Abstract
Background
Elevated plasma levels of direct low‐density lipoprotein cholesterol (LDL‐C), small dense LDL‐C (sdLDL‐C), low‐density lipoprotein (LDL) triglycerides, triglycerides, triglyceride‐rich lipoprotein cholesterol, remnant lipoprotein particle cholesterol, and lipoprotein(a) have all been associated with incident atherosclerotic cardiovascular disease (ASCVD). Our goal was to assess which parameters were most strongly associated with ASCVD risk.
Methods and Results
Plasma total cholesterol, triglycerides, high‐density lipoprotein cholesterol, direct LDL‐C, sdLDL‐C, LDL triglycerides, remnant lipoprotein particle cholesterol, triglyceride‐rich lipoprotein cholesterol, and lipoprotein(a) were measured using standardized automated analysis (coefficients of variation, <5.0%) in samples from 3094 fasting subjects free of ASCVD. Of these subjects, 20.2% developed ASCVD over 16 years. On univariate analysis, all ASCVD risk factors were significantly associated with incident ASCVD, as well as the following specialized lipoprotein parameters: sdLDL‐C, LDL triglycerides, triglycerides, triglyceride‐rich lipoprotein cholesterol, remnant lipoprotein particle cholesterol, and direct LDL‐C. Only sdLDL‐C, direct LDL‐C, and lipoprotein(a) were significant on multivariate analysis and net reclassification after adjustment for standard risk factors (age, sex, hypertension, diabetes mellitus, smoking, total cholesterol, and high‐density lipoprotein cholesterol). Using the pooled cohort equation, many specialized lipoprotein parameters individually added significant information, but no parameter added significant information once sdLDL‐C (hazard ratio, 1.42; P<0.0001) was in the model. These results for sdLDL‐C were confirmed by adjusted discordance analysis versus calculated non–high‐density lipoprotein cholesterol, in contrast to LDL triglycerides.
Conclusions
sdLDL‐C, direct LDL‐C, and lipoprotein(a) all contributed significantly to ASCVD risk on multivariate analysis, but no parameter added significant risk information to the pooled cohort equation once sdLDL‐C was in the model. Our data indicate that small dense LDL is the most atherogenic lipoprotein parameter.
Keywords: atherosclerotic cardiovascular disease, pooled cohort equations, small dense low‐density lipoprotein cholesterol
Subject Categories: Cardiovascular Disease, Primary Prevention, Risk Factors, Epidemiology
Nonstandard Abbreviations and Acronyms
- lbLDL‐C
large buoyant low‐density lipoprotein cholesterol
- LDL‐TG
low‐density lipoprotein triglycerides
- PCE
pooled cohort equation
- RLP‐C
remnant lipoprotein particle cholesterol
- sdLDL‐C
small dense low‐density lipoprotein cholesterol
- TRL‐C
triglyceride‐rich lipoprotein cholesterol
- VLDL‐C
very‐low‐density lipoprotein cholesterol
Clinical Perspective
What Is New?
In the prospective Framingham Offspring Study, we have measured multiple atherogenic lipoprotein parameters and documented that elevated small dense low‐density lipoprotein cholesterol was the best lipid measure of incident atherosclerotic cardiovascular disease risk, compared with low‐density lipoprotein triglycerides, large buoyant low‐density lipoprotein cholesterol, triglyceride‐rich lipoprotein cholesterol, remnant lipoprotein particle cholesterol, and lipoprotein(a), using multivariate analysis, and added significant risk information to the pooled cohort equation.
What Are the Clinical Implications?
Our data indicate that optimizing small dense low‐density lipoprotein cholesterol levels with lifestyle modification and cholesterol‐lowering medications, along with control of other atherosclerotic cardiovascular disease risk factors, may be a most effective way to minimize future atherosclerotic cardiovascular disease risk.
Established risk factors for atherosclerotic cardiovascular disease (ASCVD) in the pooled cohort equation (PCE) include sex, age, race, total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), systolic blood pressure, blood pressure treatment, diabetes mellitus, and current smoking. 1 It has been recommended that subjects be considered for statin therapy in addition to lifestyle modification if: (1) their 10‐year ASCVD risk is ≥7.5% based on the PCE, (2) they have established ASCVD, (3) they have diabetes mellitus (between the ages of 40 and 75 years), or (4) they have a plasma low‐density lipoprotein cholesterol (LDL‐C) concentration ≥190 mg/dL. 1 , 2 LDL‐C lowering with lifestyle modification, statin therapy, and other treatment modalities, including the use of ezetimibe and PCSK9 (proprotein convertase subtilisin kexin type 9) inhibitors, results in significant reductions in ASCVD morbidity and mortality. 3
Studies separating lipoproteins by ultracentrifugation, electrophoresis, ion mobility, or nuclear magnetic resonance have documented that patients with ASCVD were significantly more likely to have increases in low‐density lipoprotein (LDL) particle number and small dense (pattern B) LDL, as well as decreases in high‐density lipoprotein particle number and large high‐density lipoprotein, compared with control subjects. 4 , 5 , 6 , 7 These abnormalities have been associated with increased triglycerides and decreased HDL‐C levels. 4 , 5 , 6 , 7 However, assessments of plasma lipoproteins using these methods have not been shown to be independent predictors, require special instrumentation, are labor intensive, are not well standardized, and have not been adopted by standard clinical chemistry laboratories.
With the development of methods for precipitating lipoproteins containing apolipoprotein B and automated enzymatic assays for cholesterol and triglycerides, standard laboratories measured total cholesterol, triglycerides, and HDL‐C. 8 , 9 , 10 , 11 Such assays were used in the currently recommended PCE. 1 Currently, many laboratories use the Friedewald formula to calculate LDL‐C from total cholesterol, triglycerides, and HDL‐C. 12 Other formulas have also been developed to calculate LDL‐C. 13 , 14 In our view, the development of methods for directly measuring plasma or serum LDL‐C makes the use of calculated LDL‐C obsolete. 15 , 16 , 17 , 18 We recently compared the ability of 2 calculated LDL‐C methods and 2 direct LDL‐C methods to predict ASCVD and documented that only 1 direct LDL‐C method added significant information to prospective ASCVD risk prediction when added to standard risk factors in the Framingham Offspring Study (FOS). 19
Direct automated assays for small dense LDL‐C (sdLDL‐C), low‐density lipoprotein triglycerides (LDL‐TG), remnant lipoprotein particle cholesterol (RLP‐C), triglyceride‐rich lipoprotein cholesterol (TRL‐C), and lipoprotein(a) have also been developed. 20 , 21 , 22 , 23 , 24 , 25 , 26 It has been shown in both case‐control and prospective studies that subjects with ASCVD were significantly more likely to have elevated levels of these parameters, compared with control subjects. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 Our goal in this study was to determine which of these atherogenic lipoprotein parameters was most associated with ASCVD risk in univariate and multivariate analysis after controlling for standard risk factors, as well as in addition to the PCE model, using data from the prospective FOS.
METHODS
Study Population and Design
Anonymized data and materials used for this analysis have been made publicly available through the FOS and can be accessed at https://www.nhlbi.nih.gov/science/framingham‐heart‐study‐fhs. Further information can also be obtained by contacting one of the coauthors, C.‐T.L., at ctliu@bu.edu. Study subjects were participants in cycle 6 (1995–1998) of the FOS, a long‐term community‐based prospective observational study of risk factors for ASCVD consisting of the offspring of the original FHS (Framingham Heart Study) cohort and their spouses. 38 The median follow‐up period was 16 years, until December 31, 2015. The population was almost entirely White individuals. We evaluated 3094 male and female participants that: (1) were free of ASCVD at baseline, (2) had frozen plasma available from blood sampled after an overnight fast, (3) had a history and physical examination (including measurement of blood pressure, height, and weight) as part of their participation in the study, and (4) had long‐term follow‐up data available. All subjects provided information about their medical history and use of medications and supplements. Hypertension was defined as a blood pressure >140/90 mm Hg or being on medications for hypertension. Diabetes mellitus was defined as a fasting glucose >125 mg/dL or being on medications for diabetes mellitus. Smoking was defined as cigarette smoking within the past year. At baseline, 15.1% of the subjects were taking lipid‐lowering medications. All studies were performed with the approval of the human Institutional Review Boards of Boston University School of Medicine, Boston, MA; Tufts University School of Medicine, Boston, MA; and the FHS, National Heart, Lung, and Blood Institute, National Institutes of Health, Framingham, MA, and Bethesda, MD. Written informed consent was obtained from all study participants.
Criteria for Atherosclerotic Cardiovascular Events
For prospective ASCVD end points in this analysis, we used the following criteria: the development of myocardial infarction (recognized with diagnostic ECG; recognized without diagnostic ECG but including enzymes and history; recognized without diagnostic ECG but with autopsy evidence; unrecognized silent; unrecognized not silent; or recognized at autopsy), stroke (atherothrombotic brain infarction, cerebral embolism, intracerebral hemorrhage, subarachnoid hemorrhage, or definite or other cardiovascular accident), and death from either myocardial infarction or stroke (sudden death from coronary heart disease; death from coronary heart disease within 1 hour, within 1–23 hours, within 24–47 hours, or ≥48 hours; death from cerebrovascular accident; or death from other cardiovascular disease), coronary revascularization (angioplasty or coronary artery bypass grafting), carotid artery surgery, and/or peripheral artery surgery during the follow‐up period, as well as the presence of angina pectoris, coronary insufficiency, and/or transient ischemic attacks. All subjects included in this analysis were free of ASCVD, as defined above, at the time of examination 6. Only the first event over the entire median follow‐up time of 16 years was used in the analysis. In this analysis, there were 624 subjects with incident ASCVD and 2470 subjects without incident ASCVD. For comparison with the PCE model, follow‐up for events was truncated to 10 years, as specified by the model, and only hard ASCVD end points were used. 1 Hard ASCVD included all criteria listed above, except for coronary, carotid, and peripheral artery revascularization procedures, and the presence of angina pectoris, coronary insufficiency, and/or transient ischemic attacks. In this latter analysis, there were 364 subjects with incident hard ASCVD events and 2730 subjects without incident events. Those subjects whose events occurred after 10 years were counted as nonincident events during the 10‐year follow‐up period. The survival time was censored at 10 years.
Laboratory Measurements
Fasting plasma samples that were stored at −80°C and never thawed were used for the analysis. Plasma levels of total cholesterol, triglycerides, and HDL‐C were determined by standard enzymatic methods using assay kits from Roche Diagnostics, as previously described. 4 , 18 These assays were standardized with the lipid standardization program of the Centers for Disease Control and Prevention, Atlanta, GA. Direct LDL‐C, sdLDL‐C, LDL‐TG, RLP‐C, TRL‐C, and lipoprotein(a) were measured on an Olympus AU400 automated chemistry analyzer using assay kits obtained from Denka Corporation (Niigata, Japan), as previously described. 16 , 21 , 22 , 23 , 26 For all assays, the within‐run and between‐run coefficients of variation were <5.0%. Analyses were run between 2015 and 2017. The long‐term stability of direct lipoprotein cholesterol measurements has been documented for >7 years, provided plasma or serum samples have been kept continuously at −80°C and never thawed until use. 4 , 16 , 18 , 19 , 21 , 22 , 23 Large buoyant LDL‐C (lbLDL‐C), non–HDL‐C, and very‐LDL‐C (VLDL‐C) were calculated as follows: (1) lbLDL‐C=direct LDL‐C−sdLDL‐C; (2) non–HDL‐C=total cholesterol−HDL‐C; and (3) VLDL‐C=total cholesterol−(direct LDL‐C+HDL‐C). For comparison purposes, LDL‐C was also calculated using the Friedewald formula: total cholesterol−HDL‐C−triglycerides/5. 12
Statistical Analysis
H.I. and colleagues at Tufts University generated the laboratory data using numbered anonymized samples. E.L., L.A.C., and C.‐T.L. had full access to all the data in the study, performed independent data analysis, and take responsibility for this analysis and data integrity. All statistical analyses were performed using R software, version 3.6.0. Continuous variables were expressed as median values with interquartile ranges (25th–75th percentile values), and categorical variables were expressed as frequencies and percentages (Table 1). All data in Table 1 are unadjusted.
Table 1.
Characteristics of Inclusive ASCVD Cases Compared With Noncases
| Characteristic | Non‐ASCVD (n=2470) | ASCVD (n=624) | Hazard Ratio (95% CI)* | P Value † |
|---|---|---|---|---|
| Standard parameters | ||||
| Diabetes mellitus, n (%) | 159 (6.4) | 102 (15.1) | 2.57 (2.08–3.17) | 1.9×10−18 |
| Age, y | 56.0 (14.0) | 63.0 (14.0) | 2.50 (2.23–2.81) | 8.7×10−54 |
| Hypertension, n (%) | 827 (33.5) | 373 (55.1) | 2.39 (2.06–2.79) | 2.5×10−29 |
| Hypertension medication, n (%) | 521 (21.1) | 250 (36.9) | 2.16 (1.84–2.52) | 6.2×10−22 |
| Men, n (%) | 1025 (41.5) | 375 (55.4) | 1.72 (1.48–2.00) | 2.1×10−12 |
| Cholesterol‐lowering medication, n (%) | 203 (8.2) | 92 (13.6) | 1.70 (1.37–2.12) | 2.1×10−6 |
| HDL‐C, mg/dL ‡ | 51.0 (21.0) | 45.0 (19.5) | 1.64 (1.46–1.83) | 8.6×10−18 |
| Triglycerides, mg/dL § | 111 (83.0) | 133 (96.0) | 1.49 (1.35–1.64) | 5.9×10−15 |
| Smoking, n (%) | 352 (14.3) | 131 (19.4) | 1.48 (1.22–1.79) | 5.8×10−5 |
| Non–HDL‐C, mg/dL ‖ | 150 (52.0) | 162 (50.0) | 1.36 (1.24–1.49) | 3.8×10−11 |
| Calculated LDL‐C, mg/dL ¶ | 125 (44.0) | 132 (43.0) | 1.24 (1.12–1.36) | 1.4×10−5 |
| BMI, kg/m2 | 26.8 (6.0) | 28.2 (6.3) | 1.21 (1.12–1.32) | 1.6×10−5 |
| Total cholesterol, mg/dL | 203 (50.0) | 209 (49.0) | 1.17 (1.06–1.29) | 0.00147 |
| Advanced lipid biomarkers | ||||
| sdLDL‐C, mg/dL § | 41.7 (28.3) | 50.7 (29.0) | 1.64 (1.46–1.85) | 4.8×10−16 |
| LDL‐TG, mg/dL § | 16.7 (8.0) | 18.8 (8.0) | 1.53 (1.38–1.69) | 1.2×10−16 |
| TRL‐C, mg/dL § | 45.2 (29.4) | 51.8 (32.9) | 1.42 (1.28–1.58) | 9.1×10−11 |
| RLP‐C, mg/dL § | 5.9 (8.0) | 8.0 (10.20) | 1.38 (1.24–1.53) | 3.4×10−9 |
| Direct LDL‐C, mg/dL | 131 (45.4) | 139 (44.9) | 1.34 (1.21–1.48) | 9.9×10−9 |
| Lipoprotein(a), mg/dL § | 12.6 (28.8) | 13.6 (36.6) | 1.13 (1.00–1.28) | 0.052 |
| lbLDL‐C, mg/dL # | 86.1 (31.1) | 88.3 (33.0) | 1.10 (0.99–1.23) | 0.064 |
| VLDL‐C, mg/dL § , ** | 17.7 (15.6) | 19.4 (18.4) | 1.08 (0.99–1.17) | 0.077 |
Values are median (interquartile range) for continuous variables or number (percentage) for categorical variables. ASCVD indicates atherosclerotic cardiovascular disease; BMI, body mass index; HDL‐C, high‐density lipoprotein cholesterol; lbLDL‐C, large buoyant LDL‐C; LDL‐C, low‐density lipoprotein cholesterol; LDL‐TG, low‐density lipoprotein triglycerides; RLP‐C, remnant lipoprotein particle cholesterol; sdLDL‐C, small dense LDL‐C; TRL‐C, triglyceride‐rich lipoprotein cholesterol; and VLDL‐C, very‐LDL‐C.
Unadjusted hazard ratios for continuous variables represent comparison across interquartile range, the 75th percentile vs the 25th percentile. Variables not normally distributed were log transformed before regression analysis.
P value for comparison of inclusive ASCVD cases vs noncases.
The unadjusted hazard ratio for HDL‐C was calculated as lower HDL‐C (25th percentile) vs higher HDL‐C (75th percentile) based on the interquartile range.
Variable was log transformed before regression analysis.
Value is calculated using the following equation: total cholesterol−HDL‐C.
Value is calculated using the Friedewald equation: (total cholesterol−HDL‐C)−(triglycerides/5).
Value is calculated using the following equation: direct LDL‐C−sdLDL‐C.
Value is calculated using the following equation: total cholesterol−(direct LDL‐C+HDL‐C).
Quartile analyses unadjusted and adjusted for age and sex (model 1), and for age, sex, total cholesterol, HDL‐C, hypertension, antihypertensive medication use, smoking, and diabetes mellitus status (model 2) were done (Table S1). Quartile analysis was performed to determine the incident ASCVD risk for a given parameter, comparing subjects with values in the top quartile, intermediate quartiles, and the bottom quartile for a given biochemical parameter. This type of analysis is widely used to determine the risk associated with commonly used clinical cut‐points for such variables and has been used in prior analyses. 32 , 33 Unadjusted Kaplan‐Meier survival analyses by quartiles were also done for direct LDL‐C, sdLDL‐C, LDL‐TG, triglycerides, TRL‐C, and RLP‐C (Figure 1). Because all quartile risk relationships were no longer statistically significant when adjusted for total cholesterol and HDL‐C, we performed Pearson correlation coefficient analyses to assess correlations between biochemical parameters (Table 2).
Figure 1. Unadjusted Kaplan‐Meier survival analysis by quartiles: apolipoprotein B–containing lipoprotein particle biomarkers.
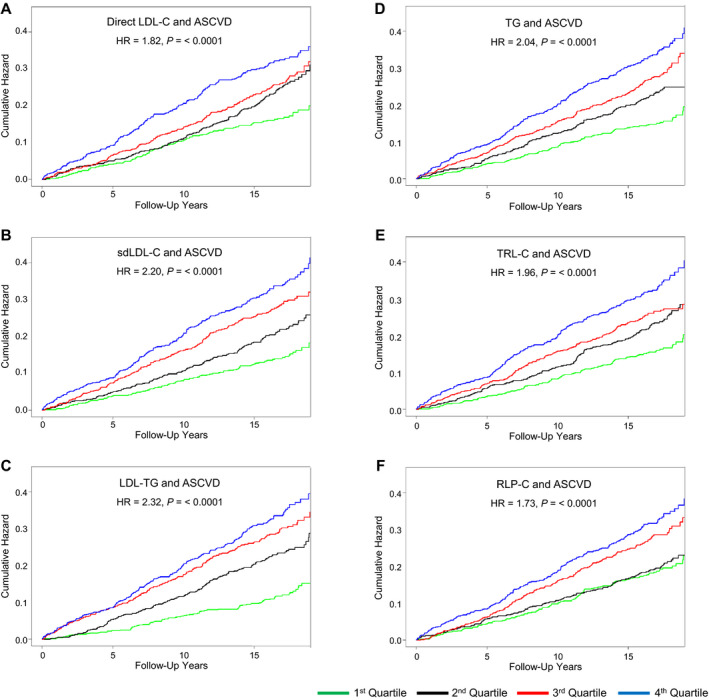
A, Direct low‐density lipoprotein cholesterol (LDL‐C) and incident atherosclerotic cardiovascular disease (ASCVD). B, Small dense LDL‐C (sdLDL‐C) and incident ASCVD. C, Low‐density lipoprotein triglycerides (LDL‐TG) and incident ASCVD. D, Fasting triglycerides (TG) and incident ASCVD. E, Triglyceride‐rich lipoprotein cholesterol (TRL‐C) and incident ASCVD. F, Remnant lipoprotein particle cholesterol (RLP‐C) and incident ASCVD. Hazard ratio (HR) and P value compared fourth quartile (top, blue line) with first quartile (bottom, green line). Quartile cut‐point values are shown in Table S1.
Table 2.
Pearson Correlation Coefficient Matrix Analysis of Standard and Advanced Lipid and Lipoprotein Measurements
| Variable | Direct LDL‐C | Log sdLDL‐C | lbLDL‐C | Log LDL‐TG | Log TRL‐C | Log RLP‐C | Log Lipoprotein(a) |
|---|---|---|---|---|---|---|---|
| TC | 0.867 | 0.697 | 0.653 | 0.501 | 0.499 | 0.324 | 0.133 |
| HDL‐C | −0.170 | −0.292 | −0.001* | −0.307 | −0.472 | −0.328 | 0.047* |
| Non–HDL‐C | 0.920† | 0.805 | 0.641 | 0.619 | 0.684 | 0.453 | 0.111 |
| Log triglycerides | 0.314 | 0.690 | −0.119 | 0.695 | 0.843 | 0.882 | −0.051* |
| Calculated LDL‐C | 0.950† | 0.658 | 0.814 | 0.432 | 0.456 | 0.165 | 0.151 |
| Direct LDL‐C | 1.00 | 0.739 | 0.818 | 0.517 | 0.472 | 0.225 | 0.135 |
| Log sdLDL‐C | 0.739 | 1.00 | 0.246 | 0.759 | 0.727 | 0.629 | 0.022* |
| Log lipoprotein(a) | 0.135 | 0.022* | 0.184 | 0.046* | 0.003* | −0.079 | 1.00 |
| Log VLDL‐C | 0.016 | 0.280 | −0.218 | 0.287 | 0.592 | 0.555 | −0.027* |
Data indicate variables with r>0.700 to r<0.900, unless otherwise indicated. HDL‐C indicates high‐density lipoprotein cholesterol; lbLDL‐C, large buoyant LDL‐C; LDL‐C, low‐density lipoprotein cholesterol; LDL‐TG, low‐density lipoprotein triglycerides; RLP‐C, remnant lipoprotein particle cholesterol; sdLDL‐C, small dense LDL‐C; TC, total cholesterol; TRL‐C, triglyceride‐rich lipoprotein cholesterol; and VLDL‐C, very‐LDL‐C.
P>0.0001. All other correlations were statistically significant at P<0.0001.
Data indicate variables with r≥0.900.
Unadjusted and adjusted Cox proportional hazards regression analysis was conducted to identify parameters significantly associated with the incident ASCVD in the absence (Table 1) or presence of other ASCVD risk factors (Table 3). These results are expressed as a hazard ratio (HR) based on the interquartile range interval, as previously described. 39 , 40 We calculated the HR from models with the continuous variable by comparing the risk for the 75th percentile value with that of the 25th percentile value. P<0.05 was considered statistically significant.
Table 3.
Association of Incident ASCVD With Atherogenic Lipoprotein Parameters as Continuous Variables
| Variable | Hazard Ratio (95% CI) | P Value | C Statistic |
|---|---|---|---|
| Log sdLDL‐C | 1.64 (1.46–1.85)† | <0.0001 | 0.600 |
| Model 1 | 1.48 (1.30–1.69) | <0.0001 | 0.728 |
| Model 2 | 1.35 (1.18–1.54) | <0.0001 | 0.734 |
| Model 3 | 1.28 (1.04–1.58) | 0.021 | 0.734 |
| Direct LDL‐C | 1.34 (1.21–1.48)† | <0.0001 | 0.568 |
| Model 1 | 1.31 (1.17–1.45) | <0.0001 | 0.723 |
| Model 2 | 1.28 (1.15–1.42) | <0.0001 | 0.731 |
| Model 3 | 1.33 (1.02–1.72) | 0.034 | 0.731 |
| Log LDL‐TG | 1.53 (1.38–1.69)† | <0.0001 | 0.600 |
| Model 1 | 1.39 (1.25–1.55) | <0.0001 | 0.729 |
| Model 2 | 1.26 (1.12–1.42) | 0.0001 | 0.733 |
| Model 3 | 1.16 (1.00–1.34) | 0.048 | 0.733 |
| Log lipoprotein(a) | 1.13 (1.00–1.28)† | 0.052 | 0.526 |
| Model 1 | 1.18 (1.04–1.33) | 0.011 | 0.719 |
| Model 2 | 1.18 (1.04–1.34) | 0.008 | 0.730 |
| Model 3 | 1.15 (1.01–1.30) | 0.031 | 0.734 |
| Log triglycerides | 1.49 (1.35–1.64)† | <0.0001 | 0.587 |
| Model 1 | 1.30 (1.17–1.44) | <0.0001 | 0.724 |
| Model 2 | 1.12 (0.99–1.27) | 0.071 | 0.729 |
| Model 3 | 1.00 (0.87–1.15) | 0.950 | 0.732 |
| Log TRL‐C | 1.42 (1.28–1.58)† | <0.0001 | 0.579 |
| Model 1 | 1.27 (1.13–1.42) | <0.0001 | 0.724 |
| Model 2 | 1.10 (0.97–1.25) | 0.157 | 0.729 |
| Model 3 | 0.91 (0.77–1.06) | 0.228 | 0.733 |
| Log RLP‐C | 1.38 (1.24–1.53)† | <0.0001 | 0.570 |
| Model 1 | 1.23 (1.10–1.38) | 0.0003 | 0.722 |
| Model 2 | 1.08 (0.96–1.22) | 0.217 | 0.729 |
| Model 3 | 0.99 (0.87–1.13) | 0.856 | 0.733 |
| lbLDL‐C | 1.10 (0.99–1.23)† | 0.064 | 0.522 |
| Model 1 | 1.12 (1.00–1.24) | 0.044 | 0.716 |
| Model 2 | 1.16 (1.04–1.29) | 0.007 | 0.727 |
| Model 3 | 1.04 (0.91–1.19) | 0.580 | 0.729 |
Hazard ratio (95% CI) is expressed as the risk for the 75th percentile vs the 25th percentile. Model 1 was adjusted by age, sex, smoking, hypertension, and antihypertensive medication use. Model 2 was model 1 plus diabetes mellitus status and high‐density lipoprotein cholesterol. Model 3 was model 2 plus total cholesterol and cholesterol‐lowering medication use (C statistic, 0.716). ASCVD indicates atherosclerotic cardiovascular disease; lbLDL‐C, large buoyant LDL‐C; LDL‐C, low‐density lipoprotein cholesterol; LDL‐TG, low‐density lipoprotein triglycerides; RLP‐C, remnant lipoprotein particle cholesterol; sdLDL‐C, small dense LDL‐C; and TRL‐C, triglyceride‐rich lipoprotein cholesterol.
Variable is unadjusted for any other risk factors.
Cox proportional hazards regression analysis was also used to determine whether any parameters added information after the PCE model was applied to our data (Figure 2). The variables in the PCE model were summed using the published regression parameters. 1 The PCE model includes age, sex, systolic blood pressure, blood pressure treatment, diabetes mellitus, smoking, total cholesterol, and HDL‐C. 1 These values for these variables were then entered into the published equation to generate an estimated 10‐year ASCVD risk for each subject, providing a weighted score as a predictor. Specialized lipid parameters were then added to this score to determine whether they added ASCVD risk information. Parameters that were not normally distributed were log transformed before regression analyses. These parameters included triglycerides, TRL‐C, RLP‐C, VLDL‐C, sdLDL‐C, and lipoprotein(a). Net reclassification analysis was used to determine if an individual parameter contributed independently to ASCVD risk, as previously described, after controlling for all risk factors, which included age, sex, hypertension, hypertension treatment, smoking, diabetes mellitus, total cholesterol, HDL‐C, and use of cholesterol‐lowering medication (Table 4). 39 , 40
Figure 2. Association with 10‐year atherosclerotic cardiovascular disease (ASCVD) risk when atherogenic biomarker is added to the pooled cohort equation (PCE).
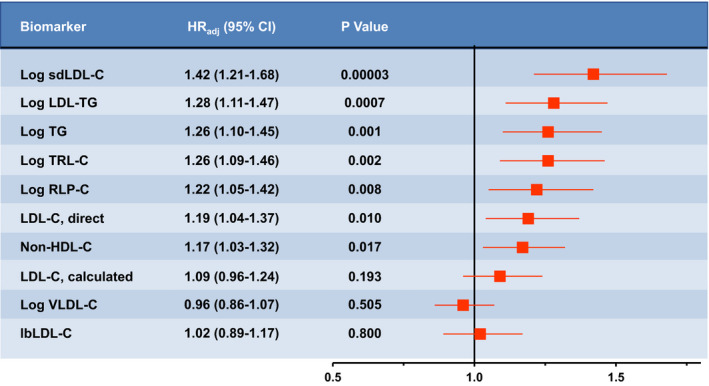
The C statistic for the PCE model (age, sex, total cholesterol, high‐density lipoprotein cholesterol [HDL‐C], systolic blood pressure, antihypertension medication, diabetes mellitus status, and smoking) was 0.6898. The C statistic increased to 0.6966 (P=0.005 vs PCE model) when log small dense low‐density lipoprotein cholesterol (sdLDL‐C) was added. The other parameters added no significant information about ASCVD risk after log sdLDL‐C was entered. When log low‐density lipoprotein triglycerides (LDL‐TG) was first entered into the model, followed by log sdLDL‐C, log LDL‐TG was no longer significant, whereas the P value for log sdLDL‐C was 0.0028. The fully adjusted hazard ratio (HRadj) (95% CI) is expressed as the 10‐year ASCVD risk for the 75th percentile vs the 25th percentile when the parameter is added to the PCE. Variables not normally distributed were log transformed before statistical analysis. lbLDL‐C indicates large buoyant LDL‐C; LDL‐C, low‐density lipoprotein cholesterol; RLP‐C, remnant lipoprotein particle cholesterol; TG, triglycerides; TRL‐C, triglyceride‐rich lipoprotein cholesterol; and VLDL‐C, very‐LDL‐C.
Table 4.
NRI Analysis
| Parameters Added to Model | Mean NRI (95% CI) | P Value |
|---|---|---|
| sdLDL‐C* | 0.077 (−0.0008 to 0.128) | 0.052 |
| Direct LDL‐C | 0.080 (−0.031 to 0.124) | 0.104 |
| LDL‐TG* | 0.030 (−0.108 to 0.092) | 0.363 |
| Lipoprotein(a)* | 0.044 (−0.032 to 0.091) | 0.172 |
| sdLDL‐C+direct LDL‐C+lipoprotein(a)* | 0.104 (0.028 to 0.152) | 0.008 |
| sdLDL‐C+direct LDL‐C+lipoprotein(a)+LDL‐TG* | 0.099 (0.041 to 0.149) | 0.008 |
Model was adjusted by age, sex, smoking, hypertension, antihypertensive medication use, diabetes mellitus status, high‐density lipoprotein cholesterol, total cholesterol, and cholesterol‐lowering medication use. LDL‐C indicates low‐density lipoprotein cholesterol; LDL‐TG, low‐density lipoprotein triglycerides; NRI, net reclassification improvement; and sdLDL‐C, small dense LDL‐C.
sdLDL‐C, lipoprotein(a), and LDL‐TG were log transformed before analysis.
To further elucidate the interrelationships between sdLDL‐C, LDL‐TG, and non–HDL‐C, we performed discordance analysis, as previously described. 19 , 41 , 42 We modeled discordance by using residuals from linear regression models to reflect the discordance between expected and observed (measured) values for sdLDL‐C and LDL‐TG based on non–HDL‐C values. This approach identified positive residuals (higher than expected sdLDL‐C and LDL‐TG values based on non–HDL‐C) and negative residuals (lower than expected sdLDL‐C and LDL‐TG values based on non–HDL‐C values). To compare risk, we examined ASCVD risk among those with positive residuals (≥75th percentile) or negative residuals (≤25th percentile), compared with those with “concordant” and intermediate residuals (25th‒75th percentile). Examination of the highest and lowest quartiles was chosen to evaluate ASCVD risk (Figure 3). The purpose of the analysis was to determine whether subjects with sdLDL‐C or LDL‐TG values above the 75th percentile or below the 25th percentile as well as non–HDL‐C values within the 25th to 75th percentile range had different ASCVD risk than those in whom the values were congruent (ie, both sdLDL‐C and LDL‐TG within the 25th–75th percentile range).
Figure 3. Discordant analysis of small dense low‐density lipoprotein cholesterol (sdLDL‐C) and low‐density lipoprotein triglycerides (LDL‐TG) relative to non–high‐density lipoprotein cholesterol (non–HDL‐C).
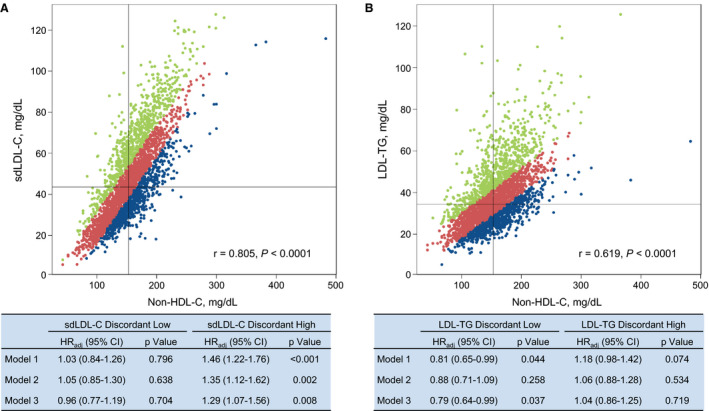
A, Discordance between sdLDL‐C and non–HDL‐C. B, Discordance between LDL‐TG and non–HDL‐C. Discordant high (>75th percentile) sdLDL‐C (23.3%) and LDL‐TG (23.5%) values are depicted in green; discordant low (<25th percentile) sdLDL‐C (23.5%) and LDL‐TG (23.5%) values are depicted in blue; and concordant (25th–75th percentile) sdLDL‐C (53.2%) and LDL‐TG (53.0%) values are depicted in red. Tables show the atherosclerotic cardiovascular disease risk, expressed as adjusted hazard ratio (HRadj) (95% CI), for discordant low and discordant high sdLDL‐C and LDL‐TG values vs the concordant values in 3 models. Model 1 was adjusted by age, sex, smoking, hypertension, and antihypertensive medication use. Model 2 was model 1 plus diabetes mellitus status and HDL‐C. Model 3 was model 2 plus total cholesterol and cholesterol‐lowering medication use.
RESULTS
Population Description and Univariate Analysis
Of the 3094 men and women studied, 624 (20.2%) had an incident ASCVD event (Table 1). All standard risk factors and other parameters were significantly (P<0.01) different at baseline between subjects who developed ASCVD and those who did not develop ASCVD over the 16‐year follow‐up period. The unadjusted associations for standard risk factors with incident ASCVD based on HR values in descending order were as follows: diabetes mellitus, age, hypertension, hypertension treatment, male sex, cholesterol‐lowering medication, low HDL‐C, triglycerides, non–HDL‐C, calculated LDL‐C, body mass index, smoking, and total cholesterol. For advanced lipoprotein parameters unadjusted for other covariates, the following parameters were significantly associated with incident ASCVD in descending order of HR: sdLDL‐C, LDL‐TG, TRL‐C, RLP‐C, and direct LDL‐C. In this unadjusted analysis, lipoprotein(a), lbLDL‐C, and calculated VLDL‐C did not reach statistical significance.
Association of Atherogenic Lipoproteins With ASCVD Risk Based on Quartile Analysis
Unadjusted quartile analysis indicated that the strongest relative significance with regard to incident ASCVD for atherogenic lipoprotein parameters, comparing top quartile versus bottom quartile values, were in descending order: LDL‐TG, sdLDL‐C, triglycerides, TRL‐C, direct LDL‐C, and RLP‐C (Table S1). VLDL‐C top quartile values were only modestly associated with increased ASCVD risk, compared with bottom quartile values. There was no significant risk relationship associated with lipoprotein(a) or lbLDL‐C. Adjustment for age and sex only modestly affected these relationships, with the notable exception being that top quartile lipoprotein(a) values became significant compared with bottom quartile values. Unadjusted Kaplan‐Meier survival analyses of the most significant relationships are presented in Figure 1. Quartile cut‐point data are provided (see figure footnote and Table S1). It can clearly be seen that the relationships between sdLDL‐C, LDL‐TG, and triglycerides and incident ASCVD were graded based on quartile groups; for direct LDL‐C and TRL‐C, the middle quartiles had similar risk; and for RLP‐C, the bottom 2 quartiles had similar risk (Figure 1). After adjustment for all ASCVD risk factors, including total cholesterol and HDL‐C, top quartile values for none of these parameters were still significantly associated with risk compared with bottom quartile values (Table S1). Only for sdLDL‐C (HR, 1.44; P=0.064) and triglycerides (HR, 1.29; P=0.084) were the relationships close to being statistically significant.
Associations Between Atherogenic Lipoprotein Parameters
Correlation coefficient analysis was used to assess interrelationships between biochemical parameters (Table 2). As expected, non–HDL‐C showed strong positive correlations with most atherogenic lipid traits, especially calculated LDL‐C, direct LDL‐C, total cholesterol, log sdLDL‐C, log TRL‐C, lbLDL‐C, log LDL‐TG, and log triglycerides (all r>0.50; P<0.001). Log sdLDL‐C values were strongly correlated with log LDL‐TG values (r=0.76; P<0.001). There were also significant (P<0.001) inverse correlations between HDL‐C and triglycerides (r=−0.43), TRL‐C (r=−0.47), and RLP‐C (r=−0.33).
Cox Proportional Hazards Analysis
We assessed the significance of each specialized continuous atherogenic biomarker for ASCVD risk without adjustment and after adjustment for standard risk factors, using 3 different models using multivariate analysis (Table 3). Model 1 included age, sex, smoking, hypertension, and hypertension treatment. Model 2 included age, sex, smoking, hypertension, hypertension treatment, diabetes mellitus, and HDL‐C. Model 3 included age, sex, smoking, hypertension, hypertension treatment, diabetes mellitus, HDL‐C, total cholesterol, and cholesterol‐lowering medication. After multivariate adjustment for the parameters in model 1, all atherogenic biomarkers, except VLDL‐C, remained significant. Once diabetes mellitus and HDL‐C entered into the model (model 2), the associations of log triglycerides, log TRL‐C, and log RLP‐C with incident ASCVD were no longer significant. In the fully adjusted model (model 3), which included total cholesterol and the use of cholesterol‐lowering medication, only log sdLDL‐C, direct LDL‐C, log LDL‐TG, and log lipoprotein(a) remained significant (P<0.05). The C statistic for model 3 alone was 0.716, and increased to 0.734 (P<0.001) when log sdLDL‐C was added, increased to 0.731 when direct LDL‐C was added, and increased to 0.734 when log lipoprotein(a) was added to model 3. Because almost all subjects in the FOS were White individuals, we did not have the opportunity to explore potential race/ethnicity ASCVD risk differences. It is well known that there are significant sex differences in ASCVD risk. Therefore, we controlled for sex in all models used.
Net Reclassification Analysis
None of the biochemical parameters, including sdLDL‐C, alone significantly affected the net reclassification index. The parameters specifically assessed in detail, as shown in Table 4, included log sdLDL‐C, direct LDL‐C, log LDL‐TG, and log lipoprotein(a). When the composite of log sdLDL‐C, direct LDL‐C, and log lipoprotein(a) was added to model 3 with all standard risk factors included, the net reclassification index for ASCVD was improved significantly by ≈10%. The addition of log LDL‐TG either alone or together with other variables did not significantly affect net reclassification.
PCE Analysis
We also assessed the extent to which atherogenic lipoprotein parameters provided incremental value in the prediction of ASCVD risk, above and beyond that provided using the PCE model. 1 With the PCE model alone, the C statistic was 0.6898 for ASCVD (Figure 2). Significant information about ASCVD risk on top of the PCE model was added individually by log sdLDL‐C, log triglycerides, log LDL‐TG, log TRL‐C, and log RLP‐C (P<0.05). When log sdLDL‐C was added to the PCE model, the C statistic was increased to 0.6966 (P=0.005). No other parameter added significantly to ASCVD risk once log sdLDL‐C was in the model. Moreover, when other variables entered the model, the addition of sdLDL‐C caused these variables to be removed, based on lack of statistical significance; and sdLDL‐C remained significant.
Discordance Analysis
Figure 3 shows that subjects with discordant high sdLDL‐C values (>75th percentile, 23.3%) versus sdLDL‐C values concordant with values expected from non–HDL‐C values (53.2%) had a significantly increased HR of 1.46, which decreased to 1.29 (95% CI, 1.07–1.56; P=0.008) after adjustment for all parameters. No such differences were seen for discordant low sdLDL‐C or discordant high or low LDL‐TG.
DISCUSSION
LDL Subfraction Composition and Metabolism
LDL particles by weight percentage contain ≈25% protein (almost all apolipoprotein B‐100), 45% cholesterol, 20% phospholipid, and 10% triglycerides. We have previously documented that as LDL size decreases from the largest to smallest LDL, there are significant decreases in the relative content of cholesteryl ester (40%–25%), free cholesterol (10%–5%), and phospholipid (24%–19%), along with significant increases in triglycerides (5%–20%) and protein content (20%–30%). 43 These data explain why there is a strong correlation between sdLDL‐C and LDL‐TG. These compositional alterations in LDL particles were estimated to cause major changes in apolipoprotein B‐100 conformation on the surface of LDL based on calculations by Dr Donald Small of Boston University, which, in turn, were predicted to cause decreases in LDL receptor binding affinity, plasma clearance, and resistance to oxidation. 43
More than a decade later, our subsequent studies confirmed some of these predictions. 44 When we studied human apolipoprotein B‐100 metabolism within VLDL (density, <1.019 g/mL), lbLDL (density, 1.019–1.044 g/mL), and sdLDL (density, 1.044–1.063 g/mL) with stable isotope methods, we documented that after VLDL apolipoprotein B‐100 production, most of lbLDL apolipoprotein B‐100 was derived from VLDL conversion and much of sdLDL apolipoprotein B‐100 was derived from lbLDL conversion. Some sdLDL apolipoprotein B‐100 was also derived directly from VLDL apolipoprotein B‐100 conversion. Of greatest importance was that the plasma residence time of sdLDL apolipoprotein B‐100 (≈74 hours) was much greater than that observed for lbLDL apolipoprotein B‐100 (≈40 hours). 44 In our view, sdLDL is substantially more atherogenic than lbLDL because of its smaller size and its greater plasma residence time. 44 These properties of sdLDL allow more time for plasma modification and oxidation, greater penetration into the artery wall, and subsequent enhanced uptake by modified LDL receptors on macrophage surfaces.
Atherogenic Lipoproteins and ASCVD Risk
Unadjusted analyses indicated that sdLDL‐C, LDL‐TG, direct LDL‐C, triglycerides, TRL‐C, and RLP‐C were all significantly associated with increased ASCVD risk. These findings were supported by the unadjusted Kaplan‐Meier plots by quartile. However, after adjustment for all risk factors, including total cholesterol and HDL‐C, top quartile values for these parameters were no longer significantly associated with risk compared with bottom quartile values. We have documented that there are significant positive and inverse correlations between various lipid and lipoprotein parameters. As expected, total cholesterol and non–HDL‐C were strongly and positively correlated with all atherogenic lipoprotein parameters except lipoprotein(a). We also noted that sdLDL‐C and LDL‐TG were strongly correlated. Triglyceride values were strongly and positively correlated with TRL‐C, RLP‐C, LDL‐TG, and sdLDL‐C, and were inversely correlated with HDL‐C. It has been previously documented that patients with premature ASCVD often have elevated triglycerides >150 mg/dL, decreased HDL‐C <40 mg/dL, and elevated small dense LDL based on gradient gel analysis. 5 , 6
On multivariate analyses, the significant relationships of atherogenic lipoprotein parameters were retained when adjusted for age, sex, hypertension, hypertension treatment, and smoking. However, once diabetes mellitus and HDL‐C were entered into the model along with the prior variables, the association of triglycerides, TRL‐C, and RLP‐C with incident ASCVD was no longer significant, presumably because of inverse associations with HDL‐C and positive associations with diabetes mellitus. When adjustments were made for total cholesterol and cholesterol‐lowering medication in addition to the other risk factors, only sdLDL‐C, direct LDL‐C, LDL‐TG, and lipoprotein(a) remained significant, although the strength of these associations was significantly attenuated. The C statistic was only marginally improved by the addition of sdLDL‐C, direct LDL‐C, and lipoprotein(a), and was not altered by adding LDL‐TG. Net reclassification analysis confirmed these findings.
Our data support the observations of Saeed and colleagues about the importance of both RLP‐C and LDL‐TG as ASCVD risk factors using univariate analysis, and the finding that only LDL‐TG is significant after adjustment for all risk factors. 37 However, our data also indicate that LDL‐TG and sdLDL‐C are highly correlated, and that once sdLDL‐C is added to the multivariate model or to the PCE, LDL‐TG no longer added significant risk information. These findings were further confirmed by discordance analysis, which documented that, after adjustment for all other standard risk factors, discordant top quartile sdLDL‐C as related to non–HDL‐C values was associated with significant increased ASCVD risk, compared with subjects who had concordant values. This was not the case for discordant bottom quartile sdLDL‐C or for discordant top quartile or bottom quartile LDL‐TG. We have previously used discordance analysis in assessing direct LDL‐C versus non–HDL‐C in the FOS, and other investigators have used this type of analysis in the WHS (Women’s Health Study). 19 , 41 , 42
Our studies support the findings reported from the ARIC (Atherosclerosis Risk in Communities) Study, the MESA (Multi‐Ethnic Study of Atherosclerosis), and the CGPS (Copenhagen General Population Study) that sdLDL‐C is significantly related to incident ASCVD. 32 , 33 , 34 However, our findings also extend their conclusions to indicate that sdLDL‐C provides additional information about ASCVD risk, even after controlling for all standard risk factors, including HDL‐C, total cholesterol, and cholesterol‐lowering medication, in contrast to other studies. We have documented this finding to be the case in univariate analysis, multivariate analysis, discordance analysis, and on top of the PCE. Therefore, our overall data support the concept that sdLDL‐C is the most atherogenic lipoprotein parameter and is worth measuring in subjects at increased ASCVD risk. Both direct LDL‐C and sdLDL‐C are assays approved by the Food and Drug Administration, are relatively inexpensive, and are available from reference laboratories in the United States and other countries. These parameters can be lowered by >50% with the combination of lifestyle modification and intensive statin therapy. 45
Study Strengths and Limitations
Strengths of the present study include its prospective nature and the wide variety of state‐of‐the‐art lipoprotein particle assays used. A limitation of the analysis was the significant correlations of many of the biochemical variables with each other, especially total cholesterol and non–HDL‐C with direct LDL‐C and sdLDL‐C. Despite these correlations, sdLDL‐C remained significant on multivariate analysis, but not in the adjusted quartile analysis. Our study population was largely White individuals; and, therefore, the results cannot be generalized to other ethnic groups. In the future, pooling of data across prospective studies should strengthen our ability to test which of these atherogenic biomarkers should be measured for more precise ASCVD risk assessment in various ethnic groups.
Conclusions
Our data indicate that sdLDL‐C is the most atherogenic lipoprotein parameter in our prospective assessment. Although sdLDL‐C, direct LDL‐C, and lipoprotein(a) all added significant ASCVD risk information with adjusted multivariate analysis, only sdLDL‐C provided such information when added to the PCE model. In our view, optimizing sdLDL‐C levels may be paramount in ASCVD risk reduction, along with optimizing blood pressure and glucose levels and smoking cessation.
Sources of Funding
Dr Ikezaki was supported by research grants from the Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad Program, Tokyo, Japan, and from the Denka Corporation, Niigata, Japan, to the Dyslipidemia Foundation of Boston, MA. The statistical consultation and analysis performed by Drs Lim, Liu, and Cupples was supported in part by a grant from the Denka Corporation to the Dyslipidemia Foundation. Drs Lim, Liu, Cupples, and the FOS were supported by National Institutes of Health (NIH) grant National Heart, Lung, and Blood Institute N01‐HC 25195 and HHSN268201500001I. Drs Asztalos and Schaefer were supported by the United States Department of Agriculture–Agricultural Research Service Specific Cooperative Agreements 58‐1950‐0‐014 and 58‐1950‐4‐003 and by NIH grants P50 HL083813‐01 and HL117933. The sponsors had no role in the data analysis or interpretation of this study. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of Tufts University, Kyushu University, Boston University, the NIH, the United States Department of Agriculture Research Service, or the Denka Corporation.
Disclosures
Dr Schaefer is a part‐time employee of Boston Heart Diagnostics, Framingham, MA, and has served as a consultant for the Denka Corporation, Niigata, Japan. The remaining authors have no disclosures to report.
Supporting information
Table S1
Acknowledgments
We dedicate this article to the memory of Dr Donald M. Small, former professor and chairman of the Department of Physiology and Biophysics, Boston University School of Medicine, who collaborated with us in our studies of low‐density lipoprotein subfraction composition and made major contributions to plasma lipoprotein and bile acid physiology. He died January 25, 2019, at the age of 87 years. The authors thank Katalin V. Horvath for technical assistance; Drs Yasuki Ito and Asako Machida, of the Denka Corporation, Niigata, Japan, for developing and providing the research assays for this study; and Dr Margaret R. Diffenderfer, of Tufts University, for assistance in manuscript preparation.
(J Am Heart Assoc. 2021;10:e019140. DOI: 10.1161/JAHA.120.019140.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019140
This research was presented in part at the American Heart Association Scientific Sessions, November 16, 2019, in Philadelphia, PA.
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 2. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta‐analysis. JAMA. 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 4. Schaefer EJ, Tsunoda F, Diffenderfer MR, Polisecki EA, Thai N, Asztalos BF. The measurement of lipids, lipoproteins, apolipoproteins, fatty acids, and sterols, and next generation sequencing for the diagnosis and treatment of lipid disorders. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com Inc; 2016:1–69. Available at: https://www.endotext.org/section/lipids. Accessed September 1, 2020. [Google Scholar]
- 5. Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype: a proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495–506. [DOI] [PubMed] [Google Scholar]
- 6. Campos H, Genest JJ, Blijlevens E, McNamara JR, Jenner J, Ordovas JM, Wilson PWF, Schaefer EJ. Low density lipoprotein particle size and coronary artery disease. Arterioscler Thromb. 1992;12:187–195. [DOI] [PubMed] [Google Scholar]
- 7. Asztalos BF, Cupples LA, Demissie S, Horvath KV, Cox CE, Batista MC, Schaefer EJ. High‐density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants in the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2004;24:2181–2187. [DOI] [PubMed] [Google Scholar]
- 8. Burstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970;11:583–595. [PubMed] [Google Scholar]
- 9. Manual of Laboratory Operations: Lipid Research Clinics Program: Lipid and Lipoprotein Analysis. DHEW publication (NIH). Bethesda, MD: National Institutes of Health; 1975:75–628. [Google Scholar]
- 10. McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. [DOI] [PubMed] [Google Scholar]
- 11. Sugiuchi H, Uji Y, Okabe H, Irie T, Uekama K, Kayahara N, Miyauchi K. Direct measurement of high‐density lipoprotein cholesterol in serum with polyethylene glycol‐modified enzymes and sulfated α‐cyclodextrin. Clin Chem. 1995;41:717–723. [PubMed] [Google Scholar]
- 12. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.DOI: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 13. Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR. Comparison of a novel method vs the Friedewald equation for estimating low‐density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061–2068.DOI: 10.1001/jama.2013.280532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meeusen JW, Lueke AJ, Jaffe AS, Saenger AK. Validation of a proposed novel equation for estimating LDL cholesterol. Clin Chem. 2014;60:1519–1523.DOI: 10.1373/clinchem.2014.227710. [DOI] [PubMed] [Google Scholar]
- 15. McNamara JR, Cole TG, Contois JH, Ferguson CA, Ordovas JM, Schaefer EJ. Immunoseparation method for measuring low‐density lipoprotein cholesterol directly from serum evaluated. Clin Chem. 1995;41:232–240. [PubMed] [Google Scholar]
- 16. Okada M, Matsui H, Ito Y, Fujiwara A, Inano K. Low density lipoprotein cholesterol can be chemically measured; a new superior method. J Lab Clin Med. 1998;132:195–201.DOI: 10.1016/S0022-2143(98)90168-8. [DOI] [PubMed] [Google Scholar]
- 17. Sakaue T, Hirano T, Yoshino G, Sakai K, Takeuchi H, Adachi M. Reactions of direct LDL‐cholesterol assays with pure LDL fraction and IDL: comparison of three homogeneous methods. Clin Chim Acta. 2000;295:97–106.DOI: 10.1016/S0009-8981(00)00200-X. [DOI] [PubMed] [Google Scholar]
- 18. Otokozawa S, Ai M, Asztalos BF, White CC, Demissie‐Banjaw S, Cupples LA, Nakajima K, Wilson PW, Schaefer EJ. Direct assessment of plasma low‐density lipoprotein and high‐density lipoprotein cholesterol and coronary heart disease: results from the Framingham Offspring Study. Atherosclerosis. 2010;213:251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ikezaki H, Fisher VA, Lim E, Ai M, Liu CT, Cupples LA, Nakajima K, Asztalos BF, Furusyo N, Schaefer EJ. Direct versus calculated low‐density lipoprotein cholesterol and C reactive protein in cardiovascular disease risk assessment in the Framingham Offspring Study. Clin Chem. 2019;65:1102–1114. [DOI] [PubMed] [Google Scholar]
- 20. Hirano T, Ito Y, Saegusa H, Yoshino G. A novel and simple method for quantification of small dense LDL. J Lipid Res. 2003;44:2193–2201.DOI: 10.1194/jlr.D300007-JLR200. [DOI] [PubMed] [Google Scholar]
- 21. Ito Y, Fujimura M, Ohta M, Hirano T. Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem. 2011;57:57–65.DOI: 10.1373/clinchem.2010.149559. [DOI] [PubMed] [Google Scholar]
- 22. Ito Y, Ohta M, Ikezaki H, Hirao Y, Machida A, Schaefer EJ, Furusyo N. Development and population results of a fully automated homogeneous assay for LDL triglyceride. J Appl Lab Med. 2018;2:746–756.DOI: 10.1373/jalm.2017.024554. [DOI] [PubMed] [Google Scholar]
- 23. Muraba Y, Koga T, Shimomura Y, Ito Y, Hirao Y, Kobayashi J, Kimura T, Nakajima K, Murakami M. The role of plasma lipoprotein lipase, hepatic lipase and GPIHBP1 in the metabolism of remnant lipoproteins and small dense LDL in patients with coronary artery disease. Clin Chim Acta. 2018;476:146–153. [DOI] [PubMed] [Google Scholar]
- 24. Schaefer EJ, Lamon‐Fava S, Jenner JL, McNamara JR, Ordovas JM, Davis CE, Abolafia JM, Lippel K, Levy RI. Lipoprotein(a) levels and risk of coronary heart disease in men: the Lipid Research Clinics Coronary Primary Prevention Trial. JAMA. 1994;271:999–1003.DOI: 10.1001/jama.1994.03510370051031. [DOI] [PubMed] [Google Scholar]
- 25. Emerging Risk Factors Collaboration , Erqou S, Kaptoge S, Perry PL, DiAngelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lamon‐Fava S, Marcovina SM, Albers JJ, Kennedy H, DeLuca C, White CC, Cupples LA, McNamara JR, Seman LJ, Bongard V, et al. Lipoprotein(a) levels, apo(a) isoform size, and coronary heart disease risk in the Framingham Offspring Study. J Lipid Res. 2011;52:1181–1187.DOI: 10.1194/jlr.M012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirano T, Ito Y, Koba S, Toyoda M, Ikejiri A, Saegusa H, Yamazaki J, Yoshino G. Clinical significance of small dense low‐density lipoprotein cholesterol levels determined by the simple precipitation method. Arterioscler Thromb Vasc Biol. 2004;24:558–563.DOI: 10.1161/01.ATV.0000117179.92263.08. [DOI] [PubMed] [Google Scholar]
- 28. Koba S, Yokota Y, Hirano T, Ito Y, Ban Y, Tsunoda F, Sato T, Shoji M, Suzuki H, Geshi E, et al. Small LDL‐cholesterol is superior to LDL‐cholesterol for determining severe coronary atherosclerosis. J Atheroscler Thromb. 2008;15:350–360.DOI: 10.5551/jat.E572. [DOI] [PubMed] [Google Scholar]
- 29. Ai M, Otokozawa S, Asztalos BF, Ito Y, Nakajima K, Shite CC, Cupples LA, Wilson PW, Schaefer EJ. Small dense LDL cholesterol and coronary heart disease: results from the Framingham Offspring Study. Clin Chem. 2010;56:967–976.DOI: 10.1373/clinchem.2009.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nishikura T, Koba S, Yokota Y, Hirano T, Tsunoda F, Shoji M, Hamazaki Y, Suzuki H, Itoh Y, Katagiri T, et al. Elevated small dense low‐density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb. 2014;21:755–767.DOI: 10.5551/jat.23465. [DOI] [PubMed] [Google Scholar]
- 31. Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, Okamura T, Miyamato Y. Small dense low‐density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb. 2013;20:195–203.DOI: 10.5551/jat.14936. [DOI] [PubMed] [Google Scholar]
- 32. Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, Remaley AT. New automated assay of small dense low‐density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi‐Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:196–201.DOI: 10.1161/ATVBAHA.113.302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, et al. Small dense low‐density lipoprotein‐cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34:1069–1077.DOI: 10.1161/ATVBAHA.114.303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Balling M, Nordestgaard BG, Langsted A, Varbo A, Kamstrup PR, Afzal S. Small dense low‐density lipoprotein cholesterol predicts atherosclerotic cardiovascular disease in the Copenhagen General Population Study. J Am Coll Cardiol. 2020;75:2873–2875.DOI: 10.1016/j.jacc.2020.03.072. [DOI] [PubMed] [Google Scholar]
- 35. McNamara JR, Shah PK, Nakajima K, Cupples LA, Wilson PW, Ordovas JM, Schaefer EJ. Remnant‐like particle (RLP) cholesterol is an independent cardiovascular disease risk factor in women: results from the Framingham Heart Study. Atherosclerosis. 2001;154:229–236.DOI: 10.1016/S0021-9150(00)00484-6. [DOI] [PubMed] [Google Scholar]
- 36. Schaefer EJ, McNamara JR, Shah PK, Nakajima K, Cupples LA, Ordovas JM, Wilson PW; Framingham Offspring Study . Elevated remnant‐like particle cholesterol and triglyceride levels in diabetic men and women in the Framingham Offspring Study. Diabetes Care. 2002;25:989–994.DOI: 10.2337/diacare.25.6.989. [DOI] [PubMed] [Google Scholar]
- 37. Saeed A, Feofanova EV, Yu B, Sun W, Virani SS, Nambi V, Coresh J, Guild CS, Boerwinkle E, Ballantyne CM, et al. Remnant‐like particle cholesterol, low‐density lipoprotein triglycerides, and incident cardiovascular disease. J Am Coll Cardiol. 2018;72:156–169.DOI: 10.1016/j.jacc.2018.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290.DOI: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 39. Pencina MJ, Steyerberg EW, D’Agostino RB Sr. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21.DOI: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pencina MJ, D'Agostino RB Sr. Evaluating discrimination of risk prediction models: the C statistic. JAMA. 2015;314:1063–1064.DOI: 10.1001/jama.2015.11082. [DOI] [PubMed] [Google Scholar]
- 41. Mora S, Buring JE, Ridker PM. Discordance of low‐density lipoprotein (LDL) cholesterol with alternative LDL‐related measures and future coronary events. Circulation. 2014;129:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lawler PR, Akinkuolie AO, Ridker PM, Sniderman AD, Buring JE, Glynn RJ, Chasman DI, Mora S. Discordance between circulating atherogenic cholesterol mass and lipoprotein particle concentration in relation to future coronary events in women. Clin Chem. 2017;63:870–879.DOI: 10.1373/clinchem.2016.264515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McNamara JR, Small DM, Li Z, Schaefer EJ. Differences in LDL subspecies involve alterations in lipid composition and conformational changes in apolipoprotein B. J Lipid Res. 1996;37:1924–1935. [PubMed] [Google Scholar]
- 44. Thongtang N, Diffenderfer MR, Ooi EMM, Barrett PHR, Turner SM, Le NA, Brown WV, Schaefer EJ. Metabolism and proteomics of large and small dense LDL in combined hyperlipidemia: effects of rosuvastatin. J Lipid Res. 2017;58:1315–1324.DOI: 10.1194/jlr.M073882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ai M, Otokozawa S, Asztalos BF, Nakajima K, Stein E, Jones PH, Schaefer EJ. Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low‐density lipoprotein cholesterol levels. Am J Cardiol. 2008;101:315–318.DOI: 10.1016/j.amjcard.2007.08.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


