Abstract
Background
Patients with transposition of the great arteries corrected by an atrial switch operation experience major clinical events during adulthood, mainly heart failure (HF) and arrhythmias, but data on the emerging risks remain scarce. We assessed the risk for events during the clinical course in adulthood, and provided a novel risk score for event‐free survival.
Methods and Results
This multicenter study observed 167 patients with transposition of the great arteries corrected by an atrial switch operation (61% Mustard procedure; age, 28 [interquartile range, 24–36] years) for 13 (interquartile range, 9–16) years, during which 16 (10%) patients died, 33 (20%) had HF events, defined as HF hospitalizations, heart transplantation, ventricular assist device implantation, or HF‐related death, and 15 (9%) had symptomatic ventricular arrhythmias. Five‐year risk of mortality, first HF event, and first ventricular arrhythmia increased from 1% each at age 25 years, to 6% (95% CI, 4%–9%), 23% (95% CI, 17%–28%), and 5% (95% CI, 2%–8%), respectively, at age 50 years. Predictors for event‐free survival were examined to construct a prediction model using bootstrapping techniques. A prediction model combining age >30 years, prior ventricular arrhythmia, age >1 year at repair, moderate or greater right ventricular dysfunction, severe tricuspid regurgitation, and mild or greater left ventricular dysfunction discriminated well between patients at low (<5%), intermediate (5%–20%), and high (>20%) 5‐year risk (optimism‐corrected C‐statistic, 0.86 [95% CI, 0.82–0.90]). Observed 5‐ and 10‐year event‐free survival rates in low‐risk patients were 100% and 97%, respectively, compared with only 31% and 8%, respectively, in high‐risk patients.
Conclusions
The clinical course of patients undergoing atrial switch increasingly consists of major clinical events, especially HF. A novel risk score stratifying patients as low, intermediate, and high risk for event‐free survival provides information on absolute individual risks, which may support decisions for pharmacological and interventional management.
Keywords: adult, atrial switch, Mustard repair, prediction model, Senning repair, transposition of the great arteries
Subject Categories: Congenital Heart Disease, Heart Failure, Arrhythmias, Prognosis, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- AtrSO
atrial switch operation
- TGA
transposition of the great arteries
- TR
tricuspid regurgitation
- VA
ventricular arrhythmia
Clinical Perspective
What Is New?
We studied the risk of major clinical events, defined as heart failure, ventricular arrhythmia, and death, in adults after atrial switch for transposition of the great arteries up to the sixth decade of life.
The risk of first heart failure event increased most rapidly to a 5‐year risk of 23% at the age of 50 years.
We presented a risk model for major clinical events (combining age >30 years, prior ventricular arrhythmia, age >1 year at repair, moderate or greater right ventricular dysfunction, severe tricuspid regurgitation, and mild or greater left ventricular dysfunction) that could discriminate well between patients at low, intermediate, and high risk, and provide estimates of the absolute risk after 5 and 10 years of follow‐up.
What Are the Clinical Implications?
The risk score may assist in counseling patients about their absolute risk, help determine follow‐up intensity, and support management decisions on prevention and treatment of the prevailing complications.
The atrial switch operation (AtrSO) significantly improved survival for patients with transposition of the great arteries (TGA), but left them with a challenging clinical course, including heart failure (HF), arrhythmias, and premature mortality. 1 , 2 Several studies have described late events up to the age of 30 to 40 years, 2 , 3 but accurate data on the emerging risks at older ages remain scarce. Furthermore, risk prediction remains difficult, as no models to predict absolute risks exist. New markers, such as B‐type natriuretic peptide, 4 myocardial fibrosis, 5 and strain, 6 are mainly studied on an individual basis with short follow‐up, whereas large long‐term studies have mainly focused on characteristics from early childhood, such as TGA complexity or details of the repair surgery. 1 , 3 , 7 Accordingly, counseling adult patients is still difficult as knowledge on absolute individual risks of clinical events is limited. This hampers adequate management of the clinical course during adulthood, 8 , 9 because risk assessment at the individual level is needed to interfere timely in patients at highest risk without overtreating patients at limited risk. Therefore, this study aimed to provide insight in the incidence of mortality, HF, and ventricular arrhythmias (VAs) in adult patients with TGA long after AtrSO, uncover clinical predictors for event‐free survival, and provide a risk score to improve patient counseling in daily clinical practice.
Methods
Using the Dutch CONCOR registry, which includes adults (aged ≥18 years) with congenital heart disease, we assessed survival and risk of events in adult patients with TGA after AtrSO. The CONCOR registry conforms to the Declaration of Helsinki, and was approved by the ethics boards of all participating centers. 10 The data will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population and Data Collection
This study consisted of patients included in the CONCOR registry in 5 tertiary medical centers with simple or complex TGA (defined as TGA with concomitant ventricular septal defect, left ventricular (LV) outflow tract obstruction, or coarctation of the aorta) after AtrSO. Patients were followed up from the first hospital visit after written informed consent for inclusion in the CONCOR registry (earliest inclusion in 2001) until 2019, heart transplantation, ventricular assist device implantation, or death. Medical records were reviewed for baseline and follow‐up data on events, clinical status, described as New York Heart Association functional class, ECGs, imaging (echocardiography, cardiovascular magnetic resonance, and computed tomography) and exercise testing performed during routine follow‐up. Echocardiographic systemic right ventricular (RV) and subpulmonary LV function levels were visually graded by cardiologists at each participating center as normal or mildly, moderately, or severely impaired. 11 Tricuspid regurgitation (TR) severity was graded, according to the European guidelines, from absent/trivial to severe TR. 12 Baffle obstruction was suspected in case of flow velocity of >1.5 m/s, turbulence, or loss of biphasic flow in the conduits. 11
Outcome Definitions
Clinical events were scored, specifically (1) HF events, defined as hospitalizations for HF, heart transplantation, ventricular assist device implantation, or HF as cause of death; (2) VAs, defined as symptomatic nonsustained VA, sustained VA, and sudden death; and (3) all‐cause mortality.
The primary end point used in prediction analysis was time to the first event of the composite of HF events, arrhythmia events, and all‐cause mortality.
In addition, RV dysfunction, defined as moderate or greater RV dysfunction on echocardiography, and New York Heart Association functional class at last follow‐up were reported to analyze clinical decline during adulthood.
Statistical Analysis
Data are expressed as numbers (percentages), mean±SD, or median (interquartile range), as appropriate. Kaplan‐Meier analysis with age as a time scale, using a delayed entry method for left‐truncated, right‐censored data, was conducted to provide survival estimates of event‐naive patients. 13 , 14 Five‐year risk of first events was estimated using discrete‐time survival methods.
Multiple imputation, based on baseline characteristics (maximum percentage missing, 29%) and the end points, was used to address missing data, creating 100 data sets. Characteristics derived from cardiovascular magnetic resonance/computed tomography and cardiopulmonary exercise tests were not included in the imputation models, because they entailed many missing values (54%–61%) as they were performed less frequently during routine follow‐up, especially in the earlier years. Cox proportional hazards regression was used to analyze whether patient characteristics were associated with event‐free survival.
Prediction Model
A prediction model based on clinical characteristics, ECG, and echocardiography measurements at baseline was derived with backward stepwise selection based on the Akaike Information Criterion, in 100 bootstrap samples per imputed data set. Entry criterion into multivariable analysis was P<0.05 in univariable analysis. Of highly correlated characteristics (symptoms/prior HF hospitalization, prior VA/implantable cardioverter‐defibrillator [ICD], and pacemaker/ventricular pacing), the variable with highest C‐statistic in univariable analysis was included in multivariable analysis. Age was kept in all models because of its clinical significance. Continuous variables that had a nonlinear relation with the outcome were dichotomized on the basis of individual receiver operating characteristic curves. Variables selected in >60% of the bootstrap samples of >60% of the imputed data sets were included in the final prediction model 15 , 16 (Table S1). Risk factors were awarded score points based on the β in the final model. Internal validation was performed using bootstrapping techniques, assessing model calibration using the calibration slope and model discrimination using the optimism‐corrected C‐statistic penalized for overfitting.
Statistical analyses were performed in RStudio V.1.1.453 (RStudio Team, Boston, MA) using R‐version 3.6.1 (R Core Team, Vienna, Austria), with the mice, 17 survival, 18 and bootStepAIC 19 packages. P<0.05 was considered statistically significant.
Results
Baseline Characteristics
In total, 167 patients with TGA after atrial switch were included (62% men; 61% Mustard procedure; median age, 28 [interquartile range, 24–36] years at inclusion) (baseline characteristics in Table 1). At inclusion, 28 (17%) used β blockers, 45 (27%) used angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, and 14 (8%) used diuretics.
Table 1.
Baseline Characteristics and Univariable Cox Regression for Event‐Free Survival
| Characteristics | All Patients (n=167) | Patients With Events (n=41) | HR (95% CI) | P Value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Aged >30 y | 74 (44) | 30 (73) | 5.21 (2.59–10.5) | <0.001 |
| Men | 104 (62) | 22 (54) | 0.73 (0.40–1.36) | 0.33 |
| Complex TGA | 51 (31) | 16 (39) | 1.61 (0.86–3.02) | 0.14 |
| Mustard procedure | 101 (61) | 35 (85) | 5.16 (2.16–12.3) | <0.001 |
| Aged >1 y at AtrSO | 89 (53) | 36 (88) | 8.40 (3.29–21.4) | <0.001 |
| Symptoms (NYHA functional class ≥2) (n=162) | 39 (25) | 16 (41) | 2.97 (1.56–5.67) | 0.001 |
| Body mass index, kg/m2 (n=159) | 24±5 | 25±6 | 1.06 (1.00–1.12) | 0.058 |
| Systolic blood pressure, mm Hg (n=160)a | 121±14 | 118±12 | 0.88 (0.69–1.12) | 0.29 |
| Prior heart failure hospitalization | 8 (5) | 6 (15) | 8.61 (3.53–21.0) | <0.001 |
| Prior ventricular arrythmia | 13 (8) | 9 (22) | 4.23 (2.01–8.90) | <0.001 |
| Prior baffle procedure | 26 (16) | 7 (17) | 1.54 (0.68–3.50) | 0.30 |
| ICD | 5 (3) | 3 (7) | 4.72 (1.45–15.4) | 0.010 |
| Pacemaker | 41 (25) | 17 (42) | 2.91 (1.56–5.45) | <0.001 |
| Prior supraventricular tachyarrhythmia | 58 (35) | 21 (51) | 2.12 (1.15–3.91) | 0.017 |
| ECG | ||||
| Heart rate, /min (n=138)a | 68±15 | 73±15 | 1.31 (1.07–1.60) | 0.009 |
| QRS duration >120 ms (n=118) | 31 (26) | 16 (53) | 4.50 (2.29–8.81) | <0.001 |
| Loss of sinus rhythm (n=163) | 42 (26) | 12 (29) | 1.30 (0.66–2.56) | 0.45 |
| Ventricular pacing | 17 (10) | 8 (20) | 2.83 (1.30–6.15) | 0.009 |
| Echocardiography | ||||
| Moderate or greater impairment of RV function (n=157) | 38 (24) | 17 (43) | 4.08 (2.11–7.90) | <0.001 |
| Severe tricuspid regurgitation (n=159) | 12 (8) | 10 (24) | 7.00 (3.24–15.1) | <0.001 |
| Mild or greater impairment of LV function (n=162) | 8 (5) | 6 (15) | 7.16 (2.80–18.3) | <0.001 |
| Signs of baffle obstruction (n=154) | 27 (18) | 6 (16) | 0.94 (0.39–2.28) | 0.90 |
| CMR/CT | ||||
| LVEDV, mL (n=67)a | 142±45 | 137±30 | 1.06 (0.95–1.18) | 0.32 |
| LVESV, mL (n=65)a | 63±25 | 61±16 | 1.14 (0.97–1.34) | 0.12 |
| LVEF, % (n=67) | 57±9 | 56±7 | 0.97 (0.92–1.01) | 0.17 |
| RVEDV, mL (n=76)a | 209±65 | 223±59 | 1.08 (1.01–1.15) | 0.015 |
| RVESV, mL (n=73)a | 120±49 | 133±46 | 1.10 (1.03–1.18) | 0.007 |
| RVEF, % (n=76) | 42±9 | 40±6 | 0.96 (0.91–1.01) | 0.082 |
| Exercise testing | ||||
| Peak heart rate, /min (n=68)a | 169 (150–184) | 157 (127–174) | 0.86 (0.74–1.00) | 0.049 |
| Peak systolic blood pressure, mm Hg (n=62)a | 170±31 | 166±32 | 1.01 (0.88–1.16) | 0.88 |
Data described as frequency (percentage), mean±SD, or median (interquartile range). AtrSO indicates atrial switch operation; CMR, cardiovascular magnetic resonance; CT, computed tomography; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; LV, left ventricular; LVEDV, LV end‐diastolic volume; LVEF, LV ejection fraction; LVESV, LV end‐systolic volume; NYHA, New York Heart Association; RV, right ventricular; RVEDV, RV end‐diastolic volume; RVEF, RV ejection fraction; RVESV, RV end‐systolic volume; and TGA, transposition of the great arteries.
HR per 10‐unit increase.
Clinical Course
Patients were prospectively followed for a median of 13 (interquartile range, 9–16) years, with a median age at last follow‐up of 41 (interquartile range, 36–46) years. Fourteen patients were followed up over the age of 50 years. During follow‐up, 30 (18%) patients were hospitalized for HF, heart transplantation was performed in 2 patients, 3 patients got a ventricular assist device as destination therapy, and 15 (9%) patients had VAs. In total, 16 (10%) patients died (7 of HF, 5 sudden deaths, 3 of other cardiac causes, and 1 noncardiac death) (distribution of events in Table 2). Forty‐one (25%) patients reached the primary end point, with a median event‐free survival of event‐naive patients surviving into adulthood of 50 years (Kaplan‐Meier curves in Figure S1). Median survival until death or transplant/ventricular assist device of adults with TGA after atrial switch was 54 years. Risks of first HF event, first arrhythmia, and mortality were all only 1% at the age of 25 to 30 years. At the age of 50 to 55 years, this increased to 23% (95% CI, 17%–28%), 5% (95% CI, 2%–8%), and 6% (95% CI, 4%–9%), respectively (Figure 1). In the age group 30 to 40 years, 30% were diagnosed to have moderate or greater RV dysfunction, which increased to 49% for those aged between 40 and 50 years and to 53% for those aged >50 years. Symptoms of HF were present in 22% of the patients aged 30 to 40 years, in 52% of patients aged 40 to 50 years, and in 67% of patients aged >50 years. In line with clinical deterioration, a subset of patients underwent interventions during follow‐up (22 baffle and 10 tricuspid interventions).
Table 2.
Distribution of Events
| Variable | Patients With Event, N | Included in Primary End Point, Na |
|---|---|---|
| (A) Event‐free survival (primary end point) | 41 | |
| (B) All scored events | ||
| Heart failure | 33 | |
| Heart failure hospitalization | 30 | 25 |
| HTX/VAD | 5 | 2 |
| Heart failure as cause of death | 7 | 1 |
| Ventricular arrhythmia | 15 | |
| Symptomatic nonsustained VT | 4 | 4 |
| Sustained VT | 5 | 3 |
| Ventricular fibrillation | 4 | 3 |
| Cardiac arrest/sudden death b | 5 | 2 |
| All‐cause mortality | 16 | |
| Heart failure | 7 | |
| Sudden death | 5 | |
| Other cardiac | 3 | |
| Noncardiac death | 1 | 1 |
(A) Number of patients who reached the primary end point. (B) Number of patients per subtype of scored events. Numbers of subtypes do not add up to the event total as patients may have had multiple subtypes of events (eg, heart failure hospitalization and subsequently HTX). HTX indicates heart transplantation; VAD, ventricular assist device; and VT, ventricular tachycardia.
In patients with multiple events, the first event was included in the primary end point. Heart failure–related and sudden deaths were scored under heart failure and ventricular arrhythmia, respectively.
Cardiac arrest with unclear underlying rhythm from patient records or unexplained sudden death.
Figure 1. Five‐year risk of first events.
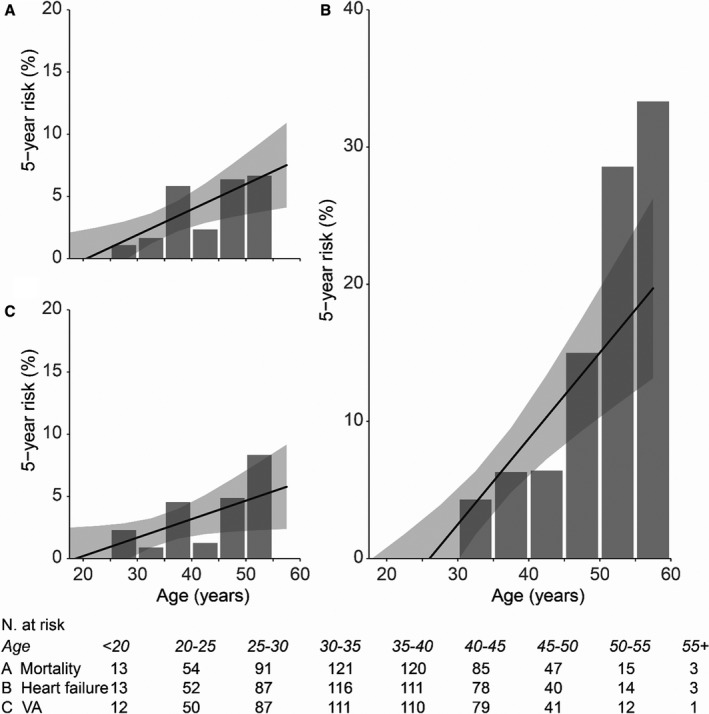
Five‐year risk of all‐cause mortality (A), first heart failure event (B), and first ventricular arrhythmia (VA) event (C). The bars show the observed incidence in percentage of patients at risk per age group, and the black line shows the predicted risk based on the observed incidences, assuming a linear model. N. indicates number.
Risk Predictors
Table 1 lists univariable associations between baseline characteristics and event‐free survival. Multiple characteristics were significant predictors in univariable analysis, including age, surgical type, age at AtrSO, symptoms, history of supraventricular arrhythmias or VAs, heart rate, QRS duration, echocardiographic measures of RV function, LV function, and TR, and peak heart rate during exercise. RV volumes, measured by cardiovascular magnetic resonance/computed tomography, were also significantly associated with event‐free survival.
Prediction Model
Table 3 shows the results of multivariable Cox regression analysis and the final prediction model, which included age >30 years, age >1 year at AtrSO, prior VA, moderate or greater RV dysfunction, severe TR, and mild or greater LV dysfunction (score chart in Figure 2A). The risk model satisfied the proportional hazard assumption, had a calibration slope of 0.97 (95% CI, 0.74–1.19) (Figure S2), and discriminated well between patients who did and did not reach the primary end point (optimism‐corrected C‐statistic, 0.86 [95% CI, 0.82–0.90]). Patients were categorized into low, intermediate, and high risk, with 5‐year predicted risk of events of <5%, 5% to 20%, and >20%, respectively (Figure 2B). In patients with a 5‐year predicted risk of <5% (score points ≤2; n=91 [54%]), observed event‐free survival was 100%, 97%, and 94% after 5, 10, and 15 years of follow‐up, respectively (Figure 2C). Risk for events was significantly increased in patients with higher scores (hazard ratio [HR], 10.5 [95% CI, 4.0–27.9] [P<0.001]; and HR, 87.7 [95% CI, 29.0–265.4] [P<0.001] for intermediate and high risk, respectively). Event‐free survival was 90% and 72% at 5‐ and 10‐year follow‐up, respectively, in intermediate‐risk patients (score points 2.5–3.5; n=62 [37%]). In high‐risk patients, with predicted 5‐year risk of >20% (score points ≥4; n=14 [8%]), observed 5‐ and 10‐year event‐free survival was only 32% and 8%, respectively. The model also performed well for prediction of all‐cause mortality alone (optimism‐corrected C‐statistic, 0.89 [95% CI, 0.82–0.95]), with observed 10‐year survival of 100%, 90%, and 37% in the low‐, intermediate‐, and high‐risk groups, respectively (Figure S3). Apart from the independent risk factors, patients at high and intermediate predicted risk more often had HF symptoms, ICD or pacemaker devices, and prior supraventricular tachyarrhythmias compared with patients in the low‐risk group (Table S2).
Table 3.
Multivariable Prediction Model and Score Points for Event‐Free Survival
| Predictors | Full Model | Final Model | Points | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Aged >30 y | 1.95 (0.79–4.77) | 0.15 | 2.75 (1.20–6.29) | 0.017 | 1 |
| Mustard procedure | 1.90 (0.71–5.07) | 0.20 | |||
| Aged >1 y at AtrSO | 4.82 (1.64–14.2) | 0.004 | 4.58 (1.64–12.8) | 0.004 | 1.5 |
| Symptoms (NYHA functional class ≥2) | 1.90 (0.88–4.11) | 0.11 | |||
| Prior ventricular arrhythmia | 4.65 (1.80–12.0) | 0.001 | 2.89 (1.22–6.81) | 0.015 | 1 |
| Pacemaker | 0.65 (0.24–1.73) | 0.38 | |||
| Prior supraventricular arrhythmia | 0.54 (0.24–1.20) | 0.13 | |||
| QRS duration >120 ms | 1.93 (0.76–4.89) | 0.17 | |||
| Heart rate, beats/min | 1.07 (0.81–1.41) | 0.64 | |||
| Moderate or greater RV dysfunction | 2.78 (1.16–6.64) | 0.021 | 2.79 (1.35–5.75) | 0.005 | 1 |
| Severe tricuspid regurgitation | 3.56 (1.25–10.2) | 0.018 | 3.55 (1.40–9.01) | 0.008 | 1.5 |
| Mild or greater LV dysfunction | 3.32 (1.16–9.46) | 0.025 | 4.46 (1.70–11.7) | 0.002 | 1.5 |
Of highly correlated characteristics (symptoms/prior heart failure hospitalization, prior ventricular arrhythmia/implantable cardioverter‐defibrillator, and pacemaker/ventricular pacing), the variable with highest C‐statistic in univariable analysis was included in multivariable analysis. Variables selected in >60% of the bootstrap samples of >60% of the imputed data sets (Table S1) were included in the final prediction model. AtrSO indicates atrial switch operation; HR, hazard ratio; LV, left ventricular; NYHA, New York Heart Association; and RV, right ventricular.
Figure 2. Observed event‐free survival by predicted risk category.
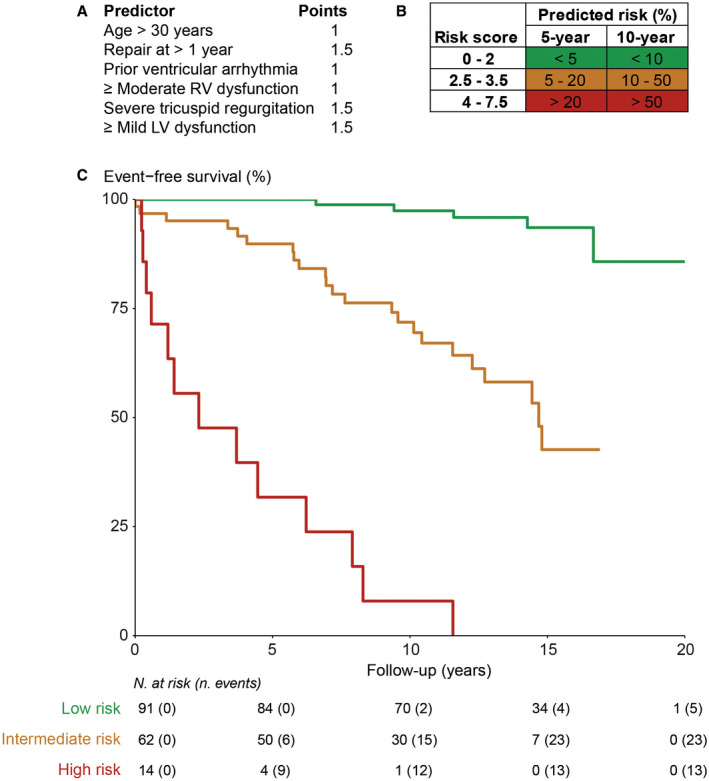
A, Score points of the risk model. B, Predicted risk for the combined end point (heart failure, ventricular arrhythmias, and mortality) based on the risk score. C, Observed event‐free survival of patients with predicted low (<5% in 5 years), intermediate (5%–20% in 5 years), and high risk (>20% in 5 years). LV indicates left ventricular; N., number; and RV, right ventricular.
Discussion
This large cohort study with comprehensive patient characteristics and long‐term follow‐up provides estimates of absolute risks of major events during the clinical course of adults after AtrSO. To our knowledge, this is the first study presenting a practical risk score based on multiple markers that can easily be obtained during routine outpatient visits, stratifying adult patients undergoing AtrSO into low‐, intermediate‐, and high‐risk groups.
Clinical Course
We present absolute risks of major clinical events during adulthood, up to the sixth decade of life. This expands current knowledge on population‐level relative risks and of studies including children to the present adult population on an individual basis. As illustrated in Figure 3, asymptomatic RV dysfunction was already present within the third decade of life, whereas more than half of patients in their fourth and fifth decade experience HF symptoms. A continuously increasing portion of patients have major clinical events with older age. In line with previous work, most major events at young adult age consisted of VAs and sudden death, but after the age of 40 years, HF prevalence had increased, and concurrently HF‐related death became the major cause of death. 20 , 21 Because the AtrSO was largely abandoned in favor of the arterial switch in the 1980s, most of the worldwide population with TGA who underwent AtrSO will be 40 to 60 years old in the coming decade. For both patients and physicians, risk assessment for the individual patient is needed to discriminate between patients at risk, who might benefit from intensive medical care and clinical interventions, and patients who can have a more relaxed regimen. The clinical tools provided in this study will be useful to support decisions on prevention and treatment of the prevailing complications.
Figure 3. Clinical course during adulthood in patients undergoing atrial switch.
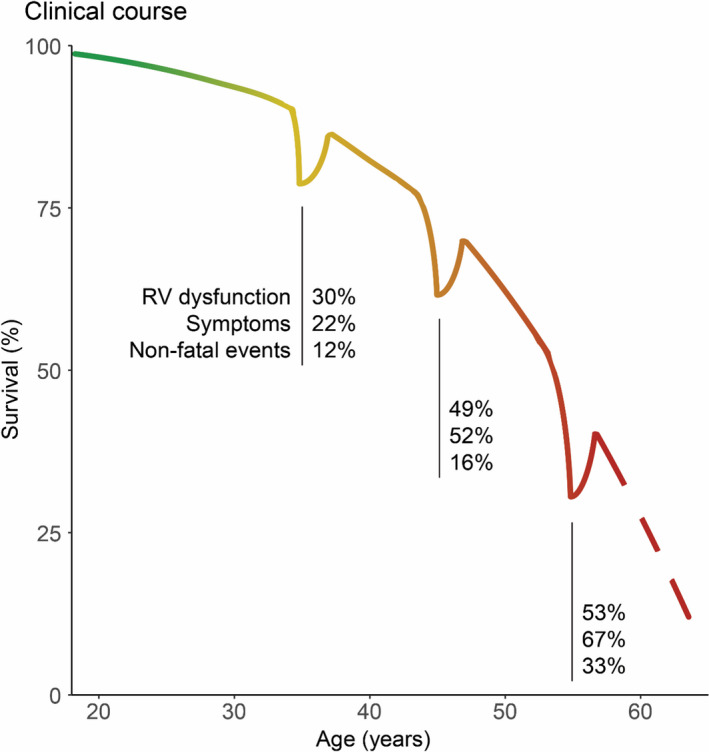
Illustration based on survival during adulthood of patients undergoing atrial switch in the CONCOR registry. 13 The percentage of patients with right ventricular (RV) dysfunction (defined as moderate or greater RV dysfunction on echocardiography), symptoms, and nonfatal clinical events (heart failure hospitalizations, transplant/ventricular assist device implantation, and symptomatic ventricular arrhythmias) is depicted for the third, fourth, and fifth decade of life. The dips in the curve signify the percentage of surviving patients enduring nonfatal clinical events during the decade. The figure illustrates that both morbidity and mortality rates are increasing as these patients grow older.
Prediction Model
Currently, management of patients with TGA corrected by an AtrSO is hampered by the lack of evidence‐based recommendations. 8 , 9 Randomized clinical trials are difficult to perform because of the limited population size. Second best are large cohort studies investigating outcomes and providing tools for risk assessment. Our study is the first to provide a risk score that can help to counsel patients and guide clinicians in their management strategies. Compared with previously published general risk models for sudden death 22 and HF 23 in adults with congenital heart disease, the presented model adds TGA‐AtrSO–specific information (ie, age at AtrSO repair and TR severity). The model discriminated well between patients at low, intermediate, and high risk for both the composite of major clinical events and all‐cause mortality alone.
The low‐risk group comprised 54% of patients who experienced no clinical events during the first 5 years of follow‐up. Moreover, during a median of 13 years of follow‐up, only 5 events occurred. The low‐risk patients were mostly young, with only mild RV dysfunction and no HF symptoms. Current US guidelines advise yearly outpatient visits in all patients with TGA corrected by an AtrSO, even those without symptoms, 9 but it seems justified to prolong the regular follow‐up interval (eg, to 2 years) and defer medical treatment with uncertain benefit in these low‐risk patients.
One third of patients comprised the intermediate‐risk group, with a 5‐year event‐free survival of 90%. In these patients, management considerations to improve event‐free survival should be considered on an individual basis (eg, yearly follow‐up with imaging for timely identification of RV deterioration and initiation of angiotensin‐converting enzyme inhibition to prevent clinical HF when symptoms appear). 24 In select cases, ICD implantation or tricuspid valve surgery may be considered. Indeed, severe TR largely contributed to the risk of events, in line with prior research. 1 , 4 , 25 , 26 , 27 TR is usually secondary to annular dilatation and may further aggravate RV dysfunction. Tricuspid valve replacement can stabilize RV function and improve functional class for several years, but timely consideration is crucial, as reduced ejection fraction of the systemic RV is a major risk factor for surgical mortality. 28
Of patients, 8% were identified as high‐risk patients, with an event‐free 5‐year survival of only 56%. Moreover, 30% had events within 1 year, and mortality was >50% within 10 years of follow‐up. These findings stress that a comprehensive clinical evaluation with frequent follow‐up (eg, every 6 months) is indicated in these patients. Within 10 years of follow‐up, 6 of the 14 high‐risk patients had had VAs. Guidelines recommend ICD implantation in patients with advanced systemic RV dysfunction, especially in presence of nonsustained VA, HF symptoms, or severe TR. 8 , 29 As most high‐risk patients meet these criteria, the presented risk model underscores that ICD implantation should be considered in these patients at highest risk. Supraventricular tachyarrhythmias and prolonged QRS duration, which have previously been associated with sudden death, 22 , 26 , 30 , 31 may also be acknowledged in the judgement for ICD therapy. Prior supraventricular tachyarrhythmias and QRS duration >120 ms were associated with our primary end point in univariable analysis. Yet, these variables did not improve the presented multivariable model, probably as 68% of our primary end point events concerned HF instead of VA. Large registries of patients with TGA corrected by an AtrSO will improve identification of those who will benefit most from ICD implantation.
HF was especially prevalent from the fourth decade on. Cardiac resynchronization therapy could be considered to decrease the contribution of dyssynchronous myocardial contractions to progressive RV failure, especially in patients with long QRS duration. 32 , 33 Progressive backward failure of the RV can also lead to dysfunction of the LV, although it is anatomically suited to sustain high pressures. Therefore, patients with overt LV dysfunction appeared to be already far down the path of deterioration. In all high‐risk patients, HF medication and timely referral for transplantation or assist device should be considered to decrease risk of premature (HF‐related) death.
Methodological Issues
Limitations inherent to the observational design include follow‐up of a selected group of patients in tertiary medical centers, with visually graded data on ventricular function as clinically customary. Evaluating the effect of interventions during follow‐up and assessing the pathogenesis of the risk model and RV deterioration were not part of our study. The sample size was limited to 167 patients, with only 41 reaching the primary end point. Although we could not perform external validation, this risk model was based on multicenter data and performed well after model stability analysis and internal validation, which is a reliable estimate of good external predictive performance. 34 We focused on robust end points that are consistently recorded in patient records. This strengthens the risk model, as it predicts major events, instead of preceding events, such as supraventricular arrhythmias. We did not study new markers, such as NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), as these were acquired less often in the earlier eras, because we preferred long‐term prediction on robust outcomes over short‐term follow‐up with new markers. Future studies may determine whether additional measurements, such as blood biomarkers, myocardial strain, or myocardial fibrosis on cardiovascular magnetic resonance, have added value in risk stratification. 6 , 25 , 35 , 36
Conclusions
The current study provides an easily applicable clinical risk model that identifies patients at low, intermediate, and high absolute risk of major clinical events. The resulting individual risk stratification can assist in counseling patients about their risk, help determine follow‐up intensity, and support decisions on invasive strategies or pharmacological treatment, thereby aiding clinicians to improve the clinical course of patients with TGA corrected by an AtrSO.
Sources of Funding
This work was supported by the Dutch Heart Foundation (CVON 2014‐18 project CONCOR‐genes) and Amsterdam University Fund (8532).
Disclosures
None.
Supporting information
Tables S1–S2
Figures S1–S3
Acknowledgments
The authors thank the CONCOR registry participants, Lia Engelfriet, and Sylvia Mantels. The CONCOR registry is part of Parelsnoer clinical biobanks at Health‐RI.
(J Am Heart Assoc.2021;10:e018565. DOI: 10.1161/JAHA.120.018565.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018565
REFERENCES
- 1. Vejlstrup N, Sorensen K, Mattsson E, Thilen U, Kvidal P, Johansson B, Iversen K, Sondergaard L, Dellborg M, Eriksson P. Long‐term outcome of Mustard/Senning correction for transposition of the great arteries in Sweden and Denmark. Circulation. 2015;132:633–638. [DOI] [PubMed] [Google Scholar]
- 2. Cuypers JA, Eindhoven JA, Slager MA, Opic P, Utens EM, Helbing WA, Witsenburg M, van den Bosch AE, Ouhlous M, van Domburg RT, et al. The natural and unnatural history of the Mustard procedure: long‐term outcome up to 40 years. Eur Heart J. 2014;35:1666–1674. [DOI] [PubMed] [Google Scholar]
- 3. Moons P, Gewillig M, Sluysmans T, Verhaaren H, Viart P, Massin M, Suys B, Budts W, Pasquet A, De Wolf D, et al. Long term outcome up to 30 years after the Mustard or Senning operation: a nationwide multicentre study in Belgium. Heart. 2004;90:307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Westhoff‐Bleck M, Podewski E, Tutarel O, Wenzel D, Cappello C, Bertram H, Bauersachs J, Widder J. Prognostic value of NT‐proBNP in patients with systemic morphological right ventricles: a single‐centre experience. Int J Cardiol. 2013;169:433–438. [DOI] [PubMed] [Google Scholar]
- 5. Rydman R, Gatzoulis MA, Ho SY, Ernst S, Swan L, Li W, Wong T, Sheppard M, McCarthy KP, Roughton M, et al. Systemic right ventricular fibrosis detected by cardiovascular magnetic resonance is associated with clinical outcome, mainly new‐onset atrial arrhythmia, in patients after atrial redirection surgery for transposition of the great arteries. Circ Cardiovasc Imaging. 2015;8:e002628. DOI: 10.1161/CIRCIMAGING.114.002628. [DOI] [PubMed] [Google Scholar]
- 6. Woudstra OI, van Dissel AC, van der Bom T, de Bruin‐Bon RHACM, van Melle JP, van Dijk APJ, Vliegen HW, Mulder BJM, Tanck MWT, Meijboom FJ, et al. Myocardial deformation in the systemic right ventricle: strain imaging improves prediction of the failing heart. Can J Cardiol. 2020;36:1525–1533. [DOI] [PubMed] [Google Scholar]
- 7. Gelatt M, Hamilton RM, McCrindle BW, Connelly M, Davis A, Harris L, Gow RM, Williams WG, Trusler GA, Freedom RM. Arrhythmia and mortality after the Mustard procedure: a 30‐year single‐center experience. J Am Coll Cardiol. 1997;29:194–201. [DOI] [PubMed] [Google Scholar]
- 8. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke‐Baerwolf C, Kaemmerer H, Kilner P, et al. ESC guidelines for the management of grown‐up congenital heart disease (new version 2010). Eur Heart J. 2010;31:2915–2957. [DOI] [PubMed] [Google Scholar]
- 9. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 10. van der Velde ET, Vriend JW, Mannens MM, Uiterwaal CS, Brand R, Mulder BJ. CONCOR, an initiative towards a national registry and DNA‐bank of patients with congenital heart disease in the Netherlands: rationale, design, and first results. Eur J Epidemiol. 2005;20:549–557. [DOI] [PubMed] [Google Scholar]
- 11. Li W, West C, McGhie J, van den Bosch AE, Babu‐Narayan SV, Meijboom F, Mongeon F‐P, Khairy P, Kimball TR, Beauchesne LM, et al. Consensus recommendations for echocardiography in adults with congenital heart defects from the International Society of Adult Congenital Heart Disease (ISACHD). Int J Cardiol. 2018;272:77–83. DOI: 10.1016/j.ijcard.2018.07.058. [DOI] [PubMed] [Google Scholar]
- 12. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14:611–644. DOI: 10.1093/ehjci/jet105. [DOI] [PubMed] [Google Scholar]
- 13. van der Bom T, Mulder BJ, Meijboom FJ, van Dijk AP, Pieper PG, Vliegen HW, Konings TC, Zwinderman AH, Bouma BJ. Contemporary survival of adults with congenital heart disease. Heart. 2015;101:1989–1995. DOI: 10.1136/heartjnl-2015-308144. [DOI] [PubMed] [Google Scholar]
- 14. Lamarca R, Alonso J, Gomez G, Munoz A. Left‐truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci. 1998;53:M337–M343. DOI: 10.1093/gerona/53A.5.M337. [DOI] [PubMed] [Google Scholar]
- 15. Kuijpers JM, Koolbergen DR, Groenink M, Peels KCH, Reichert CLA, Post MC, Bosker HA, Wajon EMCJ, Zwinderman AH, Mulder BJM, et al. Incidence, risk factors, and predictors of infective endocarditis in adult congenital heart disease: focus on the use of prosthetic material. Eur Heart J. 2017;38:2048–2056. DOI: 10.1093/eurheartj/ehw591. [DOI] [PubMed] [Google Scholar]
- 16. Musoro JZ, Zwinderman AH, Puhan MA, ter Riet G, Geskus RB. Validation of prediction models based on lasso regression with multiply imputed data. BMC Med Res Methodol. 2014;14:116. DOI: 10.1186/1471-2288-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Buuren S, Groothuis‐Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 18. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. [Google Scholar]
- 19. Rizopoulos D. Bootstepaic: bootstrap stepaic. R package version 1.2‐0. 2009.
- 20. Venkatesh P, Evans AT, Maw AM, Pashun RA, Patel A, Kim L, Feldman D, Minutello R, Wong SC, Stribling JC, et al. Predictors of late mortality in D‐transposition of the great arteries after atrial switch repair: systematic review and meta‐analysis. J Am Heart Assoc. 2019;8:e012932. DOI: 10.1161/JAHA.119.012932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diller GP, Kempny A, Alonso‐Gonzalez R, Swan L, Uebing A, Li W, Babu‐Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow‐up at a large tertiary centre. Circulation. 2015;132:2118–2125. DOI: 10.1161/CIRCULATIONAHA.115.017202. [DOI] [PubMed] [Google Scholar]
- 22. Vehmeijer JT, Koyak Z, Zwinderman AH, Harris L, Peinado R, Oechslin EN, Silversides CK, Bouma BJ, Budts W, van Gelder IC, et al. PREVENTION‐ACHD: prospective study on implantable cardioverter‐defibrillator therapy and sudden cardiac death in adults with congenital heart disease: rationale and design. Neth Heart J. 2019;27:474–479. DOI: 10.1007/s12471-019-1297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang F, Harel‐Sterling L, Cohen S, Liu A, Brophy JM, Paradis G, Marelli AJ. Heart failure risk predictions in adult patients with congenital heart disease: a systematic review. Heart. 2019;105:1661–1669. DOI: 10.1136/heartjnl-2019-314977. [DOI] [PubMed] [Google Scholar]
- 24. van Dissel AC, Winter MM, van der Bom T, Vliegen HW, van Dijk APJ, Pieper PG, Sieswerda GT, Roos‐Hesselink JW, Zwinderman AH, Mulder BJM, et al. Long‐term clinical outcomes of valsartan in patients with a systemic right ventricle: follow‐up of a multicenter randomized controlled trial. Int J Cardiol. 2019;278:84–87. DOI: 10.1016/j.ijcard.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 25. Geenen LW, van Grootel RWJ, Akman K, Baggen VJM, Menting ME, Eindhoven JA, Cuypers J, Boersma E, van den Bosch AE, Roos‐Hesselink JW. Exploring the prognostic value of novel markers in adults with a systemic right ventricle. J Am Heart Assoc. 2019;8:e013745. DOI: 10.1161/JAHA.119.013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwerzmann M, Salehian O, Harris L, Siu SC, Williams WG, Webb GD, Colman JM, Redington A, Silversides CK. Ventricular arrhythmias and sudden death in adults after a Mustard operation for transposition of the great arteries. Eur Heart J. 2009;30:1873–1879. DOI: 10.1093/eurheartj/ehp179. [DOI] [PubMed] [Google Scholar]
- 27. Dos L, Teruel L, Ferreira IJ, Rodriguez‐Larrea J, Miro L, Girona J, Albert DC, Goncalves A, Murtra M, Casaldaliga J. Late outcome of Senning and Mustard procedures for correction of transposition of the great arteries. Heart. 2005;91:652–656. DOI: 10.1136/hrt.2003.029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koolbergen DR, Ahmed Y, Bouma BJ, Scherptong RW, Bruggemans EF, Vliegen HW, Holman ER, Mulder BJ, Hazekamp MG. Follow‐up after tricuspid valve surgery in adult patients with systemic right ventricles. Eur J Cardiothorac Surg. 2016;50:456–463. DOI: 10.1093/ejcts/ezw059. [DOI] [PubMed] [Google Scholar]
- 29. Priori SG, Blomström‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. DOI: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 30. Khairy P, Harris L, Landzberg MJ, Fernandes SM, Barlow A, Mercier L‐A, Viswanathan S, Chetaille P, Gordon E, Dore A, et al. Sudden death and defibrillators in transposition of the great arteries with intra‐atrial baffles: a multicenter study. Circ Arrhythm Electrophysiol. 2008;1:250–257. DOI: 10.1161/CIRCEP.108.776120. [DOI] [PubMed] [Google Scholar]
- 31. Kempny A, Gruebler M, Dimopoulos K, Tutarel O, Agra‐Bermejo RM, Piatek P, Swan L, Diller GP, Wort SJ, Gatzoulis MA. QRS duration predicts life‐threatening ventricular arrhythmia and death in adults with a systemic right ventricle. Eur Heart J. 2013;34:P2095. [Google Scholar]
- 32. Yin Y, Dimopoulos K, Shimada E, Lascelles K, Griffiths S, Wong T, Gatzoulis MA, Babu‐Narayan SV, Li W. Early and late effects of cardiac resynchronization therapy in adult congenital heart disease. J Am Heart Assoc. 2019;8:e012744. DOI: 10.1161/JAHA.119.012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forsha D, Risum N, Smith PB, Kanter RJ, Samad Z, Barker P, Kisslo J. Frequent activation delay‐induced mechanical dyssynchrony and dysfunction in the systemic right ventricle. J Am Soc Echocardiogr. 2016;29:1074–1083. [DOI] [PubMed] [Google Scholar]
- 34. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 35. Broberg CS, Valente AM, Huang J, Burchill LJ, Holt J, Van Woerkom R, Powell AJ, Pantely GA, Jerosch‐Herold M. Myocardial fibrosis and its relation to adverse outcome in transposition of the great arteries with a systemic right ventricle. Int J Cardiol. 2018;271:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van der Bom T, Winter MM, Groenink M, Vliegen HW, Pieper PG, van Dijk AP, Sieswerda GT, Roos‐Hesselink JW, Zwinderman AH, Mulder BJ, et al. Right ventricular end‐diastolic volume combined with peak systolic blood pressure during exercise identifies patients at risk for complications in adults with a systemic right ventricle. J Am Coll Cardiol. 2013;62:926–936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S3


