of Z score.
Statistical Analysis
Statistical analyses were conducted with Statistical Packages for Social Sciences (version 22; SPSS Inc., Chicago, IL) and R software (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria). Normality of the continuous variables was assumed because of the size of the sample according to the central limit theorem. The univariate analysis was performed using the χ2 test for categorical variables and t‐test for continuous variables. Binary logistic regression models were developed. Putative confounders based on previous literature were added to the models to adjust them. Multicollinearity among the predictor variables has been checked with the variance inflation factor. Firth correction was applied (“logistf” package in R) when constructing the binary logistic regression models for ADHF to minimize the estimates of the coefficients, as there is a low rate of events.
To avoid overfitting the models with individual biomarkers, the best biomarker combination for the prediction of the defined complications was selected using PanelomiX algorithm, 11 optimizing the combination by accuracy with a maximum of 3 biomarkers. The analyses for the selection of the best biomarker combination were carried out separately and independently in each of the complications (ADHF and RTI). Briefly, PanelomiX uses the iterative combination of biomarkers and thresholds method to select thresholds for each biomarker that provide the optimal classification performance and studies the robustness of the constructed panels through receiver operating characteristic (ROC) curves and cross‐validation, performing a 10‐fold cross‐validation. After the 10‐fold cross‐validation and for the evaluation of the performance, ROC curves of the cross‐validation are built as the mean of centered predictions over the 10 cross‐validation folds. Then, the ROC curve is compared through De Long’s method with the cross‐validation of the individual biomarkers.
To evaluate the additional value of the selected biomarker combination to the logistic regression models with the clinical predictors, De Long’s method was used to compare areas under the curve of ROC curves. An internal validation of the constructed logistic regression models was performed through a 10‐fold cross‐validation with 3 repeats, evaluating the same parameters than in the cross‐validation performed by PanelomiX. A P‐value <0.05 was considered statistically significant in all tests.
Results
Nine hundred thirty‐eight patients experiencing ischemic stroke from the Stroke‐Chip study were included in this analysis. ADHF occurred in 19 of these patients (2%) and RTI in 86 patients (9.1%). Nine patients were noted with both complications. Patients with 1 or both complications were older, had higher rates of atrial fibrillation, higher baseline National Institute of Health Stroke Scale score, and higher previous modified Rankin scale score. Higher rates of in‐hospital mortality and 3‐month disability (Table 1) were also reported in these groups of patients. Patients with ADHF also had higher rates of coronary disease, and patients with RTI had higher values of admission glycemia.
Table 1.
Univariate Analysis for ADHF and RTI After Stroke
| ADHF | RTI | |||||
|---|---|---|---|---|---|---|
| No (N=919) | Yes (N=19) | P Value | No (N=852) | Yes (N=86) | P Value | |
| Age, y | 72.52 (±13) | 81.1 (±13.5) | <0.0001* | 72.2 (±13) | 77.2 (±12.3) | 0.001 † |
| Sex, female | 420 (45.7) | 11 (57.9) | 0.291 | 388 (45.6) | 42 (48.8) | 0.572 |
| Hypertension | 675 (73.4) | 13 (68.4) | 0.624 | 617 (72.6) | 69 (80.2) | 0.127 |
| Dyslipemia | 451 (49.1) | 9 (47.4) | 0.883 | 421 (49.5) | 38 (44.2) | 0.345 |
| Diabetes mellitus | 237 (25.8) | 2 (10.5) | 0.184 | 214 (25.2) | 25 (29.1) | 0.430 |
| Tobacco | 149 (16.2) | 3 (15.8) | 0.999 | 144 (16.9) | 8 (9.3) | 0.067 |
| Alcohol | 64 (7) | 1 (5.3) | 0.999 | 64 (7.5) | 1 (1.2) | 0.027 ‡ |
| Atrial fibrillation | 318 (34.6) | 11 (57.9) | 0.035 ‡ | 282 (33.2) | 46 (53.5) | <0.0001* |
| Coronary disease | 145 (15.8) | 7 (36.8) | 0.023 ‡ | 132 (15.5) | 19 (22.1) | 0.115 |
| Previous stroke | 158 (17.2) | 5 (26.3) | 0.353 | 144 (16.9) | 18 (20.9) | 0.351 |
| Previous mRS | 0 (0–1) | 1.5 (0–3) | 0.001* | 0 (0–1) | 1 (0–3) | <0.0001* |
| Baseline NIHSS | 9 (±7.3) | 15 (±4.9) | <0.0001* | 8.5 (±7.1) | 15.5 (±6) | <0.0001* |
| TOAST | ||||||
| CE | 330 (40.7) | 11 (84.6) | 0.050 | 337 (40.1) | 46 (55.4) | 0.007 † |
| LAA | 116 (14.3) | 0 (0) | 115 (13.7) | 10 (12) | ||
| Lacunar | 109 (11.9) | 0 (0) | 119 (14.1) | 2 (2.4) | ||
| Undetermined | 240 (29.6) | 2 (15.4) | 255 (30.3) | 22 (26.5) | ||
| Other | 15 (1.9) | 0 (0) | 15 (1.8) | 3 (3.6) | ||
| In‐hospital mortality | 108 (9.9) | 8 (42.1) | <0.0001* | 44 (5.2) | 30 (34.9) | <0.0001* |
| 3‐mo disability | 366 (42.2) | 16 (84.32) | <0.0001* | 221 (32.6) | 67 (89.3) | <0.0001* |
| Apolipoprotein CIII | 4.02 (±0.97) | 3.68 (±0.98) | 0.144 | 4.01 (±0.98) | 3.99 (±0.93) | 0.778 |
| d‐dimer | 4.08 (±0.95) | 4.60 (±0.79) | 0.020 ‡ | 4.05 (±0.94) | 4.53 (±0.93) | <0.0001* |
| Endostatin | 4.11 (±0.94) | 4.50 (±0.89) | 0.073 | 4.09 (±0.93) | 4.35 (±1.08) | 0.015 ‡ |
| GROA | 3.99 (±0.97) | 3.96 (±0.87) | 0.881 | 4.00 (±0.98) | 3.94 (±0.93) | 0.582 |
| Interleukin‐6 | 4.00 (±0.96) | 4.24 (±0.77) | 0.265 | 3.95 (±0.94) | 4.49 (±1.02) | <0.0001* |
| NT‐proBNP | 4.12 (±0.95) | 5.03 (±0.87) | <0.0001* | 4.11 (±0.95) | 4.45 (±0.91) | 0.001 * |
| VAP‐1 | 4.05 (±0.99) | 4.79 (±1.05) | 0.001* | 4.05 (±0.98) | 4.19 (±1.12) | 0.199 |
| vWF | 4.02 (±0.97) | 4.25 (±0.88) | 0.306 | 3.97 (±0.97) | 4.49 (±0.89) | <0.0001* |
| IGFBP‐3 | 4.00 (±0.98) | 3.91 (±1.07) | 0.694 | 3.99 (±0.98) | 4.10 (±1.05) | 0.330 |
| Fas ligand | 3.96 (±0.99) | 4.40 (±0.72) | 0.056 | 3.95 (±0.99) | 4.16 (±0.99) | 0.065 |
| TNF‐R1 | 4.04 (±0.98) | 4.36 (±0.52) | 0.038 ‡ | 4.04 (±0.97) | 4.17 (±1.03) | 0.235 |
| NCAM | 3.98 (±0.99) | 4.36 (±0.76) | 0.090 | 3.99 (±0.98) | 3.91 (±1.03) | 0.498 |
| S100B | 4.03 (±0.98) | 3.72 (±0.87) | 0.233 | 4.02 (±0.99) | 3.95 (±0.91) | 0.558 |
| Hsc70 | 4.04 (±0.95) | 4.18 (±1.09) | 0.567 | 4.04 (±0.95) | 4.13 (±0.92) | 0.414 |
Values are reported as n (%) or mean (±SD). Biomarker values are standardized. ADHF indicates acute decompensated heart failure; CE, cardiac embolism; DBP, diastolic blood pressure; GROA, growth‐related oncogene‐α; Hsc70, heat shock 70 kDa protein‐8; IGFBP‐3, insulin‐like growth factor‐binding protein‐3; LAA, large‐artery atherothrombosis; mRS, modified rankin scale; NCAM, neuron cell adhesion molecule; NIHSS, National Institutes of Health Stroke Scale; NTproBNP, N‐terminal pro‐B‐type natriuretic peptide; RTI, respiratory tract infection; S100B, S100 calcium‐binding protein B; SBP, systolic blood pressure; TNF‐R1, tumor necrosis factor receptor‐1; TOAST, Trial of Org 10172 in Acute Stroke Treatment; and VAP‐1, vascular adhesion protein‐1.
P<0.001.
P<0.01.
P<0.05.
Among the analyzed molecules, patients with ADHF had higher plasma levels of d‐dimer, NT‐proBNP, tumor necrosis factor receptor‐1, and vascular adhesion protein‐1 than those without this complication, whereas patients with RTI had higher levels of d‐dimer, endostatin, interleukin‐6, NT‐proBNP, and vWF compared with patients without this complication. The values of each biomarker and the clinical variables are reported in Table 1.
A panel including vascular adhesion protein‐1 >5.67, NT‐proBNP >4.98, and d‐dimer >5.38 was selected by the PanelomiX software as the most accurate for the prediction of ADHF. The panel was positive when any 1 of the markers was above the cutoff (specificity, 71.5%; sensitivity, 89.5%). For the prediction of RTI, the software selected a panel including interleukin‐6>3.97, vWF >3.67, and d‐dimer >4.58. In this case, the panel was positive when at least 2 of the markers were above the cutoff (specificity, 59.8%; sensitivity, 82.9%) (Figure 1). Of the 9 patients with both complications, 7 were positive for both panels, 1 patient was positive for only the RTI panel, and the other patient was negative for both panels.
Figure 1. ROC curves of the biomarkers alone and in combination for each complication.
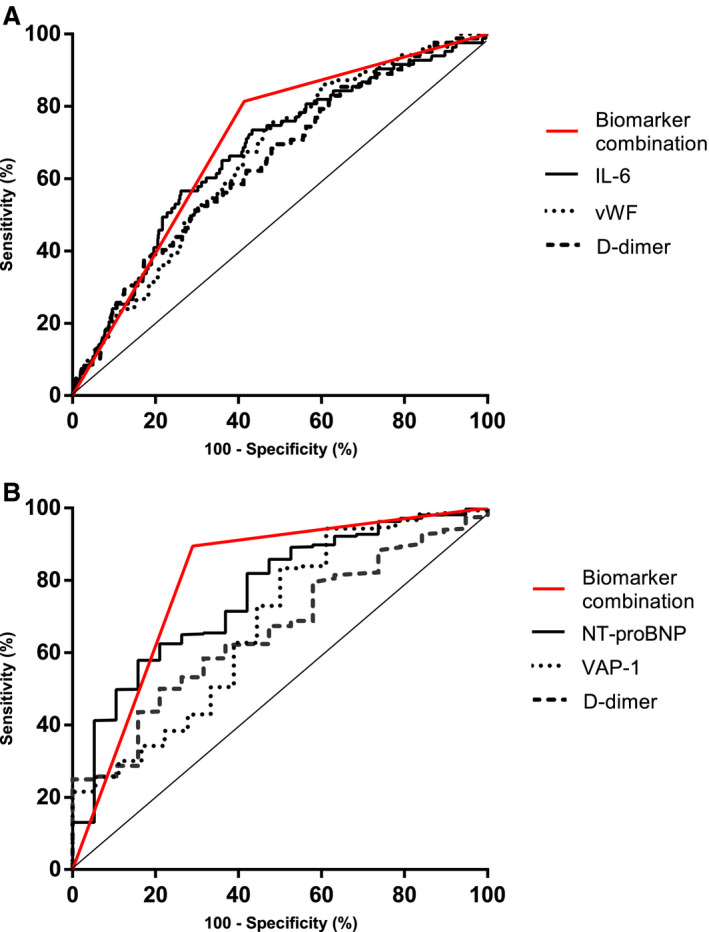
A, ROC curve for RTI biomarkers panel (black line), D‐dimer (dotted line), vWF (gray) and interleukin‐6 (dashed line). B, ROC curve for ADHF biomarker panel (black line), VAP‐1 (dotted line), NT‐proBNP (gray line), and d‐dimer (dashed line). ADHF indicates acute decompensated heart failure; IL‐6, interleukin‐6; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; ROC, receiver operating characteristic; RTI, respiratory tract infection; VAP‐1, vascular adhesion protein‐1 and vWF, von Willebrand factor.
Two different binary logistic regression models were developed, one for each complication. The selected biomarker panels independently predicted ADHF and RTI, respectively (odds ratio [OR], 11.859 [95% CI, 2.67–53.74], P=0.001 for ADHF; OR, 3.73 [1.95–7.15], P<0.0001 for RTI) after adjusting by selected clinical variables (Table 2). Age, sex, and National Institute of Health Stroke Scale were selected as confounders in both models because of their influence in the development of both complications. 12 In case of ADHF, coronary disease was also added to the model, as it has been reported as a risk factor for ADHF. 13 Multicollinearity was not detected in any of the models. The addition of the biomarker panels to the logistic regression models improved the accuracy of the clinical model for both complications in terms of area under the curve, from 0.80 (95% CI, 0.70–0.88) to 0.88 (95% CI, 0.83–0.93) (P=0.038) in ADHF and; from 0.78 (95% CI, 0.73–0.82) to 0.81 (95% CI, 0.77–0.88) (P=0.048) in the case of RTI (Table 2).
Table 2.
Logistic Regression Analyses and Additional Predictive Value of Blood Biomarkers for ADHF and RTI
| ADHF | RTI | |||
|---|---|---|---|---|
| Clinical Model | Clinical Model+Biomarkers Panel | Clinical Model | Clinical Model+Biomarkers Panel | |
| Logistic regression, OR | ||||
| NIHSS at admission | 1.09 (1.02–1.16), P=0.006* | 1.06 (1–1.14), P=0.048 † | 1.12 (1.09–1.16), P<0.0001 ‡ | 1.10 (1.07–1.14), P<0.0001 ‡ |
| Age | 1.04 (0.99–1.11), P=0.07 | 1.02 (0.98–1.08), P=0.26 | 1.02 (1–1.04), P=0.02 † | 1.01 (0.99–1.03), P=0.2 |
| Coronary disease | 3.24 (1.18–8.44), P=0.02 † | 2.8 (1.01–7.36), P=0.047 † | … | … |
| Sex, female | 1.36 (0.5–3.83), P=0.52 | 1.16 (0.43–3.26), P=0.76 | 0.8 (0.51–1.35), P=0.38 | 0.8 (0.5–1.3), P=0.37 |
| Biomarker combination | … | 10.1 (3–52.2), P<0.0001 ‡ | … | 3.73 (2.06–6.75), P<0.0001 ‡ |
| ROC curve | ||||
| AUC | 0.80 (0.70–0.88) | 0.88 (0.83–0.93) | 0.78 (0.73–0.82) | 0.81 (0.77–0.88) |
| De Long test | … | P=0.038 † | … | P=0.048 † |
| Cross‐validation AUC | 0.79 (0.72–0.87) | 0.87 (0.82–0.92) | 0.78 (0.74–0.83) | 0.82 (0.78–0.85) |
| De Long test | … | P=0.02 † | … | P=0.002* |
The table represents the comparison between predictive models, with or without biomarker combinations. For logistic regression models, OR (95% CI) and P values are given. For the evaluation of the performance of the models, the AUC (95% CI) of the models without doing cross‐validation and carrying out a 10‐fold cross‐validation is represented. AUC indicates area under the curve; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; and ROC, receiver operator characteristic.
P<0.01.
P<0.05.
P<0.001.
These increments of the area under the curve when adding the biomarker combinations to the clinical models were also observed after the internal validation of these results through a 10‐fold cross‐validation. The results of the cross‐validation are shown in Table 2, and all the ROC curves are represented in Figure 2.
Figure 2. Performance of the constructed models for the 2 complications.
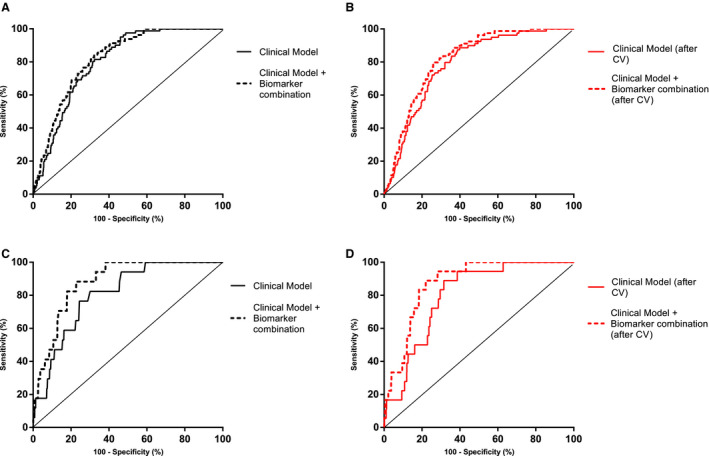
A and B represent the performance of the models constructed for RTI, and C and D represent the performance of the models constructed for ADHF. A, ROC curve overlapping the clinical model and the clinical model plus the biomarker combination for RTI. The AUC increase is significant (P=0.048). B, ROC curve overlapping the clinical model and the clinical model plus the biomarker combination for RTI after the cross‐validation. The AUC increase is significant (P=0.002). C, ROC curve overlapping the clinical model and the clinical model plus the biomarker combination for ADHF. The AUC increase is significant (P=0.038). D, ROC curve overlapping the clinical model and the clinical model plus the biomarker combination for RTI after the cross‐validation. The AUC increase is significant (P=0.02). ADHF indicates acute decompensated heart failure; AUC, area under the curve; ROC, receiver operating characteristic; and RTI, respiratory tract infection.
Discussion
The present study demonstrates that the occurrence of 2 common post‐stroke complications during hospitalization, ADHF and RTI, could be predicted at admission by using 2 different biomarker panels in combination with clinical factors.
The use of blood biomarkers for the prediction of post‐stroke complications has generated increasing research in the past years. 14 , 15 However, individual markers tested so far have not demonstrated enough predictive accuracy to be implemented in clinical practice. In this article, rather than testing individual markers, we tested automatically derived biomarker panels, which might be complementary tools to achieve a better prediction. 16 In fact, biomarker panels might be more valuable to reflect the complexity of the disease and its consequences, with several impaired biological pathways that might be reflected by automatically selected biomarkers. In fact, we obtained 2 different biomarker panels with individual markers from diverse pathways implicated in stroke and post‐stroke complications.
Of the biomarkers proposed for the ADHF prediction after stroke, NT‐proBNP has been widely associated with the diagnosis of ADHF. 17 Vascular adhesion protein‐1, a marker of endothelial dysfunction, has also been related with congestive HF, being elevated in these patients when compared with controls, 18 and an independent prognostic marker for mortality in chronic HF patients. 19 d‐dimer is a product of fibrin degradation. In healthy subjects, high levels of d‐dimer have been associated with incident HF with reduced ejection fraction. 20 Moreover, in patients with known HF, higher levels of d‐dimer predicted mortality and incident atrial fibrillation. 21
The proteins from the RTI biomarker panel are more related with the acute phase. An increase in the levels of the proinflammatory cytokine interleukin‐6 in patients with stroke‐associated pneumonia has been reported, 22 , 23 , 24 which can predict the risk of stroke‐associated infections and mortality. Regarding the other 2 biomarkers of the panel, d‐dimer has been associated with community‐acquired pneumonia. Increased plasma levels of this molecule were correlated with the severity of the disease. 25 vWF is the only protein that has not been related with RTIs previously. Its major biological functions are related with adhesion of platelets and factor VII protection. 26 But in the past years, new functions of this molecule have emerged. Interactions between platelets and bacterial infections have been reported, 27 as well as the role of vWF with the immune cells, as leukocyte extravasation. 28
Our results, if confirmed, might have clinical implications. We propose 2 different biomarker panels that could predict at admission the appearance of ADHF and RTI after stroke. Both biomarker panels have a high sensitivity, resulting in a detection of most of the true‐positive patients. After stroke onset, predicting these 2 complications could help clinicians to monitor and treat these 2 kinds of patients as early as possible. Consequently, with these 2 biomarker combinations in clinical practice and the adequate tools to treat these patients, there could be a reduction of the in‐hospital length of stay or even a decrease in mortality. In the case of RTI, there is no evidence that antibiotic prophylaxis prevents the onset of these infections or reduces the mortality of these patients, 29 but whether selective antibiotic prophylaxis in high‐risk patients or alternative prophylactic measures such as immunomodulatory therapies are effective is being studied.
As mentioned in the introduction, dyspnea is a common symptom of ADHF and RTI, and this can sometimes delay the diagnosis of the complication. We suggest that these 2 biomarker combinations could also be useful for the differentiation of both diseases, and clinicians would have additional tools for an early diagnosis or treatment. But to confirm this hypothesis, a prospective study measuring these markers in patients with dyspnea should be performed.
The study has certain limitations. First, the fact that data were collected by reviewing clinical registries might lead to some missing data. In fact, the incidence of ADHF in our study is much lower than reported. Second, the low number of events in the case of ADHF, even after the use of Firth correction, leads to wide CIs for the combination of blood biomarkers, making these results less reliable. Third, data on previous history of HF were not available and, therefore, we cannot rule out whether the predictive value of the biomarker panels might be modified by the existence of previous HF. Likewise, relevant data related to RTI were also not available, such as dysphagia or severe facial palsy at admission. An external validation or replication of our results has not been possible because of the inability to obtain a large cohort of stroke patients with acute blood samples. Furthermore, the clinical value added by the biomarkers combination in the clinical models is rather low. In addition, the use of study‐derived cutoff points for biomarker measurement, even when is automatically selected by the PanelomiX software and not driven by the investigators, might overestimate the precision of our observations. However, given the preliminary nature of the present study, the selected cutoffs might serve as a reference for replication purposes in further studies. Finally, the measured biomarkers had other intended uses; therefore, our scores might be improved using other biomarkers more related to our end points such as C‐reactive protein, 30 procalcitonin, 31 or serum amyloid A for RTI 32 or midregional proatrial natriuretic peptide, troponin, interleukin‐1 receptor–like 1 or galectin‐3 for ADHF. 17
These limitations confer to our study a hypothesis‐generating nature, and future studies are needed to establish the practical usefulness of those markers. These future prospective studies should include patients experiencing stroke at admission, collecting data of cardiac and respiratory symptoms, and, ideally, serial measurements of the biomarker panels in all included patients, to determine the specificity and sensitivity of each combination for the prediction between both complications. Dyspnea should also be reported, to explore the capacity of the biomarker combinations to differentiate between the 2 conditions in these patients. The measurement of specific biomarkers of each complication, as the commented in the previous paragraph, might help to improve the prediction capacity of the panels.
Conclusions
In conclusion, blood biomarkers could be useful for the early prediction of 2 of the major complications during the in‐hospital stay of stroke patients: ADHF and RTI. Future studies should focus on the validation of these results as well as the discriminative power of the panels in patients with dyspnea.
Sources of Funding
This project received funding from Instituto de Salud Carlos III (ISCIII) [DTS14/00004, PI17/02130], co‐financed by the European Regional Development Fund (FEDER), and from Fundació La Marató de TV3 [201706] and the European Union's Horizon 2020 research and innovation program [754517]. Neurovascular Research Laboratory takes part into the Spanish stroke research network INVICTUS+ (RD16/0019/0021). The funders had no role in the study design and conduction.
Disclosures
None.
Acknowledgments
Author contributions: Drs Bustamante, Dávalos, and Montaner conceived the study. Drs Bustamante, López‐Cancio, and García‐Berrocoso managed the study. Drs Bustamante, Reverté, Millán, Castellanos, Lara‐Rodríguez, Zaragoza, Hernández‐Pérez, Eendenburg, Cardona, López‐Cancio, Cánovas, Serena, and Rubiera acquired the data. J. Faura and O. Ventura performed the statistical analysis. J. Faura and Drs Bustamante, López‐Cancio, and Montaner interpreted the data. J. Faura drafted the manuscript. All authors performed a critical review of the manuscript.
(J Am Heart Assoc. 2021;10:e018946. DOI: 10.1161/JAHA.120.018946.)
For Sources of Funding and Disclosures, see page 7.
References
- 1. Béjot Y, Bailly H, Durier J, Giroud M Epidemiology of stroke in Europe and trends for the 21st century. Presse Med. 2016;45:e391–e398. DOI: 10.1016/j.lpm.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 2. Kumar S, Selim MH, Caplan LR Medical complications after stroke. Lancet Neurol. 2010;9:105–118. DOI: 10.1016/S1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- 3. Bustamante A, Giralt D, García‐Berrocoso T, Rubiera M, Álvarez‐Sabín J, Molina C, Serena J, Montaner J The impact of post‐stroke complications on in‐hospital mortality depends on stroke severity. Eur Stroke J. 2017;2:54–63. DOI: 10.1177/2396987316681872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gheorghiade M, Pang PS Acute heart failure syndromes. J Am Coll Cardiol. 2009;53:557–573. DOI: 10.1016/j.jacc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 5. Burkot J, Kopec G, Pera J, Slowik A, Dziedzic T Decompensated heart failure is a strong independent predictor of functional outcome after ischemic stroke. J Card Fail. 2015;21:642–646. DOI: 10.1016/j.cardfail.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 6. Badve MS, Zhou Z, van de Beek D, Anderson CS, Hackett ML Frequency of post‐stroke pneumonia: systematic review and meta‐analysis of observational studies. Int J Stroke. 2019;14:125–136. DOI: 10.1177/1747493018806196. [DOI] [PubMed] [Google Scholar]
- 7. Teh WH, Smith CJ, Barlas RS, Wood AD, Bettencourt‐Silva JH, Clark AB, Metcalf AK, Bowles KM, Potter JF, Myint PK Impact of stroke‐associated pneumonia on mortality, length of hospitalization, and functional outcome. Acta Neurol Scand. 2018;138:293–300. DOI: 10.1111/ane.12956. [DOI] [PubMed] [Google Scholar]
- 8. Bustamante A, López‐Cancio E, Pich S, Penalba A, Giralt D, García‐Berrocoso T, Ferrer‐Costa C, Gasull T, Hernández‐Pérez M, Millan M, et al. Blood biomarkers for the early diagnosis of stroke: the Stroke‐Chip study. Stroke. 2017;48:2419–2425. DOI: 10.1161/STROKEAHA.117.017076. [DOI] [PubMed] [Google Scholar]
- 9. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non–ST‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. DOI: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 10. Horan TC, Andrus M, Dudeck MA CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. DOI: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M PanelomiX: a threshold‐based algorithm to create panels of biomarkers. Transl Proteomics. 2013;1:57–64. DOI: 10.1016/j.trprot.2013.04.003. [DOI] [Google Scholar]
- 12. Smith CJ, Bray BD, Hoffman A, Meisel A, Heuschmann PU, Wolfe CDA, Tyrrell PJ, Rudd AG Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. J Am Heart Assoc. 2015;4:1–9.e001307. DOI: 10.1161/JAHA.114.001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lala A, Desai AS The role of coronary artery disease in heart failure. Heart Fail Clin. 2014;10:353–365. DOI: 10.1016/j.hfc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 14. Bustamante A, Díaz‐Fernández B, Pagola J, Blanco‐Grau A, Rubiera M, Penalba A, García‐Berrocoso T, Montaner J Admission troponin‐I predicts subsequent cardiac complications and mortality in acute stroke patients. Eur Stroke J. 2016;1:205–212. DOI: 10.1177/2396987316654337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zapata‐Arriaza E, Mancha F, Bustamante A, Moniche F, Pardo‐Galiana B, Serrano‐Gotarredona P, Navarro‐Herrero S, Pallisa E, Faura J, Vega‐Salvatierra Á, et al. Biomarkers predictive value for early diagnosis of Stroke‐Associated Pneumonia. Ann Clin Transl Neurol. 2019;6:1882–1887.DOI: 10.1002/acn3.50849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adamcova M, Šimko F Multiplex biomarker approach to cardiovascular diseases perspective. Acta Pharmacol Sin. 2018;39:1068–1072. DOI: 10.1038/aps.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mallick A, Januzzi JL Jr Biomarkers in acute heart failure. Rev Esp Cardiol. 2015;68:514–525. [DOI] [PubMed] [Google Scholar]
- 18. Boomsma F, Van Veldhuisen DJ, De Kam PJ, Man In ’T Veld AJ, Mosterd A, Lie KI, Schalekamp MADH Plasma semicarbazide‐sensitive amine oxidase is elevated in patients with congestive heart failure. Cardiovasc Res. 1997;33:387–391. DOI: 10.1016/S0008-6363(96)00209-X. [DOI] [PubMed] [Google Scholar]
- 19. Boomsma F, De Kam PJ, Tjeerdsma G, Van Den Meiracker AH, Van Veldhuisen DJ Plasma semicarbazide‐sensitive amine oxidase (SSAO) is an independent prognostic marker for mortality in chronic heart failure. Eur Heart J. 2000;21:1859–1863. DOI: 10.1053/euhj.2000.2176. [DOI] [PubMed] [Google Scholar]
- 20. de Boer RA, Nayor M, deFilippi CR, Enserro D, Bhambhani V, Kizer JR, Blaha MJ, Brouwers FP, Cushman M, Lima JAC, et al. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. 2018;3:215–224. DOI: 10.1001/jamacardio.2017.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wannamethee SG, Whincup PH, Papacosta O, Lennon L, Lowe GD Associations between blood coagulation markers, NT‐proBNP and risk of incident heart failure in older men: the British Regional Heart Study. Int J Cardiol. 2017;230:567–571. DOI: 10.1016/j.ijcard.2016.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwan J, Horsfield G, Bryant T, Gawne‐Cain M, Durward G, Byrne CD, Englyst NA IL‐6 is a predictive biomarker for stroke associated infection and future mortality in the elderly after an ischemic stroke. Exp Gerontol. 2013;48:960–965. DOI: 10.1016/j.exger.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 23. Hoffmann S, Harms H, Ulm L, Nabavi DG, Mackert B‐M, Schmehl I, Jungehulsing GJ, Montaner J, Bustamante A, Hermans M, et al. Stroke‐induced immunodepression and dysphagia independently predict stroke‐associated pneumonia—The PREDICT study. J Cereb Blood Flow Metab. 2017;37:3671–3682. DOI: 10.1177/0271678X16671964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bustamante A, Sobrino T, Giralt D, García‐Berrocoso T, Llombart V, Ugarriza I, Espadaler M, Rodríguez N, Sudlow C, Castellanos M, et al. Prognostic value of blood interleukin‐6 in the prediction of functional outcome after stroke: a systematic review and meta‐analysis. J Neuroimmunol. 2014;274:215–224. DOI: 10.1016/j.jneuroim.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 25. Arslan S, Ugurlu S, Bulut G, Akkurt I The association between plasma d‐dimer levels and community‐acquired pneumonia. Clinics. 2010;65:593–597. DOI: 10.1590/S1807-59322010000600006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luo GP, Ni B, Yang X, Wu YZ Von Willebrand factor: more than a regulator of hemostasis and thrombosis. Acta Haematol. 2012;128:158–169. DOI: 10.1159/000339426. [DOI] [PubMed] [Google Scholar]
- 27. Hamzeh‐Cognasse H, Damien P, Chabert A, Pozzetto B, Cognasse F, Garraud O Platelets and infections—complex interactions with bacteria. Front Immunol. 2015;6:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petri B, Broermann A, Li H, Khandoga AG, Zarbock A, Krombach F, Goerge T, Schneider SW, Jones C, Nieswandt B, et al. Von Willebrand factor promotes leukocyte extravasation. Blood. 2010;116:4712–4719. DOI: 10.1182/blood-2010-03-276311. [DOI] [PubMed] [Google Scholar]
- 29. Badve MS, Zhou Z, Anderson CS, Hackett ML Effectiveness and safety of antibiotics for preventing pneumonia and improving outcome after acute stroke: systematic review and meta‐analysis. J Stroke Cerebrovasc Dis. 2018;27:3137–3147. DOI: 10.1016/j.jstrokecerebrovasdis.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 30. Bustamante A, Vilar‐Bergua A, Guettier S, Sánchez‐Poblet J, García‐Berrocoso T, Giralt D, Fluri F, Topakian R, Worthmann H, Hug A, et al. C‐reactive protein in the detection of post‐stroke infections: systematic review and individual participant data analysis. J Neurochem. 2017;141:305–314. DOI: 10.1111/jnc.13973. [DOI] [PubMed] [Google Scholar]
- 31. Fluri F, Morgenthaler NG, Mueller B, Christ‐Crain M, Katan M Copeptin, procalcitonin and routine inflammatory markers—predictors of infection after stroke. PLoS One. 2012;7:e48309. DOI: 10.1371/journal.pone.0048309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azurmendi L, Lapierre‐Fetaud V, Schneider J, Montaner J, Katan M, Sanchez JC Proteomic discovery and verification of serum amyloid A as a predictor marker of patients at risk of post‐stroke infection: a pilot study. Clin Proteomics. 2017;14:14–27. DOI: 10.1186/s12014-017-9162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


