Abstract
Background
Studies on intact abdominal aortic aneurysms mainly focus on treated patients, and data on untreated patients are sparse. The objective was to investigate sex differences among untreated patients regarding rupture and mortality rates and to determine predictors for these events. Sex‐specific causes of death were evaluated.
Methods and Results
All patients ≥40 years diagnosed from 2001 to 2015 (n=32 393) with intact abdominal aortic aneurysms were identified in national registries; 60% (n=19 569) were untreated. Comorbid loads, crude rupture, and mortality rates were assessed. Predictors of 5‐year rupture and mortality were analyzed in Cox models (sex, age, comorbidities, income, and marital status). The proportion of men and women with multiple comorbidities was similar. Within 5 years, 798 ruptures occurred (9.7% women versus 6.9% men, P<0.001). Ruptures were independently predicted by female sex (hazard ratio [HR], 1.23; 95% CI, 1.07–1.42; P=0.004), chronic obstructive pulmonary disease (HR, 1.36; 95% CI, 1.15–1.62; P<0.001), age (HR, 11.49; 95% CI, 5.68–23.25 for ≥80 years; P<0.001), and income (HR, 0.63; 95% CI, 0.53–0.75 for highest tertile; P<0.001). After 5 years, 56.5% women and 50.4% men were deceased. Mortality was not independently predicted by female sex. Rupture was the third most common cause of death (11.9% women versus 8.7% men; P<0.001). The median time‐to‐events was 2.8 years.
Conclusions
A considerable proportion of patients with intact abdominal aortic aneurysms in surveillance remain untreated. Despite surveillance algorithms, the healthcare system fails to prevent a high number of ruptures, especially among women. The time‐to‐event data highlight the urgency to develop more individualized surveillance.
Keywords: abdominal aortic aneurysm, mortality, rupture, sex‐specific
Subject Categories: Aneurysm, Epidemiology, Risk Factors, Treatment, Women
Nonstandard Abbreviations and Acronyms
- CDR
cause of death register
- NPR
national patient register
Clinical Perspective
What Is New?
This population‐based study on 32 393 patients with intact abdominal aortic aneurysms defines the high proportion (60%) of diagnosed but untreated patients in a nationwide setting.
Although outcomes of treated women and men have been extensively studied, the true sex‐specific risks of rupture and death among untreated patients in surveillance are unknown; the rates of both rupture and death were higher for female patients at all time points up to 5 years after diagnosis.
Female sex predicted rupture, but not death. Ruptures were the third most common cause of death among both sexes, and the median time to both rupture and death was 2.8 years.
What Are the Clinical Implications?
Untreated status is common among men and women with intact abdominal aortic aneurysms even after several years of surveillance.
Despite standardized follow‐up algorithms for all patients, 7 out of 100 men and 10 out of 100 women had a rupture, equaling to a total of 798 ruptures during the first 5 years.
The relatively short median time‐to‐events provides information that could improve the clinical care trajectory for patients with abdominal aortic aneurysms in surveillance.
An intact abdominal aortic aneurysm (iAAA) is a permanent progressive widening of the infrarenal aorta and can potentially rupture. 1 Intact AAAs affect men and women by a 4 to 6:1 ratio, 2 by contrast, the sex difference in the incidence of ruptured aneurysms (rAAA) is known to be less pronounced (2:1). This suggests that AAAs in women may to a lesser degree be found or repaired when still intact. 3 , 4 , 5 , 6 Nearly all trend analyses on AAAs are limited to databases reporting on treated patients and, thus, the risk profiles of the untreated cohort remain unexplored. 3 , 4 , 7 , 8 , 9 Several patient series that included treated AAAs have witnessed poorer survival among women. 10 , 11 , 12 , 13 , 14 , 15 This exclusive reporting on treated patients poses a true challenge for healthcare strategizing and resource allocation. For untreated intact AAAs, data on risk profiles are limited to surveillance studies on small AAAs 16 , 17 or single‐center experiences 18 , 19 , 20 , 21 as well as 1 meta‐analysis 22 depicting the natural history of noneligible large AAAs. In a real‐world setting at any given vascular clinic, however, the untreated cohort is heterogeneous and encompasses patients at all stages of standard AAA care flow. Moreover, although survival and rupture‐free survival of untreated AAAs have been investigated in diameter strata, 17 , 18 , 20 , 22 , 23 sex‐specific rupture and mortality rates are lacking. 18 , 22 For the female patient, the event of rupture is even more detrimental, as women with rAAA face a higher risk of turndown. 3 , 4 , 6 , 7 , 15 , 24 When treated for rAAA, outcomes for women have been reported to be worse. 3 , 6 , 25
The nearly quadrupled risk of rupture for female patients detected in the UKSAT (United Kingdom Small Aneurysm Trial) including 1167 nonrandomized patients 17 and the RESCAN 16 meta‐analysis is often cited. There was considerable interstudy variation to the crude rupture rates, reflecting the risks of disparate patient samples.
Accurate risk profiling of men and women with untreated iAAAs is essential in an individualized and safe AAA management program and should include factors besides the maximum aneurysm diameter. The primary objective of this study was to report possible sex differences among surveilled but untreated patients with AAA regarding their rupture and mortality rates and to identify predictors for these fatal events. The secondary objective was to determine sex‐specific causes of death.
METHODS
The Swedish Population, Health Care, and Screening
The Swedish population reached 9.8 million in 2015 (50.0% women; Statistics Sweden 26 ). The average life span for men and women is estimated at 80.7 and 84.1 years, respectively. There are 33 hospitals providing vascular care in the country. 27 Hospital registries thrive on the prevailing policy of mandatory reimbursement, and arterial procedures are performed only within the public healthcare sector.
A 1‐time ultrasound screening program for AAAs was selectively introduced among elderly men in 2006. 28 National coverage was reached in 2015 for all 65‐year‐old men. Of all screening‐detected AAAs, 60% to 70% measure less than 40 mm at primary diagnosis. 28 , 29 , 30
A generalized algorithm with predefined imaging protocols is used for the surveillance of all patients irrespective of age, sex, smoking status, and other patient‐level factors. Most vascular services in Sweden adhere to similar protocols, commonly with ultrasound imaging (30–39 mm: 2‐ to‐3‐year interval, 40–44 mm: 1‐year interval, 45–49 mm: 6‐month interval; at 50 mm women are considered for surgery, ≥50 mm: 3‐ to 6‐month interval in men and some women, ≥55 mm surgical evaluation).
Data Sources and Patient Cohort
The data set contained all unique cases of AAAs (iAAA and rAAA) per definition of the International Classification of Diseases, Ninth Revision and Tenth Revision (ICD‐9/ICD‐10: 441E/I71.4, respectively, for intact and 441D/I71.3 for ruptured AAAs) in Sweden between 2001 and 2015 (n=41 222). Swedish vascular surgeons conform to the 30 mm threshold for AAA diagnosis. 31 For all individuals, hospital episode data and outpatient events had been extracted between 1995 and 2015 from the NPR (National Patient Register) managed by the National Board of Health and Welfare. Data on the cause of death were extracted from the Swedish CDR (Cause of Death Registry). 32 Patients diagnosed between 1995 and 1999 were excluded to ensure that the index event indeed represented the first AAA event, and those diagnosed in 2000 were excluded in order to collect complete annual data on income before diagnosis. In total, 32 393 such people were identified with a diagnosed iAAA at index. Patients undergoing treatment during the study period were analyzed (n=12 824) with regard to timing of treatment to ensure a representative population of untreated patients. Within 5 years, 92.4% and 91.7% of all electively treated men and women, respectively, had received treatment.
All patients with rAAA as index diagnosis were excluded (n=7968) as were patients <40 years at diagnosis (n=7). A subset of patients (CDR) with iAAA as a contributing cause of death (n=854) were excluded, as person‐time at risk for rupture and death was unknown.
Ruptures that occurred after the index event of iAAA were identified from the NPR and the CDR (nonfatal and fatal ruptures). Dates of rupture and death were collected. Data on comorbidities (both primary and contributing diagnoses) were extracted from the NPR through ICD codes and encompassed all entries up to 5 years before index.
Potential Predictors of Rupture and Death
Age, sex, comorbidities, disposable income, marital status, and inclusion year were considered as potential predictors of rupture and death. Comorbidities were divided into 5 major groups: cardiovascular/renal, chronic obstructive pulmonary disease (COPD), malignancies, neurological disorders, and diabetes mellitus. Cardiovascular/renal was defined as a composite variable of cardiac and renal disease (including chronic ischemic heart disease, prior myocardial infarction, history of coronary artery bypass graft surgery and/or heart failure, concomitant thoracic aortic aneurysm, aortic dissection, and renal failure). Malignancies include all subtypes of malignant cancer. Stroke and dementia were classified as neurological disorders. Hypertension, hyperlipidemia, peripheral artery disease, and arrhythmia were defined as “other” comorbidities. In the absence of all mentioned target comorbidities, the comorbidity status was marked as “none or unknown.” The 5 comorbidity groups were incorporated into further analyses and comorbid loads (accounting for 3 of the major groups: cardiovascular disease/renal failure, COPD, any malignancy) were illustrated by Venn diagrams.
Patient‐specific socioeconomic data were acquired from the longitudinal integrated database for health insurance and labor market studies 33 managed by Statistics Sweden. Two variables were studied: annual disposable income and marital status the year before diagnosis. Disposable income for each patient denoted the weighted income based on consumption units in the household. Next, an income tertile (low, middle, high) was ascribed to each patient. Patients were also subdivided according to married (married or civil union), unmarried (never married or divorced), or widowed marital status.
The Swedish personal identity number functions as a person‐specific identifier and can be used for linkage of data from national registries. 34 Personal identity numbers were used by the National Board of Health and Welfare to retrieve and match individual data and to assemble the study population.
Causes of Death
Primary causes of death were collected from the CDR and assessed for patients who died within 5 years after iAAA diagnosis. The proportion of deaths related to each group of underlying causes was recorded and ranked by sex.
Outcomes
The primary outcomes were aneurysm rupture (whether fatal or nonfatal) and overall mortality within 5 years of iAAA diagnosis among untreated men and women. The secondary outcome was sex‐specific causes of death.
Control Analysis of Diameters in Outpatient Setting
A complementary retrospective chart analysis was performed in order to compare AAA diameters of all women and men under surveillance at a vascular department. Some 120 women diagnosed with an AAA between January 2012 and December 2014 and with follow‐up at the Karolinska University Hospital (Stockholm, Sweden) were consecutively included if at least 2 imaging examinations were available. Similarly, men were consecutively included from year 2012 onwards: 120 men were identified by July 2013. Details on patient and aneurysm demographics were extracted from medical charts.
Ethical Considerations
The study was approved by the Regional Ethics Committee in Stockholm (registration number 2015/2108‐31/5) and it was conducted in accordance with the Helsinki Declaration. Informed consent was not required because of the population‐based design including anonymized data only. The complementary diameter analysis of outpatients was approved as a regional student project (2013/1277‐31/3). The reporting of this paper abides by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) 35 statement.
Statistical Analysis
Categorical values are shown as numbers and percentages, continuous variables as means with SDs, or medians with interquartile ranges. The Pearson's chi‐square test was used for nominal comparisons; continuous comparisons used the Student's t test or Mann‐Whitney U test where appropriate. Distributions were assessed using histograms. Temporal trends were described by 3‐year periods (2001–2003, 2004–2006, 2007–2009, 2010–2012, 2013–2015).
Crude rupture and mortality rates at 1, 3, and 5 years of follow‐up were recorded for patients with complete follow‐up data. Crude survival and survival free from rupture within 5 years after diagnosis were analyzed in sex strata using the Kaplan‐Meier method. Cox proportional hazards regression models were employed to examine the effect of each predictor on the risk of rupture (regardless of outcome) and death. Individuals were censored at a positive outcome (rupture, death). In the model with rupture as outcome, patients were censored at the event of non‐rupture‐related death. In both models, subjects were censored at 5 years of follow‐up or on December 31, 2015 irrespective of the preceding time at exposure. Confounding was addressed by adjusting for the following covariates: age (analyzed in 4 categories: <60, 60–69, 70–79, ≥80 years), sex, comorbidity groups, disposable income, marital status, and inclusion period. The Cox models were then performed anew after excluding the primary target group of screening (all 65‐year‐old men detected 2006 or later). In the cause of death analyses, all patients deceased within 5 years were included regardless of year of index.
Null hypotheses were rejected at a 2‐tailed P value <0.05. The R programming environment version 3.6.3 (R Core Team [2020], R Foundation for Statistical Computing, Vienna, Austria) 36 was used for data processing and statistical computing. Quasi‐proportional Venn diagrams were created using the nVenn algorithm and interface provided in the nVennR‐package (version 0.2.1, published 2018‐05‐11).
Results
Baseline Demographics and Comorbidity Loads
Of all patients with an identified index event of iAAA between 2001 and 2015, a majority remained untreated at the date of last follow‐up (19 569 of 32 393 [60%]). The proportion of women was lower among treated compared with untreated patients (17% versus 23%). At baseline, untreated women were on average 3.1 years older compared with men (76.1 [8.5] years versus 73.0 [8.5] years, Table S1). Women were overrepresented in the lowest income tertile (52.4% women versus 29.6% men). Female patients were more often widowed (40.5% versus 11.5%).
The comorbid loads of untreated men and women with iAAAs are shown in Figure 1A and 1B. The presence of synchronous comorbidities (2 or more) was equally common among men and women (15.9% for men versus 15.3% for women, P=0.46). All 3 comorbidity groups were present in 1.8% men and 1.9% women (P=0.64). Women more often suffered from COPD, whereas single cardiovascular disease was more common among men. The proportion of patients with neither major comorbidities (5 groups) nor other target conditions was 25.9% among men and 23.9% among women.
Figure 1.
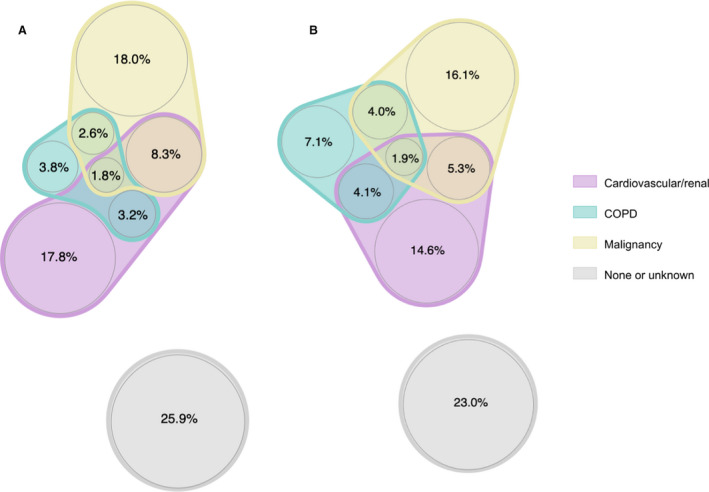
The baseline comorbid loads and proportion of patients with synchronous comorbidities for untreated (A) Men (n=15 020) and (B) Women (n=4549). The grey scale represents patients with none of the major comorbidity groups nor any other extracted comorbidities. COPD indicates chronic obstructive pulmonary disease.
Time‐to‐Event Data
The overall median times to rupture and death among untreated women and men were 2.80 years (interquartile range, 1.2–5.0) and 2.84 years (interquartile range, 1.1–5.1), respectively. The distributions of time to rupture and death were both positively skewed and similar between the sexes (P=0.065 for rupture and P=0.068 for death). The sex‐specific cumulative probabilities of both survival free from rupture and all‐cause survival significantly diverged (P<0.0001 for both, Figure 2A and 2B) with higher risks of rupture and poorer survival among untreated female patients at all time points up to 5 years after diagnosis (Figure 2A and 2B; Table 1). The sex discrepancy in overall survival prevailed in the middle 2 age categories but was not detected among the youngest (<60 years, P=0.32) and the oldest (≥80 years, P=0.56) patients (Figure S1).
Figure 2.
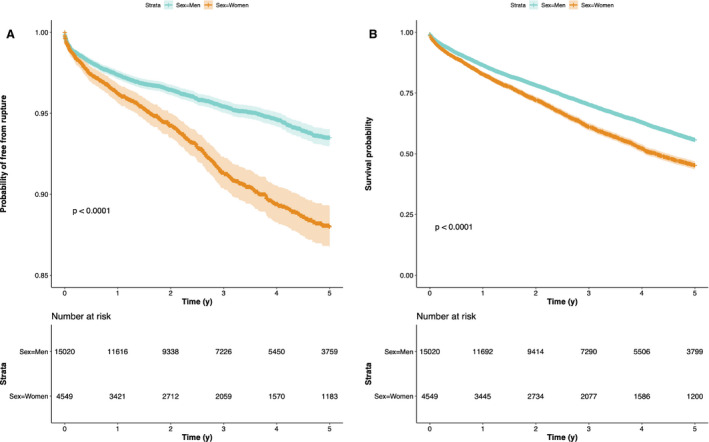
Kaplan‐Meier curves illustrating (A) Survival free from rupture and (B) Overall survival for untreated men and women with iAAAs up to 5 years after diagnosis. iAAA indicates intact abdominal aortic aneurysm.
Table 1.
Crude Cumulative Rupture and Mortality Rates Within 1, 3, and 5 years by Sex in The Cohort of Untreated Patients With iAAAs
| Total | Men | Women | P value* | |
|---|---|---|---|---|
| Rupture | ||||
| 1‐y | 500 (2.8) | 351 (2.6) | 149 (3.6) | 0.001 |
| 3‐y | 752 (5.3) | 495 (4.6) | 257 (7.4) | <0.001 |
| 5‐y | 798 (7.7) | 531 (6.9) | 267 (9.7) | <0.001 |
| Mortality | ||||
| 1‐y | 2607 (14.7) | 1875 (13.8) | 732 (17.5) | <0.001 |
| 3‐y | 4754 (33.6) | 3384 (31.7) | 1370 (39.7) | <0.001 |
| 5‐y | 5424 (52.0) | 3862 (50.4) | 1562 (56.5) | <0.001 |
| Complete follow‐up | ||||
| 1‐y | 17 747 (90.1) | 13 568 (90.3) | 4179 (91.9) | |
| 3‐y | 14 131 (72.2) | 10 680 (71.1) | 3451 (75.9) | |
| 5‐y | 10 429 (53.3) | 7666 (51.0) | 2763 (60.7) | |
iAAA indicates abdominal aortic aneurysm.
X2 test.
Rupture and Mortality Rates by Sex
Within 5 years of follow‐up, there were 798 (7.7%) ruptures in the group of untreated patients (Table 1) corresponding to 22.6 ruptures per 1000 person‐years within 5 years. Among these patients, 69% died following aneurysm rupture (68% for women versus 70% for men, P=0.57). The cumulative 1‐, 3‐, and 5‐year rupture rates (Table 1) were higher for women than men (P≤0.001 for all comparisons). At 5 years, the crude cumulative rupture rate reached 9.7% for women as opposed to 6.9% for men (P<0.001, crude rupture rate of 29.6 per 1000 female person‐years versus 20.2 for men). The age distribution of women with rupture was tilted toward older age categories: 60.5% of the ruptures occurred in women aged 80 or more compared with 55.0% in men (Figure S2). Young patients (<60 years) constituted only 0.8% among both sexes.
More than half (52.0%) of all untreated patients (n=5424, 56.5% women versus 50.4% men) with complete follow‐up data were deceased within 5 years (Table 1). Again, the overall mortality rates of women surpassed those of men at all follow‐up intervals (P<0.001 for all comparisons).
Predictors of Rupture and Mortality Within 5 Years
Female sex predicted rupture after adjusting for confounders with a relative risk increase of 23% (95% CI, 1.07–1.42; P=0.004, Figure 3A and Table S2). An increased hazard of rupture was also captured for patients with COPD (adjusted hazard ratio [HR], 1.36; 95% CI, 1.15–1.62; P<0.001). Advancing age independently predicted rupture, whereas middle and high income as well as later inclusion periods lowered the risk (Figure 3A).
Figure 3.
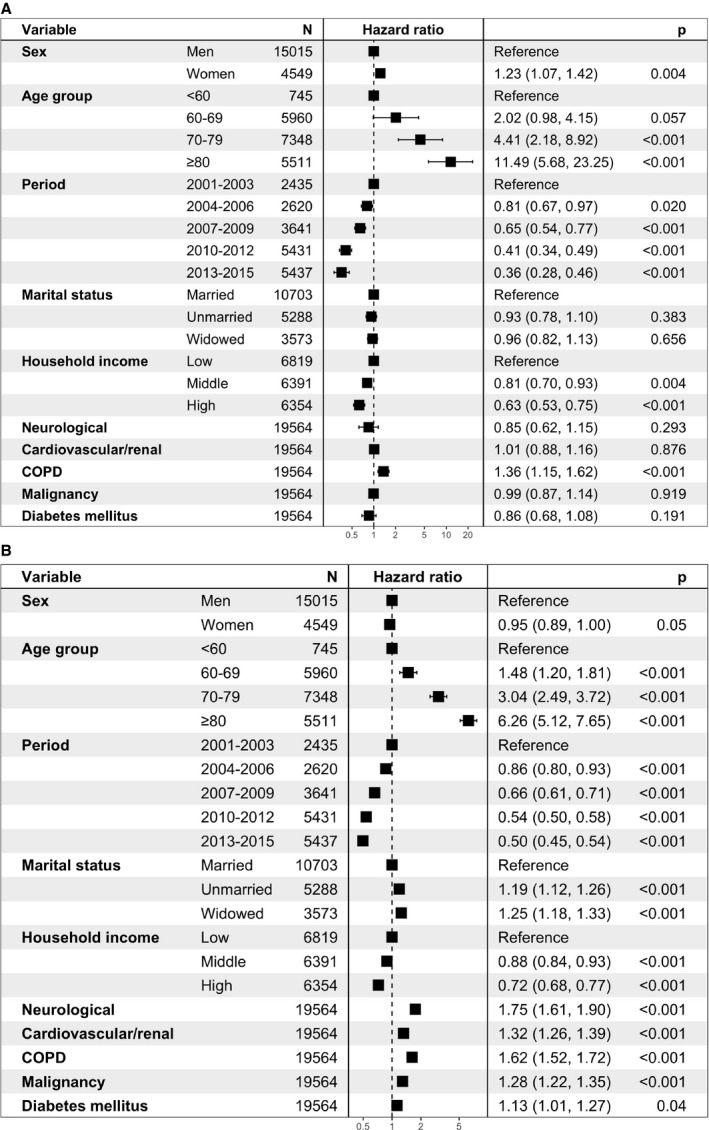
The adjusted associations between baseline demographics and the risks of (A) Rupture (1016 events) and (B) Death (7231 events) within 5 years of diagnosis among untreated patients with iAAAs. , *Five observations excluded because of missing marital status. †Adjusting for all other listed baseline variables. iAAA indicates intact abdominal aortic aneurysm; and COPD, chronic obstructive pulmonary disease.
Women experienced a higher unadjusted hazard of death (HR, 1.36; 95% CI, 1.29–1.43; P<0.001, Table S3). After adjustments for other demographics, however, no sex difference was detected in the risk of all‐cause mortality up to 5 years after diagnosis (adjusted HR, 0.95; 95% CI, 0.89–1.00; P=0.05, Figure 3B). All comorbidities independently increased the risk of death.
Sensitivity Analyses Excluding Screened Men
Male person‐years accumulated owing to the smaller aneurysm sizes and, consequently, longer follow‐up times of screening‐detected AAAs could imply diluted risk hazards for men. As sensitivity analyses, the models were run after excluding all (potentially) screening‐detected AAAs (n=1911). In terms of rupture, the predictive capacities of female sex (adjusted HR, 1.21; 95% CI, 1.05‐1.39; P=0.009), COPD (adjusted HR, 1.35; 95% CI, 1.14‐1.61; P<0.001), age, and household income were not markedly affected (no data shown). Mortality was slightly lower for women in this model (adjusted HR, 0.94; 95% CI, 0.89‐0.99; P=0.03). Other hazards of death remained unchanged.
Sex‐Specific Causes of Death
Recorded causes of death among untreated patients who succumbed within 5 years of iAAA diagnosis are outlined in Table 2. The leading cause of death for both men and women was cardiac disease (25.2% versus 20.9% for men and women, respectively; P<0.001). Malignancies were the second most common underlying cause, with a larger proportion of deaths due to malignancies among men. In the third place was ruptured AAAs: 9.6% of all the deaths within 5 years were ascribed to rAAAs. This proportion was significantly higher among women (11.9% versus 8.7%; P<0.001). Overall, 59.8% of the deaths in the cohort were due to the top 5 causes (Table 2 and Figure S3). The ICD codes used to extract the causes of death are listed in Figure S3).
Table 2.
Causes of Death for Untreated Men and Women Deceased Within 5 years of Intact AAA Diagnosis
| Rank | CoD |
Overall (7233/19 569)* % of with 95% CI |
Men (5168/15 020)* % with 95% CI |
Women (2065/4549)* % with 95% CI |
P value † |
|---|---|---|---|---|---|
| 1 | Cardiac disease | 23.9 (22.9–24.9) | 25.2 (24.0–26.4) | 20.9 (19.2–22.7) | <0.001 |
| 2 | Malignancy | 18.0 (17.1–18.9) | 20.1 (19.0–21.2) | 12.5 (11.1–13.9) | <0.001 |
| 3 | Ruptured AAA | 9.6 (8.9–10.3) | 8.7 (7.9–9.5) | 11.9 (10.5–13.3) | <0.001 |
| 4 | Stroke | 4.6 (4.1–5.1) | 4.7 (4.1–5.3) | 4.3 (3.4–5.2) | 0.468 |
| 4 | Chronic obstructive pulmonary disease | 3.7 (3.3–4.1) | 3.3 (2.8–3.8) | 4.5 (3.6–5.4) | 0.017 |
| % of all CoDs | 69.5 | 71.5 | 64.9 |
AAA indicates abdominal aortic aneurysm; and CoD indicates cause of death.
All patients deceased within 5 years included regardless of year of diagnosis.
X2 test.
Similar Diameters in Control Analysis
The review of medical records from 240 patients in an outpatient setting showed similar diameters for male and female AAAs (41.5 mm for women versus 43.00 mm for men; P=0.21, Table S4). Female patients were older but had similar comorbidity profiles. The body size areas were smaller in women resulting in larger aortic size indices (2.4 versus 2.1; P<0.05).
Discussion
A considerable proportion of diagnosed patients in surveillance remained untreated in this nationwide study on patients with intact AAAs during a 15‐year period. Untreated status was even more common among women, despite similar comorbid loads registered at diagnosis. Among both women and men, the median time‐to‐event was unexpectedly short at less than 3 years for both rupture and death. Ruptures were independently predicted by female sex, COPD, older age, and low income. Alarmingly, rupture was the third most common cause of death among both sexes.
The untreated population at hand is unique and not easily compared with cohorts of prior AAA literature. It does, however, resemble a typical patient population with AAA, confirmed by the distribution of age and comorbidities and proportion of women. 7 , 11 , 37 , 38 The rupture rate of 22.6 (per 1000 person‐years) lies within the range of recorded rates in studies on small (0.73–11.03) 16 and large (26.0–79.0) 18 , 22 AAAs. The 3‐year rupture incidence (5.3%) recorded in this study corresponds to the cumulative annual estimate of 5.3% (95% CI, 3.1–7.5) among noneligible AAAs ≥55 mm reported in the meta‐analysis by Parkinson et al. 22
Window of Opportunity after Diagnosis
Here, the untreated population represents a real‐world setting faced by vascular caregivers globally, as patients from all steps of management were included: those with newly diagnosed AAAs discovered incidentally or by screening, those awaiting surgery, and patients with continued follow‐up despite reaching the treatment threshold. 31 , 39 Yet, the median times to rupture and death were all shorter than 3 years with similar distributions for both sexes. This could be implicative of a “window of opportunity” with greatest potential for individualized surveillance. The notion of 5/100 patients suffering from rupture within 3 years could also be interpreted as a marker of adequate clinical decision‐making in general where patients with large AAAs but low life expectancy are conservatively managed.
Untreated Women and the Risk of Rupture
At the time of diagnosis, the proportions of patients with 2 or more coexisting conditions was similar between the sexes. This contradicts the prevailing view of more comorbid and frail female patients with AAA, accounting for sex differences in treatment rates as noted here and by others 3 , 4 , 7 , 9 , 15 , 19 , 20 , 21 , 24 as well as outcomes after treatment. 6 , 11 , 12 , 13 , 14 , 15 Ulug et al 9 summarized in a recent meta‐analysis that among patients with intact AAAs, 30% of women as opposed to 20% of men were turned down (pooled odds ratio of 2.27; 95% CI, 1.21–4.23). By comparison, screening studies have reported treatment rates of 24–29% within 4 to 5 years 28 , 40 explained by the smaller size of screening‐detected AAAs. 28 , 29 , 30
The finding of female sex as a predictor of rupture is not entirely novel. 16 , 17 , 18 , 37 , 41 Previously, the impact of sex on the risk of rupture has been investigated in surveillance studies predominantly including small AAAs, 16 , 17 with results indicating a female hazard ratio of 3.0 to 4.5. Therefore, the increased risk of 23% captured here for women after multivariate adjustments most likely represents a true excess risk among modern populations with AAA in surveillance.
It has been noted that female AAAs at any given diameter represent a more advanced stage of the disease with higher aortic size indices, 37 , 42 , 43 justifying a specific threshold for repair in women. 17 , 31 , 39 , 44 Therefore, in an underserved community, 4 , 9 , 19 , 20 , 21 AAAs of women could potentially rupture at an increased rate before elective repair. This is further supported by evidence of smaller diameters among ruptured female AAAs. 17 , 37 In this study, a remarkable proportion (11.9%) of deceased women had rupture as the cause of death. Likewise, the proportion dying of rupture was high (18%) in the UK study with 7 years of follow‐up including 103 ruptures (of which 39 were in women). 17 Brown et al 18 used serial measurements to investigate patients unfit for treatment with AAAs ≥50 mm. As expected, the 1‐year risks of rupture were higher at 6.6% (compared with 2.6%) for men and 11.7% (compared with 3.6%) for women. Among unfit patients, several studies have reported on mortalities due to rupture 19 , 20 and rupture rates 19 , 20 , 21 , 22 , 23 ; however, none provide these data by sex.
Registry‐based studies cannot disentangle noneligibility arising from aneurysm‐related and other reasons, as morphology data are not available within administrative databases. Likewise, obtained results rely on similar mean diameters between the sexes. Whether smaller diameters of female patients could explain the lower rate of treatment and, less intuitively, the higher risk of rupture observed for women is a pertinent question. Of note, all included men and women were diagnosed according to the same international consensus criterion of 30 mm, 1 , 42 and no evidence indicates slower growth of female AAAs. 16 , 45 In this material, 1 single center contributed with Swedish surveillance data: female AAAs are found to be of similar caliber as male counterparts yet equal to higher aortic size indices, corroborating results from other databases such as the Vascular Quality Initiative 37 and from New Zealand 42 . In the clinical setting, a variety of parameters beyond AAA diameter are pivotal for clinical considerations and preoperative evaluations, including age, sex, aneurysm morphology, renal function, concurrent conditions (cardiac disease, COPD, malignancies), and patient frailty.
Other Predictors of Rupture
The largest increase in the risk of rupture (36%) apart from older age was found in patients with COPD. Although COPD acts as a proxy for smoking in administrative data sets, this finding in general corroborates those of previous studies. 16 , 41 , 46 Current smoking has been found to approximately double the risk of rupture. 16 , 41 The prevalence and progression of AAAs have been demonstrated to be inversely associated with diabetes mellitus 16 , 47 or metformin prescription. 48 , 49 , 50 Although several attempts to enhance our knowledge on the underlying pathophysiological association have been performed, as yet, few explanations can be found. In terms of the rupture risk, most studies point toward no protective effect of diabetes mellitus 5 , 16 , 37 , 41 , 46 , 51 in line with the current results. Older age has been reported to predict rupture by some 16 , 41 but not others. 17 Advancing age could also function as a surrogate variable for increasing AAA diameters 5 and biological changes within the aneurysm wall. 1 The risk of rupture successively decreased by later time periods, possibly reflecting improvements in AAA and cardiovascular risk management but most likely also shorter follow‐up times. The predictive capacity of low income at a patient‐specific level could stem from differences in lifestyle‐related factors such as smoking, but it could also be implicative of surveillance deficiency or less effective measures of secondary prevention among socially deprived populations.
No Sex Difference in Adjusted Overall Mortality
There is a lack of comparative reports regarding the detected sex discrepancy in the survival of middle‐aged patients (Figure S1). However, parallels can be drawn between these findings and the reported higher mortality for women in most treated AAA cohorts. 10 , 11 , 12 , 15 , 37 The sex difference did not persist after multivariate adjustments, and one can therefore presume that the few women who do develop an AAA have especially unfavorable profiles with regard to, for example, socioeconomic factors that generally are difficult to catch in chart reports. 3 It could also be that the estimated comorbid loads are biased by undiagnosed and undertreated conditions that could be more prevalent among women.
As expected, the measured 3‐year survival rate of 66.4% here exceeds estimates among patients turned down for treatment (39% 19 and 35% 21 after 2 years). The detected 3‐year survival rate is, however, poorer than the corresponding rate of 84% reported by Scott et al 20 where patients with any severe comorbidities or short life expectancy (<1 year) had been excluded.
Strengths and Limitations
This study profits from the population‐based design with high data quality within the NPR 34 and minimal selection bias, but there are also some inherent limitations. Coding errors and misclassification regarding patient‐level factors, treatment status, and outcomes cannot be excluded, especially as patients diagnosed during the last period will not have a complete follow‐up of 5 years. This could lead to misclassification of untreated patients in the case of elective treatment after the end of the study. However, this misclassification is unlikely to have occurred in a sex‐dependent manner. Moreover, because a majority of 60% are untreated in the nationwide cohort of iAAAs (32 393 patients) – combined with the median time to treatment of 1.6 years – this limitation is unlikely to influence sex differences observed in this material.
It is not possible to obtain verified causes of death for population‐based samples. In Sweden, the autopsy rate has been declining and was relatively low at 15% for men and 7% for women in 2014. 32 The quality of and potential sex differences in therapeutic strategies for comorbidities such as hypertension and hyperlipidemia could not be evaluated in this data set. Finally, register‐based analyses cannot adjust for aneurysm size data. The median diameter of female AAAs could be smaller and this introduces the possibility of underestimating the impact of female sex on the risk of rupture. On the other hand, female AAAs have been observed to rupture at smaller diameters. 17 , 37
CONCLUSIONS
For the first time, the entire cohort of untreated AAAs from all stages of surveillance was included in an attempt to address the paucity of data for untreated women and men. This study reveals that even after years of surveillance, most women and men will remain untreated. Still, untreated status is often overlooked in trend analyses and care trajectories within the field of AAA. The short median times to rupture and death among over 19 500 untreated patients may imply a window of opportunity for improved AAA surveillance. Women, elderly people, and patients with COPD or low income were found to be at the greatest risk of rupture. Despite a functioning surveillance service, the healthcare system still fails to prevent a high number of fatalities caused by AAA. These findings strongly advocate individualized surveillance policies for high‐risk patients and patients with complicated risk‐benefit assessments, at least for the first 3 years after diagnosis.
Sources of Funding
The research project has been financially supported by the Swedish Heart‐Lung Foundation (20180506, 20190553, Hultgren) and the regional ALF agreement (Stockholm County Council/Karolinska Institutet, Hultgren/Roy/Talvitie) regarding financial compensation for work combining clinical research and medical education. The funding sources had no role in study design, data collection, analysis, interpretation, or decision to submit for publication.
Disclosures
None.
Supporting information
Tables S1–S4
Figures S1–S3
Acknowledgments
The authors extend their gratitude to Max Vikström and the Institute of Environmental Medicine, Karolinska Institutet, for statistical expertise and support.
(J Am Heart Assoc. 2021;10:e019592. DOI: 10.1161/JAHA.120.019592.)
Supplementary Materials for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019592
For Sources of Funding and Disclosures, see page 10.
References
- 1. Sakalihasan N, Michel JB, Katsargyris A, Kuivaniemi H, Defraigne J‐O, Nchimi A, Powell JT, Yoshimura K, Hultgren R. Abdominal aortic aneurysms. Nat Rev Dis Primers. 2018;4:34. DOI: 10.1038/s41572-018-0030-7. [DOI] [PubMed] [Google Scholar]
- 2. Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg. 2002;89:283–285. DOI: 10.1046/j.0007-1323.2001.02014.x. [DOI] [PubMed] [Google Scholar]
- 3. Zommorodi S, Bottai M, Hultgren R. Sex differences in repair rates and outcomes of patients with ruptured abdominal aortic aneurysm. Br J Surg. 2019;106:1480–1487. DOI: 10.1002/bjs.11258. [DOI] [PubMed] [Google Scholar]
- 4. Aber A, Tong TS, Chilcott J, Thokala P, Maheswaran R, Thomas SM, Nawaz S, Walters S, Michaels J. Sex differences in national rates of repair of emergency abdominal aortic aneurysm. Br J Surg. 2019;106:82–89. DOI: 10.1002/bjs.11006. [DOI] [PubMed] [Google Scholar]
- 5. Da Silva ES, Gornati VC, Casella IB, Aun R, Estenssoro AE, Puech‐Leão P, De Luccia N. The similarities and differences among patients with abdominal aortic aneurysms referred to a tertiary hospital and found at necropsy. Vascular. 2015;23:411–418. DOI: 10.1177/1708538114552095. [DOI] [PubMed] [Google Scholar]
- 6. Semmens JB, Norman PE, Lawrence‐Brown MM, Holman CD. Influence of gender on outcome from ruptured abdominal aortic aneurysm. Br J Surg. 2000;87:191–194. DOI: 10.1046/j.1365-2168.2000.01346.x. [DOI] [PubMed] [Google Scholar]
- 7. Kuhnl A, Erk A, Trenner M, Salvermoser M, Schmid V, Eckstein HH. Incidence, treatment and mortality in patients with abdominal aortic aneurysms. Dtsch Arztebl Int. 2017;114:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Englund R, Perera D, Hanel KC. Outcome for patients with abdominal aortic aneurysms that are treated non‐surgically. Aust N Z J Surg. 1997;67:260–263. DOI: 10.1111/j.1445-2197.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- 9. Ulug P, Sweeting MJ, von Allmen RS, Thompson SG, Powell JT, Ulug P, Sweeting MJ, Thompson SG, Powell JT, Jones E, et al. Morphological suitability for endovascular repair, non‐intervention rates, and operative mortality in women and men assessed for intact abdominal aortic aneurysm repair: systematic reviews with meta‐analysis. Lancet. 2017;389:2482–2491. DOI: 10.1016/S0140-6736(17)30639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bulder RMA, Talvitie M, Bastiaannet E, Hamming J, Benson L, Leander K, Hultgren R, Lindeman JHN. Long‐term prognosis after elective AAA is poor in women and men: the challenges remain. Ann Surg. 2020;272:773–778. DOI: 10.1097/SLA.0000000000004182. [DOI] [PubMed] [Google Scholar]
- 11. Sidloff DA, Saratzis A, Sweeting MJ, Michaels J, Powell JT, Thompson SG, Bown MJ. Sex differences in mortality after abdominal aortic aneurysm repair in the UK. Br J Surg. 2017;104:1656–1664. DOI: 10.1002/bjs.10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicolini F, Vezzani A, Corradi F, Gherli R, Benassi F, Manca T, Gherli T. Gender differences in outcomes after aortic aneurysm surgery should foster further research to improve screening and prevention programmes. Eur J Prev Cardiol. 2018;25:32–41. DOI: 10.1177/2047487318759121. [DOI] [PubMed] [Google Scholar]
- 13. Mehta M, Byrne WJ, Robinson H, Roddy SP, Paty PSK, Kreienberg PB, Feustel P, Darling RC 3rd. Women derive less benefit from elective endovascular aneurysm repair than men. J Vasc Surg. 2012;55:906–913. DOI: 10.1016/j.jvs.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 14. Tomee SM, Lijftogt N, Vahl A, Hamming JF, Lindeman JHN. A registry‐based rationale for discrete intervention thresholds for open and endovascular elective abdominal aortic aneurysm repair in female patients. J Vasc Surg. 2018;67:735–739. DOI: 10.1016/j.jvs.2017.07.123. [DOI] [PubMed] [Google Scholar]
- 15. Trenner M, Kuehnl A, Reutersberg B, Salvermoser M, Eckstein HH. Nationwide analysis of risk factors for in‐hospital mortality in patients undergoing abdominal aortic aneurysm repair. Br J Surg. 2018;105:379–387. 10.1002/bjs.10714 [DOI] [PubMed] [Google Scholar]
- 16. Sweeting MJ, Thompson SG, Brown LC, Powell JT. Meta‐analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655–665. DOI: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 17. Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK small aneurysm trial participants. Ann Surg. 1999;230:296–297. DOI: 10.1097/00000658-199909000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown PM, Zelt DT, Sobolev B. The risk of rupture in untreated aneurysms: the impact of size, gender, and expansion rate. J Vasc Surg. 2003;37:280–284. DOI: 10.1067/mva.2003.119. [DOI] [PubMed] [Google Scholar]
- 19. Whittaker JD, Meecham L, Summerour V, Khalil S, Layton G, Yousif M, Jennings A, Wall M, Newman J. Outcome after turndown for elective abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg. 2017;54:579–586. DOI: 10.1016/j.ejvs.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 20. Scott SW, Batchelder AJ, Kirkbride D, Naylor AR, Thompson JP. Late survival in nonoperated patients with infrarenal abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2016;52:444–449. DOI: 10.1016/j.ejvs.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 21. Karthikesalingam A, Nicoli TK, Holt PJ, Hinchliffe RJ, Pasha N, Loftus IM, Thompson MM. The fate of patients referred to a specialist vascular unit with large infra‐renal abdominal aortic aneurysms over a two‐year period. Eur J Vasc Endovasc Surg. 2011;42:295–301. DOI: 10.1016/j.ejvs.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 22. Parkinson F, Ferguson S, Lewis P, Williams IM, Twine CP. Rupture rates of untreated large abdominal aortic aneurysms in patients unfit for elective repair. J Vasc Surg. 2015;61:1606–1612. DOI: 10.1016/j.jvs.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 23. Lederle FA, Johnson GR, Wilson SE, Ballard DJ, Jordan WD Jr, Blebea J, Littooy FN, Freischlag JA, Bandyk D, Rapp JH, et al. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA. 2002;287:2968–2972. DOI: 10.1001/jama.287.22.2968. [DOI] [PubMed] [Google Scholar]
- 24. McPhee JT, Hill JS, Eslami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001–2004. J Vasc Surg. 2007;45:891–899. DOI: 10.1016/j.jvs.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 25. Laine MT, Laukontaus SJ, Sund R, Aho PS, Kantonen I, Albäck A, Venermo M. A population‐based study of abdominal aortic aneurysm treatment in Finland 2000 to 2014. Circulation. 2017;136:1726–1734. DOI: 10.1161/CIRCULATIONAHA.117.028259. [DOI] [PubMed] [Google Scholar]
- 26. Swedish National Board of Health and Welfare (NBHW): Population statistics, Statistical Database (SCB) of Statistics Sweden. Available at: http://www.statistikdatabasen.scb.se/pxweb/en/ssd/. Accessed 2018.
- 27. Swedish Society for Vascular Surgery . SWEDVASC Annual Report. https://www.ucr.uu.se/swedvasc/arsrapporter. Published 2001‐2015. Accessed 2018.
- 28. Wanhainen A, Hultgren R, Linné A, Holst J, Gottsäter A, Langenskiöld M, Smidfelt K, Björck M, Svensjö S, Lyttkens L, et al. Outcome of the Swedish nationwide abdominal aortic aneurysm screening program. Circulation. 2016;134:1141–1148. DOI: 10.1161/CIRCULATIONAHA.116.022305. [DOI] [PubMed] [Google Scholar]
- 29. Svensjö S, Björck M, Gürtelschmid M, Djavani Gidlund K, Hellberg A, Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65‐year‐old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124:1118–1123. DOI: 10.1161/CIRCULATIONAHA.111.030379. [DOI] [PubMed] [Google Scholar]
- 30. Svensjö S, Björck M, Wanhainen A. Current prevalence of abdominal aortic aneurysm in 70‐year‐old women. Br J Surg. 2013;100:367–372. DOI: 10.1002/bjs.8984. [DOI] [PubMed] [Google Scholar]
- 31. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:e72. DOI: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 32. Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, Feychting M, Ljung R. The Swedish cause of death register. Eur J Epidemiol. 2017;32:765–773. DOI: 10.1007/s10654-017-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ludvigsson JF, Svedberg P, Olén O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34:423–437. 10.1007/s10654-019-00511-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667. DOI: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. DOI: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 36. R Core Team . R: A language and environment for statistical computing, Vienna, Austria: R Foundation for Statistical Computing. 2020. https://www.R‐project.org/. Accessed. [Google Scholar]
- 37. Lo RC, Lu B, Fokkema MT, Conrad M, Patel VI, Fillinger M, Matyal R, Schermerhorn ML, Vascular Study Group of New England . Relative importance of aneurysm diameter and body size for predicting abdominal aortic aneurysm rupture in men and women. J Vasc Surg. 2014;59:1209–1216. DOI: 10.1016/j.jvs.2013.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Desai M, Choke E, Sayers RD, Nath M, Bown MJ. Sex‐related trends in mortality after elective abdominal aortic aneurysm surgery between 2002 and 2013 at National Health Service hospitals in England: less benefit for women compared with men. Eur Heart J. 2016;37:3452–3460. DOI: 10.1093/eurheartj/ehw335. [DOI] [PubMed] [Google Scholar]
- 39. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, Dick F, van Herwaarden J, Karkos C, Koelemay M, et al. Editor's choice ‐ European society for vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto‐iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57:8–93. DOI: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 40. Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RAP, Thompson SG, Walker NM, Multicentre Aneurysm Screening Study Group . The multicentre aneurysm screening study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–1539. DOI: 10.1016/S0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 41. Gokani VJ, Sidloff D, Bath MF, Bown MJ, Sayers RD, Choke E. A retrospective study: factors associated with the risk of abdominal aortic aneurysm rupture. Vascul Pharmacol. 2015;65–66:13–16. DOI: 10.1016/j.vph.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 42. Jones GT, Sandiford P, Hill GB, Williams MJA, Khashram M, Tilyard MW, Hammond‐Tooke GD, Krysa J, Van Rij AM. Correcting for body surface area identifies the true prevalence of abdominal aortic aneurysm in screened women. Eur J Vasc Endovasc Surg. 2019;57:221–228. DOI: 10.1016/j.ejvs.2018.08.048. [DOI] [PubMed] [Google Scholar]
- 43. Forbes TL, Lawlor DK, DeRose G, Harris KA. Gender differences in relative dilatation of abdominal aortic aneurysms. Ann Vasc Surg. 2006;20:564–568. DOI: 10.1007/S10016-006-9079-y. [DOI] [PubMed] [Google Scholar]
- 44. Sweeting MJ, Masconi KL, Jones E, Ulug P, Glover MJ, Michaels JA, Bown M, Powell JT, Thompson SG. Analysis of clinical benefit, harms, and cost‐effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487–495. DOI: 10.1016/S0140-6736(18)31222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mofidi R, Goldie VJ, Kelman J, Dawson AR, Murie JA, Chalmers RT. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg. 2007;94:310–314. DOI: 10.1002/bjs.5573. [DOI] [PubMed] [Google Scholar]
- 46. Kristensen KL, Rasmussen LM, Hallas J, Lindholt JS. Diabetes is not associated with the risk of rupture among patients with abdominal aortic aneurysms ‐ Results from a large danish register based matched case control study from 1996 to 2016. Eur J Vasc Endovasc Surg. 2020;60:36–42. DOI: 10.1016/j.ejvs.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 47. Xiong J, Wu Z, Chen C, Wei Y, Guo W. Association between diabetes and prevalence and growth rate of abdominal aortic aneurysms: a meta‐analysis. Int J Cardiol. 2016;221:484–495. DOI: 10.1016/j.ijcard.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 48. Golledge J, Moxon J, Pinchbeck J, Anderson G, Rowbotham S, Jenkins J, Bourke M, Bourke B, Dear A, Buckenham T, et al. Association between metformin prescription and growth rates of abdominal aortic aneurysms. Br J Surg. 2017;104:1486–1493. DOI: 10.1002/bjs.10587. [DOI] [PubMed] [Google Scholar]
- 49. Hsu CY, Su YW, Chen YT, Tsai SH, Chang CC, Li SY, Huang PH, Chen JW, Lin SJ. Association between use of oral‐antidiabetic drugs and the risk of aortic aneurysm: a nested case‐control analysis. Cardiovasc Diabetol. 2016;15:125. DOI: 10.1186/s12933-016-0447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fujimura N, Xiong J, Kettler EB, Xuan H, Glover KJ, Mell MW, Xu B, Dalman RL. Metformin treatment status and abdominal aortic aneurysm disease progression. J Vasc Surg. 2016;64:e48. DOI: 10.1016/j.jvs.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fillinger MF, Racusin J, Baker RK, Cronenwett JL, Teutelink A, Schermerhorn ML, Zwolak RM, Powell RJ, Walsh DB, Rzucidlo EM. Anatomic characteristics of ruptured abdominal aortic aneurysm on conventional CT scans: implications for rupture risk. J Vasc Surg. 2004;39:1243–1252. DOI: 10.1016/j.jvs.2004.02.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S3


