Abstract
Background
This study compared the efficacy and safety between catheter‐directed thrombolysis (CDT) and systemic thrombolysis for patients with acute pulmonary embolism (PE) with midterm follow‐up.
Methods and Results
We conducted a prospective open cohort study by using data from the Taiwan National Health Insurance Research Database for 2001 to 2013. Patients who were first admitted for PE and were treated by either systemic thrombolysis or CDT were included and compared. Inverse probability of treatment weighting, based on the propensity score, was used to mitigate possible selection bias. A total of 145 CDT‐treated and 1158 systemic thrombolysis–treated patients with PE were included. The in‐hospital mortality rate was significantly lower in the CDT group (12.7% versus 21.4%; odds ratio, 0.49; 95% CI, 0.36–0.67) after inverse probability of treatment weighting. No significant differences between the groups were observed for the safety (bleeding) outcomes. In patients who survived the index PE admission, the 1‐year all‐cause mortality rate was significantly lower in the CDT group after inverse probability of treatment weighting (12.2% versus 13.2%; hazard ratio [HR], 0.73; 95% CI, 0.56–0.94). Treatment with CDT was also associated with lower risks of recurrent PE (9.3% versus 17.5%; subdistribution HR, 0.52; 95% CI, 0.41–0.66). The difference remained through the last follow‐up.
Conclusions
Among patients with PE requiring reperfusion therapy, those accepting CDT had lower all‐cause mortality and recurrent PE over both short‐term and midterm follow‐up periods than those receiving systemic thrombolysis. The bleeding risk was similar for both groups. These findings should be cautiously validated in future randomized trials.
Keywords: catheter‐directed thrombolysis, intravenous infusion, pulmonary embolism, thrombolytic therapy
Subject Categories: Embolism, Thrombosis
Nonstandard Abbreviations and Acronyms
- CDT
catheter‐directed thrombolysis
- CTEPH
chronic thromboembolic pulmonary hypertension
- IPTW
inverse probability of treatment weighting
- NHI
National Health Insurance
- NHIRD
National Health Insurance Research Database
- ST
systemic thrombolysis
- USAT
ultrasound‐assisted thrombolysis
Clinical Perspective
What Is New?
This is the first and largest observational study undertaken to compare the safety, short‐term efficacy, and midterm outcomes of catheter‐directed thrombolysis with those of systemic thrombolysis in patients with acute pulmonary embolism.
Among patients with pulmonary embolism requiring reperfusion therapy, those accepting catheter‐directed thrombolysis had lower all‐cause mortality and recurrent pulmonary embolism over both short‐term and midterm follow‐up periods than those receiving systemic thrombolysis.
What Are the Clinical Implications?
Catheter‐directed thrombolysis should be considered in high‐risk patients with pulmonary embolism because of its efficacy and safety.
Acute pulmonary embolism (PE) is one of the leading causes of cardiovascular death worldwide, with 34% of patients dying suddenly or within hours after the symptoms of PE developed. 1 , 2 Therefore, timely diagnosis and effective therapies are crucial to saving the lives of patients with acute PE. According to the recent guideline for PE management, published by the European Society of Cardiology, the severity of PE can be stratified into 3 categories: low, intermediate, and high. 3 The initiation of anticoagulant therapy without delay is recommended for most patients in all risk groups, whereas reperfusion treatment is reserved only for patients in shock or exhibiting hemodynamic deterioration on anticoagulation treatment.
For patients with PE receiving reperfusion therapy, current guidelines advocate the systemic administration of thrombolytic drugs as the treatment of choice. 3 , 4 Systemic thrombolysis (ST) may promptly restore pulmonary circulation and reduce mortality, but it also carries higher bleeding risks, including a 2% risk of intracranial hemorrhage, which is 4‐ to 5‐fold higher than that for anticoagulation treatment. 5 Moreover, the rates of bleeding complications increase with age, especially for patients aged >65 years. 5 Hence, ST has a limited role in patients with PE who are thought to be at unusually elevated risk for bleeding or intracranial hemorrhage.
Catheter‐directed thrombolysis (CDT) provides an alternative way to deliver thrombolytic agents to patients with PE. Because CDT can achieve a higher local concentration of thrombolytic agents by directly infusing drugs into the pulmonary trunk, it may require a lower dose of thrombolytic agents than ST. This lower‐dose regimen is expected to entail a lower risk of bleeding, which is advocated by guidelines for patients with higher bleeding risk. 3 , 4 Despite these expected advantages of CDT, however, there has been a lack of randomized trials directly comparing the safety and efficacy of CDT and ST. Observational studies comparing these 2 strategies were also rare, and only provided short‐ and intermediate‐term prognosis. 6 , 7 , 8 , 9 Relatedly, because there is no clear evidence for using CDT instead, the current guidelines still suggest ST as the first‐line therapy for patients with PE requiring reperfusion therapy.
The present study aimed to compare the efficacy and safety of CDT with ST in patients with acute PE both in short‐term and midterm prognosis. We enrolled patients from the largest cohort in Asia using a nationwide database, including nearly 100% of the adult patients with PE in Taiwan. The patients were subjected to propensity score matching, according to their clinical characteristics.
Methods
Data Source
Data that support the findings of this study are available from the corresponding author on reasonable request. We conducted a prospective open cohort study by using data collected retrospectively from the Taiwan National Health Insurance Research Database (NHIRD) for 2001 through 2013. Taiwan’s National Health Insurance (NHI) program is a single‐payer system that was established in March 1995 and currently provides coverage to >99.8% of the population of Taiwan (≈23.7 million people at present). Moreover, the NHIRD contains data on the patients covered by the NHI program from 1995 through 2013. Because enrollment in the NHI program is mandatory and affordable, the vast majority of patients receive appropriate long‐term follow‐up care and evaluations. To protect privacy, all personal information contained in the NHIRD is deidentified and anonymized, so a full review by the Ethics Institutional Review Board of Taiwan University Hospital was not needed for the present study. Further information on the NHI program and the NHIRD has been provided in previous publications. 10 , 11 , 12
Study Patients
Patients who were first admitted for PE (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM], code 415.1) between January 1, 2001, and December 31, 2013, were identified. We then excluded any patients who (1) had missing demographical data (<0.1%), (2) were aged <20 years, or (3) were not treated by thrombolysis during the index PE admission. Those patients who were ultimately included were separated into 2 groups: the ST group and the CDT group (Figure 1). This definition of PE was frequently reported in previous NHIRD studies. 13 , 14 , 15
Figure 1. Study patient selection.
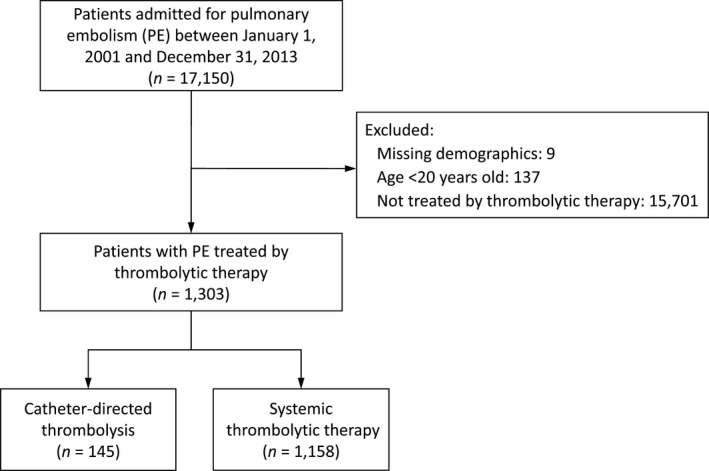
PE indicates pulmonary embolism.
Study Group and Thrombolytic Exposure
The included patients with PE with thrombolytic agent use were separated into 2 groups, according to their delivery method. The patients who received thrombolytic agents through multi–side‐hole catheters were assigned to the CDT group, whereas those who received thrombolytic agents without multi–side‐hole catheters were assigned to the systemic thrombolytic group. The thrombolytic agents consisted of tPA (tissue‐type plasminogen activator), urokinase, and streptokinase. The information on the above therapies of interest was extracted using the Taiwan reimbursement codes included in the inpatient claims data.
Outcomes
The clinical outcomes in this study included in‐hospital and follow‐up outcomes. The in‐hospital outcomes were in‐hospital death, length of stay in intensive care unit, cardiovascular complications (ie, heart failure and new‐onset atrial fibrillation), and the safety (bleeding) outcomes (ie, major bleeding, gastrointestinal bleeding, intracranial hemorrhage, and bleeding requiring transfusion). The follow‐up outcomes were all‐cause mortality, recurrent PE, heart failure, and new‐onset pulmonary hypertension. All‐cause mortality was defined by a withdrawal from the NHI program. 16 Admission for recurrent PE or heart failure was detected using the principle inpatient diagnosis. New‐onset pulmonary hypertension was ascertained by using 2 outpatient diagnoses or any single inpatient diagnosis. Patients were followed up from the discharge date to the date of death, date of event occurrence, or December 31, 2013, whichever came first.
Covariates
The covariates were age, sex, previous venous thromboembolism history, 17 comorbidities, recent traumatic injuries or major surgeries (ie, orthopedic, gastroenterological, and gynecological injuries or surgeries), Charlson Comorbidity Index score, pregnancy in the previous year, 11 kinds of medication after discharge, and in‐hospital conditions or treatments (including extracorporeal membrane oxygenation, cardiogenic shock with use of inotropic agents, and intubation). We used the ICD‐9‐CM diagnostic and ICD‐9‐CM procedure codes and the Taiwan NHI reimbursement codes to obtain the baseline characteristics and surgical details of the patients based on the outpatient and inpatient claims data. Details of the ICD‐9‐CM diagnostic codes used in this study are provided in Table S1.
Statistical Analysis
To mitigate against possible confounding and selection bias when comparing outcomes between the CDT and systemic thrombolytic groups, we calculated the inverse probability of treatment weighting (IPTW) based on the propensity score. The propensity score was estimated using a multivariable logistic regression model without interaction terms in which the study group (1, CDT; and 0, ST) was regressed on all of the covariates listed in Table 1 with a force entry, except that the follow‐up year was replaced with the date of index admission. To prevent the impact of extreme values of the estimated propensity scores, we used a stabilized weight to mitigate the influence of outliers. The quality of weighting was checked using the absolute value of standardized difference between the groups after weighting, where a value of <0.1 was considered a negligible difference and a value of <0.2 was considered a small effect size of group difference. 17 , 18
Table 1.
Baseline Characteristics of the Patients With PE Treated by CDT or ST Before and After Propensity Score Weighting
| Before IPTW* | After IPTW† | |||||
|---|---|---|---|---|---|---|
| Characteristics |
CDT (n=145) |
ST (n=1158) |
STD | CDT | ST | STD |
| Age, y | 61.5±16.0 | 62.9±15.7 | −0.09 | 63.4±16.5 | 62.8±15.7 | 0.04 |
| Male sex | 56 (38.6) | 527 (45.5) | −0.14 | 39.0 | 44.6 | −0.11 |
| Venous thromboembolism history | ||||||
| Deep‐vein thrombosis | 1 (0.7) | 20 (1.7) | −0.10 | 1.3 | 1.6 | −0.03 |
| PE | 2 (1.4) | 13 (1.1) | 0.02 | 0.5 | 1.1 | −0.07 |
| Cardiometabolic disease | ||||||
| Hypertension | 70 (48.3) | 593 (51.2) | −0.06 | 52.4 | 50.8 | 0.03 |
| Diabetes mellitus | 32 (22.1) | 299 (25.8) | −0.09 | 23.3 | 25.3 | −0.05 |
| Hyperlipidemia | 28 (19.3) | 236 (20.4) | −0.03 | 26.8 | 20.3 | 0.15 |
| Peripheral arterial disease | 8 (5.5) | 71 (6.1) | −0.03 | 5.3 | 6.0 | −0.03 |
| Gout | 6 (4.1) | 114 (9.8) | −0.23 | 9.7 | 9.2 | 0.02 |
| Ischemic heart disease | 37 (25.5) | 296 (25.6) | <0.01 | 24.4 | 25.5 | −0.03 |
| Previous myocardial infarction | 3 (2.1) | 40 (3.5) | −0.08 | 3.3 | 3.3 | <0.01 |
| Atrial fibrillation | 6 (4.1) | 79 (6.8) | −0.12 | 6.4 | 6.5 | <0.01 |
| Previous stroke | 13 (9.0) | 127 (11.0) | −0.07 | 6.4 | 10.7 | −0.15 |
| Chronic kidney disease | 25 (17.2) | 196 (16.9) | 0.01 | 14.3 | 16.9 | −0.07 |
| Provoking factor of PE | ||||||
| Paralysis (immobilization) | 19 (13.1) | 75 (6.5) | 0.22 | 6.9 | 7.2 | −0.01 |
| Cancer | 19 (13.1) | 154 (13.3) | −0.01 | 12.0 | 13.3 | −0.04 |
| Pregnant in the previous year | 1 (0.7) | 27 (2.3) | −0.13 | 2.7 | 2.2 | 0.03 |
| Trauma injury in the previous month | 8 (5.5) | 61 (5.3) | 0.01 | 5.0 | 5.3 | −0.01 |
| Major surgery in the previous month | 4 (2.8) | 17 (1.5) | 0.09 | 1.7 | 1.6 | 0.01 |
| Other comorbidity | ||||||
| COPD | 11 (7.6) | 161 (13.9) | −0.21 | 10.9 | 13.2 | −0.07 |
| Liver disease | 16 (11.0) | 140 (12.1) | −0.03 | 11.0 | 12.0 | −0.03 |
| Congestive heart failure | 9 (6.2) | 114 (9.8) | −0.13 | 7.9 | 9.4 | −0.05 |
| Pulmonary hypertension | 7 (4.8) | 45 (3.9) | 0.05 | 6.0 | 4.1 | 0.09 |
| Autoimmune disease | 3 (2.1) | 21 (1.8) | 0.02 | 1.2 | 1.8 | −0.05 |
| Charlson Comorbidity Index score | 2.2±2.4 | 2.4±2.6 | −0.07 | 2.1±2.5 | 2.4±2.6 | −0.11 |
| Medication after discharge | ||||||
| Statin | 17 (11.7) | 104 (9.0) | 0.09 | 14.3 | 9.3 | 0.15 |
| ACEI/ARB | 31 (21.4) | 232 (20.0) | 0.03 | 24.2 | 20.2 | 0.09 |
| β‐Blocker | 29 (20.0) | 190 (16.4) | 0.09 | 18.6 | 16.7 | 0.05 |
| CCB | 26 (17.9) | 221 (19.1) | −0.03 | 22.0 | 18.9 | 0.08 |
| Diuretics | 18 (12.4) | 192 (16.6) | −0.12 | 15.4 | 16.1 | −0.02 |
| Antiplatelet | 21 (14.5) | 201 (17.4) | −0.08 | 17.7 | 17.0 | 0.02 |
| OHA | 16 (11.0) | 136 (11.7) | −0.02 | 11.2 | 11.7 | −0.01 |
| Insulin | 4 (2.8) | 39 (3.4) | −0.04 | 5.1 | 3.3 | 0.09 |
| Antidepressants | 8 (5.5) | 87 (7.5) | −0.08 | 8.4 | 7.3 | 0.04 |
| Hormone therapy | 3 (2.1) | 10 (0.9) | 0.10 | 0.7 | 1.0 | −0.03 |
| Anticoagulant | 102 (70.3) | 743 (64.2) | 0.13 | 70.2 | 64.9 | 0.11 |
| In‐hospital condition or treatment | ||||||
| Cardiogenic shock | 62 (42.8) | 433 (37.4) | 0.11 | 36.0 | 37.9 | −0.04 |
| ECMO | 12 (8.3) | 44 (3.8) | 0.19 | 3.9 | 4.2 | −0.02 |
| Intubation | 38 (26.2) | 288 (24.9) | 0.03 | 20.6 | 24.9 | −0.10 |
| Follow‐up time, y | 3.8±4.0 | 3.4±3.6 | 0.10 | 4.1±3.7 | 3.4±3.7 | 0.18 |
| Follow‐up time, y | 2.1 (0.3– 7.2) | 2.2 (0.2–5.6) | … | 3.3 (0.7–6.7) | 2.2 (0.2–5.6) | … |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CDT, catheter‐directed thrombolysis; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; IPTW, inverse probability of treatment weighting; OHA, oral hypoglycemic agent; PE, pulmonary embolism; ST, systemic thrombolysis; and STD, standardized difference.
Data are presented as frequency (percentage), mean±SD, or median (25th–75th percentile).
Data are presented as percentage, mean±SD, or median (25th–75th percentile).
The in‐hospital outcomes during the admission between groups (CDT versus ST) were compared by logistic regression for binary outcomes or by linear regression for continuous outcomes. As to the follow‐up outcomes, the mortality rates between the groups were compared by a Cox proportional hazard model. The incidences of other time‐to‐event outcomes (eg, recurrent PE) between the groups were compared by the Fine and Gray subdistribution hazard model, with death during the follow‐up period being considered a competing risk. Because of the absolute values of standardized difference >0.1 after IPTW, the following covariates were additionally adjusted in the regression analyses to account for the possibility of residual confounding: sex, hyperlipidemia, hyperthyroidism, previous stroke, Charlson Comorbidity Index score, statin and anticoagulant use, and intubation during admission. For all the regression models, the interactions between the treatment variable and covariates were not considered.
The assumption of proportional hazard was tested using a time‐varying variable by creating an interaction of the study group (CDT versus ST) and the natural logarithm of survival time. Subgroup analysis was further conducted to evaluate the consistency of the observed treatment effect on outcomes across different levels of subgroup variables. The outcomes for the subgroup analysis were in‐hospital death, 1‐year mortality, including in‐hospital death, all‐cause mortality by the end of follow‐up, and recurrent PE. The subgroup variables were age (dichotomized by 65 years), sex, chronic kidney disease, cancer, Charlson Comorbidity Index score (dichotomized by 3 scores), cardiogenic shock, and intubation during the index admission. A 2‐sided P value <0.05 was considered to be statistically significant, and no adjustment of multiple testing (multiplicity) was made in this study. All the statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient Characteristics
A total of 145 CDT‐treated and 1158 ST‐treated patients with PE, seen from January 1, 2001, to December 31, 2013, were included. The mean follow‐up years were 3.8 and 3.4 years for the CDT and ST groups, respectively. Overall, the patients with PE who received thrombolysis were predominantly women (61.4% in the CDT group and 54.5% in the ST group). The CDT group had a higher incidence of cardiogenic shock (42.8% versus 37.4%) and extracorporeal membrane oxygenation (8.3% versus 3.8%) than the ST group. After propensity score weighting, the 2 study groups were well balanced in terms of most of the characteristics (Table 1).
In‐Hospital Outcomes
During the index hospitalization, 22 (15.2%) patients in the CDT group died and 250 (21.6%) patients in the ST group died. The in‐hospital mortality rate was significantly lower in the CDT group (12.7% versus 21.4%; odds ratio [OR], 0.49; 95% CI, 0.36–0.67) after IPTW. The incidence of heart failure events in the CDT cohort was also lower than that in the ST cohort after IPTW (6.9% versus 10.0%; OR, 0.68; 95% CI, 0.51–0.90). However, no significant differences between the 2 groups were observed in terms of intensive care unit stay, new‐onset atrial fibrillation, or the safety (bleeding) outcomes (Table 2).
Table 2.
In‐Hospital Complications and Events of the Patients With PE Treated by CDT or ST Before and After Propensity Score Weighting
| Data Before IPTW* | Data After IPTW † | |||||
|---|---|---|---|---|---|---|
| Outcome |
CDT (n=145) |
ST (n=1158) |
CDT | ST |
B or OR of CDT (95% CI) ‡ |
P Value |
| In‐hospital death | 22 (15.2) | 250 (21.6) | 12.7 | 21.4 | 0.49 (0.36 to 0.67) | <0.001 |
| ICU length of stay, d | 5.6±6.6 | 4.8±6.6 | 4.8±5.8 | 4.8±6.6 | 0.29 (−0.32 to 0.91) | 0.349 |
| Cardiovascular complication | ||||||
| Heart failure | 9 (6.2) | 117 (10.1) | 6.9 | 10.0 | 0.68 (0.51 to 0.90) | 0.008 |
| Acute myocardial infarction | 2 (1.4) | 19 (1.6) | 1.1 | 1.7 | 0.71 (0.36 to 1.41) | 0.326 |
| New‐onset atrial fibrillation | 2 (1.4) | 22 (1.9) | 1.3 | 1.8 | 0.73 (0.39 to 1.38) | 0.332 |
| Safety outcome | ||||||
| Major bleeding | 13 (9.0) | 91 (7.9) | 7.6 | 7.8 | 1.02 (0.75 to 1.37) | 0.913 |
| Bleeding requiring transfusion | 2 (1.4) | 20 (1.7) | 1.7 | 1.7 | 1.21 (0.64 to 2.26) | 0.561 |
| Gastrointestinal bleeding | 11 (7.6) | 70 (6.0) | 5.9 | 6.0 | 0.99 (0.71 to 1.38) | 0.944 |
| Intracranial hemorrhage | 2 (1.4) | 21 (1.8) | 1.7 | 1.8 | 1.19 (0.64 to 2.23) | 0.582 |
B indicates regression coefficient; CDT, catheter‐directed thrombolysis; ICU, intensive care unit; IPTW, inverse probability of treatment weighting; OR, odds ratio; PE, pulmonary embolism; and ST, systemic thrombolysis.
Data are presented as frequency (percentage) or mean±SD.
Data are presented as percentage or mean±SD.
Adjusted for sex, hyperlipidemia, hyperthyroidism, previous stroke, Charlson Comorbidity Index score, statin use, anticoagulant use, and intubation.
Follow‐Up Outcomes in Patients Who Survived the Index PE Admission
A total of 1031 patients who survived the index hospitalization, comprising 123 CDT‐treated patients and 908 ST‐treated patients, were eligible for further analysis. There were 16 (13%) of these patients in the CDT group who died and 122 (13.4%) patients in the ST group who died within 1 year after the index hospitalization. The 1‐year all‐cause mortality rate was significantly lower in the CDT group after IPTW (12.2% versus 13.2%; hazard ratio [HR], 0.73; 95% CI, 0.56–0.94). Treatment with CDT was also associated with lower risks of recurrent PE (9.3% versus 17.5%; subdistribution HR, 0.52; 95% CI, 0.41–0.66). In addition, the CDT group had a lower incidence of new‐onset pulmonary hypertension (0.3% versus 1.6%; subdistribution HR, 0.20; 95% CI, 0.06–0.72) (Table 3).
Table 3.
Time‐to‐Event Outcomes of the Patients With PE Who Survived the Index Hospitalization Treated by CDT or ST Before and After Propensity Score Weighting
| Data Before IPTW* | Data After IPTW † | |||||
|---|---|---|---|---|---|---|
| Outcome | CDT (n=123) | ST (n=908) | CDT | ST | HR or SHR of CDT (95% CI) ‡ | P Value |
| 1‐y Outcome | ||||||
| All‐cause mortality | 16 (13.0) | 122 (13.4) | 12.2 | 13.2 | 0.73 (0.56–0.94) | 0.015 |
| Recurrent PE | 12 (9.8) | 160 (17.6) | 9.3 | 17.5 | 0.52 (0.41–0.66) | <0.001 |
| Heart failure | 3 (2.4) | 31 (3.4) | 3.6 | 3.4 | 1.17 (0.68–1.99) | 0.576 |
| New‐onset pulmonary hypertension | 1 (0.8) | 15 (1.7) | 0.3 | 1.6 | 0.20 (0.06–0.72) | 0.013 |
| As of the last follow‐up | ||||||
| All‐cause mortality | 34 (27.6) | 303 (33.4) | 29.5 | 32.8 | 0.84 (0.72–0.98) | 0.031 |
| Recurrent PE | 22 (17.9) | 219 (24.1) | 22.2 | 23.9 | 0.81 (0.68–0.97) | 0.021 |
| Heart failure | 4 (3.3) | 71 (7.8) | 4.2 | 7.6 | 0.55 (0.37–0.80) | 0.002 |
| New‐onset pulmonary hypertension | 5 (4.1) | 29 (3.2) | 1.8 | 3.1 | 0.58 (0.33–1.03) | 0.065 |
CDT indicates catheter‐directed thrombolysis; HR, hazard ratio; IPTW, inverse probability of treatment weighting; PE, pulmonary embolism; SHR, subdistribution HR; and ST, systemic thrombolysis.
Values are given as number (percentage).
Values are given as percentage.
Adjusted for sex, hyperlipidemia, hyperthyroidism, previous stroke, Charlson Comorbidity Index score, statin use, anticoagulant use, and intubation.
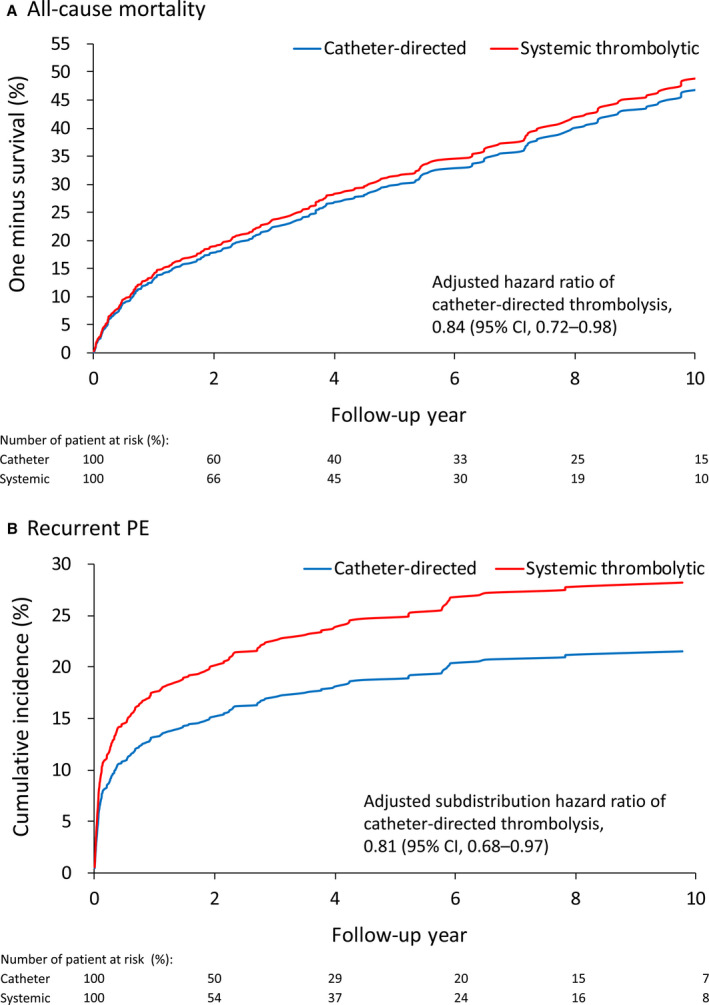
As of the last follow‐up, the all‐cause mortality rate and the recurrence rate of PE remained significantly lower in the CDT group than in the ST group after IPTW (Figure 2A and 2B). The incidence of heart failure was also lower in the CDT group, whereas there was no statistical significance in the difference in new‐onset pulmonary hypertension between the 2 groups (Table 3). In addition, the results showed that the assumption of proportional hazard was not violated with the statistical significance for the interaction term (time‐varying variable) of 0.750, 0.244, 0.755, and 0.457 for all‐cause mortality, recurrent PE, heart failure hospitalization, and new‐onset pulmonary hypertension, respectively.
Figure 2. Fitted survival curves of all‐cause mortality (A) and fitted cumulative incidence function of recurrent pulmonary embolism (PE) (B) of patients treated with catheter‐directed thrombolysis or systemic thrombolytic therapy in the inverse probability of treatment weighting–adjusted cohort.
Subgroup Analysis
The subgroup analysis of in‐hospital death showed that the beneficial effect of CDT was more apparent in patients with chronic kidney disease and patients with cardiogenic shock or intubation during the PE admission (P for interaction <0.05; Figure 3A). The subgroup analysis of 1‐year mortality (including in‐hospital death) and all‐cause mortality showed that female patients, patients with chronic kidney disease, and patients having cancer tended to benefit more from CDT (P for interaction <0.05; Figure 3B and 3C). The subgroup analysis of recurrent PE showed that the beneficial effect of CDT was more obvious in patients with cancer, patients with more comorbidities, and patients with cardiogenic shock during the PE admission (P for interaction <0.05; Figure 3D). However, the sample size of patients undergoing CDT was only 145; therefore, the aforementioned subgroup analysis may be underpowered.
Figure 3. Subgroup analysis comparing catheter‐directed thrombolysis with systemic thrombolytic therapy in terms of the risks of in‐hospital death (A), 1‐year mortality, including in‐hospital death (B), all‐cause mortality by the end of follow‐up (C), and recurrent pulmonary embolism (PE) (D) in the inverse probability of treatment weighting–adjusted cohort.
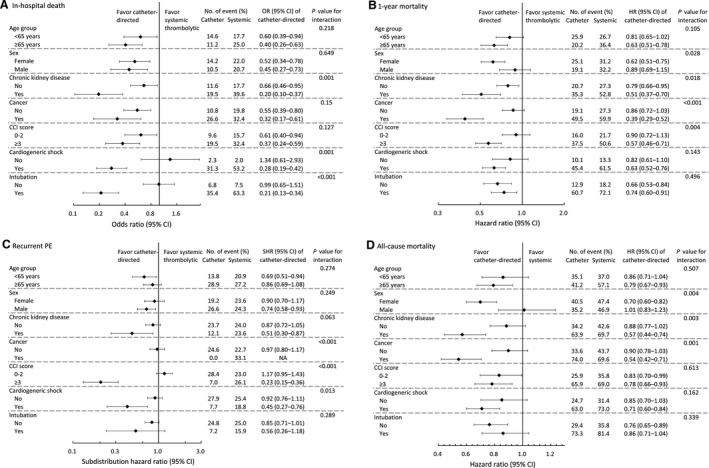
CCI indicates charlson comorbidity index; HR, hazard ratio; OR, odds ratio; and SHR, subdistribution HR.
Discussion
This is the first and largest observational study undertaken to compare the safety, short‐term efficacy, and midterm outcomes of CDT with those of ST in patients with acute PE. Previous studies showed that CDT could effectively reduce pulmonary artery pressures and improve right ventricular function, whereas the long‐term outcomes were less certain. 19 , 20 , 21 , 22 Most trials reported only up to 90‐day outcomes, and they either had no control group or merely compared CDT with anticoagulation alone. There were even rare observational studies comparing these 2 strategies, and they were only in short‐ and intermediate‐term prognosis 6 , 7 , 8 , 9 . In the current study, we clearly showed that the CDT group had both lower in‐hospital mortality and 1‐year mortality than the ST group. In fact, the survival benefit of CDT was maintained throughout the whole follow‐up period (Figure 2A). In contrast, Konstantinides et al reported that ST had no impact on mortality or symptoms at 3 years with long‐term follow‐up. 23 This different outcome‐related finding may come from the fundamental difference in the delivery of thrombolytic agents between CDT and ST. Because CDT can achieve a higher local concentration of thrombolytic agents, its efficacy in terms of thrombus resolution is greater. Our study showed that the CDT group had lower rates of recurrent PE and pulmonary hypertension, which were both important risk factors for mortality. It is important to treat acute PE as completely as possible at the first attack, or it may lead to grave cardiovascular outcomes.
Recurrent PE with subsequent pulmonary hypertension is another important issue after an acute PE episode. Recurrent PE may come from irregular or early termination of anticoagulation use. The latest European Society of Cardiology guideline now suggests extended anticoagulation therapy in almost all patients with acute PE to reduce the risk of recurrent PE. 3 However, Frederikus et al reported that the risk of recurrent PE events is still high even under long‐term anticoagulation use, especially in patients with unprovoked venous thromboembolic events. 24 Other than anticoagulation maintenance, the resolution of thrombus as early as possible seems to be a reasonable strategy to prevent further PE episodes. Previous studies showed that there was considerable residual thrombus after acute PE treatment, even when patients were symptom free with normal right atrium/right ventricle size in echocardiography. 25 , 26 The end point for acute PE treatment has yet to be standardized in current guidelines or the various previous studies. 25 , 26 Theoretically, CDT has higher local concentration and efficiency to clear thrombus in the pulmonary artery. In our study, we found that the recurrence rate of PE was significantly lower in the CDT cohort than in the ST cohort throughout the follow‐up period, which was similar to the finding with respect to all‐cause mortality. It is thus reasonable to suppose that the early resolution of pulmonary thrombus may prevent further PE events.
Beyond the risk of death and recurrent PE, previous studies showed that chronic thromboembolic pulmonary hypertension (CTEPH) may develop in survivors of PE. Vitorrio et al reported that the cumulative incidence of symptomatic CTEPH after acute PE treated with either naticoagulation or thrombolysis was 3.1% at 1 year and 3.8% at 2 years. 27 It is generally believed that CTEPH comes from repeated PE, which finally leads to pulmonary hypertension. Although CTEPH is now regarded as a critical disease leading to grave outcomes, it is important to treat patients early and even prevent them from developing CTEPH. We found that patients with acute PE accepting CDT not only had a lower recurrence of PE but also a lower rate of new‐onset pulmonary hypertension than those receiving ST. According to the definition of CTEPH, recurrent PE with pulmonary hypertension is not necessarily equivalent to but is highly associated with CTEPH. From the current study, it can be supposed that CDT may be an effective therapy for the prevention of CTEPH after acute PE, but this should be validated in future prospective studies.
Surprisingly, the expected superiority of CDT to ST in patients with PE in terms of bleeding events was not observed in our study. As mentioned above, the current guidelines reserve CDT for patients with PE requiring reperfusion therapy with higher bleeding risk. In our study, all the uses of CDT were performed with multi–side‐hole catheters, but the use of ultrasound‐assisted thrombolysis (USAT) was allowed in the PERFECT (Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis) trial. 20 USAT is a modified CDT using a proprietary system of local ultrasound waves to dissociate the thrombus, allowing for deeper penetration of lytic medication. Kuo et al reported that USAT was more efficient with reduced dosages of thrombolytic agents and shorter CDT courses than traditional CDT in treating patients with acute PE. Therefore, a lower major bleeding rate among patients with PE treated with USAT is expected, which may explain the difference between our study and the PERFECT trial.
Current guidelines suggest systemic thrombolytic therapy, not CDT, as the first‐line therapy for patients with PE requiring reperfusion therapy. In the current study, there was significant interaction in the subgroup analysis for in‐hospital mortality in certain critical circumstances, including chronic kidney disease, intubation, and shock. On the basis of our results, the application of CDT could be considered more often than the current guidelines suggest. There were similar benefits of CDT in the subgroup analysis for chronic kidney disease and cancer with respect to 1‐year and all‐cause mortality. Previous studies showed that there were 5 to 7 times the risk of venous thromboembolism and 3 times the rate of fatal PE in patients with cancer than in those without cancer. 28 , 29 , 30 , 31 Furthermore, thrombosis was confirmed to be one of the leading complications and the second leading cause of death in patients with cancer. 32 , 33
Limitations
This study had several limitations. First, the diseases in the NHIRD were identified using ICD‐9‐CM codes, and the database does not provide hemodynamic data, laboratory test, or image information. Accordingly, we could not stratify our patients into distinct risk groups as current guidelines suggest. However, we still detected cardiogenic shock, extracorporeal membrane oxygenation use, and intubation, which are related to the severity of PE. Second, there was no standard protocol of CDT with respect to the dosage and infusion time of thrombolytic agents, and it was impossible to extract the drug dosing data from our database. The different dosages and infusion times of thrombolytic agents might influence the efficacy and safety of CDT. Third, all the uses of CDT were performed with multi–side‐hole catheters, and there were no cases involving USAT included in our study. Meanwhile, given that newer generations of CDT devices for acute PE have propagated rapidly, our results may not truly reflect the current status of CDT. Moreover, the subgroup analysis may be underpowered because of the small sample size of patients undergoing CDT. Last, this was a retrospective observational database study, and the existence of numerous confounding factors in our study cohort may have influenced the results.
Conclusions
In our analysis, we found that for patients with PE, CDT might be more effective than ST in terms of reducing in‐hospital mortality, all‐cause mortality, and recurrent PE, whereas the bleeding risk of both forms of treatment is essentially equal. This result should be cautiously validated in further randomized trials to confirm our findings and guide clinical practice.
Sources of Funding
This research was supported by the Ministry of Science and Technology of Taiwan (MOST 108‐2221‐E‐002‐163 and MOST 109‐2221‐E‐002‐083) and National Taiwan University Hospital (107‐EDN11, 108‐N4406, 108EDN02, 109‐O20, 109‐S4579, and 109‐EDN11).
Disclosures
None.
Supporting information
Table S1
Acknowledgments
We thank Alfred Hsing‐Fen Lin for his assistance with the statistical analysis during the completion of the manuscript.
(J Am Heart Assoc. 2021;10:e019296. DOI: 10.1161/JAHA.120.019296.)
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Heng‐Hsu Lin, Email: henghsulin@gmail.com.
Jen‐Kuang Lee, Email: b85401104@gmail.com.
References
- 1. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118:1340–1347. DOI: 10.1161/CIRCRESAHA.115.306841. [DOI] [PubMed] [Google Scholar]
- 2. Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, Greer IA, Heit JA, Hutchinson JL, Kakkar AK, et al. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–764. [DOI] [PubMed] [Google Scholar]
- 3. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G‐J, Harjola V‐P, Huisman MV, Humbert M, Jennings CS, Jiménez D, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2019;54:1–61. DOI: 10.1183/13993003.01647-2019. [DOI] [PubMed] [Google Scholar]
- 4. Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. DOI: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 5. Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, Kumbhani DJ, Mukherjee D, Jaff MR, Giri J. Thrombolysis for pulmonary embolism and risk of all‐cause mortality, major bleeding, and intracranial hemorrhage: a meta‐analysis. JAMA. 2014;311:2414–2421. DOI: 10.1001/jama.2014.5990. [DOI] [PubMed] [Google Scholar]
- 6. Geller BJ, Adusumalli S, Pugliese SC, Khatana SAM, Nathan A, Weinberg I, Jaff MR, Kobayashi T, Mazurek JA, Khandhar S, et al. Outcomes of catheter‐directed versus systemic thrombolysis for the treatment of pulmonary embolism: a real‐world analysis of national administrative claims. Vasc Med. 2020;25:334–340. DOI: 10.1177/1358863X20903371. [DOI] [PubMed] [Google Scholar]
- 7. Beyer SE, Shanafelt C, Pinto DS, Weinstein JL, Aronow HD, Weinberg I, Yeh RW, Secemsky EA, Carroll BJ. Utilization and outcomes of thrombolytic therapy for acute pulmonary embolism: a Nationwide Cohort Study. Chest. 2020;157:645–653. DOI: 10.1016/j.chest.2019.10.049. [DOI] [PubMed] [Google Scholar]
- 8. Arora S, Panaich SS, Ainani N, Kumar V, Patel NJ, Tripathi B, Shah P, Patel N, Lahewala S, Deshmukh A, et al. Comparison of in‐hospital outcomes and readmission rates in acute pulmonary embolism between systemic and catheter‐directed thrombolysis (from the National Readmission Database). Am J Cardiol. 2017;120:1653–1661. DOI: 10.1016/j.amjcard.2017.07.066. [DOI] [PubMed] [Google Scholar]
- 9. Patel N, Patel NJ, Agnihotri K, Panaich SS, Thakkar B, Patel A, Savani C, Patel N, Arora S, Deshmukh A, et al. Utilization of catheter‐directed thrombolysis in pulmonary embolism and outcome difference between systemic thrombolysis and catheter‐directed thrombolysis. Catheter Cardiovasc Interv. 2015;86:1219–1227. DOI: 10.1002/ccd.26108. [DOI] [PubMed] [Google Scholar]
- 10. Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan national health insurance research database. JAMA Intern Med. 2015;175:1527–1529. DOI: 10.1001/jamainternmed.2015.3540. [DOI] [PubMed] [Google Scholar]
- 11. Lin LY, Warren‐Gash C, Smeeth L, Chen PC. Data resource profile: the National Health Insurance Research Database (NHIRD). Epidemiol Health. 2018;40:e2018062. DOI: 10.4178/epih.e2018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, Lai EC. Taiwan’s national health insurance research database: past and future. Clin Epidemiol. 2019;11:349–358. DOI: 10.2147/CLEP.S196293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung WS, Lin CL, Kao CH. Diabetes increases the risk of deep‐vein thrombosis and pulmonary embolism. Thromb Haemost. 2015;114:812–818. DOI: 10.1160/TH14-10-0868. [DOI] [PubMed] [Google Scholar]
- 14. Hsu WY, Lane HY, Lin CL, Kao CH. A population‐based cohort study on deep vein thrombosis and pulmonary embolism among schizophrenia patients. Schizophr Res. 2015;162:248–252. DOI: 10.1016/j.schres.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 15. Wang IK, Shen TC, Muo CH, Yen TH, Sung FC. Risk of pulmonary embolism in patients with end‐stage renal disease receiving long‐term dialysis. Nephrol Dial Transplant. 2017;32:1386–1393. DOI: 10.1093/ndt/gfw272. [DOI] [PubMed] [Google Scholar]
- 16. Wu CY, Chen YJ, Ho HJ, Hsu YC, Kuo KN, Wu MS, Lin JT. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1913. DOI: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 17. Normand SLT, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following an acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. DOI: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 18. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388–3414. DOI: 10.1002/sim.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Furfaro D, Stephens RS, Streiff MB, Brower R. Catheter‐directed thrombolysis for intermediate‐risk pulmonary embolism. Ann Am Thorac Soc. 2018;15:134–144. DOI: 10.1513/AnnalsATS.201706-467FR. [DOI] [PubMed] [Google Scholar]
- 20. Kuo WT, Banerjee A, Kim PS, DeMarco FJ, Levy JR, Facchini FR, Unver K, Bertini MJ, Sista AK, Hall MJ, et al. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest. 2015;148:667–673. DOI: 10.1378/chest.15-0119. [DOI] [PubMed] [Google Scholar]
- 21. Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV. Catheter‐directed therapy for the treatment of massive pulmonary embolism: systematic review and meta‐analysis of modern techniques. J Vasc Interv Radiol. 2009;20:1431–1440. DOI: 10.1016/j.jvir.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 22. Mostafa A, Briasoulis A, Telila T, Belgrave K, Grines C. Treatment of massive or submassive acute pulmonary embolism with catheter‐directed thrombolysis. Am J Cardiol. 2016;117:1014–1020. DOI: 10.1016/j.amjcard.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 23. Konstantinides SV, Vicaut E, Danays T, Becattini C, Bertoletti L, Beyer‐Westendorf J, Bouvaist H, Couturaud F, Dellas C, Duerschmied D, et al. Impact of thrombolytic therapy on the long‐term outcome of intermediate‐risk pulmonary embolism. J Am Coll Cardiol. 2017;69:1536–1544. DOI: 10.1016/j.jacc.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 24. Klok FA, Zondag W, van Kralingen KW, van Dijk AP, Tamsma JT, Heyning FH, Vliegen HW, Huisman MV. Patient outcomes after acute pulmonary embolism: a pooled survival analysis of different adverse events. Am J Respir Crit Care Med. 2010;181:501–506. DOI: 10.1164/rccm.200907-1141OC. [DOI] [PubMed] [Google Scholar]
- 25. Pesavento R, Filippi L, Palla A, Visonà A, Bova C, Marzolo M, Porro F, Villalta S, Ciammaichella M, Bucherini E, et al. Impact of residual pulmonary obstruction on the long‐term outcome of patients with pulmonary embolism. Eur Respir J. 2017;49:1601980. DOI: 10.1183/13993003.01980-2016. [DOI] [PubMed] [Google Scholar]
- 26. Robin P, Eddy M, Sikora L, Le Roux P‐Y, Carrier M, Couturaud F, Planquette B, Pesavento R, Rodger M, Salaun P‐Y, et al. Residual pulmonary vascular obstruction and recurrence after acute pulmonary embolism: protocol for a systematic review and meta‐analysis of individual participant data. BMJ Open. 2018;8:e023939. DOI: 10.1136/bmjopen-2018-023939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pengo V, Lensing AWA, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, Biasiolo A, Pegoraro C, Iliceto S, et al. Thromboembolic pulmonary hypertension study group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–2264. DOI: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 28. Næss IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population‐based study. J Thromb Haemost. 2007;5:692–699. DOI: 10.1111/j.1538-7836.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 29. Connolly GC, Francis CW. Cancer‐associated thrombosis. Hematology Am Soc Hematol Educ Program. 2013;2013:684–691. DOI: 10.1182/asheducation-2013.1.684. [DOI] [PubMed] [Google Scholar]
- 30. Agnelli G, Verso M. Management of venous thromboembolism in patients with cancer. J Thromb Haemost. 2011;9:316–324. DOI: 10.1111/j.1538-7836.2011.04346.x. [DOI] [PubMed] [Google Scholar]
- 31. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. DOI: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 32. Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6:401–410. DOI: 10.1016/S1470-2045(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 33. Noble S, Pasi J. Epidemiology and pathophysiology of cancer‐associated thrombosis. Br J Cancer. 2010;102:S2–S9. DOI: 10.1038/sj.bjc.6605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


