Lipoprotein(a) (Lp[a]) is an independent, highly heritable risk factor for incident atherosclerotic cardiovascular disease and mortality. 1 , 2 Approximately 1 in 5 individuals have Lp(a) >150 nmol/L, which is associated with 1.5‐fold risk for coronary artery disease (CAD). 1 American cholesterol guidelines note that family history of premature atherosclerotic cardiovascular disease is a relative indication for Lp(a) assessment for primary prevention and support using elevated Lp(a) as a risk‐enhancing factor to reinforce statin recommendations. 3 However, the incident CAD risk of elevated Lp(a) in those without a family history of heart disease is poorly understood.
The UK Biobank is a prospective, observational cohort of ~500 000 UK residents, 40 to 69 years old, recruited in 2006 to 2010 as previously described. 4 Researchers can apply for access to the data at: https://www.ukbiobank.ac.uk/register‐apply/. We studied participants with no CAD at enrollment, no self‐reported family history of heart disease in a sibling or parent, and with Lp(a) measured. Prevalent CAD status was determined by self‐reported history of myocardial infarction, coronary artery bypass grafting, or coronary artery angioplasty during an interview with a nurse at the baseline visit or corresponding hospital billing codes (International Classification of Diseases, Tenth Revision [ICD‐10]: I21, I22, I23, I24.1, I25.2; ICD‐9: 410, 411, 412; OPCS‐4: K40, K41, K45, K49, K50.2, K75) from hospital medical records dated before recruitment. Lp(a) was measured at baseline by immunoturbidimetric assay with polyclonal antibodies targeting apolipoprotein(a) epitopes (Denka Seiken, Coventry, UK) (Field 30790). Out of assay range values previously not provided were included (Return 2321). Incident CAD, our primary outcome, was determined by the presence of CAD ICD‐10 codes dated after recruitment. Participants were censored by date of outcome, death, or last follow‐up, whichever came first. We used Cox regression to estimate incident risk of CAD conferred by Lp(a) levels with natural cubic splines adjusting for age, sex, self‐reported ethnicity (White, Black, Asian, or other), type 2 diabetes mellitus, smoking status, low‐density lipoprotein cholesterol, and statin or ezetimibe use. We compared the risk of incident CAD to those with a family history of heart disease, and secondarily looked at risk of incident atherosclerotic cardiovascular disease (CAD, peripheral arterial disease, or ischemic stroke). To satisfy the proportional hazards assumption, subjects with a follow‐up time longer than 9 years were excluded from the analyses. Type 2 diabetes mellitus was determined by self‐report or corresponding ICD‐10 codes during hospitalization. Low‐density lipoprotein cholesterol was measured at baseline. Smoking status and statin or ezetimibe use were determined by self‐report. Model fit was determined by Akaike's information criterion. An α threshold of 0.05 was considered statistically significant. Analyses were performed in R (version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria). These analyses were approved by the Massachusetts General Hospital Institutional Review Board. Participants in the UK Biobank gave informed consent.
We identified 343 728 individuals without prevalent CAD and with Lp(a) measured, of whom 86.8% (n=298 461) did not report a family history of heart disease, and 153 228 had a follow‐up time of <9 years. The present participants had a mean (standard deviation) age of 58.4 years (7.9 years), 80 043 (52.2%) were female, and the median (interquartile range) Lp(a) was 18.6 nmol/L (7.4–72.9 nmol/L). The median (interquartile range) follow‐up period was 8.2 years (7.8–8.6 years). We modeled Lp(a) concentration and risk of incident CAD with a natural cubic spline, adjusting for age, sex, ethnicity, and cardiovascular risk factors; the relationship was approximately linear above 50 nmol/L (Figure). The hazard ratio for incident CAD per 50 nmol/L Lp(a) among those with a family history was 1.18 (95% CI: 1.11–1.26; P<0.001) and was 1.15 (95% CI: 1.13–1.17; P<0.001) among those without (P[interaction]=0.73). In the secondary analyses, the hazard ratio for incident atherosclerotic cardiovascular disease per 50 nmol/L Lp(a) in those with a family history was 1.15 (95% CI: 1.09–1.21; P<0.001) and 1.12 (95% CI: 1.10–1.14; P<0.001) for those without (P[interaction]=0.66).
Figure 1. Incident risk for coronary artery disease among individuals without a self‐reported family history of cardiovascular disease.
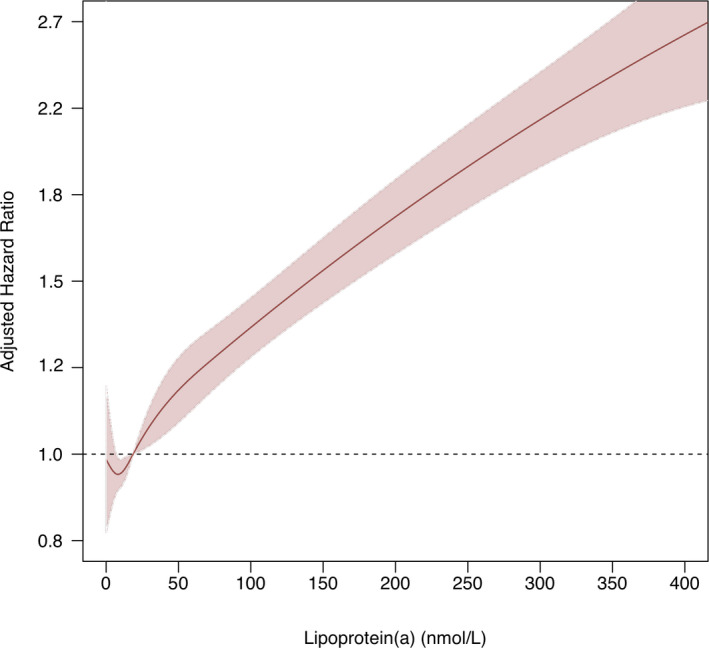
Cox regression was used to estimate hazard ratios and 95% CI by lipoprotein(a) (Lp[a]) concentration, modeled by natural cubic spline, in reference to an individual with the median Lp(a) concentration of 18.6 nmol/L. The model was adjusted for age, sex, ethnicity, type 2 diabetes mellitus, smoking status, low‐density lipoprotein cholesterol, and statin or ezetimibe use. The x axis was truncated at 400.
In the study cohort, 19 170 (12.5%) had Lp(a) of 150 nmol/L or greater. Compared with Lp(a) <150 nmol/L, the hazard ratio for incident CAD risk associated with Lp(a) >150 nmol/L for those with a family history was 2.00 (95% CI: 1.46–2.74; P<0.001) versus without was 1.68 (95% CI: 1.55–1.82; P<0.001) (P[interaction]=0.68).
Our study's key strengths are in its size and uniform ascertainment of Lp(a) and family history. Although self‐report of family history may be imprecise, the true negative rate is >90% in similar studies. 5 Lp(a)‐associated risk outside of the present study's age and follow‐up time range requires further study. Additionally, although only baseline Lp(a) values are considered, given uniquely high heritability and current lack of medicines substantially altering values, Lp(a) values are anticipated to largely be stable throughout life.
In conclusion, elevated Lp(a) was associated with an increased risk for incident CAD in the absence of heart disease in first‐degree family members, with effects similar to those observed in the general population. Our analyses show that Lp(a) measurement may be beneficial in refining CAD risk in primary prevention patients without a family history of heart disease.
Sources of Funding
This work was supported by UK Biobank application 7089, and the authors would like to thank the UK Biobank study staff and participants. Dr Peloso and Dr Natarajan are supported by a grant from the National Heart, Lung, and Blood Institute (R01HL142711). Dr Natarajan is also supported by grants from the National Heart, Lung, and Blood Institute (R01HL148565, R01HL148050) and Fondation Leducq (TNE‐18CVD04), and a Hassenfeld Scholar Award from the Massachusetts General Hospital. Dr Patel and Dr Paruchuri are supported by grant T32HL007208 from the National Heart, Lung, and Blood Institute.
Disclosures
Dr Natarajan reports grant support from Amgen, Apple, and Boston Scientific, consulting income from Apple, Blackstone Life Sciences, and Novartis, and spousal employment at Vertex all unrelated to the present work. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2021;10:e017470. DOI: 10.1161/JAHA.120.017470.)
For Sources of Funding and Disclosures, see page 3.
REFERENCES
- 1. Tipping RW, Ford CE, Simpson LM, Walldius G, Jungner I, Folsom AR, Chambless L, Panagiotakos D, Pitsavos C, Chrysohoou C, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kamstrup PR, Tybjærg‐Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. DOI: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 3. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1046–e1081. [DOI] [PubMed] [Google Scholar]
- 4. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. DOI: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson BJ, Qureshi N, Santaguida P, Little J, Carroll JC, Allanson J, Raina P. Systematic review: family history in risk assessment for common diseases. Ann Intern Med. 2009;151:878–885. DOI: 10.7326/0000605-200912150-00177. [DOI] [PubMed] [Google Scholar]


