Abstract
Background
Patients treated for breast cancer have a high incidence of cardiovascular complications. In this study, we evaluated the impact of breast cancer on cardiac function and cardiomyocyte Ca2+‐handling protein expression. We also investigated whether exercise training (ET) would prevent these potential alterations.
Methods and Results
Transgenic mice with spontaneous breast cancer (mouse mammary tumor virus–polyomavirus middle T antigen [MMTV‐PyMT+], n=15) and littermate mice with no cancer (MMTV‐PyMT−, n=14) were studied. For the ET analysis, MMTV‐PyMT+ were divided into sedentary (n=10) and exercise‐trained (n=12) groups. Cardiac function was evaluated by echocardiography with speckle‐tracking imaging. Exercise tolerance test was conducted on a treadmill. Both studies were performed when the tumor became palpable and when it reached 1 cm3. After euthanasia, Ca2+‐handling protein expression (Western blot) was evaluated. Exercise capacity was reduced in MMTV‐PyMT+ compared with MMTV‐PyMT− (P interaction=0.031). Longitudinal strain (P group <0.001) and strain rate (P group=0.030) were impaired. Cardiomyocyte phospholamban was increased (P=0.011), whereas phospho‐phospholamban and sodium/calcium exchanger were decreased (P=0.038 and P=0.017, respectively) in MMTV‐PyMT+. No significant difference in sarcoplasmic or endoplasmic reticulum calcium 2 ATPase (SERCA2a) was found. SERCA2a/phospholamban ratio was reduced (P=0.007). ET was not associated with increased exercise capacity. ET decreased left ventricular end‐systolic diameter (P group=0.038) and end‐diastolic volume (P group=0.026). Other morphological and functional cardiac parameters were not improved by ET in MMTV‐PyMT+. ET did not improve cardiomyocyte Ca2+‐handling protein expression.
Conclusions
Breast cancer is associated with decreased exercise capacity and subclinical left ventricular dysfunction in MMTV‐PyMT+, which is at least partly associated with dysregulation of cardiomyocyte Ca2+ handling. ET did not prevent or reverse these changes.
Keywords: breast cancer, Ca2+ handling, cardiac function
Subject Categories: Animal Models of Human Disease, Basic Science Research, Contractile function, Pathophysiology, Calcium Cycling/Excitation-Contraction Coupling
Nonstandard Abbreviations and Acronyms
- EjT
ejection time
- ERK
extracellular signal‐regulated kinase
- ET
exercise training
- ETT
exercise tolerance test
- HER2
human epidermal growth factor receptor type 2
- IVCT
isovolumetric contraction time
- IVRT
isovolumetric relaxation time
- IVS
interventricular septum
- JNK
c‐Jun N‐terminal kinase
- LVEDD
left ventricular end‐diastolic diameter
- LVESD
left ventricular end‐systolic diameter
- LVESV
left ventricular end‐systolic volume
- LVPW
posterior wall thickness
- MAPK
mitogen‐activated protein kinase
- MMTV
mouse mammary tumor virus
- PI3K
phosphoinositide 3‐kinase
- p‐Phospholamban
phospho‐phospholamban
- PyMT
polyomavirus middle T antigen
- SERCA2a
sarcoplasmic or endoplasmic reticulum calcium 2 ATPase
Clinical Perspective
What Is New?
Breast cancer is associated with subclinical left ventricular dysfunction in transgenic mice with spontaneous breast cancer at the end stage of this disease.
This cardiac dysfunction is associated with dysregulation of cardiomyocyte Ca2+ handling.
Exercise training alone is not an effective strategy to prevent or reverse the cardiac function provoked by breast cancer.
What Are the Clinical Implications?
These findings highlight that cancer is a systemic disease that gradually compromises body homeostasis as the disease progresses.
Moreover, it is suggested that patients with cancer are more susceptible to organ dysfunction, including cardiac dysfunction.
Breast cancer is the most diagnosed cancer among women, and it was responsible for more than 2 million new cancer cases worldwide in 2018. 1 Multimodality treatment, which includes surgical resection, radiation, and systemic therapy, is the current standard of care for this disease. 2
Systemic agents commonly used to treat breast cancer, such as anthracyclines, are known to be associated with cardiovascular events, a phenomenon known as cardiovascular toxicity. 3 Endothelial dysfunction, 4 acute coronary syndrome, 5 supraventricular and ventricular arrhythmias, 6 and ventricular dysfunction, 7 leading to heart failure, 8 have all been described in patients during or after chemotherapy treatment. However, little is known about the impact of breast cancer itself on cardiac function, which can be important to clarify the impact of cancer as a systemic disease that can negatively affect the cardiovascular physiology.
Exercise training (ET) promotes many benefits to the cardiovascular system both in healthy and chronically ill individuals. Previous studies from our group demonstrated that ET improves cardiac function in hypercaloric obese rats and in a mouse model of heart failure induced by sympathetic hyperactivity. 9 , 10 In addition, ET substantially increases physical capacity 9 , 10 and is considered a marker of ET adaptation.
In this study, we hypothesized that breast cancer would promote cardiac dysfunction and decrease exercise tolerance. We also hypothesized that ET would attenuate or prevent cancer‐induced cardiac dysfunction. To understand the effects of breast cancer on cardiac function, we used a mouse model of breast cancer. We used this same model to explore the effects of ET on cardiac function, and to test whether the changes in cardiac function were associated with deregulation of Ca2+ handling in the mouse cardiomyocyte.
METHODS
The data, analytical methods, and study materials that support the conclusions of this study will be available to other researchers for the purpose of replicating the procedures and reproducing the results through reasonable request by contacting the corresponding author.
Animal Model
Female mice carrying the polyomavirus middle T antigen (mouse mammary tumor virus [MMTV]‐PyMT+), a transgenic mouse model of spontaneous breast cancer with C57BL6/J genetic background, from University of São Paulo Medical School, were used in the study, and their respective healthy offspring (MMTV‐PyMT−) served as controls. In these mice, the development of spontaneous breast cancer in 4 distinctly identifiable stages of tumor progression occurs in a single primary tumor focus and it resembles the pathology of human breast cancer in relation to hyperplasia, adenoma, and early and late carcinoma. 11 , 12 , 13 In addition, the loss of estrogen and progesterone receptors and the overexpression of human epidermal growth factor receptor type 2 (HER2) are similar to HER2‐amplified human breast cancer.
Mice were kept in the animal facilities of the University of São Paulo Medical School, placed in a standard mouse polypropylene box (30×20×13 cm) with a meshed lid and lined with sterile wood shavings. The animals were housed 4 per cage and kept at controlled temperatures of 22 to 24°C, with relative humidity of 45% to 50%, and reversed light‐dark cycle in 12‐hour day and night periods. Meals included standard mice chow and water ad libitum. All animal procedures were approved by the Scientific Committee for Animal Research from the University of São Paulo Medical School (N°061/16). The experiment was conducted in concordance with the National Council for Animal Experimentation Control.
Experimental Protocol
In a pilot study to test the hypothesis regarding cardiac function analysis, the sample size (n=4 per group) was reconsidered because of the variability in the results. In addition, some histological and molecular techniques widely used in previous studies highlight a minimum number of 5 animals for tissue biodistribution. 14 The sample size calculation based on the longitudinal strain of the first 5 animals of each group from the substudy 1 (pilot) showed the average value of −10.3% with 1.9% (SD) variability in the group with cancer (MMTV‐PyMT+) after the period of tumor development and the average value of −12.9% with 2.6% (SD) variability in the group without cancer (MMTV‐PyMT−) after the corresponding period of the experimental protocol. Expecting that these differences would be maintained in the total sampling with a power of 80% and confidence of 95% (α of 5%), the sample size would be 13 animals in each group. Thus, 14 and 15 mice were included in the substudy 1. In the complementary study (substudy 2), 10 to 12 mice were involved in the experiment. All animals were monitored by high‐resolution echocardiographic imaging and exercise tolerance test (ETT), with the exception of 1 animal in the sedentary group, which died during the experimental protocol.
The mice were matched for age under the same environmental conditions. This experiment began when the tumor became palpable, which occurred around the 12th week of age and 20 g body weight and ended when the tumor reached 1 cm3 (average: 22 weeks), according to regulations from the Scientific Committee for Animal Research of the University of São Paulo Medical School. Body weight was assessed every week and tumor growth every 2 to 3 days. 15 Echocardiography and ETT were conducted at the beginning and at the end of the experiment. When tumors reached 1 cm3, the animals were euthanized after lethal dose by isoflurane (5%) inhalation. After loss of consciousness and motor activity, there was exsanguination and removal of tissues for analysis. Lungs, liver, spleen, kidneys, white and brown fat, anterior tibial and soleus muscles, and tumors were weighed and banked (Figure 1). The heart was only deposited.
Figure 1. Experimental protocol.
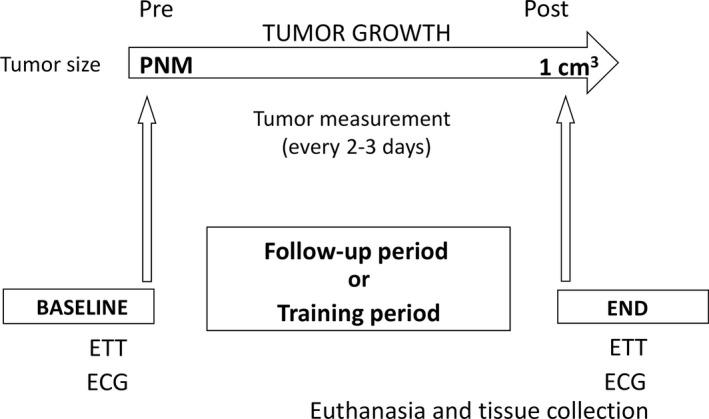
ETT indicates exercise tolerance test; PNM, palpable and not measurable tumor; Pre, pre phase tumor growth (BASELINE); and Post, post phase tumor growth (END).
Next, we randomized MMTV‐PyMT+ mice into 2 groups: sedentary (n=10) and exercise‐trained (n=12). The animals were matched for age and selected in a systematic random manner. ECG and ETT evaluation were performed when the tumor became apparent and when it reached 1 cm3. In addition, the ETT was performed every 4 weeks during the experimental protocol to readjust training intensity. The test was performed in the control group in the same period. Body weight was assessed every week and tumor growth every 2 to 3 days. 15 At the end of the protocol, all animals were euthanized after lethal dose by isoflurane (5%) inhalation. After loss of consciousness and motor activity, there was exsanguination and removal of tissues for analysis. Heart, liver, spleen, kidneys, white and brown fat, anterior tibial and soleus muscles, and tumors were weighed and banked for analysis (Figure 1). The lungs were only inflated and stored for further analysis.
The researchers analyzing the study samples were blinded to the experimental arms to assure both animal experiments were performed in a blinded manner.
Measures and Procedures
During the experimental protocol, measurements were performed in the morning, while the functional procedures were performed in the afternoon.
Exercise Tolerance Test
ETT was performed on a motor treadmill as previously described. 16 In brief, exercise started with a 6‐m/min speed increment of 3 m/min every 3 minutes up to the maximal speed tolerated. Exhaustion was determined when the mice could no longer run despite further speed increment. The maximal distance, maximal velocity, and duration of the exercise were registered.
ET Protocol
ET was performed on a treadmill at 50% to 60% of the maximal speed achieved in the ETT. Training sessions occurred 5 times a week, 16 with a session duration of 60 minutes.
High‐Resolution Echocardiography
To assess cardiac morphology and function, transthoracic high‐resolution echocardiography was performed using a Vevo 2100 system (Visual Sonics) with an MS400 (30‐MHz centerline frequency) probe. In brief, mice were anesthetized with isoflurane (Zoetis IsoFlo; induction 3.0% and maintenance 1%–3%) and hair was removed from the chest. Mice were subsequently placed in the supine position on a heated table with embedded ECG leads and the core temperature maintained at 37°C. Anesthesia was maintained with 1% to 3% isoflurane throughout the study. Image acquisition was performed by 2‐dimensional mode in the parasternal long‐axis view followed by the short‐axis and apical 4‐chamber views. Two‐dimensional guided M‐mode imaging was used to measure left ventricular (LV) end‐diastolic diameter (LVEDD), LV end‐systolic diameter (LVESD), interventricular septum (IVS), and posterior wall thickness (LVPW), corrected by body weight. Also, the LV end‐diastolic volume (LVEDV), LV end‐systolic volume (LVESV) and mass , both corrected by body weight, fractional shortening , and ejection fraction by Teichholz method were calculated from M‐mode echocardiography. 17 Left atrium (LA) diameter was acquired from 2‐dimensional parasternal long‐axis view. Diastolic function was evaluated using conventional echocardiography with tissue Doppler imaging and pulsed‐wave Doppler techniques. Transmitral inflow velocities were recorded from an apical long‐axis view by setting the sample volume in the tip of the mitral leaflets. From the pulsed‐wave Doppler spectral waveforms, the peak early‐ and late‐diastolic transmitral velocities (E and A waves) to obtain the E/A ratio were measured. E‐wave deceleration time and isovolumetric relaxation time (IVRT) were corrected by heart rate dividing them by the square root of the R‐R interval. Isovolumetric contraction time (IVCT) and ejection time (EjT) were measured to obtain myocardium performance index . From the tissue Doppler spectral waveforms, E′ (early diastolic mitral annular peak velocity at the septum) was measured and E/E′ ratio was calculated. 17
Speckle‐tracking imaging analyses were performed on parasternal long‐axis and short‐axis view (papillary level) using a Visual Sonics Vevo 2100 system (Visual Sonics). Image depth, width, and gain settings were optimized to improve image quality. Two‐dimensional loops with a frame rate ≥200 frame/s were utilized for speckle‐tracking imaging. All images were digitally stored in cine loops consisting of 300 frames, acquired, and then analyzed using Vevo Strain Software (Vevo LAB1.7.1). Strain, which evaluates length changes relative to initial length, was calculated either in the radial short‐ and long‐axis (thickening of the LV wall along radius axis) or longitudinally (shortening of the LV wall along long axis) and circumferentially (reduction in the circumference of the LV cavity). Strain rate, defined as the rate of change of this deformation over time, was also measured. 18
Cardiac Morphological and Structural Analysis
Heart sections were fixed in 10% buffered formalin for 48 hours. After routine histologic processing, paraffin‐embedded tissue was sectioned (5‐µm‐thick sections) and the histologic slides were stained with hematoxylin‐eosin and Sirius red for quantitative analysis of cardiomyocyte diameters and of collagen rate, respectively. Measurement of the transverse diameter of the cardiomyocytes was performed in a computerized system (LEICA QUANTIMET 500) coupled with a ×40 magnification optical microscope. Cardiomyocyte diameter was calculated from an average of all fields of a single cut of the LV per animal, considering the fibers intact. The degree of myocardial fibrosis was analyzed by means of a complete ventricular wall scan performed under a polarized light optical microscope (OLYMPUS BX‐41) at ×40 magnification. The type I collagen (yellowish orange and reddish orange) and type III collagen (green or yellowish green) were evaluated by staining. The analysis was performed by ImageJ software (National Institutes of Health) using the threshold color plug‐in to obtain the collagen percentage by analyzing the stained pixels according to the area measurement. Threshold color values for each collagen type were standardized: matrix 0 to 40 for collagen type I red color and 45 to 120 for collagen type III green color, with saturation 0 to 255 and brightness 5 to 225 for both.
Expression of Protein Levels Analysis by Western Blot
The protein levels of sodium/calcium exchanger, sarcoplasmic or endoplasmic reticulum calcium ATPase cardiac isoform 2a (SERCA2a), and phospholamban and phospho‐phospholamban (p‐phospholamban) in the heart muscle lysate were analyzed by Western blot. Frozen samples were homogenized in cell lysis buffer (100 mmol/L TrisHCl, 50 mmol/L NaCl, 1% Triton X‐100) and protease and phosphatase inhibitor cocktail (1:100; Sigma‐Aldrich). Heart tissue debris was removed by centrifugation at 3000g, 4°C, for 10 minutes. Samples were loaded and subjected to SDS‐PAGE on polyacrylamide gels (8%–15%) depending on the protein molecular weight. After electrophoresis, proteins were electrotransferred to a nitrocellulose membrane (BioRad Biosciences). Equal loading of samples (30 μg) and even transfer efficiency were monitored with the use of 0.5% Ponceau staining of the blot membrane. The blot membrane was then incubated in a blocking buffer (5% bovine serum albumin, 10 mmol/L Tris‐HCl [pH 7.6], 150 mmol/L NaCl, and 0.1% Tween 20) for 2 hours at room temperature and then incubated overnight at 4°C with anti‐sodium/calcium exchanger (Thermo Fisher, #MA3‐926), anti‐SERCA2 ATPase (Thermo Fisher, #MA3‐919), anti‐phospholamban (Thermo Fisher, #MA3‐922), and anti–p‐phospholambanSer16,Thr17 (Thermo Fisher, #711401) antibodies. Binding of the primary anti‐sodium/calcium exchanger, anti‐SERCA2 ATPase, anti‐phospholamban, anti–p‐phospholambanSer16,Thr17, and anti‐GAPDH was detected by secondary antibody (IRDye 800CW anti‐mouse [#926‐32210] or IRDye 800CW anti‐rabbit [#926‐32211], and LI‐COR Biosciences), and detection was performed by LI‐COR (LI‐COR Biosciences). The bands were analyzed using Image Studio Lite software (LI‐COR Biosciences). The results of protein expression were normalized by Ponceau staining. They are expressed as a percentage of control expression for each group. Of note, the Western blot assays were run in triplicate for all of the Ca2+‐handling proteins, with the exception of the p‐phospholamban protein, performed in duplicate.
Citrate Synthase Activity
Activity of citrate synthase enzyme in the anterior tibial and soleus muscles was evaluated as a marker of ET effectiveness. The enzyme activity was determined by means of commercial citrate synthase assay kit (Sigma‐Aldrich).
Statistical Analysis
Values are expressed as means±SD or medians and interquartile range (IQR). Animal body weights were evaluated weekly, but only the values of every 2 weeks were used for the analysis. The echocardiogram and speckle tracking measures were compared according to group and time of evaluation using generalized estimation equations with normal marginal distribution and identity link function, assuming a first‐order autoregressive correlation matrix between moments. 19 In case of significant difference, the results were followed by Bonferroni multiple comparisons to identify between group and time differences. 20 The nonparametric parameters or those with a sample size of <15 animals were compared by using Mann‐Whitney test. 21 The analyses were performed using SPSS for Windows version 20.0 software (IBM) and tabulated using Microsoft Excel 2003 software. The tests were performed with a 5% significance level.
RESULTS
Breast Cancer Provokes Physical Characteristic Changes
Body weight increased in both MMTV‐PyMT− and MMTV‐PyMT+ mice (Figure S1, P time<0.001). Despite significant interaction (P interaction=0.013), it was not possible to identify difference between groups throughout the study. Body weight after euthanasia was higher in MMTV‐PyMT+ mice (P<0.001). This difference was caused by tumor burden, since body weight without tumor was similar between groups (Figure S1). Anterior tibial muscle, lung, and brown and white fat weight were not different between MMTV‐PyMT+ and MMTV‐PyMT− mice. Soleus muscle and kidney weight was lower and liver and spleen weight were higher in MMTV‐PyMT+ mice compared with MMTV‐PyMT− mice (Table S1).
Breast Cancer Changes Physical Capacity
Time to exhaustion (P interaction=0.031) and treadmill velocity (P interaction=0.037) in the maximal exercise test were significantly lower in the MMTV‐PyMT+ mice. Distance in the maximal exercise test was statistically lower in the MMTV‐PyMT+ mice (P group=0.003) regardless of the time evaluated. These results are summarized in Table 1.
Table 1.
Physical Capacity Obtained in the Maximal Exercise Test in MMTV‐PyMT− and MMTV‐PyMT+ Mice at the Beginning and End of Experimental Protocol
| Variable | MMTV‐PyMT− | MMTV‐PyMT+ | P Group | P Time | P Interaction | ||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| Distance, m | 703.4±128.5 | 599.1±224.7 | 653.1±95.9 | 384.9±187.6 | 0.003* | <0.001* | 0.079 |
| Time, min | 35.9±3.4 | 32.9±6.2 | 34.6±2.5 | 25.7±6.4 | 0.001* | <0.001* | 0.031* |
| Velocity, m/min | 36.2±3.3 | 33.7±6.3 | 34.9±2.8 | 26.6±6.6 | 0.002* | <0.001* | 0.037* |
Generalized estimation equations with normal distribution and identity link function using AR(1) correlation matrix between evaluations. Values are expressed as means±SD. One mouse in the mouse mammary tumor virus–polyomavirus middle T antigen (MMTV‐PyMT)− group and 1 mouse in the MMTV‐PyMT+ group were not included in exercise tolerance test. The MMTV‐PyMT− mouse refused to run on the treadmill, while the MMTV‐PyMT+ mouse had its stride motion compromised by the tumor. Thus, 13 mice in the MMTV‐PyMT− and 14 mice in the MMTV‐PyMT+ groups were included in this study.
Significant difference.
Breast Cancer Alters Cardiac Morphology and Function
The pooled data (beginning and end of the study) of the LVEDD (PyMT− pre: 0.185±0.019 and post: 0.168±0.01 mm/g versus PyMT+ pre: 0.177±0.014 and post: 0.151±0.014 mm/g [P group<0.001, P interaction=0.417]) and LVEDV (PyMT− pre: 2.99±0.41 and post: 2.64±0.38 μL/g versus PyMT+ pre: 2.61±0.35 and post: 2.45±0.34 μL/g [P group=0.003, P interaction=0.437]) were lower in the MMTV‐PyMT+ mice when compared with MMTV‐PyMT− mice (Figure 2). LVESD and LVESV were similar between MMTV‐PyMT +and MMTV‐PyMT− mice. The LA responses were statistically different between groups throughout the experimental protocol (P interaction=0.004). The comparison between groups showed that at the beginning of the study, the LA was not different between the MMTV‐PyMT+ and MMTV‐PyMT− groups; however, at the end of the study, the LA was lower in MMTV‐ PyMT+ mice (0.079±0.001 versus 0.091±0.008 mm/g, P=0.030) (Figure 2 and Table S2). The pooled data (beginning and end of the study) of LV mass was lower in MMTV‐PyMT+ mice than in MMTV‐PyMT− mice (PyMT− pre: 4.297±0.798 and post: 3.396±0.496 mg/g versus PyMT+ pre: 3.454±0.649 and post: 2.968±0.601 mg/g [P group<0.001, P interaction=0.365]) (Figure 2 and Table S2). E/E′ wave, E‐wave deceleration time, and IVRT values were not different between groups. Similarly, heart rate, fractional shortening, ejection fraction, and myocardium performance index were not different in MMTV‐PyMT− and MMTV‐PyMT+ mice.
Figure 2. Morphological and functional cardiac parameters in mouse mammary tumor virus–polyomavirus middle T antigen (MMTV‐PyMT)− and MMTV‐PyMT+ mice in the pre‐experimental and post‐experimental protocol.
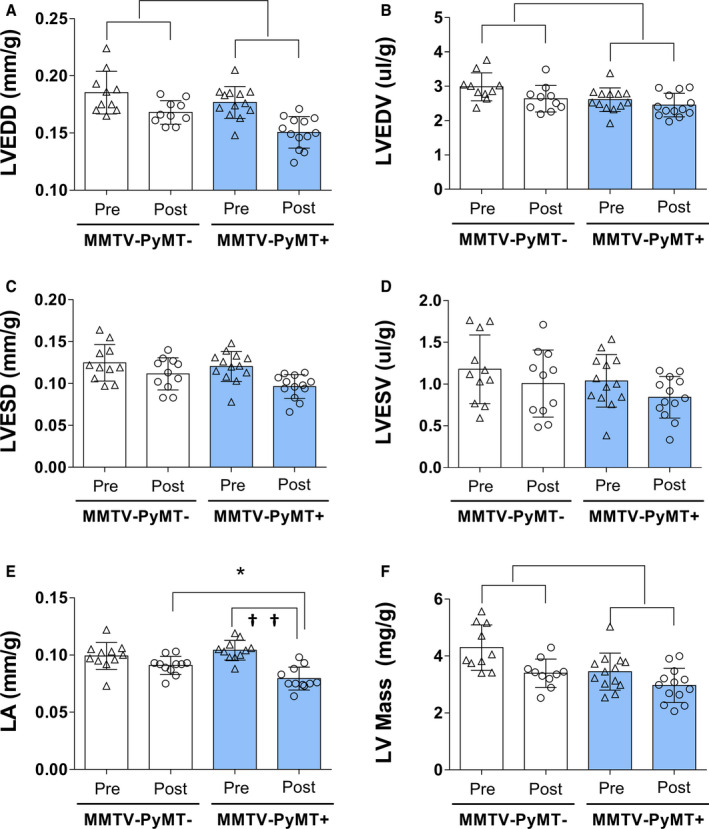
Left‐ventricular (LV) end‐diastolic diameter (LVEDD) (A); LV end‐diastolic volume (LVEDV) (B); LV end‐systolic diameter (LVESD) (C); LV end‐systolic volume (LVESV) (D); left atrium (LA) (E); and LV mass (F). Parameters were corrected by weight. LVEDD: P group<0.001, P interaction=0.417; LVEDV: P group=0.003, P interaction=0.437; LA: P interaction=0.004; post hoc test: * P=0.030, between group difference and †† P<0.001, within MMTV‐PyMT+ group difference; and LV mass: P group<0.001, P interaction=0.365. Generalized estimation equations with normal distribution and identity link function using AR(1) correlation matrix between evaluations followed by Bonferroni multiple comparison. Values are expressed as means and SD. Four mice in the MMTV‐PyMT− and 2 mice in the MMTV‐PyMT+ were excluded from this analysis because of poor imaging quality on echocardiography. Thus, 10 mice in the MMTV‐PyMT− and 13 mice in the MMTV‐PyMT+ were involved in this analysis. Of note, 2 LA measures were not obtained in the MMTV‐PyMT+ mice.
Longitudinal strain and strain rate at the beginning and at the end of the study are shown in Figure 3. The pooled data (beginning and end of the study) of the longitudinal strain (PyMT− pre: −14.4±4.8 and post: −14.5±2.7% versus PyMT+ pre: −12.4±2.4 and post: −9.8±1.7% [P group<0.001, P interaction=0.126]) and strain rate (PyMT− pre: −5.07±2.27 and post: −6.03±3.56 s−1 versus PyMT+ pre: −4.39±1.47 and post: −3.66±0.92 s−1 [P group=0.030, P interaction=0.168]) were impaired in the MMTV‐PyMT+ mice when compared with MMTV‐PyMT− mice. Both the longitudinal strain and strain rate were statistically lower in absolute value in animals with cancer than in control animals. Radial strain and strain rate, radial and circumferential short systolic axis strain, and strain rate were similar between MMTV‐PyMT− and MMTV‐PyMT+ mice (Table S2).
Figure 3. Cardiac function by speckle tracking in mouse mammary tumor virus–polyomavirus middle T antigen (MMTV‐PyMT)− and MMTV‐PyMT+—longitudinal long‐axis strain (A) and strain rate (B).
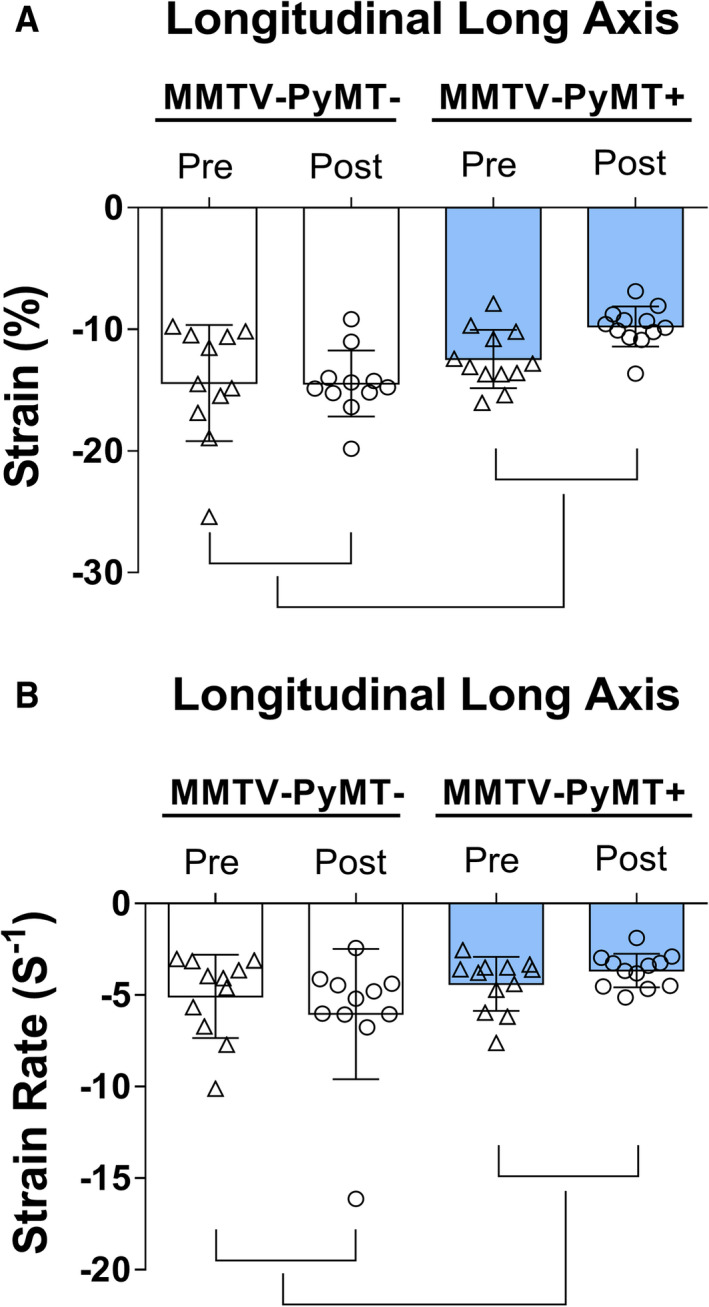
Longitudinal strain (P group<0.001, P interaction=0.126) and strain rate (P group=0.030, P interaction=0.168) were lower in MMTV‐PyMT+ mice when compared with MMTV‐PyMT− mice. Pre: pre phase tumor growth and post: post phase tumor growth. Generalized estimation equations with normal distribution and identity link function using AR(1) correlation matrix between evaluations. Values are expressed as means and SD. Three mice in the MMTV‐PyMT− and 3 mice in the MMTV‐PyMT+ groups were excluded from the speckle‐tracking analysis because of poor imaging quality. Thus, 11 mice in the MMTV‐PyMT− and 12 mice in the MMTV‐PyMT+ were involved in this analysis.
Cardiac myocyte diameter and cardiac collagen volume fraction were similar between MMTV‐PyMT− and MMTV‐PyMT+ mice (Table 2).
Table 2.
Cardiac Morphology in MMTV‐PyMT− and MMTV‐PyMT+
| Variable | MMTV‐PyMT− | MMTV‐PyMT+ | P Value | ||
|---|---|---|---|---|---|
| Median (IQR) | N | Median (IQR) | N | ||
| Myocyte diameter, µm | 16.9 (15.8–20.7) | 7 | 16.7 (16.3–18) | 8 | >0.999 |
| Type 1 collagen, µm2 | 339.3 (93.2–488.1) | 7 | 381.7 (222.5–497.3) | 7 | 0.535 |
| Type 3 collagen, µm2 | 403.4 (107–921.4) | 7 | 902.5 (285–1000.8) | 7 | 0.535 |
| Type 1+3 collagen, µm2 | 911 (194.9–1261.6) | 7 | 1125 (621.2–1498.1) | 7 | 0.383 |
Mann‐Whitney test. Values are expressed as medians and interquartile range (IQR). MMTV‐PyMT indicates mouse mammary tumor virus–polyomavirus middle T antigen.
Breast Cancer Changes Myocyte Ca2+‐Handling Protein Expression
In an attempt to understand the molecular basis of the alterations in the longitudinal strain and strain rate in the MMTV‐PyMT+ mice at end of the study protocol, we studied Ca2+‐handling proteins in the cardiac myocyte (Figure 4). In the MMTV‐PyMT+ mice, phospholamban protein expression was increased (247.8% [IQR, 192.3%–360.4%] versus 105.4% [IQR, 76.8%–123.7%], P=0.011), whereas sodium/calcium exchanger protein expression was decreased (40.5% [IQR, 11%–66.6%] versus 101.5% [IQR, 64.2%–143.5%], P=0.017) compared with MMTV‐PyMT− mice. SERCA2a protein expression was not different between the MMTV‐PyMT+ and MMTV‐PyMT− groups (51.1% [IQR, 37%–84.5%] versus 91.3% [IQR, 55.2%–159.7%], P=0.165); however, the SERCA2a/phospholamban ratio was reduced in the MMTV‐PyMT+ mice (0.23% [IQR, 0.15%–0.61%] versus 0.86% [IQR, 0.65%–1.41%], P=0.007). We also observed that p‐phospholamban was lower in the MMTV‐PyMT+ compared with the MMTV‐PyMT− mice (31.2% [IQR, 3.5%–69.6%] versus 83.8 [IQR, 47.9%–163.3%], P=0.038), but because of the lack of a sufficient sample we were able to test this marker only 2 times.
Figure 4. Cardiac myocyte Ca2+‐handling proteins in mouse mammary tumor virus–polyomavirus middle T antigen (MMTV‐PyMT)− and MMTV‐PyMT+ mice—phospholamban (A); SERCA2a (sarcoplasmic or endoplasmic reticulum calcium 2 ATPase) (B); phosphorilated phospholamban (p‐phospholamban) (C); sodium/calcium exchanger (NCX) (D); and SERCA2a/phospholambam ratio (E).
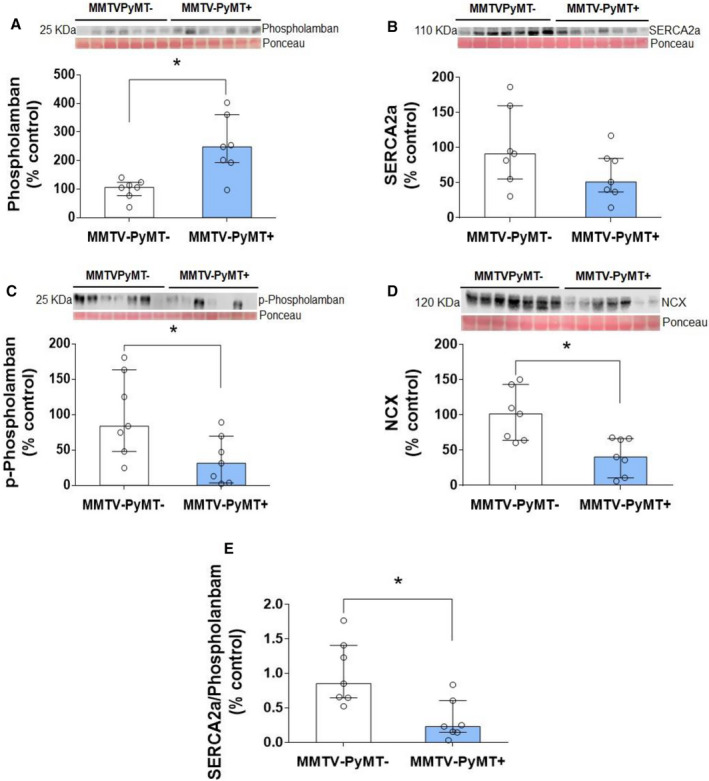
Western blot bands are shown for each protein. In the MMTV‐PyMT+ mice, phospholamban protein expression was increased (P=0.011) and SERCA2a protein expression was not different between the MMTV‐PyMT+ and MMTV‐PyMT− groups (P=0.165). SERCA2a/phospholamban ratio was reduced in the MMTV‐PyMT+ mice (P=0.007). p‐Phospholamban and NCX protein expression were decreased (P=0.038 and P=0.017, respectively) compared with MMTV‐PyMT− mice. Mann‐Whitney test. Values are expressed as medians and interquartile ranges (IQRs). Animals per group: 7 MMTV‐PyMT− and 7 MMTV‐PyMT+.
ET Does Not Reverse Morphologic and Physiologic Changes in MMTV‐PyMT+ Mice and Does Not Delay Time to Tumor Growth
Next, we assessed the impact of ET in modulating the changes in exercise capacity, cardiac function, and tumor growth in the MMTV‐PyMT+ mice. ET had no effect on tumor growth rate. Time of tumor growth to reach 1 cm3 was not significantly different between sedentary and exercise‐trained mice (11.0±1.3 versus 12.8±0.6 weeks, P=0.215).
ET did not affect animal body weight (Figure S2; P interaction=0.930) or weight of the heart, anterior tibial and soleus muscles, liver, spleen, kidneys, and brown and white fat (Table S3; P>0.05). ET did not seem to prevent the reduction in physical capacity provoked by cancer (Figure S3). There was a significant reduction in the distance covered in the ETTs performed during the experimental protocol in both groups (P time<0.001), although no significant difference in running distance was observed between the sedentary and exercise‐trained groups at 0, 1, 2, and 3 months (P interaction=0.069). This occurred despite a significant increase in citrate synthase enzyme activity in both anterior tibial and soleus muscle (Figure S4; P<0.001).
Data of cardiac morphology and function in sedentary and exercise‐trained MMTV‐PyMT+ mice are shown in Figure S5 and Table S4. ET decreased LVESD (P group=0.038, P interaction=0.226) and LVEDV (P group=0.026, P interaction=0.431), but did not change the other parameters associated with cardiac morphology (Table S4). Despite the average difference in longitudinal strain rate responses throughout the experimental protocol (P interaction=0.032; Table S4), it was not possible to identify differences between sedentary and exercise‐trained MMTV‐PyMT+ mice.
ET did not improve phospholamban protein expression. Likewise, ET did not change SERCA2a, p‐phospholamban, and sodium‐calcium exchanger protein expression. In addition, ET did not alter SERCA2a/phospholamban ratio (Figure S6). Additional results can be found in Data S1.
DISCUSSION
Previous studies have shown that chemotherapy agents used for treatment of breast cancer are associated with cardiotoxicity. 7 , 22 Changes in QT interval, 6 , 23 ventricular arrhythmias, 6 , 24 acute coronary syndrome, 5 , 25 and systolic and diastolic LV dysfunction have been reported in patients undergoing chemotherapy. However, the impact of cancer progression in cardiovascular function remains poorly addressed. In this regard, our study shows for the first time that breast cancer itself, in an advanced stage, is associated with myocardial dysfunction. In MMTV‐PyMT+ mice, we show that the presence of breast cancer was associated with worse longitudinal long‐axis strain and strain rate, known subclinical markers of cardiac dysfunction, and impaired exercise capacity, both at least partly related to Ca2+protein‐handling deregulation.
The evaluation of ventricular function by speckle‐tracking imaging has been highly used in clinical practice. Ventricular longitudinal deformation alterations have been used for early detection of ventricular impairment before changes in circumferential and radial deformations in most cardiac diseases with high sensitivity and specificity. 26 In addition, longitudinal deformation changes are prognostic of cardiotoxicity 26 even in the presence of preserved ejection fraction. 7 Our study shows for the first time that breast cancer is associated with impairment in LV longitudinal strain and strain rate, both consistent with subclinical myocardial dysfunction. 27 Furthermore, these myocardial alterations are associated with an increase in phospholamban expression and a decrease in sodium/calcium exchanger, p‐phospholamban expression, and SERCA2a/phospholamban ratio in the cardiac myocyte of MMTV‐PyMT+ mice, all consistent with altered myocardial Ca2+ handling. Ca2+‐related proteins play an important role in myocardial contraction and relaxation. Phospholamban binds SERCA2a in a 1:1 heterodimeric regulatory complex, which results in inhibition of SERCA2a activity. 28 , 29 Reduction in phosphorylation of phospholamban at Ser16 by protein kinase A and Thr17 by calmodulin‐dependent protein kinase II increases the inhibition of phospholamban on SERCA2a. 30 These responses reduce Ca2+ reuptake into the sarcoplasmic reticulum during the muscle relaxation phase and the load of Ca2+ necessary for myocardial contraction in the subsequent cardiac cycle, 31 which could explain the impairment in LV longitudinal strain and strain rate. Although our findings do not establish a definitive causal effect, they strongly suggest that Ca2+‐handling deregulation plays at least a partial role in breast cancer–induced cardiac dysfunction.
Another important finding in our study was the association between breast cancer and changes to cardiac morphology. The reductions in LV diameter and volume associated with lower LV mass and LA size is suggestive of cardiac remodeling in consequence of breast cancer. These morphological changes can predispose to progression of ventricular dysfunction and malignant arrhythmias. 32 Cancer cachexia has been associated with loss of cardiac mass and, consequently, cardiac dysfunction 33 and sarcopenia. The sarcopenia has been described in 25% of patients with metastatic breast cancer. 34 , 35 In fact, we found decreased soleus muscle in MMTV‐PyMT+ mice. Despite the changes in cardiac morphology, we found no changes in cardiomyocyte diameter and fibrosis. The histological adaptations related to heart mass loss seem to occur as a late compensation event, along with manifestations of overt clinical cardiac dysfunction, such as a fall in ejection fraction and fractional shortening. 33
Other than cardiac dysfunction, our findings in the MMTV‐PyMT mouse model highlight that the systemic effects of cancer can affect other organs as well. Some findings include increased liver and spleen dimensions and decreased kidneys size compared with MMTV‐PyMT− mice. Despite no functional studies having been performed in these organs, hepatomegaly and kidney atrophy are well‐establish signs of organ dysfunction. 36 , 37 In addition, splenomegaly has been associated with changes in immune function 38 , 39 that can further affect the prognosis. 40 Spleen enlargement was associated with an increase in the percentage of CD3+T lymphocytes and CD8+T lymphocytes. 41 Of note, ET did not impact the weight of these organs in MMTV‐PyMT+ mice.
Our group has previously reported that ET improves the net balance of cardiac Ca2+‐handling–related proteins in heart failure, 42 and similar findings were observed in obese rat models. 9 This improvement seems to be related to a reduction in cardiac phospho–Thr17‐phospholamban and phospho–Ser2808‐Ryanodine protein expression, which ultimately improves cardiac function. 9 Based on these findings, we hypothesized that ET would prevent cancer‐induced cardiac dysfunction, but this was not the case. ET decreased LVESD and LVEDV, but physical capacity was not overall changed. Likewise, ET did not change cardiomyocyte Ca2+‐handling protein expression. It is likely that the lack of anti‐tumor effect of ET alone in the absence of specific anti‐cancer therapy to halt cancer progression is at least partly if not completely associated with these findings. This is supported by the fact that ET alone did not impact the tumor growth rate in the MMTV‐PyMT+ mice. Another possibility is that our exercise paradigm was not adequate to prevent the changes in cardiac function in MMTV‐PyMT+ mice. Despite forced treadmill exercise being an accepted model of animal training, it may be insufficient or inadequate for animals with ongoing cancer progression. We are convinced that studies involving ET in cancer models should continue. However, we recognize that these studies should be performed in the context of anti‐cancer treatment to understand how ET may sinergize with specific anti‐cancer therapies to promote cancer control and/or preservation of body homeostasis. This is an area of ongoing investigation in our group as well as others.
Study Limitations
The animals were euthanized when the tumor reached 1 cm3, ie, at the late stage of breast cancer. Thus, our study does not provide information on the effects of breast cancer on cardiac function during the development of the disease. The reduction in caveolin‐1 following transformation of activated oncogenes, including the polyomavirus middle T antigen (PyMT), 43 and the known role of caveolin‐1 in several cardiovascular alterations 44 could lead someone to raise the hypothesis that the cardiac alterations in the present study were due, in part, to the reduction in caveolin‐1. The activation in mitogen‐activated protein kinase (MAPK) and phosphoinositide 3‐kinase (PI3K) pathways associated with PyMT 45 , 46 might have also contributed to the cardiac alterations in the present study. The role of extracellular signal‐regulated kinase (ERK), c‐Jun N‐terminal kinase (JNK), and p38 MAPK has been established in animal models and humans with heart failure. 47 , 48 The increase in total PI3K activity can negatively regulate Ca2+ transients, which results in decreasing of positive inotropic effects of β‐adrenergic agonists. 49 Unfortunately, the caveolin‐1, MAPK, and PI3K were not investigated in our study. This is an interesting topic for further investigations.
The study was entirely conducted in animals, which limits its extrapolation to humans with breast cancer. In addition, we analyzed a breast cancer model (MMTV‐PyMT) that only resembles HER2‐positive breast cancer in humans. Thus, our findings cannot be generalized to other tumor types. However, the MMTV‐PyMT mouse is a unique transgenic model of multifocal breast adenocarcinoma because cancer development occurs similarly to that of invasive ductal carcinoma of the human breast. Cancer spontaneously develops and gradually progresses from hyperplasia, dysplasia, adenoma, and carcinoma because of expression of the polyoma middle oncoprotein (PyMT). 12 Therefore, we believe that our study will provide important information that will be useful for future investigations in the clinical setting.
Conclusions
Breast cancer is associated with decreased exercise capacity and subclinical LV dysfunction in MMTV‐PyMT+ mice, which is directly associated with dysregulation of cardiomyocyte Ca2+‐handling. ET alone is not an effective strategy to prevent or reverse these changes.
Sources of Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP#2015/22814‐5). Gomes‐Santos and Passos received scholarship from FAPESP (#2014/13690‐8 and #15/19076‐2, respectively). Brum, Oliveira, Salemi and C. E. Negrao were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ#306261/2016‐2; #313479/2017‐8; #307227/2018‐9 and; #303573/2015‐5).
Disclosures
None.
Supporting information
Data S1
Tables S1–S4
Figures S1–S6
(J Am Heart Assoc. 2021;10:e018076. DOI: 10.1161/JAHA.120.018076.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018076
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. GLOBOCAN 2020: new global cancer data|UICC. Available at: https://www.uicc.org/news/globocan‐2020‐new‐global‐cancer‐data. Accessed January 13, 2021.
- 2. Kesson EM, Allardice GM, George WD, Burns HJG, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344:e2718. DOI: 10.1136/bmj.e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. New Engl J Med. 2016;375:1457–1467. DOI: 10.1056/NEJMra1100265. [DOI] [PubMed] [Google Scholar]
- 4. Chow AY, Chin C, Dahl G, Rosenthal DN. Anthracyclines cause endothelial injury in pediatric cancer patients: a pilot study. J Clin Oncol. 2006;24:925–928. DOI: 10.1200/JCO.2005.03.5956. [DOI] [PubMed] [Google Scholar]
- 5. Herrmann J, Yang EH, Iliescu CA, Marmagkiolis K. Response by Herrmann et al. to Letter Regarding Article, “vascular toxicities of cancer therapies: the old and the new—an evolving avenue”. Circulation. 2016;133:1272–1289. DOI: 10.1161/CIRCULATIONAHA.115.018347. [DOI] [PubMed] [Google Scholar]
- 6. Buza V, Rajagopalan B, Curtis AB. Cancer treatment‐induced arrhythmias: focus on chemotherapy and targeted therapies. Circ Arrhythm Electrophysiol. 2017;10:1–13. DOI: 10.1161/CIRCEP.117.005443. [DOI] [PubMed] [Google Scholar]
- 7. Bloom MW, Hamo CE, Cardinale D, Ky B, Nohria A, Baer L, Skopicki H, Lenihan DJ, Gheorghiade M, Lyon AR, et al. Cancer therapy‐related cardiac dysfunction and heart failure part 1: definitions, pathophysiology, risk factors, and imaging. Circ Hear Fail. 2017;9:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hershman DL, Till C, Shen S, Wright JD, Ramsey SD, Barlow WE, Unger JM. Association of cardiovascular risk factors with cardiac events and survival outcomes among patients with breast cancer enrolled in SWOG clinical trials. J Clin Oncol. 2018;36:2710–2717. DOI: 10.1200/JCO.2017.77.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paulino EC, Ferreira JC, Bechara LR, Tsutsui JM, Mathias W, Lima FB, Casarini DE, Cicogna AC, Brum PC, Negrão CE. Exercise training and caloric restriction prevent reduction in cardiac Ca2+‐handling protein profile in obese rats. Hypertension. 2010;56:629–635. [DOI] [PubMed] [Google Scholar]
- 10. Medeiros A, Rolim NPL, Oliveira RSF, Rosa KT, Mattos KC, Casarini DE, Irigoyen MC, Krieger EM, Krieger JE, Negrão CE, et al. Exercise training delays cardiac dysfunction and prevents calcium handling abnormalities in sympathetic hyperactivity‐induced heart failure mice. J Appl Physiol. 2008;104:103–109. DOI: 10.1152/japplphysiol.00493.2007. [DOI] [PubMed] [Google Scholar]
- 11. Duivenvoorden HM, Spurling A, O’Toole SA, Parker BS. Discriminating the earliest stages of mammary carcinoma using myoepithelial and proliferative markers. PLoS One. 2018;13:1–12. DOI: 10.1371/journal.pone.0201370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. DOI: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park MK, Lee CH, Lee H. Mouse models of breast cancer in preclinical research. Lab Anim Res. 2018;34:160–165. DOI: 10.5625/lar.2018.34.4.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eckelman WC, Kilbourn MR, Joyal JL, Labiris R, Valliant JF. Justifying the number of animals for each experiment. Nucl Med Biol. 2007;34:229–232. DOI: 10.1016/j.nucmedbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 15. Parkins KM, Dubois VP, Hamilton AM, Makela AV, Ronald JA, Foster PJ. Multimodality cellular and molecular imaging of concomitant tumour enhancement in a syngeneic mouse model of breast cancer metastasis. Sci Rep. 2018;8:1–10. DOI: 10.1038/s41598-018-27208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferreira JC, Rolim NP, Bartholomeu JB, Gobatto CA, Kokubun E, Brum PC. Maximal lactate steady state in running mice: effect of exercise training. Clin Exp Pharmacol Physiol. 2007;34:760–765. DOI: 10.1111/j.1440-1681.2007.04635.x. [DOI] [PubMed] [Google Scholar]
- 17. Salemi VM, Bilate AM, Ramires FJ, Picard MH, Gregio DM, Kalil J, Neto EC, Mady C. Reference values from M‐mode and Doppler echocardiography for normal Syrian hamsters. Eur J Echocardiogr. 2005;6:41–46. DOI: 10.1016/j.euje.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 18. De Lucia C, Wallner M, Eaton DM, Zhao H, Houser SR, Koch WJ. Echocardiographic strain analysis for the early detection of left ventricular systolic/diastolic dysfunction and dyssynchrony in a mouse model of physiological aging. J Gerontol A Biol Sci Med Sci. 2019;74:455–461. DOI: 10.1093/gerona/gly139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCullagh P, Nelder JA. Generalized Linear Models. 2nd ed. London, England: Chapman and Hall; 1989:1–532. [Google Scholar]
- 20. Neter J, Kutner M, Wasserman W, Nachtsheim C. Applied Linear Statistical Models. 4th ed. New York, NY: McGraw‐Hill; 1996:1–1408. [Google Scholar]
- 21. Kirkwood BR, Sterne JA. Essential Medical Statistics. 2nd ed. New York, NY: John Wiley & Sons; 2003:1–512. [Google Scholar]
- 22. Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. DOI: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 23. Accordino MK, Neugut AI, Hershman DL. Cardiac effects of anticancer therapy in the elderly. J Clin Oncol. 2014;32:2654–2661. DOI: 10.1200/JCO.2013.55.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farmakis D, Parissis J, Filippatos G. Insights into onco‐cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63:945–953. DOI: 10.1016/j.jacc.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 25. Peretto G, Lazzeroni D, Sartorio CL, Camici PG. Cardiotoxicity in oncology and coronary microcirculation: future challenges in theranostics. Front Biosci (Landmark Ed). 2017;22:1760–1773. [DOI] [PubMed] [Google Scholar]
- 26. Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab‐induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–498. DOI: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 27. Nahum J, Bensaid A, Dussault C, Macron L, Clémence D, Bouhemad B, Monin JL, Rande JL, Gueret P, Lim P. Impact of longitudinal myocardial deformation on the prognosis of chronic heart failure patients. Circ Cardiovasc Imaging. 2010;3:249–256. DOI: 10.1161/CIRCIMAGING.109.910893. [DOI] [PubMed] [Google Scholar]
- 28. Cantilina T, Sagara Y, Inesi G, Jones LR. Comparative studies of cardiac and skeletal sarcoplasmic reticulum ATPases. J Biol Chem. 1993;268:17018–17025. [PubMed] [Google Scholar]
- 29. Sasaki T, Inui M, Kimura Y, Kuzuya T, Tada M. Molecular regulation of Ca2+ pump ATPase by phospholamban in cardiac sarcoplasmic reticulum. J Biol Chem. 1992;267:1674–1679. [PubMed] [Google Scholar]
- 30. Mattiazzi A, Mundiña‐Weilenmann C, Guoxiang C, Vittone L, Kranias E. Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions. Cardiovasc Res. 2005;68:366–375. DOI: 10.1016/j.cardiores.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 31. Díaz ME, Graham HK, O’Neill SC, Trafford AW, Eisner DA. The control of sarcoplasmic reticulum Ca content in cardiac muscle. Cell Calcium. 2005;38:391–396. DOI: 10.1016/j.ceca.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 32. Azevedo PS, Polegato BF, Minicucci MF, Paiva SA, Zornoff LA. Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq Bras Cardiol. 2016;106:62–69. DOI: 10.5935/abc.20160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Springer J, Tschirner A, Haghikia A, von Haehling S, Lal H, Grzesiak A, Kaschina E, Palus S, Pötsch M, von Websky K, et al. Prevention of liver cancer cachexia‐induced cardiac wasting and heart failure. Eur Heart J. 2014;35:932–941. DOI: 10.1093/eurheartj/eht302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang R, Bhat‐nakshatri P, Padua MB, Prasad MS, Anjanappa M, Jacobson M, Finnearty C, Sefcsik V, McElyea K, Redmond R, et al. Pharmacological dual inhibition of tumor and tumor‐induced functional limitations in transgenic model of breast cancer. Mol Cancer Ther. 2017;16:2747–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rossi F, Valdora F, Bignotti B, Torri L, Succio G, Tagliafico AS. Evaluation of body computed tomography‐determined sarcopenia in breast cancer patients and clinical outcomes: a systematic review. Cancer Treat Res Commun. 2019;21:1–7. DOI: 10.1016/j.ctarc.2019.100154. [DOI] [PubMed] [Google Scholar]
- 36. Collett JA, Paulose JK, Cassone VM, Osborn JL. Kidney‐specific reduction of oxidative phosphorylation genes derived from spontaneously hypertensive rat. PLoS One. 2015;10:1–19. DOI: 10.1371/journal.pone.0136441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goncalvesová E. [Liver in heart failure]. Vnitr Lek. 2014;60:298–303. [PubMed] [Google Scholar]
- 38. Mu J, Jeyanathan M, Shaler CR, Horvath C, Damjanovic D, Zganiacz A, Kugathasan K, McCormick S, Xing Z. Respiratory mucosal immunization with adenovirus gene transfer vector induces helper CD4 T cell‐independent protective immunity. J Gene Med. 2010;12:693–704. DOI: 10.1002/jgm.1487. [DOI] [PubMed] [Google Scholar]
- 39. Zanetti M, Castiglioni P, Ingulli E. Principles of memory CD8 T‐cells generation in relation to protective immunity. Adv Exp Med Biol. 2010;684:108–125. [DOI] [PubMed] [Google Scholar]
- 40. Marshall JC, Charbonney E, Gonzalez PD. The immune system in critical illness. Clin Chest Med. 2008;29:605–616. DOI: 10.1016/j.ccm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 41. Fang JJ, Zhu ZY, Dong H, Zheng GQ, Teng AG, Liu AJ. Effect of spleen lymphocytes on the splenomegaly in hepatocellular carcinoma‐bearing mice. Biomed Environ Sci. 2014;27:17–26. [DOI] [PubMed] [Google Scholar]
- 42. Rolim NPL, Medeiros A, Rosa KT, Mattos KC, Irigoyen MC, Krieger EM, Krieger JE, Negrão CE, Brum PC. Exercise training improves the net balance of cardiac Ca2+ handling protein expression in heart failure. Physiol Genomics. 2007;29:246–252. [DOI] [PubMed] [Google Scholar]
- 43. Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA. 1995;92:1381–1385. DOI: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wunderlich C, Schober K, Lange SA, Drab M, Braun‐Dullaeus RC, Kasper M, Schwencke C, Schmeisser A, Strasser RH. Disruption of caveolin‐1 leads to enhanced nitrosative stress and severe systolic and diastolic heart failure. Biochem Biophys Res Commun. 2006;340:702–708. DOI: 10.1016/j.bbrc.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 45. Wada M, Canals D, Adada M, Coant N, Salama MF, Helke KL, Arthur JS, Shroyer KR, Kitatani K, Obeid LM, et al. P38 delta MAPK promotes breast cancer progression and lung metastasis by enhancing cell proliferation and cell detachment Masayuki. Oncogene. 2018;36:6649–6657. DOI: 10.1038/onc.2017.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sai J, Owens P, Novitskiy SV, Hawkins OE, Vilgelm AE, Yang J, Sobolik T, Lavender N, Johnson AC, McClain C, et al. PI3K inhibition reduces mammary tumor growth and facilitates anti‐tumor immunity and anti‐PD1 responses. Clin Cancer Res. 2018;23:3371–3384. DOI: 10.1158/1078-0432.CCR-16-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677. DOI: 10.1161/01.CIR.103.5.670. [DOI] [PubMed] [Google Scholar]
- 48. Muslin AJ. MAPK signaling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin Sci. 2009;115:203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ghigo A, Laffargue M, Li M, Hirsch E. PI3K and calcium signaling in cardiovascular disease. Circ Res. 2017;121:282–292. DOI: 10.1161/CIRCRESAHA.117.310183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S4
Figures S1–S6


