Abstract
Background
Pre‐pregnancy hypertension and hypertensive disorders of pregnancy (HDP; preeclampsia, eclampsia, gestational hypertension) are major health risks for maternal morbidity and mortality. However, it is unknown if racial/ethnic differences exist. We aimed to determine the impact of HDP and pre‐pregnancy hypertension on maternal coronary heart disease, stroke, and mortality risk ≤1, 3, and 5 years post‐delivery and by race/ethnicity ≤5 years.
Methods and Results
This retrospective cohort study included women aged 12 to 49 years with a live, singleton birth between 2004 to 2016 (n=254 491 non‐Hispanic White; n=137 784 non‐Hispanic Black; n=41 155 Hispanic). Birth and death certificates and International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD‐9‐CM and ICD‐10‐CM) diagnosis codes in hospitalization/emergency department visit data defined HDP, pre‐pregnancy hypertension, incident coronary heart disease and stroke, and all‐cause mortality. During at least 1 pregnancy of the 433 430 women, 2.3% had pre‐pregnancy hypertension with superimposed HDP, 15.7% had no pre‐pregnancy hypertension with HDP, and 0.4% had pre‐pregnancy hypertension without superimposed HDP, whereas 81.6% had neither condition. Maternal deaths from coronary heart disease, stroke, and all causes totaled 2136. Within 5 years of delivery, pre‐pregnancy hypertension, and HDP were associated with all‐cause mortality (hazard ratio [HR], 2.21; 95% CI, 1.61–3.03), incident coronary heart disease (HR, 3.79; 95% CI, 3.09–4.65), and incident stroke (HR, 3.10; 95% CI, 2.09–4.60). HDP alone was related to all outcomes. Race/ethnic differences were observed for non‐Hispanic Black and non‐Hispanic White women, respectively, in the associations of pre‐pregnancy hypertension and HDP with all‐cause mortality within 5 years of delivery (HR, 2.34 [95% CI, 1.58–3.47]; HR, 2.11 [95% CI, 1.23–3.65]; P interaction=0.001).
Conclusions
Maternal cardiovascular outcomes including mortality were increased ≤5 years post‐delivery in HDP, pre‐pregnancy hypertension, or pre‐pregnancy hypertension with superimposed HDP. The race/ethnic interaction for all‐cause mortality ≤5 years of delivery warrants further research.
Keywords: cardiovascular disease, disparities, hypertensive disorders of pregnancy, maternal outcomes, mortality, pre‐pregnancy hypertension, race/ethnicity, stroke
Subject Categories: Preeclampsia, Epidemiology, Pregnancy, Race and Ethnicity, Women
Nonstandard Abbreviations and Acronyms
- HDP
hypertensive disorders of pregnancy
- NHB
non‐Hispanic Black
- NHW
non‐Hispanic White
Clinical Perspective
What Is New?
In this retrospective cohort study among women aged 12 to 49 years with a live, singleton birth in South Carolina between 2004 to 2016, those exposed to hypertensive disorders of pregnancy (HDP) without pre‐pregnancy hypertension (“HDP alone”), and pre‐pregnancy hypertension with superimposed HDP (“both conditions”) were at greater risk of incident stroke, incident coronary heart disease, and all‐cause mortality within 5 years of delivery.
Non‐Hispanic Black women with both conditions and HDP alone, non‐Hispanic White women with both conditions, and Hispanic women with pre‐pregnancy hypertension alone experienced increased all‐cause mortality risk within 5 years of delivery (P interaction=0.001); there were no significant differences for incident coronary heart disease or stroke.
What Are the Clinical Implications?
These patients should be watched more closely after delivery during the high‐risk period and monitored for cardiovascular risk factors.
Our data suggest that HDP persist after pregnancy and may lead to changes possibly increasing the risk of cardiovascular events later in life.
Hypertensive disorders of pregnancy (HDP) including preeclampsia, eclampsia, and gestational hypertension, affect ≈10% of all pregnancies and are associated with increased morbidity and mortality for the mother and the baby. 1 New‐onset hypertension usually experienced after 20 weeks of gestation characterizes gestational hypertension. New‐onset hypertension with proteinuria and/or significant end‐organ dysfunction characterizes preeclampsia, whereas eclampsia is characterized by new‐onset seizures (ie, tonic‐clonic, focal, or multifocal) without a causative condition(s). 2 , 3 Preeclampsia occurs among 3% to 5% of all pregnancies. 4 Risk factors identified for preeclampsia include: advanced age (>40 years), 5 Black race, 6 , 7 gestational diabetes mellitus, 5 history of preeclampsia, 5 certain preexisting medical conditions (eg, preexistent hypertension), 5 , 8 , 9 and high pre‐pregnancy body mass index (BMI) (>29.0 kg/m2). 5 Preexisting hypertension, which is known to increase the risk of superimposed preeclampsia, occurs in ≈1% to 4% of all pregnancies. 10 , 11 , 12
Significant maternal and perinatal morbidity and mortality have included preterm delivery (16–36 weeks gestational age) in ≈11% of women with preeclampsia, increasing the risk of premature‐related complications for the neonate. 13 In severe preeclampsia and HDP, respectively, 16.4% and 15.0% of women experienced iatrogenic preterm delivery (22–36 weeks gestational age). 14 Additional maternal outcomes associated with HDP include stroke, seizure, coagulopathy, renal failure, hemorrhage, and death. 15 , 16 , 17 , 18 , 19
A significant proportion of maternal deaths in developed countries (16.1%) and developing countries (25.7% in the Caribbean and Latin America) are attributed to HDP. 20 Compared with White women in the United States, Black women experience a 2.7‐fold higher case‐fatality rate for preeclampsia, 21 and a 2‐fold higher case‐fatality rate for preeclampsia or eclampsia. 22 Racial/ethnic differences in cause‐specific pregnancy‐related mortality were observed during 2007 to 2016 with a higher proportion of deaths attributed to HDP for Black (8.2%) and Hispanic (9.7%) women than White women (6.7%; P<0.05). 23 Preexisting hypertension without superimposed preeclampsia has also been associated with maternal complications, such as end‐organ complications, 24 delivery at <37 or <34 weeks of gestation, 25 cesarean delivery, 26 post‐partum hemorrhage, 26 and stroke. 27
More recently, preeclampsia and HDP have been linked to long‐term morbidity and mortality. A recent meta‐analysis reported associations between maternal risk of coronary heart disease (CHD), stroke, and death from CHD and cardiovascular disease (CVD) with preeclampsia following covariate adjustment. 28 However, parity, length of follow‐up time, and risk factor adjustment (eg, sociodemographic and clinical characteristics) were inconsistent among the studies. 28 There are significant gaps in the literature as evidence is lacking on the role of established risk factors (eg, BMI, family history of CVD, smoking) as potential confounders in the relationship between HDP and CVD. 29 , 30 Furthermore, previous studies 19 , 20 , 21 , 22 assessing long‐term outcomes have been unable to examine racial health disparities in stroke, ischemic heart disease, venous thromboembolism, or mortality among women with preeclampsia. 13 , 30 , 31 , 32 , 33
We hypothesized that HDP regardless of preexisting or chronic hypertension before pregnancy (referred to hereafter as “pre‐pregnancy hypertension”) is associated with increased maternal risk of all‐cause mortality, incident CHD, and incident stroke, and the increased cardiovascular risks associated with HDP are greater in non‐Hispanic Black (NHB) and Hispanic women than in non‐Hispanic White (NHW) women. The objectives of the current study were to investigate the association between HDP and pre‐pregnancy hypertension and maternal cardiovascular outcomes specifically CHD, stroke, and mortality within 1, 3, and 5 years of delivery as well as any time across the follow‐up period (up to 14 years). We also examined potential racial/ethnic differences in these cardiovascular outcomes and mortality within 5 years of delivery.
Methods
Because of policies of the South Carolina (SC) Revenue and Fiscal Affairs Office and the SC Department of Health and Environmental Control, it is not possible to share the data used in this study.
Study Design
A retrospective cohort study was conducted using data from statewide birth certificates, death certificates, and hospitalization and emergency department (ED) visits in SC to examine the relationship between HDP and the risk of maternal cardiovascular outcomes including mortality. Hospital discharge records and ED visit data were obtained from the SC Revenue and Fiscal Affairs, Health and Demographics Section. Birth and death certificate data were obtained from the SC Department of Health and Environmental Control. Institutional review board approval was received from the Medical University of South Carolina, and no informed consent was required.
Study Population
Live births between 2004 to 2016 in SC were identified by birth certificates. Maternal procedure and diagnosis codes were available from hospital discharge records and ED visit data. Maternal mortality data were available from death certificates and included the primary (ie, underlying) cause of death and comorbid causes of death. Deliveries identified through birth certificates were linked to hospital discharge records for the mother by the SC Revenue and Fiscal Affairs using a unique identifier; 97.5% of deliveries had a corresponding record in the hospitalization data.
Inclusion and Exclusion Criteria
Figure 1 displays delivery and maternal‐level inclusion and exclusion criteria for the study. Our inclusion criteria were live, singleton births as reported on the birth certificate identified through December 2016 to allow at least 1 year of follow‐up through 2017. Exclusion criteria included data entry errors (ie, multiple death records and negative time to death), newborn deaths the day of delivery, and births of multifetal gestations. We excluded mothers with missing age or <12 and >49 years of age, residing outside of SC, with a history of kidney or other transplants, pre‐pregnancy weight of <92 or >320 lbs, or pre‐pregnancy BMI of <16.0 or >52.8 kg/m2. Deliveries with implausible size for gestational age or birth weight <500 g or >6000 g according to the national fetal growth curve developed by Alexander et al 34 were also excluded.
Figure 1. Flowchart of study inclusion and exclusion criteria.
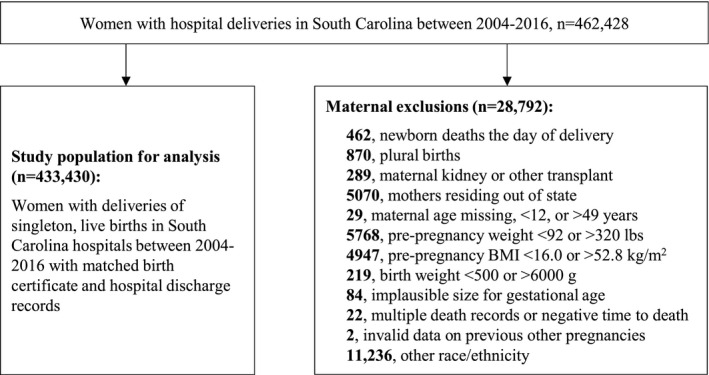
BMI indicates body mass index; and HDP, hypertensive disorders of pregnancy.
Definitions
Sociodemographic characteristics included maternal age at delivery, race/ethnicity, education, and urban/rural residential status based on Rural‐Urban Commuting Area codes. Median household income per year (<$36 000; $36 000–<$54 000; ≥$54 000) was based on US Census zip code residential level data. Eligibility for the Women, Infants, and Children (WIC) program was during pregnancy. Primary payer during pregnancy was available from birth records. Based on self‐report of race and ethnicity, women were categorized into NHW, NHB, or Hispanic. Women self‐reporting as “Other race/ethnicity” were excluded from the analysis because of low numbers.
The birth certificate data contained information on pre‐pregnancy smoking, smoking during pregnancy, and clinical characteristics including pre‐pregnancy or gestational diabetes mellitus, and pre‐pregnancy BMI (kg/m2). Gestational diabetes mellitus was also defined using hospitalization/ED visit data based on the International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD‐9‐CM and ICD‐10‐CM) codes. The Revised‐Graduated Prenatal Care Utilization Index (R‐GINDEX) was used to classify adequacy of prenatal care based on the total number of visits for prenatal care, as well as the gestational age (weeks) and the trimester at which prenatal care was initiated. 35 , 36
The exposures and maternal outcomes of interest between 2004 to 2017 were identified from birth certificates and hospital/ED visit discharge data based on ICD‐9‐CM and ICD‐10‐CM diagnosis and procedure codes (Table S1). The 4 exposure groups consisted of women: (1) without pre‐pregnancy hypertension or HDP (referred to throughout as “neither condition”), (2) with pre‐pregnancy hypertension without superimposed HDP (referred to throughout as “pre‐pregnancy hypertension alone”), (3) with HDP without pre‐pregnancy hypertension (referred to throughout as “HDP alone”), and (4) with pre‐pregnancy hypertension with superimposed HDP (referred to throughout as “both conditions”). The exposure, HDP, was defined as reported on the birth certificate or a diagnosis in the hospitalization/ED visit data based on ICD‐9‐CM/ICD‐10‐CM codes for gestational hypertension, preeclampsia or eclampsia (642.3–642.7; O11.x, O13.x‐O16.x), or gestational hypertension (also used for preeclampsia or eclampsia) as reported on the birth certificate. Pre‐pregnancy hypertension was defined by ICD‐9‐CM/ICD‐10‐CM codes at delivery (642.0–642.2; O10.0) or as indicated on the birth certificate. Having both conditions (pre‐pregnancy hypertension with superimposed HDP) was defined by ICD‐9‐CM/ICD‐10‐CM codes at delivery (642.7; O13.x‐O15.x), a combination of diagnosis codes, or indication on the birth certificate of gestational hypertension and/or pre‐pregnancy hypertension.
For women with pre‐pregnancy hypertension and/or HDP, the index pregnancy was defined as the first pregnancy with either or both condition(s). For women without HDP or pre‐pregnancy hypertension in any pregnancy, the index pregnancy was defined as the first pregnancy noted in the data set.
The outcomes, maternal fatal and non‐fatal incident CHD and stroke, were defined using hospitalization/ED visit data based on ICD‐9‐CM and ICD‐10‐CM codes, and death certificates based on ICD‐10‐CM codes (Table S1). Maternal all‐cause mortality was also available from death certificates and defined using ICD‐10‐CM codes.
Statistical Analysis
Maternal sociodemographic and clinical characteristics were compared between the 4 groups of women based on HDP and pre‐pregnancy hypertension status. Chi‐square tests were used for categorical variables reported as frequencies (percentages). ANOVA was used for continuous variables reported as means ± SD). Hazard ratios (HR) and corresponding 95% CIs were estimated for incident CHD events, incident stroke events, and all‐cause mortality within 1, 3, and 5 years of delivery and across the total time of follow‐up (up to 14 years) using Cox proportional hazards models. Time from delivery to the event/death served as the time variable in the models. Examining different time periods within 1, 3, and 5 years of delivery as well as any time across the study period was hypothesized a priori because of our interest in post‐delivery maternal outcomes, both short‐ (within 1 year) and long‐term. Schoenfeld residuals were used to test the Cox proportional hazard model assumption of proportionality for all variables, for which the assumption was met. The models adjusted for sociodemographic (maternal age at delivery, race/ethnicity, education, urban/rural residence, median income, payer during pregnancy; and WIC program eligibility during pregnancy), behavioral (pre‐pregnancy smoking), and clinical characteristics (pre‐pregnancy BMI and pre‐pregnancy or gestational diabetes mellitus).
Given our interest in long‐term, post‐delivery outcomes and because of the number of events, our a priori hypotheses included examination of racial/ethnic differences within 5 years of delivery. Interactions between race/ethnicity and HDP/pre‐pregnancy hypertension groups were tested to determine whether race/ethnicity modified the association between HDP and/or pre‐pregnancy hypertension and maternal all‐cause mortality, incident CHD, and incident stroke within 5 years of delivery with the presence of an interaction indicated by a P value <0.05. Kaplan‒Meier analyses were performed to calculate differences in time to incident CHD, incident stroke, and all‐cause mortality in the 4 exposure groups. A sensitivity analysis limited to women known to receive medical care through an ED visit or hospitalization >5 years post‐delivery in the data set, because of either experiencing an outcome of interest or based on their last visit, was performed. The results did not change the HRs >12% and the point estimates were within the 95% CIs of the original estimates. SAS Version 9.4 (SAS Institute Inc., Cary, NC) 37 was used to conduct the analyses. Stata Version 13 38 was used to create graphs and test the Cox proportional hazard models assumption.
Results
Of the 462 428 women who gave birth in SC between 2004 to 2016 (representing n=702 119 deliveries), a total of 433 430 (93.7%) were eligible for inclusion in the current analysis, including NHW (n=254 491), NHB (n=137 784), and Hispanic (n=41 155) women. The 4 exposure groups consisted of women without pre‐pregnancy hypertension or HDP (neither condition; n=353 575), with pre‐pregnancy hypertension without superimposed HDP (pre‐pregnancy hypertension alone; n=1591), with HDP without pre‐pregnancy hypertension (HDP alone; n=68 212), and with pre‐pregnancy hypertension and superimposed HDP (both conditions; n=10 052). Mean (±SD) follow‐up time was 8.4 (±3.8) years. A total of 2136 maternal deaths were observed from CHD (n=86), stroke (n=48), and all causes (n=2002).
Sociodemographic, behavioral, and clinical characteristics are shown in Table 1 with comparisons based on Chi‐square tests or ANOVA. Compared with those with neither condition, women with both conditions were more likely to be older, NHB, have a lower income, receive Medicaid, have WIC program eligibility during pregnancy, and have a higher pre‐pregnancy BMI. Women with both conditions were also more likely to have pre‐pregnancy or gestational diabetes mellitus than those with neither condition. Women with HDP alone were slightly older and more likely to be NHB, have a lower income, receive Medicaid, be WIC program eligible, have pre‐pregnancy or gestational diabetes mellitus, and have a higher pre‐pregnancy BMI than those with neither condition. Table S2 presents maternal characteristics overall stratified by racial/ethnic group with comparisons based on Chi‐square tests or ANOVA.
Table 1.
Demographic and Clinical Characteristics of Mothers at Index Birth in South Carolina Overall and by HDP and Pre‐Pregnancy Hypertension Status, 2004 to 2016 * , † , ‡ , §
| Characteristic n (%) or Mean±SD | Total | Neither Pre‐Pregnancy Hypertension nor HDP | Pre‐Pregnancy Hypertension Alone | HDP Alone | Both Pre‐Pregnancy Hypertension and HDP | P Value ‖ | |
|---|---|---|---|---|---|---|---|
| n=433 430 | n=353 575 (81.6) | n=1591 (0.4) | n=68 212 (15.7) | n=10 052 (2.3) | |||
| Maternal age at delivery, y | 27.7±5.9 | 27.6±5.9 | 30.1±6.2 | 27.9±6.0 | 30.5±6.0 | <0.0001 | |
| Race/ethnicity | |||||||
| Non‐Hispanic White | 254 491 (58.7) | 211 871 (59.9) | 716 (45.0) | 37 544 (55.0) | 4360 (43.4) | <0.0001 | |
| Non‐Hispanic Black | 137 784 (31.8) | 105 092 (29.7) | 761 (47.8) | 26 581 (39.0) | 5350 (53.2) | ||
| Hispanic | 41 155 (9.5) | 36 612 (10.4) | 114 (7.2) | 4087 (6.0) | 342 (3.4) | ||
| Education | |||||||
| <High school | 75 492 (17.4) | 62 745 (17.7) | 258 (16.2) | 11 131 (16.3) | 1358 (13.5) | <0.0001 | |
| High school graduate | 107 347 (24.8) | 86 310 (24.4) | 427 (26.8) | 17 933 (26.3) | 2677 (26.6) | ||
| Some college | 111 735 (25.8) | 89 789 (25.4) | 444 (27.9) | 18 693 (27.4) | 2809 (27.9) | ||
| ≥College graduate | 138 856 (32.0) | 114 731 (32.4) | 462 (29.0) | 20 455 (30.0) | 3208 (31.9) | ||
| Rural residence | 114 332 (26.4) | 92 973 (26.3) | 438 (27.5) | 18 117 (26.6) | 2804 (27.9) | 0.0015 | |
| Annual household income | |||||||
| <$36 000 | 118 037 (27.2) | 93 974 (26.6) | 571 (35.9) | 19 921 (29.2) | 3571 (35.5) | <0.0001 | |
| $36 000 to <$54 000 | 204 675 (47.2) | 166 230 (47.0) | 601 (37.8) | 33 282 (48.8) | 4562 (45.4) | ||
| ≥$54 000 | 101 708 (23.5) | 85 168 (24.1) | 274 (17.2) | 14 400 (21.1) | 1866 (18.6) | ||
| Primary payer during pregnancy | |||||||
| Medicaid | 211 909 (48.9) | 170 004 (48.1) | 797 (50.1) | 35 927 (52.7) | 5181 (51.5) | <0.0001 | |
| Private | 171 562 (39.6) | 140 029 (39.6) | 653 (41.0) | 26 745 (39.2) | 4135 (41.1) | ||
| Self‐pay | 22 224 (5.1) | 19 740 (5.6) | 60 (3.8) | 2168 (3.2) | 256 (2.5) | ||
| Other | 24 604 (5.7) | 21 165 (6.0) | 61 (3.8) | 2970 (4.4) | 408 (4.1) | ||
| WIC eligibility in pregnancy | 216 671 (50.0) | 174 279 (49.3) | 864 (54.3) | 35 961 (52.7) | 5567 (55.4) | <0.0001 | |
| Pre‐pregnancy smoking | 66 727 (15.4) | 54 707 (15.5) | 245 (15.4) | 10 331 (15.1) | 1444 (14.4) | 0.005 | |
| Pre‐pregnancy BMI, kg/m2 | 27.5±6.8 | 26.7±6.4 | 32.0±8.0 | 30.4±7.6 | 33.7±7.9 | <0.0001 | |
| Pre‐pregnancy or gestational diabetes mellitus | 30 159 (7.0) | 19 556 (5.5) | 330 (20.7) | 8033 (11.8) | 2240 (22.3) | <0.0001 | |
BMI indicates body mass index; HDP, hypertensive disorders of pregnancy; and WIC, Women, Infants, and Children.
Data include person‐level diagnostic codes at time of discharge or birth certificate. Hypertensive disorders of pregnancy were defined as gestational hypertension, preeclampsia, or eclampsia (International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification [ICD‐9‐CM/ICD‐10‐CM]: 642.3–642.7; O11.x, O13.x‐16.x) based on hospitalization/emergency department (ED) visit data, or gestational hypertension as reported on the birth certificate. Pre‐pregnancy hypertension was based on hospitalization/ED visit data or birth certificates.
For pre‐pregnancy smoking, ≤0.1% of data are missing and not shown.
Variables with >0.1% missing data included: annual household income, n=9010; primary payer during pregnancy, n=3131; and Women, Infants, and Children eligibility in pregnancy, n=6583.
Pre‐pregnancy smoking, pre‐pregnancy body mass index, pre‐pregnancy diabetes mellitus, and gestational diabetes mellitus were available from the birth certificate. Gestational diabetes mellitus was also defined using hospitalization/ED visit data based on ICD‐9‐CM and ICD‐10‐CM codes.
P‐values were calculated by Chi‐square tests for categorical variables or ANOVA for continuous variables.
Additional clinical characteristics at the index birth including prenatal data, complications, and delivery variables are displayed in Table S3 overall and by HDP and pre‐pregnancy hypertension status with comparisons based on Chi‐square tests or ANOVA.
Unadjusted Kaplan‒Meier survival estimates within 5 years of delivery are presented in Figure 2A through 2C by HDP and pre‐pregnancy hypertension status (note atypical scale). Survival time for all‐cause mortality appeared to differ by exposure group with survival lowest for women with both conditions, followed by those with pre‐pregnancy hypertension alone. Survival for incident CHD was most striking as it was lowest among women with both conditions, and similar between women with pre‐pregnancy hypertension alone and HDP alone. For incident stroke, survival was also lowest among women with both conditions.
Figure 2. Unadjusted survival since delivery for (A) all‐cause mortality, (B) incident coronary heart disease, and (C) incident stroke for women by hypertensive disorders of pregnancy and pre‐pregnancy hypertension status.
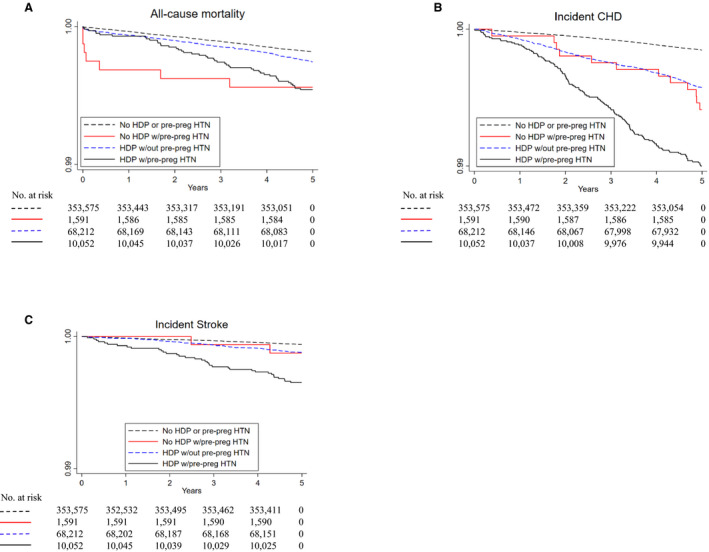
Note: Scale is not a standard survival scale. CHD indicates coronary heart disease; HDP, hypertensive disorders of pregnancy; HTN, hypertension; and pre‐preg, pre‐pregnancy.
Maternal Incident CHD, Incident Stroke, and All‐Cause Mortality by Pre‐Pregnancy Hypertension and/or HDP Exposure
Event rates and the hazards of incident CHD, incident stroke, and all‐cause mortality based on multivariable Cox proportional hazards modeling are presented in Table 2 within 1, 3, and 5 years of delivery and across the total potential follow‐up time (up to 14 years). While the number of incident CHD, stroke, and all‐cause mortality events was low, differences were observed in event rates for the 4 exposure groups. The incident CHD event rate per 1000 person‐years was 4.04 (n=344) among women with both conditions compared with 1.77 (n=1001) among women with HDP alone, 1.90 (n=25) among women with pre‐pregnancy hypertension alone, and 0.67 (n=1970) among women with neither condition. With regard to all‐cause mortality, the event rate per 1000 person‐years was 1.21 (n=104) among women with both conditions, 0.68 (n=385) among women with HDP alone, 1.29 (n=17) among women with pre‐pregnancy hypertension alone, and 0.50 (n=1496) among women with neither condition.
Table 2.
Adjusted Hazard Ratios Comparing Women With and Without Pre‐Pregnancy Hypertension by HDP Status for All‐Cause Mortality, Fatal and Non‐Fatal Incident CHD, and Fatal and Non‐Fatal Incident Stroke Within 1, 3, and 5 Years of Delivery, and Across the Entire Study Period, 2004 to 2017
| All‐Cause Mortality | Incident CHD | Incident Stroke | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Event | Event Rate* | HR (95% CI) † | Event | Event Rate* | HR (95% CI) † | Event | Event Rate* | HR (95% CI) † | |
| ≤1 y of delivery | |||||||||
| Neither pre‐preg hypertension/HDP | 132 | 0.37 | referent | 103 | 0.29 | referent | 43 | 0.12 | referent |
| Pre‐pregnancy hypertension alone | 5 | 3.15 | 4.92 (1.55–15.7) | <5 | 0.63 | 1.41 (0.20–10.2) | 0 | 0.00 | … |
| HDP alone | 43 | 0.63 | 1.67 (1.16–2.41) | 66 | 0.97 | 2.80 (2.02–3.88) | 10 | 0.15 | 0.97 (0.48–1.98) |
| Both pre‐pregnancy hypertension and HDP | 7 | 0.70 | 1.65 (0.71–3.83) | 15 | 1.49 | 2.67 (1.46–4.90) | 7 | 0.70 | 2.63 (1.05–6.59) |
| ≤3 y of delivery | |||||||||
| Neither pre‐pregnancy hypertension/HDP | 384 | 0.36 | referent | 353 | 0.33 | referent | 113 | 0.11 | referent |
| Pre‐pregnancy hypertension alone | 6 | 1.26 | 2.14 (0.80–5.76) | 5 | 1.05 | 2.10 (0.86–5.09) | <5 | 0.21 | 1.39 (0.19–9.99) |
| HDP alone | 101 | 0.49 | 1.32 (1.05–1.67) | 214 | 1.05 | 2.67 (2.23–3.19) | 44 | 0.22 | 1.63 (1.13–2.35) |
| Both pre‐pregnancy hypertension and HDP | 26 | 0.86 | 2.16 (1.42–3.29) | 76 | 2.53 | 4.36 (3.32–5.73) | 23 | 0.76 | 4.07 (2.47–6.73) |
| ≤5 y of delivery | |||||||||
| Neither pre‐pregnancy hypertension/HDP | 646 | 0.37 | referent | 695 | 0.39 | referent | 217 | 0.12 | referent |
| Pre‐pregnancy hypertension alone | 7 | 0.88 | 1.55 (0.64–3.74) | 12 | 1.51 | 2.48 (1.40–4.40) | <5 | 0.25 | 1.38 (0.34–5.56) |
| HDP alone | 175 | 0.51 | 1.35 (1.13–1.60) | 372 | 1.09 | 2.32 (2.03–2.65) | 82 | 0.24 | 1.59 (1.22–2.08) |
| Both pre‐pregnancy hypertension and HDP | 46 | 0.92 | 2.21 (1.61–3.03) | 129 | 2.58 | 3.79 (3.09–4.65) | 35 | 0.70 | 3.10 (2.09–4.60) |
| Entire study period | |||||||||
| Neither pre‐pregnancy hypertension/HDP | 1496 | 0.50 | referent | 1970 | 0.67 | referent | 660 | 0.22 | referent |
| Pre‐pregnancy hypertension alone | 17 | 1.29 | 2.00 (1.20–3.33) | 25 | 1.90 | 1.75 (1.17–2.62) | 12 | 0.91 | 2.81 (1.58–4.99) |
| HDP alone | 385 | 0.68 | 1.27 (1.13–1.43) | 1001 | 1.77 | 2.16 (1.99–2.34) | 273 | 0.48 | 1.86 (1.61–2.16) |
| Both pre‐pregnancy hypertension and HDP | 104 | 1.21 | 2.03 (1.65–2.51) | 344 | 4.04 | 3.47 (3.07–3.92) | 103 | 1.20 | 3.17 (2.53–3.98) |
CHD indicates coronary heart disease; HDP, hypertensive disorders of pregnancy; and HR, hazard ratio.
Per 1000 person‐years.
Adjusted for sociodemographic (maternal age; race/ethnicity; education; rural/urban residence; median income; payer; Women, Infants, and Children), behavioral (pre‐pregnancy smoking), and clinical characteristics (pre‐pregnancy BMI, pre‐pregnancy or gestational diabetes mellitus).
Within 1 year of delivery, women with both conditions (HR, 2.67; 95% CI, 1.46–4.90) and with HDP alone (HR, 2.80; 95% CI, 2.02–3.88) were at increased risk for incident CHD compared with those with neither condition after adjustment for sociodemographic, behavioral, and clinical characteristics. Within 5 years of delivery, the risk of incident CHD was elevated further for women with both conditions (HR, 3.79; 95% CI, 3.09–4.65), and the risk attenuated but remained significant for women with HDP alone (HR, 2.32; 95% CI, 2.03–2.65) and with pre‐pregnancy hypertension alone (HR, 2.48; 95% CI, 1.40–4.40) compared with neither condition.
The risk of incident stroke was elevated for women with both conditions within 1 year of delivery (HR, 2.63; 95% CI, 1.05–6.59). Within 5 years of delivery, there was an increased risk of incident stroke among women who experienced both conditions (HR, 3.10; 95% CI, 2.09–4.60) and women with HDP alone (HR, 1.59; 95% CI, 1.22–2.08). Women with pre‐pregnancy hypertension alone had a 2.81‐fold (95% CI,1.58–4.99) increased risk of incident stroke across the entire study period, although the number of events was small limiting statistical power.
With regard to all‐cause mortality, potential increased risks were observed within 1 year of delivery for women with pre‐pregnancy hypertension alone (HR, 4.92; 95% CI, 1.55–15.7) as well as for those with HDP alone (HR, 1.67; 95% CI, 1.16–2.41), although there were a small number of events among women with pre‐pregnancy hypertension alone. Women with both conditions experienced increased risk of all‐cause mortality within 5 years of delivery (HR, 2.21; 95% CI, 1.61–3.03). In addition, women with HDP alone were at increased risk of all‐cause mortality within 5 years of delivery (HR, 1.35; 95% CI, 1.13–1.60), as were women with pre‐pregnancy hypertension alone across the entire study period (HR, 2.00; 95% CI, 1.20–3.33).
Racial/Ethnic Differences in Maternal Incident CHD, Incident Stroke, and All‐Cause Mortality by Pre‐Pregnancy Hypertension and HDP Exposure
Race/ethnic specific estimates for the hazards of incident CHD, incident stroke, and all‐cause mortality within 5 years of delivery after adjustment for covariates are shown in Table 3 based on multivariable Cox proportional hazards modeling. A significant interaction was observed between the exposure and race/ethnic groups for all‐cause mortality (P=0.001). There was an increased risk of all‐cause mortality among NHW (HR, 2.11; 95% CI, 1.23–3.65) and NHB (HR, 2.34; 95% CI, 1.58–3.47) women with both conditions; no Hispanic women with both conditions died within 5 years of delivery. All‐cause mortality risk was also elevated for NHB women with HDP alone (HR, 1.70; 95% CI, 1.34–2.15) and non‐significantly elevated for NHW women (HR, 1.08; 95% CI, 0.82–1.42). Among women with pre‐pregnancy hypertension alone, an increased risk of all‐cause mortality was observed for Hispanic women (HR, 18.4; 95% CI, 4.17–80.9), but not for NHW or NHB women.
Table 3.
Adjusted Hazard Ratios Comparing Women With HDP and/or Pre‐Pregnancy Hypertension to Women Without HDP and Pre‐Pregnancy Hypertension for All‐Cause Mortality, Fatal and Non‐Fatal Incident CHD, and Fatal and Non‐Fatal Incident Stroke Stratified by Racial‐Ethnic Group Within 5 Years of Delivery, 2004 to 2017
| All‐Cause Mortality | Incident CHD | Incident Stroke | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Event | Event Rate* | HR (95% CI) † | Event | Event Rate* | HR (95% CI) † | Event | Event Rate* | HR (95% CI) † | |
| Non‐Hispanic White | |||||||||
| Neither pre‐pregnancy hypertension/HDP | 359 | 0.34 | referent | 367 | 0.35 | referent | 120 | 0.11 | referent |
| Pre‐pregnancy hypertension alone | <5 | 0.28 | 0.86 (0.12–6.12) | 5 | 1.40 | 3.06 (1.26–7.43) | 0 | … | … |
| HDP alone | 65 | 0.35 | 1.08 (0.82–1.42) | 164 | 0.88 | 2.31 (1.90–2.81) | 37 | 0.20 | 1.49 (1.02–2.19) |
| Both pre‐pregnancy hypertension and HDP | 14 | 0.64 | 2.11 (1.23–3.65) | 34 | 1.57 | 3.24 (2.24–4.69) | 9 | 0.41 | 1.92 (0.88–4.20) |
| Non‐Hispanic Black | |||||||||
| Neither pre‐pregnancy hypertension/HDP | 256 | 0.49 | referent | 307 | 0.59 | referent | 90 | 0.17 | referent |
| Pre‐pregnancy hypertension alone | <5 | 1.06 | 1.03 (0.26–4.15) | 6 | 1.58 | 1.95 (0.86–4.39) | <5 | 0.53 | 2.35 (0.57–9.65) |
| HDP alone | 107 | 0.81 | 1.70 (1.34–2.15) | 201 | 1.52 | 2.33 (1.94–2.80) | 42 | 0.32 | 1.67 (1.14–2.46) |
| Both pre‐pregnancy hypertension and HDP | 32 | 1.20 | 2.34 (1.58–3.47) | 93 | 3.51 | 4.02 (3.13–5.18) | 26 | 0.97 | 4.00 (2.48–6.45) |
| Hispanic | |||||||||
| Neither pre‐pregnancy hypertension/HDP | 31 | 0.17 | referent | 21 | 0.11 | referent | 7 | 0.04 | referent |
| Pre‐pregnancy hypertension alone | <5 | 3.55 | 18.4 (4.17–80.9) | <5 | 1.77 | 16.0 (2.10–122) | 0 | … | … |
| HDP alone | <5 | 0.15 | 0.30 (0.04–2.20) | 7 | 0.34 | 2.68 (1.06–6.81) | <5 | 0.15 | 2.15 (0.43–10.8) |
| Both pre‐pregnancy hypertension and HDP | 0 | … | … | <5 | 1.18 | 9.38 (2.10–41.8) | 0 | … | … |
| P value § | 0.001 | 0.61 | 0.92 | ||||||
CHD indicates coronary heart disease; HDP, hypertensive disorders of pregnancy; and HR, hazard ratio.
Per 1000 person‐years.
Adjusted for sociodemographic (maternal age; education;, rural/urban residence; median income; payer; Women, Infants, and Children), behavioral (pre‐pregnancy smoking), and clinical characteristics (pre‐pregnancy body mass index, pre‐pregnancy or gestational diabetes mellitus).
P value for interaction between hypertensive disorders of pregnancy and pre‐pregnancy hypertension status and racial/ethnic group.
With regard to incident CHD within 5 years of delivery, there was no evidence of an interaction. Women with both compared with neither condition experienced race/ethnic specific group HRs for incident CHD that varied substantially ranging from 3.24 (95% CI, 2.24–4.69) to 9.38 (95% CI, 2.10–41.8), but the interaction did not reach statistical significance. Race/ethnic specific HRs for incident CHD among women who experienced HDP alone were similar ranging from 2.31 (95% CI, 1.90–2.81) to 2.68 (95% CI, 1.06–6.81). Among women who experienced pre‐pregnancy hypertension alone, the HR for incident CHD was 3.06 (95% CI, 1.26–7.43) for NHW women, 1.95 (95% CI, 0.86–4.39) for NHB women, and 16.0 (95% CI, 2.10–122) for Hispanic women, although the interaction did not reach statistical significance.
There was also no evidence of an interaction for incident stroke within 5 years of delivery. Among women who experienced both conditions, the HRs for incident stroke ranged from 1.92 (95% CI, 0.88–4.20) to 4.00 (95% CI, 2.48–6.45), although the interaction did not reach statistical significance. Race/ethnic specific HRs for incident stroke for women with HDP alone were similar ranging from 1.49 (95% CI, 1.02–2.19) to 2.15 (95% CI, 0.43–10.8). Among women with pre‐pregnancy hypertension alone, only NHB women experienced incident stroke events with a HR of 2.35 (95% CI, 0.57–9.65) for incident stroke, but the interaction did not reach statistical significance.
Discussion
Principal Findings
We observed significantly increased short‐ and long‐term risks of maternal cardiovascular outcomes and all‐cause mortality among women with both conditions, with HDP alone, and with pre‐pregnancy hypertension alone in a statewide, retrospective cohort study following adjustment for potential confounders. Specifically, incident fatal and nonfatal CHD, incident fatal and nonfatal stroke, and all‐cause mortality in a racially/ethnically diverse sample were significantly higher in women with a single or a combined hypertensive disorder within 1, 3, and 5 years of delivery, and any time across the study period (up to 14 years).
This research was conducted in SC, a state where minority groups make up ≈34% of the population and Black people, who have the highest poor birth outcomes and infant mortality rates nationally, comprise the largest minority group. Our study observed variations in the risk of all‐cause mortality within 5 years of delivery by racial/ethnic group with significant elevations for NHB women with both conditions as well as those with HDP alone, NHW women with both conditions, and Hispanic women with pre‐pregnancy hypertension alone. No significant differences were observed by race/ethnic group for incident CHD or incident stroke; however, statistical power to detect race‐ethnic group interactions was limited because of a small number of events.
Our findings add novel scientific information on the relationship between pre‐pregnancy hypertension and HDP with maternal CVD. This is the first study to our knowledge evaluating racial/ethnic differences in maternal cardiovascular outcomes and all‐cause mortality by exposure to HDP and/or pre‐pregnancy hypertension using 4 exposure groups. We assessed maternal CVD using combined cardiovascular outcomes (ie, incident CHD and incident stroke) while additionally evaluating all‐cause mortality. We also adjusted for risk factors (ie, sociodemographic, behavioral, and clinical characteristics) as possible. Furthermore, given the large, diverse study population, our study had the ability to adjust for race/ethnicity in the overall models as well as construct race/ethnic‐specific models to consider potential racial/ethnic disparities.
It is important to examine differences in pregnancy‐related outcomes by race/ethnicity because while birth rates in SC and the United States in 2017 were 11.4 and 11.8 births per 1000 women, respectively, 39 SC birth rates were highest among Hispanic (17.8) followed by NHB (12.6) and NHW (10.1) women. 40 SC’s overall and race/ethnic‐specific birth rates were comparable with those of other southern US states such as North Carolina, 41 Florida, 42 Alabama, 43 and Tennessee 44 varying slightly by race/ethnic group. However, birth rates were only reported for White and Black women in Florida and Tennessee. The overall infant mortality rate was slightly higher for SC than the United States in 2017 (6.51 and 5.79 deaths per 1000 live births, respectively). 45 Race/ethnic‐specific infant mortality rates were also slightly higher for SC compared with the United States at 4.99 versus 4.61 for NHW women, 10.12 versus 11.46 for NHB women, and 4.98 versus 5.35 deaths per 1000 live births for Hispanic women, respectively. 45
Previous Studies of Maternal Cardiovascular Outcomes and Mortality among Women With HDP
A previous meta‐analysis of 22 studies published between January 2005 and August 2015 reported an increased risk of CHD (risk ratio [RR], 2.50; 95% CI, 1.43–4.37), stroke (RR, 1.81; 95% CI, 1.29–2.55), death from CHD (RR, 2.10; 95% CI, 1.25–3.51), and death from CVD (RR, 2.21; 95% CI, 1.83–2.66) in women with preeclampsia after controlling for covariates associated with HDP. 28 Women with preeclampsia were also found to have the highest risks of CHD (RR, 3.10; 95% CI, 1.56–6.16) and stroke (RR, 2.22; 95% CI, 1.73–2.85) <1 year of delivery in sensitivity analyses for follow‐up time (<1, 1–10, and >10 years). 28 In our study, the risk of incident CHD within 1 year of delivery was highest for women with HDP alone (2.80‐fold), and the risk of incident stroke was highest among women with both conditions (2.63‐fold) compared with neither condition. Overall, these results in conjunction with an increased risk in mortality suggest there may be associations between pre‐pregnancy hypertension and/or HDP and short‐term risks of maternal cardiovascular outcomes including mortality within the first year following delivery.
Past studies investigating the association between maternal cardiovascular outcomes and HDP have varied with regard to the outcome(s) and exposure(s) of interest, inclusion/exclusion criteria, length of follow‐up, and covariates. Moreover, most studies have involved homogeneous populations, limiting generalizability. Overall, outcomes examined have included CVD, CHD, ischemic heart disease, stroke, heart failure, thromboembolic events, myocardial infarction, CVD risk factors, as well as all‐cause and cause‐specific mortality (eg, CVD, CHD, stroke, ischemic heart disease, circulatory disease). 28 , 52 Exposures have included HDP and the individual disorders (preeclampsia, eclampsia, gestational hypertension, and preexisting hypertension). With regard to reported inclusion/exclusion criteria, studies have differed by maternal age, parity, index pregnancy definition, and pre‐clinical conditions (preexisting or subsequent). Other studies have not been able to assess long‐term outcomes or examine racial/ethnic health disparities in stroke, ischemic heart disease, venous thromboembolism, or mortality among women with preeclampsia. 13 , 30 , 31 , 32 , 33 Almost all studies controlled for sociodemographic factors (eg, age, household income, insurance), whereas some adjusted for clinical characteristics (eg, parity, gestational diabetes mellitus, BMI, hypertension) and behavioral characteristics such as smoking. Differences in study findings may therefore be expected because of differing methodologies.
Because previous studies have been inconsistent in evaluating the exposure of interest and given the potential role of certain preexisting conditions such as hypertension in pregnancy‐related complications and outcomes, our study included 4 exposure groups based on the presence or absence of HDP and/or pre‐pregnancy hypertension. In this way, it was possible to assess the impact of each exposure including the joint effect of HDP and pre‐pregnancy hypertension on the outcomes of interest. Inclusion of the 4 exposure groups also allowed for consideration of an established risk factor (hypertension) as a potential effect modifier, filling an important gap in the literature. The previously described meta‐analysis reported an increased risk of death from CVD among women with preeclampsia between 1 to 10 and >10 years after delivery (2.30‐ and 2.21‐fold, respectively). 28 In our study, the risk of incident CHD within 3 and 5 years of delivery was highest for women with both conditions (4.36‐fold and 3.79‐fold, respectively). Given the large sample size of our study and availability of individual‐level data, we were able to adjust for sociodemographic and clinical covariates that are established risk factors and potential confounders related to the metabolic syndrome. Our findings suggest potential relationships between exposure to HDP or pre‐pregnancy hypertension and long‐term cardiovascular outcomes including all‐cause mortality 3 and 5 years subsequent to delivery, as well as throughout the entire study period of up to 14 years. However, the number of events was small for women in the pre‐pregnancy hypertension alone exposure group across all time points, thereby limiting our statistical power.
Few prior studies of preeclampsia and long‐term maternal outcomes have been conducted in populations that included NHB or Hispanic women, and no studies to date, to our knowledge, have assessed racial/ethnic differences in the relationship between HDP and long‐term cardiovascular outcomes. International studies have observed increased risks of long‐term maternal CVD morbidity or mortality among women with preeclampsia, although generalizability of the findings is limited because of inclusion of homogeneous racial/ethnic populations (eg, Denmark, 47 Iceland, 31 Scotland, 32 Israel, 53 Norway 54 ). However, a recent US study by Miller et al examined the relationship between race/ethnicity and maternal stroke during delivery hospitalization by hypertension status (normotensive, chronic hypertension, pregnancy‐induced hypertension) using the National Inpatient Sample from 1998 to 2014. Higher stroke risk was observed among women from minority groups (Black, Hispanic, Asian/Pacific Islander) compared with White women and was modified by hypertensive status (interaction P<0.0001). 55
While several studies have included race and/or ethnicity as a covariate in the relationship between maternal CVD outcomes and HDP, 29 , 46 , 51 , 52 , 56 they did not test for interactions by race/ethnic group. Potential disparities are important to consider since vascular disease and many risk factors are present at different rates among different race/ethnic groups. Preeclampsia also differs by race/ethnic group with rates higher among NHB women. Findings from our stratified analyses suggest racial/ethnic disparities in long‐term maternal all‐cause mortality up to 5 years after delivery for women with pre‐pregnancy hypertension or HDP with an interaction detected for all‐cause mortality, although we did not detect interactions for incident CHD or incident stroke.
Strengths and Limitations
Our population‐based study included all live births in the state of SC over a 13‐year period providing a large, diverse population in which it was possible to consider potential differences by race/ethnic group. Over the 13‐year period of 2004 to 2016, a total of 462 428 women gave birth in SC; 433 430 of whom were included in the current study: 254 491 NHW, 137 784 NHB, and 41 155 Hispanic women. Similar to other studies and because of the quality of existing data, we included women with live, singleton births and excluded births during which the baby died. 57 , 58 , 59 , 60 HDP was ever diagnosed in 18.1% of women in the data set. We found evidence of an interaction between HDP and pre‐pregnancy hypertension and race/ethnicity for all‐cause mortality. To our knowledge, the current study is among the first to examine potential racial/ethnic health disparities in maternal outcomes in HDP, which is critical in terms of the risk and clinical course of HDP such as preeclampsia and long‐term disease outcomes and mortality. Our findings add significantly to the body of knowledge related to HDP and pre‐pregnancy hypertension and maternal cardiovascular outcomes.
While up to 14 years of follow‐up time was available, the overall event numbers and rates of incident CHD, stroke, and all‐cause mortality events were low in our study population of relatively young women of child‐bearing age. Moreover, stroke may take longer to manifest from hypertension than CHD and has a lower incident rate. Examination of the event rates per 1000 person‐years by exposure group helps to place the results in context in terms of the absolute risk. The incident CHD event rate among women with both conditions was 4.04, with HDP alone was 1.77, with pre‐pregnancy hypertension alone was 1.90, and with neither condition was 0.67. The all‐cause mortality event rate among women with both conditions was 1.21, with HDP alone was 0.68, with pre‐pregnancy hypertension alone was 1.29, and with neither condition was 0.50.
It is difficult to make comparisons between groups with a small number of events. Therefore, potential findings for all‐cause mortality and incident stroke as well as findings among women with pre‐pregnancy hypertension alone and Hispanic women should be interpreted with caution and results may not be generalizable. Stroke subtypes could not be examined given the data source and lack of information related to ischemic or hemorrhagic etiology.
Because of the use of administrative data available for the current study period, lifetime history of pregnancy and information on potential diagnoses that may have occurred before the index pregnancy including CVD are not included. The first pregnancy with either/both exposure (ie, pre‐pregnancy hypertension and/or HDP) or the first pregnancy for women without the aforementioned exposure(s) in the data set defined the index pregnancy. This may not have been the actual first pregnancy, although information on previous births was available from the birth certificates. Women who did not experience an event or were not seen in the ED/hospital were assumed to be healthy and not lost to follow‐up. To address this limitation of our study, we conducted a sensitivity analysis limited to those with either events or receipt of ED/inpatient medical care more than 5 years post‐delivery in the data set. Results of the sensitivity analysis were similar to our initial analysis for incident CHD, incident stroke, and all‐cause mortality.
Some studies have reported low sensitivity (72%) of birth certificates for maternal characteristics. 61 Our study was able to successfully link 97.5% of birth certificates to the corresponding hospital discharge records. Further, a validation study conducted in Washington state among 3701 women with live births reported a true positive fraction of 73.5 (95% CI, 63.8–83.3) for pregnancy‐induced hypertension based on comparison of birth certificates, hospital discharge data, and medical records in 2000. 62 Similar to Washington state, SC birth certificates use a check box system for indications such as gestational hypertension, which is also used for preeclampsia and eclampsia. Although SC birth certificates are unable to differentiate between preeclampsia and eclampsia, they do differentiate among pre‐pregnancy hypertension, pre‐pregnancy diabetes mellitus, and gestational diabetes mellitus. While historical data on individual‐level blood pressure and smoking were limited, pre‐pregnancy smoking, smoking during pregnancy, as well as gestational hypertension and pre‐pregnancy hypertension were available from birth certificate data. Past diagnoses included as covariates (eg, pre‐pregnancy) were limited to codes at delivery available from the birth certificates.
Conclusions
Relationships between pre‐pregnancy hypertension and HDP and short‐ and long‐term risks of maternal cardiovascular outcomes including mortality are supported by our findings. Further, racial/ethnic differences in mortality were observed within 5 years post‐delivery. Pre‐pregnancy hypertension with superimposed HDP was associated with a 3.79‐fold increase in incident CHD, a 3.10‐fold increase in incident stroke, and a 2.21‐fold increase in all‐cause mortality within 5 years of delivery. The presence of HDP alone or pre‐pregnancy hypertension alone was also associated with future cardiovascular outcomes up to 5 years post‐delivery. Our findings could help to further improve clinical practice and public health prevention efforts such as close monitoring and management of modifiable cardiovascular risk factors as well as extension of health care beyond the 6‐week postpartum period in women with history of HDP or pre‐pregnancy hypertension for prevention and early detection of associated events to prevent poor short‐ and long‐term maternal outcomes.
Sources of Funding
This work was supported by a grant from the National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI) (K01 HL138273‐02). This project was also supported in part by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health under Grant Number UL1 TR001450. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the NCATS.
Disclosures
None.
Supporting information
Tables S1–S3
(J Am Heart Assoc. 2021;10:e018155. DOI: 10.1161/JAHA.120.018155.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018155
For Sources of Funding and Disclosures, see page 12.
References
- 1. Antza C, Cifkova R, Kotsis V. Hypertensive complications of pregnancy: a clinical overview. Metabolism. 2018;86:102–111.DOI: 10.1016/j.metabol.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 2. Homer CS, Brown MA, Mangos G, Davis GK. Non‐proteinuric pre‐eclampsia: a novel risk indicator in women with gestational hypertension. J Hypertens. 2008;26:295–302.DOI: 10.1097/HJH.0b013e3282f1a953. [DOI] [PubMed] [Google Scholar]
- 3. Acog practice bulletin no. 202: Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–e25. [DOI] [PubMed] [Google Scholar]
- 4. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7.DOI: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 5. Duckitt K, Harrington D. Risk factors for pre‐eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. DOI: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanaka M, Jaamaa G, Kaiser M, Hills E, Soim A, Zhu M, Shcherbatykh IY, Samelson R, Bell E, Zdeb M, et al. Racial disparity in hypertensive disorders of pregnancy in New York State: a 10‐year longitudinal population‐based study. Am J Public Health. 2007;97:163–170.DOI: 10.2105/AJPH.2005.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samadi AR, Mayberry RM, Zaidi AA, Pleasant JC, McGhee N Jr, Rice RJ. Maternal hypertension and associated pregnancy complications among african‐american and other women in the United States. Obstet Gynecol. 1996;87:557–563.DOI: 10.1016/0029-7844(95)00480-7. [DOI] [PubMed] [Google Scholar]
- 8. Bramham K, Briley AL, Seed PT, Poston L, Shennan AH, Chappell LC. Pregnancy outcome in women with chronic kidney disease: a prospective cohort study. Reproductive sciences. 2011;18:623–630.DOI: 10.1177/1933719110395403. [DOI] [PubMed] [Google Scholar]
- 9. Nevis IF, Reitsma A, Dominic A, McDonald S, Thabane L, Akl EA, Hladunewich M, Akbari A, Joseph G, Sia W, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587–2598.DOI: 10.2215/CJN.10841210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panaitescu AM, Syngelaki A, Prodan N, Akolekar R, Nicolaides KH. Chronic hypertension and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol. 2017;50:228–235.DOI: 10.1002/uog.17493. [DOI] [PubMed] [Google Scholar]
- 11. Bateman BT, Bansil P, Hernandez‐Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206:e131–e138.DOI: 10.1016/j.ajog.2011.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banhidy F, Acs N, Puho EH, Czeizel AE. The efficacy of antihypertensive treatment in pregnant women with chronic and gestational hypertension: a population‐based study. Hypertens Res. 2010;33:460–466.DOI: 10.1038/hr.2010.17. [DOI] [PubMed] [Google Scholar]
- 13. Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre‐eclampsia: population based cohort study. BMJ. 2001;323:1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gretarsdottir AS, Aspelund T, Steingrimsdottir T, Bjarnadottir RI, Einarsdottir K. Preterm births in iceland 1997–2016: preterm birth rates by gestational age groups and type of preterm birth. Birth. 2019;47:105–114.DOI: 10.1111/birt.12467. [DOI] [PubMed] [Google Scholar]
- 15. Sibai BM. Magnesium sulfate prophylaxis in preeclampsia: lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520–1526.DOI: 10.1016/j.ajog.2003.12.057. [DOI] [PubMed] [Google Scholar]
- 16. Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181–192. [DOI] [PubMed] [Google Scholar]
- 17. American College of Obstetricians Gynecologists. Task Force on Hypertension in Pregnancy . Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' task force on hypertension in pregnancy. Obstetrics and gynecology. 2013;122:1122‐1131. [DOI] [PubMed] [Google Scholar]
- 18. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre‐eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. [DOI] [PubMed] [Google Scholar]
- 19. Mustafa R, Ahmed S, Gupta A, Venuto RC. A comprehensive review of hypertension in pregnancy. J Pregnancy. 2012;2012:105918. DOI: 10.1155/2012/105918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. Who analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074.DOI: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 21. Tucker MJ, Berg CJ, Callaghan WM, Hsia J. The black‐white disparity in pregnancy‐related mortality from 5 conditions: differences in prevalence and case‐fatality rates. Am J Public Health. 2007;97:247–251.DOI: 10.2105/AJPH.2005.072975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. MacKay AP, Berg CJ, Atrash HK. Pregnancy‐related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97:533–538. [DOI] [PubMed] [Google Scholar]
- 23. Petersen EE, Davis NL, Goodman D, Cox S, Syverson C, Seed K, Shapiro‐Mendoza C, Callaghan WM, Barfield W. Racial/ethnic disparities in pregnancy‐related deaths—United States, 2007–2016. MMWR Morb Mortal Wkly Rep. 2019;68:762–765.DOI: 10.15585/mmwr.mm6835a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magee LA, Abalos E, von Dadelszen P , Sibai B, Walkinshaw SA, Group CS . Control of hypertension in pregnancy. Curr Hypertens Rep. 2009;11:429–436.DOI: 10.1007/s11906-009-0073-y. [DOI] [PubMed] [Google Scholar]
- 25. Magee LA, von Dadelszen P, Singer J, Lee T, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, et al.; Group* CS . The CHIPS randomized controlled trial (Control of Hypertension in Pregnancy Study): is severe hypertension just an elevated blood pressure? Hypertension. 2016;68:1153–1159.DOI: 10.1161/HYPERTENSIONAHA.116.07862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vanek M, Sheiner E, Levy A, Mazor M. Chronic hypertension and the risk for adverse pregnancy outcome after superimposed pre‐eclampsia. Int J Gynaecol Obstet. 2004;86:7–11.DOI: 10.1016/j.ijgo.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 27. James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106:509–516.DOI: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 28. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew‐Graham CA, et al. Preeclampsia and future cardiovascular health: a systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. DOI: 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- 29. Kestenbaum B, Seliger SL, Easterling TR, Gillen DL, Critchlow CW, Stehman‐Breen CO, Schwartz SM. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am J Kidney Dis. 2003;42:982–989. DOI: 10.1016/j.ajkd.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 30. Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845. DOI: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jonsdottir LS, Arngrimsson R, Geirsson RT, Sigvaldason H, Sigfusson N. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand. 1995;74:772–776.DOI: 10.3109/00016349509021195. [DOI] [PubMed] [Google Scholar]
- 32. Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357:2002–2006. DOI: 10.1016/S0140‐6736(00)05112‐6. [DOI] [PubMed] [Google Scholar]
- 33. Wikstrom AK, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG: an international journal of obstetrics and gynaecology. 2005;112:1486–1491.DOI: 10.1111/j.1471-0528.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 34. Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168.DOI: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 35. Alexander GR, Kotelchuck M. Quantifying the adequacy of prenatal care: a comparison of indices. Public Health Rep. 1996;111:408–418.discussion 419 [PMC free article] [PubMed] [Google Scholar]
- 36. Tayebi T, Hamzehgardeshi Z, Ahmad Shirvani M, Dayhimi M, Danesh M. Relationship between revised graduated index (R‐GINDEX) of prenatal care utilization & preterm labor and low birth weight. Glob J Health Sci. 2014;6:131–137. DOI: 10.5539/gjhs.v6n3p131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. SAS Institute . Statistical analytical software. 2012.
- 38. StataCorp . Stata statistical software. 2013.
- 39. Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2017. Natl Vital Stat Rep. 2018;67:1–50. [PubMed] [Google Scholar]
- 40. South Carolina Department of Health and Environmental Control . South carolina community assessment network (scan) birth certificate data. 2019;2019.
- 41. North Carolina State Center for Health Statistics . Selected vital statistics for 2017 and 2013–2017. 2019;2019.
- 42. Florida Department of Health . Florida vital statistics annual report 2017. Live Births. 2017;2019:1–48. [Google Scholar]
- 43. Alabama Department of Public Health . Center for Health Statistics. Alabama vital statistics: Division of Statistical Analysis; 2017. [Google Scholar]
- 44. Tennessee Department of Health DoVRaS . Number of live births with rates per 1,000 population, by race of mother, for counties of tennessee, resident data, 2017. 2019.
- 45. Kochanek KDMS, Xu JQ, Arias E. Deaths: final data for 2017. Natl Vital Stat Rep. 2019;68:1–77. [PubMed] [Google Scholar]
- 46. Theilen LH, Meeks H, Fraser A, Esplin MS, Smith KR, Varner MW. Long‐term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am J Obstet Gynecol. 2018;219:107.e1–107.e6.DOI: 10.1016/j.ajog.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lykke JA, Langhoff‐Roos J, Lockwood CJ, Triche EW, Paidas MJ. Mortality of mothers from cardiovascular and non‐cardiovascular causes following pregnancy complications in first delivery. Paediatr Perinat Epidemiol. 2010;24:323–330.DOI: 10.1111/j.1365-3016.2010.01120.x. [DOI] [PubMed] [Google Scholar]
- 48. Brown DW, Dueker N, Jamieson DJ, Cole JW, Wozniak MA, Stern BJ, Giles WH, Kittner SJ. Preeclampsia and the risk of ischemic stroke among young women: results from the stroke prevention in young women study. Stroke. 2006;37:1055–1059.DOI: 10.1161/01.STR.0000206284.96739.ee. [DOI] [PubMed] [Google Scholar]
- 49. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre‐eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta‐analysis. BMJ. 2007;335:974. DOI: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bhattacharya S, Prescott GJ, Iversen L, Campbell DM, Smith WC, Hannaford PC. Hypertensive disorders of pregnancy and future health and mortality: a record linkage study. Pregnancy Hypertens. 2012;2:1–7.DOI: 10.1016/j.preghy.2011.08.116. [DOI] [PubMed] [Google Scholar]
- 51. Arnaout R, Nah G, Marcus G, Tseng Z, Foster E, Harris IS, Divanji P, Klein L, Gonzalez J, Parikh N. Pregnancy complications and premature cardiovascular events among 1.6 million california pregnancies. Open Heart. 2019;6:e000927. DOI: 10.1136/openhrt-2018-000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James‐Todd TM, Rich‐Edwards JW. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med. 2018;169:224–232.DOI: 10.7326/M17-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kessous R, Shoham‐Vardi I, Pariente G, Sergienko R, Sheiner E. Long‐term maternal atherosclerotic morbidity in women with pre‐eclampsia. Heart. 2015;101:442–446.DOI: 10.1136/heartjnl-2014-306571. [DOI] [PubMed] [Google Scholar]
- 54. Riise HKR, Sulo G, Tell GS, Igland J, Egeland G, Nygard O, Selmer R, Iversen AC, Daltveit AK. Hypertensive pregnancy disorders increase the risk of maternal cardiovascular disease after adjustment for cardiovascular risk factors. Int J Cardiol. 2019;282:81–87.DOI: 10.1016/j.ijcard.2019.01.097. [DOI] [PubMed] [Google Scholar]
- 55. Miller EC, Zambrano Espinoza MD, Huang Y, Friedman AM, Boehme AK, Bello NA, Cleary KL, Wright JD, D'Alton ME. Maternal race/ethnicity, hypertension, and risk for stroke during delivery admission. J Am Heart Assoc. 2020;9:e014775. DOI: 10.1161/JAHA.119.014775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leon LJ, McCarthy FP, Direk K, Gonzalez‐Izquierdo A, Prieto‐Merino D, Casas JP, Chappell L. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: a CALIBER study. Circulation. 2019;140:1050–1060.DOI: 10.1161/CIRCULATIONAHA.118.038080. [DOI] [PubMed] [Google Scholar]
- 57. Ellerbe CN, Gebregziabher M, Korte JE, Mauldin J, Hunt KJ. Quantifying the impact of gestational diabetes mellitus, maternal weight and race on birthweight via quantile regression. PLoS One. 2013;8:e65017. DOI: 10.1371/journal.pone.0065017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hunt KJ, Marlow NM, Gebregziabher M, Ellerbe CN, Mauldin J, Mayorga ME, Korte JE. Impact of maternal diabetes on birthweight is greater in non‐hispanic blacks than in non‐Hispanic whites. Diabetologia. 2012;55:971–980.DOI: 10.1007/s00125-011-2430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nash MC, Kip K, Wang W, Custer M, O'Rourke K. Deployment among active‐duty military women and pregnancy‐related hypertensive disorders. Mil Med. 2019;184:e278–e283.DOI: 10.1093/milmed/usy228. [DOI] [PubMed] [Google Scholar]
- 60. Savitz DA, Danilack VA, Elston B, Lipkind HS. Pregnancy‐induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol. 2014;180:41–44.DOI: 10.1093/aje/kwu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Howland RE, Madsen AM, Toprani A, Gambatese M, Mulready‐Ward C, Begier E. How well do birth records serve maternal and child health programs? Birth registration system evaluation, New York City, 2008–2011. Matern Child Health J. 2015;19:1559–1566.DOI: 10.1007/s10995-015-1664-7. [DOI] [PubMed] [Google Scholar]
- 62. Lydon‐Rochelle MT, Holt VL, Cardenas V, Nelson JC, Easterling TR, Gardella C, Callaghan WM. The reporting of pre‐existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol. 2005;193:125–134.DOI: 10.1016/j.ajog.2005.02.096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


