Abstract
In patients with cardiovascular disease, the use of antibody or serological testing is frequently encountered as the coronavirus disease 2019 pandemic continues to evolve. Antibody testing detects one form of the acquired immunological response to a pathogenic antigen. Once the immune system recognizes a viral antigen or a protein as foreign, a humoral immune response is initiated, which is generally detected by laboratory testing in 5 to 10 days after the initial exposure. While this information is critical from a public health perspective to implement surveillance systems and measures to limit infectivity and transmission rate, the misinterpretation of serologic testing in clinical practice has generated much confusion in the medical community because some attempted to apply these strategies to individual patient's treatment schemes.
In this mini‐review, we examine the different serologic‐based testing strategies, how to interpret their results, and their public health impact at the population level, which are critical to contain the transmission of the virus in the community within a busy cardiovascular practice. Further, this review will also be particularly helpful as vaccination and immune therapy for coronavirus disease 2019 become available to the society as a whole.
Keywords: cardiovascular diseases, COVID‐19, immunity, serologic test
Subject Categories: Biomarkers, Inflammation, Pathophysiology
Nonstandard Abbreviations and Acronyms
- COVID‐19
Coronavirus Disease 2019
- FDA
Food and Drug Administration
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
The World Health Organization declared coronavirus disease 2019 (COVID‐19) a pandemic with millions of people infected resulting in substantial morbidity and mortality worldwide. Cardiovascular practitioners are now frequently asked to interpret the results from an array of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) tests. Serologic testing in particular has been controversial with different strategies implemented in hospital‐, office‐, and community‐based settings. In this mini‐review, we critically appraise serologic‐based testing and strategies for clinical and research applications.
Background on Serologic Testing
Antibody (or serology) testing detects one form of the acquired immunological response to a pathogenic antigen. Serology depends on the immune system recognizing an antigen (typically a protein) as foreign and eliciting a humoral response, which is generally detectable 5 to 10 days after the infection. Although it is generally accepted that immunoglobulin M (IgM) is detectable after 5 to 10 days and immunoglobulin G (IgG) total antibody after 11 to 14 days, for anti‐SARS‐CoV‐2, there is controversy about the sequence of antibody subtype response after acute COVID‐19. 1 For example, Long et al reported that the median day of conversion for both IgM and IgG titers was 13 days, but 3 types of seroconversion were observed: (1) synchronous seroconversion of IgG and IgM; (2) IgM seroconversion earlier than that of IgG; and (3) IgM seroconversion later than that of IgG. 2 Because of the humoral response's delay, anti‐SARS‐CoV‐2 testing is not useful for diagnosis of acute COVID‐19. Rather, the indication for serologic testing includes: (1) understanding COVID‐19 epidemiology; (2) assessing an individual's previous SARS‐CoV‐2 exposure; and (3) assessing neutralizing potential of specimens or identification of convalescent plasma donors.
Assays for Anti‐SARS‐CoV‐2
Anti‐SARS‐CoV‐2 assays can evaluate for presence and quantity of IgG, IgM, IgA, or total antibody by “binding”; or “functional” assays, which can determine the presence of neutralizing antibodies. The foundation of anti‐SARS‐CoV‐2 testing is binding assay detection of antibodies in a person's blood to one of several protein‐antigens of SARS‐CoV‐2 including the nucleocapsid phosphoprotein, spike full‐length protein, and receptor binding domain (RBD) (Figure 1). For measurement to be optimally informative, the binding assay must have high specificity for SARS‐CoV‐2 antibodies. The nucleocapsid protein is the virus' most abundant protein‐antigen. 3 However, cross‐reactivity with other coronaviruses is a concern. 3 The SARS‐CoV‐2 spike protein has been championed as a highly specific target because it deviates most among coronaviruses with a number of unique epitopes. The spike protein shares only 75% genome sequence identity with SARS‐CoV and the similarity with the common cold coronaviruses spike proteins is only 50% to 60%. 3 Spike protein is critical in viral entry to the host cell by the receptor‐binding domain of the S1 subunit of the spike protein. 4 Through the S2 subunit, fusing the virus to the host membrane occurs. 4 Therefore, the binding assays targeting RBD regions of the spike protein and their subunits (S1 and S2) may also have the closest association with the findings from functional assays measuring the presence of neutralizing antibodies. 4 , 5 It should be noted that some "binding" assays are only qualitative, and the manufacturer may recommend that positive results must be confirmed with another method.
Figure 1. Serology‐based testing to SARS‐COV‐2.

The figure illustrates the protein antigens of the severe acute respiratory syndrome coronavirus 2: nucleocapsid phosphoprotein, spike full‐length protein, and receptor binding domain and the 2 most common serology‐based testing strategies: enzyme‐linked immunosorbent assay and chemiluminescent immunoassay. PRNT50 indicates 50% plaque reduction neutralization test; and SARS‐COV‐2, severe acute respiratory syndrome coronavirus 2. The nucleocapsid protein is not shown in the illustration because it is attached to the viral RNA. The illustration only shows the surface proteins; the orange proteins represent the phospholipid bilayer. The blue/purple 5‐part proteins represent viroporin pentamers, also called E proteins.
Two general types of binding antibody tests are available that can detect total SARS‐CoV‐2 antibodies or specific subtypes (ie, IgG): (1) point‐of‐care lateral flow assays that bind antibodies in whole blood from a finger stick or venipuncture; or (2) manual or highly automated ELISA‐type assays that provide results as a color, fluorescence or sensitive chemiluminescent signal that quantifies the amount of antigen‐antibody complex formation directly or after serial dilution. Generally lateral flow tests perform poorly with respect to specificity compared with more quantitative measures making their use uncertain. 6 Currently no tests for anti‐SARS‐CoV‐2 are Food and Drug Administration (FDA)‐cleared (ie, meet stringency requirements of laboratory tests used in clinical practice like troponin), but a substantial number have received FDA Emergency Use Authorization.
Neutralization assays are in vitro methods that assess immunity to viral illness by quantifying functional anti‐SARS‐CoV‐2 activity. ELISA are first performed by serial dilutions of the patient serum sample and values from the dilution curve are used to determine the area under the curve to quantify the anti‐SARS‐CoV‐2 activity. 7 Figure 1 shows that neutralization tests are performed by incubating the patient sample with a live SARS‐CoV‐2 viral (or a pseudo‐virus expressing SARS‐CoV‐2 proteins) suspension at serially diluted sample concentrations. The reported value is the greatest dilution at which antibody concentration is able to reduce the virus' infectivity in vitro by 50% PRNT50. Such levels of antibodies reflect the ability of the patient to neutralize viral activity. Functional neutralization assays are time consuming and not yet FDA approved.
Clinical Application of Serologic Testing for COVID‐19
The wide availability of serologic binding assays, their ability to rapidly detect an active immune response at a low‐cost are characteristics that make them ideal for population screening and monitoring of immune response. An interactive example of longitudinal serosurveys and how they can be used to predict asymptomatic infection was recently published by the Centers for Disease Control and Prevention and can provide regional insights as to what to expect for positive viral testing before invasive cardiac procedures. To maximize the specificity to distinguish those with true positives, 3 testing strategies have been proposed. First, a single strategy test includes using a serologic test with high specificity ≥95%, which results in acceptable positive predictive value, but it is challenged when there is a low population prevalence of prior exposure. Second, test only those with a high pre‐test probability of COVID‐19. Third, an orthogonal test strategy includes an initial serologic‐based test and among those who test positive, a second test for antibodies to a different viral epitope is subsequently performed. Orthogonal testing strategies are used to achieve maximum specificity while retaining high sensitivity. 8
Antibody testing turns positive 5 to 10 days after exposure but remains positive for months or years after initial infection. This information is useful for public health monitoring, as previously expressed, because the orthogonal testing strategy is highly sensitive for population transmission. Global immunological observatory or surveillance systems to detect signals of infection on a population level and related seasonality can limit the transmission of the virus in the community. Because viral or molecular testing remains positive in an infected individual for only a short period of time, testing at infrequent intervals from the population level can result in an underestimation of the point prevalence of COVID‐19. However, the use of serologic testing in a population at risk may allow a more accurate assessment of the point prevalence because the viral antibodies remain detectable in the blood for months or potentially years after the initial exposure. From a cost perspective, serologic testing is much cheaper than molecular testing, which is particularly relevant when large segments of the population are being tested.
Combing the information obtained from molecular and serologic testing together from one population at risk can also be helpful to contain the COVID‐19 infection. For example, estimates on a point prevalence of 4% based molecular testing combined with high estimates from serologic testing can potentially mean that the infection is likely decreasing in the population as a whole because the population had already developed an immune response to the virus (ie, had a prior infection). However, a similar point prevalence of 4% based on molecular testing combined with low estimates of infection based on serologic testing in the population at risk can potentially mean that the infection will rise in the near future because the population at risk had not been previously exposed to the virus, and more public health measures should be implemented to limit the infectivity and transmission rates in the community as a whole.
Serologic Testing in Areas With Low Disease Prevalence
The burden and clinical implication of COVID‐19 on the society as a whole remains substantial, but the prevalence of the disease is variable depending on exposure and transmission rates within different regions of the United States. In large areas with low disease prevalence, the Centers for Disease Control and Prevention recommends a 2‐stage specimen pooling strategy, or orthogonal testing for population surveillance. 9 According to the Centers for Disease Control and Prevention, Public Health surveillance is an “ongoing systematic collection, analysis, and interpretation of health‐related data essential for planning implementation, and evaluation of public health practice, closely integrated with the timely dissemination of these data to those responsible for prevention and control”. 10 This surveillance system is important because it (1) evaluates the overall public health status of a communicable disease; (2) defines priorities where public health interventions are needed; (3) evaluates the effectiveness of public health programs (eg, vaccinations and immune therapies); and (4) serves as a pivot for future research. 10 When 2 orthogonal testing strategies are used in the community with low prevalence of COVID‐19, the positive predictive value of the test is maximized (Figure 2). The use of orthogonal testing strategies will further preserve resources, reduce the amount of time and effort aimed at obtaining a large number of specimens, and limit overall testing cost. 9
Figure 2. Positive predictive value for 2 testing strategies by prevalence of COVID‐19 in a hypothetical situation.
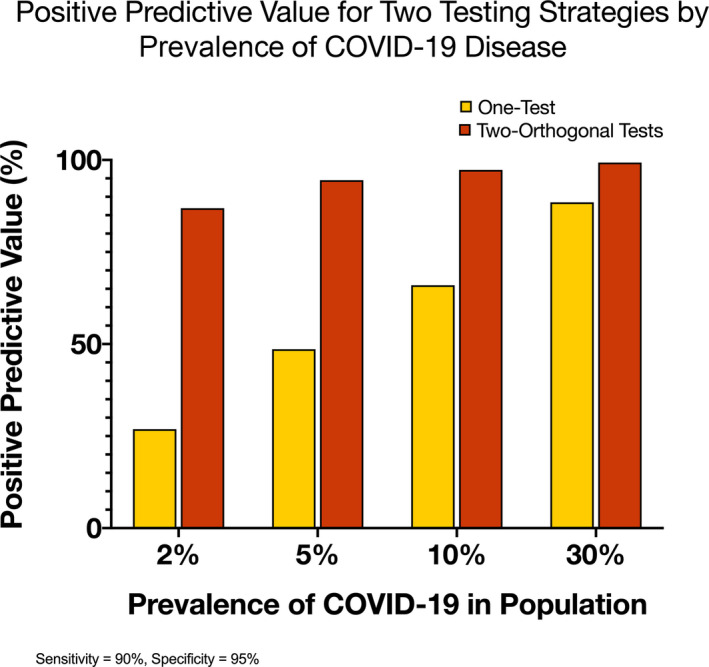
COVID‐19 indicates coronavirus disease 2019. Data derived from https://www.cdc.gov/coronavirus/2019‐ncov/lab/resources/antibody‐tests‐guidelines.html/.
Long‐Term Immune Response to COVID‐19
While some parts of the world are recovering from the effects of the pandemic, the United States and Europe are facing a large number of COVID‐19 cases. Among the survivals, the long‐term immune response to the virus remain an area of active investigation. Early reports indicated that among asymptomatic patients or those with mild symptoms, there was an immune signal at 3‐months after onset of symptoms. 11 , 12 , 13 , 14 The immune response with anti‐SARS‐CoV‐2 spike and nucleocapsid IgG levels correlated with the neutralization titers during follow‐up (Table). 12 However, more recent data on the sustainability of the immune response after the onset of symptoms were contradictory. 15 , 16 , 17 Long et al showed that there was a median percentage decrease of 71.1% in IgG levels 2 months after the onset of illness, 2 and this finding was recently corroborated by other studies. 17 , 18 Other investigators reported sustained IgM and IgG levels in symptomatic participants against spike RBD, S2, with these neutralizing antibodies remaining detectable 5 to 7 months after the onset of symptoms (Table). This gap in knowledge has critical implications on vaccination development and immunotherapies to limit the spread of the virus. Longer term data on sustainability of serologic response to SARS‐CoV‐2 will be necessary to evaluate the efficacy of immune therapies in the society as a whole.
Table 1.
Recent Clinical Studies That Evaluated Anti‐SARS‐COV‐2 Antibodies in COVID‐19
| Author | Publication Date | Study Design (n) | Test | Patient Population | Longitudinal Follow‐Up | Main Results | Limitation |
|---|---|---|---|---|---|---|---|
| Premkumar et al 19 | June 11, 2020 | Prospective cohort (n=63) | IgG/IgM binding to spoke RBD antigen and SARS‐CoV‐2 neutralization assay | Symptomatic PCR confirmed subjects with SARS‐CoV‐2 | 39 d | Strong correlation between levels of RBD‐binding antibodies and SARS‐CoV‐2 neutralizing antibodies |
Single center Small sample Short surveillance period |
| Steensels et al 11 | June 15, 2020 | Cross‐sectional (n=4125) | IgG/IgM Lateral flow assay against nucleocapsid protein* | Asymptomatic workers at Hospital East‐Limburg | 8 d | 6.4% had IgG antibodies to SARS‐CoV‐2 |
Single center No long‐term follow‐up Short‐surveillance period Only 74% of total sample enrolled |
| Long et al 2 | June 18, 2020 | Matched case‐control (n=178) | IgG and IgM ELISA against spike protein | Asymptomatic cases, defined as individuals with a positive nucleic acid test result with without clinical symptoms | 8 wk |
20.8% had asymptomatic infection (37/178) Median percentage decrease in IgG level was 71.1% (range, 32.8%–88%) Neutralizing antibodies decreased by 81.1% |
Inaccurate estimate of asymptomatic infection in general population Variability in sensitivity/specificity Confounding because of prior SARS infections |
| Wang et al 12 | July 7, 2020 | (n=23) | IgG and IgM ELISA to spike, S1, S2, RBD, and nucleocapsid proteins | Symptomatic cases (12 severely ill and 11 mildly ill) from 3 hospitals | 6 wk after symptom onset (baseline was measured during symptom onset) |
Lower level of IgM was observed in mildly ill patients, but similar IgG responses in mildly and severely ill patients Anti‐SARS‐CoV‐2 spike and nucleocapsid IgG levels correlated with neutralization titers |
Small sample Short follow‐up period |
| Wajnberg et al 13 | July 17, 2020 | Prospective cohort (n=51 829) | IgG ELISA against spike protein | Confirmed SARS‐CoV‐2 infection by PCR or suspected disease | 82 d (range of interval after symptom onset 52–104 d) |
38% had ELISA antibody test IgG spike protein at baseline |
Follow‐up beyond 3‐mo not available |
| Wu et al 20 | July 24, 2020 | Prospective cohort (n=349) | IgM and IgG ELISA RBD of the spike protein | Symptomatic patients with COVID‐19 | 26 wk |
IgG against spike and nucleocapsid was maintained at high positive rates and titers at 6 mo IgG positively correlated with neutralizing activity |
Missing samples at 9 and 11 wk Not all samples were assessed in virus neutralizing tests |
| Rodda et al 14 | August 15, 2020 | Prospective case‐control (n=15) | IgM, IgA, and IgG ELISA against RBD spike protein | Mildly symptomatic PCR‐confirmed COVID‐19 | 3 mo following symptoms onset (median=86 d) | Sustained immunity (including neutralizing antibodies) against SARS‐CoV‐2 | Small samples |
| Gudbjartsson et al 15 | September 1, 2020 | Prospective cohort (n=1797) | Pan‐immunoglobulin assays, antibodies against nucleocapsid, RBD, S1 | Symptomatic participants recovered from COVID‐19 | 3 mo after recovery |
Over 90% of qPCR‐positive patients tested positive with both pan‐Ig antibody and remained positive at 120 d after diagnosis Some diminution of antibody titer was observed |
Low prevalence of infection in Iceland |
| Patel et al 16 | September 4, 2020 | Prospective cohort (n=249) † | IgG ELISA against spike protein | Convenience sample of healthcare personnel at Vanderbilt University Medical Center | 60 d |
7.6% had anti‐SARS‐CoV‐2 antibodies at baseline 42% had antibodies that persisted at 60 d All participants who were positive at baseline had antibody titers decrease at 60 d |
Single center Small sample Convenience sampling Lacking information on timing of infection |
| Ibarrondo et al 17 | September 10, 2020 | Prospective cohort (n=34) | IgG ELISA against spike protein | Participants recovered from mild COVID‐19 infection | Mean 86 d (range, 44–119 d) | The estimated mean change in IgG level was an estimated half‐life of 36 d |
Short follow‐up of ≈3 mo Small sample size |
| Bolke et al 18 | September 23, 2020 | Prospective cohort (n=151) | IgA and IgG antibodies | Symptomatic participants | 120 d after the onset of symptoms | IgA and IgG levels remained unchanged |
Small sample Limited reported data |
| Terpos et al 18 | September 23, 2020 | Phase 2 prospective cohort (n=259) | IgG and IgA antibodies against spike protein S1 | Symptomatic participants or +PCR | 100 d | Rapid reduction in anti‐SARS‐CoV‐2 antibody in patients recovered from COVID‐19 | Limited data |
| Katsuna et al 18 | September 23, 2020 | Prospective cohort (n=81) | ELISA antibodies against spike protein | Symptomatic participants (mild, moderate, and severe disease) | 60 d |
Titers were higher in those with severe disease All patients showed decreased antibody titers after 60 d of symptom onset |
Limited data |
| Ripperger et al 21 | October 5, 2020 | Prospective cohort (serum samples 75) | ELISA antibodies against RBD and S2 | Symptomatic and asymptomatic PCR confirmed patients with COVID‐19 | 3 mo post disease onset |
Spike RBD and S2 and neutralizing antibodies remained detectable 5–7 mo post‐onset α‐nucleocapsid capsid titers diminished |
Seroconversion from (+) to (−) before testing No data beyond 226 d |
| Wajnberg et al 22 | October 28, 2020 | Prospective cohort (n=30 082) | IgG ELISA against spike protein | Mild‐to‐moderate confirmed SARS‐CoV‐2 infection by PCR or suspected disease or exposure to SARS‐COV‐2 | 5 mo | Anti‐spike binding titers significantly correlate with neutralization of authentic SARS‐CoV‐2 | No follow‐up date beyond 5 mo |
| Ladhani et al 23 | November 6, 2020 | Prospective cohort (n=518) | IgG ELISA against spike protein and nucleocapsid and neutralization assay | Asymptomatic and Symptomatic | Median=36 d | Most participants had neutralizing antibodies during follow‐up regardless of age and symptoms |
Survival bias Lack of serial testing to infected individuals |
| Dan et al 24 | November 16, 2020 |
Cross‐sectional (n=185) Prospective follow‐up (n=41) |
IgG ELISA against spike protein | Asymptomatic‐Mild‐moderate‐severe COVID‐19 cases | 6 mo | Spike IgG was relatively stable >6+ mo |
Many of original sample were lost to follow‐up Serial measurements with 3 time points are lacking |
| Dan et al 25 | January 6, 2020 | Prospective cohort (n=188) |
IgG ELISA against spike protein RBD IgG SARS‐CoV‐2 neutralizing antibodies |
Asymptomatic‐Mild‐moderate‐severe COVID‐19 cases | 6 mo | Spike IgG was relatively stable >6+ mo |
Serial measurements with 3 time points are lacking |
COVID‐19 indicates coronavirus disease 2019; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM,immunoglobulin M; PCR, polymerase chain reaction; RBD, receptor binding domain; and SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Immunoglobulin M results were excluded.
Six‐hundred healthcare personnel were eligible.
RNA‐Based COVID‐19 Vaccine and Serologic Testing
Walsh et al have reported on the safety and immunogenicity of 2 mRNA‐based COVID‐19 vaccine candidates. 26 To increase immunogenicity, the BNT162b1 that encodes the SARS‐CoV‐2 RBD was trimerized by adding T4 fibritin foldon domain. The BNT162b2 that encodes the SARS‐CoV‐2 full‐length spike protein was modified by 2 proline mutations. 26 , 27 Both vaccine candidates had similar immunologic response with antigen‐antibody binding and neutralizing antibodies. 26 The highest 90% neutralization titers were observed 7 or 14 days after the second dose elicited by BNT162b1 and BNT162b2. 26 The BNT162b2 was moved forward into a phase 2/3 clinical trial and now had FDA emergency use authorization (Pfizer/Biontech). The Moderna COVID‐19 vaccine also received emergency use authorization by the FDA. This vaccine uses the virus's mRNA, which instructs the human cells to produce copies of the spike protein and immune response against SARS‐CoV‐2. 28 Among participants who were between ages 56 to 70 years and those >70 years, the pseudovirus neutralization assay showed that the 50% inhibitory dilution was the highest at 43 days when 100‐μg dose of the mRNA‐1273 was administered. 28
These vaccination programs appear to have excellent efficacy with antibody titers, which is typically 100‐fold greater than convalescent plasma with associated greater neutralization capability and an acceptable safety profile. Therefore, with either of these vaccines at least early after administration, one would expect titers 100‐fold higher than those who have had native infections. The durability of these titers after several months and what this indicates for immunity remains to be determined. Because these programs illicit immunologic response against the RBD and full‐length spike protein, immunologic testing against the nucleocapsid may have an important role as orthogonal test for sero‐surveys of the population to determine if infections still take place and could reflect a warning of mutation against the first generation of vaccine‐produced antibodies. Lastly, recent preliminary evidence associated with the use of monoclonal antibody treatment in symptomatic ambulatory patients diagnosed with COVID‐19, identified that the absence of endogenous serum antibodies to SARS‐CoV‐2 (negative to both IgG and IgA anti‐S1 domain of the spike protein, and IgG anti‐nucleocapsid protein), but not the time after the onset of symptoms (all treated within 7 days), as critical to identifying patients who had a significant drop in viral loads and reduced medical encounters versus symptomatic patients with measurable serum antibodies. 29 Whether serology studies should be evaluated before the administration of monoclonal antibodies, an expensive and limited resource, will need to be determined in a larger study. 19 , 20 , 21 , 22 , 23 , 24 , 25
Limitation of Serologic Testing
The Centers for Disease Control and Prevention has stated “Serologic testing by itself should not be used to establish the presence or absence of SARS‐CoV‐2 infection or reinfection". Antibodies may not be present among those tested early in illness before antibodies develop or among those who never develop detectable antibodies following infection. In addition, the presence of antibodies may reflect previous infection and may be unrelated to the current illness. It is therefore important to recognize while knowledge continues to evolve that the presence or absence of detectable antibodies to SARS‐CoV‐2 can't be used as a surrogate to determine if a patient is potentially infectious for COVID‐19 and this needs to be determined by molecular tests to detect the virus. Despite wide availability and relatively rapid turnaround of the test results, assessment of the exact levels of antibody needed to elicit a protective immune response with neutralization ability remains unknown. Many antibody assays are available and the FDA's approval by Emergency Use Authorization means assessment isn't nearly as rigorous as the typical 510k process. Cross‐reactivity with other respiratory viral pathogens can result in false‐positive results, but these can be overcome with orthogonal testing strategies. Generally large instrument in‐vitro diagnostic manufacturers have created serology assays with high sensitivity and specificity. Finally, the heterogeneity of the immune response and waning of immunity with time that can be tracked with serology assays are fundamental issues that will influence the development of therapies, including vaccines, aimed to modulate the immune response to SARS‐CoV‐2 infection. 3
CONCLUSIONS
Understanding the clinical implications for serologic testing are critical for cardiovascular practice in the near future as vaccination and immune therapy for COVID‐19 become available in clinical practice. This in turn will affect how we manage the urgent and perhaps even eventually the elective care of cardiovascular patients. Many serologic assays that received FDA Emergency Use Authorization can be used to detect previous infections with COVID‐19 by detecting the acquired immune response to SARS‐CoV‐2, but conflicting data exist on the sustainability of this immune response to the virus. In current cardiovascular practice, antibody‐binding tests with high specificity ≥95% can provide insights into the regional probability of positive molecular tests for SARS‐CoV‐2 infection in patients undergoing cardiovascular procedures. Further research is needed to determine if a positive serology result in asymptomatic patients should trigger an evaluation for subclinical SARS‐CoV‐2 induced cardiovascular disease.
Sources of Funding
Dr Damluji receives research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging P30‐AG021334 and the National Institutes of Health‐National Heart, Lung, and Blood Institute K23‐HL153771‐01. Dr deFilippi receives funding from the National Center for Advancing Translational Science of the National Institutes of Health Award UL1TR003015.
Disclosures
Dr Christenson discloses honoraria, consulting, and scientific advisory board participation with Siemens Healthineers, Roche Diagnostic (and Medscape), Beckman‐Coulter, PixCell, Quidel Corporation, BD Diagnostics and Sphingotec. Dr Christenson receives research funding from Abbott Diagnostics, Roche Diagnostics, Siemens Healthineers, Beckman‐Coulter, Mitsubushi, Abbott Diagnostics, Quidel Corporation, Becton Dickinson, Sphingotech (NexusDx), Ortho Clinical Diagnostics and PixCell. Dr deFilippi reports consulting fees from Abbott Diagnostics, FujiRebio, Quidel, Ortho Diagnostics, Roche Diagnostics, and Siemens Healthineers. Dr Damluji has no disclosures to report.
Acknowledgments
The authors acknowledge Ms. Devon Stuart for her assistance with medical illustrations.
(J Am Heart Assoc. 2021;10:e019506. DOI: 10.1161/JAHA.120.019506.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. Peeling RW, Wedderburn CJ, Garcia PJ, Boeras D, Fongwen N, Nkengasong J, Sall A, Tanuri A, Heymann DL. Serology testing in the covid‐19 pandemic response. Lancet Infect Dis. 2020;20:e245–e249. DOI: 10.1016/S1473-3099(20)30517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Long Q‐X, Tang X‐J, Shi Q‐L, Li Q, Deng H‐J, Yuan J, Hu J‐L, Xu W, Zhang Y, Lv F‐J, et al. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med. 2020;26:1200–1204. DOI: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 3. Petherick A. Developing antibody tests for SARS‐CoV‐2. Lancet. 2020;395:1101–1102. DOI: 10.1016/S0140-6736(20)30788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L. Characterization of the receptor‐binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17:613–620. DOI: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bryant JE, Azman AS, Ferrari MJ, Arnold BF, Boni MF, Hayford K, Luquero FJ, Mina MJ, Rodriguez‐Barraquer I, et al. Serology for SARS‐CoV‐2: apprehensions, opportunities, and the path forward. Sci Immunol. 2020;5:eabc6347. DOI: 10.1126/sciimmunol.abc6347. [DOI] [PubMed] [Google Scholar]
- 6. Lisboa Bastos M, Tavaziva G, Abidi SK, Campbell JR, Haraoui L‐P, Johnston JC, Lan Z, Law S, MacLean E, Trajman A, et al. Diagnostic accuracy of serological tests for covid‐19: systematic review and meta‐analysis. BMJ. 2020;370:m2516. DOI: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, Jiang K, Arunkumar GA, Jurczyszak D, Polanco J, et al. A serological assay to detect SARS‐CoV‐2 seroconversion in humans. Nat Med. 2020;26:1033–1036. DOI: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Current Status of Antibody Testing in the United States. Available at: https://www.Cdc.Gov/coronavirus/2019‐ncov/lab/resources/antibody‐tests‐guidelines.Html#anchor_1590280385631. Accessed January 21, 2021.
- 9.Interim Guidance for Use of Pooling Procedures in SARS‐CoV‐2 Diagnostic, Screening, and Surveillance Testing. Available at: https://www.Cdc.Gov/coronavirus/2019‐ncov/lab/pooling‐procedures.Html. Accessed January 21, 2021.
- 10.Introduction to Public Health Surveillance. Available at: https://www.Cdc.Gov/publichealth101/surveillance.Html. Accessed January 21, 2021.
- 11. Steensels D, Oris E, Coninx L, Nuyens D, Delforge ML, Vermeersch P, Heylen L. Hospital‐wide SARS‐CoV‐2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324:195–197. DOI: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Zhang LU, Sang L, Ye F, Ruan S, Zhong B, Song T, Alshukairi AN, Chen R, Zhang Z, et al. Kinetics of viral load and antibody response in relation to COVID‐19 severity. J Clin Invest. 2020;130:5235–5244. DOI: 10.1172/JCI138759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wajnberg A, Amanat F, Firpo A, Altman D, Bailey M, Mansour M, McMahon M, Meade P, Mendu DR, Muellers K, et al. SARS‐CoV‐2 infection induces robust, neutralizing antibody responses that are stable for at least three months. medRxiv. 2007:2020.2007.2014.20151126.
- 14. Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PM, Thouvenel C, Takehara KK, Eggenberger J, Hemann EA, Waterman HR, et al. Functional SARS‐CoV‐2‐specific immune memory persists after mild COVID‐19. medRxiv. 2020:2020.2008.2011.20171843. [DOI] [PMC free article] [PubMed]
- 15. Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E, Arnthorsson AO, Helgason D, Bjarnadottir K, Ingvarsson RF, et al. Humoral immune response to SARS‐CoV‐2 in Iceland. N Engl J Med. 2020;383:1724–1734. DOI: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel MM, Thornburg NJ, Stubblefield WB, Talbot HK, Coughlin MM, Feldstein LR, Self WH. Change in antibodies to SARS‐CoV‐2 over 60 days among health care personnel in Nashville, Tennessee. JAMA. 2020;324:1781–1782. DOI: 10.1001/jama.2020.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, Elliott J, Hofmann C, Hausner MA, Ferbas KG, Tobin NH, Aldrovandi GM, Yang OO. Rapid decay of anti–SARS‐CoV‐2 antibodies in persons with mild Covid‐19. N Engl J Med. 2020;383:1085–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loss of anti–SARS‐CoV‐2 antibodies in mild Covid‐19. N Engl J Med. 2020;383:1697–1698. DOI: 10.1056/NEJMc2027051. [DOI] [PubMed] [Google Scholar]
- 19. Premkumar L, Segovia‐Chumbez B, Jadi R, Martinez DR, Raut R, Markmann A, Cornaby C, Bartelt L, Weiss S, Park Y, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS‐CoV‐2 patients. Sci Immunol. 2020;5:eabc8413. DOI: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu J, Liang B, Chen C, Wang H, Fang Y, Shen S, Yang X, Wang B, Chen L, Chen Q, et al. SARS‐CoV‐2 infection induces sustained humoral immune responses in convalescent patients following symptomatic covid‐19. medRxiv. 2020:2020.2007.2021.20159178. [DOI] [PMC free article] [PubMed]
- 21. Ripperger TJ, Uhrlaub JL, Watanabe M, Wong R, Castaneda Y, Pizzato HA, Thompson MR, Bradshaw C, Weinkauf CC, Bime C, et al. Orthogonal SARS‐CoV‐2 serological assays enable surveillance of low‐prevalence communities and reveal durable humoral immunity. Immunity. 2020;53:925–933.e924. DOI: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, McMahon M, Meade P, Mendu DR, Muellers K, et al. Robust neutralizing antibodies to SARS‐CoV‐2 infection persist for months. Science. 2020;370:1227–1230. DOI: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ladhani SN, Jeffery‐Smith A, Patel M, Janarthanan R, Fok J, Crawley‐Boevey E, Vusirikala A, Fernandez Ruiz De Olano E, Perez MS, Tang S, et al. High prevalence of SARS‐CoV‐2 antibodies in care homes affected by COVID‐19: prospective cohort study, England. EClinicalMedicine. 2020;28:100597. DOI: 10.1016/j.eclinm.2020.100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dan JM, Mateus J, Kato Y, Hastie KM, Faliti C, Ramirez SI, Frazier A, Yu ED, Grifoni A, Rawlings SA, et al. Immunological memory to SARS‐CoV‐2 assessed for greater than six months after infection. bioRxiv. 2020:2020.2011.2015.383323.
- 25. Dan JM, Mateus J, Kato YU, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021:eabf4063. DOI: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. DOI: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603–2615. DOI: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, et al. Safety and immunogenicity of SARS‐CoV‐2 mRNA‐1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. DOI: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, et al. REGN‐COV2, a neutralizing antibody cocktail, in outpatients with Covid‐19. N Engl J Med. 2021;384:238–251. DOI: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]


