Abstract
Background
Preeclampsia is a prominent risk factor for long‐term development of cardiovascular disease. Although existing studies report a strong correlation between preeclampsia and heart failure, the underlying mechanisms are poorly understood. One possibility is the glycoprotein growth factor activin A. During pregnancy, elevated activin A levels are associated with impaired cardiac global longitudinal strain at 1 year, but whether these changes persist beyond 1 year is not known. We hypothesized that activin A levels would remain increased more than 1 year after a preeclamptic pregnancy and correlate with impaired cardiac function.
Methods and Results
To test our hypothesis, we performed echocardiograms and measured activin A levels in women approximately 10 years after an uncomplicated pregnancy (n=25) or a pregnancy complicated by preeclampsia (n=21). Compared with women with a previously normal pregnancy, women with preeclampsia had worse global longitudinal strain (−18.3% versus −21.3%, P=0.001), left ventricular posterior wall thickness (0.91 mm versus 0.80 mm, P=0.003), and interventricular septal thickness (0.96 mm versus 0.81 mm, P=0.0002). Women with preeclampsia also had higher levels of activin A (0.52 versus 0.37 ng/mL, P=0.02) and activin/follistatin‐like 3 ratio (0.03 versus 0.02, P=0.04). In a multivariable model, the relationship between activin A levels and worsening global longitudinal strain persisted after adjusting for age at enrollment, mean arterial pressure, race, and body mass index (P=0.003).
Conclusions
Our findings suggest that both activin A levels and global longitudinal strain are elevated 10 years after a pregnancy complicated by preeclampsia. Future studies are needed to better understand the relationship between preeclampsia, activin A, and long‐term cardiac function.
Keywords: cardiac dysfunction, global longitudinal strain, hypertension, preeclampsia/pregnancy, pregnancy and postpartum
Subject Categories: Pregnancy, Echocardiography, Biomarkers
Nonstandard Abbreviations and Acronyms
- FSTL3
follistatin‐like 3
- GLS
global longitudinal strain
Clinical Perspective
What Is New?
Ten years after a pregnancy complicated by preeclampsia, women are found to have worse global longitudinal strain and higher levels of the profibrotic glycoprotein activin A than women who did not have preeclampsia.
Our study demonstrates a correlation between activin A level and worsening global longitudinal strain 10 years postpartum.
What Are the Clinical Implications?
The potential role of activin A in postpartum cardiac dysfunction raises the possibility that therapies targeted against activin A, need further investigation.
Preeclampsia is a hypertensive disorder that affects up to 5% of all pregnant women and is the most common medical complication of pregnancy. 1 Although preeclampsia was previously considered only a peripartum disease, it is now recognized as a risk factor for the long‐term development of cardiovascular disease. In a 2017 meta‐analysis of 22 studies and 6.4 million women, preeclampsia was associated with a fourfold increase in the development of postpartum heart failure and a twofold increased risk of coronary heart disease, stroke, and death caused by cardiovascular disease. 2 In addition, a 2019 study of 149 712 women likewise reported a 2.77% rate of first‐time cardiovascular events including heart failure, stroke, and cardiovascular deaths in the first 9 years after a delivery complicated by preeclampsia as opposed to a 1.4% rate in women after an uncomplicated pregnancy. 3 We have previously observed that women diagnosed with hypertensive disorders of pregnancy such as preeclampsia are twice as likely to be admitted with new‐onset heart failure within 90 days of delivery as women with normotensive pregnancies. 4
The mechanisms underlying preeclampsia‐induced postpartum heart failure are poorly understood. Although biomarkers such as soluble fms‐like tyrosine kinase 1 and placental growth factor are associated with antepartum cardiac dysfunction, they are unlikely to play a mechanistic role in the development of postpartum cardiac dysfunction, as their levels return to normal within 72 hours after a delivery complicated by preeclampsia. 5
One mechanistic possibility is dysregulated activin A signaling. Activin A is a glycoprotein growth factor normally involved in cellular differentiation and proliferation. Abnormal activin A regulation may contribute to fibrosis in preclinical models, 6 , 7 and lead clinically to hypertension and heart failure. 8 , 9 , 10 During pregnancy, activin A is produced by the placenta and by other cell types, such as macrophages, and circulating levels are highest at term. The role of activin A in the development of postpartum cardiac dysfunction, however, remains unclear.
Follistatin‐like 3 (FSTL3) is a coregulatory factor of activin A that binds to and antagonizes the effect of activin A by binding activin A through its N‐terminal domain. Because FSLT3/activin A binding may affect activin A levels, measurement of FSTL3 levels may play an important role in understanding the effect of activin. 11
Global longitudinal strain (GLS) with speckle tracking echocardiography is a sensitive measure of subclinical cardiac dysfunction. Because GLS differentiates active from passive contraction and is less sensitive to loading conditions than ejection fraction, it is sensitive to the early onset of subclinical systolic heart failure in patients with preeclampsia. 12 , 13 , 14 In patients with preserved ejection fraction, GLS can detect early subclinical cardiac dysfunction before a decline in ejection fraction. 15 GLS also assesses subendocardial longitudinal fibers, which are particularly sensitive to states with reduced afterload such as pregnancy. 12 , 16
Despite this high incidence of serious cardiovascular morbidity and mortality after a preeclamptic pregnancy, no mechanistic biomarker has been identified that is elevated both during a preeclamptic pregnancy and postpartum, and that correlates with ongoing cardiac dysfunction. Further echocardiographic manifestations of long‐term cardiac dysfunction after a preeclamptic pregnancy in the United States remain poorly described.
We hypothesized that after a pregnancy complicated by preeclampsia, levels of circulating activin A would be high and correlate with worsened GLS. To test our hypothesis, we studied women with preeclampsia and patients with normotensive pregnancies 10 years after their index pregnancy. Our prespecified primary analysis was to determine whether activin A levels are elevated 10 years after a preeclamptic pregnancy. We secondarily assessed the correlation between activin A levels and GLS in this cohort, and measured activin A/FSTL3 ratios, as FSTL3 is a coregulatory factor of activin.
METHODS
Study Design and Oversight
This cohort study was approved by the institutional review board at the University of Chicago Medical Center (Chicago, IL) with written informed consent and performed in accordance with all institutional policies (#IRB18‐0248). All data and supporting materials have been provided with the published article.
Human Subjects
Eligible patients were women who delivered at the University of Chicago between 2007 and 2011. The first pregnancy was defined as the index pregnancy for study participation. These women were identified based on retrospective review of the perinatal database, which collects demographics, diagnoses, and outcomes for all deliveries at the University of Chicago. Once identified, patients from this database had diagnoses confirmed by a senior maternal fetal medicine specialist (S.R.) based on the 2019 American College of Obstetricians and Gynecologists definition of preeclampsia. 17 The list of eligible patients was sorted using a random‐number generator. Mailing address information was abstracted from the electronic medical record.
For the index pregnancy, we excluded patients with a history of chronic hypertension, gestational hypertension, advanced maternal age (>35 years), substance abuse, diabetes mellitus, chronic kidney disease, myocardial infarction, stroke, lupus, any vasculopathy, and moderate to severe asthma. Eligible women were then contacted in batches by postal mail and asked to call the study coordinator if they were interested in participating. Overall, 1036 letters were mailed, 212 letters were undeliverable, and calls back were received from 79 women. When we attempted to follow up with interested participants, 50 could be reached after 3 attempts. Four additional patients were determined to be ineligible at the time of the study visit. In the final study cohort of 46 participants, 21 had been diagnosed with preeclampsia at the time of the index pregnancy, and 25 had had a normotensive pregnancy.
Data Collection at the Index Pregnancy
Demographic information was collected from electronic medical records at the time of the index pregnancy. Preeclampsia was defined as systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg occurring after 20 weeks of gestation with proteinuria. The blood pressure readings were documented on at least 2 occasions, 4 hours to 2 weeks apart. Proteinuria was defined as urinary excretion of ≥0.3 grams of protein in a 24‐hour period. Each diagnosis was confirmed by a senior author (S.R.) based on clinical data available at the time of delivery for the index pregnancy.
Data Collection at Study Enrollment (Follow‐Up)
Intervening history about subsequent medical problems and medications were collected at the time of enrollment by the study staff via an interview with the patient. The study staff collecting clinical information were blinded to the patients' echocardiogram results and biomarker information. Systolic and diastolic blood pressure were obtained using standardized blood pressure cuff measurements at the time of echocardiogram during the study follow‐up visit.
Echocardiography
All echoes were by a certified sonographer in an Intersocietal Commission for the Accreditation of Echocardiography Laboratories Certified Cardiology Echocardiography Laboratory at the University of Chicago. A comprehensive 2‐dimensional echocardiographic examination was performed using a commercial ultrasound system (i33 or Epiq7; Philips Healthcare, Andover, MA) equipped with an X5‐1 probe by trained sonographers blinded to the patient’s diagnosis and to the levels of activin A. Transthoracic echocardiograms were performed and reported according to American Society of Echocardiography guidelines. 18 The sonographer performed a comprehensive examination including a complete 2‐dimensional and color‐flow Doppler valvular assessment along with measurement of indices of both systolic and diastolic function.
Ejection fraction and left atrial volume were calculated using the Simpson biplane disc method. Left ventricular mass index was calculated using the area length method. 19 GLS measurement was performed using fully automated TOMTEC software (AutoStrain, Tomtec Image Arena 1.2; Unterschleissheim, Germany), a vendor‐independent software package that utilizes a computer learning algorithm to facilitate endocardial border detection and has been validated against manual strain measurements. 20 Unlike manual analysis of GLS, automated analysis reduces variability with repeated measurements and minimizes inter‐ and intraobserver variability. 20 Measurement and interpretation of all echocardiography indices were performed in Dr. Lang's laboratory. All personnel performing and interpreting the echocardiograms were blinded to the patient’s diagnosis and biomarker levels.
Measurement of Circulating Activin A and FSTL3
Venous blood samples were collected from the subject at the time of their echocardiogram. Blood samples were centrifuged for 8 minutes at −4°C, and the plasma was then aliquoted, labeled with a study identification, and stored at −80°C. Because the regulatory hormone FSTL3 binds to and inactivates activin A, we also measured FSTL3 levels and calculated activin A/FSTL3 ratios to estimate free activin A levels. 21 A single operator (J.D.), blinded to clinical and echocardiographic information, performed activin A and FSTL3 assays on each plasma sample in duplicate using commercially available kits (Ansh Labs, Webster, TX). The activin A assay has an intra‐assay coefficient of variation of 4.23±0.06 ng/mL and an interassay coefficient of variation of 2.29±0.02 ng/mL.
Statistical Analysis
Descriptive statistics of the data were assessed and reported. Continuous data are presented as a mean±standard deviation or median (quartile 1, quartile 3) depending on variable distribution and assessed with a t test or Wilcoxon rank sum test, as appropriate. Normality of continuous parameters was assessed with a Shapiro–Wilk test. Categorical data are presented as frequencies and proportions, and assessed with a χ2 test, or in the event of small cell counts, Fisher exact test. Multivariable linear regression was employed to assess the relationship between GLS and both activin and preeclamptic pregnancy status after adjusting for potential confounders. In the final model adjustment, variables included age at enrollment, mean arterial pressure (MAP), race, and body mass index (BMI). These variables were selected because of their biological plausibility, clinical relevance, and because of baseline differences observed between groups at enrollment. Given the nonparametric distribution of biomarker data, log transformations were applied to meet model testing assumptions. In all analyses, 2‐sided P values <0.05 were considered statistically significant. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). No a priori power calculation was performed.
RESULTS
Demographics
A total of 46 women were included in the analysis. Baseline characteristics and data from the index pregnancy are shown in Table 1. Of the 46 women, 21 (45.6%) were preeclamptic during the index pregnancy. Although some divergences were observed, patients with preeclampsia did not statistically differ from patients without preeclampsia with regard to race, ethnicity, and smoking status at the time of the index pregnancy. Women with preeclampsia during their index delivery were younger, had a higher BMI at the follow‐up visit, and were more likely to carry a diagnosis of hypertension with higher MAP.
Table 1.
Characteristics of the Index Delivery
| Index Delivery Characteristics | All Patients, N=46 | Patients Without Preeclampsia, n=25 | Patients With Preeclampsia, n=21 | P Value |
|---|---|---|---|---|
| Age at index delivery, y | 28.04±5.33 | 28.92±5.51 | 27.00±5.05 | 0.23 |
| Race | 0.16 | |||
| White | 6 (13.04) | 5 (20.00) | 1 (4.76) | |
| Black | 35 (76.09) | 16 (64.00) | 19 (90.48) | |
| Other | 5 (10.87) | 4 (16.00) | 1 (4.76) | |
| Ethnicity | 0.49 | |||
| Hispanic | 2 (4.35) | 2 (8.00) | 0 (0) | |
| Non‐Hispanic | 43 (93.48) | 22 (88.00) | 21 (100.00) | |
| Unknown/refuse to answer | 1 (2.17) | 1 (4.00) | 0 (0) | |
| Smoking status at index pregnancy | 0.24 | |||
| Never smoked | 37 (80.43) | 22 (88.00) | 15 (71.43) | |
| Past/quit before pregnancy | 3 (6.52) | 2 (8.00) | 1 (4.76) | |
| Past/quit early pregnancy | 3 (6.52) | 1 (4.00) | 2 (9.52) | |
| Smoker | 3 (6.52) | 0 (0) | 3 (14.29) | |
| Gravida | 4 (3, 6) | 4 (3, 5) | 4 (3, 7) | 0.57 |
| Parity | 3 (2, 3) | 2 (2, 3) | 3 (2, 4) | 0.54 |
| Other preeclampsia pregnancy | 9 (20.00) | 0 (0) | 9 (45.00) | 0.0002 |
| Fetal sex | 0.57 | |||
| Male | 24 (52.17) | 14 (56.00) | 10 (47.62) | |
| Female | 22 (47.83) | 11 (44.00) | 11 (52.38) | |
| Mode of delivery | 0.09 | |||
| Vaginal | 29 (63.04) | 19 (76.00) | 10 (47.62) | |
| Vaginal birth after cesarean | 1 (2.17) | 0 (0) | 1 (4.76) | |
| Cesarean section | 16 (34.78) | 6 (24.00) | 10 (47.62) | |
| Blood loss, mL | 500 (250, 800) | 375 (250, 800) | 550 (500, 725) | 0.55 |
Data are presented as mean±standard deviation, median (quartile 1, quartile 3), or n (%) depending on variable type and distribution.
Data at the time of study enrollment are depicted in Table 2. At the time of enrollment, corresponding to the long‐term follow‐up, women with preeclampsia during their index delivery had a higher BMI at the follow‐up visit and were more likely to carry a diagnosis of hypertension with higher MAP.
Table 2.
Patient Characteristics at the Time of Study Enrollment (Follow‐Up)
| Follow‐up Characteristics | All Patients, N=46 | Patients Without Preeclampsia, n=25 | Patients With Preeclampsia, n=21 | P Value |
|---|---|---|---|---|
| Age at follow‐up, y | 37.91±6.11 | 39.72±6.02 | 35.76±5.62 | 0.03 |
| Current weight, kg | 79.1 (67.5, 103.3) | 76.2 (62.7, 93.3) | 83.2 (71.1, 119.0) | 0.16 |
| Current height, in | 64.43±3.13 | 65.22±3.00 | 63.50±3.09 | 0.06 |
| Current body mass index, kg/m2 | 30.70 (24.03, 38.65) | 27.79 (22.40, 36.45) | 33.70 (26.54, 43.79) | 0.03 |
| Smoking status at follow‐up | 0.09 | |||
| Never smoked | 43 (93.48) | 25 (100.00) | 18 (85.71) | |
| Past/quit before pregnancy | 3 (6.52) | 0 (0) | 3 (14.29) | |
| Hypertension | 9 (20.93) | 0 (0) | 9 (47.37) | 0.0002 |
| Mean arterial blood pressure | 95.5±14.8 | 88.2±12.3 | 104.5±12.6 | 0.0001 |
| Heart rate, beats per minute | 76.3±13.7 | 71.8±11.9 | 82.4±13.9 | 0.01 |
Data are presented as mean±standard deviation, median (quartile 1, quartile 3), or n (%) depending on variable type and distribution.
Echocardiography Measurements
At the time of enrollment (follow‐up), GLS was numerically higher (worse) in women whose prior pregnancy was complicated with preeclampsia than in control women whose delivery was normotensive (−18.31±2.68 versus −21.29±2.70, P=0.001; Figure 1). Left ventricular mass index was also higher in women with preeclampsia (median 70.12 [IQR 59.83, 80.77] versus 62.74 [IQR 54.82, 69.02], P=0.04), as was left ventricular posterior wall thickness (0.91 [IQR 0.84, 1.00] versus 0.80 [IQR 0.69, 0.88] mm, P=0.003) and interventricular septal thickness (0.96±0.14 versus 0.81±0.11 mm, P=0.002; Table 3). In a multivariable model adjusting for clinically relevant variables including MAP and BMI, the relationship between preeclampsia and GLS persisted (P=0.04).
Figure 1. Global Longitudinal Strain.
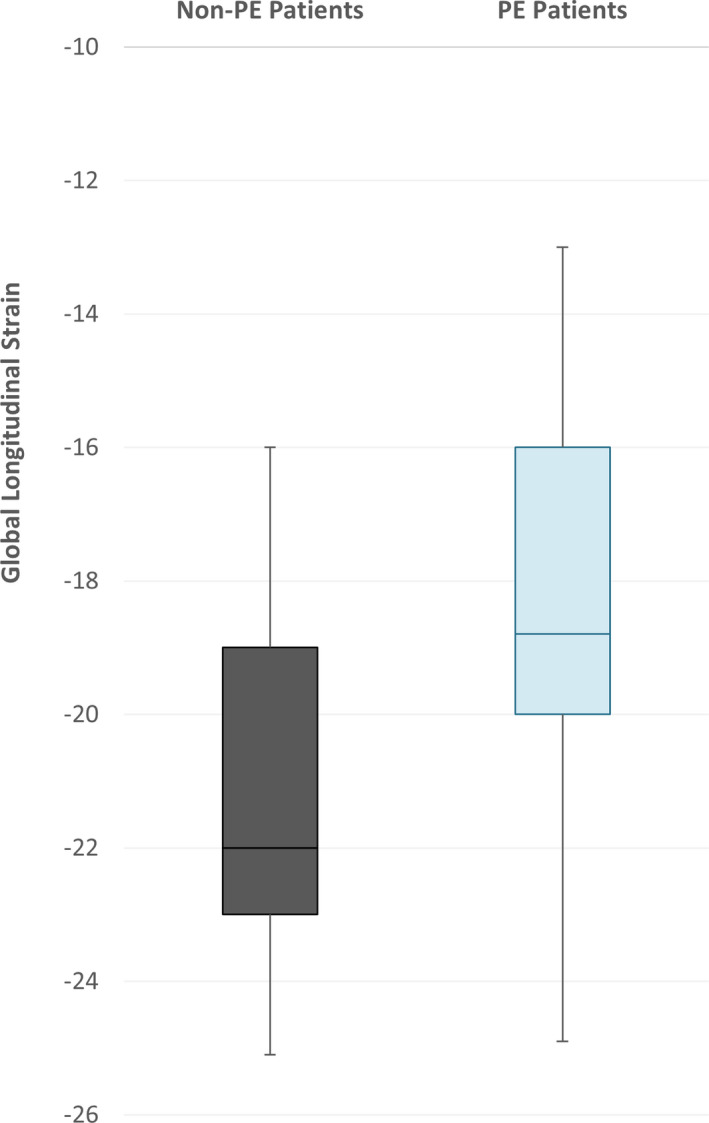
Global longitudinal strain is presented between those with (blue) and without (grey) preeclampsia (PE). Values that are lower (more negative) imply better cardiac function.
Table 3.
Echocardiographic Characteristics of Patients
| All Patients, N=46 | Patients Without Preeclampsia, n=25 | Patients With Preeclampsia, n=21 | P Value | |
|---|---|---|---|---|
| GLS, % | ‐19.93±3.05 | ‐21.29±2.70 | ‐18.31±.68 | 0.001 |
| LVEF, % | 60.45±.41 | 61.24±4.06 | 59.52±4.71 | 0.19 |
| lVSd, cm | 0.88±0.14 | 0.81±0.11 | 0.96±.14 | 0.0002 |
| LVID, cm | 4.6 (4.2, 4.8) | 4.6 (4.3, 4.8) | 4.6 (4.1, 4.8) | 0.47 |
| LVPWd, cm | 0.85 (0.75, 0.97) | 0.80 (0.69, 0.88) | 0.91 (0.84, 1.00) | 0.003 |
| LVMI, g/m2 | 65.96 (56.40, 73.04) | 62.74 (54.82, 69.02) | 70.12 (59.83, 80.77) | 0.04 |
| LVED volume, mL | 91.94±26.30 | 95.27±19.75 | 87.98±32.52 | 0.38 |
| Stroke volume, mL | 55.32±14.96 | 58.33±12.42 | 51.73±17.13 | 0.14 |
| E, cm/s | 84.41±15.63 | 87.17±15.08 | 81.10±16.01 | 0.20 |
| A, cm/s | 54.26±21.29 | 51.41±14.66 | 57.69±27.25 | 0.36 |
| E/A | 1.50 (1.25, 1.75) | 1.65 (1.50, 2.10) | 1.30 (1.05, 1.50) | 0.002 |
| DT, ms | 186.63±37.60 | 192.08±38.97 | 179.74±35.61 | 0.29 |
| Septal E, cm/s | 10.18±1.55 | 10.35±1.52 | 9.97±1.60 | 0.42 |
| Septal A, cm/s | 9.05 (7.25, 10.40) | 8.55 (7.20, 10.40) | 9.40 (7.65, 10.45) | 0.44 |
| E/E′ septal | 8.10 (7.00, 9.80) | 8.25 (7.00, 10.30) | 7.85 (7.10, 9.05) | 0.42 |
| Average E′, cm/s | 10.20±3.04 | 10.99±2.95 | 9.24±2.94 | 0.06 |
| LAVI, mL/m2 | 27.91 (24.34, 32.67) | 27.96 (25.17, 32.67) | 27.57 (22.88, 32.38) | 0.74 |
| Left atrial strain, % | 39.02±11.42 | 39.62±11.35 | 38.31±11.75 | 0.70 |
Data are presented as mean±standard deviation, median (quartile 1, quartile 3), or n (%) depending on variable type and distribution. A indicates atrial contraction; DT, deceleration time; E, early filling; E/A, ratio of early filling/ atrial contraction; E/E', ratio fo early filling/early‐diastolic septal mitral annular velocity; GLS, global longitudinal strain; IVSd, intraventricular septal diameter; LAV, left atrial volume; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LVID, left ventricular internal diameter; LV mass, left ventricular mass; LVMI, left ventricular mass index; LVPWd, left ventricular posterior wall diameter; Septal A, atrial mitral annular motion; and Septal E, early septal mitral annular motion.
Activin A, Follistatin, and FSTL3 Measurements
Preeclamptic women had higher levels of activin A (median 0.52 [IQR 0.41, 0.65] versus 0.37 [IQR 0.22, 0.48] ng/mL, P=0.02) and higher activin/FSTL3 ratio levels (0.03 [IQR 0.02, 0.04] versus 0.02 [IQR 0.01, 0.04]; P=0.04) when compared with normal women without preeclampsia (Table 4). FSLT3 levels alone did not differ between groups (14.81 [IQR 11.37, 17.34] versus 16.21 [IQR 12.48, 18.48], P=0.58).
Table 4.
Biomarker Characteristics of Patients
| All Patients, N=46 | Patients Without Preeclampsia, n=25 | Patients With Preeclampsia, n=21 | P Value | |
|---|---|---|---|---|
| Activin, ng/mL | 0.43 (0.25, 0.56) | 0.37 (0.22, 0.48) | 0.52 (0.41, 0.65) | 0.02 |
| FSTL3, ng/mL | 15.76 (11.37, 18.06) | 16.21 (12.48, 18.48) | 14.81 (11.37, 17.34) | 0.58 |
| Activin/FSTL3 ratio | 0.03 (0.02, 0.04) | 0.02 (0.01, 0.04) | 0.03 (0.02, 0.04) | 0.04 |
Data are presented as median (quartile 1, quartile 3). FSTL3 indicates follistatin‐like 3.
Correlation Between Activin A Levels and Echocardiography Measurements
Among all participants, increased activin A levels correlated with worsened GLS (r=0.46, P=0.001; Figure 2). A similar correlation was also observed between worsened GLS and increasing log of the activin A/FSTL3 ratio (r=0.44, P=0.002). In a multivariable model, the relationship between activin A levels and worsening GLS persisted after adjusting for age at enrollment, MAP, race, and BMI (P=0.003).
Figure 2. Association Between Global Longitudinal Strain and Activin.
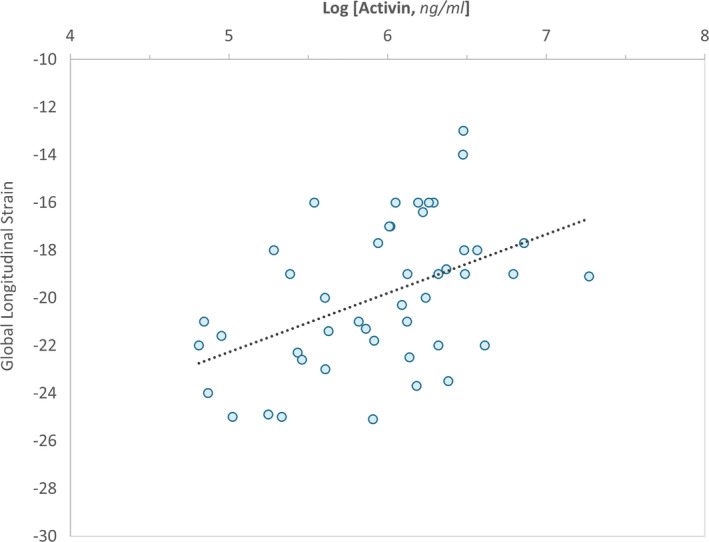
The correlation between global longitudinal strain and activin A is presented. Given the skewed distribution of activin A, the natural logarithm is employed.
Discussion
In this prospective cohort study of cardiac function and activin A levels in patients 10 years after delivery, we found that women with pregnancies complicated by preeclampsia had increased levels of circulating activin A and worsened GLS than control women with normal pregnancies. We further observed that changes in GLS correlated with circulating levels of activin A, suggesting a mechanistic role for activin A in the delayed development of cardiac dysfunction in women after a pregnancy with preeclampsia. Our findings are particularly clinically relevant, as many of our subjects were Black, with increased BMI and hypertensive, thus at higher risk of cardiovascular disease and death related to preeclampsia. 22
Our findings also support previous work on cardiac function in preeclampsia at the time of pregnancy and acute postpartum period, 23 , 24 , 25 , 26 and our previous observations that antepartum women with preeclampsia exhibit both elevated antepartum activin A levels and a worse than normal GLS at 1‐year postpartum. 12 In this study, we extend our previous findings to note that activin A levels and GLS in women after a preeclamptic pregnancy correlate at 10 years postpartum.
Our data suggest a potential mechanism to explain how a pregnancy complicated by preeclampsia may lead to subsequent development of cardiovascular disease. Previous work in a mouse model has demonstrated a direct cause and effect relationship between experimentally induced increases in activin A levels and systolic and diastolic dysfunction. In addition, blocking activin type 2 receptor activity attenuated cardiac dysfunction in mouse models of heart failure. 6 Clinically, we have demonstrated that activin A levels correlate with worsening GLS during both the peri‐ and immediate postpartum periods, suggesting that activin A may play a role in cardiovascular disease among women with preeclampsia. 12 , 27
A sustained increase in activin A levels during and after a preeclamptic pregnancy is plausible. During pregnancy, activin A is produced by the placenta and other cell types, such as macrophages, and undergoes endocytic degradation by binding to heparan sulfate on the cell surface. 28 Extraplacental sources of activin A identified in preeclampsia include endothelial cells and monocytes, which both remain activated postpartum and likely continue to secrete activin A. 29 , 30 , 31 Gene expression profiles have also identified oxidative stress, inflammation‐mediated cytokines and chemokines, and interleukins as factors common to both preeclampsia and cardiovascular disease. 32 These pathways are all potent stimulators of activin A secretion. 33
Obesity increases the overall risk of preeclampsia by approximately two‐ to threefold. In addition, preeclampsia and subsequent cardiovascular disease, apart from sharing common risk factors like obesity, also share common pathogenic features including endothelial dysfunction, oxidative stress, and increased inflammatory activation. 34 Interestingly, activin A is highly expressed in adipose tissue and in women with preeclampsia and elevated in cardiovascular disease, thus possibly providing a pathogenic link between obesity, preeclampsia, and subsequent cardiovascular disease. 35
The potential role of activin A in postpartum cardiac dysfunction raises the possibility that therapies targeted against activin A may mitigate the development of cardiac dysfunction after a preeclamptic pregnancy. Aspirin, for example, blocks heparanase, which subsequently increases heparan sulfate for activin A binding. 36 We have previously observed that antepartum aspirin therapy in the second trimester reduced serum activin A levels and improved GLS in preeclamptic patients. 27 The American College of Obstetricians and Gynecologists guidelines recommend the use of low‐dose aspirin (81 mg/d) to mitigate the risk of preeclampsia in women at high risk. 37 Current guidelines also recommend discontinuing aspirin at 36 weeks or at the time of delivery unless indicated for thrombotic conditions. These observations suggest that continuing aspirin in patients after a preeclamptic pregnancy may potentially modulate activin A signaling. More research is needed to evaluate whether this modulation can reduce the risk of long‐term cardiac dysfunction.
Our study has several limitations. Because study enrollment required that women respond to our inquiry, response bias may have affected our results. Previous studies have observed that respondents to a voluntary study are older, have higher education levels, and report fewer risky behaviors. 38 , 39 , 40 Our data may thus be confounded by inherent differences between responders and nonresponders. Our study sample size is also small, which may limit the reproducibility of our findings. Our results should be interpreted with caution bearing this in mind. Future studies evaluating this initial finding in larger sample sizes will thus be needed to confirm the observations. Furthermore, it is possible that hypertension itself may have affected GLS independently. We found, however, that after adding MAP to our multivariable model, the relationship persisted, such that worsening GLS was associated with a history of preeclampsia. In addition, although we adjusted for baseline differences between groups, it is possible that our results may be confounded by between‐group differences that we were unable to account for. However, we excluded patients who had chronic hypertension or diabetes mellitus before their index pregnancy, suggesting that postpartum hypertension had developed after the preeclampsia pregnancy, similar to previous large studies. 41 , 42
CONCLUSIONS
We report that among women 10 years after a pregnancy complicated by preeclampsia, activin A levels were higher and GLS was worse than in a non‐preeclampsia cohort. In addition, we found among patients with preeclampsia that activin A correlated with worsened GLS, suggesting a possible etiologic role for activin A. Further work is needed to better understand the factors that drive the development of long‐term cardiovascular disease after a pregnancy complicated by preeclampsia to develop strategies to mitigate that risk.
Sources of Funding
This work was supported by the Chicago Lying‐in Board of Directors Grant, Department of Obstetrics and Gynecology at University of Chicago, 1R21HL14848801, and R01 HL14819101A1.
Disclosures
None.
(J Am Heart Assoc.2021;10:e018526. DOI: 10.1161/JAHA.120.018526.)
For Sources of Funding and Disclosures, see page 8.
References
- 1. Ananth CV, Keyes KM, Wapner RJ. Pre‐eclampsia rates in the united states, 1980–2010: age‐period‐cohort analysis. BMJ. 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew‐Graham CA, et al. Preeclampsia and future cardiovascular health: a systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. DOI: 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- 3. Leon LJ, McCarthy FP, Direk K, Gonzalez‐Izquierdo A, Prieto‐Merino D, Casas JP, Chappell L. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: a caliber study. Circulation. 2019;140:1050–1060. [DOI] [PubMed] [Google Scholar]
- 4. Nizamuddin J, Gupta A, Patel V, Minhaj M, Nizamuddin S, Mueller A, Naseem H, Tung A, Rana S. Hypertensive diseases of pregnancy increase risk of readmission with heart failure: a national readmissions database study. Mayo Clin Proc. 2018;94:811‐819. [DOI] [PubMed] [Google Scholar]
- 5. Saleh L, van den Meiracker AH, Geensen R, Kaya A, Roeters van Lennep JE, Duvekot JJ, Verdonk K, Steegers EAP, Russcher H, Danser AHJ, et al. Soluble fms‐like tyrosine kinase‐1 and placental growth factor kinetics during and after pregnancy in women with suspected or confirmed pre‐eclampsia. Ultrasound Obstet Gynecol. 2018;51:751–757. [DOI] [PubMed] [Google Scholar]
- 6. Roh JD, Hobson R, Chaudhari V, Quintero P, Yeri A, Benson M, Xiao C, Zlotoff D, Bezzerides V, Houstis N, et al. Activin type II receptor signaling in cardiac aging and heart failure. Sci Transl Med. 2019;11:eaau8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castillero E, Akashi H, Najjar M, Ji R, Brandstetter LM, Wang C, Liao X, Zhang X, Sperry A, Gailes M, et al. Activin type ii receptor ligand signaling inhibition after experimental ischemic heart failure attenuates cardiac remodeling and prevents fibrosis. Am J Physiol Heart Circ Physiol. 2020;318:H378–H390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vasquez C, Benamer N, Morley GE. The cardiac fibroblast: functional and electrophysiological considerations in healthy and diseased hearts. J Cardiovasc Pharmacol. 2011;57:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu J, Wang X, Wei SM, Tang YH, Zhou Q, Huang CX. Activin A stimulates the proliferation and differentiation of cardiac fibroblasts via the ERK1/2 and p38‐MAPK pathways. Eur J Pharmacol. 2016;789:319–327. [DOI] [PubMed] [Google Scholar]
- 11. Shimano M, Ouchi N, Nakamura K, Oshima Y, Higuchi A, Pimentel DR, Panse KD, Lara‐Pezzi E, Lee SJ, Sam F, et al. Cardiac myocyte‐specific ablation of follistatin‐like 3 attenuates stress‐induced myocardial hypertrophy. J Biol Chem. 2011;286:9840–9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shahul S, Ramadan H, Nizamuddin J, Mueller A, Patel V, Dreixler J, Tung A, Lang RM, Weinert L, Nasim R, et al. Activin A and late postpartum cardiac dysfunction among women with hypertensive disorders of pregnancy. Hypertension. 2018;72:188–193. [DOI] [PubMed] [Google Scholar]
- 13. Shahul S, Ramadan H, Mueller A, Nizamuddin J, Nasim R, Lopes Perdigao J, Chinthala S, Tung A, Rana S. Abnormal mid‐trimester cardiac strain in women with chronic hypertension predates superimposed preeclampsia. Pregnancy Hypertens. 2017;10:251–255. [DOI] [PubMed] [Google Scholar]
- 14. Shahul S, Medvedofsky D, Wenger JB, Nizamuddin J, Brown SM, Bajracharya S, Salahuddin S, Thadhani R, Mueller A, Tung A, et al. Circulating antiangiogenic factors and myocardial dysfunction in hypertensive disorders of pregnancy. Hypertension. 2016;67:1273–1280. [DOI] [PubMed] [Google Scholar]
- 15. Onishi T, Saha SK, Delgado‐Montero A, Ludwig DR, Onishi T, Schelbert EB, Schwartzman D, Gorcsan J 3rd. Global longitudinal strain and global circumferential strain by speckle‐tracking echocardiography and feature‐tracking cardiac magnetic resonance imaging: comparison with left ventricular ejection fraction. J Am Soc Echocardiogr. 2015;28:587–596. [DOI] [PubMed] [Google Scholar]
- 16. Sengupta SP, Bansal M, Hofstra L, Sengupta PP, Narula J. Gestational changes in left ventricular myocardial contractile function: new insights from two‐dimensional speckle tracking echocardiography. Int J Cardiovasc Imaging. 2017;33:69–82. [DOI] [PubMed] [Google Scholar]
- 17. ACOG . ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133:e1–e25. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1–39):e14. [DOI] [PubMed] [Google Scholar]
- 20. Knackstedt C, Bekkers SC, Schummers G, Schreckenberg M, Muraru D, Badano LP, Franke A, Bavishi C, Omar AM, Sengupta PP. Fully automated versus standard tracking of left ventricular ejection fraction and longitudinal strain: the FAST‐EFs multicenter study. J Am Coll Cardiol. 2015;66:1456–1466. [DOI] [PubMed] [Google Scholar]
- 21. Hedger MP, de Kretser DM. The activins and their binding protein, follistatin‐diagnostic and therapeutic targets in inflammatory disease and fibrosis. Cytokine Growth Factor Rev. 2013;24:285–295. DOI: 10.1016/j.cytogfr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 22. Shahul S, Tung A, Minhaj M, Nizamuddin J, Wenger J, Mahmood E, Mueller A, Shaefi S, Scavone B, Kociol RD, et al. Racial disparities in comorbidities, complications, and maternal and fetal outcomes in women with preeclampsia/eclampsia. Hypertens Pregnancy. 2015;34:506–515. DOI: 10.3109/10641955.2015.1090581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 1979;2011(57):85–93. [DOI] [PubMed] [Google Scholar]
- 24. Shahul S, Rhee J, Hacker MR, Gulati G, Mitchell JD, Hess P, Mahmood F, Arany Z, Rana S, Talmor D. Subclinical left ventricular dysfunction in preeclamptic women with preserved left ventricular ejection fraction: a 2d speckle‐tracking imaging study. Circ Cardiovasc Imaging. 2012;5:734–739. DOI: 10.1161/CIRCIMAGING.112.973818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Melchiorre K, Sutherland GR, Watt‐Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertens Pregnancy. 2012;31:454–471. DOI: 10.3109/10641955.2012.697951. [DOI] [PubMed] [Google Scholar]
- 26. Melchiorre K, Sutherland G, Sharma R, Nanni M, Thilaganathan B. Mid‐gestational maternal cardiovascular profile in preterm and term pre‐eclampsia: a prospective study. BJOG. 2013;120:496–504. DOI: 10.1111/1471‐0528.12068. [DOI] [PubMed] [Google Scholar]
- 27. Naseem H, Dreixler J, Mueller A, Tung A, Dhir R, Chibber R, Fazal A, Granger JP, Bakrania BA, deMartelly V, et al. Antepartum aspirin administration reduces Activin A and cardiac global longitudinal strain in preeclamptic women. J Am Heart Assoc. 2020;9:e015997. DOI: 10.1161/JAHA.119.015997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hashimoto O, Nakamura T, Shoji H, Shimasaki S, Hayashi Y, Sugino H. A novel role of follistatin, an activin‐binding protein, in the inhibition of activin action in rat pituitary cells. Endocytotic degradation of activin and its acceleration by follistatin associated with cell‐surface heparan sulfate. J Biol Chem. 1997;272:13835–13842. DOI: 10.1074/jbc.272.21.13835. [DOI] [PubMed] [Google Scholar]
- 29. van Rijn BB, Bruinse HW, Veerbeek JH, Post Uiterweer ED, Koenen SV, van der Bom JG, Rijkers GT, Roest M, Franx A. Postpartum circulating markers of inflammation and the systemic acute‐phase response after early‐onset preeclampsia. Hypertension. 1979;2016(67):404–414. [DOI] [PubMed] [Google Scholar]
- 30. Yndestad A, Ueland T, Øie E, Florholmen G, Halvorsen B, Attramadal Håvard, Simonsen S, Frøland SS, Gullestad L, Christensen G, et al. Elevated levels of Activin A in heart failure: potential role in myocardial remodeling. Circulation. 2004;109:1379–1385. DOI: 10.1161/01.CIR.0000120704.97934.41. [DOI] [PubMed] [Google Scholar]
- 31. Tannetta DS, Muttukrishna S, Groome NP, Redman CW, Sargent IL. Endothelial cells and peripheral blood mononuclear cells are a potential source of extraplacental Activin A in preeclampsia. J Clin Endocrinol Metab. 2003;88:5995–6001. DOI: 10.1210/jc.2002‐021924. [DOI] [PubMed] [Google Scholar]
- 32. Sitras V, Fenton C, Acharya G. Gene expression profile in cardiovascular disease and preeclampsia: a meta‐analysis of the transcriptome based on raw data from human studies deposited in gene expression omnibus. Placenta. 2015;36:170–178. [DOI] [PubMed] [Google Scholar]
- 33. Mandang S, Manuelpillai U, Wallace EM. Oxidative stress increases placental and endothelial cell Activin A secretion. J Endocrinol. 2007;192:485–493. DOI: 10.1677/JOE‐06‐0061. [DOI] [PubMed] [Google Scholar]
- 34. Jeyabalan A. Epidemiology of preeclampsia: Impact of obesity. Nutr Rev. 2013;71:S18–25. DOI: 10.1111/nure.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeller J, Krüger C, Lamounier‐Zepter V, Sag S, Strack C, Mohr M, Loew T, Schmitz G, Maier L, Fischer M, et al. The adipo‐fibrokine Activin A is associated with metabolic abnormalities and left ventricular diastolic dysfunction in obese patients. ESC Heart Fail. 2019;6:362–370. DOI: 10.1002/ehf2.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dai X, Yan J, Fu X, Pan Q, Sun D, Xu Y, Wang J, Nie L, Tong L, Shen A, et al. Aspirin inhibits cancer metastasis and angiogenesis via targeting heparanase. Clin Cancer Res. 2017;23:6267–6278. DOI: 10.1158/1078‐0432.CCR‐17‐0242. [DOI] [PubMed] [Google Scholar]
- 37. Committee on Obstetric Practice Society for Maternal–Fetal Medicine . ACOG committee opinion no. 743 summary: low‐dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:254–256. [DOI] [PubMed] [Google Scholar]
- 38. Cheung KL, Ten Klooster PM, Smit C, de Vries H, Pieterse ME. The impact of non‐response bias due to sampling in public health studies: a comparison of voluntary versus mandatory recruitment in a Dutch national survey on adolescent health. BMC Public Health. 2017;17:276. DOI: 10.1186/s12889‐017‐4189‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jooste PL, Yach D, Steenkamp HJ, Botha JL, Rossouw JE. Drop‐out and newcomer bias in a community cardiovascular follow‐up study. Int J Epidemiol. 1990;19:284–289. 10.1093/ije/19.2.284. [DOI] [PubMed] [Google Scholar]
- 40. Criqui MH, Barrett‐Connor E, Austin M. Differences between respondents and non‐respondents in a population‐based cardiovascular disease study. Am J Epidemiol. 1978;108:367–372. [DOI] [PubMed] [Google Scholar]
- 41. Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, Thilaganathan B, Boyd HA. Risk of post‐pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078. DOI: 10.1136/bmj.j3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 1979;2011(58):709–715. [DOI] [PubMed] [Google Scholar]


