Abstract
Background
The significant morbidity associated with systolic heart failure makes it imperative to identify patients with a reversible cause. We thus sought to evaluate the proportion of patients who received an ischemic evaluation after a hospitalization for new‐onset systolic heart failure.
Methods and Results
Patients admitted with a new diagnosis of heart failure and a reduction in left ventricular ejection fraction (≤40%) were identified in the VA Healthcare System from January 2006 to August 2017. Among those who survived 90 days without a readmission, we evaluated the proportion of patients who underwent an ischemic evaluation. We identified 9625 patients who were admitted with a new diagnosis of systolic heart failure with a concomitant reduction in ejection fraction. A minority of patients (3859, 40%) underwent an ischemic evaluation, with significant variation across high‐performing (90th percentile) and low‐performing (10th percentile) sites (odds ratio, 3.79; 95% CI, 2.90–4.31). Patients who underwent an evaluation were more likely to be treated with angiotensin‐converting enzyme inhibitors (75% versus 64%, P<0.001) or beta blockers (92% versus 82%, P<0.001) and subsequently undergo percutaneous (8% versus 0%, P<0.001) or surgical (2% versus 0%, P<0.001) revascularization. Patients with an ischemic evaluation also had a significantly lower adjusted hazard of all‐cause mortality (hazard ratio, 0.54; 95% CI, 0.47–0.61) compared with those without an evaluation.
Conclusions
Ischemic evaluations are underutilized in patients admitted with heart failure and a new reduction in left ventricular systolic function. A focused intervention to increase guideline‐concordant care could lead to an improvement in clinical outcomes.
Keywords: coronary artery disease, ischemic evaluation, revascularization, systolic heart failure
Subject Categories: Quality and Outcomes, Health Services, Cardiomyopathy
Nonstandard Abbreviations and Acronyms
- IE
ischemic evaluation
- VA
Veterans Affairs
Clinical Perspective
What Is New?
Ischemic evaluations are performed in a minority of patients admitted for new‐onset systolic heart failure, with significant variation across institutions.
The performance of an ischemic evaluation in this population is associated with an increased use of guideline‐indicated medical therapy for heart failure.
The performance of an ischemic evaluation in this population is associated with a reduction in the adjusted hazard ratio for all‐cause mortality.
What Are the Clinical Implications?
There is an opportunity to standardize practices to improve guideline‐concordant care—including routine ischemic evaluations—for patients admitted with new‐onset systolic heart failure.
Heart failure is associated with significant morbidity and mortality, resulting in a large burden to our healthcare system. Previous research has demonstrated that there is a significant overall lifetime risk of developing systolic heart failure among adults in the United States. 1 Concordant with this finding, the prevalence of heart failure has dramatically increased over the past 2 decades, resulting in substantially higher rates of hospitalization and mortality. 2 , 3 The costs in caring for affected patients are also projected to significantly increase in the United States, from $21 to $53 billion over the next 20 years. 4 Identifying the underlying cause of heart failure is thus imperative to ensure that the most appropriate medical and procedural therapies are initiated to reduce the overall burden of this disease.
Coronary artery disease is among the most common causes of heart failure, affecting >50% of patients with this condition. 5 , 6 A recent meta‐analysis suggests that both percutaneous and surgical revascularizations are associated with a reduction in mortality among those with significant coronary artery disease and heart failure with reduced ejection fraction. 7 , 8 With this in mind, the current professional society guidelines strongly recommend an ischemic evaluation (IE) for all patients with newly diagnosed systolic dysfunction. 9 Despite this recommendation, preliminary studies have suggested a gross underutilization of both noninvasive and invasive modalities to evaluate for coronary artery disease in these patients. 10 A more granular investigation evaluating the site‐level variation in IEs and its downstream consequences in clinical management and outcomes has not yet been performed in a national healthcare system.
With this in mind, we sought to evaluate the rates of IE among those diagnosed with heart failure with reduced ejection fraction. Further investigations were designed to evaluate site‐level variation in testing as well as the association between an IE and clinical outcomes among patients with this condition. To do so, we leveraged data from the largest integrated healthcare system in the United States: the Veterans Affairs (VA) Healthcare System. 11
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request, though they will be subject to the stringent data privacy rules of the VA Healthcare System and US Government.
Population
All patients presenting to Veterans Health Administration Hospitals with a new diagnosis of systolic heart failure from January 2006 to August 2017 were included in the analysis. For the purpose of this project, hospital admission for a new heart failure diagnosis was determined using administrative billing codes. A new heart failure admission was defined as a primary discharge diagnosis of heart failure using the International Classification of Diseases, Ninth Revision (ICD‐9: 428.XX) or 10th Revision (ICD‐10: I50.1/I50.20/I50.23/I50.30–3/I50.40–3/I50.9) codes, consistent with prior studies. 10 , 12 Subjects who met these criteria were then queried for documentation of newly reduced left ventricular systolic function via echocardiography that occurred 90 days before their admission or 10 days after their admission, defined as an ejection fraction ≤40% consistent with the recommendations in current professional society guidelines. 9 A new decline was defined as a reduction in the reported ejection fraction compared with a prior echocardiogram within the VA Healthcare System. Patients without a prior echocardiogram and a reported ejection fraction of ≤40% were considered to have a new diagnosis of heart failure with reduced ejection fraction. Only the index report of an ejection fraction ≤40% was included, to ensure that patients with a chronic reduction in ejection fraction documented over multiple studies were not repeatedly incorporated into the cohort. Patients with a diagnosis of diastolic heart failure were excluded, as were patients who had an IE within 1 year before the index hospitalization. Patients who were admitted within the study period (2006–2017) and who had a prior hospital admission for heart failure since 2002 were also excluded, because the hospitalization measured in this study would not reflect the index admission for their condition. Finally, patients who were discharged with hospice or were rehospitalized or died within 90 days of the index hospitalization were excluded from the subsequent analysis to ensure that only patients who were healthy enough to benefit from an IE were ultimately included in the analytic cohort. This project was approved by the Colorado Multiple Institution Review Board, which includes the Rocky Mountain Regional VA Medical Center, with a waiver of informed consent.
Measurements
Patient characteristics were abstracted from the linked electronic medical record, including documentation of left ventricular ejection fraction by echocardiography. An IE was assessed within 90 days of the qualifying hospital admission for new heart failure with reduced ejection fraction, to allow a reasonable period of time from diagnosis to evaluation. Previously validated procedural codes 13 were used to determine whether an IE was performed within this time frame, which included exercise stress tests, nuclear stress tests, echocardiographic stress tests, computed tomography angiography or coronary angiography (Table S1), and could occur on an inpatient or outpatient basis. A revascularization procedure was also considered to include an IE, including percutaneous coronary intervention or coronary artery bypass grafting (Table S2), because it would be unlikely that a patient would undergo revascularization without a concomitant IE. All outcomes were assessed within the VA Healthcare System as well as outside the VA Healthcare System using fee‐basis data. Coronary angiography and percutaneous revascularization within the VA Healthcare System were ascertained using data from the VA Clinical Assessment, Reporting and Tracking Program as previously described. 14
Outcomes
Clinical outcomes were assessed among patients with new heart failure with reduced ejection fraction, including rehospitalization for heart failure, rehospitalization for myocardial infarction (MI), and mortality (Table S3). Mortality was ascertained from the VA Information Resource Center Vital Status File which includes data from the Beneficiary Identification Record Locator Subsystem Death File, VA Medicare Vital Status File, and the Social Security Administration Death Master File. 15
Statistical Analysis
The temporal trends in patients admitted with heart failure were reported by calendar year, stratified by the performance of any IE. Subsequently, the demographic and clinical characteristics were compared among those with and without an IE. To assess the comparative effectiveness of the treatment (receiving an IE within 90 days of diagnosis) on the outcomes (mortality and the composite outcome of mortality or readmission for heart failure or MI), inverse probability weighting was implemented to create a balanced cohort, followed by Cox proportional hazard models to assess the effect of the treatment. Inverse probability weighting creates a data set where the covariates between treated and controlled are balanced, thus adjusting for confounding, by weighting individuals according to their propensity score. Site variation was further assessed with the reference effect measure, to demonstrate the effect of site compared with the effect of the treatment. To calculate the reference effect measure, a random effect for site is added to the logistic regression model with the outcomes of interest as dependent variables and same covariates used to calculate the propensity score as dependent variables. Using the variance of this random effect, the difference in odds ratios between a high‐performing (90th percentile) and low‐performing (10th percentile) site is calculated as previously described. 16 Clinical outcomes were assessed after 90 days from the index presentation, using inverse probability weighting to control for potential confounders. Gradient boosted machines were used to estimate propensity scores, with demographic information (age/sex/race/ethnicity/body mass index) and medical comorbidities (cerebrovascular disease/chronic obstructive pulmonary disease/chronic kidney disease/coagulopathy/diabetes mellitus/depression/ejection fraction/hyperlipidemia/hypertension/prior bypass/prior IE/prior percutaneous intervention/peripheral artery disease/substance abuse) included as covariates. Additional covariates were included to account for the prior use of home health services to control for patient frailty, as well as the year of presentation to account for temporal trends. Data were trimmed to remove individuals with extreme propensity scores (<0.05 or >0.95) as depicted in Figure S1. Differences between the 2 weighted subpopulations were assessed with absolute standardized differences, with a value <0.1 indicating balance. 17 Using this data set, the estimated hazard ratio (HR) for the effect of IE on death or a composite including death and rehospitalization for heart failure or MI was calculated with a Cox proportional hazard model using robust standardized errors. A sensitivity analysis was subsequently performed to evaluate the effect of an unmeasured confounder as previously described. 18 To do so, we calculated an adjusted HR and 95% CI from a model that assumed the presence of a hypothetical confounder with known prevalence and association with the outcome, to determine the magnitude of influence that would be needed to completely account for the observed relationship. 18 All analyses were performed with R 3.5.1 (The R Foundation for Statistical Computing, Vienna, Austria). The Toolkit for Weighting and Analysis of Nonequivalent Groups (TWANG) R package was used in the calculation of propensity scores. A P<0.05 was considered statistically significant.
Results
Population
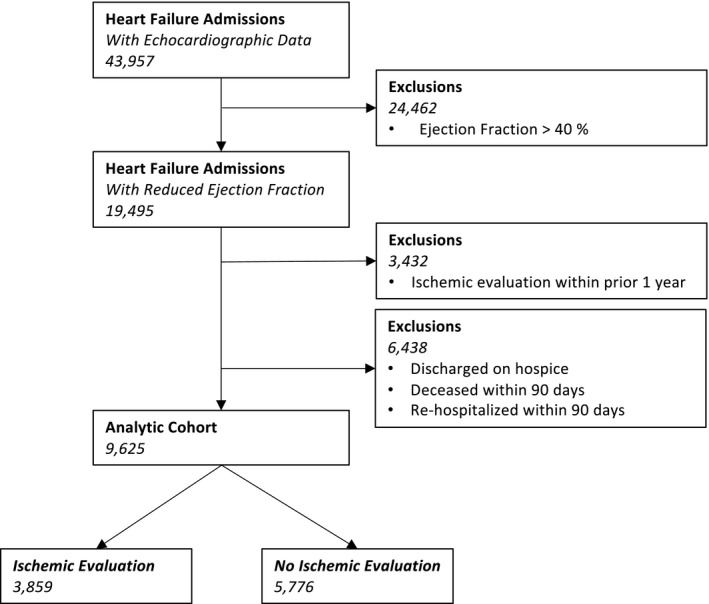
During the time period under investigation, there were 43 957 patients admitted with a primary diagnosis of heart failure and a documented echocardiogram during the index admission. A minority (19 495, 44%) were found to have a new reduction in left ventricular systolic function. After excluding patients with a prior IE within 1 year (3432) and those who died or were rehospitalized within 90 days of the index presentation (6438), we identified 9625 patients who constituted the analytic cohort. A minority of patients in the analytic cohort (3859, 40%) underwent an IE within 90 days of hospitalization (Figure 1).
Figure 1. Cohort construction.
Temporal Trends
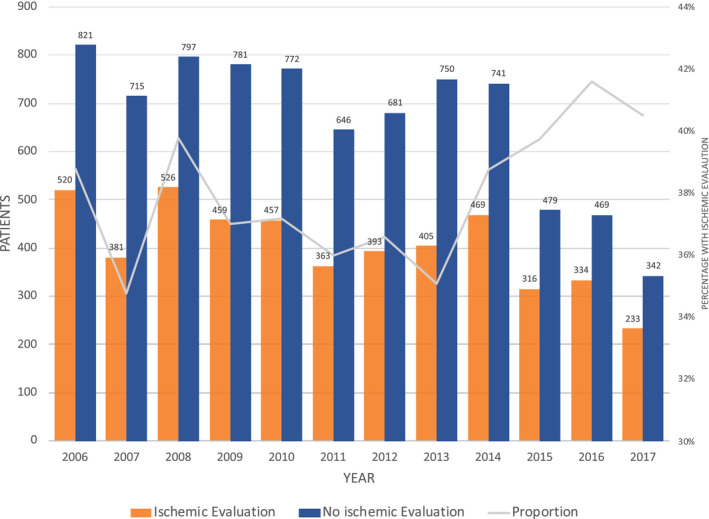
The temporal trends in hospital admission for a new diagnosis of heart failure with reduced ejection fraction are shown in Figure 2, stratified by the performance of an IE. As shown, annual admissions for new systolic heart failure in this cohort declined 26.3 patients/y between 2006 and 2017 (linear trend P=0.01), though the proportion of patients who underwent an IE slightly increased from 38% at the beginning of the study to 41% at its conclusion.
Figure 2. Temporal trends in admissions for heart failure with reduced ejection fraction, showing the number of patients admitted with and without an ischemic evaluation as well as the proportion (line) stratified by time.
Patient Characteristics
The patient characteristics with newly diagnosed heart failure with reduced ejection fraction are demonstrated in Table 1, stratified by IE. As shown, unweighted patients who underwent an IE were younger (66 versus 71 years, P<0.001) and were less likely to have cerebrovascular disease (7% versus 12%, P<0.001), chronic kidney disease (14% versus 22%, P<0.001), diabetes mellitus (39 versus 42, P<0.001) or previously receive home health services (5% versus 6%, P=0.010) compared with those who did not undergo an IE. Propensity weighting decreased the differences in these subpopulations, with all characteristics exhibiting an absolute standardized difference <0.10 after weighting.
Table 1.
Demographic and Clinical Characteristics
| Unweighted | Weighted | |||||
|---|---|---|---|---|---|---|
| No Ischemic Evaluation | Ischemic Evaluation | P Value | No Ischemic Evaluation | Ischemic Evaluation | Standardized Difference | |
| N=5766 | N=3859 | N=9155 | N=8600 | |||
| Age, y | 71 (62–81) | 65 (59–74) | <0.001 | 68 (61–79) | 68 (61–78) | 0.076 |
| Male | 5696 (99) | 43 796 (98) | 0.102 | 9022 (98) | 8478 (99) | 0.003 |
| Race | ||||||
| White | 4083 (71) | 2650 (69) | 0.026 | 6346 (69) | 6007 (70) | 0.011 |
| Hispanic | 288 (5) | 214 (6) | 0.253 | 453 (5) | 463 (5) | 0.020 |
| Medical comorbidities | ||||||
| Cerebrovascular disease | 667 (12) | 285 (7) | <0.001 | 941 (10) | 783 (9) | 0.040 |
| Chronic kidney disease | 1253 (22) | 527 (14) | <0.001 | 1748 (19) | 1462 (17) | 0.054 |
| Dementia | 347 (6) | 84 (2) | <0.001 | 468 (5) | 242 (3) | 0.118 |
| Obstructive lung disease | 549 (10) | 244 (6) | <0.001 | 772 (8) | 621 (7) | 0.046 |
| Coagulopathy | 1509 (26) | 722 (19) | <0.001 | 2188 (24) | 1883 (22) | 0.048 |
| Depression | 435 (8) | 290 (8) | 0.989 | 694 (8) | 647 (8) | 0.002 |
| Diabetes mellitus | 2443 (42) | 1522 (39) | 0.005 | 3797 (42) | 3490 (41) | 0.018 |
| Hypertension | 4185 (73) | 2411 (63) | <0.001 | 6366 (70) | 5797 (67) | 0.046 |
| Hyperlipidemia | 3173 (55) | 1833 (48) | <0.001 | 4859 (53) | 4401 (51) | 0.038 |
| Peripheral artery disease | 872 (15) | 401 (10) | <0.001 | 1251 (14) | 1053 (12) | 0.042 |
| Substance abuse | 277 (5) | 190 (5) | 0.827 | 441 (5) | 374 (4) | 0.022 |
| Prior cardiovascular studies | ||||||
| Prior ischemic evaluation (>1 y) | 709 (12) | 297 (8) | <0.001 | 990 (11) | 809 (9) | 0.047 |
| Prior ejection fraction | 322 (6) | 451 (12) | <0.001 | 642 (7) | 732 (9) | 0.010 |
| Prior revascularization | ||||||
| Prior bypass surgery | 40 (1) | 12 (1) | 0.018 | 56 (1) | 30 (1) | 0.037 |
| Prior percutaneous intervention | 74 (1) | 34 (1) | 0.082 | 104 (1) | 102 (1) | 0.005 |
| Prior home health services | 338 (6) | 179 (5) | 0.010 | 508 (6) | 433 (5) | 0.023 |
Data are presented as median (interquartile range) or number (percent).
Ischemic Evaluation and Site Variation
A minority of patients (3859, 40%) underwent an IE, with the largest proportion of patients undergoing coronary angiography (45%) or isolated noninvasive stress testing (40%) as shown in Table 2. Among patients who underwent an IE, the majority (2197, 63%) had the testing performed during the index presentation. There was significant site variation in the performance of an IE after adjusting for patient characteristics, wherein a patient treated at a site more likely to perform an evaluation (90th percentile) had 3.79 (95% CI, 2.90–4.33) greater odds of undergoing the evaluation compared with a similar patient treated at a site less likely (10th percentile) to undergo an evaluation. The proportion of patients who underwent an IE was significantly greater at a site with a cardiac catheterization laboratory (42%) than the proportion of patients who presented to a site without this service (34%, P<0.001).
Table 2.
Ischemic Evaluation, Subsequent Management, and Clinical Outcomes
| No Ischemic Evaluation (N=5766) | Ischemic Evaluation (N=3849) | P Value | |
|---|---|---|---|
| Ischemic evaluation | |||
| Ischemic evaluation <90 d | 0 (0) | 3849 (100) | <0.001 |
| Invasive evaluation | 0 (0) | 1742 (45) | |
| Noninvasive evaluation | 0 (0) | 1545 (40) | |
| Both | 0 (0) | 572 (15) | |
| None | 5766 (100) | 0 (0) | |
| Medical management | |||
| ACE inhibitors | 3707 (64) | 2884 (75) | <0.001 |
| Beta blockers | 4727 (82) | 3556 (92) | <0.001 |
| Statins | 3142 (55) | 2449 (64) | <0.001 |
| Revascularization (within 90 d) | |||
| Percutaneous intervention | 0 (0) | 291 (8) | <0.001 |
| Coronary artery bypass grafting | 0 (0) | 90 (2) | <0.001 |
| Mortality (1 y) | 1108 (19) | 355 (9) | <0.001 |
| Composite (1 y) | 2014 (35) | 916 (24) | <0.001 |
| Hospitalization heart failure | 1197 (21) | 640 (17) | <0.001 |
| Hospitalization myocardial infarction | 107 (2) | 63 (2) | 0.462 |
Data are presented as number (percent). ACE indicates angiotensin‐converting enzyme.
Subsequent Management
Patients who underwent an IE were more likely to be treated with guideline‐directed medical therapy for cardiomyopathy, with an increasing proportion of patients receiving beta blockers (92% versus 82%, P<0.001) and angiotensin‐converting enzyme inhibitors (75% versus 64%, P<0.001) within 90 days of discharge compared with those who did not undergo an IE. Similarly, percutaneous (8% versus 0%, P<0.001) or surgical revascularization (2% versus 0%, P<0.001) within 90 days was also significantly higher among those who underwent an IE after their index hospitalization (Table 2).
Clinical Outcomes
The overall mortality for the analytic cohort was high (15%), with a significantly lower mortality rate among those who received an IE (9%) compared with those who did not (19%, P<0.001) at 1 year of follow‐up (Table 2). The unadjusted hazard for mortality between the 2 groups was similar (HR, 0.45; 95% CI, 0.40–0.51) and persisted after propensity weighting (HR, 0.54; 95% CI, 0.47–0.61), as those with an IE had a significantly lower adjusted hazard of all‐cause mortality compared with those without an IE (Figure 3). Similarly, the proportion of patients who had a composite outcome of death and rehospitalization for heart failure or MI was significantly lower among those with an IE (25%) compared with those without one (34%, P<0.001) at 1 year of follow‐up (Table 2). The unadjusted hazard for the composite outcome between the 2 groups followed these trends (HR, 0.63; 95% CI, 0.58–0.68) and persisted after propensity weighting (HR, 0.69; 95% CI, 0.64–0.75), as those with an IE had a significantly lower hazard of composite of rehospitalization or death compared with those without an IE (Figure 4). The relationship between site‐level IE by quartiles and clinical outcomes is reproduced in Figure S2, demonstrating a consistent benefit for patients who underwent an IE across all sites.
Figure 3. Mortality among propensity‐weighted patients admitted with heart failure and reduced ejection fraction, stratified by an ischemic evaluation.
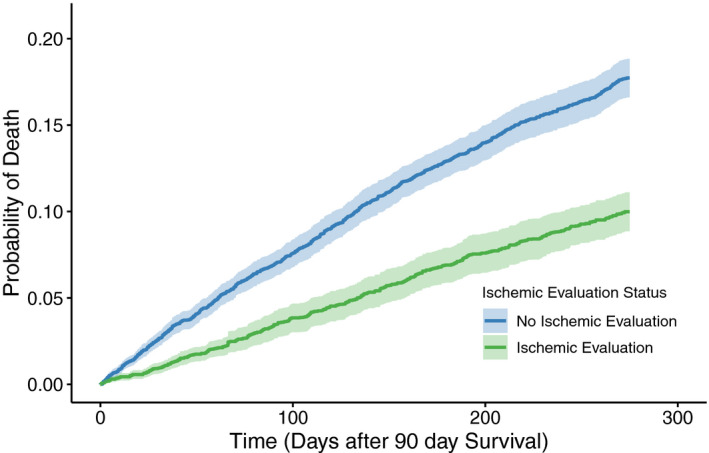
The hazard ratio (HR) for mortality was reduced 46% (HR, 0.54; 95% CI, 0.47–0.61) among patients with an ischemic evaluation compared with those without an ischemic evaluation, with the comparison beginning 90 days after the index presentation to mitigate the immortal time bias.
Figure 4. Composite of mortality and rehospitalization for heart failure or myocardial infarction among propensity‐weighted patients admitted with heart failure and reduced ejection fraction, stratified by an ischemic evaluation.
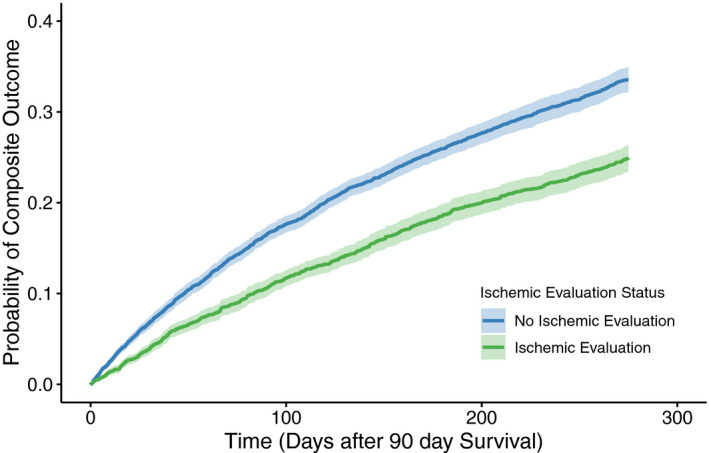
The hazard ratio (HR) for the composite was reduced 31% (HR, 0.69; 95% CI, 0.64–0.75) among patients with an ischemic evaluation compared with those without an ischemic evaluation, with the comparison beginning 90 days after the index presentation to mitigate the immortal time bias.
Sensitivity Analysis
A sensitivity analysis was performed to assess the impact of an unmeasured confounder for mortality. Using the measured HR for mortality, it would require an unmeasured confounder with a HR of 3.999 and a prevalence of 25% in the non‐IE subpopulation to completely negate the mortality difference between the 2 groups (Figure S3).
Discussion
The present study evaluated the proportion of patients who received an IE after a hospitalization for new‐onset systolic heart failure, and the association of this evaluation with clinical outcomes. As the data demonstrate, a minority of patients hospitalized for new systolic heart failure undergo an IE within 90 days of their index presentation with significant variation across sites. The performance of an IE had important downstream consequences, because those who received ischemic testing had a much greater likelihood of being treated with guideline‐indicated medical therapies for cardiomyopathy and undergoing percutaneous or surgical coronary revascularization. Furthermore, propensity‐weighted patients who underwent an IE had a 46% reduction in the hazard for mortality and a 31% reduction in the hazard for death and rehospitalization for heart failure or MI compared with patients who were not assessed for underlying obstructive coronary artery disease. These data reinforce the current guidelines supporting IEs in patients with new systolic heart failure, and emphasize the importance of developing standardized practices to improve the overall quality of care patients with heart failure receive.
An IE is performed in a minority of patients presenting with new‐onset systolic heart failure. Previous research has demonstrated that coronary artery disease remains one of the most common causes of systolic heart failure in the developed world. 5 , 6 , 19 Because of this, professional society guidelines strongly recommend an IE for all patients with newly diagnosed systolic dysfunction. 9 These recommendations have not been widely embraced, however, because preliminary data utilizing administrative billing codes have demonstrated that a minority of patients with heart failure undergo ischemic testing in the community. 10 It is important to note that these billing codes, however, may have identified a heterogeneous population with multiple medical conditions rather than a specific population with systolic heart failure. A recently published analysis utilizing echocardiographic data to identify a homogeneous population of older patients with heart failure with reduced ejection fraction did confirm that a minority of patients (39%) underwent an IE. 20 The present study corroborates this in a national healthcare system among all age groups, whereby a similar minority of patients (40%) hospitalized with a new diagnosis of heart failure and reduced ejection fraction undergo an IE within 90 days of their hospitalization. Furthermore, the performance of an IE is subject to significant site‐level variation, whereas a similar patient could have greater than a 4‐fold increased chance of undergoing an IE simply by presenting to an alternative site. This is similar to the site‐level variation identified in another community‐based cohort of patients with heart failure over a decade ago, suggesting little progress in standardizing care for this population. 21 These data reinforce the importance of developing systems of care that improve guideline‐concordant practices for patients with heart failure, especially in light of the downstream consequences for patients without ischemic testing.
An IE is associated with improved clinical outcomes among patients admitted with a new diagnosis of systolic heart failure. A diagnostic test is unlikely to significantly improve clinical outcomes unless it is associated with downstream therapeutic interventions. Previous research has suggested that performance of an IE during the index hospitalization for acute heart failure is associated with an increased utilization of guideline‐directed medical therapy for cardiovascular disease. 22 , 23 Furthermore, revascularization is unlikely to occur unless a patient with heart failure has been evaluated for coronary artery disease, and both percutaneous and surgical revascularization are associated with an improvement in left ventricular systolic function and decrease in mortality among those with systolic heart failure. 7 , 8 , 24 Supporting this, the present study demonstrates that the proportion of patients treated with guideline‐indicated medical therapies for cardiomyopathy was significantly greater among those who received an IE. Similarly, patients who underwent an IE were also significantly more likely to later undergo percutaneous or surgical revascularization. The increased utilization of appropriate medical and procedural therapies for heart failure among those who received an IE may have contributed to an improvement in clinical outcomes, because the data demonstrated a 46% reduction in the hazard for mortality and a 31% reduction in the hazard for a composite that included rehospitalization for heart failure or MI compared with those who did not undergo similar testing. These results are after excluding those who had a rehospitalization or death within the first 90 days of the index presentation, reducing the impact of immortal time bias. A sensitivity analysis suggested that a significant unmeasured confounder (HR, 3.999) with a high prevalence (25%) would be necessary to completely negate these findings. Given the substantial differences in clinical outcomes, it is imperative to better understand the lack of IEs in patients with heart failure.
The reasons for avoiding ischemic testing in patients with heart failure are likely multifactorial. Previous data have suggested that patients with heart failure have a high mortality rate, with recent declines in cardiovascular mortality offset by increases in noncardiovascular mortality. 25 Some patients with heart failure may be deemed too sick to undergo further diagnostic testing, including an evaluation for the underlying cause of their heart failure. The present analysis attempted to account for these patients by excluding anyone who died or was rehospitalized within 90 days of the index presentation from the analytic cohort. Individuals discharged to hospice care after the index presentation were also excluded. Despite this, the utilization of ischemic testing remains low even after excluding those patients with poor prognoses. A lack of ischemic testing in the remaining cohort may be a result of the services available, because ischemic testing was significantly less common for patients who presented to sites without a cardiac catheterization laboratory. Discoordination of care as a patient transits from the acute inpatient setting to chronic outpatient setting may also play a role, because the majority of patients who received an IE did so during their index hospitalization. Programs developed to enhance the transition for patients with heart failure may improve the rates of IE after discharge, and overall evidence‐based medical therapy for cardiomyopathy. 26 These factors obscure the larger issue, though, because the data suggest that patients admitted with new‐onset heart failure and a reduction in ejection fraction are not receiving guideline‐concordant care. Prior studies have suggested that physician concordance with guidelines is diminished because of decreased awareness and familiarity with their recommendations. 27 The present study should highlight the importance of guideline‐recommended IEs for patients with new systolic heart failure and its potential to improve clinical outcomes.
Limitations
These data should be interpreted in the context of several limitations. Patients were initially identified using administrative billing codes, which may be subject to errors. The data for the present study were augmented with clinical information derived from echocardiogram reports, increasing the specificity of the patients included in the analytic cohort. It is important to note that we do not have access to data from echocardiograms that were performed outside the integrated healthcare system, such that a small proportion of patients included in the cohort could have had a reduced ejection fraction identified at another healthcare facility before their index presentation. Among those included in the analytic cohort, propensity weighting sought to balance the medical comorbidities among those with and without an IE. Covariates for hard‐to‐determine markers of frailty such as the utilization of home health care were also included in attempts to provide a balanced population for comparison. The possibility of residual confounding remains, particularly among clinical characteristics that are not easily captured in administrative and clinical data. We attempted to create a more homogeneous population for comparison by excluding highly morbid patients with heart failure, by eliminating patients who died or were subsequently rehospitalized within 3 months of the index presentation. Though a difference in death rates was identified between the 2 groups, the cause of death is not readily available. Because of this, we are not able to accurately ascertain whether the difference in mortality is because of cardiovascular‐ or noncardiovascular‐related deaths. Finally, the data represent the clinical practice of the VA Healthcare System and may not reflect other clinical settings. Further studies in alternative data sets or prospective clinical trials would serve to confirm or refute our findings.
Conclusions
Ischemic evaluations are underutilized in patients admitted with heart failure and a new reduction in left ventricular systolic function despite current professional society guidelines. The association of an IE with clinical outcomes suggests an opportunity for a focused intervention to improve care for this patient population.
Sources of Funding
None.
Disclosures
Dr Valle receives unrelated consulting fees from Philips Medical, Medtronic, and Cardiovascular Systems Incorporated. Dr Ho is supported by grants from NHLBI, VA HSR&D, and University of Colorado School of Medicine. He has a research agreement with Bristol‐Myers Squibb through the University of Colorado. He serves as the Deputy Editor for Circulation: Cardiovascular Quality and Outcomes. Dr Waldo receives unrelated investigator‐initiated research support from Abiomed, Cardiovascular Systems Incorporated, Merck Pharmaceuticals, Janssen Pharmaceuticals, and the National Institutes of Health. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figures S1–S3
Acknowledgments
The authors thank Gary Grunwald, PhD and Anna Baron, PhD for their assistance with statistical analysis. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
(J Am Heart Assoc. 2021;10:e019452. DOI: 10.1161/JAHA.120.019452.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019452
For Sources of Funding and Disclosures, see page 9.
References
- 1. Lloyd‐Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D; Framingham Heart Study . Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. DOI: 10.1161/01.CIR.0000039105.49749.6F. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 3. Anderson HV, Weintraub WS, Radford MJ, Kremers MS, Roe MT, Shaw RE, Pinchotti DM, Tcheng JE. Standardized cardiovascular data for clinical research, registries, and patient care: a report from the Data Standards Workgroup of the National Cardiovascular Research Infrastructure project. J Am Coll Cardiol. 2013;61:1835–1846. DOI: 10.1016/j.jacc.2012.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, et al.; American Heart Association Advocacy Coordinating Committee, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Stroke Council . Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. DOI: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ling LF, Marwick TH, Flores DR, Jaber WA, Brunken RC, Cerqueira MD, Hachamovitch R. Identification of therapeutic benefit from revascularization in patients with left ventricular systolic dysfunction: inducible ischemia versus hibernating myocardium. Circ Cardiovasc Imaging. 2013;6:363–372. DOI: 10.1161/CIRCIMAGING.112.000138. [DOI] [PubMed] [Google Scholar]
- 6. Ammirati E, Rimoldi OE, Camici PG. Is there evidence supporting coronary revascularization in patients with left ventricular systolic dysfunction? Circ J. 2011;75:3–10. DOI: 10.1253/circj.CJ-10-1164. [DOI] [PubMed] [Google Scholar]
- 7. Wolff G, Dimitroulis D, Andreotti F, Kołodziejczak M, Jung C, Scicchitano P, Devito F, Zito A, Occhipinti M, Castiglioni B, et al. Survival benefits of invasive versus conservative strategies in heart failure in patients with reduced ejection fraction and coronary artery disease: a meta‐analysis. Circ Heart Fail. 2017;10:e003255. DOI: 10.1161/CIRCHEARTFAILURE.116.003255. [DOI] [PubMed] [Google Scholar]
- 8. DeVore AD, Velazquez EJ. Rethinking revascularization in left ventricular systolic dysfunction. Circ Heart Fail. 2017;10:e003770. DOI: 10.1161/CIRCHEARTFAILURE.116.003770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. DOI: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 10. Doshi D, Ben‐Yehuda O, Bonafede M, Josephy N, Karmpaliotis D, Parikh MA, Moses JW, Stone GW, Leon MB, Schwartz A, et al. Underutilization of coronary artery disease testing among patients hospitalized with new‐onset heart failure. J Am Coll Cardiol. 2016;68:450–458. DOI: 10.1016/j.jacc.2016.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cowper DC, Hynes DM, Kubal JD, Murphy PA. Using administrative databases for outcomes research: select examples from VA Health Services Research and Development. J Med Syst. 1999;23:249–259. [DOI] [PubMed] [Google Scholar]
- 12. Goff DC, Pandey DK, Chan FA, Ortiz C, Nichaman MZ. Congestive heart failure in the United States: is there more than meets the I(CD code)? The Corpus Christi Heart Project. Arch Intern Med. 2000;160:197–202. DOI: 10.1001/archinte.160.2.197. [DOI] [PubMed] [Google Scholar]
- 13. Shah NR, Ahmed ST, Winchester DE, Ramsey DJ, Akeroyd JM, Wu W‐C, Waldo SW, Schofield RS, Ballantyne CM, Petersen LA, et al. Facility‐level variation in stress test utilization in veterans with ischemic heart disease. JACC Cardiovasc Imaging. 2019;12:1292–1293. DOI: 10.1016/j.jcmg.2019.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maddox TM, Plomondon ME, Petrich M, Tsai TT, Gethoffer H, Noonan G, Gillespie B, Box T, Fihn SD, Jesse RL, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114:1750–1757. DOI: 10.1016/j.amjcard.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 15. US Department of Veterans Affairs . VIReC Home [Internet]. 2016. Available at: http://www.virec.research.va.gov/index.htm. Accessed July 27, 2016.
- 16. Glorioso TJ, Grunwald GK, Ho PM, Maddox TM. Reference effect measures for quantifying, comparing and visualizing variation from random and fixed effects in non‐normal multilevel models, with applications to site variation in medical procedure use and outcomes. BMC Med Res Methodol. 2018;18:74. DOI: 10.1186/s12874-018-0517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Austin P. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. DOI: 10.1080/03610910902859574. [DOI] [Google Scholar]
- 18. Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54:948–963. DOI: 10.2307/2533848. [DOI] [PubMed] [Google Scholar]
- 19. Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. DOI: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Connor KD, Brophy T, Fonarow GC, Blankstein R, Swaminathan RV, Xu H, Matsouaka RA, Albert NM, Velazquez EJ, Yancy CW, et al. Testing for coronary artery disease in older patients with new‐onset heart failure: findings from Get With The Guidelines‐Heart Failure. Circ Heart Fail. 2020;13:e006963. DOI: 10.1161/CIRCHEARTFAILURE.120.006963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farmer SA, Lenzo J, Magid DJ, Gurwitz JH, Smith DH, Hsu G, Sung SH, Go AS. Hospital‐level variation in use of cardiovascular testing for adults with incident heart failure: findings from the cardiovascular research network heart failure study. JACC Cardiovasc Imaging. 2014;7:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, et al.; COURAGE Trial Research Group . Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. DOI: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 23. Flaherty JD, Rossi JS, Fonarow GC, Nunez E, Stough WG, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, Yancy CW, et al. Influence of coronary angiography on the utilization of therapies in patients with acute heart failure syndromes: findings from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF). Am Heart J. 2009;157:1018–1025. DOI: 10.1016/j.ahj.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 24. Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta‐analysis. J Am Coll Cardiol. 2002;39:1151–1158. DOI: 10.1016/S0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 25. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, et al. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet. 2018;391:572–580. DOI: 10.1016/S0140-6736(17)32520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albert NM, Barnason S, Deswal A, Hernandez A, Kociol R, Lee E, Paul S, Ryan CJ, White‐Williams C; American Heart Association Complex Cardiovascular Patient and Family Care Committee of the Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research . Transitions of care in heart failure: a scientific statement from the American Heart Association. Circ Heart Fail. 2015;8:384–409. DOI: 10.1161/HHF.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 27. Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. DOI: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S3


