Abstract
Background
The natural history of autonomic alterations following catheter ablation of drug‐refractory paroxysmal atrial fibrillation is poorly defined, largely because of the historical reliance on non‐invasive intermittent rhythm monitoring for outcome ascertainment.
Methods and Results
The study included 346 patients with drug‐refractory paroxysmal atrial fibrillation undergoing pulmonary vein isolation using contemporary advanced‐generation ablation technologies. All patients underwent insertion of a Reveal LINQ (Medtronic) implantable cardiac monitor before ablation. The implantable cardiac monitor continuously recorded physical activity, heart rate variability (measured as the SD of the average normal‐to‐normal), daytime heart rate, and nighttime heart rate. Longitudinal autonomic data in the 2‐month period leading up to the date of ablation were compared with the period from 91 to 365 days following ablation. Following ablation there was a significant decrease in SD of the average normal‐to‐normal (mean difference versus baseline of 19.3 ms; range, 12.9–25.7; P<0.0001), and significant increases in daytime and nighttime heart rates (mean difference versus baseline of 9.6 bpm; range, 7.4–11.8; P<0.0001, and 7.4 bpm; range, 5.4–9.3; P<0.0001, respectively). Patients free of arrhythmia recurrence had significantly faster daytime (11±11 versus 8±12 bpm, P=0.001) and nighttime heart rates (8±9 versus 6±8 bpm, P=0.049), but no difference in SD of the average normal‐to‐normal (P=0.09) compared with those with atrial fibrillation recurrence. Ablation technology and cryoablation duration did not influence these autonomic nervous system effects.
Conclusions
Pulmonary vein isolation results in significant sustained changes in the heart rate parameters related to autonomic function. These changes are correlated with procedural outcome and are independent of the ablation technology used.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01913522.
Keywords: atrial fibrillation, atrial fibrillation arrhythmia, autonomic, autonomic function, autonomic nervous system
Subject Categories: Atrial Fibrillation, Catheter Ablation and Implantable Cardioverter-Defibrillator, Electrophysiology
Nonstandard Abbreviations and Acronyms
- ANS
autonomic nervous system
- DHR
daytime heart rate
- HRV
heart rate variability
- ICM
implantable cardiac monitor
- NHR
nighttime heart rate
- PV
pulmonary vein
- PVI
pulmonary vein isolation
- SDANN
SD of the average normal‐to‐normal
Clinical Perspective
What Is New?
Atrial fibrillation ablation is associated with a significant increase in daytime and nighttime heart rates and significant decreases in heart rate variability (measured as SD of average normal‐to‐normal intervals); these changes were dynamic over the first 3 months and persisted for >1‐year post‐ablation.
Patients free of arrhythmia recurrence had significantly faster daytime and nighttime heart rates, but no difference in SD of the average normal‐to‐normal, compared with those with atrial fibrillation recurrence.
Ablation technology and cryoablation duration did not influence these autonomic nervous system effects, suggesting that the autonomic nervous system changes are unrelated to the extent of ablation and are more a function of direct influence of ablation on the ganglionated plexi.
What Are the Clinical Implications?
Patients and providers should be aware that pulmonary vein isolation results in significant sustained changes in the heart rate parameters related to autonomic function.
The increase in heart rate and decrease in heart rate variability following ablation may result from elevated sympathetic activity, decreased parasympathetic activity, or a combination of these factors.
Catheter ablation for atrial fibrillation (AF) offers an efficacious means to maintaining sinus rhythm when antiarrhythmic drugs are ineffective, contraindicated, or not tolerated. 1 , 2 , 3 Foundational to these catheter ablation procedures is the achievement of pulmonary vein (PV) isolation (PVI). More recently it has been increasingly recognized that ablation in the vicinity of the left atrial‐PV junction results in modification of the cardiac autonomic nervous system (ANS), predominantly through ablation of the adrenergic and cholinergic nerves present within the ganglionated plexi. 4 , 5
While there is considerable evidence that the ANS contributes to the initiation and maintenance of AF, it is uncertain whether ganglionated plexi ablation influences post‐ablation arrhythmia outcomes. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Moreover, the natural history of autonomic alterations following catheter ablation is poorly defined, largely because of the historical reliance on non‐invasive intermittent rhythm monitoring for outcome ascertainment. 11 , 12 , 13 , 14 , 15 , 16 In addition to lacking sensitivity in detecting sporadic arrhythmias, the use of non‐invasive monitoring is unable to comprehensively evaluate the progression and/or resolution of post‐ablation autonomic alterations. A continuous monitoring strategy addresses these limitations and offers the opportunity to precisely establish the extent of autonomic changes following catheter ablation of AF.
We sought to define the natural history of post‐ablation autonomic alterations and determine the clinical relevance of post‐PVI autonomic alterations in symptomatic patients with drug‐refractory paroxysmal AF undergoing ablation using contemporary advanced‐generation AF ablation technologies.
Methods
Study Design and Procedure Overview
The CIRCA‐DOSE (Cryoballoon vs Irrigated Radiofrequency Catheter Ablation) study (ClinicalTrials.gov #NCT01913522) was a multicenter, prospective, parallel‐group, single‐blinded randomized clinical trial, with blinded end‐point ascertainment conducted at 8 clinical centers in Canada. 17 Details of the protocol have been reported. 18 The study was approved by the appropriate national authorities and the ethics committee at each center. A steering committee was responsible for the study design, conduct, and reporting. Data monitoring, collection, and primary data analysis were performed by the Montreal Health Innovations Coordinating Centre. All patients provided written informed consent. The data that support the findings of this analysis are available from the corresponding author upon reasonable request.
In brief, 346 patients with paroxysmal AF refractory to at least 1 class I or class III antiarrhythmic drug were randomized in a 1:1:1 ratio to: (1) Contact‐force guided point‐by‐point radiofrequency ablation; (2) Short 2‐minute cryoballoon ablation duration; and (3) Standard 4‐minute cryoballoon ablation duration. Patients were blinded to their randomization assignment.
Patients randomized to the contact‐force guided point‐by‐point radiofrequency ablation group underwent circumferential PVI guided by a 3‐dimensional, non‐fluoroscopic mapping system (CARTO3, Biosense Webster) using an irrigated‐tip contact‐force sensing radiofrequency ablation catheter (Thermocool SmartTouch or SmartTouch Surround Flow, Biosense Webster). The pre‐specified contact force targeted before lesion delivery was 20 g (acceptable range, 10–40 g), with a minimum individual lesion duration of 400 g‐seconds force‐time integral. Circumferential ablation lesions were delivered around each of the PV ostia until each vein was electrically isolated from the left atrium. Patients randomized to cryoballoon ablation underwent PVI using a 23‐ or 28‐mm cryoballoon (Arctic Front Advance, Medtronic) using standardized techniques. The balloon was placed at each PV until it was occluded and then the tissue was cooled until conduction block was achieved. The cryoablation lesion was interrupted if the cryoballoon temperature was warmer than −35°C or PVI was not achieved within 60 seconds. Following PVI, a single additional cryoapplication was delivered after the rewarming phase (to +20°C). Cryoablation was performed with a lesion duration of 4 minutes, or 2 minutes depending on treatment allocation. A single “bonus” freeze was delivered to each vein following the rewarming phase of the successful lesion. In all groups, the procedure was deemed complete when bidirectional conduction block was achieved in all PVs. No additional left atrial lesions were permitted.
Follow‐Up
Antiarrhythmic drugs (except amiodarone) were allowed following ablation but were discontinued 5 half‐lives before the end of the 3‐month post‐ablation blanking period. Patients were followed for 1 year following ablation with clinical visits, a 12‐lead ECG, and a 24‐hour ambulatory Holter monitor at 3, 6, and 12 months.
All patients underwent insertion of a Reveal LINQ (Medtronic) implantable cardiac monitor (ICM) a minimum of 30 days before the index ablation procedure. The ICM was programmed to standardized arrhythmia detection settings (AF detection threshold—Balanced Sensitivity, Ectopy rejection—Nominal, Episode storage threshold—All; Record ECG of 2 minutes). 18 Automatic transmissions from the ICM were obtained on a daily basis. Patients recorded symptomatic arrhythmia episodes via use of the patient activator. Arrhythmia events meeting these criteria were stored for adjudication by an independent, blinded clinical end‐point committee.
Atrial tachyarrhythmia recurrence was defined as the time to first recurrence of symptomatic or asymptomatic atrial tachyarrhythmia (AF, atrial flutter, or atrial tachycardia) between days 91 and 365 post‐ablation, or a repeat ablation procedure between days 0 and 365 post‐ablation. An atrial tachyarrhythmia qualified as an arrhythmia if it lasted 30 seconds or longer. A standard 90‐day blanking period for early AF recurrences was employed. 19
In addition, the ICM continuously recorded physical activity (activity), heart rate variability (HRV), daytime heart rate (DHR), and nighttime heart rate (NHR). Activity was defined as the number of active minutes per day, where an active minute is defined by the number of accelerometer deflections over a minute exceeding the activity threshold (70 steps/min). HRV was defined based on the SD of the median interbeat V‐V interval values measured for each of the 5‐minute segments during a 24‐hour period, which is analogous to the SD of the average normal‐to‐normal (SDANN) and is reported in milliseconds. DHR and NHR are defined as the average ventricular rate, with “day” defined as the 12‐hour period between 8:00 am and 8:00 pm, and “night” defined as the 4‐hour period between midnight and 4:00 am (as indicated by the device clock). DHR, NHR, and SDANN calculations were not computed during AF episodes. During AF episodes the device calculated the mean and maximum heart rate in AF, as well as quantified the atrial fibrillation burden as hours per day.
Statistical Analysis
Continuous variables are presented using mean±SD or median (interquartile range) depending on the distribution. Categorical variables are presented as the number and percentages. Normality of data distributions were evaluated with Kolmogorov‒Smirnov test. Group differences were compared using the Mann‐Whitney and Chi‐square tests for continuous and discrete variables. The significance of autonomic indices within subjects was determined by 1‐way ANOVA for repeated measures. Group comparisons were evaluated by unpaired t test if the data followed a normal distribution, or the non‐parametric Mann‐Whitney U test, if otherwise.
In addition, the longitudinal autonomic data were classified into 2 observation windows: pre‐ and post‐ablation. We defined the end of the pre‐ablation period as the 2‐month period leading up to the date of ablation. The post‐ablation period was defined as the period from 91 to 365 days following ablation.
Analyses were performed using a combination of SAS software version 9.4 (SAS Institute, Cary, NC) and MATLAB 2018a (The Mathworks, Natick, 2018).
Results
A total of 346 patients were enrolled between September 2014 and July 2017. Characteristics of the study cohort are presented in Table. ICM insertion occurred in a median of 73.5 days (interquartile range, 50.0‒98.3) before ablation. A total of 265 624 days of ICM monitoring were evaluated.
Table 1.
Baseline Characteristics of the Study Cohort
| n=346 | |
|---|---|
| Age, y | 58.8±10.0 |
| Female sex | 115 (33.2%) |
| Height, cm | 173.8±9.7 |
| Weight, kg | 87.9±16.4 |
| Body mass index, kg/m2 | 29.1±5.3 |
| Systolic blood pressure, mm Hg | 130.6±17.3 |
| Diastolic blood pressure, mm Hg | 77.0±10.4 |
| CHADS2 * Score | 0 (0‒1) |
| CHA2DS2‐VASc Score † | 1 (0‒2) |
| Congestive heart failure | 6 (1.7%) |
| Hypertension | 120 (34.7%) |
| Diabetes mellitus | 29 (8.4%) |
| Ischemic heart disease | 29 (8.4%) |
| Chronic obstructive pulmonary disease | 7 (2.0%) |
| Sleep apnea | 45 (13.0%) |
| Previous stroke or transient ischemic attack | 16 (4.6%) |
| Thyroid dysfunction | 34 (9.8%) |
| Tobacco use | 17 (4.9%) |
| Antiarrhythmic drugs failed before enrollment | 2 (1‒2) |
| Left atrial volume, mL/m2 | 35.2±15.2 |
| Left ventricular ejection fraction, % | 59.2±6.1 |
| Diastolic dysfunction | 44 (16.7%) |
| Daytime heart rate, bpm | 68.18±0.57 |
| Nighttime heart rate, bpm | 60.39±0.50 |
| Heart rate variability ‡ , ms | 122.26±1.66 |
| Mean ventricular response in AF, bpm | 100.47±1.29 |
Data are mean±SD, median (interquartile range), or n (%).
The CHADS2 score is a clinical estimation of the risk of stroke in patients with atrial fibrillation, with scores ranging from 0 to 6, with higher scores indicating a greater risk.
The CHA2DS2‐VASc score is a clinical estimation of the risk of stroke in patients with atrial fibrillation, with scores ranging from 0 to 9, with higher scores indicating a greater risk.
Heart rate variability was measured as the SD of the average normal‐to‐normal intervals on continuous cardiac monitoring.
Autonomic Alterations for the Study Cohort
Before ablation the mean DHR was 69.2±0.6 bpm, the mean NHR was 60.4±0.5 bpm, and the mean SDANN was 122.26±1.66 ms.
Following PVI there was an immediate increase in DHR (≈5 bpm), which remained relatively stable at this level for the first 50 days following ablation (Figure 1, Figure S1). From days 50 to 100 post‐ablation there was a gradual increase in DHR before stabilizing at 78.7±0.6 bpm (ΔDHR, mean increase versus baseline of 9.6 bpm; range, 7.4–11.8; P<0.0001). DHR persisted at this level for the remainder of the year post‐ablation (mean difference in ΔDHR of −0.3 bpm, −0.5 bpm, −0.8 bpm for months 6, 9, and 12 versus month 3, respectively; P=0.99, P=0.97, P=0.86 respectively).
Figure 1. Autonomic alterations pre‐ and post‐pulmonary vein isolation in the study cohort.
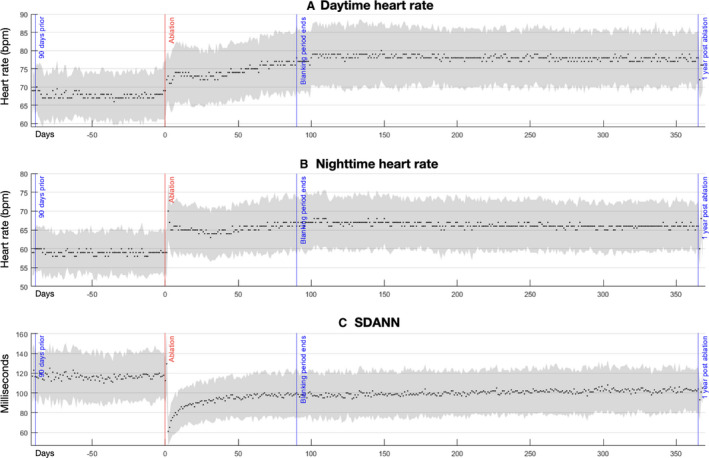
Pictured are median and interquartile ranges of the daytime heart rate (A), nighttime heart rate (B), and the SD of the average normal‐to‐normal intervals (C). Date of ablation is noted as time zero on the y‐axis, with a range of −91 days before ablation, and +366 days post‐ablation. SDANN indicates SD of the average normal‐to‐normal intervals.
NHR broadly followed the pattern of DHR with the exception of an immediate spike (immediate sharp increase of 11 bpm post‐ablation, followed by a rapid decrease within the first post‐ablation week to a level 5–6 bpm above baseline). Over the first 50 days post‐ablation there was a further 1 to 2 bpm increase in NHR. NHR then increased by 2 to 3 bpm over the subsequent 50 days, before stabilizing at 67.7±0.5 bpm (ΔNHR, mean difference versus baseline of 7.4 bpm; range, 5.4–9.3; P<0.0001). Thereafter there was no significant change in NHR for the remainder of the year (mean difference in ΔNHR of −0.8 bpm, −1.0 bpm, −1.2 bpm for months 6, 9, and 12 versus month 3, respectively; P=0.80, P=0.66, P=0.42, respectively).
Similar to NHR, the SDANN exhibited a similar immediate post‐ablation peak, decreasing by ≈60 ms following ablation, with rapid recalibration over the first 10 post‐ablation days (increase of 20–25 ms). From post‐ablation days 10 to 40 there was another 10 ms increase, and another 10 ms increase from post‐ablation days 50 to 100, where it stabilized at 103.0±1.7 ms (ΔSDANN, mean difference versus baseline of 19.3 ms; range, 12.9–25.7; P<0.0001). Thereafter there was no significant change in SDANN for the remainder of the year (mean difference in ΔSDANN of 2.2 ms, 3.7 ms, 4.7 ms for months 6, 9, and 12 versus month 3, respectively; P=0.87, P=0.53, P=0.28, respectively).
There was no difference in DHR, NHR, or SDANN between those treated with radiofrequency ablation and those treated with cryoablation at any time point (Figure 2).
Figure 2. Autonomic alterations pre‐ and post‐pulmonary vein isolation, stratified by randomized group.
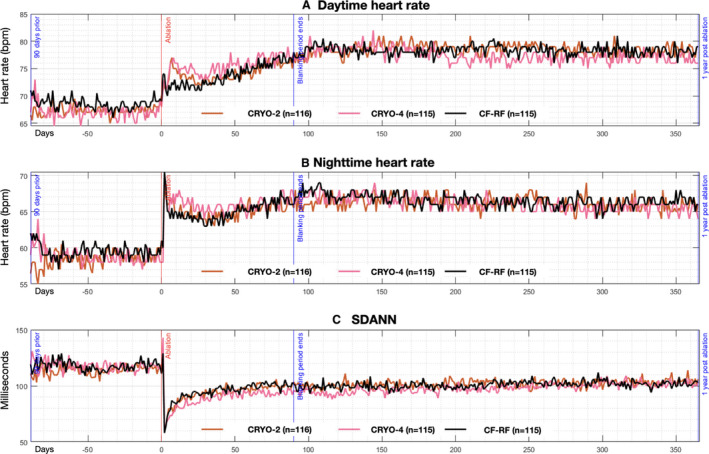
Pictured are median values of the daytime heart rate (A), nighttime heart rate (B), and the SD of the average normal‐to‐normal intervals (C). Date of ablation is noted as time zero on the Y axis, with a range of −91 days before ablation, and +366 days post‐ablation. Black line depicts patients randomized to contact‐force radiofrequency ablation, red line depicts those randomized to short duration cryoablation, and pink line depicts those randomized to standard duration cryoablation. CRYO indicates cryoballoon ablation; SDANN, SD of the average normal‐to‐ normal.
Sex‐Based Differences in Autonomic Alterations
Of the 346 trial participants, 33% were women. Compared with men, women were older (mean age 60.9±9.1 years versus 57.7±10.3 years, P=0.004) with a more frequent history of thyroid dysfunction (20.9% versus 4.3%, P<0.0001), but otherwise no significant differences in patient characteristics (Table S1). 20
Men and women participants demonstrated similar pre‐ and post‐ablation DHR and NHR, with no important differences in the pattern and natural history (Figure S2). In contrast, women had lower SDANN pre‐and post‐ablation compared with men. While there was no significant difference in the magnitude of change between the sexes, women had a significantly lower SDANN at all time points.
Age‐Based Differences in Autonomic Alterations
The age range of trial participants was 31 to 85 years, with 92 patients aged >65 years, 137 patients aged 56 to 65 years, and 117 patients younger than 56 years. When compared with younger patients, older patients had significantly higher systolic blood pressure (respectively, 136.1±19.6, 129.7±17.3, and 127.3±14.3 mm Hg; P<0.001) and more frequent history of hypertension (45.7% versus 39.4% and 20.5%, P<0.001), diabetes mellitus (13.0% versus 9.5% and 3.4%, P=0.037), coronary artery disease (13.0% versus 9.5% and 3.4%, P=0.037), and heart failure (4.3% versus 0.0% and 1.7%, P=0.047) (Table S2).
At baseline, older patients had a significantly lower DHR and NHR (Figure S3). This age‐based difference in DHR and NHR was amplified post‐ablation. Specifically, there was less relative change in ΔNHR and ΔDHR post‐ablation in older age categories. In contrast, SDANN did not differ between age categories at any time.
Autonomic Alterations Relative to the Success of Ablation
At 12 months, freedom from a documented recurrence of any (symptomatic or asymptomatic) atrial tachyarrhythmia after a single ablation procedure had occurred in 182 of 346 (52.6%) participants. Those with documented recurrence of atrial fibrillation, atrial flutter, or atrial tachycardia were older (60.3±9.6 years versus 57.4±10.1 years, P=0.006), and had a larger left atrial volume (37.8±16.1 versus 33.1±14.1 mL/m2, P=0.027) (Table S3).
When compared with those free of atrial tachyarrhythmia, those with documented recurrence had a lower baseline DHR but similar NHR and SDANN (Figure 3). Post‐ablation patients free of atrial tachyarrhythmia had higher DHR and NHR, with a larger relative change from baseline in ΔDHR (11±11 versus 8±12 bpm, P=0.001) and ΔNHR (8±9 versus 6±8 bpm, P=0.049). SDANN did not differ between those free of atrial tachyarrhythmia and those with documented recurrence (−12±31.5 versus −18±24 ms change, P=0.091).
Figure 3. Autonomic alterations pre‐ and post‐pulmonary vein isolation, stratified by procedural outcome.
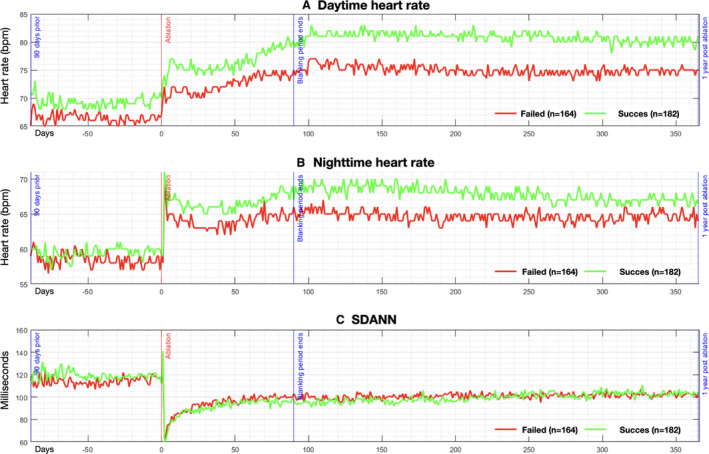
Pictured are median values of the daytime heart rate (A), nighttime heart rate (B), and the SD of the average normal‐to‐normal intervals (C). Date of ablation is noted as time zero on the y‐axis, with a range of −100 days before ablation, and +365 days post‐ablation. Red line depicts patients with recurrence of atrial fibrillation/atrial flutter/atrial tachycardia, and green line depicts patients free of arrhythmia recurrence.
Discussion
There is considerable evidence that the cardiac ANS contributes to the initiation and maintenance of AF through a combination of parasympathetic‐mediated shortening of action potential duration and the effective refractory period, sympathetic‐mediated calcium‐loading, and promotion of delayed afterdepolarizations, and sympathoadrenal discharge induced early afterdepolarizations in the PV and atrial myocardium. 3 These effects are predominantly facilitated by the intrinsic cardiac ANS, which consists of clusters of autonomic neurons located within the ganglionated plexi. 4 These ganglionated plexi contain afferent neurons from the atrial myocardium and the central ANS (ie, the extrinsic cardiac ANS), efferent adrenergic and cholinergic neurons, as well as an extensive network of interconnecting neurons. These efferent parasympathetic and sympathetic neurons heavily innervate the PV and atrial myocardium surrounding the ganglionated plexi, effectively modulating impulse formation from the PV triggers as well as impulse propagation in the left atrial substrate.
Although it remains uncertain whether additional targeted ablation of the ANS inputs impacts arrhythmia outcomes, 6 , 7 , 8 , 9 , 10 circumferential PVI often destroys the ganglionated plexi given their localization within 5‐mm of the pulmonary venous‐left atrial junction. 4 , 5 , 21 Observed post‐ablation alterations in autonomic activity include elevation of heart rate, reduction in HRV, as well as a decrease in deceleration capacity and acceleration capacity. 11 , 12 , 13 , 14 , 16 , 22 , 23 , 24 These alterations have been described as transient in some reports (lasting <6 months), 11 , 12 , 13 but prolonged in others. 14 , 15 , 16 However, these reports were limited by small numbers of patients as well as a reliance on non‐invasive intermittent rhythm monitoring, which is unable to comprehensively evaluate the progression and/or resolution of post‐ablation autonomic alterations. While these monitoring methods are widely used, these short‐term measurements of HRV are predominantly driven by parasympathetically‐mediated respiratory sinus arrhythmia, which correlate poorly with 24‐hour indices and lack predictive ability. 25 In contrast, the use of long‐term implantable monitoring in the current study has enabled us to comprehensively assess HRV in response to a greater range of environmental stimulation, 26 in addition to facilitating one of the most robust evaluations of the natural history and clinical impact of autonomic alterations following ablation.
The current study demonstrates the following key findings. (1) Post‐ablation there is a significant decrease in HRV (measured as SDANN), and significant increases in daytime and nighttime heart rates; (2) These changes are dynamic over the first 3 months following ablation, and persist for >1 year post‐ablation; (3) Ablation technology and cryoablation duration did not influence these ANS effects, suggesting that the ANS changes are unrelated to the extent of ablation and are more a function of direct influence of ablation on the ganglionated plexi; (4) Patients free of arrhythmia recurrence had significantly faster daytime and nighttime heart rates, but no difference in SDANN compared with those with AF recurrence; (5) Women have similar daytime and nighttime heart rates as men, however their SDANN is lower at all time points; (6) Older patients have significantly faster daytime and nighttime heart rates but similar SDANN, compared with younger patients.
The increase in heart rate and decrease in HRV following ablation may result from elevated sympathetic activity, decreased parasympathetic activity, or a combination of these factors. Typically, faster heart rates are associated with lower HRV because of cycle length dependence, as the faster heart rates reduce the time between successive beats and therefore the opportunity for the interbeat intervals to vary. Globally this relationship was observed in our study where the post‐ablation DHR and NHR both increased, and the HRV decreased. However, when patients were examined by treatment success this relationship was not preserved. Specifically, patients free of arrhythmia recurrence had a significantly greater rise in DHR and NHR when compared with those with arrhythmia recurrence, yet both groups experienced an identical reduction in HRV. This relationship persisted despite correcting for antiarrhythmic drug and rate‐controlling medication use, activity level, and other relevant confounders.
Limitations
The current study was a sub‐analysis of the CIRCA‐DOSE study, which randomized patients between 3 different strategies for PV isolation. The study did not evaluate intraprocedural vagal reflexes, nor necessitate their elimination. Second, the measure of HRV employed in the current study was the SD of the average normal‐to‐normal intervals for each of the 5‐minute segments during a 24‐hour recording (SDANN). SDANN is highly correlated to the ultra‐low‐frequency power band and provides information on the interplay of the sympathetic and parasympathetic branches of the ANS, particularly in reference to slow‐acting biological processes such as circadian rhythms, core body temperature, metabolism, and the renin–angiotensin system. 25 However, the wide time intervals (5 minutes) mean that SDANN is insensitive to changes in the high frequency power band, which measures efferent vagal activity (eg, parasympathetically‐mediated heart rate variations related to the respiratory cycle). 25 , 27
Conclusions
PV isolation results in significant sustained changes in the heart rate parameters related to autonomic function. These changes are correlated with procedural outcome and are independent of the ablation technology used.
Sources of Funding
The CIRCA‐DOSE study was funded by a peer‐reviewed grant from the Heart and Stroke Foundation of Canada (grant number G‐13‐0003121), with additional financial support from Medtronic. Drs Andrade and Deyell are supported by a Michael Smith Foundation for Health Research Scholar Award. Dr Khairy is supported by the André Chagnon Research Chair in Electrophysiology and Congenital Heart Disease. Dr Hawkins is the Dr Charles Kerr Distinguished Scholar in Heart Rhythm Management. Dr Ho reports funding from the Huawei‐University of British Columbia's Data Science Institute Research Program. The funding sources had no role in the design of this study and did not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Disclosures
Dr Andrade reports grants and personal fees from Medtronic, grants from Baylis, and personal fees from Biosense‐Webster; Dr Verma reports grants and personal fees from Medtronic and Biosense‐Webster; Dr Deyell reports grants from Biosense‐Webster; Dr Macle reports personal fees from Medtronic, grants and personal fees from St. Jude Medical/Abbott and Biosense‐Webster. The remaining authors have no disclosures to report.
Supporting information
Appendix S1
Tables S1–S3
Figures S1–S3
(J Am Heart Assoc. 2021;10:e018610. DOI: 10.1161/JAHA.120.018610.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018610
For Sources of Funding and Disclosures, see page 8.
References
- 1. Oral H, Knight BP, Tada H, Özaydın M, Chugh A, Hassan S, Scharf C, Lai SWK, Greenstein R, Pelosi F Jr, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–1081. DOI: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 2. Karch MR, Zrenner B, Deisenhofer I, Schreieck J, Ndrepepa G, Dong J, Lamprecht K, Barthel P, Luciani E, Schömig A, et al. Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: a randomized comparison between 2 current ablation strategies. Circulation. 2005;111:2875–2880. DOI: 10.1161/CIRCULATIONAHA.104.491530. [DOI] [PubMed] [Google Scholar]
- 3. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. DOI: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 4. Nakagawa H, Scherlag BJ, Patterson E, Ikeda A, Lockwood D, Jackman WM. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm. 2009;6:S26–S34. DOI: 10.1016/j.hrthm.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 5. Tan AY, Li H, Wachsmann‐Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein‐atrial junction: implications for catheter ablation of atrial‐pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–143. DOI: 10.1016/j.jacc.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 6. Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. 2011;8:672–678. DOI: 10.1016/j.hrthm.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 7. Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–2325. [DOI] [PubMed] [Google Scholar]
- 8. Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Shirokova N, Karaskov A, Mittal S, Steinberg JS. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long‐standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm. 2013;10:1280–1286. [DOI] [PubMed] [Google Scholar]
- 9. Driessen AHG, Berger WR, Krul SPJ, van den Berg NWE, Neefs J, Piersma FR, Chan Pin Yin D, de Jong J, van Boven WP, de Groot JR. Ganglion plexus ablation in advanced atrial fibrillation: the AFACT study. J Am Coll Cardiol. 2016;68:1155–1165. [DOI] [PubMed] [Google Scholar]
- 10. Berger WR, Neefs J, van den Berg NWE, Krul SPJ, van Praag EM, Piersma FR, de Jong J, van Boven WP, Driessen AHG, de Groot JR. Additional ganglion plexus ablation during thoracoscopic surgical ablation of advanced atrial fibrillation: intermediate follow‐up of the AFACT study. JACC Clin Electrophysiol. 2019;5:343–353. [DOI] [PubMed] [Google Scholar]
- 11. Hsieh MH, Chiou CW, Wen ZC, Wu CH, Tai CT, Tsai CF, Ding YA, Chang MS, Chen SA. Alterations of heart rate variability after radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. Circulation. 1999;100:2237–2243. DOI: 10.1161/01.CIR.100.22.2237. [DOI] [PubMed] [Google Scholar]
- 12. Yamada T, Yoshida N, Murakami Y, Okada T, Yoshida Y, Muto M, Inden Y, Murohara T. The difference in autonomic denervation and its effect on atrial fibrillation recurrence between the standard segmental and circumferential pulmonary vein isolation techniques. Europace. 2009;11:1612–1619. DOI: 10.1093/europace/eup330. [DOI] [PubMed] [Google Scholar]
- 13. Oswald H, Klein G, Koenig T, Luesebrink U, Duncker D, Gardiwal A. Cryoballoon pulmonary vein isolation temporarily modulates the intrinsic cardiac autonomic nervous system. J Interv Card Electrophysiol. 2010;29:57–62. DOI: 10.1007/s10840-010-9491-7. [DOI] [PubMed] [Google Scholar]
- 14. Bauer A, Deisenhofer I, Schneider R, Zrenner B, Barthel P, Karch M, Wagenpfeil S, Schmitt C, Schmidt G. Effects of circumferential or segmental pulmonary vein ablation for paroxysmal atrial fibrillation on cardiac autonomic function. Heart Rhythm. 2006;3:1428–1435. DOI: 10.1016/j.hrthm.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 15. Wang K, Chang D, Chu Z, Yang Y, Gao L, Zhang S, Xia Y, Dong Y, Yin X, Cong P, et al. Denervation as a common mechanism underlying different pulmonary vein isolation strategies for paroxysmal atrial fibrillation: evidenced by heart rate variability after ablation. ScientificWorldJournal. 2013;2013:569564. DOI: 10.1155/2013/569564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marinkovic M, Mujovic N, Vucicevic V, Steffel J, Potpara TS. A square root pattern of changes in heart rate variability during the first year after circumferential pulmonary vein isolation for paroxysmal atrial fibrillation and their relation with longterm arrhythmia recurrence. Kardiol Pol. 2020;78:209–218. [DOI] [PubMed] [Google Scholar]
- 17. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle L, Leong‐Sit P, Novak P, Badra‐Verdu M, Sapp J, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779–1788. [DOI] [PubMed] [Google Scholar]
- 18. Andrade JG, Deyell MW, Badra M, Champagne J, Dubuc M, Leong‐Sit P, Macle L, Novak P, Roux JF, Sapp J, et al. Randomised clinical trial of cryoballoon versus irrigated radio frequency catheter ablation for atrial fibrillation‐the effect of double short versus standard exposure cryoablation duration during pulmonary vein isolation (CIRCA‐DOSE): methods and rationale. BMJ Open. 2017;7:e017970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yao RJR, Macle L, Deyell MW, Tang L, Hawkins NM, Sedlak T, Nault I, Verma A, Khairy P, Andrade JG, et al. Impact of female sex on clinical presentation and ablation outcomes in the circa‐dose study. JACC Clin Electrophysiol. 2020;6:945–954. DOI: 10.1016/j.jacep.2020.04.032. [DOI] [PubMed] [Google Scholar]
- 21. Scherschel K, Hedenus K, Jungen C, Lemoine MD, Rübsamen N, Veldkamp MW, Klatt N, Lindner D, Westermann D, Casini S, et al. Cardiac glial cells release neurotrophic S100B upon catheter‐based treatment of atrial fibrillation. Sci Transl Med. 2019;11:eaav7770. DOI: 10.1126/scitranslmed.aav7770. [DOI] [PubMed] [Google Scholar]
- 22. Goff ZD, Laczay B, Yenokyan G, Sivasambu B, Sinha SK, Marine JE, Ashikaga H, Berger RD, Akhtar T, Spragg DD, et al. Heart rate increase after pulmonary vein isolation predicts freedom from atrial fibrillation at 1 year. J Cardiovasc Electrophysiol. 2019;30:2818–2822. DOI: 10.1111/jce.14257. [DOI] [PubMed] [Google Scholar]
- 23. Miyazaki S, Nakamura H, Taniguchi H, Hachiya H, Kajiyama T, Watanabe T, Igarashi M, Ichijo S, Hirao K, Iesaka Y. Autonomic nervous system modulation and clinical outcome after pulmonary vein isolation using the second‐generation cryoballoon. J Cardiovasc Electrophysiol. 2017;28:1015–1020. DOI: 10.1111/jce.13262. [DOI] [PubMed] [Google Scholar]
- 24. Kuyumcu MS, Ozeke O, Cay S, Ozcan F, Bayraktar MF, Kara M, Vicdan M, Acar B, Aydogdu S, Topaloglu S, et al. The short‐term impact of the catheter ablation on noninvasive autonomic nervous system parameters in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2017;40:1193–1199. DOI: 10.1111/pace.13179. [DOI] [PubMed] [Google Scholar]
- 25. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. DOI: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 27. Roach D, Wilson W, Ritchie D, Sheldon R. Dissection of long‐range heart rate variability: controlled induction of prognostic measures by activity in the laboratory. J Am Coll Cardiol. 2004;43:2271–2277. DOI: 10.1016/j.jacc.2004.01.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Tables S1–S3
Figures S1–S3


